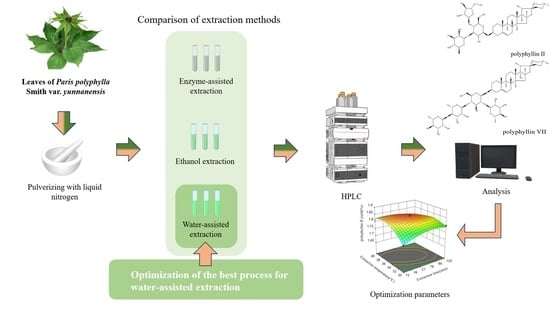
Response Surface Optimization for Water-Assisted Extraction of Two Saponins from Paris polyphylla var. yunnanensis Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pre-Experimentation
2.2. Single-Factor Experiment
2.3. Establishment of the Regression Equation and ANOVA
0.0268A2 − 0.0473B2 − 0.0342C2
0.0883A2 − 0.1744B2 − 0.0298C2
2.4. Optimization of the Extraction Conditions and Verification of the Mode
2.5. Response Surface Methodology Extracted Variables Optimization and Validation
3. Materials and Methods
3.1. Materials and Reagents
3.2. HPLC Analysis of Polyphyllin II and Polyphyllin VII
3.3. Pre-Experimentation
3.4. Single Factor Experiment
3.5. Response Surface Design
3.6. Statistical Analysis and Validation of Data
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, Y.L.; Yu, Z.Y.; Tang, X.M.; Zhao, Y.; Yuan, X.L.; Wang, S.; Ma, B.P.; Cong, Y.W. Pennogenin glycosides with a spirostanol structure are strong platelet agonists: Structural requirement for activity and mode of platelet agonist synergism. J. Thromb. Haemost. 2008, 6, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Cui, Y.; Huang, J.J.; Zhang, Y.Z.; Nie, Z.; Wang, L.F.; Yan, B.Z.; Tang, Y.L.; Liu, Y. Immuno-stimulating properties of diosgenyl saponins isolated from Paris polyphylla. Bioorg. Med. Chem. Lett. 2007, 17, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gong, G.Y.; Ma, L.L.; Wang, Z.Q.; Song, D.; Fang, M.Y. Anti-cancer effects of Polyphyllin I: An update in 5 years. Chem. Biol. Interact. 2020, 316, 108936. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Li, J.; Qiu, P.; Liu, J.; Liu, Z.; Ma, L.; Gao, W. Inhibition of lung cancer in diethylnitrosamine-induced mice by Rhizoma paridis saponins. Mol. Carcinog. 2017, 56, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.J.; Chen, P.; Zhu, F.Y.; Di, K.; Zhou, C.; Zhuang, J.; Cao, X.J.; Yang, J.; Deng, L.J.; Sun, C.G. Effect of Rhizoma paridis total saponins on apoptosis of colorectal cancer cells and imbalance of the JAK/STAT3 molecular pathway induced by IL-6 suppression. Genet. Mol. Res. 2015, 14, 5793–5803. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Man, S.; Li, J.; Zhang, Y.; Meng, X.; Gao, W. Inhibition of diethylnitrosamine-induced liver cancer in rats by Rhizoma paridis saponin. Environ. Toxicol. Pharmacol. 2016, 46, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Xu, L.; Li, J.; Zhai, Y.; Sun, Z.; Shi, W.; Jiang, Y.; Ma, C.; Lin, H.; Guo, Y.; et al. Polyphyllin II inhibits human bladder cancer migration and invasion by regulating EMT-associated factors and MMPs. Oncol. Lett. 2020, 20, 2928–2936. [Google Scholar] [CrossRef]
- Cheng, G.; Xue, Y.Y.; Fang, F.; Sun, G.Q.; Lu, Y.Y.; Ji, Y.Q.; Qiu, P.C.; Tang, H.F. Promotion of Ros-mediated Bax/Cyt-c apoptosis by polyphyllin II leads to suppress growth and aggression of glioma cells. Transl. Cancer Res. 2021, 10, 3894–3905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, X.; Wang, K.; Bao, J.; Li, P.; Chen, M.; Wan, J.B.; Su, H.; Mei, Z.; He, C. Polyphyllin VII Induces an Autophagic Cell Death by Activation of the JNK Pathway and Inhibition of PI3K/AKT/mTOR Pathway in HepG2 Cells. PLoS ONE 2016, 11, e0147405. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China Part 1; China Medical Science Press: Beijing, China, 2020; p. 260. [Google Scholar]
- Liu, S.; Yu, H.; Hou, A.; Man, W.; Zhang, J.; Wang, S.; Wang, X.; Zheng, S.; Su, X.; Yang, L. A Review of the Pharmacology, Application, Ethnopharmacology, Phytochemistry, Quality Control, Processing, Toxicology, and Pharmacokinetics of Paridis Rhizoma. World J. Tradit. Chin. Med. 2022, 8, 21–49. [Google Scholar] [CrossRef]
- Thakur, U.; Shashni, S.; Thakur, N.; Rana, K.S.; Singh, A. A review on Paris polyphylla Smith: A vulnerable medicinal plant species of a global significance. J. Appl. Res. Med. Aromat. Plants 2023, 33, 100447. [Google Scholar] [CrossRef]
- Qin, X.J.; Ni, W.; Chen, C.X.; Liu, H.Y. Seeing the light: Shifting from wild rhizomes to extraction of active ingredients from above-ground parts of Paris polyphylla var. yunnanensis. J. Ethnopharmacol. 2018, 224, 134–139. [Google Scholar] [CrossRef]
- Wen, F.; Chen, S.; Wang, Y.; Wu, Q.; Yan, J.; Pei, J.; Zhou, T. The synthesis of Paris saponin VII mainly occurs in leaves and is promoted by light intensity. Front. Plant Sci. 2023, 14, 1199215. [Google Scholar] [CrossRef]
- Boulila, A.; Imed Hassen, I.; Haouari, L.; Mejri, F.; Amor, I.B.; Casabianca, H.; Hosni, K. Enzyme-assisted extraction of bioactive compounds from bay leaves (Laurus nobilis L.). Ind. Crops Prod. 2015, 74, 485–493. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Rosli Mohd Yunus, R.M. Optimization of saponins, phenolics, and antioxidants extracted from fenugreek seeds using microwave-assisted extraction and response surface methodology as an optimizing tool. Comptes Rendus. Chim. 2019, 22, 714–727. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M. Superheated water: The ultimate green solvent for separation science. Anal. Bioanal. Chem. 2006, 385, 419–421. [Google Scholar] [CrossRef]
- Zhou, F.; Hearne, Z.; Li, C.J. Water—The greenest solvent overall. Curr. Opin. Green Sustain. Chem. 2019, 18, 118–123. [Google Scholar] [CrossRef]
- Morales-Muñoz, S.; Luque-García, J.; de Castro, L. Pure and modified water assisted by auxiliary energies: An environmental friendly extractant for sample preparation. Anal. Chim. Acta. 2006, 557, 278–286. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Basak, S.; Annapure, U.S. The potential of subcritical water as a “green” method for the extraction and modification of pectin: A critical review. Food Res. Int. 2022, 161, 111849. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Said, K.A.; Mohamed Amin, M.A. Overview on the Response Surface Methodology (RSM) in Extraction Processes. J. Appl. Sci. Process. Eng. 2015, 2, 8–17. [Google Scholar] [CrossRef]
- Ferreira, S.L.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science, 1st ed.; Kayarogaman, P., Ed.; IntechOpen: London, UK, 2021; Volume 1, pp. 1–19. [Google Scholar] [CrossRef]
- Cerqueira, U.M.F.M.; Bezerra, M.A.; Ferreira, S.L.C.; de Jesus Araújo, R.; da Silva, B.N.; Novaes, C.G. Doehlert design in the optimization of procedures aiming food analysis—A review. Food Chem. 2021, 364, 130429. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Ahmad, A.; Alkharfy, K.M.; Wani, T.A.; Raish, M. Application of Box-Behnken design for ultrasonic-assisted extraction of polysaccharides from Paeonia emodi. Int. J. Biol. Macromol. 2015, 72, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Shahabi Mohammadabadi, S.; Goli, M.; Naji Tabasi, S. Optimization of Bioactive Compound Extraction from Eggplant Peel by Response Surface Methodology: Ultrasound-Assisted Solvent Qualitative and Quantitative Effect. Foods 2022, 11, 3263. [Google Scholar] [CrossRef] [PubMed]
- Aryanti, N.; Heny, D.R.; Nafiunisa, A. Optimization of ultrasound-assisted extraction of rarak saponin from Sapindus rarak DC. using response surface methodology (RSM). AIP Conf. Proc. 2020, 2197, 040007. [Google Scholar] [CrossRef]
- Zhang, W.N.; Zhang, H.L.; Lu, C.Q.; Luo, J.P.; Zha, X.Q. A new kinetic model of ultrasound-assisted extraction of polysaccharides from Chinese chive. Food Chem. 2016, 212, 274–281. [Google Scholar] [CrossRef]
- Yang, L.; Cao, Y.L.; Jiang, J.G.; Lin, Q.S.; Chen, J.; Zhu, L. Response surface optimization of ultrasound-assisted flavonoids extraction from the flower of Citrus aurantium L. var. amara. Engl. J. Sep. Sci. 2010, 33, 1349–1355. [Google Scholar] [CrossRef]
- Patil, D.M.; Akamanchi, K.G. Ultrasound-assisted rapid extraction and kinetic modelling of influential factors: Extraction of camptothecin from Nothapodytes nimmoniana plant. Ultrason. Sonochem. 2017, 37, 582–591. [Google Scholar] [CrossRef]
- Aurelio, D.L.; Edgardo, R.G.; Navarro-Galindo, S. Thermal kinetic degradation of anthocyanins in a roselle (Hibiscus sabdariffa L. cv. ‘Criollo’) infusion. Int. J. Food Sci. Technol. 2008, 43, 322–325. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Zhang, M.; Yang, H.; Li, G.; Ren, X.; Liang, S. Optimization of Oil Extraction from Rice Bran with Mixed Solvent Using Response Surface Methodology. Foods 2022, 11, 3849. [Google Scholar] [CrossRef]
- Yin, G.; Dang, Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008, 74, 603–610. [Google Scholar] [CrossRef]
- Shen, S.; Chen, D.; Li, X.; Li, T.; Yuan, M.; Zhou, Y.; Ding, C. Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Paris polyphylla. Carbohydr. Polym. 2014, 104, 80–86. [Google Scholar] [CrossRef]







| Number | A Period (min) | B Temperature (°C) | C Solid-to-Liquid Ratio (g/mL) | Polyphyllin II Yield (%) a | Polyphyllin VII Yield (%) a |
|---|---|---|---|---|---|
| 1 | 67.5 | 35 | 1:10 | 1.8547 | 4.5207 |
| 2 | 15.0 | 40 | 1:10 | 1.8123 | 4.5329 |
| 3 | 120.0 | 30 | 1:10 | 1.7986 | 4.3014 |
| 4 | 67.5 | 30 | 1:15 | 1.7855 | 4.4788 |
| 5 | 67.5 | 40 | 1:5 | 1.8071 | 4.2982 |
| 6 | 67.5 | 30 | 1:5 | 1.7226 | 4.9687 |
| 7 | 120.0 | 35 | 1:5 | 1.7434 | 4.5200 |
| 8 | 67.5 | 35 | 1:10 | 1.8436 | 4.5766 |
| 9 | 15.0 | 35 | 1:5 | 1.8155 | 4.8418 |
| 10 | 67.5 | 35 | 1:10 | 1.8614 | 4.6581 |
| 11 | 67.5 | 35 | 1:10 | 1.8455 | 4.6141 |
| 12 | 120.0 | 40 | 1:10 | 1.7554 | 3.7153 |
| 13 | 120.0 | 35 | 1:15 | 1.8198 | 3.9430 |
| 14 | 15.0 | 30 | 1:10 | 1.7440 | 4.8699 |
| 15 | 15.0 | 35 | 1:15 | 1.7840 | 4.6934 |
| 16 | 67.5 | 40 | 1:15 | 1.7656 | 3.9081 |
| 17 | 67.5 | 35 | 1:10 | 1.8535 | 4.7187 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 0.0298 | 9 | 0.0033 | 37.41 | <0.0001 *** |
| A | 0.0002 | 1 | 0.0002 | 2.11 | 0.1900 |
| B | 0.0010 | 1 | 0.0010 | 11.37 | 0.0119 * |
| C | 0.0005 | 1 | 0.0005 | 6.21 | 0.0414 * |
| AB | 0.0031 | 1 | 0.0031 | 35.15 | 0.0006 ** |
| AC | 0.0029 | 1 | 0.0029 | 32.92 | 0.0007 ** |
| BC | 0.0027 | 1 | 0.0027 | 30.82 | 0.0009 ** |
| A2 | 0.0030 | 1 | 0.0030 | 34.32 | 0.0006 ** |
| B2 | 0.0094 | 1 | 0.0094 | 106.63 | <0.0001 *** |
| C2 | 0.0049 | 1 | 0.0049 | 55.76 | 0.0001 ** |
| Residual | 0.0006 | 7 | 0.0001 | ||
| Lack of Fit | 0.0004 | 3 | 0.0001 | 2.59 | 0.1902 |
| Pure Error | 0.0002 | 4 | 0.0001 | ||
| Cor Total | 0.0304 | 16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1.90 | 9 | 0.2115 | 29.09 | <0.0001 *** |
| A | 0.7554 | 1 | 0.7554 | 103.89 | <0.0001 *** |
| B | 0.5855 | 1 | 0.5855 | 80.53 | <0.0001 *** |
| C | 0.3222 | 1 | 0.3222 | 44.31 | 0.0003 ** |
| AB | 0.0155 | 1 | 0.0155 | 2.13 | 0.1875 |
| AC | 0.0459 | 1 | 0.0459 | 6.32 | 0.0402 * |
| BC | 0.0025 | 1 | 0.0025 | 0.3425 | 0.5768 |
| A2 | 0.0329 | 1 | 0.0329 | 4.52 | 0.0711 |
| B2 | 0.1281 | 1 | 0.1281 | 17.62 | 0.0040 ** |
| C2 | 0.0037 | 1 | 0.0037 | 0.5128 | 0.4971 |
| Residual | 0.0509 | 7 | 0.0073 | ||
| Lack of Fit | 0.0280 | 3 | 0.0093 | 1.62 | 0.3178 |
| Pure Error | 0.0229 | 4 | 0.0057 | ||
| Cor Total | 1.95 | 16 |
| Number | Yield (%) | Average Yield (%) | RSD (%) | |
|---|---|---|---|---|
| Polyphyllin II | 1 | 1.940 | ||
| 2 | 1.892 | 1.895 | 2.300 | |
| 3 | 1.853 | |||
| 1 | 5.083 | |||
| Polyphyllin VII | 2 | 4.969 | 5.010 | 1.270 |
| 3 | 4.977 |
| Time (min) | Water (%) | Acetonitrile (%) |
|---|---|---|
| 0.0 | 57.0 | 43.0 |
| 13.0 | 57.0 | 43.0 |
| 14.0 | 45.0 | 55.0 |
| 25.0 | 45.0 | 55.0 |
| Level | Factor | ||
|---|---|---|---|
| A (min) | B (°C) | C (g/mL) | |
| 1 | 15.0 | 30 | 1:5 |
| 0 | 67.5 | 35 | 1:10 |
| −1 | 120.0 | 40 | 1:15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Qiao, Q.; Dong, L.; Cao, M.; Li, P.; Liu, A.; Sun, R. Response Surface Optimization for Water-Assisted Extraction of Two Saponins from Paris polyphylla var. yunnanensis Leaves. Molecules 2024, 29, 1652. https://doi.org/10.3390/molecules29071652
Jin Y, Qiao Q, Dong L, Cao M, Li P, Liu A, Sun R. Response Surface Optimization for Water-Assisted Extraction of Two Saponins from Paris polyphylla var. yunnanensis Leaves. Molecules. 2024; 29(7):1652. https://doi.org/10.3390/molecules29071652
Chicago/Turabian StyleJin, Yutian, Qing Qiao, Linmei Dong, Mokun Cao, Ping Li, Aizhong Liu, and Rui Sun. 2024. "Response Surface Optimization for Water-Assisted Extraction of Two Saponins from Paris polyphylla var. yunnanensis Leaves" Molecules 29, no. 7: 1652. https://doi.org/10.3390/molecules29071652