Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review
Abstract
:1. Introduction
2. Au NC-Involved Biosensing Applications
2.1. Ions
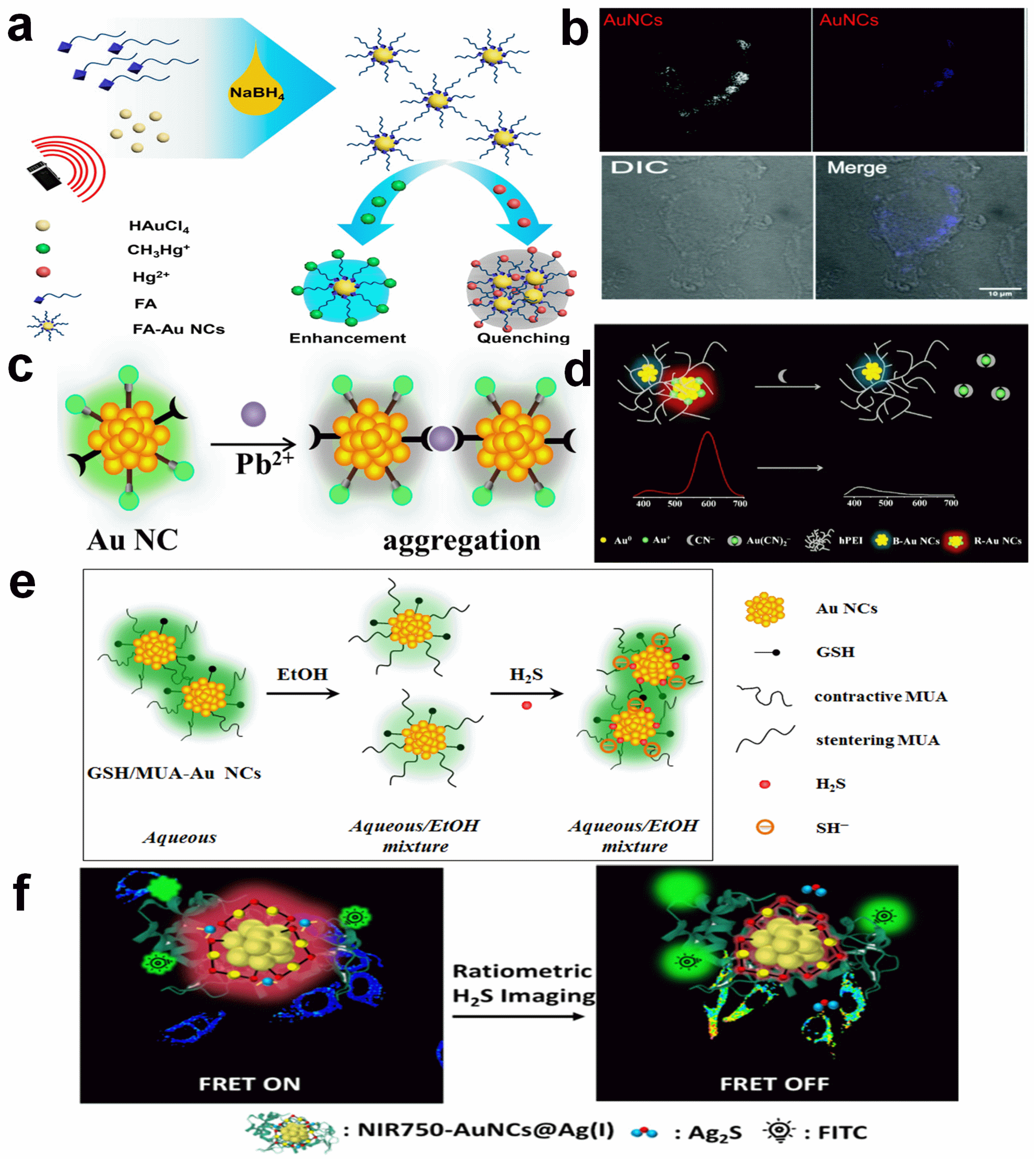
2.2. Small Molecules
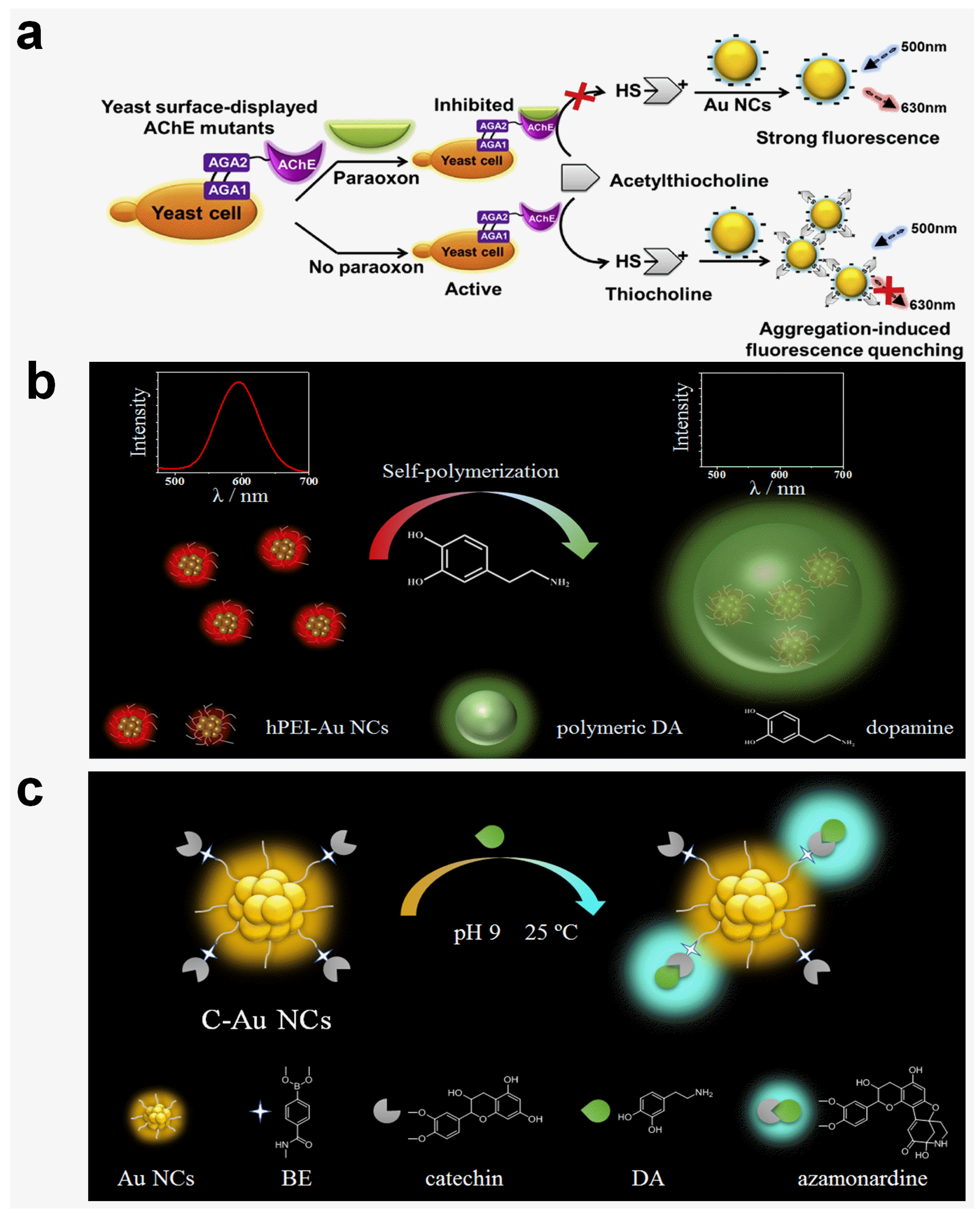
2.3. Reactive Oxygen Species
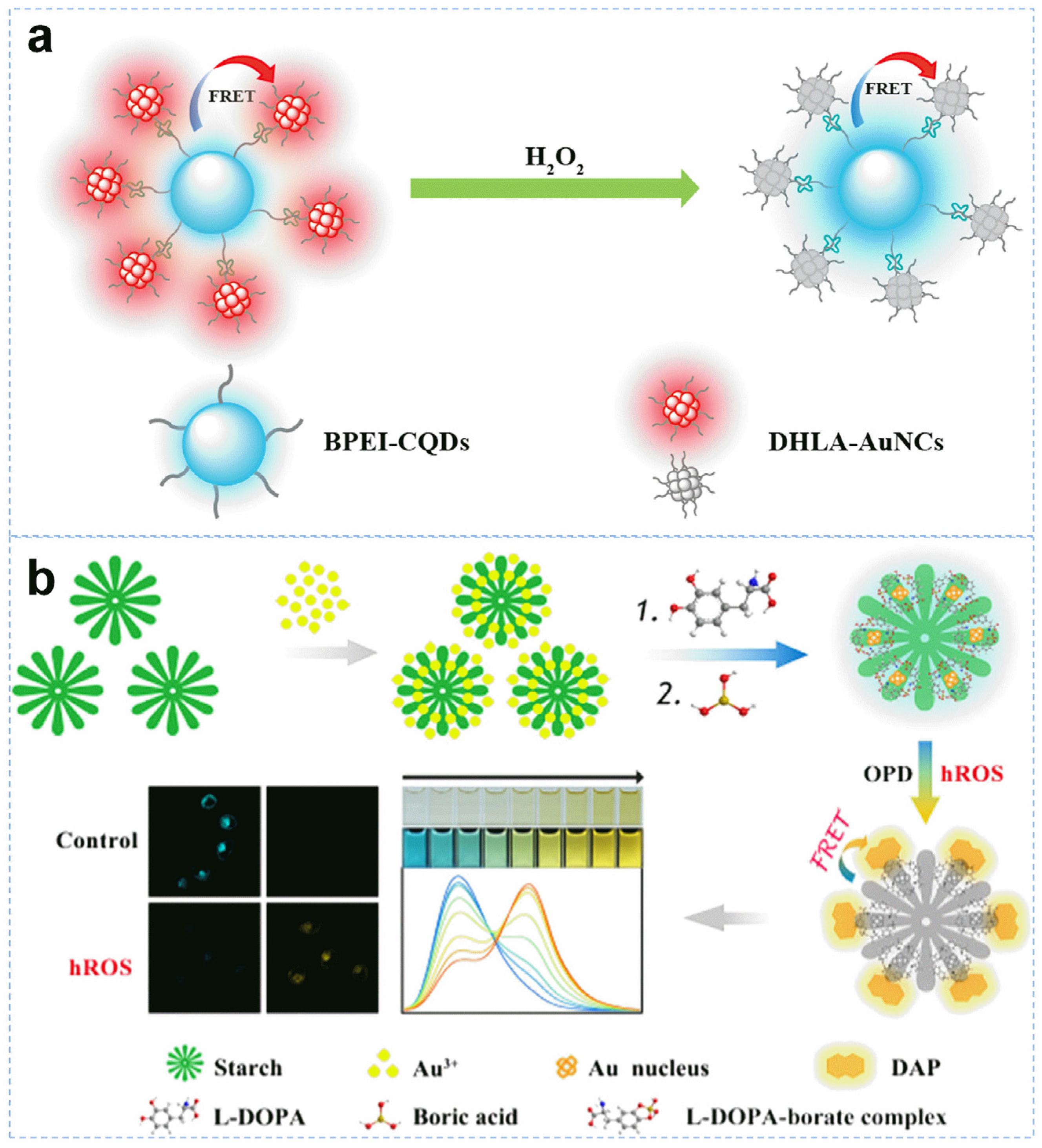
2.4. Biomacromolecules

2.5. Cancer Cells
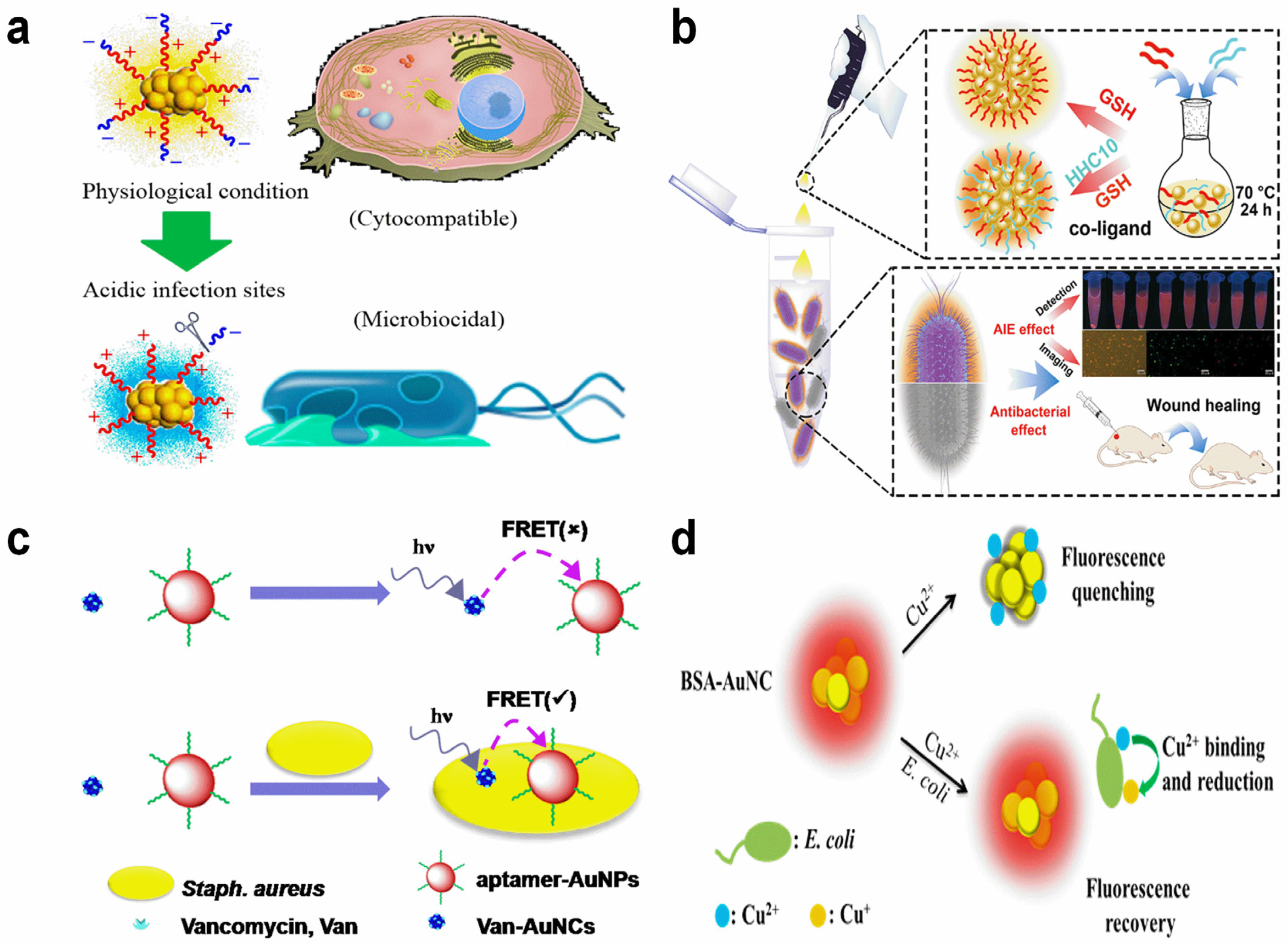
2.6. Bacteria
3. Au NC-Based Therapeutic Applications
3.1. Photodynamic Therapy
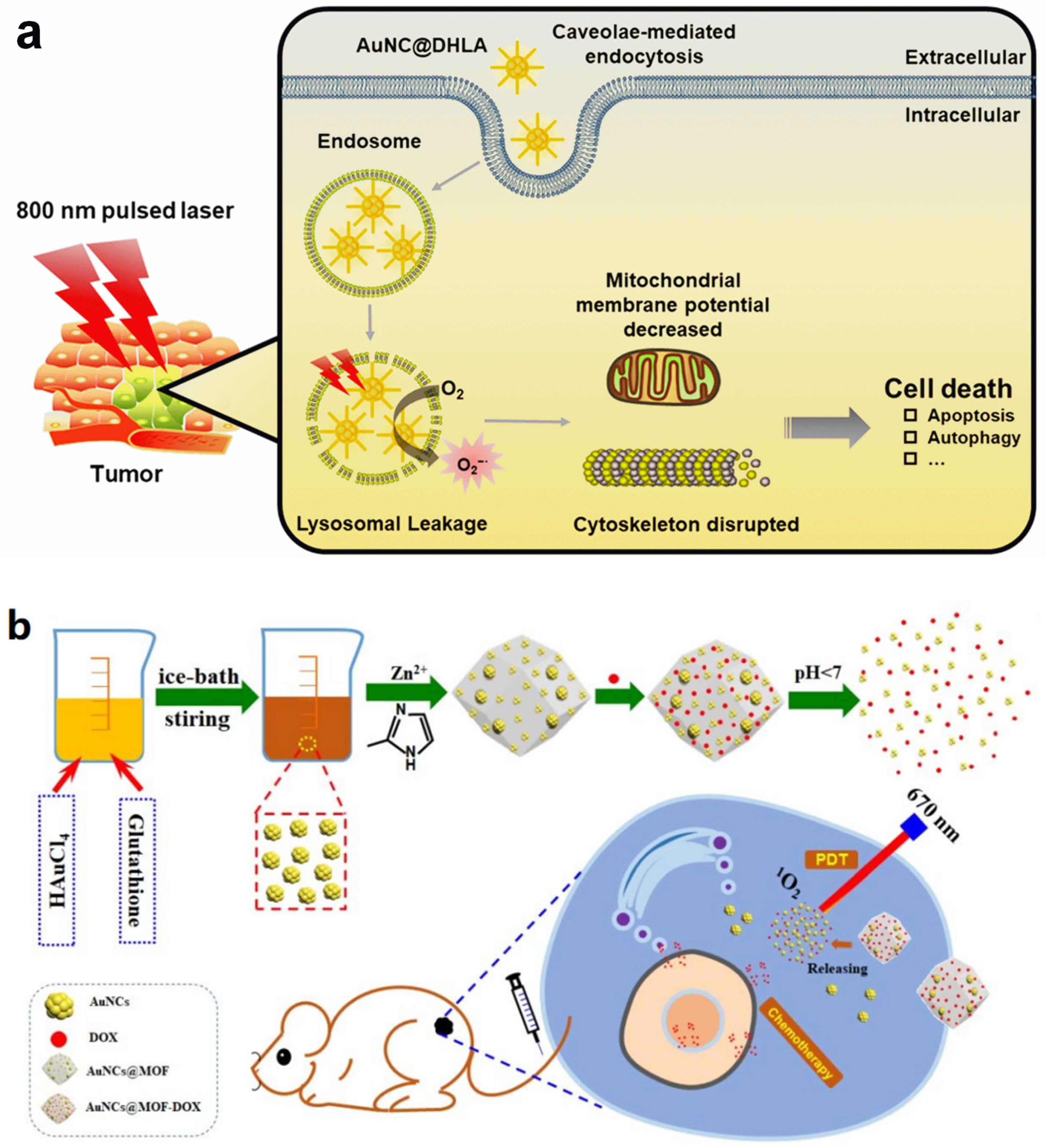
3.2. Photothermal Therapy
3.3. Drug Carrier
4. Conclusions and Perspectives
- Highly fluorescent Au NCs in the NIR II region.
- Ultrastable Au NCs against ROS and hROS.
- Super-resolution imaging of single Au NCs.
- The mechanism of the aggregation-enhanced emission (AIE) of Au NCs.
- Nano–bio interface-enhanced enzymatic analysis.
- Waterborne and precise Au NCs from hydrophobic ligand protection.
- In situ formation of Au NCs for diagnosis and therapy.
Author Contributions
Funding
Conflicts of Interest
References
- Goddard, Z.R.; Marín, M.J.; Russell, D.A.; Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 2020, 49, 8774–8789. [Google Scholar] [CrossRef] [PubMed]
- Greskovich, K.M.; Powderly, K.M.; Kincanon, M.M.; Forney, N.B.; Jalomo, C.A.; Wo, A.; Murphy, C.J. The Landscape of Gold Nanocrystal Surface Chemistry. Acc. Chem. Res. 2023, 56, 1553–1564. [Google Scholar] [CrossRef]
- Li, C.-H.; Chan, M.-H.; Chang, Y.-C.; Hsiao, M. Gold Nanoparticles as a Biosensor for Cancer Biomarker Determination. Molecules 2023, 28, 364. [Google Scholar] [CrossRef]
- Tian, M.; Yuan, Z.; Liu, Y.; Lu, C.; Ye, Z.; Xiao, L. Recent advances of plasmonic nanoparticle-based optical analysis in homogeneous solution and at the single-nanoparticle level. Analyst 2020, 145, 4737–4752. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xiong, H.; Wang, X.; Jiang, H. Gold nanomaterials: Important vectors in biosensing of breast cancer biomarkers. Anal. Bioanal. Chem. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Xi, Z.; Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Role of Surface Curvature in Gold Nanostar Properties and Applications. ACS Biomater. Sci. Eng. 2024, 10, 38–50. [Google Scholar] [CrossRef]
- Ye, J.; Wen, Q.; Wu, Y.; Fu, Q.; Zhang, X.; Wang, J.; Gao, S.; Song, J. Plasmonic anisotropic gold nanorods: Preparation and biomedical applications. Nano Res. 2022, 15, 6372–6398. [Google Scholar] [CrossRef]
- Zhou, R.; Luo, H.; Peng, C.; Guo, X.; Zhao, T.; Du, Y.; Xu, D.; Lin, Q. Rapid synthesis of concave gold nanocubes with tunable indentations and high index facets for enhanced catalytic performance. Chem. Eng. J. 2023, 470, 144044. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, L.; Jin, R. Visible to NIR-II Photoluminescence of Atomically Precise Gold Nanoclusters. Adv. Mater. 2024, 36, 2309073. [Google Scholar] [CrossRef]
- Cheng, D.; Liu, R.; Hu, K. Gold nanoclusters: Photophysical properties and photocatalytic applications. Front. Chem. 2022, 10, 958626. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, W.; Zhang, X.; Lin, D.; Wu, J. Nanomedicine as a promising strategy for the theranostics of infectious diseases. J. Mater. Chem. B 2021, 9, 7878–7908. [Google Scholar] [CrossRef]
- Guan, Z.-J.; Li, J.-J.; Hu, F.; Wang, Q.-M. Structural Engineering toward Gold Nanocluster Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202209725. [Google Scholar] [CrossRef]
- Vilian, A.E.; Mohammadi, A.; Han, S.; Tiwari, J.N.; Kumar, K.; Kumar, A.S.; Saravanan, A.; Huh, Y.S.; Han, Y.-K. Gold nanoclusters supported Molybdenum diselenide-porous carbon composite as an efficient electrocatalyst for selective ultrafast probing of chlorpyrifos-pesticide. Chem. Eng. J. 2023, 472, 145048. [Google Scholar] [CrossRef]
- Zhang, B.; Xia, C.; Hu, J.; Sheng, H.; Zhu, M. Structure control and evolution of atomically precise gold clusters as heterogeneous precatalysts. Nanoscale 2024, 16, 1526–1538. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, T.; Chen, J.; Shi, J.; Wang, L.; Shen, J.; Liu, X. Water-Dispersible Gold Nanoclusters: Synthesis Strategies, Optical Properties, and Biological Applications. Chem. Eur. J. 2022, 28, e202103736. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Yuan, Z.; Yang, Y.-Y.; Huang, C.-C.; Chang, H.-T. Photoluminescent gold nanodots: Role of the accessing ligands. RSC Adv. 2014, 4, 33629–33635. [Google Scholar] [CrossRef]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold Nanoclusters: Imaging, Therapy, and Theranostic Roles in Biomedical Applications. Bioconjug. Chem. 2022, 33, 4–23. [Google Scholar] [CrossRef]
- Cifuentes-Rius, A.; Deepagan, V.G.; Xie, J.; Voelcker, N.H. Bright Future of Gold Nanoclusters in Theranostics. Acs Appl. Mater. Interfaces 2021, 13, 49581–49588. [Google Scholar] [CrossRef]
- El-Sayed, N.; Schneider, M. Advances in biomedical and pharmaceutical applications of protein-stabilized gold nanoclusters. J. Mater. Chem. B 2020, 8, 8952–8971. [Google Scholar] [CrossRef] [PubMed]
- Santhoshkumar, S.; Madhu, M.; Tseng, W.-B.; Tseng, W.-L. Gold nanocluster-based fluorescent sensors for in vitro and in vivo ratiometric imaging of biomolecules. Phys. Chem. Chem. Phys. 2023, 25, 21787–21801. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.M.; Niazi, S.; Akhtar, W.; Yue, L.; Pasha, I.; Khan, M.K.I.; Mohsin, A.; Iqbal, M.W.; Zhang, Y.; Wang, Z. Surface functionalized AuNCs optical biosensor as an emerging food safety indicator: Fundamental mechanism to future prospects. Coord. Chem. Rev. 2023, 474, 214842. [Google Scholar] [CrossRef]
- Ni, S.; Liu, Y.; Tong, S.; Li, S.; Song, X. Emerging NIR-II Luminescent Gold Nanoclusters for In Vivo Bioimaging. J. Anal. Test. 2023, 7, 260–271. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Li, Q.; Tan, L.-L.; Liu, C.-G.; Shang, L. Metal–Organic Frameworks-Mediated Assembly of Gold Nanoclusters for Sensing Applications. J. Anal. Test. 2022, 6, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-J.; Liu, W.-C.; Lu, F.-N.; Tang, Y.; Yuan, Z.-Q. Recent Progress in Fluorescent Formaldehyde Detection Using Small Molecule Probes. J. Anal. Test. 2022, 6, 204–215. [Google Scholar] [CrossRef]
- Guo, Y.; Amunyela, H.T.N.N.; Cheng, Y.; Xie, Y.; Yu, H.; Yao, W.; Li, H.-W.; Qian, H. Natural protein-templated fluorescent gold nanoclusters: Syntheses and applications. Food Chem. 2021, 335, 127657. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.; Khan, I.M.; Akhtar, W.; ul Haq, F.; Pasha, I.; Khan, M.K.I.; Mohsin, A.; Ahmad, S.; Zhang, Y.; Wang, Z. Aptamer functionalized gold nanoclusters as an emerging nanoprobe in biosensing, diagnostic, catalysis and bioimaging. Talanta 2024, 268, 125270. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shu, T.; Su, L.; Zhang, X. Strategies of Luminescent Gold Nanoclusters for Chemo-/Bio-Sensing. Molecules 2019, 24, 3045. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Y.; Yang, T.; Wang, X.-Y.; Chen, M.-L.; Yu, Y.-L.; Wang, J.-H. Mercury Speciation with Fluorescent Gold Nanocluster as a Probe. Anal. Chem. 2018, 90, 6945–6951. [Google Scholar] [CrossRef]
- Chakraborty, S.; Nandy, A.; Ghosh, S.; Das, N.K.; Parveen, S.; Datta, S.; Mukherjee, S. Protein-templated gold nanoclusters as specific bio-imaging probes for the detection of Hg(ii) ions in in vivo and in vitro systems: Discriminating between MDA-MB-231 and MCF10A cells. Analyst 2021, 146, 1455–1463. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, M.; He, Y.; Yeung, E.S. Functionalized fluorescent gold nanodots: Synthesis and application for Pb2+ sensing. Chem. Commun. 2011, 47, 11981–11983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, W.; Yang, L.; Zhao, J.; Han, G.; Yu, X.; ShenTu, X.; Ye, Z. Surface engineered bimetallic gold/silver nanoclusters for in situ imaging of mercury ions in living organisms. Anal. Bioanal. Chem. 2022, 414, 4235–4244. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Babaee, E.; Gholivand, M.-B.; Molaabasi, F.; Mousavi, F.; Barati, A.; Hajipour Verdom, B.; Shojaedin-Givi, B.; Naderi-Manesh, H. Bright Green Light-Emitting Gold Nanoclusters Confined in Insulin as Selective Fluorescent Switch Probes for Sensing and Imaging of Copper Ions and Glutathione. ACS Appl. Nano Mater. 2023, 6, 5939–5951. [Google Scholar] [CrossRef]
- Liang, Q.-Y.; Wang, C.; Li, H.-W.; Wu, Y. A ratiometric luminescence probe for selective detection of Ag+ based on thiolactic acid–capped gold nanoclusters with near-infrared emission and employing bovine serum albumin as a signal amplifier. Microchim. Acta 2023, 190, 374. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, Y.; Liu, S.; Zhan, X.; Zhou, H.; Li, X.; Yuan, Z. Ratiometric and sensitive cyanide sensing using dual-emissive gold nanoclusters. Anal. Bioanal. Chem. 2020, 412, 5819–5826. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Q.; Wang, C.; Yang, K.; Hong, Z.; Chen, Q.; Song, J.; Song, X.; Yang, H. Near-Infrared II Gold Nanocluster Assemblies with Improved Luminescence and Biofate for In Vivo Ratiometric Imaging of H2S. Anal. Chem. 2022, 94, 2641–2647. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Yang, H.; Tang, Y.; Yu, C.-J.; Wang, G.; Yuan, Z.; Quan, H. 11-Mercaptoundecanoic acid capped gold nanoclusters with unusual aggregation-enhanced emission for selective fluorometric hydrogen sulfide determination. Microchim. Acta 2020, 187, 200. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, M.; Shi, L.; Du, Y.; Cai, N.; He, Y.; Chang, H.-T.; Yeung, E.S. Disassembly mediated fluorescence recovery of gold nanodots for selective sulfide sensing. Nanoscale 2013, 5, 4683–4686. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Zheng, L.R.; He, W.; Ren, X.; Jiang, C.; Lu, L. In Situ Formation of Phosphorescent Molecular Gold(I) Cluster in a Macroporous Polymer Film to Achieve Colorimetric Cyanide Sensing. Anal. Chem. 2014, 86, 1687–1692. [Google Scholar] [CrossRef]
- Chuang, C.-H.; Chen, W.-Y.; Tseng, W.-B.; Lin, A.; Lu, C.-Y.; Tseng, W.-L. Microwave-Mediated Synthesis of Near-Infrared-Emitting Silver Ion-Modified Gold Nanoclusters for Ratiometric Sensing of Hydrosulfide in Environmental Water and Hydrogen Sulfide in Live Cells. ACS Sustain. Chem. Eng. 2022, 10, 2461–2472. [Google Scholar] [CrossRef]
- Yang, L.; Lou, X.; Yu, F.; Liu, H. Cross-linking structure-induced strong blue emissive gold nanoclusters for intracellular sensing. Analyst 2019, 144, 2765–2772. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, Y.; Hou, G.; Ran, X.; Chi, Z.; He, Y.; Kuang, Y.; Wang, X.; Liu, R.; Guo, L. Excellent Multiphoton Excitation Fluorescence with Large Multiphoton Absorption Cross Sections of Arginine-Modified Gold Nanoclusters for Bioimaging. ACS Appl. Mater. Interfaces 2022, 14, 2452–2463. [Google Scholar] [CrossRef]
- Wu, F.-N.; Zhu, J.; Weng, G.-J.; Li, J.-J.; Zhao, J.-W. Tyrosine-Decorated Gold Nanoclusters Chelated Cerium(III) for Fluorescence Detection of Dopamine. ACS Appl. Nano Mater. 2021, 4, 13501–13509. [Google Scholar] [CrossRef]
- Liang, B.; Han, L. Displaying of acetylcholinesterase mutants on surface of yeast for ultra-trace fluorescence detection of organophosphate pesticides with gold nanoclusters. Biosens. Bioelectron. 2020, 148, 111825. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Hu, Q.; Yi, S.; Chi, Y.; Xiao, Y. Development of an Au nanoclusters based activatable nanoprobe for NIR-II fluorescence imaging of gastric acid. Biosens. Bioelectron. 2023, 224, 115062. [Google Scholar] [CrossRef]
- Lou, X.; Yu, F.; Cao, Z.; Xu, Y.; Yang, L.; Liu, H. Surface motif sensitivity of dual emissive gold nanoclusters for robust ratiometric intracellular imaging. Chem. Commun. 2020, 56, 7112–7115. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yin, H.; Li, Y.; Zheng, S.; Gan, F.; Ye, G. One-step synthesis of enzyme-stabilized gold nanoclusters for fluorescent ratiometric detection of hydrogen peroxide, glucose and uric acid. Microchem. J. 2018, 141, 431–437. [Google Scholar] [CrossRef]
- Sang, F.; Li, M.; Yin, S.; Shi, H.; Zhao, Y.; Zhang, Z. Highly sensitive and selective detection and intracellular imaging of glutathione using MnO2 nanosheets assisted enhanced fluorescence of gold nanoclusters. Spectrochim. Acta Part A 2021, 256, 119743. [Google Scholar] [CrossRef]
- Li, W.; Xingzhuo, Z.; Yan, W.; Wang, R.; Yang, Z.; Hu, Y.; Liu, Y.; Jia, Z.; Li, Y. Lysozyme-encapsulated gold nanoclusters for ultrasensitive detection of folic acid and in vivo imaging. Talanta 2023, 251, 123789. [Google Scholar] [CrossRef]
- Bai, H.-J.; Qi, D.-Y.; Li, H.-W.; Wu, Y. Assembly-Induced Emission Enhancement in Glutathione-Capped Bimetallic Gold and Copper Nanoclusters by Al3+ Ions and Further Application in Myricetin Determination. Molecules 2023, 28, 758. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, H.; Pandey, B.; James, T.D.; Yoon, J.; Anslyn, E.V. Recent advances in fluorescent and colorimetric chemosensors for the detection of chemical warfare agents: A legacy of the 21st century. Chem. Soc. Rev. 2023, 52, 663–704. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Huang, H.; Kang, X.; Yang, L.; Xi, Z.; Sun, H.; Pluth, M.D.; Yi, L. NBD-based synthetic probes for sensing small molecules and proteins: Design, sensing mechanisms and biological applications. Chem. Soc. Rev. 2021, 50, 7436–7495. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Lu, F.; Peng, M.; Wang, C.-W.; Tseng, Y.-T.; Du, Y.; Cai, N.; Lien, C.-W.; Chang, H.-T.; He, Y.; et al. Selective Colorimetric Detection of Hydrogen Sulfide Based on Primary Amine-Active Ester Cross-Linking of Gold Nanoparticles. Anal. Chem. 2015, 87, 7267–7273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Liu, Y.; Liu, W.; Lu, F.; Yuan, Z.; Lu, C. Gold Nanocluster-Encapsulated Hyperbranched Polyethyleneimine for Selective and Ratiometric Dopamine Analyses by Enhanced Self-Polymerization. Front. Chem. 2022, 10, 928607. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, J.; Zhang, X.; Zhang, X.; Yang, B.; Deng, F.; Li, Z.; Wang, K.; Yang, Y.; Wei, Y. Self-polymerization of dopamine and polyethyleneimine: Novel fluorescent organic nanoprobes for biological imaging applications. J. Mater. Chem. B 2015, 3, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Zhang, J.; Zheng, J.; Yuan, Z.; Lu, C. Catechin-inspired gold nanocluster nanoprobe for selective and ratiometric dopamine detection via forming azamonardine. Spectrochim. Acta Part A 2022, 274, 121142. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lu, F.; Sun, Y.; Yuan, Z.; Lu, C. Fluorescent Gold Nanocluster-Based Sensor Array for Nitrophenol Isomer Discrimination via an Integration of Host-Guest Interaction and Inner Filter Effect. Anal. Chem. 2018, 90, 12846–12853. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, S.; Yuan, Z.; Lu, C. Carbon quantum dot-gold nanocluster nanosatellite for ratiometric fluorescence probe and imaging for hydrogen peroxide in living cells. Sens. Actuators B-Chem. 2017, 241, 821–827. [Google Scholar] [CrossRef]
- Xie, Y.; Xianyu, Y.; Wang, N.; Yan, Z.; Liu, Y.; Zhu, K.; Hatzakis, N.S.; Jiang, X. Functionalized Gold Nanoclusters Identify Highly Reactive Oxygen Species in Living Organisms. Adv. Funct. Mater. 2018, 28, 1702026. [Google Scholar] [CrossRef]
- Fang, H.; Yu, H.; Lu, Q.; Fang, X.; Zhang, Q.; Zhang, J.; Zhu, L.; Ma, Q. A New Ratiometric Fluorescent Probe for Specific Monitoring of hROS under Physiological Conditions Using Boric Acid-Protected L-DOPA Gold Nanoclusters. Anal. Chem. 2020, 92, 12825–12832. [Google Scholar] [CrossRef]
- Quan, Z.; Xue, F.; Li, H.; Chen, Z.; Wang, L.; Zhu, H.; Pang, C.; He, H. A bioinspired ratiometric fluorescence probe based on cellulose nanocrystal-stabilized gold nanoclusters for live-cell and zebrafish imaging of highly reactive oxygen species. Chem. Eng. J. 2022, 431, 133954. [Google Scholar] [CrossRef]
- Zhuang, Q.-Q.; Deng, H.-H.; He, S.-B.; Peng, H.-P.; Lin, Z.; Xia, X.-H.; Chen, W. Immunoglobulin G-Encapsulated Gold Nanoclusters as Fluorescent Tags for Dot-Blot Immunoassays. ACS Appl. Mater. Interfaces 2019, 11, 31729–31734. [Google Scholar] [CrossRef]
- Lu, F.; Yang, H.; Yuan, Z.; Nakanishi, T.; Lu, C.; He, Y. Highly fluorescent polyethyleneimine protected Au8 nanoclusters: One-pot synthesis and application in hemoglobin detection. Sens. Actuators B 2019, 291, 170–176. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, F.; Wang, D.; Xiong, D.; Yan, S.; Liu, S.; Tang, H. Fabrication of avidin-stabilized gold nanoclusters with dual emissions and their application in biosensing. J. Nanobiotechnol. 2022, 20, 306. [Google Scholar] [CrossRef]
- Li, B.; Yu, S.; Feng, R.; Qian, Z.; He, K.; Mao, G.-J.; Cao, Y.; Tang, K.; Gan, N.; Wu, Y.-X. Dual-Mode Gold Nanocluster-Based Nanoprobe Platform for Two-Photon Fluorescence Imaging and Fluorescence Lifetime Imaging of Intracellular Endogenous miRNA. Anal. Chem. 2023, 95, 14925–14933. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Du, Y.; Tseng, Y.-T.; Peng, M.; Cai, N.; He, Y.; Chang, H.-T.; Yeung, E.S. Fluorescent Gold Nanodots Based Sensor Array for Proteins Discrimination. Anal. Chem. 2015, 87, 4253–4259. [Google Scholar] [CrossRef]
- Xu, S.; Li, W.; Zhao, X.; Wu, T.; Cui, Y.; Fan, X.; Wang, W.; Luo, X. Ultrahighly Efficient and Stable Fluorescent Gold Nanoclusters Coated with Screened Peptides of Unique Sequences for Effective Protein and Serum Discrimination. Anal. Chem. 2019, 91, 13947–13952. [Google Scholar] [CrossRef]
- Fan, D.; Ou, J.; Chen, L.; Zhang, L.; Zheng, Z.; Yu, H.; Meng, X.; Zhu, M. An Oligopeptide-Protected Ultrasmall Gold Nanocluster with Peroxidase-Mimicking and Cellular-Imaging Capacities. Molecules 2023, 28, 70. [Google Scholar] [CrossRef]
- Feng, B.; Xing, Y.; Lan, J.; Su, Z.; Wang, F. Synthesis of MUC1 aptamer-stabilized gold nanoclusters for cell-specific imaging. Talanta 2020, 212, 120796. [Google Scholar] [CrossRef]
- Hada, A.-M.; Craciun, A.-M.; Focsan, M.; Borlan, R.; Soritau, O.; Todea, M.; Astilean, S. Folic acid functionalized gold nanoclusters for enabling targeted fluorescence imaging of human ovarian cancer cells. Talanta 2020, 225, 121960. [Google Scholar] [CrossRef]
- Chandra, A.; Bhoge, P.R.; Remya, K.R.; Shanthamurthy, C.D.; Kikkeri, R. Fluorescent glyco-gold nanocluster induced EGFR mediated targeting of cancer cells. Chem. Commun. 2023, 59, 1213–1216. [Google Scholar] [CrossRef] [PubMed]
- Chiechio, R.M.; Le Guevél, R.; Ducarre, S.; Solhi, H.; Dutertre, S.; Pinson, X.; Bazureau, J.-P.; Mignen, O.; Even-Hernandez, P.; Musumeci, P.; et al. Active U11 Peptide Luminescent Gold Nanoclusters for Pancreatic Tumor Cell Targeting. ACS Appl. Nano Mater. 2023, 6, 8971–8980. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.; Wang, Y.; Xu, S.; James, T.D.; Wang, L. Stepwise-Enhanced Tumor Targeting of Near-Infrared Emissive Au Nanoclusters with High Quantum Yields and Long-Term Stability. Anal. Chem. 2022, 94, 13189–13196. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Jia, N.; Luo, J.; Li, L.; Xue, J.; Bu, H.; Xie, G.; Wan, Y. DNA Nanoribbon-Assisted Intracellular Biosynthesis of Fluorescent Gold Nanoclusters for Cancer Cell Imaging. JACS Au 2023, 3, 2566–2577. [Google Scholar] [CrossRef] [PubMed]
- Pranantyo, D.; Liu, P.; Zhong, W.; Kang, E.-T.; Chan-Park, M.B. Antimicrobial Peptide-Reduced Gold Nanoclusters with Charge-Reversal Moieties for Bacterial Targeting and Imaging. Biomacromolecules 2019, 20, 2922–2933. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zheng, C.; Wu, Q.; Wu, Q.; Jin, M.; Jiang, Y.; Huang, F.; Lou, Y.; Zheng, L. One-step synthesized antimicrobial peptide-functionalized gold nanoclusters for selective imaging and killing of pathogenic bacteria. Front. Microbiol. 2022, 13, 1003359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, B.; Zhang, Z.; Chen, Y.; Zhu, L.; Zhang, Y.; Huang, H.; Jiang, L. Multifunctional fluorescent gold nanoclusters with enhanced aggregation-induced emissions (AIEs) and excellent antibacterial effect for bacterial imaging and wound healing. Biomater. Adv. 2022, 137, 212841. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wang, H.; Fu, F.; Li, L.; Li, J.; Li, G.; Song, Y.; Swihart, M.T.; Song, E. Dual-Recognition Forster Resonance Energy Transfer Based Platform for One-Step Sensitive Detection of Pathogenic Bacteria Using Fluorescent Vancomycin-Gold Nanoclusters and Aptamer-Gold Nanoparticles. Anal. Chem. 2017, 89, 4085–4090. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Shou, Z.; Chen, J.; Wu, H.; Zhao, Y.; Qiu, L.; Jiang, P.; Mou, X.-Z.; Wang, J.; Li, Y.-Q. On-Off-On Gold Nanocluster-Based Fluorescent Probe for Rapid Escherichia coli Differentiation, Detection and Bactericide Screening. ACS Sustain. Chem. Eng. 2018, 6, 4504–4509. [Google Scholar] [CrossRef]
- Gao, N.; Zhang, Y.; Huang, P.; Xiang, Z.; Wu, F.Y.; Mao, L. Perturbing Tandem Energy Transfer in Luminescent Heterobinuclear Lanthanide Coordination Polymer Nanoparticles Enables Real-Time Monitoring of Release of the Anthrax Biomarker from Bacterial Spores. Anal. Chem. 2018, 90, 7004–7011. [Google Scholar] [CrossRef]
- Kefayat, A.; Ghahremani, F.; Motaghi, H.; Amouheidari, A. Ultra-small but ultra-effective: Folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, S.; Wang, L.; Liu, G.; Cheng, Y.; Lin, M.; Sui, K.; Zhang, H. Alginate mediated functional aggregation of gold nanoclusters for systemic photothermal therapy and efficient renal clearance. Carbohydr. Polym. 2020, 241, 116344. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ji, Q.; Wu, D.; Li, Z.; Fan, M.; Zhang, H.; Zhao, X.; Wu, A.; Cheng, L.; Zeng, L. H2O2-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for “off/on” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 14928–14937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, H.; Li, H.; Zhang, T.; Zhang, J.; Guo, W.; Fu, K.; Du, G. Magnetic and near-infrared-II fluorescence Au–Gd nanoclusters for imaging-guided sensitization of tumor radiotherapy. Nanoscale Adv. 2022, 4, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, X.; Guo, Y.; Kawasaki, H. A Review on Gold Nanoclusters for Cancer Phototherapy. ACS Appl. Bio Mater. 2023, 6, 4504–4517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Zhang, Y.; Zhang, J.; Li, L.; Liu, C.; Wu, Q. An Oxygen-Independent Photodynamic Therapy Nanoplatform for Combating Anaerobic Infection. J. Anal. Test. 2023, 7, 227–236. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, P.; Huang, Q.; Ding, X.; Wang, N.; Yao, W.; Miao, M.; Zhang, Z. Near-Infrared Light-Activatable Melanized Paclitaxel Nano–Self-Assemblies for Synergistic Anti-tumor Therapy. J. Anal. Test. 2023, 7, 204–214. [Google Scholar] [CrossRef]
- Han, R.; Zhao, M.; Wang, Z.; Liu, H.; Zhu, S.; Huang, L.; Wang, Y.; Wang, L.; Hong, Y.; Sha, Y.; et al. Super-Efficient In Vivo Two-Photon Photodynamic Therapy with a Gold Nanocluster as a Type I Photosensitizer. ACS Nano 2020, 14, 9532–9544. [Google Scholar] [CrossRef]
- Santhakumar, H.; Nair, R.V.; Govindachar, D.M.; Periyasamy, G.; Jayasree, R.S. Engineering of Tripeptide-Stabilized Gold Nanoclusters with Inherent Photosensitizing Property for Bioimaging and Photodynamic Therapy. ACS Sustain. Chem. Eng. 2023, 11, 2102–2114. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. pH-Responsive metal-organic framework encapsulated gold nanoclusters with modulated release to enhance photodynamic therapy/chemotherapy in breast cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef]
- Dan, Q.; Yuan, Z.; Zheng, S.; Ma, H.; Luo, W.; Zhang, L.; Su, N.; Hu, D.; Sheng, Z.; Li, Y. Gold Nanoclusters-Based NIR-II Photosensitizers with Catalase-like Activity for Boosted Photodynamic Therapy. Pharmaceutics 2022, 14, 1645. [Google Scholar] [CrossRef]
- Byun, J.; Kim, D.; Choi, J.; Shim, G.; Oh, Y.-K. Photosensitizer-Trapped Gold Nanocluster for Dual Light-Responsive Phototherapy. Biomedicines 2020, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bahadur, R.; Maske, P.; Gandhi, M.; Singh, D.; Srivastava, R. Preclinical safety assessment of red emissive gold nanocluster conjugated crumpled MXene nanosheets: A dynamic duo for image-guided photothermal therapy. Nanoscale 2023, 15, 2932–2947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-Nanocluster-Mediated Delivery of siRNA to Intact Plant Cells for Efficient Gene Knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Wu, M.; Guo, Z.; Tan, D.; Zhou, X.; Li, Y.; Liu, S.; Xue, L.; Lei, Y. Glucose-Responsive Gold Nanocluster-Loaded Microneedle Patch for Type 1 Diabetes Therapy. ACS Appl. Bio Mater. 2020, 3, 8640–8649. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Walker, E.; Springer, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Targeted Chemoradiotherapy of Prostate Cancer Using Gold Nanoclusters with Protease Activatable Monomethyl Auristatin E. ACS Appl. Mater. Interfaces 2022, 14, 14916–14927. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Du, B.; Huang, Y.; Yu, M.; Zheng, J. Cancer Photothermal Therapy with ICG-Conjugated Gold Nanoclusters. Bioconjug. Chem. 2020, 31, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, S.; Zhao, J.; Luo, Y.; Liang, H.; Zhao, S.; Zhang, L. Gold Nanocluster Encapsulated Nanorod for Tumor Microenvironment Simultaneously Activated NIR-II Photoacoustic/Photothermal Imaging and Cancer Therapy. Adv. Ther. 2023, 6, 2200350. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, J.; Lu, F.; Du, Y.; Cao, D.; Hu, S.; Yang, Y.; Yuan, Z. Visualization of Polymer-Surfactant Interaction by Dual-Emissive Gold Nanocluster Labeling. Biosensors 2022, 12, 686. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Z.; Zhao, Y.; Zhao, Y.; Li, X.; He, L.; Zvyagin, A.V.; Yang, B.; Lin, Q.; Ma, X. Lotus Seedpod-Inspired Crosslinking-Assembled Hydrogels Based on Gold Nanoclusters for Synergistic Osteosarcoma Multimode Imaging and Therapy. ACS Appl. Mater. Interfaces 2022, 14, 34377–34387. [Google Scholar] [CrossRef]
- Wang, W.; Kong, Y.; Jiang, J.; Xie, Q.; Huang, Y.; Li, G.; Wu, D.; Zheng, H.; Gao, M.; Xu, S.; et al. Engineering the Protein Corona Structure on Gold Nanoclusters Enables Red-Shifted Emissions in the Second Near-infrared Window for Gastrointestinal Imaging. Angew. Chem. Int. Ed. 2020, 59, 22431–22435. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Tang, S.; Guo, S.; Li, H.; Shao, N. In situ synthesis of red fluorescent gold nanoclusters with enzyme-like activity for oxidative stress amplification in chemodynamic therapy. Chin. Chem. Lett. 2022, 33, 1331–1336. [Google Scholar] [CrossRef]
- López-Domene, R.; Kumar, K.; Barcelon, J.E.; Guedes, G.; Beloqui, A.; Cortajarena, A.L. Nanozymes with versatile redox capabilities inspired in metalloenzymes. Nanoscale 2023, 15, 16959–16966. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Ye, F.; Zhang, C.; Fan, J.; Du, Z.; Zhao, W.; Yuan, Q.; Niu, W.; Gao, F.; He, B.; et al. A comparison of Remdesivir versus gold cluster in COVID-19 animal model: A better therapeutic outcome of gold cluster. Nano Today 2022, 44, 101468. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Hao, Y.; Li, X.; Zvyagin, A.V.; Whittaker, A.K.; Cui, Y.; Yang, B.; Lin, Q.; Li, Y. Ultrasmall Red Fluorescent Gold Nanoclusters for Highly Biocompatible and Long-Time Nerve Imaging. Part. Part. Syst. Charact. 2021, 38, 2100001. [Google Scholar] [CrossRef]

| No. | Target | Surface Ligand | Strategy | Linear Range | LOD | Environment | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | Hg2+ | folic-acid | Turn-off | 10–1000 nM | 28 nM | In vitro | [29] |
| 2 | Cu2+ | insulin | Turn-off | 0.05–1.7 μM | 7.5 × 10−3 μM | In vitro | [33] |
| 3 | Pb2+ | THPC/GSH | Turn-off | 5 × 10−3–5.0 μM | 2.0 × 10−3 μM | In vitro | [31] |
| 4 | CN− | hPEI | Ratiometric | 0.02–1.0 μM | 1.0 × 10−4 μM | In vitro | [35] |
| 5 | S2− | MUA | Turn-on | 0.5–4.0 μM | 3.5 × 10−4 μM | In vitro | [37] |
| 6 | H2S | GSH CDS | Turn-on | 1.10–1.55 × 10−3 μM | -- | Mouse liver | [36] |
| 7 | Cr2O72− | PAMAM | Turn-on | 0–55.0 μM | 1.9 μM | HeLa cell | [41] |
| 8 | H2O2 | CAT | Turn-on | 10–80 μM | 2.5 × 10−4 μM | In vitro | [47] |
| 9 | DA | tyrosine | Turn-off | 0.1–1000 μM | 10.85 × 10−3 μM | In vitro | [43] |
| 10 | OPs | AChE | Turn-off | 1.0 × 10−7–1.0 × 10−4 μM | 3.33 × 10−8 μM | Yeast cell | [44] |
| 11 | GSH | Manganesedioxide | On-off-on | 1–300 μM | 6.8 × 10−2 μM | Human serum | [48] |
| 12 | DA | hPEI | Ratiometric | 0–25 μM | 10 × 10−3 μM | In vitro | [54] |
| 13 | Nitrophenols | β-CDs | Array-based sensing | 1–50 μM | 5 μM | In vitro | [57] |
| 14 | H2O2 | CQD | Ratiometric | 5.0–80 nM | 2.9 nM | HeLa cell | [58] |
| 15 | ·OH | R9 | Turn-off | 0.2–100 μM | 0.1 μM | rat blood cells | [59] |
| 16 | ClO− | l-DOPA | Ratiometric | 0–350 μM | 0.50 μM | HeLa cell | [60] |
| 17 | ONOO− | CNCs | Ratiometric | 0–800 μM | 0.79 μM | cell of zebrafish | [61] |
| 18 | Immunoprotein | IgG | Turn-on | -- | 6.21 × 10−2 μM | In vitro | [62] |
| 19 | Hemoglobin | hPEI | Turn-on | 0.010–2.0 μM | 5.0 × 10−3 μM | In vitro | [63] |
| 20 | DNA | affinity hormone | Turn-on | 0.2 × 10−3–20 μM | 0.043 × 10−3 μM | In vitro | [64] |
| 21 | Proteins | GSH/MUA | Array-based sensing | -- | -- | In vitro | [68] |
| 22 | Proteins | CMMMMM | Turn-on | 0.1–50 μg/mL | -- | In vitro | [67] |
| 23 | Cancer cell | MUC1 | Turn-on | -- | -- | 4T1 cancer cells | [69] |
| 24 | Cancer cell | FA-BSA | Turn-on | -- | -- | ovarian CC | [70] |
| 25 | Cancer cell | sulfated oligo-iduronic acid | Turn-on | -- | -- | regular 2D cell | [71] |
| 26 | Tumor cell | PF | Turn-on | -- | -- | Mouse tumor cell | [73] |
| 27 | Escherichia coli | CWR11 | Turn-on | 0–712 μg/mL | 178 μg/ml | In vitro | [76] |
| 28 | Bacteria | HHC10 | Turn-on | 2.0 × 106–8 × 108 cfu/mL | 1.7 × 107 cfu/mL | In vitro | [77] |
| 29 | Bacteria | Vancomycin | Turn-on | 20–1.0 × 108 cfu/mL | 10 cfu/mL | In vitro | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Hou, P.; Wei, J.; Li, B.; Gao, A.; Yuan, Z. Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review. Molecules 2024, 29, 1574. https://doi.org/10.3390/molecules29071574
Yang L, Hou P, Wei J, Li B, Gao A, Yuan Z. Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review. Molecules. 2024; 29(7):1574. https://doi.org/10.3390/molecules29071574
Chicago/Turabian StyleYang, Lu, Pengqi Hou, Jingyi Wei, Bingxin Li, Aijun Gao, and Zhiqin Yuan. 2024. "Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review" Molecules 29, no. 7: 1574. https://doi.org/10.3390/molecules29071574
APA StyleYang, L., Hou, P., Wei, J., Li, B., Gao, A., & Yuan, Z. (2024). Recent Advances in Gold Nanocluster-Based Biosensing and Therapy: A Review. Molecules, 29(7), 1574. https://doi.org/10.3390/molecules29071574







