Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response
Abstract
:1. Introduction
2. Results and Discussion
2.1. Infrared Spectral Analysis
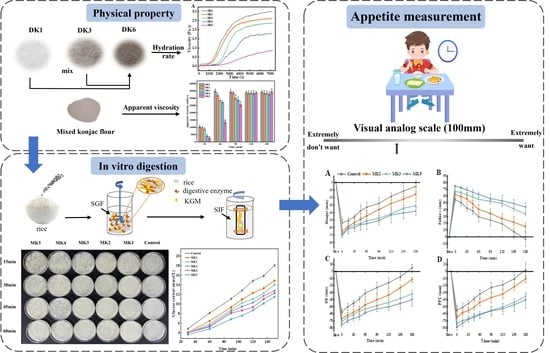
2.2. Hydration Process of Konjac Flour
2.3. Rheology Property of DKGM
2.4. Apparent Viscosity of MK
2.5. Morphological Analysis of Rice after Digestion
2.6. Texture of Digested Rice
2.7. Effects of MK’s Hydration Rate on Glucose Diffusion
2.8. Postprandial Subjective Appetite
3. Materials and Methods
3.1. Materials
3.2. Preparation of DKGM
3.3. Determination of Deacetylation Degree
3.4. Characterization of DKGM
3.4.1. Infrared Spectroscopy Analysis
3.4.2. Hydration Process Monitoring
3.4.3. Rheological Measurement
3.5. Digestion of Rice In Vitro
3.5.1. Simulated Digestive Fluid
3.5.2. Preparation of Mixed Konjac Flour with Different HRs
3.5.3. Texture Measurement of Rice
3.5.4. Glucose Diffusion Measurement
3.6. Postprandial Satiety Measurement
3.6.1. Preparation of Test Meal
3.6.2. Subjects’ Recruitment
- BMI between 18.5 and 23.9 kg/m2;
- Age between 18 and 35 years;
- Breakfast regularity with at least 5 days of breakfast per week.
- Unstable weight, weight gain, or loss of more than 3 kg in the last 3 months;
- Unhealthy dietary habits, including smoking and alcohol consumption;
- Patients with gastrointestinal disorders.
3.6.3. Subjective Appetite
3.7. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akhlaghi, M. The role of dietary fibers in regulating appetite, an overview of mechanisms and weight consequences. Crit. Rev. Food Sci. Nutr. 2022, 64, 3139–3150. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, C.; Shang, L. Research Progress in Appetite-regulating Effects of Dietary Fiber’s Viscosity. Chin. Inst. Food Sci. Technol. 2023, 23, 1–11. [Google Scholar]
- Chen, M.; Guo, L.; Nsor-Atindana, J.; Douglas, G.; Zhang, W.; Mao, J.; Zhong, F. The effect of viscous soluble dietary fiber on nu-trient digestion and metabolic responses I: In vitro digestion process. Food Hydrocoll. 2020, 107, 105971. [Google Scholar] [CrossRef]
- Capuano, E. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 2017, 57, 3543–3564. [Google Scholar] [CrossRef]
- Qadir, N.; Wani, I.A. In-vitro digestibility of rice starch and factors regulating its digestion process: A review. Carbohydr. Polym. 2022, 291, 119600. [Google Scholar] [CrossRef]
- Wee, M.S.M.; Henry, C.J. Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 670–702. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sahoo, U.; Baisakha, B.; Okpani, O.A.; Ngangkham, U.; Parameswaran, C.; Basak, N.; Kumar, G.; Sharma, S.G. Resistant starch could be decisive in determining the glycemic index of rice cultivars. J. Cereal Sci. 2018, 79, 348–353. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; Lambré, C.; et al. Re-evaluation of konjac gum (E 425 i) and konjac glucomannan (E 425 ii) as food additives. EFSA J. 2017, 15, e04864. [Google Scholar]
- Zhang, D.; Zhang, X.; Xue, Z.; Ge, W.; Feng, X.; Cao, Z. Advances on modification Technology of konjac glucomannan. J. Food Saf. Qual. 2019, 10, 8389–8398. [Google Scholar]
- Tang, H.; Bai, Y.; Li, Y.; Liu, X. Carboxymethylation and cross-linking of konjac glucomannan: Structure and properties. Iran. Polym. J. 2024, 33, 305–316. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, S.; Shang, L.; Zhou, P.; Li, J.; Li, B. An efficient and simple approach for the controlled preparation of partially degraded konjac glucomannan. Food Hydrocoll. 2020, 108, 106017. [Google Scholar] [CrossRef]
- Yin, J.; Ma, L.; Siu, K.; Wu, J. Effects of Ultrasonication on the Conformational, Microstructural, and Antioxidant Properties of Konjac Glucomannan. Appl. Sci. 2019, 9, 461. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Ge, Z.; Xu, M.; Jin, X.; Gao, S.; Zhao, G.; Zong, W. Physicochemical Properties of Deacetylated Konjac Glucomannan and Its Effects on the Quality and in Vitro Digestion Characteristics of Cold Noodles. Food Sci. 2023, 44, 85–90. [Google Scholar]
- Luo, Y.; Luo, L.; Jia, K. Preparation of Surface Deacetylated Konjac Glucomannan and Its Ability to Adsorb Cholesterol. Food Ind. 2021, 42, 91–95. [Google Scholar]
- Li, S.; Shang, L.; Wu, D.; Dun, H.; Wei, X.; Zhu, J.; Zong, A.; Li, B.; Geng, F. Sodium caseinate reduces the swelling of konjac flour: A further examination. Food Hydrocoll. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Luo, X.; He, P.; Lin, X. The mechanism of sodium hydroxide solution promoting the gelation of Konjac glucomannan (KGM). Food Hydrocoll. 2013, 30, 92–99. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, W.; Ye, S.; Hu, Y.; Wang, Y.; Xu, W.; Li, J.; Li, B. Comparative studies of konjac flours extracted from Amorphophallus guripingensis and Amorphophallus rivirei: Based on chemical analysis and rheology. Food Hydrocoll. 2016, 57, 209–216. [Google Scholar] [CrossRef]
- Wu, D.; Yu, S.; Liang, H.; He, C.; Li, J.; Li, B. The influence of deacetylation degree of konjac glucomannan on rheological and gel properties of konjac glucomannan/κ-carrageenan mixed system. Food Hydrocoll. 2020, 101, 105523. [Google Scholar] [CrossRef]
- Lin, W.; Ni, Y.; Wang, L.; Liu, D.; Wu, C.; Pang, J. Physicochemical properties of degraded konjac glucomannan prepared by laser assisted with hydrogen peroxide. Int. J. Biol. Macromol. 2019, 129, 78–83. [Google Scholar] [CrossRef]
- Munialo, C.D.; Kontogiorgos, V.; Euston, S.R.; Nyambayo, I. Rheological, tribological and sensory attributes of texture-modified foods for dysphagia patients and the elderly: A review. Int. J. Food Sci. Technol. 2020, 55, 1862–1871. [Google Scholar] [CrossRef]
- Zaremba, S.M.M.; Gow, I.F.; Drummond, S.; Mccluskey, J.T. Effects of oat β-glucan consumption at breakfast on ad libitum eating, appetite, glycemia, insulinemia and GLP-1 concentrations in healthy subjects. Appetite 2018, 128, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Ren, Y.; Ai, T.; Zhou, P.; Hu, L.; Wang, L.; Li, J.; Li, B. In vitro gastric emptying characteristics of konjac glucomannan with different viscosity and its effects on appetite regulation. Food Funct. 2020, 11, 7596–7610. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Xin, C.; Li, J.; Li, B. Ultrasonic Degradation of Konjac Glucomannan and the Effect of Freezing Combined with Alkali Treatment on Their Rheological Profiles. Molecules 2019, 24, 1860. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Xu, W.; Li, Z.; Li, J.; Zhou, B.; Zhang, C.; Li, B. Degraded konjac glucomannan by γ-ray irradiation assisted with ethanol: Preparation and characterization. Food Hydrocoll. 2014, 36, 85–92. [Google Scholar] [CrossRef]
- Ye, S.; Zhu, J.; Shah, B.R.; Wend-Soo, Z.A.; Li, J.; Zhan, F.; Li, B. Preparation and characterization of konjac glucomannan (KGM) and deacetylated KGM (DA-KGM) obtained by sonication. J. Sci. Food Agric. 2022, 102, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Dikeman, C.L.; Fahey, G.C. Viscosity as Related to Dietary Fiber: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, S.; Ak, M.M. Dynamic oscillatory shear testing of foods—Selected applications. Trends Food Sci. Technol. 2000, 11, 115–127. [Google Scholar] [CrossRef]
- Nadia, J.; Bronlund, J.E.; Singh, H.; Singh, R.P.; Bornhorst, G.M. Contribution of the proximal and distal gastric phases to the breakdown of cooked starch-rich solid foods during static in vitro gastric digestion. Food Res. Int. 2022, 157, 111270. [Google Scholar] [CrossRef]
- Park, J.-W.; Lee, S.; Yoo, B.; Nam, K. Effects of texture properties of semi-solid food on the sensory test for pharyngeal swallowing effort in the older adults. BMC Geriatr. 2020, 20, 493. [Google Scholar] [CrossRef]
- Nishinari, K.; Fang, Y. Perception and measurement of food texture: Solid foods. J. Texture Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Mennah, G.Y.A.; Bornhorst, G.M.; Singh, R.P. Acid Diffusion into Rice Boluses is Influenced by Rice Type, Variety, and Presence of α-Amylase. J. Food Sci. 2015, 80, E316–E325. [Google Scholar]
- Guo, Q.; Ye, A.; Singh, H.; Rousseau, D. Destructuring and restructuring of foods during gastric digestion. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1658–1679. [Google Scholar] [CrossRef]
- Fang, X.; Rioux, L.E.; Labrie, S.; Turgeon, S.L. Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion. Int. Dairy J. 2016, 56, 169–178. [Google Scholar] [CrossRef]
- Ma, Y.S.; Pan, Y.; Xie, Q.T.; Li, X.M.; Zhang, B.; Chen, H.Q. Evaluation studies on effects of pectin with different concentrations on the pasting, rheological and digestibility properties of corn starch. Food Chem. 2019, 274, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Cui, B.; Yuan, C.; Zou, Y.; Liu, W.; Pan, Y. Effects of konjac glucomannan on the rheological, microstructure and digestibility properties of debranched corn starch. Food Hydrocoll. 2020, 100, 105342. [Google Scholar] [CrossRef]
- Guo, L.; Goff, H.D.; Chen, M.; Zhong, F. The hydration rate of konjac glucomannan after consumption affects its in vivo glycemic response and appetite sensation and in vitro digestion characteristics. Food Hydrocoll. 2022, 122, 107102. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Zhou, X.; Li, B.; Zhang, X.; Hui, R. Structural characterization and α-glucosidase inhibitory activity of polysaccharides extracted from Chinese traditional medicine Huidouba. Int. J. Biol. Macromol. 2018, 117, 815–819. [Google Scholar] [CrossRef]
- Chi, C.; Shi, M.; Zhao, Y.; Chen, B.; He, Y.; Wang, M. Dietary compounds slow starch enzymatic digestion: A review. Front. Nutr. 2022, 9, 1004966. [Google Scholar] [CrossRef]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef]
- Butterworth, P.J.; Warren, F.J.; Ellis, P.R. Human α-amylase and starch digestion: An interesting marriage. Starch Stärke 2011, 63, 395–405. [Google Scholar] [CrossRef]
- Li, J.; Ye, T.; Wu, X.; Chen, J.; Wang, S.; Lin, L.; Li, B. Preparation and characterization of heterogeneous deacetylated konjac glucomannan. Food Hydrocoll. 2014, 40, 9–15. [Google Scholar] [CrossRef]
- Guo, L.; Yokoyama, W.; Chen, L.; Liu, F.; Chen, M.; Zhong, F. Characterization and physicochemical properties analysis of konjac glucomannan: Implications for structure-properties relationships. Food Hydrocoll. 2021, 120, 106818. [Google Scholar] [CrossRef]
- Marcano, J.; Hernando, I.; Fiszman, S. In vitro measurements of intragastric rheological properties and their relationships with the potential satiating capacity of cheese pies with konjac glucomannan. Food Hydrocoll. 2015, 51, 16–22. [Google Scholar] [CrossRef]
- Sanz, T.; Luyten, H. Effect of thickening agent in the in vitro mouth, stomach and intestine release of tyrosol from enriched custards. Food Hydrocoll. 2006, 20, 703–711. [Google Scholar] [CrossRef]
- Chang, H.; Wang, S.; Zhou, J.; Li, W.; Wang, Y.; Wang, B. Effect of in vitro simulated digestion on flavonoid and reducing ability of fresh-cut apple residue. Sci. Technol. Food Ind. 2022, 43, 39–44. [Google Scholar]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, B.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Fabek, H.; Messerschmidt, S.; Brulport, V.; Goff, H.D. The effect of invitro digestive processes on the viscosity of dietary fibres and their influence on glucose diffusion. Food Hydrocoll. 2014, 35, 718–726. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.E.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]











| Group | η0 (Pa·s) | n | R2 | η50 |
|---|---|---|---|---|
| DK1 | 104.284 | 0.1098 | 0.9996 | 2.9202 |
| DK2 | 89.7114 | 0.1401 | 0.9994 | 2.3283 |
| DK3 | 43.5777 | 0.2119 | 0.9989 | 1.4205 |
| DK4 | 28.8039 | 0.2438 | 0.9964 | 1.1225 |
| DK5 | 8.56849 | 0.4202 | 0.9953 | 0.4083 |
| Hunger | Fullness | DE | PFC | |
|---|---|---|---|---|
| Control | −5363 ± 1101.7 a | 4994 ± 1098.4 c | −4875 ± 481.9 a | −5354 ± 1013.5 a |
| MK1 | −7235 ± 1134.4 b | 7048 ± 1050.8 b | −7748 ± 893.4 b | −7319 ± 766 b |
| MK3 | −9076 ± 589.9 bc | 9860 ± 468 a | −9389 ± 986.8 c | −10,468 ± 769.7 c |
| MK5 | −9780 ± 943.9 c | 11,081 ± 800.9 a | −10,523 ± 589.4 c | −10,953 ± 1176.5 c |
| Ingredients | MK1 (g) | MK2 (g) | MK3 (g) | MK4 (g) | MK5 (g) |
|---|---|---|---|---|---|
| DK1 | 1.7 | ||||
| DK2 | 2.0 | ||||
| DK3 | 2.2 | ||||
| DK4 | 2.5 | ||||
| DK5 | 3.0 | ||||
| DK6 | 1.3 | 1.0 | 0.8 | 0.5 | |
| Total (g) | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Cheng, K.; Kang, Y.; Cheng, C.; Zhang, C.; Shang, L. Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response. Molecules 2024, 29, 1681. https://doi.org/10.3390/molecules29071681
Xu C, Cheng K, Kang Y, Cheng C, Zhang C, Shang L. Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response. Molecules. 2024; 29(7):1681. https://doi.org/10.3390/molecules29071681
Chicago/Turabian StyleXu, Chenfeng, Kaixuan Cheng, Yu Kang, Chao Cheng, Chi Zhang, and Longchen Shang. 2024. "Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response" Molecules 29, no. 7: 1681. https://doi.org/10.3390/molecules29071681
APA StyleXu, C., Cheng, K., Kang, Y., Cheng, C., Zhang, C., & Shang, L. (2024). Deacetylated Konjac Glucomannan with a Slower Hydration Rate Delays Rice Digestion and Weakens Appetite Response. Molecules, 29(7), 1681. https://doi.org/10.3390/molecules29071681