Phytochemical Constituent Analysis of Phyllanthus emblica L. Fruit Nanoherbals by LC-HRMS and Their Antimutagenic Activity and Teratogenic Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Constituent Analysis of P. emblica Fruit Nanoherbal by LC-HRMS
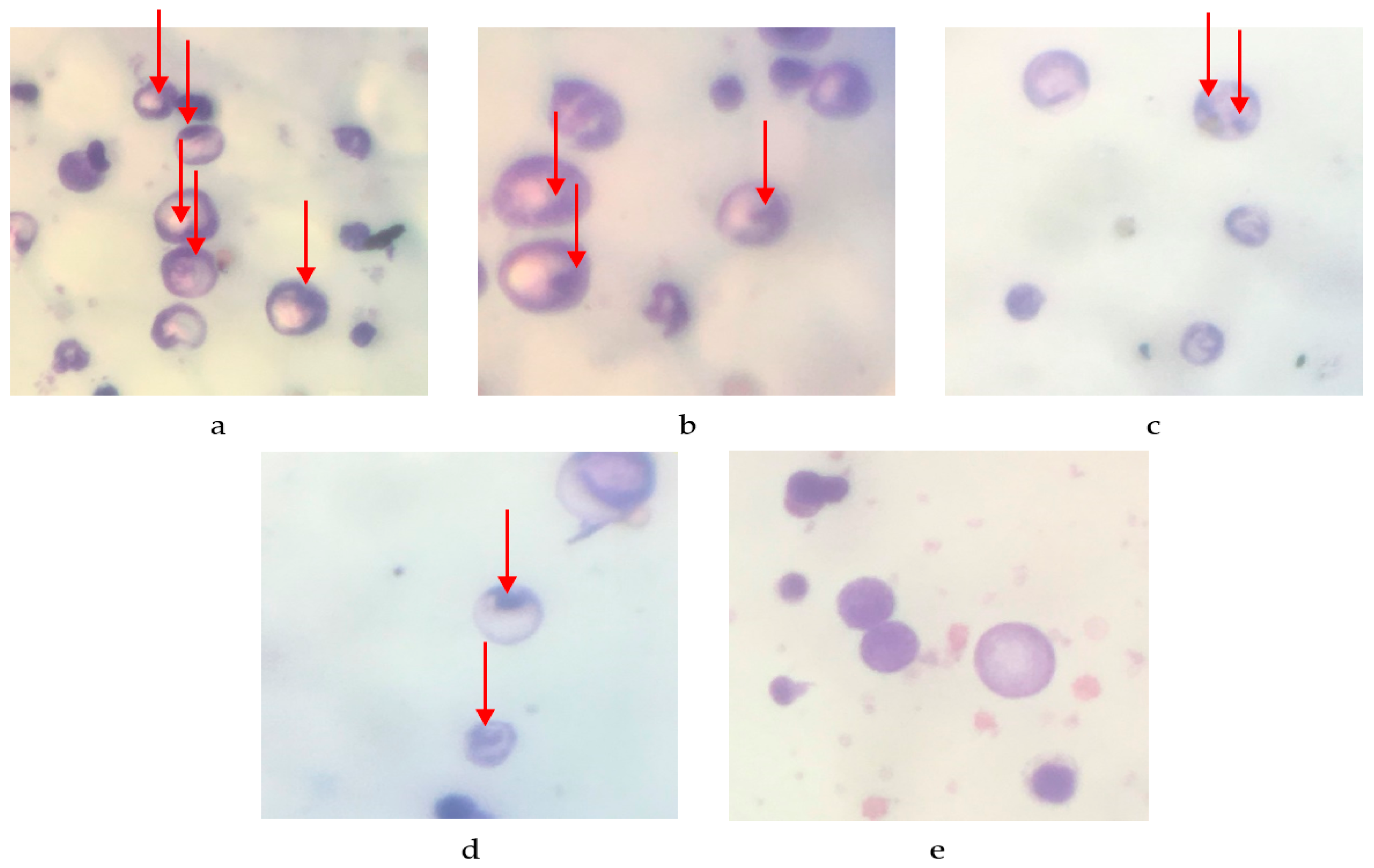
2.2. Antimutagenic Activity
2.3. Teratogenic Effects
2.3.1. Body Weight of Pregnant Rats
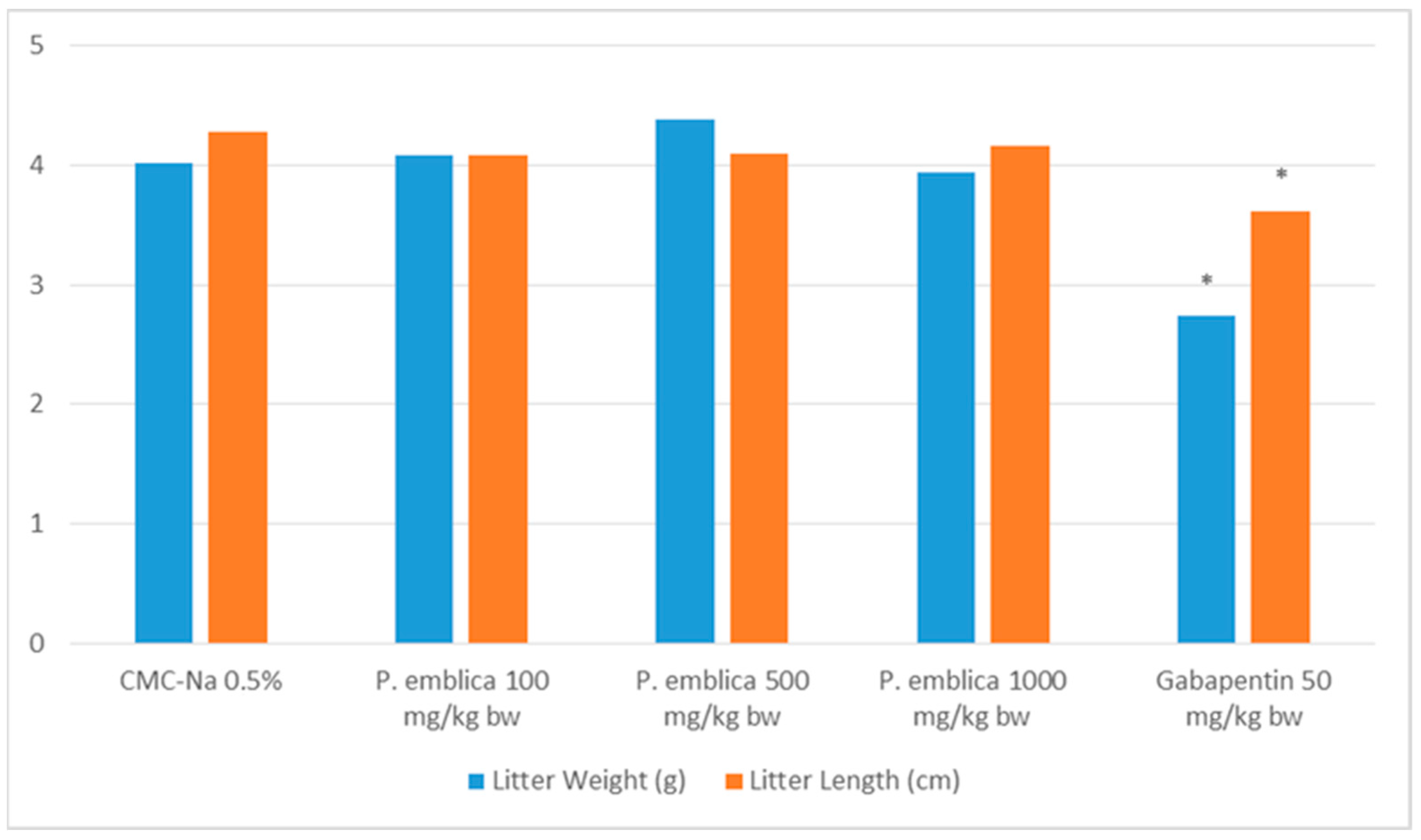
2.3.2. Litter Size
2.3.3. Litter Length and Birth Weight
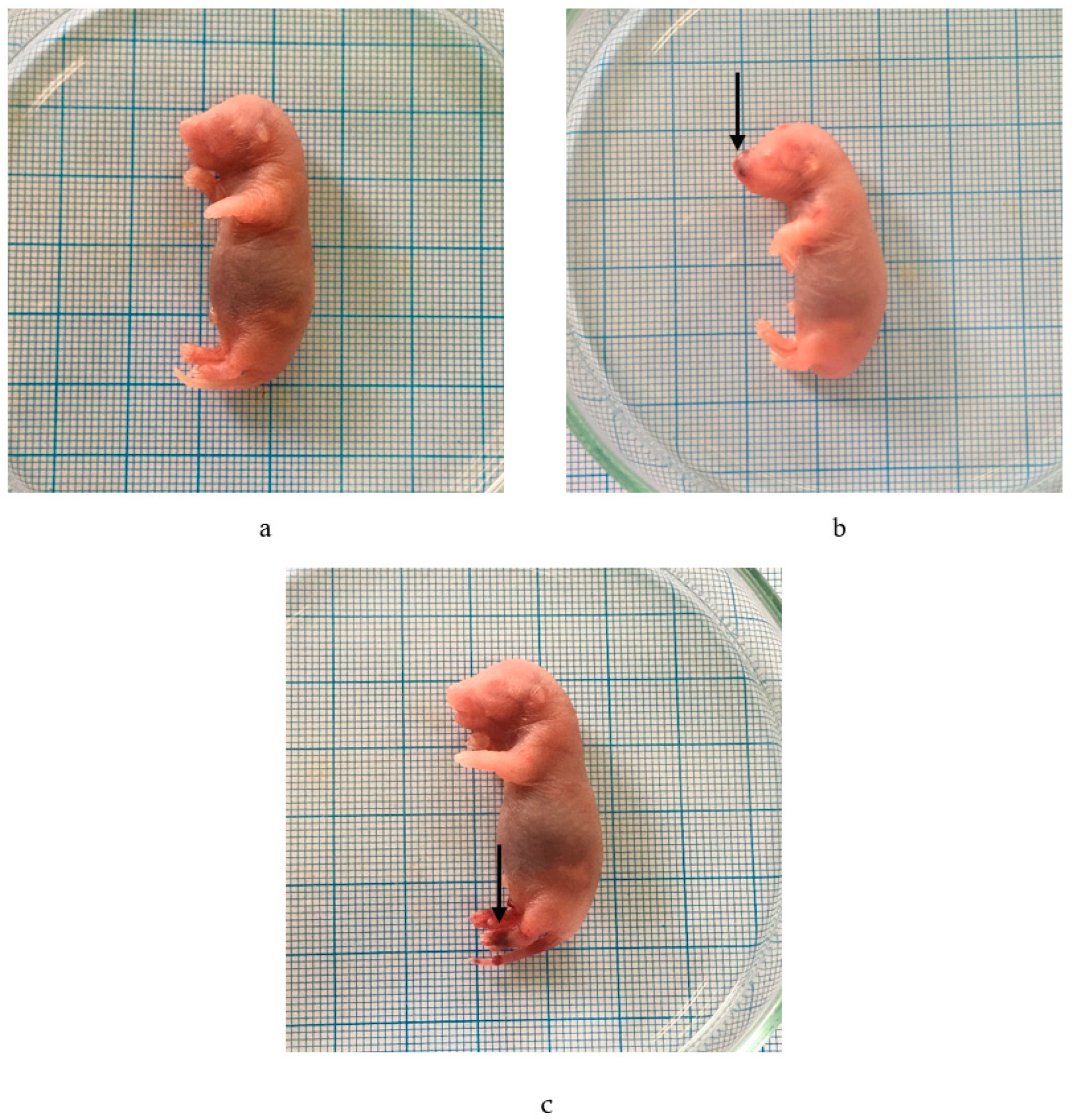
2.3.4. External Malformations
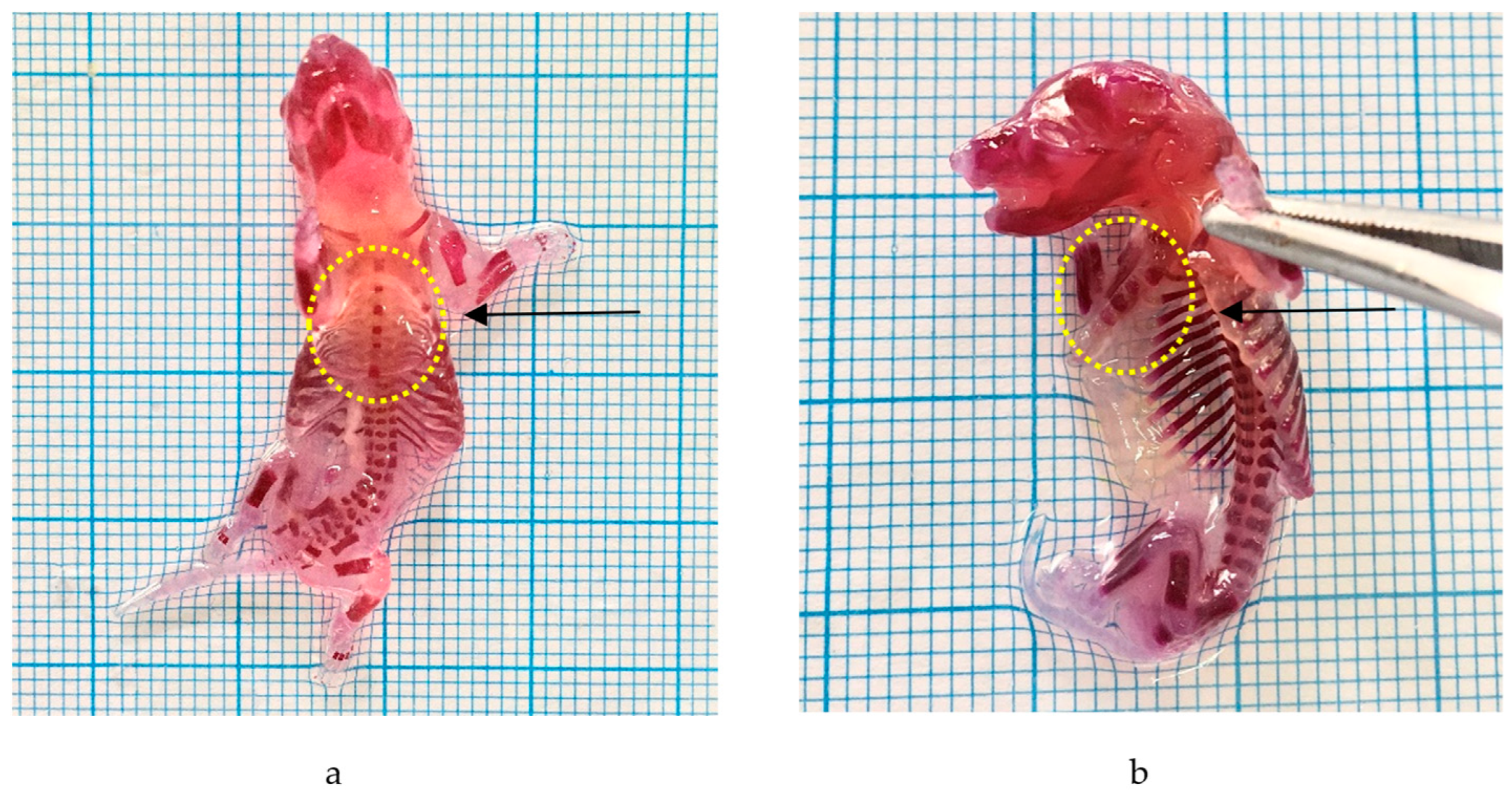
2.3.5. Skeletal Malformations
3. Materials and Methods
3.1. Materials
3.2. Animals
3.3. Phytochemical Constituent Analysis of P. emblica Fruit Nanoherbal by LC-HRMS
3.4. Antimutagenic Activity Testing
3.4.1. Testing of Antimutagenic Effects in Mice
3.4.2. Preparation of Femoral Bone Marrow Smears
3.5. Teratogenic Effect Testing
3.5.1. Confirmation of Pregnancy
3.5.2. Treatment
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almahdy, A. Experimental Teratology; Universitas Andalas Press: Padang, Indonesia, 2012. [Google Scholar]
- Singh, E.; Sharma, S.; Pareek, A.; Dwivedi, J.; Yadav, S. Phytochemistry, Traditional uses and cancer Chemopreventive Activity of Amla (Phyllanthus emblica). Sustain. J. Appl. Pharm. 2011, 2, 176–183. [Google Scholar]
- Uji, T. Species Diversity of Indigenous Fruits in Indonesia and its Potential. Biodiversitas 2007, 8, 157–167. [Google Scholar] [CrossRef]
- Masfria, M.; Mukhlisyam, M.; Permata, Y.M.; Faizar, F. Evaluation of Antihyperlipidemic and Antidiabetic Activity of Phyllanthus emblica L. Fruits. Trop. J. Nat. Prod. Res. 2021, 5, 668–672. [Google Scholar]
- Zhang, L.Z.; Zhao, W.H.; Guo, Y.J.; Tu, G.Z.; Lin, S.; Xin, L.G. Studies on Chemical Constituens in Fruits of Tibetian Medicine Phyllanthus emblica. China J. Chin. Mater. Med. 2003, 28, 940–943. [Google Scholar]
- Sudiana, I.K. Molecular Pathobiology of Cancer; Salemba Medika: Jakarta, Indonesia, 2008. [Google Scholar]
- Schmid, W. The Micronucleus Test. Mutat. Res. 1975, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Depkes, R.I. General Standard Parameters of Medicinal Plant Extracts; Dirjen POM: Jakarta, Indonesia, 2000. [Google Scholar]
- Purnamawati, D.; Ariawan, I. Consumption of Herbal Medicine for Pregnant Women as a Risk Factor for Newborn Asphyxia. Natl. J. Public Health 2012, 6, 267–272. [Google Scholar] [CrossRef]
- BPOM RI. In Vivo Nonclinical Toxicity Test Guidelines; Drug and Food Research Center of BPOM RI: Jakarta, Indonesia, 2022. [Google Scholar]
- Sumpena, Y.; Sofyan, R.; Rusilawati, R. Benzo(a)pyrene Mutagenicity Test with Micronucleus Method on Bone Marrow of Albino Mice (Mus musculus). Cermin Dunia Kedokt. 2009, 36, 23–36. [Google Scholar]
- Lakhanpal, P.; Deepak, K.R. Quercetin: A Versatile Flavonoid. J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Chen, H.C.; Roco, M.C.; Li, X.; Lin, Y.L. Trends in Nanotechnology Patents. Nat. Nanotechnol. 2008, 3, 123–125. [Google Scholar] [CrossRef]
- Vijaykumar, N.; Venkateswarlu, V.; Raviraj, P. Development of Oral tablet Dosage form Incorporating Drug Nanoparticles. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 952–955. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterial and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hong, T.; Dong, M.; Meng, Y.; Mu, J. Protective Effect of Myricetin in Dextran Sulphate Sodium-Induced Murine Ulcerative Colitis. Mol. Med. Rep. 2013, 7, 565–570. [Google Scholar] [CrossRef]
- Lu, F.C. Basic Toxicology: Principles, Organs, Objectives and Risk Assessment, 2nd ed.; Universitas Indonesia Press: Jakarta, Indonesia, 1995. [Google Scholar]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective Effects of Ursolic Acid and Luteolin against Oxidative DNA Damage include Enhancement of DNA Repair in Caco-2 cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2010, 692, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and Apoptosis inducing Activities of Ellagic Acid. Anticancer Res. 2006, 26, 3601–3606. [Google Scholar] [PubMed]
- Godarzi, S.M.; Gorji, A.V.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant Effect of p-Coumaric Acid on Interleukin 1-β and Tumor Necrosis Factor-α in Rats with Renal Ischemicreperfusion. Nefrologia 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Klaidman, L.K.; Mukherjee, S.K.; Hutchin, T.P.; Adam, J.D. Nicotinamide as a Precursor for NAD+ Prevents Apoptosis in the Mouse Brain induced by Tertiary-butylhydroperoxide. Neurosci. Lett. 1996, 206, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bogan, K.L.; Brenner, C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD+ Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008, 28, 15–30. [Google Scholar] [CrossRef]
- Kamat, J.P.; Devasagayam, T.P.A. Nicotinamide (Vitamin B3) as an Effective Antioxidant against Oxidative Damage in Rat Brain Mitochondria. Redox Rep. 1999, 4, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhao, G.; Zhu, Y.; Chen, X.; Zhang, N.; Yang, J.; Lin, H. Protective Effects of Nicotinamide Riboside on H2O2-induced Oxidative Damage in Lens Epithelial Cells. Curr. Eye Res. 2021, 46, 961–970. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, L.; Jiang, R.; Liao, C.; Xu, J.; Jiang, S.; Yang, Y.; Lin, L.; Huang, J.; Shen, Y.; et al. Nicotinic Acid against Acetaminophen-Induced Hepatotoxicity via Sirt1/Nrf2 Antioxidative Pathway in Mice. J. Nutr. Sci. Vitaminol. 2021, 67, 145–152. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, W.D.; Liu, Y.; Chen, G.F.; Jiang, J.; Li, S.H.; Feng, L.; Zhou, X.Q. Effect of Choline on Antioxidant Defenses and Gene Expressions of Nrf2 Signaling Molecule in the Spleen and Head Kidney of Juvenile Jian Carp (Cyprinus carpio var. Jian). Fish Shellfish. Immunol. 2014, 38, 374–382. [Google Scholar] [CrossRef]
- Costa, M.C.; Tayra FO, L.; Arcaro, C.A.; Inacio, M.D.; Duharte, A.B.; Carlos, I.Z.; Spolidorio, L.C.; Assis, R.P.; Brunetti, I.L.; Baviera, A.M. Trigonelline and Curcumin Alone, but not in Combination, Counteract Oxidative Stress and Inflammation and Increase Glycation Product Detoxification in the Liver and Kidney of Mice with High-Fat Diet-Induced Obesity. J. Nutr. Biochem. 2019, 76, 108303. [Google Scholar] [CrossRef] [PubMed]
- Muchtadi, D. Introduction to Nutrition; Alfabeta: Bandung, Indonesia, 2009. [Google Scholar]
- Eghbaliferiz, S.; Iranshahi, M. Prooxidant Activity of Polyphenols, Flavonoids, Anthocyanins and Carotenoids: Updated Review of Mechanisms and Catalyzing Metals. Phytother. Res. 2016, 30, 1379–1391. [Google Scholar] [CrossRef]
- Hagerman, A.E.; Riedl, K.M.; Jones, G.A.; Sovik, K.N.; Ritchard, N.T.; Hartzfeld, P.W.; Riechel, T.L. High Molecular Weight Plant Polyphenolics (Tannins) as Biological Antioxidants. J. Agric. Food Chem. 1998, 46, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Fenech, M.; Kirsch-Volders, M.; Natarajan, A.T.; Suralles, J.; Crott, J.W.; Parry, J.; Norppa, H.; Eastmond, D.A.; Tucker, J.D.; Thomas, P. Molecular Mechanisms of Micronucleus, Nucleoplasmic Bridge and Nuclear Bud Formation in Mammalian and Human Cells. Mutagenesis 2011, 26, 125–132. [Google Scholar] [CrossRef]
- Didi, J.P.; Anas, S.; Cucu, H.; Supriyatna. Antimutagenic and Antioxidant Activity of Puspa Leaves (Schima wallichii Kort). Cermin Dunia Kedokteran 2000, 127, 18–21. [Google Scholar]
- Smith, D.G.G.; Aronson, J.K. Oxford Textbook of Clinical Pharmacology and Drug Therapy; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Czyzewska, A.; Mazur, L. Suppressing Effect of WR-2721 on Micronucleus Induced by Cyclophosphamide in Mice. Teratog. Carcinongenesis Mutagen. 1995, 15, 109–114. [Google Scholar] [CrossRef]
- Ramu, K.; Perry, C.S.; Ahmed, T.; Pakenham, G.; Kehrer, J.P. Studies on the Basis for the Toxicity of Acrolein Mercapturates. Toxicol. Appl. Pharmacol. 1996, 140, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Wang, J.; Yang, B.; Jiang, Y. Antioxidant Activity of Methanolic Extract of Emblica Fruit (Phyllanthus emblica L.) from Six Regions China. J. Food Compos. Anal. 2007, 21, 219–228. [Google Scholar] [CrossRef]
- Lingga, M.N.; Rustikawati, I.; Buwono, I.D. The Effectiveness of Kecombrang Flower Extract (Nicolaia speciosa Horan) for the Prevention of Attacks of Saprolegnia Sp. on Sangkuriang Catfish. J. Fish. Mar. 2012, 3, 75–80. [Google Scholar]
- Marusin, N.; Almahdy, A.; Fitri, H. Test of Vitamin A Activity against the Teratogen Effect of Warfarin on Fetuses of White Mice. J. Pharm. Sci. Technol. 2011, 21, 617–629. [Google Scholar]
- Guyton, A.C. Textbook of Medical Physiology; Tenyadi, K.A., Translator; Part III; EGC Book of Medicine: Jakarta, Indonesia, 1994. [Google Scholar]
- Wilson, J.G.; Warkany, J. (Eds.) Teratology: Principles and Techniques; University of Chicago Press: Chicago, IL, USA, 1965. [Google Scholar]
- Olayaki, L.A.; Olatunji-Bello, I.; Soladoye, A.O.; Jimoh, O.R.; Ghazal, O.; Ighodalo, M. Effects of Aqueous Leaf Extract of Cajanus cajan on Litter Size and Serum Progesterone in Pregnant Rats. J. Pharmacogn. Phytother. 2009, 1, 021–024. [Google Scholar]
- Yantrio, A.; Sugiyanto, J.; Aida, Y. Effects of Chlorambucil on Fetal Development of White Rat (Rattus norvegicus L.) Sprague-Dawley Strains. J. Biota 2012, 7, 101–108. [Google Scholar]
- Wilson, J.G. Environment and Birth Defects; Academic Press: London, UK, 1973. [Google Scholar]
- Nogrady, T. Medical Chemistry: A Biochemical Approach; ITB Press: Bandung, Indonesia, 1992. [Google Scholar]
- Katzung, B.G. Basic and Clinical Pharmacology; Edisi 6; EGC Book of Medicine: Jakarta, Indonesia, 1997. [Google Scholar]
- Tine, Y.; Yang, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. LC-MS/MS Analysis of Flavonoid Compounds from Zanthoxylum zanthoxyloides Extracts and Their Antioxidant Activities. Nat. Prod. Commun. 2017, 12, 1934578X1701201213. [Google Scholar] [CrossRef]
- Kalil, I.C.; Gibson BA, V.; Ribeiro, C.A.; Benincá, L.S.; Brasil, G.A.; Andrade, T.U.; Endringer, D.C. Antimutagenic Activity of Carica papaya L. Assayed in vivo by Micronucleus Test. Rev. Ciências Farm. Básica Apl. 2011, 32, 419–423. [Google Scholar]
- Devi, K.R.; Reddy, K.D. Protective Effects of Solanum lycopersicum Fruit Extract on Lead Induced Micronucleus in Bone Marrow Cells of Mice. World J. Pharm. Res. 2014, 3, 523–531. [Google Scholar]
- Jain, R.A.; Agarwal, R.C.; Pandey, A.; Jain, R. Evaluation of Argemone mexicana Fruits Extract using Micronucleus Assay in Mouse Bone Marrow Cells. Bull. Pharm. Res. 2011, 1, 22–24. [Google Scholar]
- Organisation for Economic Co-Operation and Development; Test No. 414: Prenatal Developmental Toxicity Study; OECD Publishing: Berlin, Germany, 2018.
- Yuandani; Tarigan, K.S.A.; Yuliasmi, S. Teratogenic Effects of Ethanol Extract of Curcuma mangga Val. Rhizomes in Wistar Rats. Toxicol. Res. 2021, 37, 429–434. [Google Scholar] [CrossRef]
| No. | Name | Formula | Molecule Weight | Retention Time (min) |
|---|---|---|---|---|
| 1 | 3,5-di-tert-Butyl-4-hydroxybenzoic acid | C15H22O3 | 250.15 | 15.02 |
| 2 | NP-004917 | C15H26O3 | 276.17 | 13.52 |
| 3 | NP-020014 | C15H26O3 | 276.17 | 17.57 |
| 4 | 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile | C15H13ClN2S | 326.00 | 4.63 |
| 5 | 3,5-di-tert-Butyl-4-hydroxybenzaldehyde | C15H22O2 | 234.16 | 17.04 |
| 6 | Quercetin | C15H10O7 | 302.04 | 7.98 |
| 7 | 2-[(2-chlorobenzyl)sulfanyl]-4,6-dimethylnicotinonitrile | C15H13ClN2S | 326.00 | 20.33 |
| 8 | (2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl(2E)-3-phenylprop-2-enoate | C15H18O7 | 332.08 | 7.63 |
| 9 | Kojic acid | C6H6O4 | 142.02 | 2.04 |
| 10 | 4-Coumaric acid | C9H8O3 | 164.04 | 2.06 |
| 11 | Nicotinamide | C6H6N2O | 122.04 | 2.05 |
| 12 | Nicotinic acid | C6H5NO2 | 106.00 | 2.05 |
| 13 | Ellagic acid | C14H6O8 | 302.00 | 7.19 |
| 14 | 3,4-Dihydroxyphenylpropionic acid | C9H10O4 | 164.04 | 13.51 |
| 15 | Myricitrin | C21H20O12 | 464.09 | 7.14 |
| 16 | Trigonelline | C7H7NO2 | 137.04 | 1.54 |
| 17 | Betaine | C5H11NO2 | 117.07 | 1.51 |
| 18 | Choline | C5H13NO | 103.09 | 1.48 |
| 19 | D-(+)-Proline | C5H9NO2 | 115.06 | 1.55 |
| Group | Number of Micronucleus/200 Cells ± SEM | Percentage Reduction Micronucleus |
|---|---|---|
| Negative control | 136.8 ± 6.591 | 31.6 |
| Dose of 100 mg/kg BW | 71.2 ± 3.611 * | 64.4 |
| Dose of 200 mg/kg BW | 47.6 ± 3.187 * | 76.2 |
| Dose of 400 mg/kg BW | 16.8 ± 2.059 * | 91.6 |
| Normal control | 0 ± 0 * | 100 |
| Day | Body Weight (g) ± SEM (Mean ± SEM) | ||||
|---|---|---|---|---|---|
| CMC Na 0.5% | P. emblica 100 mg/kg BW | P. emblica 500 mg/kg BW | P. emblica 1000 mg/kg BW | Gabapentin 50 mg/kg BW | |
| 6 | 217.10 ± 5.52 | 238.80 ± 7.99 | 232.84 ± 9.45 | 217.920 ± 7.02 | 216.46 ± 6.62 |
| 7 | 219.88 ± 5.91 | 240.64 ± 7.47 | 235.02 ± 9.45 | 219.48 ± 7.28 | 218.94 ± 6.77 |
| 8 | 222.30 ± 5.80 | 242.24 ± 7.60 | 235.30 ± 9.86 | 222.70 ± 6.82 | 221.64 ± 6.95 |
| 9 | 224.56 ± 5.76 | 243.86 ± 7.57 | 237.10 ± 9.94 | 224.80 ± 6.81 | 223.38 ± 6.80 |
| 10 | 228.06 ± 5.48 | 245.66 ± 7.55 | 239.08 ± 9.81 | 227.7 ± 6.81 | 224.32 ± 5.29 |
| 11 | 232.92 ± 6.01 | 247.54 ± 8.75 | 240.94 ± 9.69 | 228.34 ± 6.94 | 224.50 ± 6.54 |
| 12 | 238.78 ± 4.86 | 250.12 ± 8.42 | 242.76 ± 9.78 | 230.94 ± 7.03 | 227.50 ± 6.89 |
| 13 | 243.26 ± 4.53 | 251.26 ± 9.11 | 244.84 ± 9.71 | 232.86 ± 7.38 | 225.52 ± 5.84 |
| 14 | 250.26 ± 5.10 | 252.72 ± 9.04 | 251.46 ± 9.57 | 235.64 ± 7.23 | 227.82 ± 5.47 |
| 15 | 254.94 ± 4.62 | 255.90 ± 8.98 | 260.10 ± 9.69 | 239.34 ± 7.47 | 229.84 ± 5.52 |
| 16 | 261.14 ± 6.47 | 257.98 ± 8.97 | 269.06 ± 9.65 | 246.48 ± 6.10 | 248.04 ± 11.44 |
| 17 | 265.42 ± 7.16 | 260.08 ± 8.91 | 272.92 ± 9.67 | 255.48 ± 6.53 | 253.86 ± 12.04 |
| 18 | 268.78 ± 6.93 | 265.64 ± 9.41 | 274.92 ± 9.62 | 264.08 ± 7.07 | 262.42 ± 12.05 |
| 19 | 272.48 ± 8.31 | 267.66 ± 9.32 | 285.32 ± 9.68 | 274.96 ± 5.68 | 270.18 ± 10.22 |
| Samples | Number of Pregnant Rats | Litter Size |
|---|---|---|
| CMC-Na 0.5% | 5 | 10.20 ± 0.37 |
| P. emblica 100 mg/kg BW | 5 | 9.40 ± 0.40 |
| P. emblica 500 mg/kg BW | 5 | 8.00 ± 1. 04 |
| P. emblica 1000 mg/kg BW | 5 | 9.80 ± 0.37 |
| Gabapentin 50 mg/kg BW | 5 | 9.80 ± 0.49 |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| CMC Na 0.5% | P. emblica 100 mg/kg BW | P. emblica 500 mg/kg BW | P. emblica 1000 mg/kg BW | Gabapentin 50 mg/kg BW | |
| Number of fetuses examined | 34 | 31 | 26 | 32 | 32 |
| Stunted | - | - | - | - | 8 |
| Hemorrhage | - | - | - | 6 | 8 |
| Parameters | Samples | ||||
|---|---|---|---|---|---|
| CMC Na 0.5% | P. emblica 100 mg/kg BW | P. emblica 500 mg/kg BW | P. emblica 1000 mg/kg BW | Gabapentin 50 mg/kg BW | |
| Number of fetuses examined | 17 | 16 | 14 | 17 | 17 |
| Truncus malformation | |||||
| - | - | - | 1 | 8 |
| - | - | - | - | - |
| Group | Treatment |
|---|---|
| I (negative control) | Animals were induced with 50 mg/kg BW cyclophosphamide solution on the first day, then administered CMC Na 0.5% suspension orally every day until the seventh day. |
| II (dose of 100 mg/kg BW) | In the test group, rats were induced with 50 mg/kg BW cyclophosphamide solution on the first day, then given 100 mg/kg BW P. emblica fruit nanoherbal orally every day until the seventh day. |
| III (dose of 200 mg/kg BW) | In the test group, rats were induced with 50 mg/kg BW cyclophosphamide solution on the first day, then given 200 mg/kg BW P. emblica fruit nanoherbal orally every day until the seventh day. |
| IV (dose of 400 mg/kg BW) | In the test group, rats were induced with 50 mg/kg BW cyclophosphamide solution on the first day, then given 400 mg/kg BW P. emblica fruit nanoherbal orally every day until the seventh day. |
| V (normal control) | Normal control animals received 0.5% CMC Na orally for 7 days. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masfria; Dalimunthe, A.; Suci, N.; Syahputra, H. Phytochemical Constituent Analysis of Phyllanthus emblica L. Fruit Nanoherbals by LC-HRMS and Their Antimutagenic Activity and Teratogenic Effects. Molecules 2024, 29, 1642. https://doi.org/10.3390/molecules29071642
Masfria, Dalimunthe A, Suci N, Syahputra H. Phytochemical Constituent Analysis of Phyllanthus emblica L. Fruit Nanoherbals by LC-HRMS and Their Antimutagenic Activity and Teratogenic Effects. Molecules. 2024; 29(7):1642. https://doi.org/10.3390/molecules29071642
Chicago/Turabian StyleMasfria, Aminah Dalimunthe, Nurul Suci, and Hafid Syahputra. 2024. "Phytochemical Constituent Analysis of Phyllanthus emblica L. Fruit Nanoherbals by LC-HRMS and Their Antimutagenic Activity and Teratogenic Effects" Molecules 29, no. 7: 1642. https://doi.org/10.3390/molecules29071642





