Boosting Blue Self-Trapped Exciton Emission in All-Inorganic Zero-Dimensional Metal Halide Cs2ZnCl4 via Zirconium (IV) Doping
Abstract
:1. Introduction
2. Results and Discussion
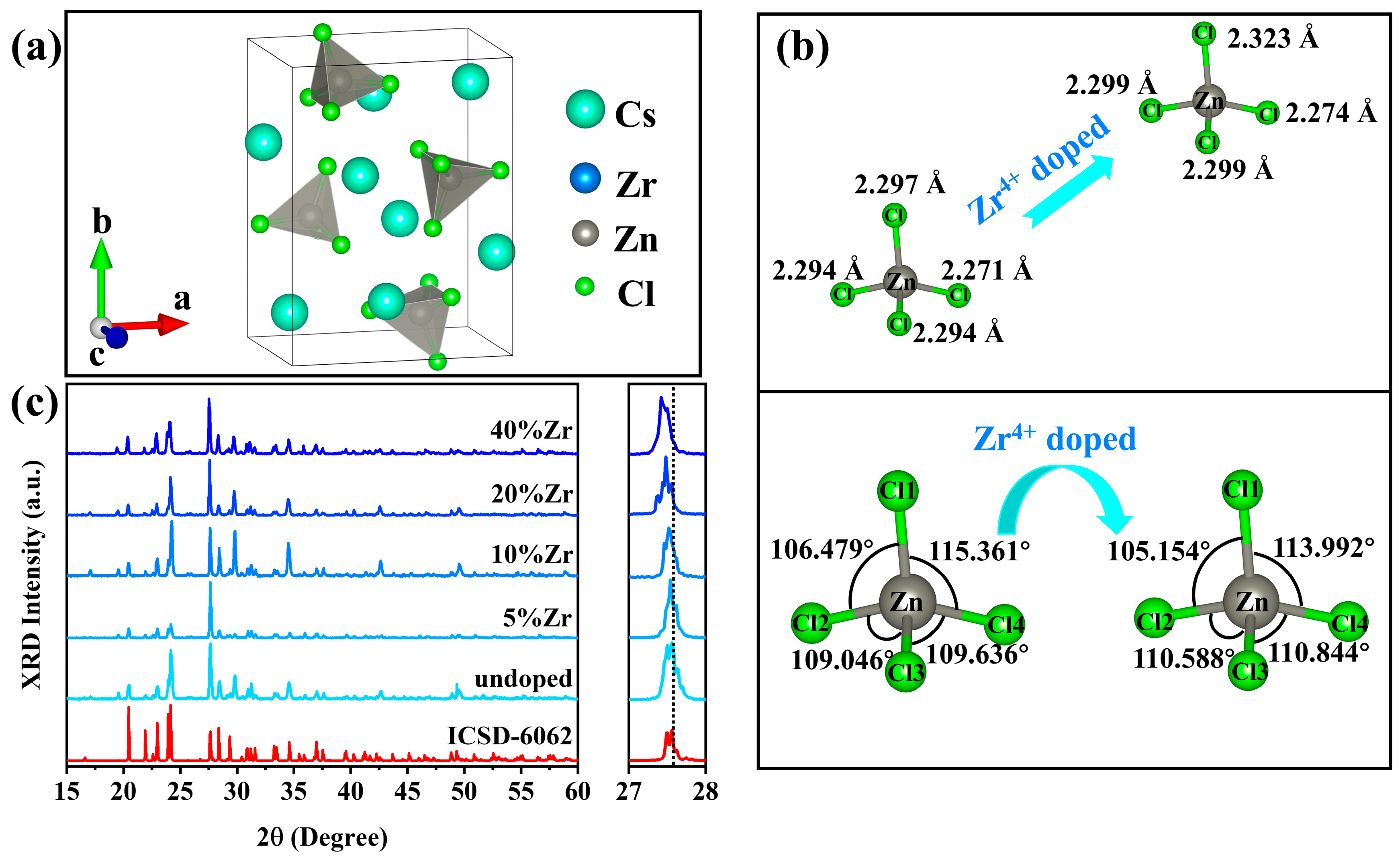
2.1. Structure and Morphology
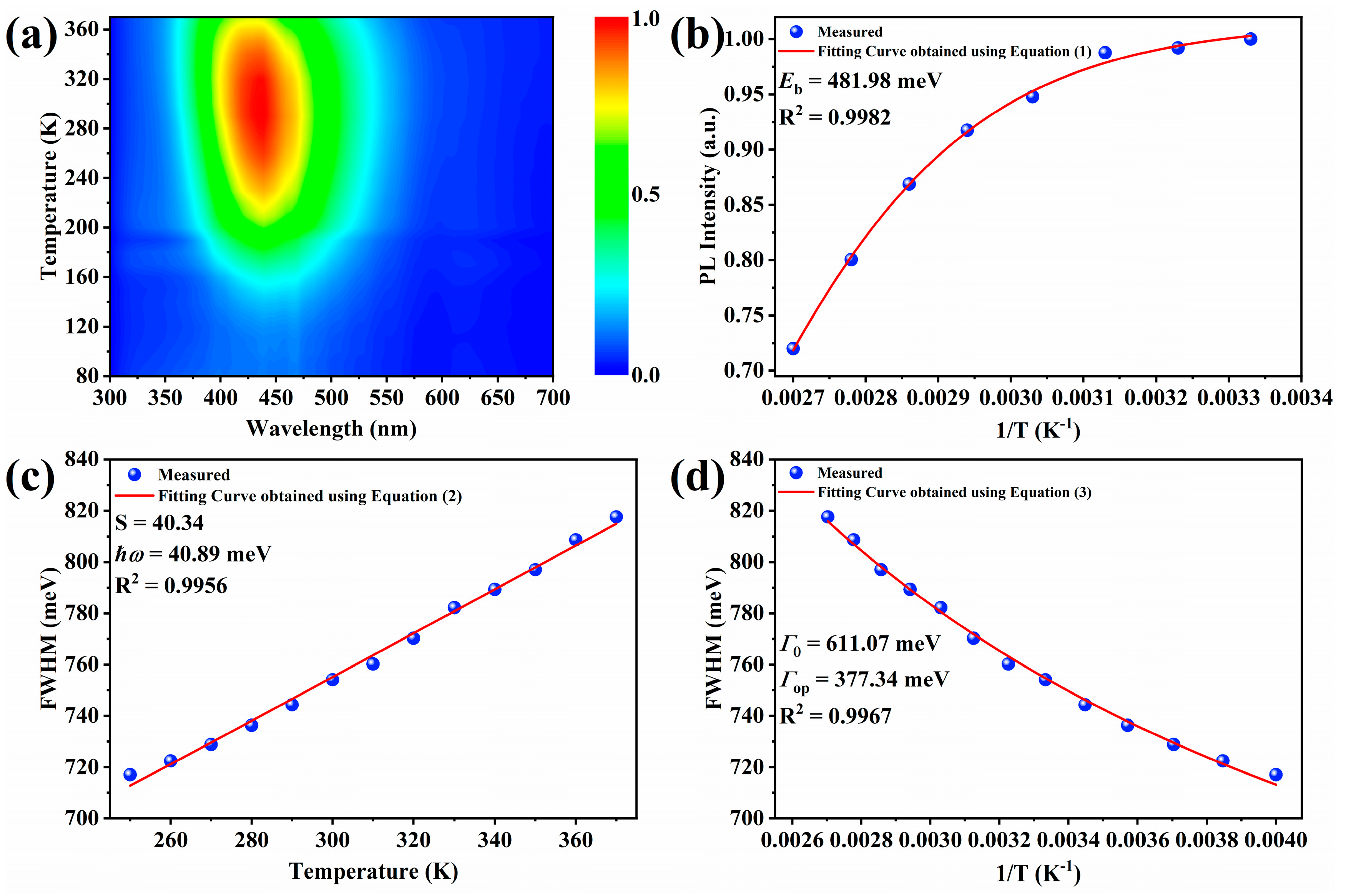
2.2. Optical Properties
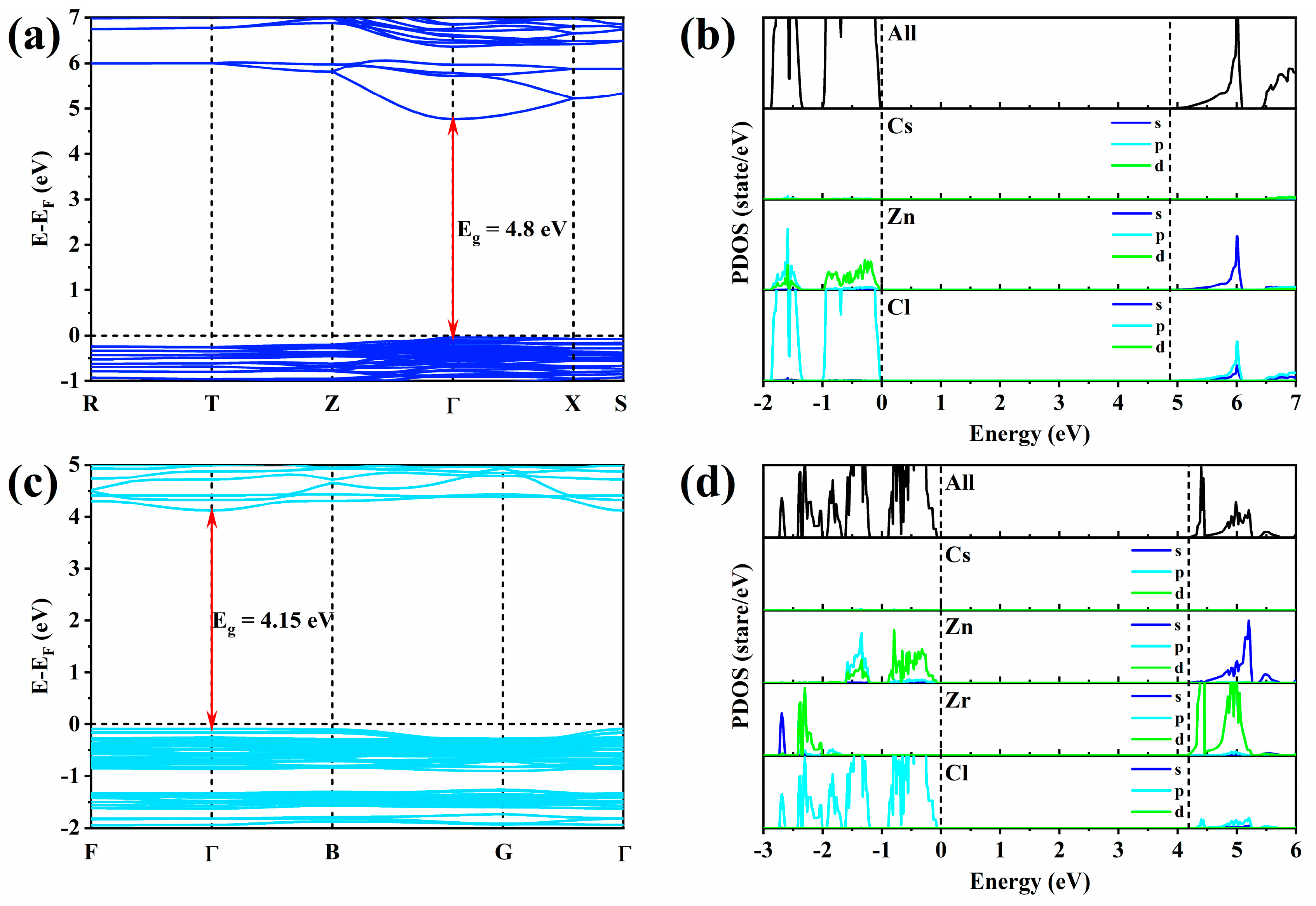
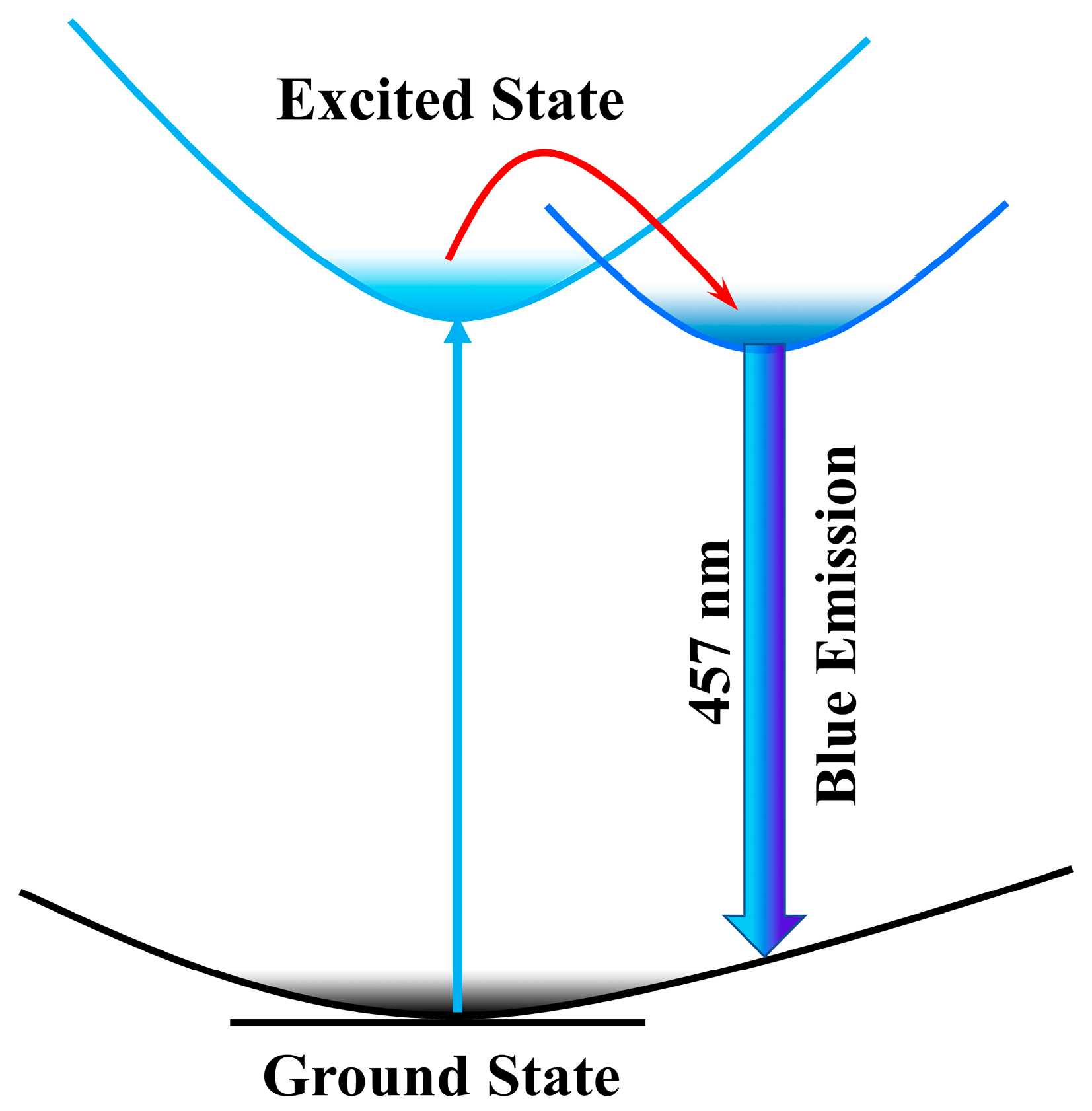
2.3. Theoretical Calculation and Photophysical Model
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ponce, F.; Bour, D. Nitride-Based Semiconductors for Blue and Green Light-Emitting Devices. Nature 1997, 386, 351–359. [Google Scholar] [CrossRef]
- Fasol, G.; Nakamura, S. The Blue Laser Diode; Springer-Verlag: Berlin/Heidelberg, Germany, 1997. [Google Scholar]
- Zhou, C.; Lin, H.; He, Q.; Xu, L.; Worku, M.; Chaaban, M.; Lee, S.; Shi, X.; Du, M.-H.; Ma, B. Low Dimensional Metal Halide Perovskites and Hybrids. Mater. Sci. Eng. R 2019, 137, 38–65. [Google Scholar] [CrossRef]
- Jiang, T.; Ma, W.; Zhang, H.; Tian, Y.; Lin, G.; Xiao, W.; Yu, X.; Qiu, J.; Xu, X.; Yang, Y. Highly Efficient and Tunable emission of Lead-Free Manganese Halides toward White Light-Emitting Diode and X-ray Scintillation Applications. Adv. Funct. Mater. 2021, 31, 2009973. [Google Scholar] [CrossRef]
- Han, P.; Luo, C.; Yang, S.; Yang, Y.; Deng, W.; Han, K. All-Inorganic Lead-Free 0D Perovskites by A Doping Strategy to Achieve A PLQY Boost from <2% to 90%. Angew. Chem. Int. Ed. 2020, 132, 12809–12813. [Google Scholar]
- Li, Z.; Li, Y.; Liang, P.; Zhou, T.; Wang, L.; Xie, R.-J. Dual-Band Luminescent Lead-Free Antimony Chloride Halides with Near-Unity Photoluminescence Quantum Efficiency. Chem. Mater. 2019, 31, 9363–9371. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Tian, Y.; Yuan, Z.; Clark, R.; Chen, B.; van de Burgt, L.J.; Wang, J.C.; Zhou, Y.; Hanson, K. Luminescent Zero-Dimensional Organic Metal Halide Hybrids with Near-Unity Quantum Efficiency. Chem. Sci. 2018, 9, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; Shi, H.; Tian, Y.; Pak, C.; Shatruk, M.; Zhou, Y.; Djurovich, P.; Du, M.H.; Ma, B. A Zero-Dimensional Organic Seesaw-Shaped Tin Bromide with Highly Efficient Strongly Stokes-Shifted Deep-Red Emission. Angew. Chem. Int. Ed. 2018, 130, 1033–1036. [Google Scholar] [CrossRef]
- Zhou, C.; Worku, M.; Neu, J.; Lin, H.; Tian, Y.; Lee, S.; Zhou, Y.; Han, D.; Chen, S.; Hao, A. Facile Preparation of Light Emitting Organic Metal Halide Crystals with Near-Unity Quantum Efficiency. Chem. Mater. 2018, 30, 2374–2378. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Li, W.G.; Chen, H.Y.; Kuang, D.B.; Su, C.Y. A Highly Red-Emissive Lead-Free Indium-Based Perovskite Single Crystal for Sensitive Water Detection. Angew. Chem. Int. Ed. 2019, 131, 5331–5335. [Google Scholar] [CrossRef]
- Morad, V.; Shynkarenko, Y.; Yakunin, S.; Brumberg, A.; Schaller, R.D.; Kovalenko, M.V. Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-ray Scintillation. J. Am. Chem. Soc. 2019, 141, 9764–9768. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, C.; Chaaban, M.; Xu, L.-J.; Zhou, Y.; Neu, J.; Worku, M.; Berkwits, E.; He, Q.; Lee, S. Bulk Assembly of Zero-Dimensional Organic Lead Bromide Hybrid with Efficient Blue Emission. ACS Mater. Lett. 2019, 1, 594–598. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Worku, M.; Neu, J.; Zhou, Y.; Tian, Y.; Lee, S.; Djurovich, P.; Siegrist, T.; Ma, B. Blue Emitting Single Crystalline Assembly of Metal Halide Clusters. J. Am. Chem. Soc. 2018, 140, 13181–13184. [Google Scholar] [CrossRef]
- Jun, T.; Sim, K.; Iimura, S.; Sasase, M.; Kamioka, H.; Kim, J.; Hosono, H. Lead-Free Highly Efficient Blue-Emitting Cs3Cu2I5 with 0D Electronic Structure. Adv. Mater. 2018, 30, 1804547. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, B.; Song, E.; Molokeev, M.S.; Xia, Z. Ultra-Broad-Band-Excitable Cu(I)-Based Organometallic Halide with Near-Unity Emission for Light-Emitting Diode Applications. Chem. Mater. 2021, 33, 4382–4389. [Google Scholar] [CrossRef]
- Creason, T.D.; Yangui, A.; Roccanova, R.; Strom, A.; Du, M.H.; Saparov, B. Rb2CuX3 (X = Cl, Br): 1D All-Inorganic Copper Halides with Ultrabright Blue Emission and Up-Conversion Photoluminescence. Adv. Opt. Mater. 2020, 8, 1901338. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, F.; Zhu, C.; Li, J.; Lv, X.; Xing, G.; Wei, Q.; Wang, G.; Dai, J.; Dong, H. Near-Unity Blue Lminance from Lad-Free Copper Halides for Light-Emitting Diodes. Nano Energy 2022, 91, 106664. [Google Scholar] [CrossRef]
- Li, H.; Lv, Y.; Zhou, Z.; Tong, H.; Liu, W.; Ouyang, G. Coordinated Anionic Inorganic Module—An Efficient Approach towards Highly Efficient Blue-Emitting Copper Halide Ionic Hybrid Structures. Angew. Chem. Int. Ed. 2022, 61, e202115225. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Li, M.; Yang, Y.; Liang, F.; Huang, Q.; Liu, X.; Gong, P.; Xia, Z.; Lin, Z. Lead-Free Tin (IV)-Based Organic–Inorganic Metal Halide Hybrids with Excellent Stability and Blue-Broadband Emission. J. Phys. Chem. Lett. 2020, 11, 1808–1813. [Google Scholar] [CrossRef] [PubMed]
- Fattal, H.; Creason, T.D.; Delzer, C.J.; Yangui, A.; Hayward, J.P.; Ross, B.J.; Du, M.-H.; Glatzhofer, D.T.; Saparov, B. Zero-Dimensional Hybrid Organic–Inorganic Indium Bromide with Blue Emission. Inorg. Chem. 2021, 60, 1045–1054. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Fu, H.-Q.; Liu, X.-L.; Sun, Y.-M.; Zhong, Q.-Q.; Xu, W.-J.; Lei, X.-W.; Liu, G.-D.; Yue, C.-Y. Zero-Dimensional Organic–Inorganic Hybrid Indium Chlorides with Intrinsic Blue Light Emissions. Inorg. Chem. 2022, 61, 8977–8981. [Google Scholar] [CrossRef]
- Li, D.-Y.; Sun, Y.-M.; Wang, X.-Y.; Wang, N.-N.; Zhang, X.-Y.; Yue, C.-Y.; Lei, X.-W. Zero-Dimensional Hybrid Indium Halides with Efficient and Tunable White-Light Emissions. J. Phys. Chem. Lett. 2022, 13, 6635–6643. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.; López-Fernández, I.; Prasanthkumar, S.; Polavarapu, L. All-Inorganic Lead-Free Doped-Metal Halides for Bright Solid-State Emission from Primary Colors to White Light. ACS Appl. Mater. Interfaces 2023, 15, 35206–35215. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Zhou, T.; Wang, Y.; Zheng, J.; Liu, R.; Hou, J.; Li, X.; Wang, L.; Jiang, W. Efficient Emission in Copper-Doped Cs3ZnX5 (X = Cl, I) for White LEDs and X-ray Scintillators. J. Mater. Chem. C 2023, 11, 9850–9859. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, M.; Zhou, Z.; Kang, C.; Wang, J.; Liu, Q. Recent Advances in Lead-Free Cs2ZrCl6 Metal Halide Perovskites and Their Derivatives: From Fundamentals to Advanced Applications. Coord. Chem. Rev. 2024, 499, 215490. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Yao, Z.; Zhao, Q.; Chabera, P.; Zheng, K.; Yang, B.; Pullerits, T.; Chen, J. Nature of Self-Trapped Exciton Emission in Zero-Dimensional Cs2ZrCl6 Perovskite Nanocrystals. J. Phys. Chem. Lett. 2023, 14, 7665–7671. [Google Scholar] [CrossRef] [PubMed]
- Dohner, E.R.; Hoke, E.T.; Karunadasa, H.I. Self-Assembly of Broadband White-Light Emitters. J. Am. Chem. Soc. 2014, 136, 1718–1721. [Google Scholar] [CrossRef]
- Mao, L.; Wu, Y.; Stoumpos, C.C.; Wasielewski, M.R.; Kanatzidis, M.G. White-Light Emission and Structural Distortion in New Corrugated Two-Dimensional Lead Bromide Perovskites. J. Am. Chem. Soc. 2017, 139, 5210–5215. [Google Scholar] [CrossRef]
- Peng, C.; Zhuang, Z.; Yang, H.; Zhang, G.; Fei, H. Ultrastable, Cationic Three-Dimensional Lead Bromide Frameworks that Intrinsically Emit Broadband White-Light. Chem. Sci. 2018, 9, 1627–1633. [Google Scholar] [CrossRef]
- Cheng, P.; Feng, L.; Liu, Y.; Zheng, D.; Sang, Y.; Zhao, W.; Yang, Y.; Yang, S.; Wei, D.; Wang, G. Doped Zero-Dimensional Cesium Zinc Halides for High-Efficiency Blue Light Emission. Angew. Chem. Int. Ed. 2020, 59, 21414–21418. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Y.; Li, S.; Guo, L.; Wang, E.; Cao, Y. Doping Behavior of Zr4+ Ions in Zr4+-Doped TiO2 Nanoparticles. J. Phys. Chem. C 2013, 117, 27120–27126. [Google Scholar] [CrossRef]
- Dohner, E.R.; Jaffe, A.; Bradshaw, L.R.; Karunadasa, H.I. Intrinsic White-Light Emission from Layered Hybrid Perovskites. J. Am. Chem. Soc. 2014, 136, 13154–13157. [Google Scholar] [CrossRef]
- Cortecchia, D.; Yin, J.; Bruno, A.; Lo, S.-Z.A.; Gurzadyan, G.G.; Mhaisalkar, S.; Brédas, J.-L.; Soci, C. Polaron Self-Iocalization in White-Light Emitting Hybrid Perovskites. J. Mater. Chem. C 2017, 5, 2771–2780. [Google Scholar] [CrossRef]
- Liu, X.; Peng, C.; Zhang, L.; Guo, D.; Pan, Y. Te4+-Doped Zro-Dimensional Cs2ZnCl4 Single Crystals for Broadband Yellow Light Emission. J. Mater. Chem. C 2022, 10, 204–209. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Liu, J.; Tang, J. Self-Trapped Excitons in All-Inorganic Halide Perovskites: Fundamentals, Status, and Potential Applications. J. Phys. Chem. Lett. 2019, 10, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liao, J.F.; Huang, Z.G.; Wei, J.H.; Wang, X.D.; Chen, H.Y.; Kuang, D.B. Intrinsic Self-Trapped Emission in 0D Lead-Free (C4H14N2)2In2Br10 Single Crystal. Angew. Chem. Int. Ed. 2019, 131, 15581–15586. [Google Scholar] [CrossRef]
- Peng, C.; Wei, Q.; Yao, S.; Meng, X.; Yu, Z.; Peng, H.; Zhong, X.; Zou, B. H2O–NH4+-Induced Emission Modulation in Sb3+-Doped (NH4)2InCl5·H2O. Inorg. Chem. 2022, 61, 12406–12414. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, L.; Niu, G.; Yuan, J.H.; Xue, K.H.; Tan, Z.; Miao, X.S.; Niu, M.; Du, X.; Song, H. Lead-Free Halide Rb2CuBr3 as Sensitive X-ray Scintillator. Adv. Mater. 2019, 31, 1904711. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liu, Y.; Jiang, X.; Molokeev, M.S.; Lin, Z.; Xia, Z. Sb3+ Dopant and Halogen Substitution Triggered Highly Efficient and Tunable Emission in Lead-Free Metal Halide Single Crystals. Chem. Mater. 2020, 32, 5327–5334. [Google Scholar] [CrossRef]
- Gong, X.; Voznyy, O.; Jain, A.; Liu, W.; Sabatini, R.; Piontkowski, Z.; Walters, G.; Bappi, G.; Nokhrin, S.; Bushuyev, O. Electron–Phonon Interaction in Efficient Perovskite Blue Emitters. Nat. Mater. 2018, 17, 550–556. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, C.; Li, B.; Lin, C.; Li, Y.; Wang, L.; Xie, R.-J. Large-Scale Room-Temperature Synthesis of High-Efficiency Lead-Free Perovskite Derivative (NH4)2SnCl6:Te Phosphor for Warm wLEDs. Chem. Eng. J. 2021, 420, 129740. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I. QUANTUM ESPRESSO: A modular and Ppen-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Wei, Q.; Duan, L.; Peng, C. Boosting Blue Self-Trapped Exciton Emission in All-Inorganic Zero-Dimensional Metal Halide Cs2ZnCl4 via Zirconium (IV) Doping. Molecules 2024, 29, 1651. https://doi.org/10.3390/molecules29071651
Tian Y, Wei Q, Duan L, Peng C. Boosting Blue Self-Trapped Exciton Emission in All-Inorganic Zero-Dimensional Metal Halide Cs2ZnCl4 via Zirconium (IV) Doping. Molecules. 2024; 29(7):1651. https://doi.org/10.3390/molecules29071651
Chicago/Turabian StyleTian, Ye, Qilin Wei, Lian Duan, and Chengyu Peng. 2024. "Boosting Blue Self-Trapped Exciton Emission in All-Inorganic Zero-Dimensional Metal Halide Cs2ZnCl4 via Zirconium (IV) Doping" Molecules 29, no. 7: 1651. https://doi.org/10.3390/molecules29071651
APA StyleTian, Y., Wei, Q., Duan, L., & Peng, C. (2024). Boosting Blue Self-Trapped Exciton Emission in All-Inorganic Zero-Dimensional Metal Halide Cs2ZnCl4 via Zirconium (IV) Doping. Molecules, 29(7), 1651. https://doi.org/10.3390/molecules29071651





