Water-Soluble Molecular Cages for Biological Applications
Abstract
1. Introduction
2. Scope of the Review
3. Main Concepts
Solubility and Stability in Water
4. Synthesis
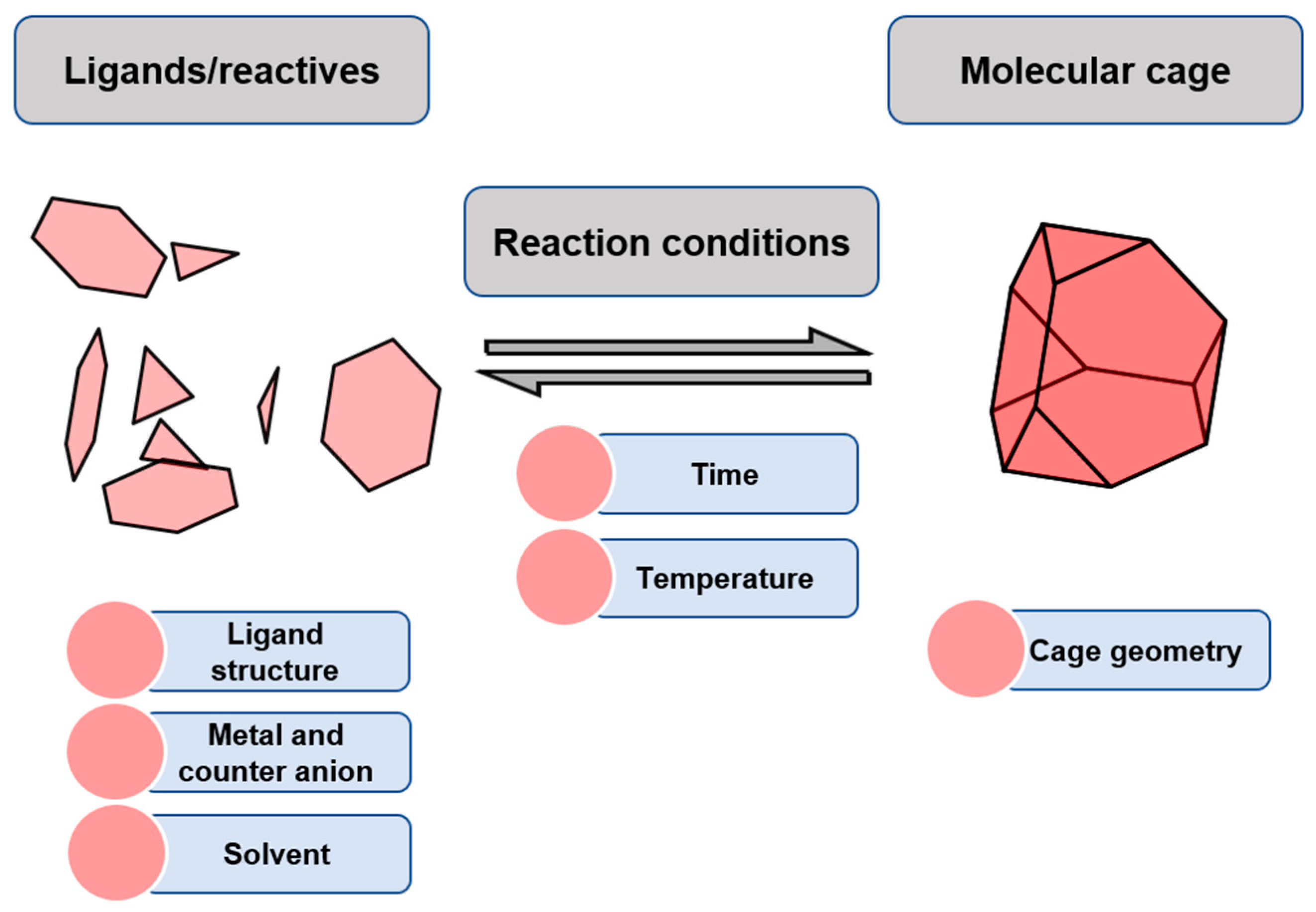
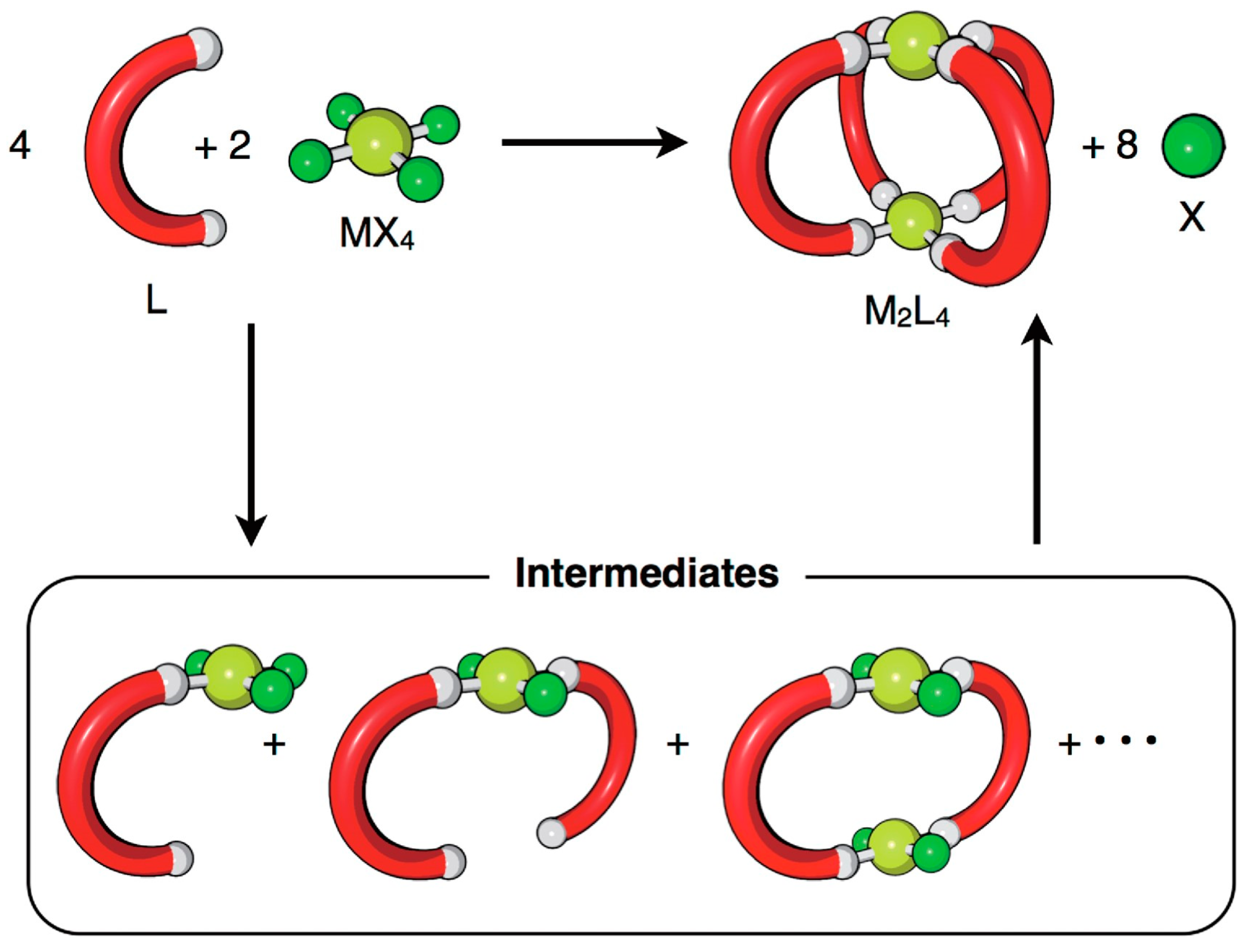
4.1. Molecular Modelling
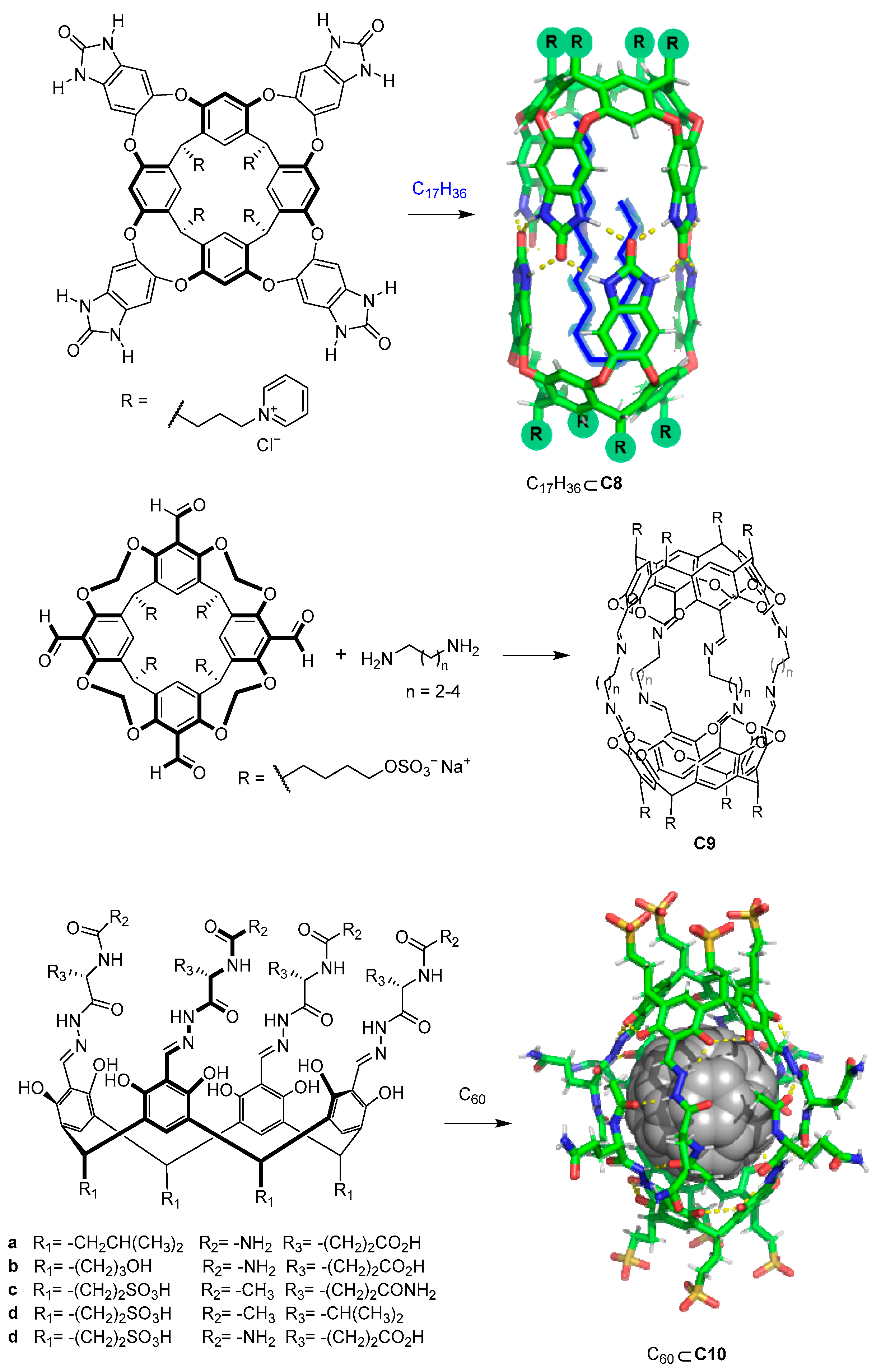
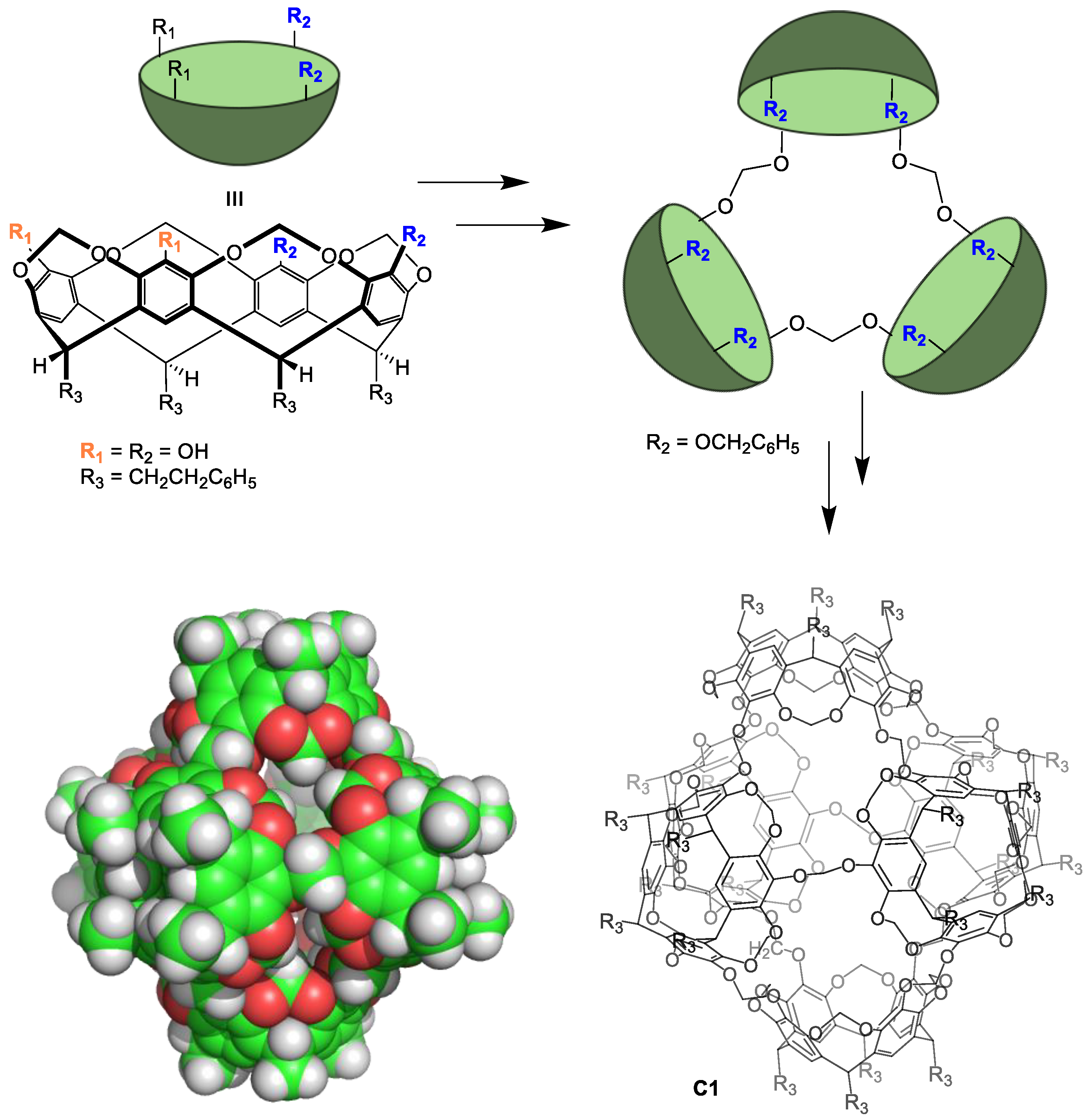
4.2. Strategies to Obtain Water-Soluble Molecular Cages
5. Biological Applications
5.1. Cancer Therapy
5.2. Other Biological Applications
6. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Lewis, J.E.M. Developing Sophisticated Microenvironments in Metal-organic Cages. Trends Chem. 2023, 5, 717–719. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Duarte, F.; Lusby, P.J. Host-Guest Chemistry of Self-Assembled Hemi-Cage Systems: The Dramatic Effect of Lost Pre-Organization. Isr. J. Chem. 2019, 59, 257–266. [Google Scholar] [CrossRef]
- Montà-González, G.; Sancenón, F.; Martínez-Máñez, R.; Martí-Centelles, V. Purely Covalent Molecular Cages and Containers for Guest Encapsulation. Chem. Rev. 2022, 122, 13636–13708. [Google Scholar] [CrossRef] [PubMed]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and Applications of Water-soluble Coordination Cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Takezawa, H.; Fujita, M. Molecular Confinement Effects by Self-assembled Coordination Cages. Bull. Chem. Soc. Jpn. 2021, 94, 2351–2369. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Lawrence, A.L.; Lusby, P.J. High Activity and Efficient Turnover by a Simple, Self-Assembled Artificial “Diels-Alderase”. J. Am. Chem. Soc. 2018, 140, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, J.M.; Rebek, J. Molecules in Confined Spaces: Reactivities and Possibilities in Cavitands. Chem 2020, 6, 1265–1274. [Google Scholar] [CrossRef]
- Pappalardo, A.; Puglisi, R.; Sfrazzetto, G.T. Catalysis inside Supramolecular Capsules: Recent Developments. Catalysts 2019, 9, 630. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.-J. Metal–organic Cages: Applications in Organic Reactions. Chemistry 2022, 4, 494–519. [Google Scholar] [CrossRef]
- Mal, P.; Breiner, B.; Rissanen, K.; Nitschke, J.R. White Phosphorus is Air-Stable within a Self-Assembled Tetrahedral Capsule. Science 2009, 324, 1697–1699. [Google Scholar] [CrossRef]
- Galan, A.; Ballester, P. Stabilization of Reactive Species by Supramolecular Encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ronson, T.K.; Zou, Y.-Q.; Nitschke, J.R. Metal–organic Cages for Molecular Separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Little, M.A.; Cooper, A.I. The Chemistry of Porous Organic Molecular Materials. Adv. Funct. Mater. 2020, 30, 1909842. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Zi, M.; Yuan, L. Recent Advances of Application of Porous Molecular Cages for Enantioselective Recognition and Separation. J. Sep. Sci. 2020, 43, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Mastalerz, M. Organic Cage Compounds-from Shape-Persistency to Function. Chem. Soc. Rev. 2014, 43, 1934–1947. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Stoddart, J.F. Emergent Behavior in Nanoconfined Molecular Containers. Chem 2021, 7, 919–947. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U.C. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in Drug Delivery: An Updated Review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Assaf, K.I.; Nau, W.M. Applications of Cucurbiturils in Medicinal Chemistry and Chemical Biology. Front. Chem. 2019, 7, 619. [Google Scholar] [CrossRef]
- Martí-Centelles, V.; Pandey, M.D.; Burguete, M.I.; Luis, S.V. Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem. Rev. 2015, 115, 8736–8834. [Google Scholar] [CrossRef]
- Martí-Centelles, V. Kinetic and thermodynamic concepts as synthetic tools in supramolecular chemistry for preparing macrocycles and molecular cages. Tetrahedron Lett. 2022, 93, 153676. [Google Scholar] [CrossRef]
- Lewis, J.E.M. Molecular Engineering of Confined Space in Metal–organic Cages. Chem. Commun. 2022, 58, 13873–13886. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, T.; Yoshimura, M.; Tokuda, S.; Matsuda, F.; Fujita, D.; Furukawa, S. Coordination/metal–organic cages inside out. Coord. Chem. Rev. 2022, 467, 214612. [Google Scholar] [CrossRef]
- Yang, X.; Ullah, Z.; Stoddart, J.F.; Yavuz, C.T. Porous Organic Cages. Chem. Rev. 2023, 123, 4602–4634. [Google Scholar] [CrossRef] [PubMed]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Sherman, J.C. A Six-Bowl Carceplex That Entraps Seven Guest Molecules. J. Am. Chem. Soc. 2005, 127, 12363–12367. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, S. Unresolved Issues that Remain in Molecular Self-Assembly. Bull. Chem. Soc. Jpn. 2018, 91, 957–978. [Google Scholar] [CrossRef]
- Kai, S.; Martí-Centelles, V.; Sakuma, Y.; Mashiko, T.; Kojima, T.; Nagashima, U.; Tachikawa, M.; Lusby, P.J.; Hiraoka, S. Quantitative Analysis of Self-assembly Process of a Pd2L4 Cage Consisting of Rigid Ditopic Ligands. Chem. Eur. J. 2018, 24, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, T.; Takahashi, S.; Okazawa, A.; Martí-Centelles, V.; Wang, J.; Kojima, T.; Lusby, P.J.; Sato, H.; Hiraoka, S. Navigated Self-Assembly of a Pd2L4 Cage by Modulation of an Energy Landscape under Kinetic Control. J. Am. Chem. Soc. 2019, 141, 19669–19676. [Google Scholar] [CrossRef]
- Tateishi, T.; Kojima, T.; Hiraoka, S. Multiple Pathways in the Self-Assembly Process of a Pd4L8 Coordination Tetrahedron. Inorg. Chem. 2018, 57, 2686–2694. [Google Scholar] [CrossRef]
- Santolini, V.; Miklitz, M.; Berardo, E.; Jelfs, K.E. Topological Landscapes of Porous Organic Cages. Nanoscale 2017, 9, 5280–5298. [Google Scholar] [CrossRef] [PubMed]
- Piskorz, T.K.; Martí-Centelles, V.; Young, T.A.; Lusby, P.J.; Duarte, F. Computational Modeling of Supramolecular Metallo-organic Cages—Challenges and Opportunities. ACS Catal. 2022, 12, 5806–5826. [Google Scholar] [CrossRef] [PubMed]
- Tarzia, A.; Jelfs, K.E. Unlocking the Computational Design of Metal–organic Cages. Chem. Comm. 2022, 58, 3717–3730. [Google Scholar] [CrossRef] [PubMed]
- Greenaway, R.L.; Jelfs, K.E. High-throughput Approaches for the Discovery of Supramolecular Organic Cages. ChemPlusChem 2020, 85, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Martí-Centelles, V.; Piskorz, T.K.; Duarte, F. Cagecavitycalc (C3): A Computational Tool for Calculating and Visualizing Cavities in Molecular Cages. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, P.S. Recent Trends in Organic Cage Synthesis: Push towards Water-soluble Organic Cages. Chem. Commun. 2022, 58, 5558–5573. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cram, D.J. The First Water-Soluble Hermicarceplexes. Chem. Commun. 1997, 118, 497–498. [Google Scholar] [CrossRef]
- Barwell, N.P.; Davis, A.P. Substituent Effects in Synthetic Lectins—Exploring the Role of CH-π Interactions in Carbohydrate Recognition. J. Org. Chem. 2011, 76, 6548–6557. [Google Scholar] [CrossRef] [PubMed]
- Howgego, J.D.; Butts, C.P.; Crump, M.P.; Davis, A.P. An Accessible Bicyclic Architecture for Synthetic Lectins. Chem. Commun. 2013, 49, 3110–3112. [Google Scholar] [CrossRef]
- Joshi, G.; Davis, A.P. New H-Bonding Patterns in Biphenyl-Based Synthetic Lectins; Pyrrolediamine Bridges Enhance Glucose Selectivity. Org. Biomol. Chem. 2012, 10, 5760–5763. [Google Scholar] [CrossRef]
- Liu, X.; Sun, J.; Warmuth, R. Water-Soluble Octahedral Polyammonium Nanocapsules: Synthesis and Encapsulation Studies. Tetrahedron 2009, 65, 7303–7310. [Google Scholar] [CrossRef]
- Gavette, J.V.; Zhang, K.D.; Ajami, D.; Rebek, J. Folded Alkyl Chains in Water-Soluble Capsules and Cavitands. Org. Biomol. Chem. 2014, 12, 6561–6563. [Google Scholar] [CrossRef] [PubMed]
- Gavette, J.V.; Petsalakis, I.D.; Theodorakopoulos, G.; Zhang, K.D.; Yu, Y.; Rebek, J. The Effects of Hexafluoroisopropanol on Guest Binding by Water-Soluble Capsule and Cavitand Hosts. Chem. Commun. 2015, 51, 17604–17606. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sun, J.; Efremovska, B.; Warmuth, R. Assembly of Water-Soluble, Dynamic, Covalent Container Molecules and Their Application in the Room-Temperature Stabilization of Protoadamantene. Chem. Eur. J. 2012, 18, 12864–12872. [Google Scholar] [CrossRef] [PubMed]
- Eichstaedt, K.; Szpotkowski, K.; Grajda, M.; Gilski, M.; Wosicki, S.; Jaskólski, M.; Szumna, A. Self-assembly and Ordering of Peptide-based Cavitands in Water and DMSO: The Power of Hydrophobic Effects Combined with Neutral Hydrogen Bonds. Chem. Eur. J. 2019, 25, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, D.; Kolarski, D.; Grüning, W.R.; Diederich, F. Resorcin[4]Arene-Based Molecular Baskets and Water-Soluble Container Molecules: Synthesis and 1H NMR Host-Guest Complexation Studies. Eur. J. Org. Chem. 2014, 2014, 3575–3583. [Google Scholar] [CrossRef]
- Tyagi, R.; Witte, C.; Haag, R.; Schröder, L. Dendronized Cryptophanes as Water-Soluble Xenon Hosts for 129Xe Magnetic Resonance Imaging. Org. Lett. 2014, 16, 4436–4439. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, N.; Holcroft, J.M.; Hartlieb, K.J.; Dale, E.J.; Vermeulen, N.A.; Stern, C.L.; Sarjeant, A.A.; Stoddart, J.F. Modulating the Binding of Polycyclic Aromatic Hydrocarbons Inside a Hexacationic Cage by Anion-π Interactions. Angew. Chem. Int. Ed. 2015, 54, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Cai, K.; Liu, Z.; Wu, G.; Shen, L.; Cheng, C.; Feng, Y.; Stern, C.L.; Stoddart, J.F.; Li, H. Guest Recognition Enhanced by Lateral Interactions. Chem. Sci. 2019, 10, 5114–5123. [Google Scholar] [CrossRef]
- Chakraborty, D.; Modak, R.; Howlader, P.; Mukherjee, P.S. De Novo Approach for Synthesis of Water-Soluble Interlocked and Non-Interloked Organic Cages. Chem. Commun. 2021, 57, 3995–3998. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, G.; Chen, L.; Tong, L.; Lei, Y.; Shen, L.; Jiao, T.; Li, H. Selective Recognition of Chloride Anion in Water. Org. Lett. 2020, 22, 4878–4882. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fang, S.; Wu, G.; Lei, Y.; Chen, Q.; Wang, H.; Wu, Y.; Lin, C.; Hong, X.; Kim, S.K.; et al. Constraining Homo- and Heteroanion Dimers in Ultraclose Proximity within a Self-Assembled Hexacationic Cage. J. Am. Chem. Soc. 2020, 142, 20182–20190. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, Y.; Wu, G.; Liu, J.R.; Cao, N.; Wang, L.; Wang, Y.; Li, X.; Hong, X.; Yang, C.; et al. Temperature-Dependent Self-Assembly of a Purely Organic Cage in Water. Chem. Commun. 2018, 54, 3138–3141. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Oguro, D.; Miyazawa, M.; Oka, H.; Yamaguchi, K.; Ogura, K. Self-Assembly of Ten Molecules into Nanometer-Sized Host Frameworks. Nature 1995, 378, 469–471. [Google Scholar] [CrossRef]
- Ibukuro, F.; Kusukawa, T.; Fujita, M. A Thermally Switchable Molecular Lock. Guest-Templated Synthesis of a Kinetically Stable Nanosized Cage. J. Am. Chem. Soc. 1998, 120, 8561–8562. [Google Scholar] [CrossRef]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Enzymelike Catalysis of the Nazarov Cyclization by Supramolecular Encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. [Google Scholar] [CrossRef] [PubMed]
- Mal, P.; Schultz, D.; Beyeh, K.; Rissanen, K.; Nitschke, J.R. An Unlockable-Relockable Iron Cage by Subcomponent Self-Assembly. Angew. Chem. Int. Ed. 2008, 47, 8297–8301. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.K.; Cremers, J.; Hogben, A.J.; Breiner, B.; Smulders, M.M.J.; Thoburn, J.D.; Nitschke, J.R. A Stimuli Responsive System of Self-Assembled Anion-Binding Fe4L68+ Cages. Chem. Sci. 2013, 4, 68–76. [Google Scholar] [CrossRef]
- Bolliger, J.L.; Belenguer, A.M.; Nitschke, J.R. Enantiopure Water-Soluble [Fe4L6] Cages: Host-Guest Chemistry and Catalytic Activity. Angew. Chem. Int. Ed. 2013, 52, 7958–7962. [Google Scholar] [CrossRef]
- Whitehead, M.; Turega, S.; Stephenson, A.; Hunter, C.A.; Ward, M.D. Quantification of Solvent Effects on Molecular Recognition in Polyhedral Coordination Cage Hosts. Chem. Sci. 2013, 4, 2744–2751. [Google Scholar] [CrossRef]
- Casini, A.; Woods, B.; Wenzel, M. The Promise of Self-Assembled 3D Supramolecular Coordination Complexes for Biomedical Applications. Inorg. Chem. 2017, 56, 14715–14729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Pan, M.; Su, C.-Y. Metal-Organic Cages for Biomedical Applications. Isr. J. Chem. 2018, 59, 209–219. [Google Scholar] [CrossRef]
- Dou, W.-T.; Yang, C.-Y.; Hu, L.-R.; Song, B.; Jin, T.; Jia, P.-P.; Ji, X.; Zheng, F.; Yang, H.-B.; Xu, L. Metallacages and Covalent Cages for Biological Imaging and Therapeutics. ACS Mater. Lett. 2023, 5, 1061–1082. [Google Scholar] [CrossRef]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal–organic Molecular Cages: Applications of Biochemical Implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Feng, X.; Zhu, X.; Wang, Y.; Yang, J. Anticancer Agents Based on Metal Organic Cages. Coord. Chem. Rev. 2024, 500, 215546. [Google Scholar] [CrossRef]
- Cruz-Nava, S.; De Jesús Valencia-Loza, S.; Percástegui, E.G. Protection and Transformation of Natural Products within Aqueous Metal-organic Cages. Eur. J. Org. Chem. 2022, 2022, e202200844. [Google Scholar] [CrossRef]
- Tapia, L.; Alfonso, I.; Solà, J. Molecular Cages for Biological Applications. Org. Biomol. Chem. 2021, 19, 9527–9540. [Google Scholar] [CrossRef]
- Zheng, Y.-R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt(iv) Prodrugs Within a Pt(ii) Cage for Drug Delivery. Chem. Sci. 2015, 6, 1189–1193. [Google Scholar] [CrossRef]
- Yue, Z.; Wang, H.; Bowers, D.J.; Gao, M.; Stilgenbauer, M.; Nielsen, F.; Shelley, J.T.; Zheng, Y.-R. Nanoparticles of Metal–organic Cages Designed to Encapsulate Platinum-based Anticancer Agents. Dalton Trans. 2018, 47, 670–674. [Google Scholar] [CrossRef]
- Bhat, I.A.; Jain, R.; Siddiqui, M.M.; Saini, D.K.; Mukherjee, P.S. Water-Soluble Pd8L4 Self-assembled Molecular Barrel as an Aqueous Carrier for Hydrophobic Curcumin. Inorg. Chem. 2017, 56, 5352–5360. [Google Scholar] [CrossRef]
- Liang, Y.; Fang, Y.; Cui, Y.; Zhou, H. A Stable Biocompatible Porous Coordination Cage Promotes In Vivo Liver Tumor Inhibition. Nano Res. 2021, 14, 3407–3415. [Google Scholar] [CrossRef]
- Fang, Y.; Lian, X.; Huang, Y.; Fu, G.; Xiao, Z.; Wang, Q.; Nan, B.; Pellois, J.; Zhou, H. Investigating Subcellular Compartment Targeting Effect of Porous Coordination Cages for Enhancing Cancer Nanotherapy. Small 2018, 14, 1802709. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, V.W.L.; Ward, C.; Wang, H.; Holbrook, J.H.; Sekera, E.R.; Cui, H.; Hummon, A.B.; Badjić, J.D. Crystalline Nanoparticles of Water-soluble Covalent Basket Cages (cbcs) for Encapsulation of Anticancer Drugs. Angew. Chem. Int. Ed. 2023, 62, e202306722. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiu, Z.; Liu, H.; Jayawardhana, A.M.D.S.; Yue, Z.; Daghlas, H.; Bowers, D.J.; Datta, B.; Zheng, Y.-R. Nanoparticles of Metal-organic Cages Overcoming Drug Resistance in Ovarian Cancer. Front. Chem. 2019, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Mihaylova, R.; Momekova, D.; Shestakova, P.; Stoykova, S.; Zaharieva, J.; Yamashina, M.; Momekov, G.; Akita, M.; Yoshizawa, M. M2L4 Coordination Capsules with Tunable Anticancer Activity upon Guest Encapsulation. Dalton Trans. 2016, 45, 13214–13221. [Google Scholar] [CrossRef] [PubMed]
- Ahmedova, A.; Momekova, D.; Yamashina, M.; Shestakova, P.; Momekov, G.; Akita, M.; Yoshizawa, M. Anticancer Potencies of Ptii- and Pdii-linked M2L4 Coordination Capsules with Improved Selectivity. Chem. Asian J. 2016, 11, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.P. Synthetic Lectins. Org. Biomol. Chem. 2009, 7, 3629–3638. [Google Scholar] [CrossRef] [PubMed]
- Barwell, N.P.; Crump, M.P.; Davis, A.P. A Synthetic Lectin for Β-glucosyl. Angew. Chem. Int. Ed. 2009, 48, 7673–7676. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Zhao, C.-W.; Zhang, Y.-F.; Guan, Q.; Wan, J.-J.; Ma, J.-P.; Li, Y.-A.; Dong, Y.-B. A metal–organic cage-based nanoagent for enhanced photodynamic antitumor therapy. Chem. Commun. 2021, 57, 7954–7957. [Google Scholar] [CrossRef]
- Burke, B.P.; Grantham, W.; Burke, M.J.; Nichol, G.S.; Roberts, D.; Renard, I.; Hargreaves, R.; Cawthorne, C.; Archibald, S.J.; Lusby, P.J. Visualizing Kinetically Robust CoIII4L6 Assemblies In Vivo: SPECT Imaging of the Encapsulated [99mTc]TcO4– Anion. J. Am. Chem. Soc. 2018, 140, 16877–16881. [Google Scholar] [CrossRef]
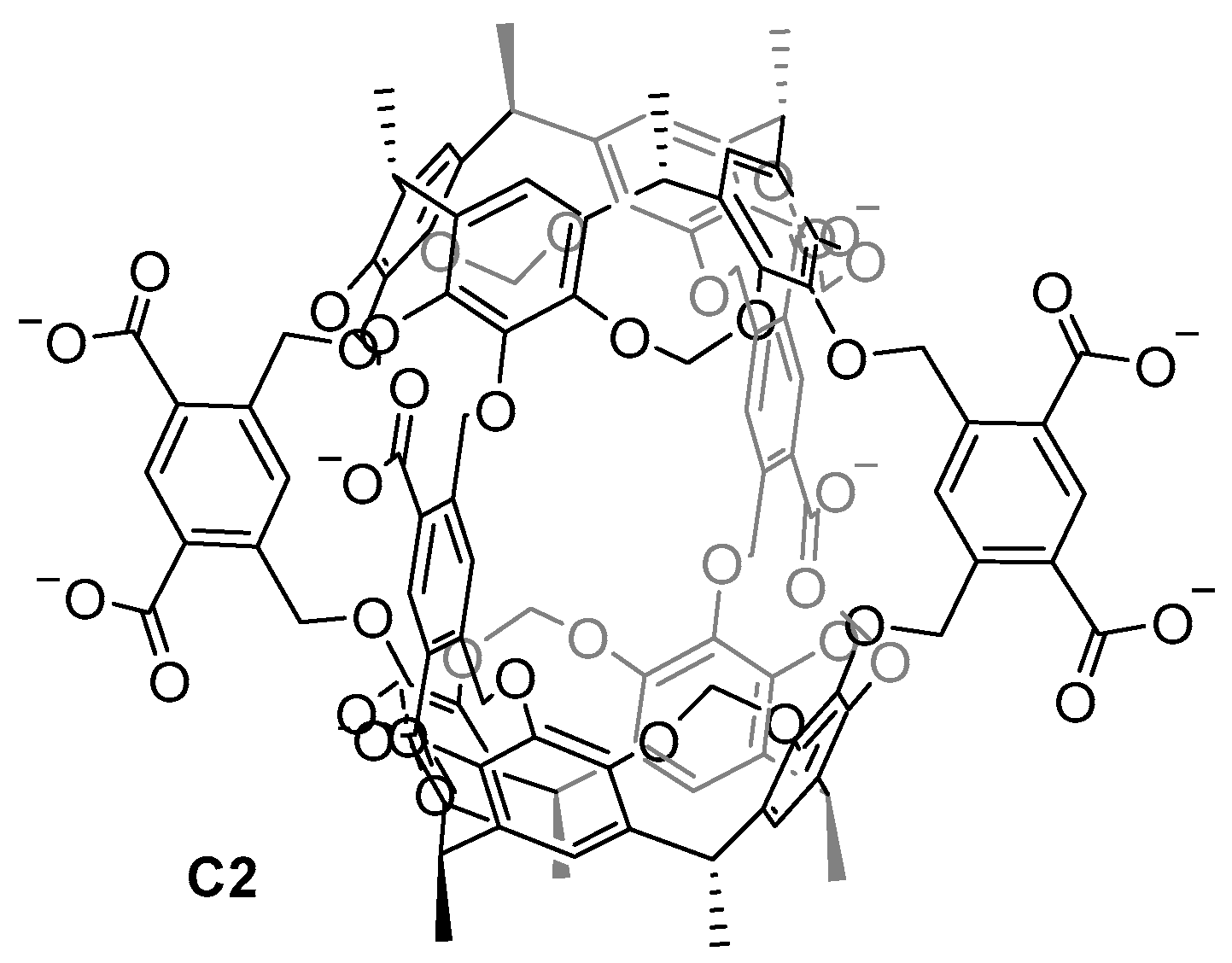
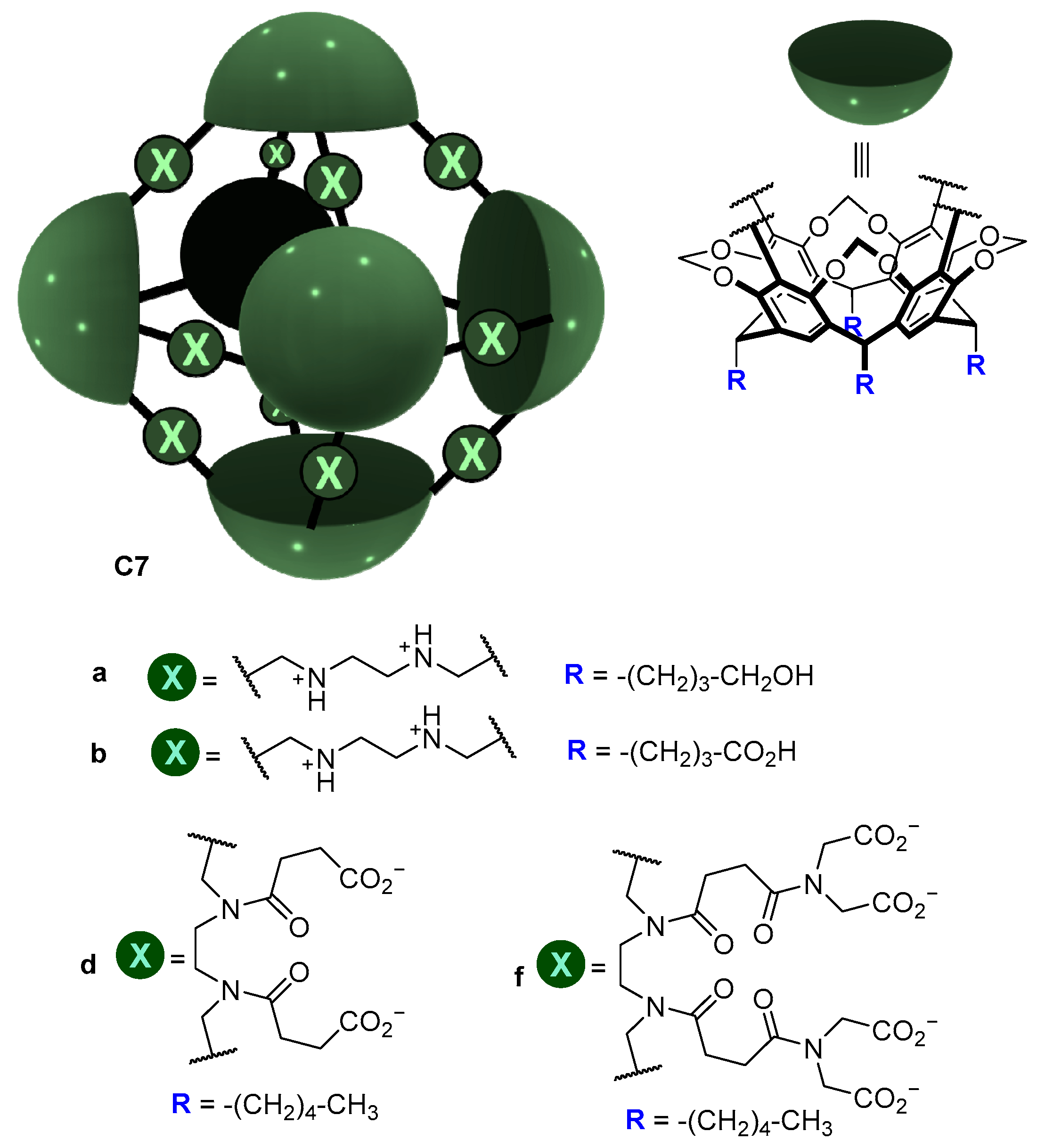
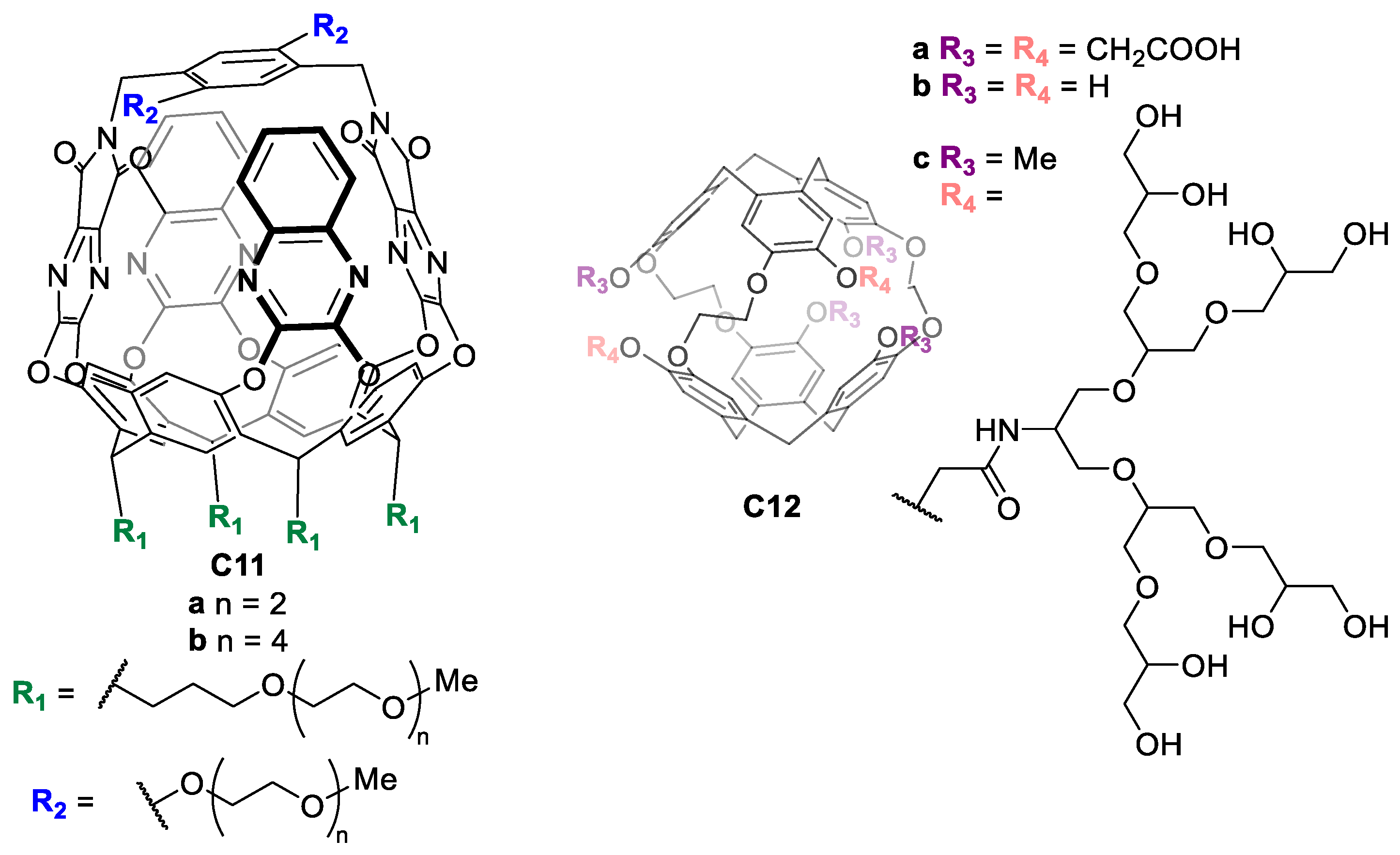
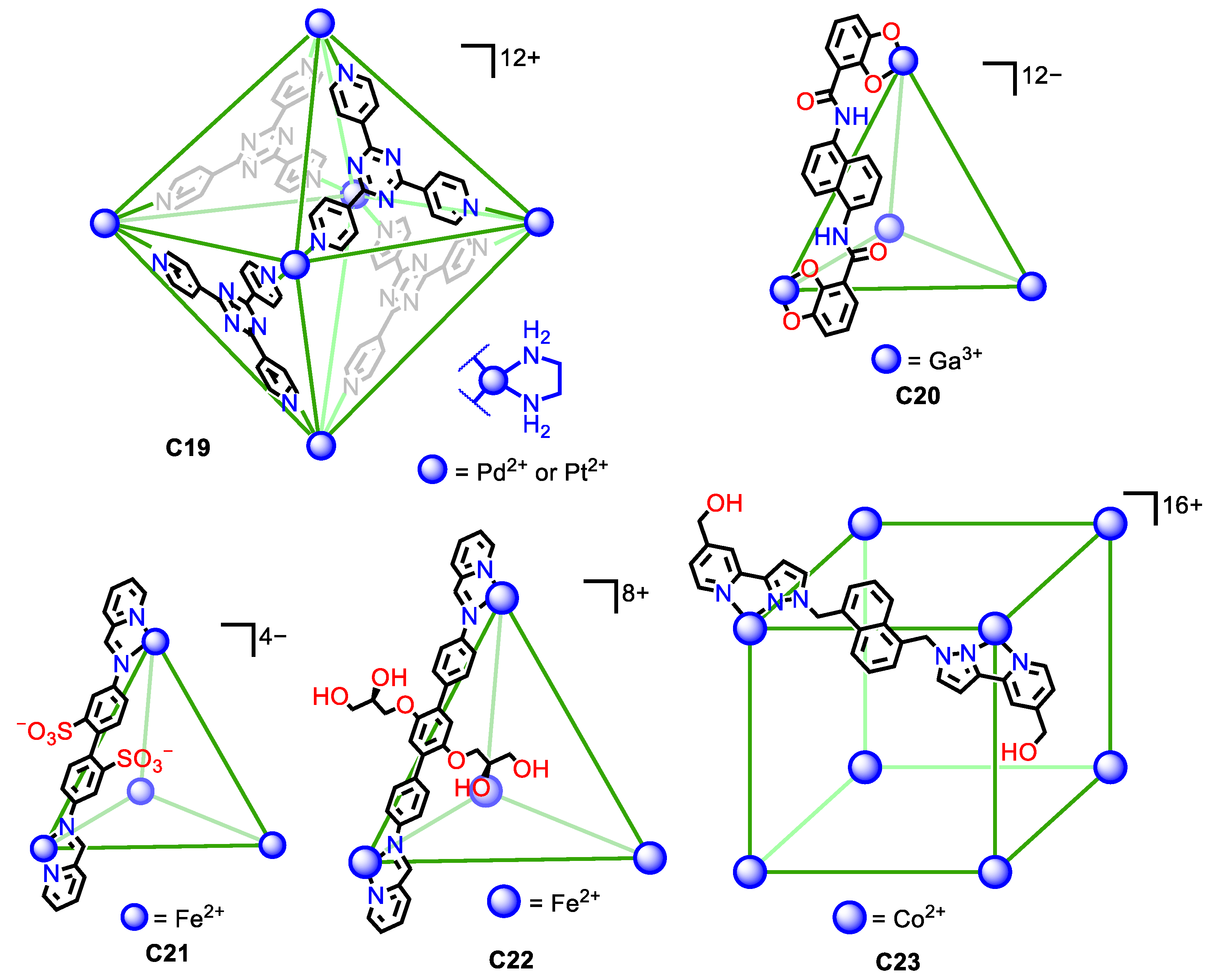
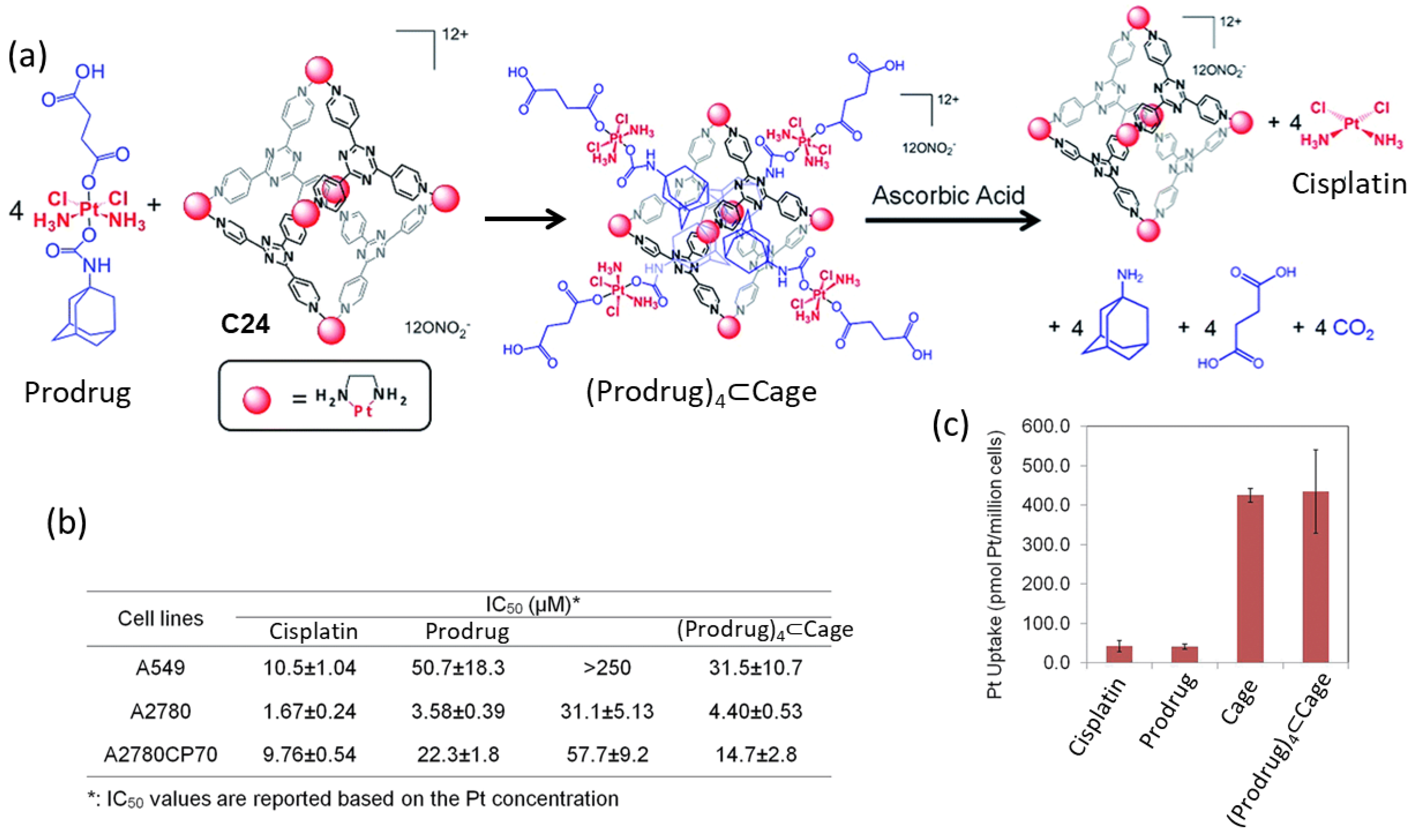
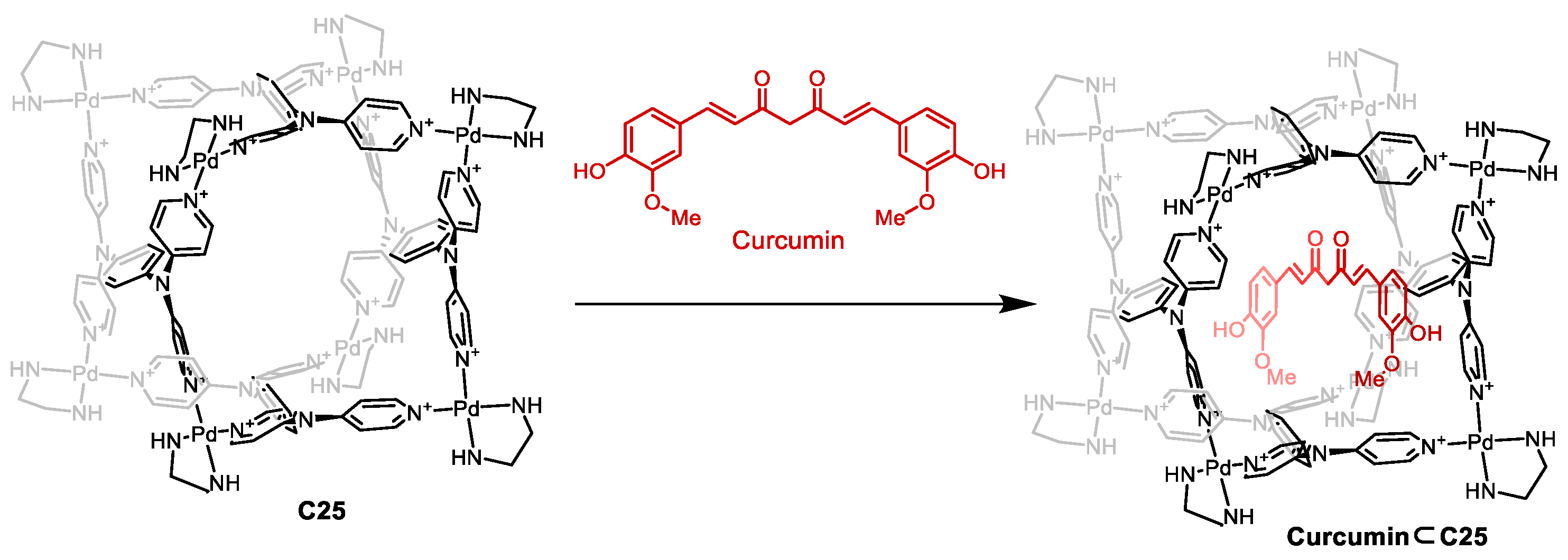
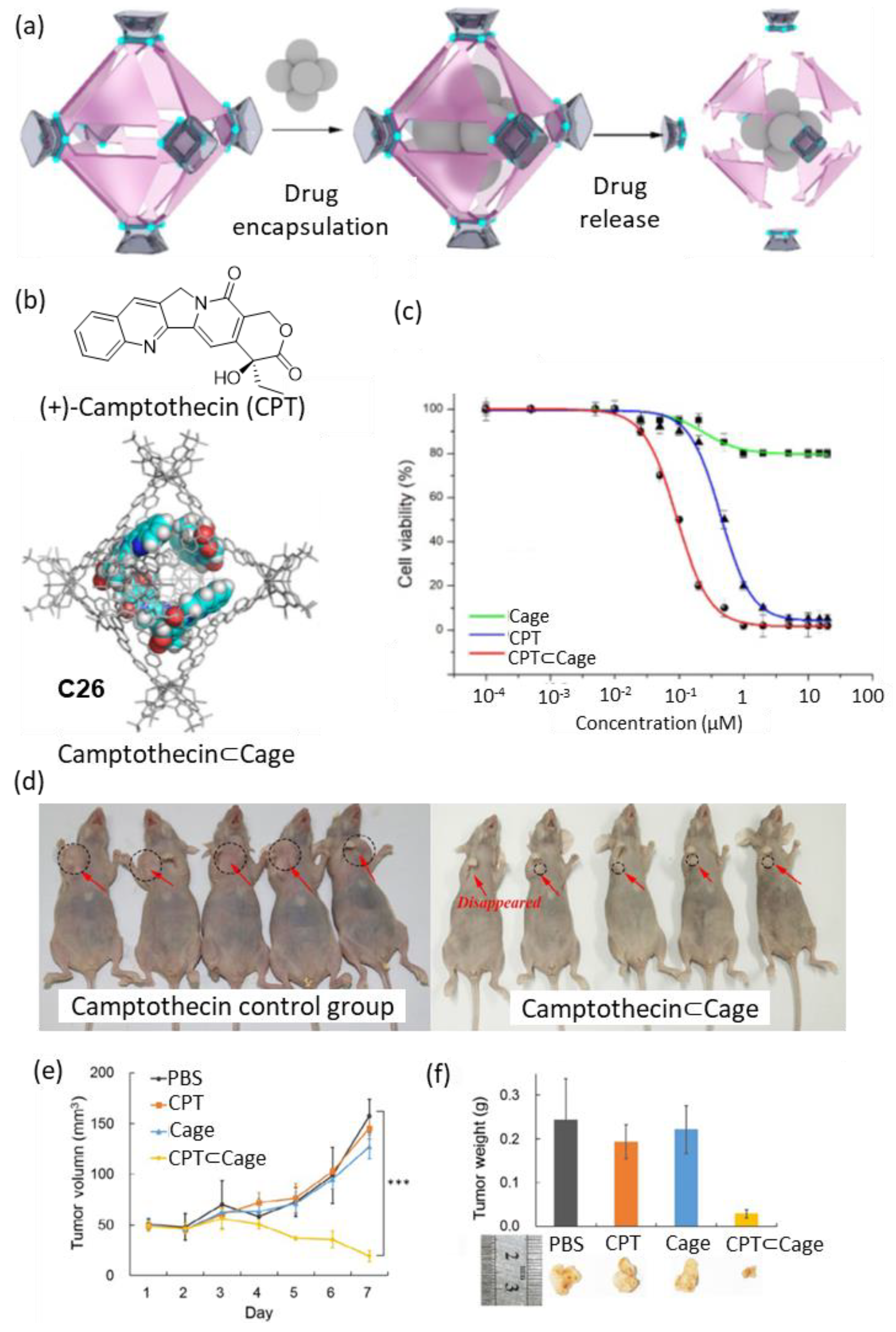
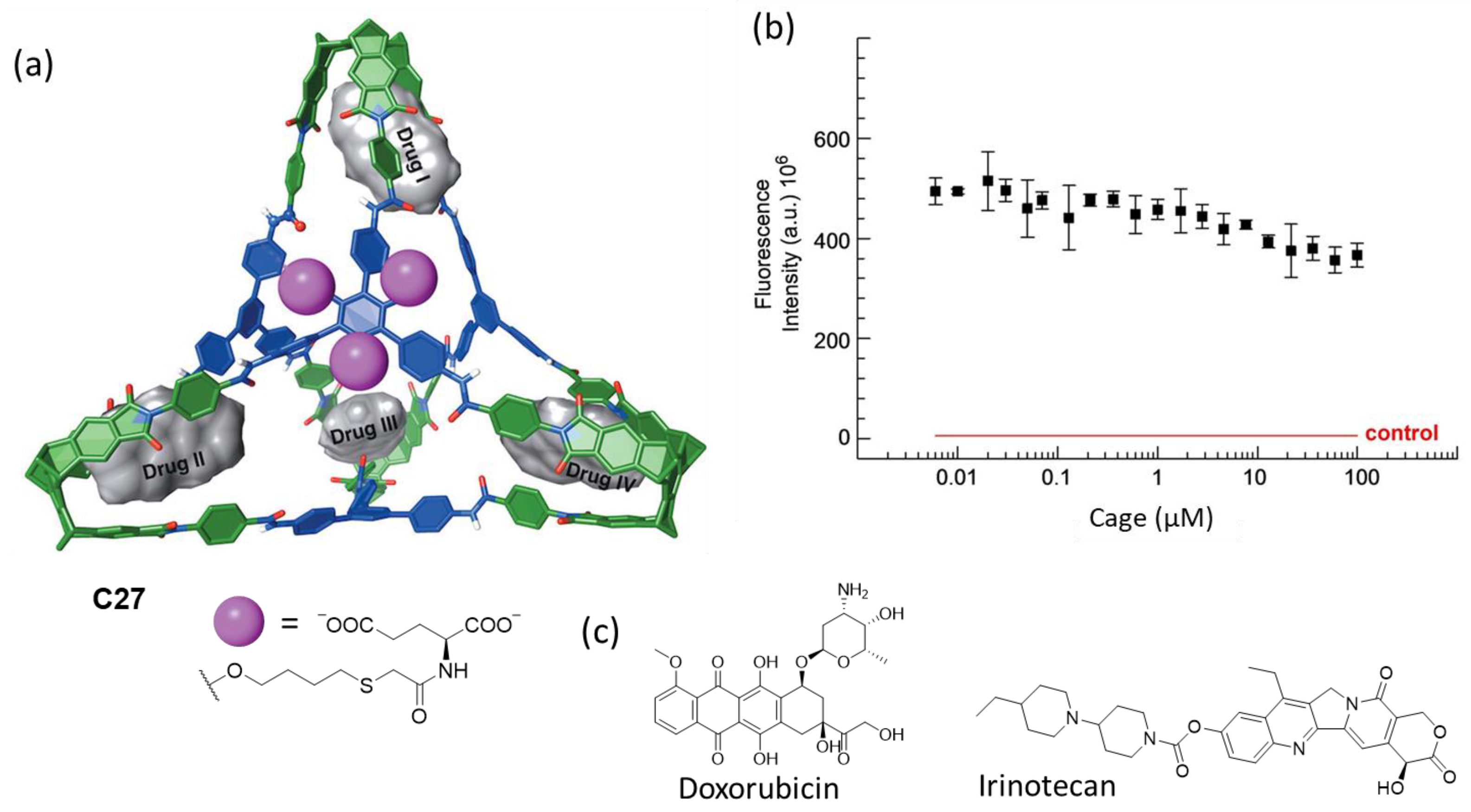
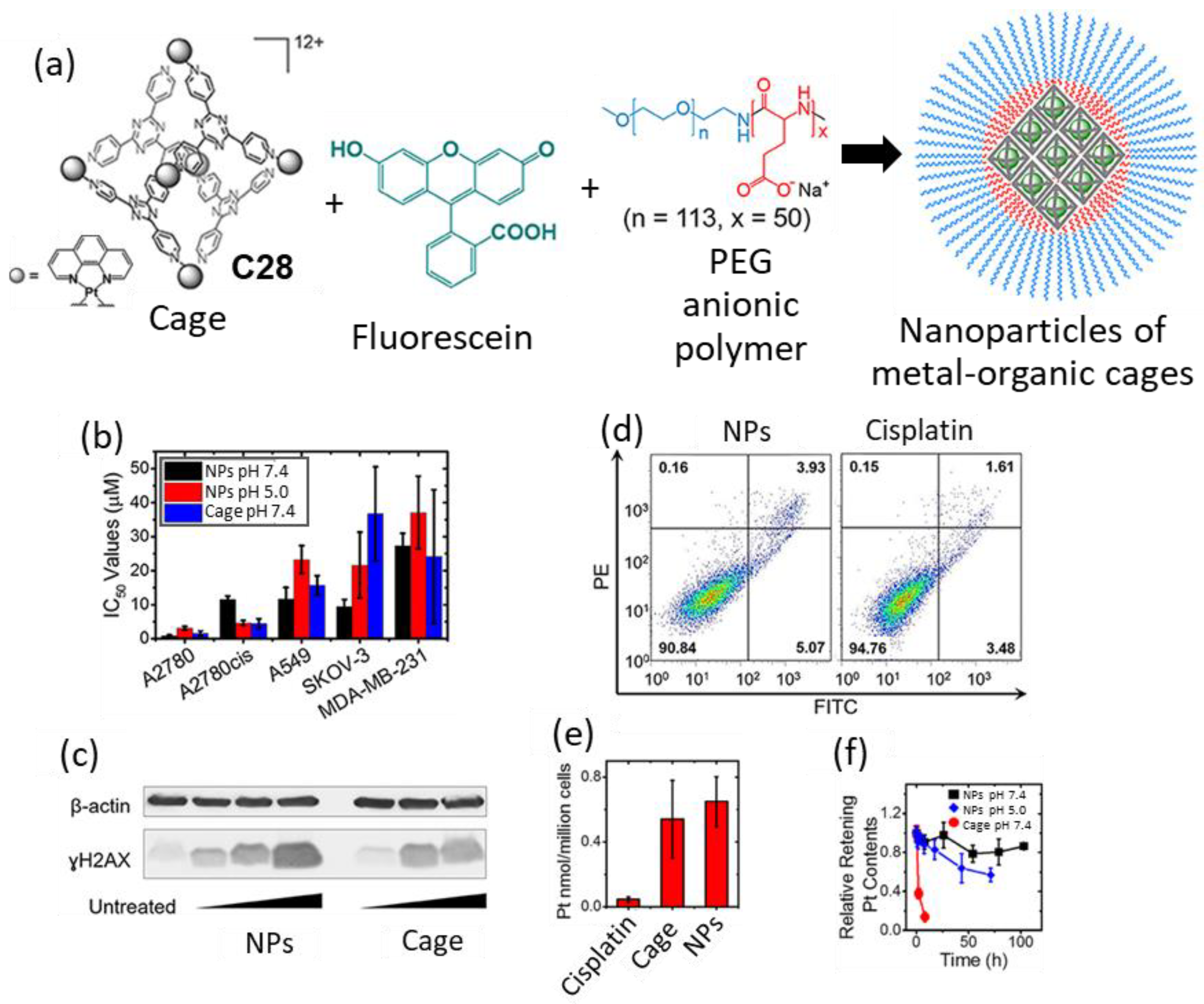
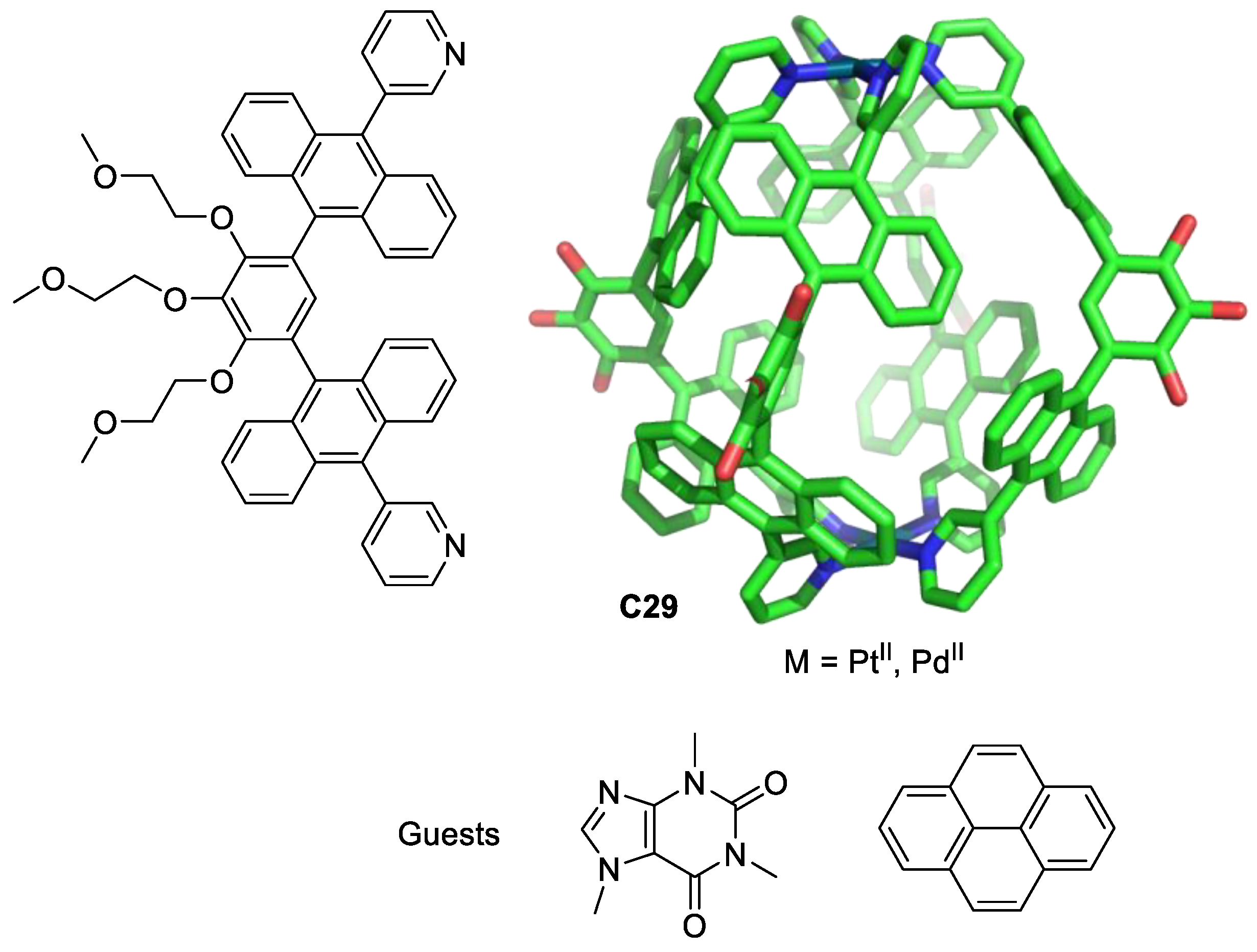

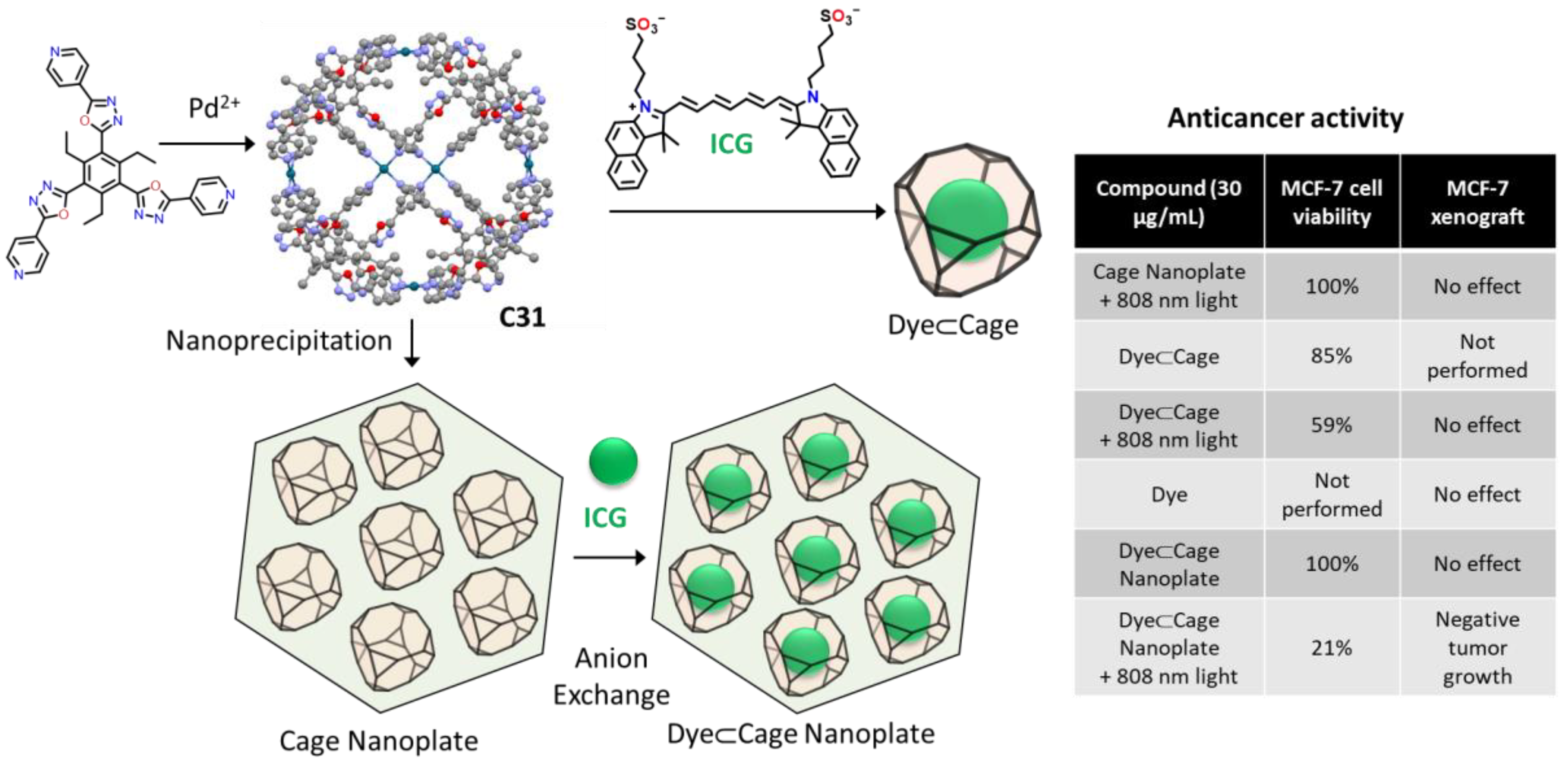
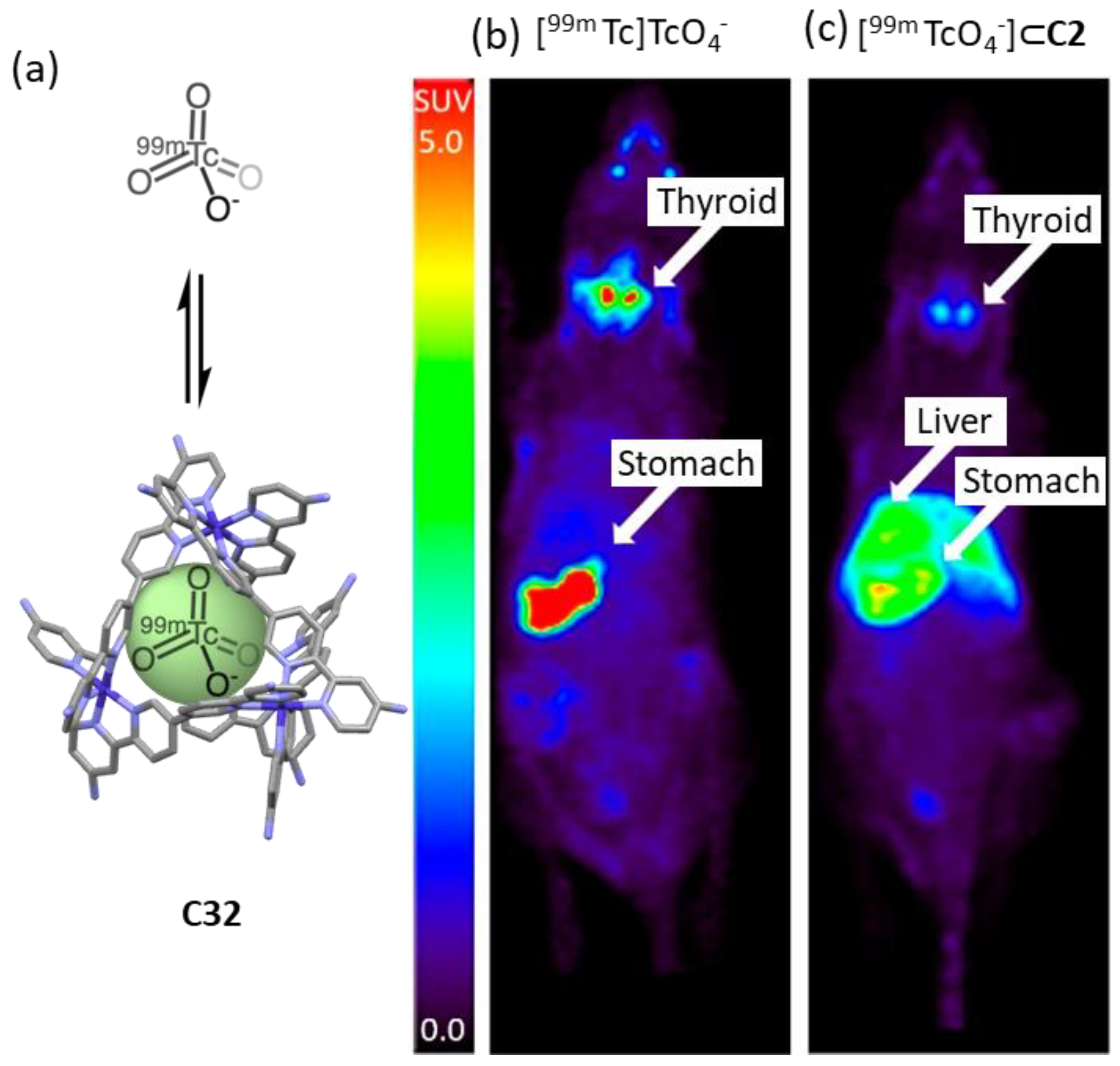
| Compound | HL-60 | HL-60/Dox | HT-29 | T-24 |
|---|---|---|---|---|
| (pyrene)2⊂C29-Pt | 22.8 | 21.5 | 109.4 | >100 |
| (caffeine)2⊂C29-Pt | 2.6 | 5.0 | 18.2 | >100 |
| C29-Pt | 5.0 | 1.0 | 0.9 | 5.3 |
| (pyrene)2⊂C29-Pd | 2.5 | 8.4 | 69.3 | >100 |
| (caffeine)2⊂C29-Pd | 0.7 | 4.7 | 23.2 | >100 |
| C29-Pd | 1.6 | 1.1 | 17.5 | 37.4 |
| Ligand, pyrene, caffeine | >100 | >100 | >100 | >100 |
| Cisplatin | 9.3 | 32.9 | 36.6 | 13.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montà-González, G.; Ortiz-Gómez, E.; López-Lima, R.; Fiorini, G.; Martínez-Máñez, R.; Martí-Centelles, V. Water-Soluble Molecular Cages for Biological Applications. Molecules 2024, 29, 1621. https://doi.org/10.3390/molecules29071621
Montà-González G, Ortiz-Gómez E, López-Lima R, Fiorini G, Martínez-Máñez R, Martí-Centelles V. Water-Soluble Molecular Cages for Biological Applications. Molecules. 2024; 29(7):1621. https://doi.org/10.3390/molecules29071621
Chicago/Turabian StyleMontà-González, Giovanni, Eduardo Ortiz-Gómez, Rocío López-Lima, Guillermo Fiorini, Ramón Martínez-Máñez, and Vicente Martí-Centelles. 2024. "Water-Soluble Molecular Cages for Biological Applications" Molecules 29, no. 7: 1621. https://doi.org/10.3390/molecules29071621
APA StyleMontà-González, G., Ortiz-Gómez, E., López-Lima, R., Fiorini, G., Martínez-Máñez, R., & Martí-Centelles, V. (2024). Water-Soluble Molecular Cages for Biological Applications. Molecules, 29(7), 1621. https://doi.org/10.3390/molecules29071621