The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid
Abstract
:1. Introduction
2. Results
2.1. Settling Inhibition Activity
2.2. Effect of EOs on Probing Behavior of R. padi
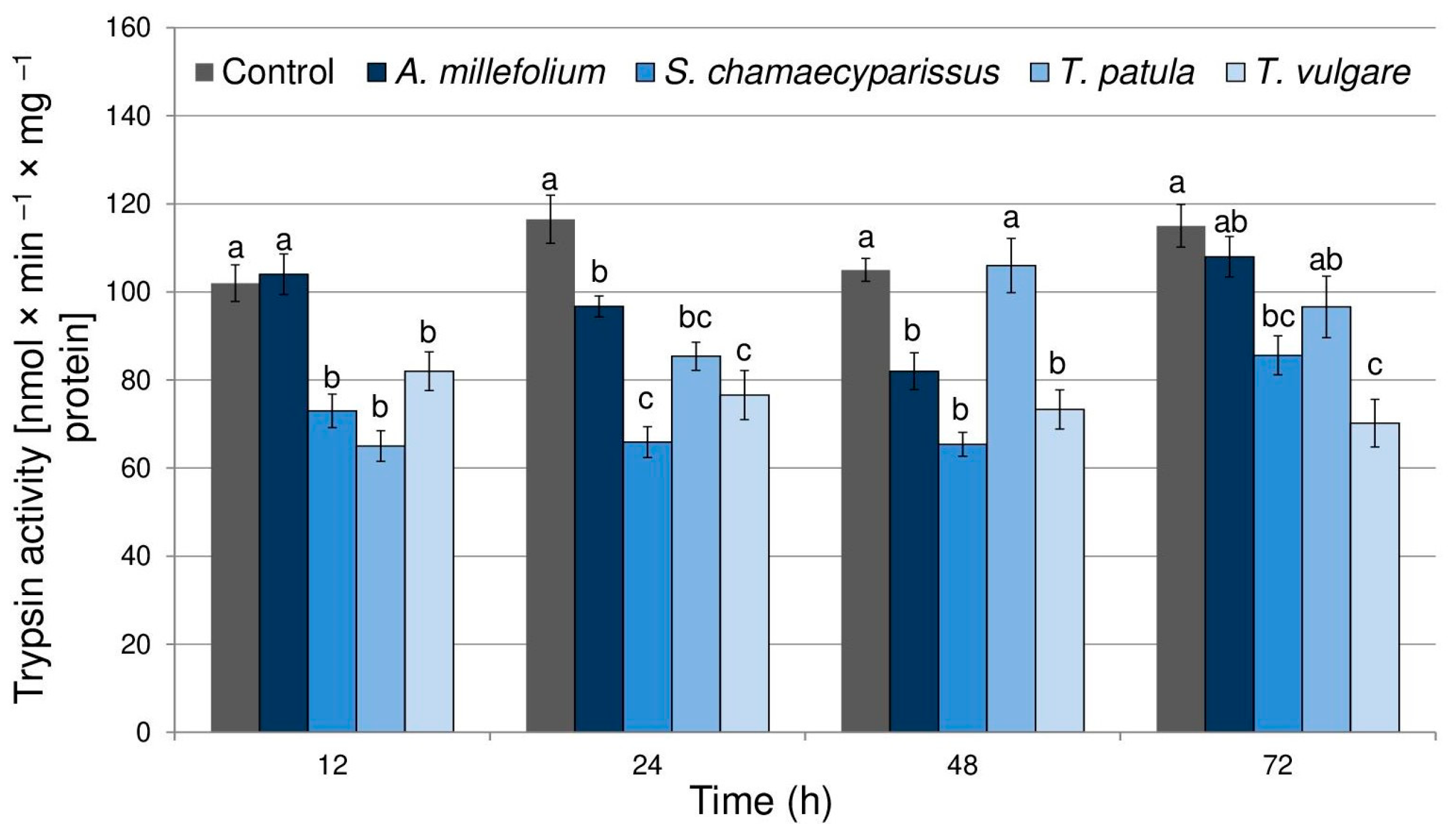
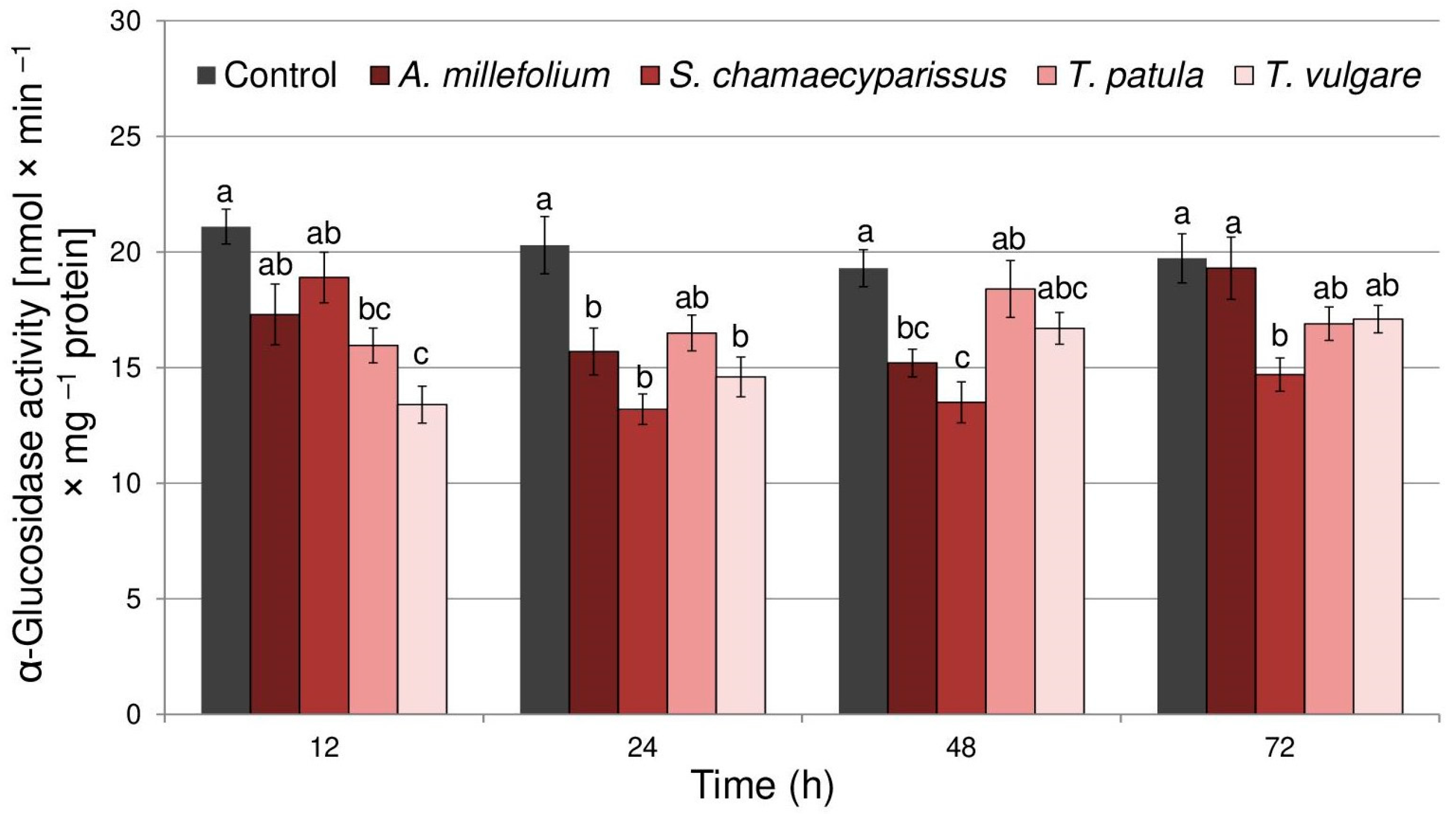
2.3. Effect of Essential Oils on Aphid Enzymes
3. Discussion
4. Materials and Methods
4.1. Aphid Culture
4.2. Plant Material and EO Extraction
4.3. Bioassays
4.3.1. Settling Inhibition Bioassays
4.3.2. Electronic Registration of Aphid Probing Behavior
4.4. Insect Treatment and Biochemical Analyses
4.4.1. Trypsin Assay
4.4.2. Pepsin Assay
4.4.3. Assay of α- and β-Glucosidase
4.4.4. Protein Content Measurement
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mossa, A.H. Green pesticides: Essential oils as biopesticides in insect-pest management. J. Environ. Sci. Technol. 2016, 9, 354–378. [Google Scholar] [CrossRef]
- Devrnja, N.; Milutinović, M.; Savić, J. When scent becomes a weapon—Plant essential oils as potent bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Phumthum, M. Fundamental chemistry of essential oils and volatile organic compounds, methods of analysis and authentication. Plants 2022, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, C.; Pavela, R. Essential oils as active ingredients of botanical insecticides against aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Wagan, T.A.; Hua, H.; Bozdoğan, H.; Khan, M.M. Repellency, contact toxicity, and anti-oviposition effects of three ethanol-extracted plant essential oils on Bemisia tabaci (Hemiptera: Aleyrodidae). Physiol. Plant 2022, 174, e13799. [Google Scholar] [CrossRef] [PubMed]
- Żukowska, G.; Durczyńska, Z. Properties and applications of essential oils: A review. J. Ecol. Eng. 2024, 25, 333–340. [Google Scholar] [CrossRef]
- Dassanayake, M.K.; Chong, C.H.; Khoo, T.-J.; Figiel, A.; Szumny, A.; Choo, C.M. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: A Review. Foods 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Jaouannet, M.; Rodriguez, P.A.; Thorpe, P.; Lenoir, C.J.; MacLeod, R.; Escudero-Martinez, C.; Bos, J.I. Plant immunity in plant-aphid interactions. Front. Plant Sci. 2014, 5, 663. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; van Emden, H., Harrington, R., Eds.; CABI: Wallingford, UK, 2007; pp. 1–29. [Google Scholar]
- Kern, M.; Meiners, T.; Schliephake, E.; Habekus, A.; Ordon, F.; Will, T. Infection of susceptible/tolerant barley genotypes with Barley yellow dwarf virus alters the host plant preference of Rhopalosiphum padi clones depending upon their ability to transmit BYDV. J. Pest Sci. 2022, 95, 215–229. [Google Scholar] [CrossRef]
- Almeida, J.; Cormier, D.; Lucas, E. Effect of Achillea millefolium strips and essential oil on the european apple sawfly, Hoplocampa testudinea (Hymenoptera: Tenthredinidea). Entomol. Ornithol. Herpetol. 2017, 6, 199. [Google Scholar] [CrossRef]
- Akbari, S.; Aramideh, S. Fumigant toxicity and sublethal effects of Achilla millefolium L. and Mentha pulegium L. essential oils on life table parameters of Aphis gossypii Glover. Iran. J. Med. Aromat. Plants 2023, 39, 188–202. [Google Scholar]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The effect of Tanacetum vulgare essential oil and its main components on some ecological and physiological parameters of Acrobasis advenella (Zinck.) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2020, 162, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, N.; Gospodarek, J.; Boligłowa, E. The effects of water extracts from tansy on pea leaf weevil and black bean aphid. J. Ecol. Eng. 2020, 21, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Elguea-Culebras, G.; Sánchez-Vioque, R.; Berruga, M.I.; Herraiz-Penalver, D.; González-Coloma, A.; Andrés, M.F.; Santana-Méridas, O. Biocidal potential and chemical composition of industrial essential oils from Hyssopus officinalis, Lavandula × intermedia var. Super, and Santolina chamaecyparissus. Chem. Biodivers. 2018, 15, e1700313. [Google Scholar] [CrossRef] [PubMed]
- Manh, H.D.; Tuyet, O.T. Larvicidal and repellent activity of Mentha arvensis L. essential oil against Aedes aegypti. Insects 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper aduncum essential oil: A promising insecticide, acaricide and antiparasitic. A review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal activity of selected Asteraceae essential oils and their effect on enzyme activities of the green peach aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G. The effect of Santolina chamaecyparissus and Tagetes patula essential oils on biochemical markers of oxidative stress in aphids. Insects 2021, 12, 360. [Google Scholar] [CrossRef]
- Gabryś, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by novel β-damascone analogues. J. Pest Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef]
- Tine-Djebbar, F.; Trad, M.; Tine, A.O.; Tine, S.; Soltani, N. Effects of menthol on nutritional physiology and enzyme activities of the lesser grain borer, Rhyzopertha dominica (F. 1792) (Coleoptera: Bostrichidae). J. Plant Dis. Prot. 2023, 130, 509–518. [Google Scholar] [CrossRef]
- Jagdale, S.; Bansode, S.; Joshi, R. Insect proteases: Structural-functional outlook. In Proteases in Physiology and Pathology; Chakraborti, S., Dhalla, N., Eds.; Springer: Singapore, 2017; pp. 451–473. [Google Scholar] [CrossRef]
- Farhoodi, N.; Kazzazi, M.; Hosseininaveh, V.; Arezi, I. Inhibitory effect of proteinaceous seed extract of three Iranian wheat cultivars on Eurygaster integriceps (Sunn pest) digestive enzymes. Arch. Phytopathol. Plant Prot. 2019, 52, 1177–1192. [Google Scholar] [CrossRef]
- Dancewicz, K.; Szumny, A.; Wawrzeńczyk, C.; Gabryś, B. Repellent and antifeedant activities of citral-derived lactones against the peach potato aphid. Int. J. Mol. Sci. 2020, 21, 8029. [Google Scholar] [CrossRef]
- Hori, M. Antifeeding, settling inhibitory and toxic activities of labiate essential oils against the green peach aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 1999, 34, 113–118. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Hardie, J.; Dawson, G.W.; Hick, A.J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Behavioral and electro-physiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol. 1991, 17, 1231–1242. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- Halbert, S.E.; Corsini, D.; Wiebe, M.; Vaughn, S.F. Plant-derived compounds and extracts with potential as aphid repellents. Ann. Appl. Biol. 2009, 154, 303–307. [Google Scholar] [CrossRef]
- Wróblewska-Kudryk, A.; Gniłka, R.; Dancewicz, K.; Grudniewska, A.; Wawrzeńczyk, C.; Gabryś, B. β-Thujone and its derivatives modify the probing behavior of the peach potato aphid. Molecules 2019, 24, 1847. [Google Scholar] [CrossRef]
- Suleiman, M.; Rugumamu, C.P.; Ibrahim, N.D. Repellency potential of some botanicals against the maize weevil, Sitophilus zeamais (Motschulsky, 1855) (Coleoptera: Curculionidae) in stored sorghum. Pol. J. Entomol. 2018, 87, 85–99. [Google Scholar] [CrossRef]
- Grudniewska, A.; Dancewicz, K.; Białońska, A.; Wawrzeńczyk, C.; Gabryś, B. Piperitone-derived saturated lactones: Synthesis and aphid behawior-modyfying activity. J. Agric. Food Chem. 2013, 61, 3364–3372. [Google Scholar] [CrossRef]
- Sundar, S.K.; Parikh, J.K. Advances and trends in encapsulation of essential oils. Int. J. Pharm. 2023, 635, 122668. [Google Scholar] [CrossRef]
- Dancewicz, K.; Sznajder, K.; Załuski, D.; Kordan, B.; Gabryś, B. Behavioral sensitivity of Myzus persicae to volatile isoprenoids in plant tissues. Entomol. Exp. Appl. 2016, 160, 229–240. [Google Scholar] [CrossRef]
- Abualfia, R.; Samara, R. Antifeedants impact of plant essential oil on green peach aphid on potato crops. J. Ecol. Eng. 2022, 23, 274–285. [Google Scholar] [CrossRef]
- Denoirjean, T.; Belhassen, D.; Doury, G.; Ameline, A.; Werrie, P.Y.; Fauconnier, M.L.; Hance, T.; Le Goff, G.J. Essential oil trunk injection into orchard trees: Consequences on the performance and preference of hemipteran pests. J. Econ. Entomol. 2023, 116, 389–398. [Google Scholar] [CrossRef]
- Wróblewska-Kudryk, A.; Nowak, L.; Dancewicz, K.; Szumny, A.; Gabryś, B. In search of biopesticides: The effect of caraway Carum carvi essential oil and its major constituents on peach potato aphid Myzus persicae probing behavior. Acta Biol. 2015, 22, 51–62. [Google Scholar] [CrossRef]
- Dardouri, T.; Gomez, L.; Ameline, A.; Costagliola, G.; Schoeny, A.; Gautier, H. Non-host volatiles disturb the feeding behavior and reduce the fecundity of the green peach aphid, Myzus persicae. Pest Manag. Sci. 2020, 77, 1705–1713. [Google Scholar] [CrossRef]
- Moreno, A.; Tjallingii, W.F.; Fernandez-Mata, G.; Fereres, A. Differences in the mechanism of inoculation between a semi-persistent and a non-persistent aphid-transmitted plant virus. J. Gen. Virol. 2012, 93, 662–667. [Google Scholar] [CrossRef]
- Jacobson, A.L.; Kennedy, G.G. Electrical penetration graph studies to investigate the effects of cyantraniliprole on feeding behavior of Myzus persicae (Hemiptera: Aphididae) on Capsicum annuum. Pest Manag. Sci. 2014, 70, 836–840. [Google Scholar] [CrossRef]
- Costa, R.R.; Moraes, J.C.; DaCosta, R.R. Feeding behavior of the greenbug Schizaphis graminum on wheat plants treated with imidacloprid and/or silicon. J. Appl. Entomol. 2011, 135, 115–120. [Google Scholar] [CrossRef]
- Taglienti, A.; Donati, L.; Dragone, I.; Ferretti, L.; Gentili, A.; Araniti, F.; Sapienza, F.; Astolfi, R.; Fiorentino, S.; Vecchiarelli, V.; et al. In vivo antiphytoviral and aphid repellency activity of essential oils and hydrosols from Mentha suaveolens and Foeniculum vulgare to control zucchini yellow mosaic virus and its vector Aphis gossypii. Plants 2023, 12, 1078. [Google Scholar] [CrossRef]
- Tatun, N.; Vajarasathira, B.; Tungjitwitayakul, J.; Sakurai, S. Inhibitory effects of plant extracts on growth, development and α-amylase activity in the red flour beetle Tribolium castaneum (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2014, 111, 181–188. [Google Scholar] [CrossRef]
- Zhou, B.G.; Wang, S.; Dou, T.T.; Liu, S.; Li, M.Y.; Hua, R.M.; Li, S.G.; Lin, H.F. Aphicidal activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in Myzus persicae (Hemiptera: Aphididae). J. Insect Sci. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Pascual-Ruiz, S.; Carrillo, L.; Alvarez-Alfageme, F.; Ruiz, M.; Castanera, P.; Ortego, F. The effects of different prey regimes on the proteolytic digestion of nymphs of the spined soldier bug, Podisus maculiventris (Hemiptera: Pentatomidae). Bull. Entomol. Res. 2009, 99, 487–491. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahebzadeh, N.; Zibaee, A. Effect of Teucrium polium (Lamiaceae) essential oil on digestive enzyme activities and energy reserves of Ephestia kuehniella (Lepidoptera: Pyralidae). Invertebr. Surviv. J. 2017, 14, 182–189. [Google Scholar] [CrossRef]
- Liu, X.; Xi, K.; Wang, Y.; Ma, J.; Huang, X.; Liu, R.; Cai, X.; Zhu, Y.; Yin, J.; Jia, Q.; et al. Evaluation of the contact toxicity and physiological mechanisms of ginger (Zingiber officinale) shoot extract and selected major constituent compounds against Melanaphis sorghi Theobald. Horticulturae 2022, 8, 944. [Google Scholar] [CrossRef]
- Rohn, S.; Rawel, H.M.; Kroll, J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002, 50, 3566–3571. [Google Scholar] [CrossRef]
- Lazarević, J.; Janković-Tomanić, M. Dietary and phylogenetic correlates of digestive trypsin activity in insect pests. Entomol. Exp. Appl. 2015, 157, 123–151. [Google Scholar] [CrossRef]
- Price, D.R.; Karley, A.J.; Ashford, D.A.; Isaacs, H.V.; Pownall, M.E.; Wilkinson, H.S.; Gatehouse, J.A.; Douglas, A.E. Molecular characterisation of a candidate gut sucrase in the pea aphid, Acyrthosiphon pisum. Insect Biochem. Mol. Biol. 2007, 37, 307–317. [Google Scholar] [CrossRef]
- Sprawka, I.; Goławska, S.; Goławski, A.; Chrzanowski, G.; Czerniewicz, P.; Sytykiewicz, H. Entomotoxic action of jackbean lectin (Con A) in bird cherry-oat aphid through the effect on insect enzymes. J. Plant Interact. 2014, 9, 425–433. [Google Scholar] [CrossRef]
- Khosravi, R.; Sendi, J.J. Toxicity, development and physiological effect of Thymus vulgaris and Lavandula angustifolia essential oils on Xanthogaleruca luteola (Coleoptera: Chrysomelidae). J. King Saud Univ. Sci. 2013, 25, 349–355. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Gutierrez, C.; Fereres, A.; Reina, M.; Cabrera, R.; Gonzales-Coloma, A. Behavioral and sublethal effects of structurally related lower terpenes on Myzus persicae. J. Chem. Ecol. 1997, 23, 1641–1650. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; pp. 95–108. [Google Scholar]
- Goławska, S.; Leszczyński, B.; Oleszek, W. Effect of low and high-saponin lines of alfalfa on pea aphid. J. Insect Physiol. 2006, 52, 737–743. [Google Scholar] [CrossRef]
- Pontual, E.V.; Napoleao, T.H.; Dias de Assis, C.R.; Souza, R.; Xavier, H.S.; Navarro, D.M.; Coelho, L.C.; Paiva, P.M. Effect of Moringa oleifera flower extract on larval trypsin and acetylcholinesterase activities in Aedes aegypti. Arch. Insect Biochem. Physiol. 2012, 79, 135–152. [Google Scholar] [CrossRef]
- Anson, M.L. The estimation of pepsin, trypsin, papain and cathepsin with haemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Houseman, J.G.; Downe, A.E.R. Cathepsin D-like activity in the posterior midgut of hemipteran insects. Comp. Biochem. Physiol. 1983, 75, 509–512. [Google Scholar] [CrossRef]
- Katagiri, C. α-D-glucosidase in the serum of the American cockroach Periplaneta Americana. Insect Biochem. 1979, 9, 199–204. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Essential Oil | Time (h) | Number of Aphids per Leaf | p | % SI | |
|---|---|---|---|---|---|
| Treated | Control | ||||
| A. millefolium | 1 | 2.2 ± 0.46 | 11.6 ± 0.94 | <0.001 | 81.0 |
| 4 | 3.1 ± 0.90 | 13.9 ± 0.92 | <0.001 | 77.7 | |
| 8 | 4.1 ± 0.60 | 14.1 ± 0.92 | <0.001 | 70.9 | |
| 24 | 4.3 ± 0.77 | 13.6 ± 0.91 | <0.001 | 68.4 | |
| S. chamaecyparissus | 1 | 2.5 ± 0.68 | 10.2 ± 1.05 | <0.001 | 75.5 |
| 4 | 3.8 ± 1.01 | 12.6 ± 1.11 | <0.001 | 69.8 | |
| 8 | 4.2 ± 0.97 | 13.3 ± 1.25 | <0.001 | 68.4 | |
| 24 | 4.8 ± 1.17 | 13.3 ± 1.32 | <0.001 | 63.9 | |
| T. patula | 1 | 5.4 ± 1.02 | 10.4 ± 1.21 | 0.005 | 48.1 |
| 4 | 5.2 ± 0.94 | 12.2 ± 0.95 | <0.001 | 57.4 | |
| 8 | 6.4 ± 0.68 | 12.6 ± 0.94 | <0.001 | 49.2 | |
| 24 | 7.4 ± 0.82 | 10.9 ± 0.84 | 0.008 | 32.1 | |
| T. vulgare | 1 | 2.2 ± 0.29 | 6.5 ± 0.99 | <0.001 | 66.1 |
| 4 | 4.8 ± 0.64 | 11.4 ± 0.67 | <0.001 | 57.9 | |
| 8 | 4.6 ± 0.65 | 11.4 ± 0.73 | <0.001 | 59.6 | |
| 24 | 5.2 ± 0.80 | 11.8 ± 1.04 | <0.001 | 55.9 | |
| EPG Parameter | Control | Essential Oils | |||
|---|---|---|---|---|---|
| A. millefolium | S. chamaecyparissus | T. patula | T. vulgare | ||
| General Aspects of Aphid Probing Behavior | |||||
| Time from start of EPG to first probe (min) | 6.3 ± 1.4 | 23.9 ± 6.3 * | 15.7 ± 3.1 * | 10.6 ± 2.5 | 8.0 ± 1.2 |
| Number of probes | 6.9 ± 0.8 | 11.1 ± 1.7 * | 10.1 ± 1.8 | 7.9 ± 1.4 | 14.4 ± 2.6 * |
| Total duration of probing (min) | 451.8 ± 4.6 | 404.2 ± 12.0 * | 426.2 ±7.8 * | 434.0 ± 6.0 * | 441.3 ± 6.3 |
| Number of pathway phases | 10.4 ± 1.0 | 15.3 ± 1.6 * | 14.5 ± 1.7 * | 11.2 ± 1.5 | 18.8 ± 2.5 * |
| Total duration of pathway phase (min) | 121.2 ± 12.8 | 173.5 ± 21.4 | 152.6 ± 20.3 | 145.7 ± 19.9 | 216.4 ± 23.6 * |
| Total duration of xylem phase (min) | 7.8 ± 3.9 | 11.9 ± 4.2 | 5.3 ± 3.0 | 9.8 ± 4.5 | 14.8 ± 5.1 |
| Total duration of phloem phase a (min) | 322.8 ± 17.8 | 218.7 ± 29.7 * | 268.2 ± 19.0 | 278.4 ± 19.7 | 210.1 ± 26.7 * |
| Phloem phase index b | 0.71 ± 0.03 | 0.48 ± 0.06 * | 0.67 ± 0.05 | 0.65 ± 0.05 | 0.49 ± 0.07 * |
| Aphid Probing Behavior Associated with Phloem Phase | |||||
| Total duration of phloem salivation phase (min) | 12.2 ± 1.9 | 13.8 ± 2.5 | 23.0 ± 2.0 * | 13.4 ± 1.6 | 17.1 ± 2.0 |
| Time from start to first salivation c (min) | 65.6 ± 11.6 | 94.8 ± 14.4 | 103.8 ± 18.9 | 79.8 ± 10.9 | 142.2 ± 16.6 * |
| Total duration of phloem ingestion (min) | 310.6 ± 17.8 | 205.0 ± 28.9 * | 245.2 ± 19.5 * | 265.0 ± 19.5 | 193.0 ± 26.7 * |
| Time from start to first sustained phloem ingestion d (min) | 104.4 ± 14.7 | 151.4 ± 19.9 | 136.1 ± 20.6 | 118.8 ± 12.9 | 184.3 ± 18.2 * |
| Number of probes before the first sustained phloem ingestion d | 4.7 ± 0.6 | 6.1 ± 0.7 | 5.4 ± 0.7 | 5.2 ± 0.8 | 7.1 ± 1.3 |
| Potential drops (pd) | |||||
| Total number of pd | 66.3 ± 6.6 | 79.8 ± 7.7 | 70.3 ± 8.9 | 68.1 ± 8.6 | 88.1 ± 7.2 * |
| Mean duration of a single pd (s) | 4.3 ± 0.07 | 4.1 ± 0.12 | 4.5 ± 0.13 | 4.4 ± 0.08 | 4.3 ± 0.09 |
| Total duration of sub-phase pd II-1 (min) | 1.9 ± 0.24 | 2.0 ± 0.20 | 2.0 ± 0.27 | 1.9 ± 0.24 | 2.3 ± 0.22 |
| Total duration of sub-phase pd II-2 (min) | 1.1 ± 0.14 | 1.4 ± 0.14 | 1.3 ± 0.18 | 1.0 ± 0.16 | 1.5 ± 0.15 |
| Total duration of sub-phase pd II-3 (min) | 1.7 ± 0.20 | 2.2 ± 0.23 | 1.9 ± 0.27 | 1.7 ± 0.23 | 2.4 ± 0.22 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czerniewicz, P.; Sytykiewicz, H.; Chrzanowski, G. The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid. Molecules 2024, 29, 1673. https://doi.org/10.3390/molecules29071673
Czerniewicz P, Sytykiewicz H, Chrzanowski G. The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid. Molecules. 2024; 29(7):1673. https://doi.org/10.3390/molecules29071673
Chicago/Turabian StyleCzerniewicz, Paweł, Hubert Sytykiewicz, and Grzegorz Chrzanowski. 2024. "The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid" Molecules 29, no. 7: 1673. https://doi.org/10.3390/molecules29071673
APA StyleCzerniewicz, P., Sytykiewicz, H., & Chrzanowski, G. (2024). The Effect of Essential Oils from Asteraceae Plants on Behavior and Selected Physiological Parameters of the Bird Cherry-Oat Aphid. Molecules, 29(7), 1673. https://doi.org/10.3390/molecules29071673






