Synthesis and Characterization of Phenazine-Based Redox Center for High-Performance Polymer Poly(aryl ether sulfone)-5,10-Diphenyl-dihydrophenazine
Abstract
1. Introduction
2. Results and Discussion
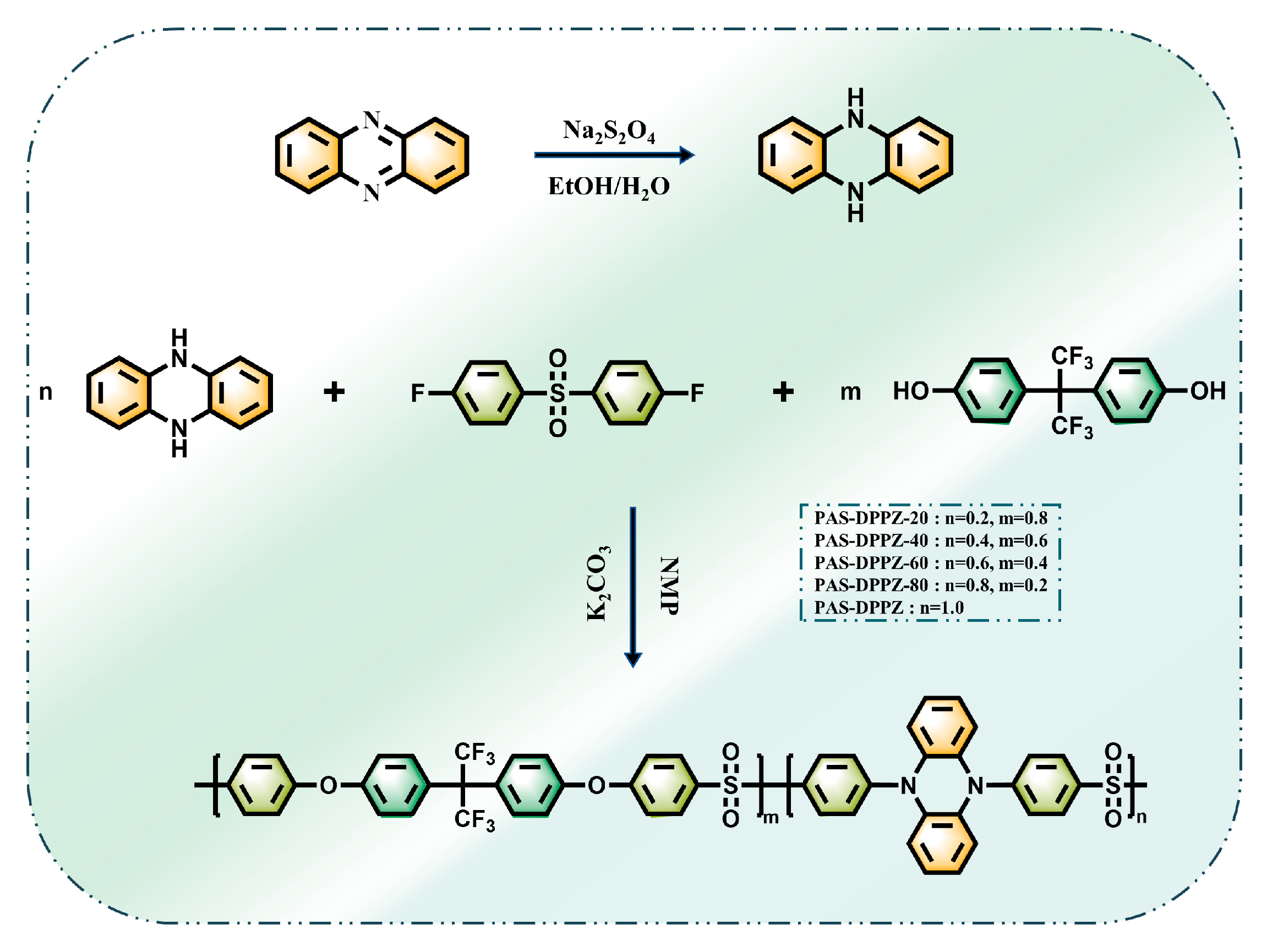
2.1. Monomer Synthesis
2.2. Polymer Synthesis and Characterization
2.3. Solubility and Molecular Weight
2.4. Thermal Properties
| Polymer Code | GPC (kDa) a | Thermal Stability (°C) | |||
|---|---|---|---|---|---|
| Mn | Mw | PDI | Tg b | Td5% c | |
| PAS-DPPZ-20 | 65.5 | 95.8 | 1.5 | 218.1 | 512.0 |
| PAS-DPPZ-40 | 92.7 | 213.5 | 2.3 | 241.9 | 486.3 |
| PAS-DPPZ-60 | 91.0 | 204.3 | 2.2 | 259.3 | 482.6 |
| PAS-DPPZ-80 | - | - | - | 272.4 | 466.1 |
| PAS-DPPZ | - | - | - | 275.5 | 434.8 |
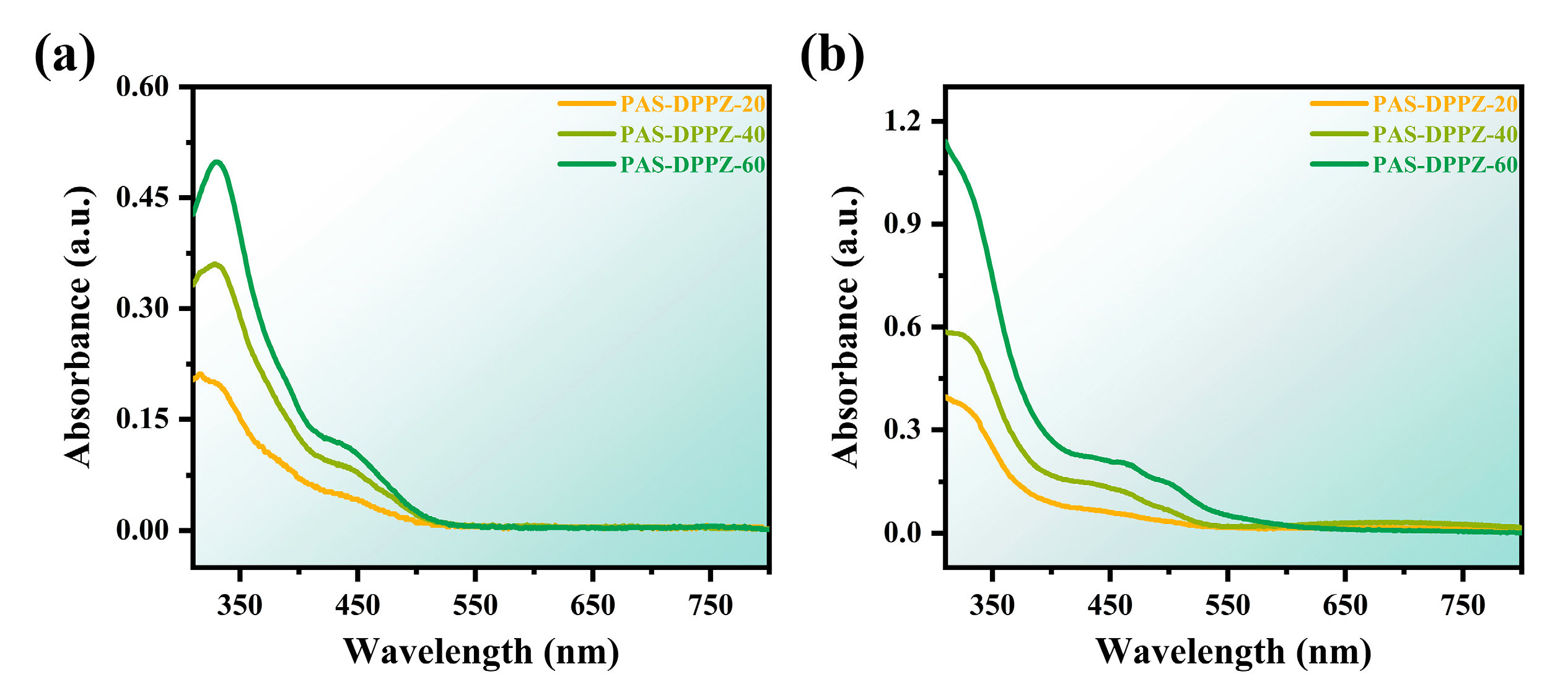
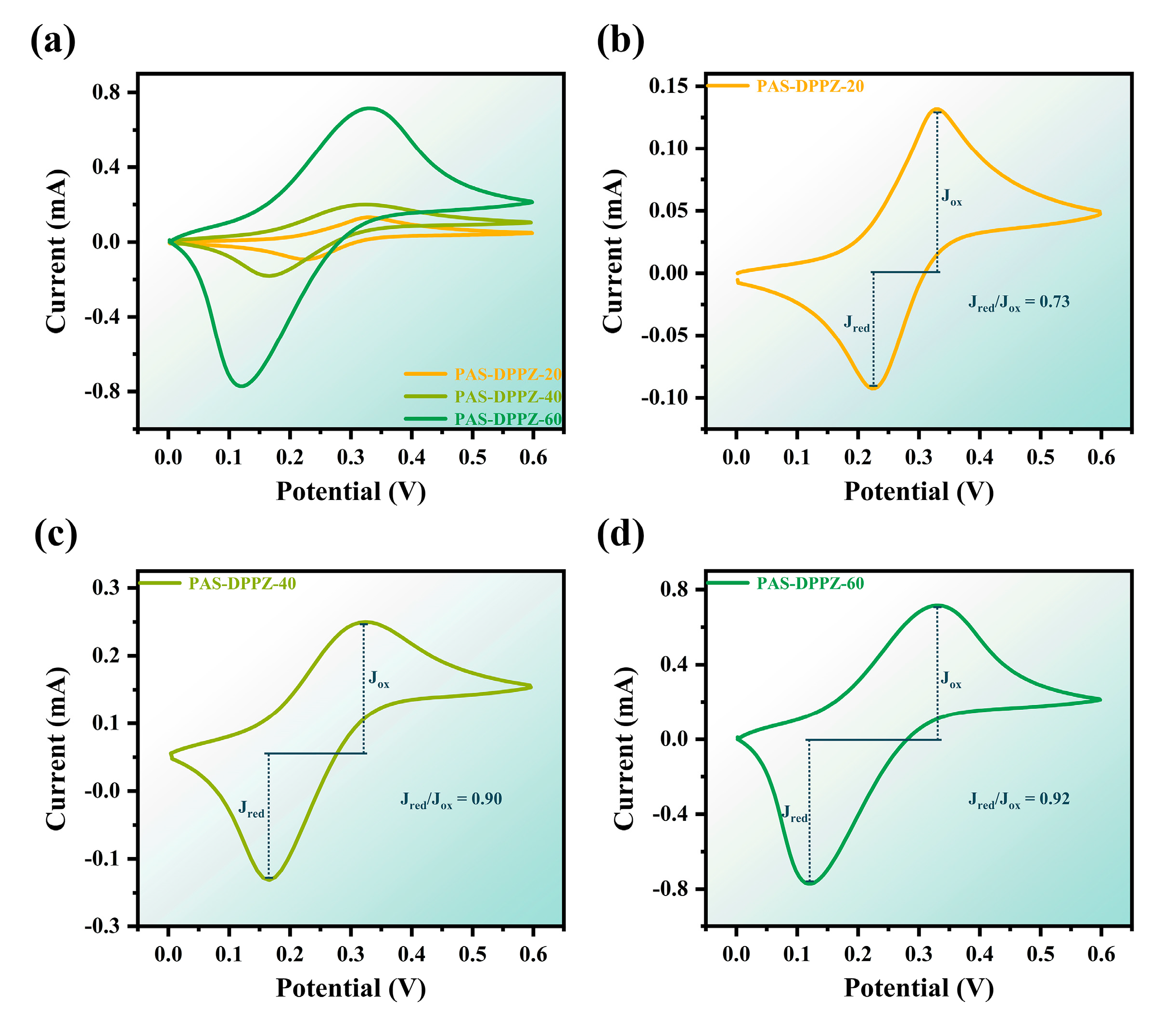
2.5. Optical and Electrochemical Properties
| Polymer Code | Solution | Film | |
|---|---|---|---|
| λmax (nm) | λmax (nm) | λonset (nm) | |
| PAS-DPPZ-20 | 316,434 | 445 | 533 |
| PAS-DPPZ-40 | 328,437 | 452 | 546 |
| PAS-DPPZ-60 | 331,439 | 460 | 565 |
| Polymer Code | Oxidation a (V) | Eg b (eV) | Energy Level c (eV) | ||
|---|---|---|---|---|---|
| Eonset | Eox1/2 | HOMO | LUMO | ||
| PAS-DPPZ-20 | 0.18 | 0.26 | 2.33 | −4.98 | −2.65 |
| PAS-DPPZ-40 | 0.13 | 0.21 | 2.27 | −4.93 | −2.66 |
| PAS-DPPZ-60 | 0.11 | 0.20 | 2.19 | −4.91 | −2.72 |
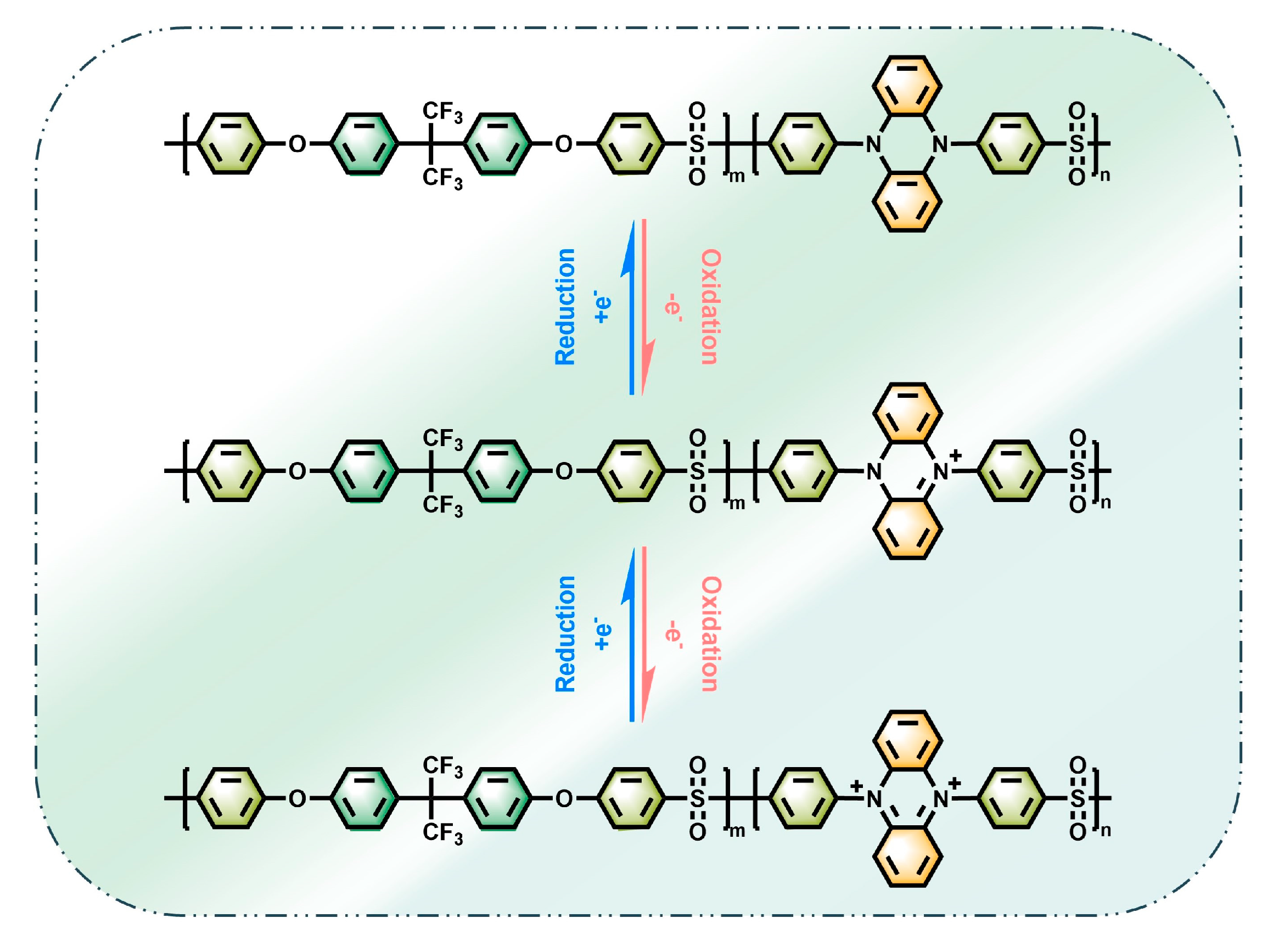
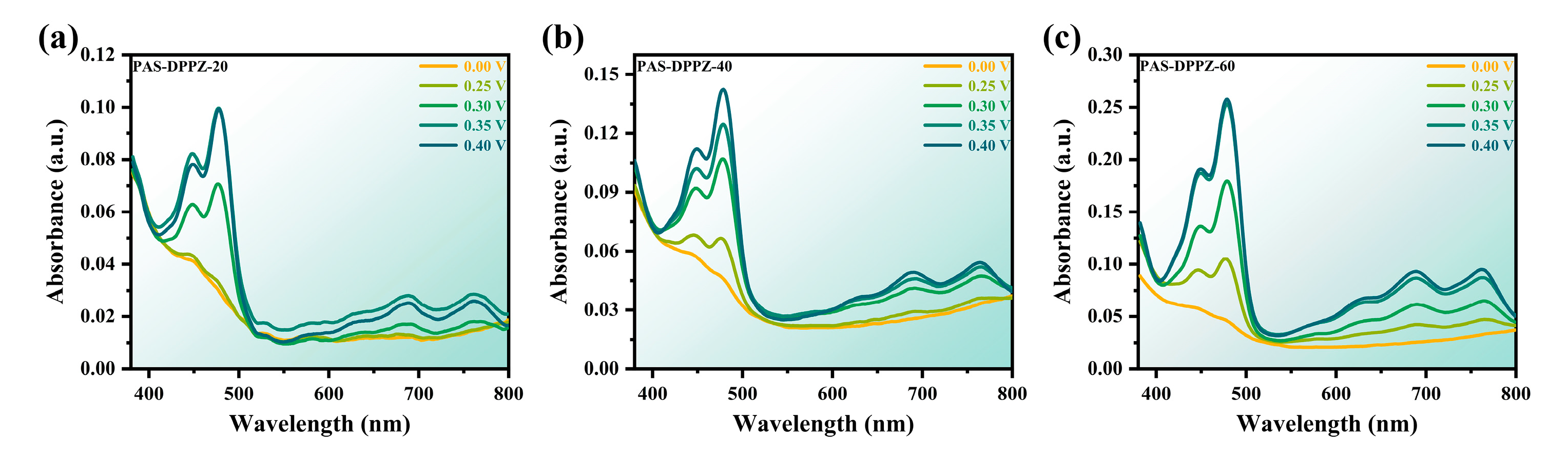
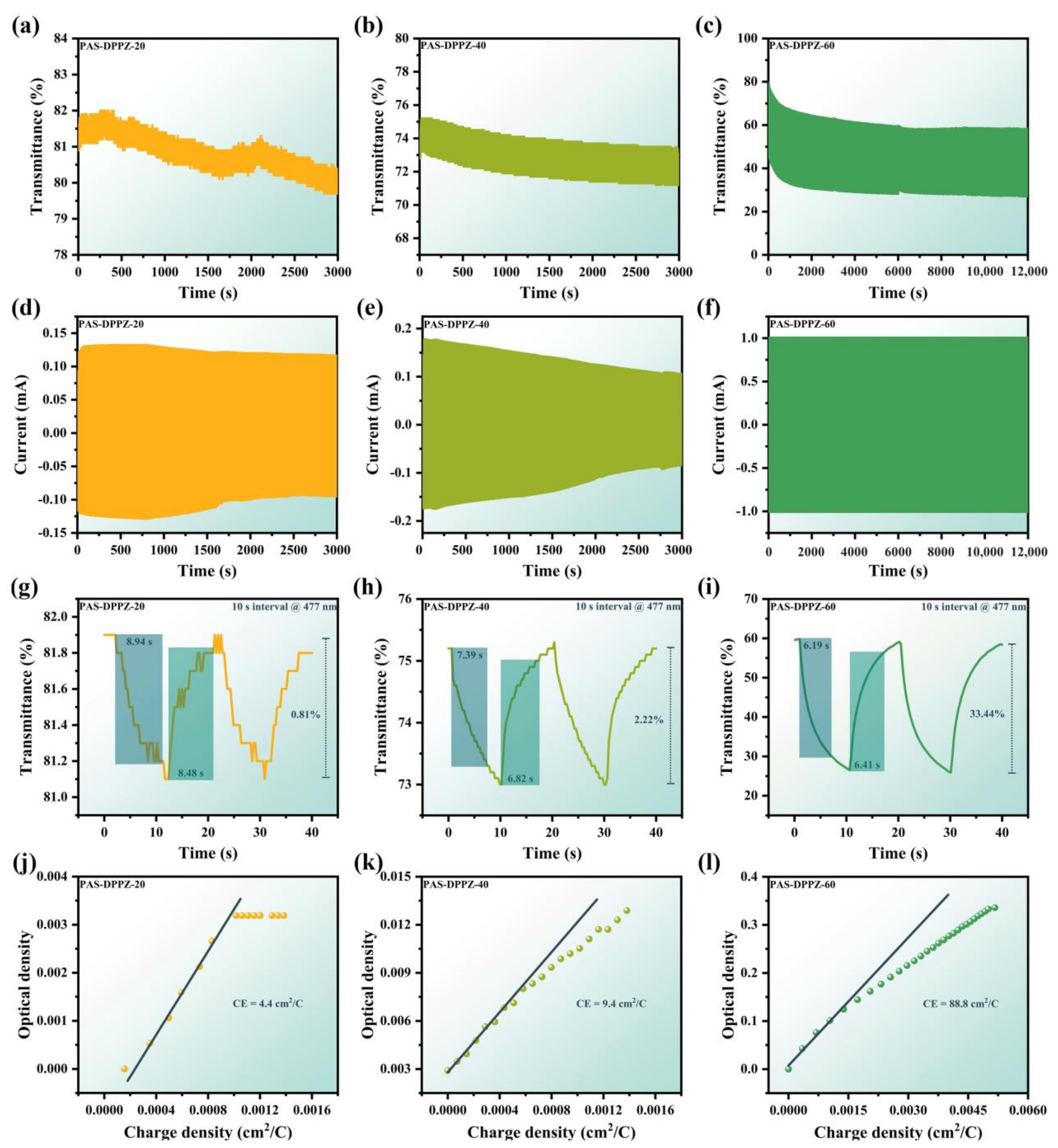
2.6. Spectroelectrochemistry and Electrochromic Properties
2.7. Battery Properties
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, J.; Cai, L.H.; Weitz, D.A. Tough self-healing elastomers by molecular enforced integration of covalent and reversible networks. Adv. Mater. 2017, 29, 1702616. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Jasinski, N.; Benitez, A.; Noack, M.; Park, D.; Goldmann, A.S.; Barner-Kowollik, C.; Walther, A. Hierarchical nacre mimetics with synergistic mechanical properties by control of molecular interactions in self-healing polymers. Angew. Chem. Int. Ed. 2015, 54, 8653–8657. [Google Scholar] [CrossRef]
- Chang, G.; Yang, L.; Yang, J.; Stoykovich, M.P.; Deng, X.; Cui, J.; Wang, D. High-performance pH-switchable supramolecular thermosets via cation–π interactions. Adv. Mater. 2018, 30, 1704234. [Google Scholar] [CrossRef] [PubMed]
- Balkenende, D.W.R.; Monnier, C.A.; Fiore, G.L.; Weder, C. Optically responsive supramolecular polymer glasses. Nat. Commun. 2016, 7, 10995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zou, W.; Zhao, N.; Xu, J. Electrically insulating PBO/MXene film with superior thermal conductivity, mechanical properties, thermal stability, and flame retardancy. Nat. Commun. 2023, 14, 5342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, B.; Fan, B.; Sun, H.; Zhang, H. Enhanced nonisothermal crystallization and heat resistance of poly(l-lactic acid) by d-sorbitol as a homogeneous nucleating agent. ACS Macro Lett. 2021, 10, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhao, L.; Yu, Q.; Lin, P.; Xu, J.; Yin, X.; Chen, S.; Wang, H.; Wang, L. Flexible and highly efficient bilayer photothermal paper for water desalination and purification: Self-floating, rapid water transport, and localized heat. ACS Appl. Mater. Interfaces 2020, 12, 11204–11213. [Google Scholar] [CrossRef] [PubMed]
- Tannous, F.; Steiner, M.; Shahin, R.; Kern, M. Retentive forces and fatigue resistance of thermoplastic resin clasps. Dent. Mater. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Choupin, T.; Fayolle, B.; Régnier, G.; Paris, C.; Cinquin, J.; Brulé, B. Macromolecular modifications of poly(etherketoneketone) (PEKK) copolymer at the melting state. Polym. Degrad. Stab. 2018, 155, 103–110. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, W.; Zhao, C. Progress in functionalized polyethersulfone membranes. J. Funct. Polym. 2021, 34, 114–143. [Google Scholar]
- Wang, Z.; Chen, T.; Xu, J. Gas and water vapor transport through a series of novel poly(aryl ether sulfone) membranes. Macromolecules 2001, 34, 9015–9022. [Google Scholar] [CrossRef]
- Kim, Y.S.; Wang, F.; Hickner, M.; McCartney, S.; Hong, Y.T.; Harrison, W.; Zawodzinski, T.A.; McGrath, J.E. Effect of acidification treatment and morphological stability of sulfonated poly(arylene ether sulfone) copolymer proton-exchange membranes for fuel-cell use above 100 °C. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2816–2828. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES–blood interactions and modification techniques. Mater. Sci. Eng. C 2015, 56, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Hajatdoost, S.; Sammon, C.; Yarwood, J. FTIR–ATR studies of diffusion and perturbation of water in polyelectrolyte thin films. Part 4. diffusion, perturbation and swelling processes for ionic solutions in SPEES/PES membranes. Polymer 2002, 43, 1821–1827. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Böhme, S.; Schwarz, G.; Krüger, R.P.; Schulz, G. Macrocycles. 18. the role of cyclization in syntheses of poly(ether-sulfone)s. Macromolecules 2001, 34, 8886–8893. [Google Scholar] [CrossRef]
- Ma, X.; Lv, Z.; Wang, D.; Guan, S.; Chen, C.; Wang, G.; Zhang, D.; Jiang, Z. Crosslinkable fluorinated poly(aryl ether ketone)s containing pendent phenylethynyl moieties for optical waveguide devices. J. Photochem. Photobiol. A Chem. 2007, 188, 43–50. [Google Scholar] [CrossRef]
- Su, Y.; Li, C.; Zhao, W.; Shi, Q.; Wang, H.; Jiang, Z.; Zhu, S. Modification of polyethersulfone ultrafiltration membranes with phosphorylcholine copolymer can remarkably improve the antifouling and permeation properties. J. Membr. Sci. 2008, 322, 171–177. [Google Scholar] [CrossRef]
- Percec, V.; Nava, H.; Auman, B.C. Functional polymers and sequential copolymers by phase transfer catalysis. VII. synthesis and characterization of alternating block copolymers of aromatic poly(ether sulfone)s with aliphatic polysulfides and aliphatic polysulfones. Polym. J. 1984, 16, 681–691. [Google Scholar] [CrossRef][Green Version]
- Iijima, T.; Hiraoka, H.; Tomoi, M. Preparation of epoxy-terminated poly(aryl ether sulfone)s and their use as modifiers for epoxy resins. J. Appl. Polym. Sci. 2003, 45, 709–721. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.-Q.; Su, Y.-L.; Jiang, Z.-Y. Antifouling ultrafiltration membrane composed of polyethersulfone and sulfobetaine copolymer. J. Membr. Sci. 2006, 280, 343–350. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Ariga, K. Redox-active polymers for energy storage nanoarchitectonics. Joule 2017, 1, 739–768. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, C.-H.; Alshehri, A.; Young, C.; Yamauchi, Y.; Kim, J.; Kuo, S.-W. Strategic design of triphenylamine- and triphenyltriazine-based two-dimensional covalent organic frameworks for CO2 uptake and energy storage. J. Mater. Chem. A 2018, 6, 19532–19541. [Google Scholar] [CrossRef]
- Qu, W.-J.; Fang, H.; An, J.-N.; Yang, H.-H.; He, J.-X.; Yao, H.; Wei, T.-B.; Lin, Q.; Zhang, Y.-M. Highly sensitive detection of mercury(II) and silver(I) ions in aqueous solution via a chromene-functionalized imidazophenazine derivative. J. Photochem. Photobiol. A Chem. 2020, 402, 112814. [Google Scholar] [CrossRef]
- Sendh, J.; Singh, M.P.; Baruah, J.B. 5-[(Pyren-9-ylmethyl)amino]isophthalic acid with nitrogen containing heterocycles: Stacking, N–H⋯π interactions and photoluminescence. Cryst. Eng. Comm. 2021, 23, 6952–6966. [Google Scholar] [CrossRef]
- Qi, X.-N.; Che, Y.-X.; Qu, W.-J.; Zhang, Y.-M.; Yao, H.; Lin, Q.; Wei, T.-B. Design and fabricating biogenic amine-responsive platform based on self-assembly property of phenazine derivative for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2021, 333, 129430. [Google Scholar] [CrossRef]
- Imato, K.; Ohira, K.; Yamaguchi, M.; Enoki, T.; Ooyama, Y. Phenazine-based photosensitizers for singlet oxygen generation. Mater. Chem. Front. 2020, 4, 589–596. [Google Scholar] [CrossRef]
- Lee, S.H.; Matula, A.J.; Hu, G.; Troiano, J.L.; Karpovich, C.J.; Crabtree, R.H.; Batista, V.S.; Brudvig, G.W. Strongly coupled phenazine–porphyrin dyads: Light-harvesting molecular assemblies with broad absorption coverage. ACS Appl. Mater. Interfaces 2019, 11, 8000–8008. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Wang, X.; Qian, Y.; Niu, Z.; Zhu, X.; Ye, J.; Zhao, Y.; Zhang, X. Manipulation of conjugation to stabilize N redox-active centers for the design of high-voltage organic battery cathode. Energy Storage Mater. 2019, 16, 236–242. [Google Scholar] [CrossRef]
- Podemska, K.; Podsiadly, R.; Orzel, A.; Sokołowska, J. The photochemical behavior of benzo[a]pyrido[2′,1′:2,3]imidazo[4,5-c]phenazine dyes. Dye. Pigment. 2013, 99, 666–672. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Fang, H.; Zhu, W.; He, J.-X.; Yao, H.; Wei, T.-B.; Lin, Q.; Qu, W.-J. Ratiometric fluorescent sensor based oxazolo-phenazine derivatives for detect hypochlorite via oxidation reaction and its application in environmental samples. Dye. Pigment. 2020, 172, 107765. [Google Scholar] [CrossRef]
- Wei, T.-B.; Yong, B.-R.; Dang, L.-R.; Zhang, Y.-M.; Yao, H.; Lin, Q. A simple water-soluble phenazine dye for colorimetric/fluorogenic dual-mode detection and removal of Cu2+ in natural water and plant samples. Dye. Pigment. 2019, 171, 107707. [Google Scholar] [CrossRef]
- Maddala, G.; Gade, R.; Ahemed, J.; Kalvapalli, S.; Simhachalam, N.B.; Chetti, P.; Pola, S.; Mitty, R. Efficient, dopant free phenazine based hole transporting materials for high performance perovskite solar cells. Sol. Energy 2021, 226, 501–512. [Google Scholar] [CrossRef]
- Kothavale, S.; Lim, J.; Lee, J.Y. Rational design of CN substituted dibenzo[a,c]phenazine acceptor for color tuning of thermally activated delayed fluorescent emitters. Chem. Eng. J. 2022, 431, 134216. [Google Scholar] [CrossRef]
- Chen, Z.; Ono, R.J.; Wiggins, K.M.; Cui, H.; Rong, C.; Bielawski, C.W.; Jiang, Z. Synthesis and characterization of polyketones containing pendant carbazoles. Polymer 2011, 52, 1731–1737. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Yang, J.; Qu, Y.; Hua, J. Colorimetric and ratiometric Near-Infrared fluorescent cyanide chemodosimeter based on phenazine derivatives. ACS Appl. Mater. Interfaces 2013, 5, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xing, Z.; Ma, P.; Li, S.; Wang, C.; Jiang, Z.; Chen, Z. Design rules for improving the cycling stability of high-performance donor–acceptor-type electrochromic polymers. ACS Appl. Mater. Interfaces 2020, 12, 7529–7538. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Wang, Y.; Han, Y.; Zhai, Y.; Tian, Y.; Qi, S.; Zhu, X.; Jiang, Z.; Chen, Z. The effect of constructing discontinuous side chain D-A structure on high-performance poly (ether sulfone)s optoelectronic materials. Dye. Pigment. 2021, 189, 109259. [Google Scholar] [CrossRef]
- Yongsheng, E.; Ping, L.; Bin, L.; Fang, Y. Preparation and properties of bisphenol AF polycarbonate. Eng. Plast. Appl. 2022, 50, 41–45. [Google Scholar]
- Wang, Q.; Zhai, Y.; Chao, D.; Chen, Z.; Jiang, Z. Preparation and electrochromic properties of benzodithiophene-isoindigo conjugated polymers with oligoethylene glycol side chains. Materials 2022, 16, 60. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lin, Y.; Sun, D.; Xing, Z.; Jiang, Z.; Chen, Z. Poly(aryl amino ketone)-based materials with excellent electrochromic and electrofluorochromic behaviors. Dye. Pigment. 2019, 163, 40–47. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Chen, P. Phenazine-based organic cathode for aqueous zinc secondary batteries. J. Power Sources 2020, 468, 228401. [Google Scholar] [CrossRef]
- Dai, G.; Gao, Y.; Niu, Z.; He, P.; Zhang, X.; Zhao, Y.; Zhou, H. Dilution of the electron density in the π-conjugated skeleton of organic cathode materials improves the discharge voltage. ChemSusChem 2020, 13, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Issi, J.P.; Devaux, J.; Billaud, D. The stability of polypyrrole and its composites. J. Mater. Sci. 1997, 32, 1515–1518. [Google Scholar] [CrossRef]
- Chhatre, S.; Ichake, A.; Harpale, K.; Patil, S.; Deshpande, A.; More, M.; Wadgaonkar, P.P. Phenazine-containing poly(phenylenevinylene): A new polymer with impressive field emission properties. J. Polym. Res. 2018, 25, 61. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Qu, Y.; Qu, W.; Zhang, X.; Hang, Y.; Ågren, H.; Hua, J. Red turn-on fluorescent phenazine-cyanine chemodosimeters for cyanide anion in aqueous solution and its application for cell imaging. Sens. Actuators B Chem. 2014, 203, 833–847. [Google Scholar] [CrossRef]
- Xiao-Ni, Q.; Dang, L.-R.; Qu, W.-J.; Zhang, Y.-M.; Yao, H.; Lin, Q.; Wei, T.-B. Phenazine derivatives for optical sensing: A review. J. Mater. Chem. C 2020, 8, 11308–11339. [Google Scholar] [CrossRef]
- Pan, B.-C.; Chen, W.-H.; Hsiao, S.-H.; Liou, G.-S. A facile approach to prepare porous polyamide films with enhanced electrochromic performance. Nanoscale 2018, 10, 16613–16620. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, S.; Zheng, Z.; Chen, Z.; Jiang, Z. Rapid and effective electrochemical strategy for the synthesis of PAASs/PAAKs electrochromic high-performance polymers. Sol. Energy Mater. Sol. Cells 2023, 254, 112256. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Wang, X.; Zhai, Y.; Zheng, Z.; Shen, H.; Han, Y.; Chen, Z.; Jiang, Z. Synthesis and Characterization of Phenazine-Based Redox Center for High-Performance Polymer Poly(aryl ether sulfone)-5,10-Diphenyl-dihydrophenazine. Molecules 2024, 29, 1618. https://doi.org/10.3390/molecules29071618
Wang Q, Wang X, Zhai Y, Zheng Z, Shen H, Han Y, Chen Z, Jiang Z. Synthesis and Characterization of Phenazine-Based Redox Center for High-Performance Polymer Poly(aryl ether sulfone)-5,10-Diphenyl-dihydrophenazine. Molecules. 2024; 29(7):1618. https://doi.org/10.3390/molecules29071618
Chicago/Turabian StyleWang, Qilin, Xuehan Wang, Yuehui Zhai, Zhibo Zheng, Huilin Shen, Yuntao Han, Zheng Chen, and Zhenhua Jiang. 2024. "Synthesis and Characterization of Phenazine-Based Redox Center for High-Performance Polymer Poly(aryl ether sulfone)-5,10-Diphenyl-dihydrophenazine" Molecules 29, no. 7: 1618. https://doi.org/10.3390/molecules29071618
APA StyleWang, Q., Wang, X., Zhai, Y., Zheng, Z., Shen, H., Han, Y., Chen, Z., & Jiang, Z. (2024). Synthesis and Characterization of Phenazine-Based Redox Center for High-Performance Polymer Poly(aryl ether sulfone)-5,10-Diphenyl-dihydrophenazine. Molecules, 29(7), 1618. https://doi.org/10.3390/molecules29071618






