Sorption of Polycyclic Aromatic Sulfur Heterocycles (PASH) on Nylon Microplastics at Environmentally Relevant Concentrations
Abstract
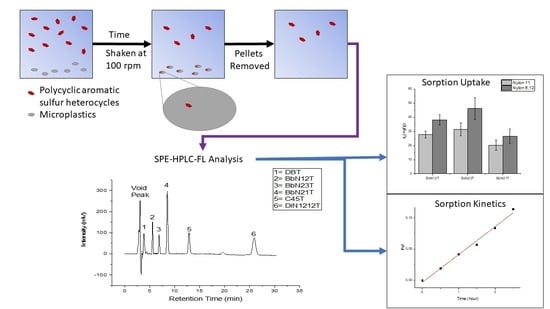
:1. Introduction
2. Results and Discussion
2.1. HPLC Analytical Figures of Merit
2.2. HPLC-SPE Analytical Figures of Merit
2.3. Sorption Uptake by Nylon Microplastics
2.4. Sorption Kinetic Modeling
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Stock Preparation
3.3. HPLC Analysis
3.4. Solution Preparation for HPLC Analytical Figures of Merit
3.5. Preparation for SPE-HPLC Analytical Figure of Merit
3.6. Sorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arthur, C.; Baker, J.; Bamford, H. (Eds.) Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Micro-Plastic Marine Debris, 9–11 September 2008. NOAA Technical Memorandum NOS-OR&R-30. 2009. Available online: https://marine-debris-site-s3fs.s3.us-west-1.amazonaws.com/s3fs-public/publications-files/TM_NOS-ORR_30.pdf?VersionId=AkHQs2er_rm6MTlJLwSTu35mauQxDuaU (accessed on 12 October 2019).
- Wagner, J.; Wang, Z.-M.; Ghosal, S.; Rochman, C.; Gassel, M.; Wall, S. Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices. Anal. Methods 2017, 9, 1479–1490. [Google Scholar] [CrossRef]
- Gao, F.; Lia, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Costigan, E.; Collins, A.; Hatinoglu, M.D.; Bhagat, K.; MacRae, J.; Perreault, F.; Apul, O. Adsorption of organic pollutants by microplastics: Overview of a dissonant literature. J. Hazard. Mater. Adv. 2022, 6, 100091. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, X.; Zhao, J.; Liu, R.; Xing, B. Interaction of Microplastics with Antibiotics in Aquatic Environment: Distribution, Adsorption, and Toxicity. Environ. Sci. Technol. 2021, 55, 15579–15595. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, Z.; Ogonowski, M.; Nybom, I.; Ek, C.; Adolfsson-Erici, M.; Barth, A.; Gorokhova, E. Microplastic-mediated transport of PCBs? A depuration study with Daphnia magna. PLoS ONE 2019, 14, e0205378. [Google Scholar] [CrossRef] [PubMed]
- Qui, Y.; Zheng, M.; Wang, L.; Zhao, L.; Lou, Y.; Shi, L.; Qu, L. Sorption of polyhalogenated carbazoles (PHCs) to microplastics. Mar. Pollut. Bull. 2019, 146, 718–728. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, M.; Sha, W.; Wang, Y.; Hao, H.; Dou, Y.; Li, Y. Sorption Behavior and Mechanisms of Organic Contaminants to Nano and Microplastics. Molecules 2020, 25, 1827. [Google Scholar] [CrossRef]
- NOAA, Technical Memorandum NMFS-NWFSC-125 Northwest Fisheries Science Center’s Analyses of Tissue, Sediment, and Water Samples for Organic Contaminants by Gas Chromatography/mass Spectrometry and Analyses of Tissue for Lipid Classes by Thin Layer Chromatography/flame Ionization Detection. Washington, DC, USA. 2014. Available online: https://repository.library.noaa.gov/view/noaa/4626 (accessed on 12 October 2019).
- European Commission, Priority Substances and Certain Other Pollutants According to Annex II of Directive 2008/105/EC. 2019. Available online: https://ec.europa.eu/environment/water/water-framework/priority_substances.htm (accessed on 12 October 2021).
- EPA United States Environmental Protection Agency. Priority Pollutant List. 2014. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/priority-pollutant-list-epa.pdf (accessed on 12 October 2021).
- Eastmond, D.A.; Booth, G.M.; Lee, M.L. Toxicity, Accumulation, and Elimination of Polycyclic Aromatic Sulfur Heterocycles in Daphnia magna. Arch. Environ. Contam. Toxicol. 1984, 13, 105–111. [Google Scholar] [CrossRef]
- Siemers, A.K.; Mänz, J.; Wolf-Ulric, P.; Ruck, W. Development and application of a simultaneous SPE-method for polycyclic aromatic hydrocarbons (PAHs), alkylated PAHs, heterocyclic PAHs (NSO-HET) and phenols in aqueous samples from German Rivers. Chemosphere 2015, 122, 105–114. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, M.J.; Hunter, J.V. Sources and fates of aromatic compounds in urban stormwater runoff. Environ. Sci. Technol. 1979, 13, 179–183. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Ambeed. Available online: https://www.ambeed.com/products/205-43-6.html (accessed on 11 January 2024).
- Chemical Properties of Benzo[b]naphtho[2,3-d]thiophene. Chemeo. Available online: https://www.chemeo.com/cid/66-382-5/Benzo-b-naphtho-2-3-d-thiophene (accessed on 11 January 2024).
- Chemical Properties of Benzo[b]naphtho[2,1-d]thiophene. Chemeo. Available online: https://www.chemeo.com/cid/42-561-2/Benzo-b-naphtho-2-1-d-thiophene (accessed on 11 January 2024).
- Farkas, O.; Heberger, K.; Zenkevich, I.G. Quantitative structure-retention relationships. XIV. Prediction of gas chromatographic retention indices for saturated O-, N-, and S heterocyclic compounds. Chemometr. Intell. Lab. Syst. 2004, 72, 173–184. [Google Scholar] [CrossRef]
- Wilson, W.B.; Hayes, H.V.; Sander, L.C.; Campiglia, A.D.; Wise, S.A. Normal-phase liquid chromatography retention behavior of polycyclic aromatic sulfur heterocycles and alkyl-substituted polycyclic aromatic sulfur heterocycle isomers on an aminopropyl stationary phase. Anal. Bioanal. Chem. 2018, 410, 1511–1524. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.R.; Prelot, B. Chapter 7—Absorption Processes for the removal of contaminants from wastewater: The perspective of nanomaterials and nanotechnology. In Nanomaterials for the Detection and Removal of Wastewater Pollutants, 1st ed.; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 9780128184905. [Google Scholar]
- Nylon 11. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/181153#product-documentation (accessed on 17 March 2024).
- Nylon 6,12. Available online: https://www.sigmaaldrich.com/US/en/product/aldrich/181145 (accessed on 17 March 2024).
- Baldi, L.D.C.; Iamazaki, E.T.; Atvars, T.D.Z. Evaluation of the polarity of polyamide surfaces using the fluorescence emission of pyrene. Dye. Pigment. 2008, 76, 669–676. [Google Scholar] [CrossRef]






| PASH | Retention Time 1 (min) | LOD 2 (ng/mL) | LOQ 3 (ng/mL) | LDR 4 (ng/mL) | % RSD 5 |
|---|---|---|---|---|---|
| DBT | 3.87 ± 0.022 | 0.9 | 3.0 | 3.0–200 | 5.64 |
| BbN12T | 5.68 ± 0.078 | 0.3 | 0.9 | 0.9–200 | 3.53 |
| BbN23T | 7.07 ± 0.135 | 0.2 | 0.8 | 0.8–200 | 0.30 |
| BbN21T | 8.78 ± 0.196 | 0.3 | 0.9 | 0.9–200 | 1.94 |
| C45T | 13.24 ± 0.346 | 0.5 | 1.8 | 1.8–200 | 4.10 |
| DiN1212T | 26.33 ± 0.868 | 0.7 | 2.2 | 2.2–200 | 3.75 |
| PASH | Retention Time 1 (min) | LOD 2 (pg/mL) | LOQ 3 (pg/mL) | LDR 4 (pg/mL) | RSD 5 (%) | Analytical Recovery 6 (%) |
|---|---|---|---|---|---|---|
| DBT | 3.73± 0.015 | 0.05 | 0.2 | 0.2–75 | 5.0 | 90.5 ± 3.1 |
| BbN12T | 5.57 ± 0.120 | 3 | 10 | 10–75 | 1.1 | 101.6 ± 1.4 |
| BbN23T | 7.02 ± 0.125 | 0.7 | 2 | 2–100 | 3.0 | 88.2 ± 3.4 |
| BbN21T | 8.72 ± 0.159 | 0.6 | 2 | 2–100 | 3.9 | 77.0 ± 2.7 |
| C45T | 13.11 ±0.405 | 2 | 8 | 8–75 | 7.4 | 68.5 ± 4.1 |
| DiN1212T | 25.89 ± 0.674 | 2 | 7 | 7–75 | 1.1 | 69.9 ± 2.5 |
| Type of Microplastics | PASHs | R2 a | b (ng/g) | c (ng/g) × h | d (g/ng) × h |
|---|---|---|---|---|---|
| Nylon 6,12 | BbN12T | 0.9778 | 23.87 | 181.8 | 0.3191 |
| BbN23T | 0.9953 | 35.46 | 714.3 | 0.5681 | |
| BbN21T | 0.9949 | 24.39 | 1111 | 1.8676 | |
| Nylon 11 | BbN12T | 0.9975 | 22.52 | 217.4 | 0.4287 |
| BbN23T | 0.9895 | 31.95 | 163.9 | 0.1606 | |
| BbN21T | 0.9515 | 17.21 | 51.81 | 0.1749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nauth, S.D.; Campiglia, A.D. Sorption of Polycyclic Aromatic Sulfur Heterocycles (PASH) on Nylon Microplastics at Environmentally Relevant Concentrations. Molecules 2024, 29, 1653. https://doi.org/10.3390/molecules29071653
Nauth SD, Campiglia AD. Sorption of Polycyclic Aromatic Sulfur Heterocycles (PASH) on Nylon Microplastics at Environmentally Relevant Concentrations. Molecules. 2024; 29(7):1653. https://doi.org/10.3390/molecules29071653
Chicago/Turabian StyleNauth, Stephanie D., and Andres D. Campiglia. 2024. "Sorption of Polycyclic Aromatic Sulfur Heterocycles (PASH) on Nylon Microplastics at Environmentally Relevant Concentrations" Molecules 29, no. 7: 1653. https://doi.org/10.3390/molecules29071653