Preparation and Application of Degradable Lignin/Poly (Vinyl Alcohol) Polymers as Urea Slow-Release Coating Materials
Abstract
:1. Introduction
2. Characterization
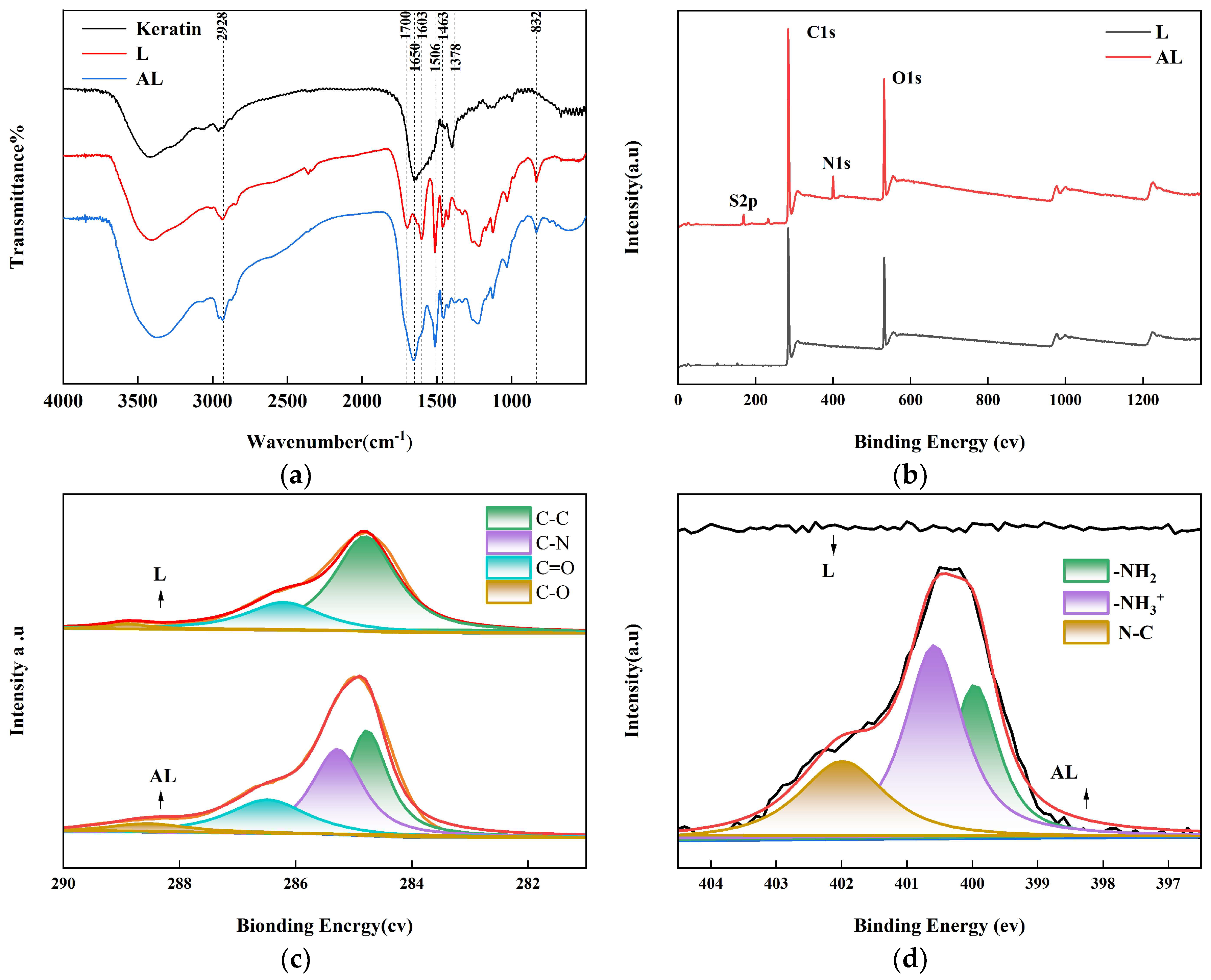
2.1. Characterization of L, AL and PL Composite Films
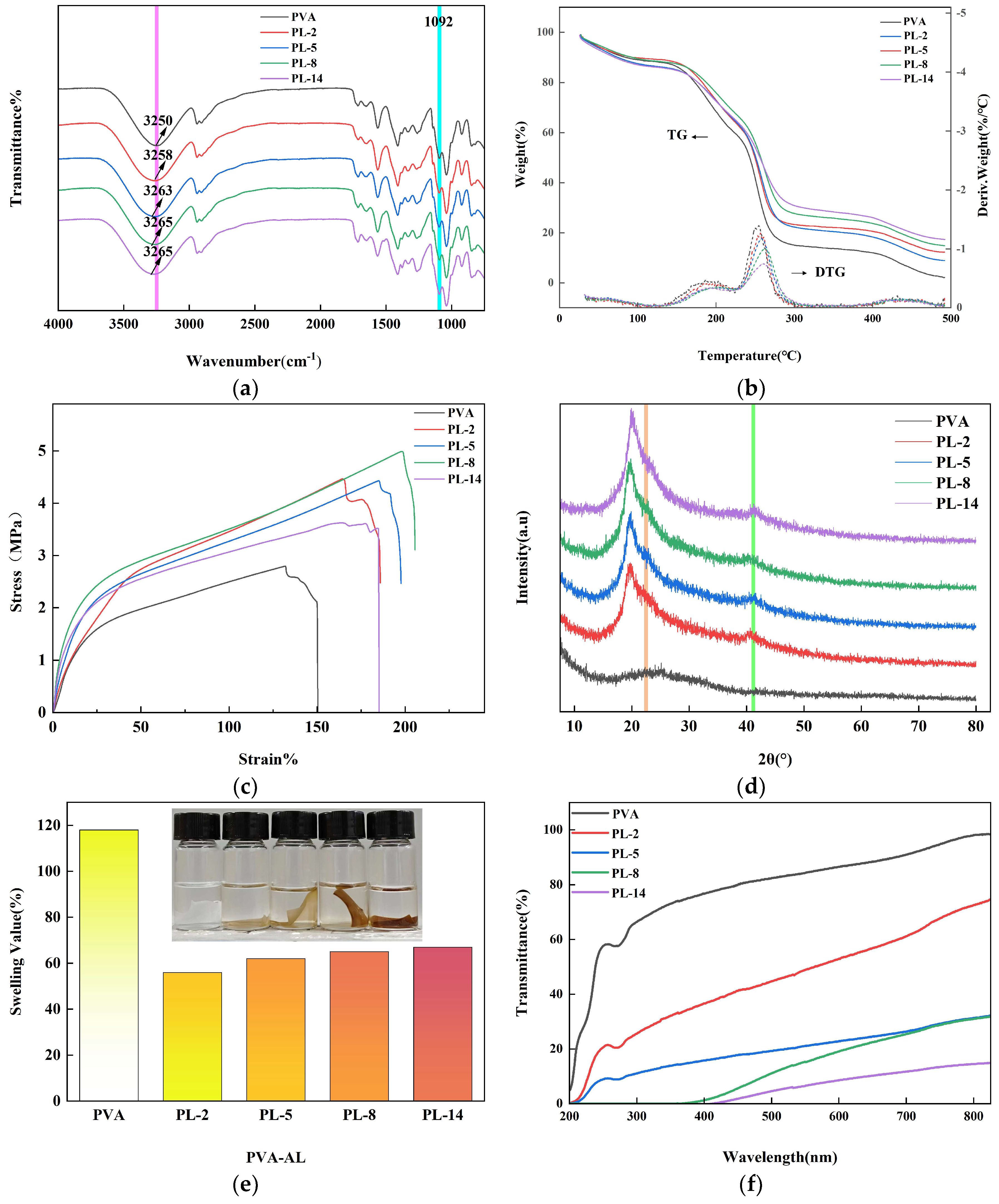
2.2. Properties of Membrane and Degradation
2.2.1. Stress–Strain of the Films
2.2.2. Water Absorption
2.2.3. UV Shielding Performance
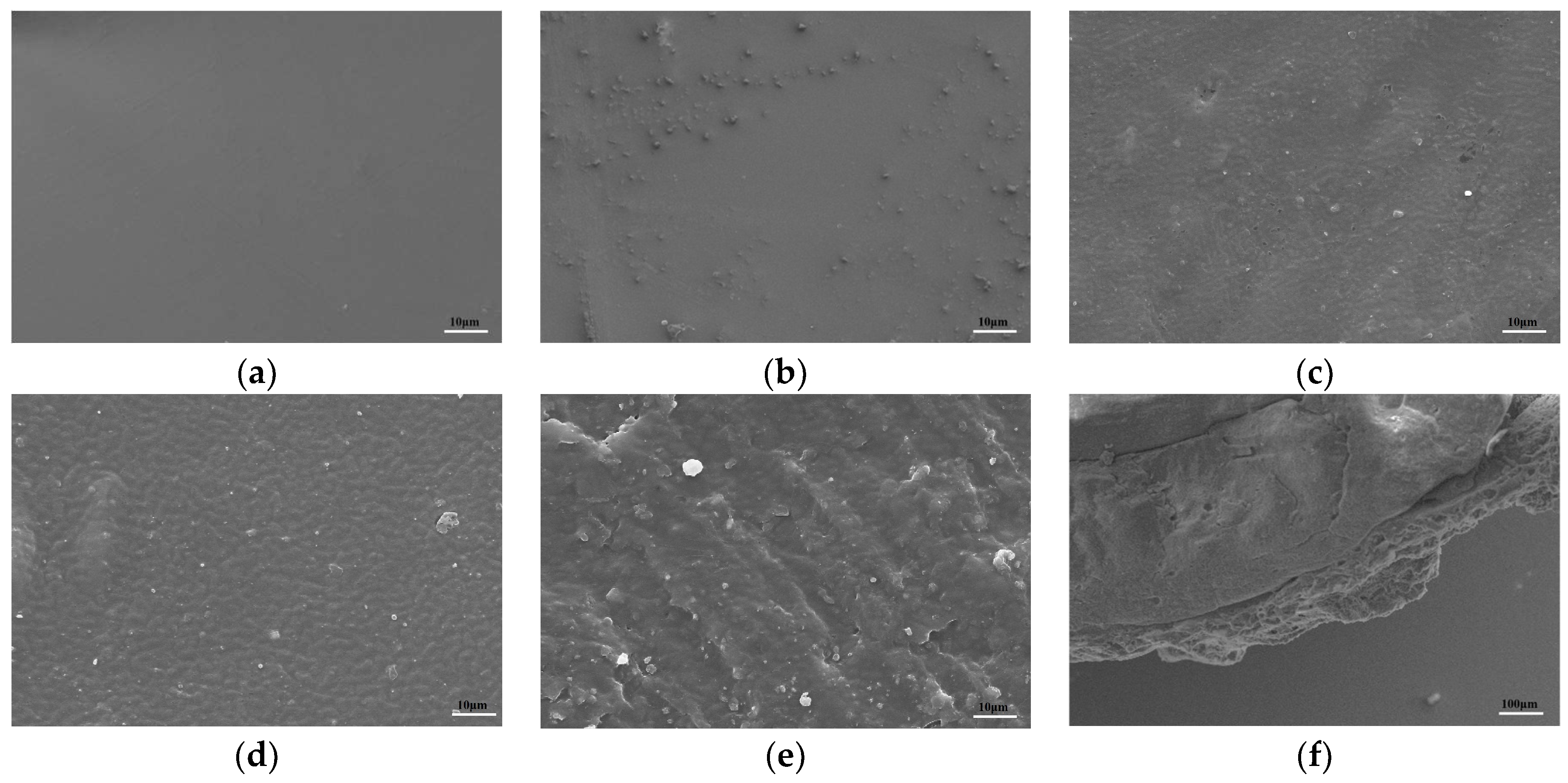
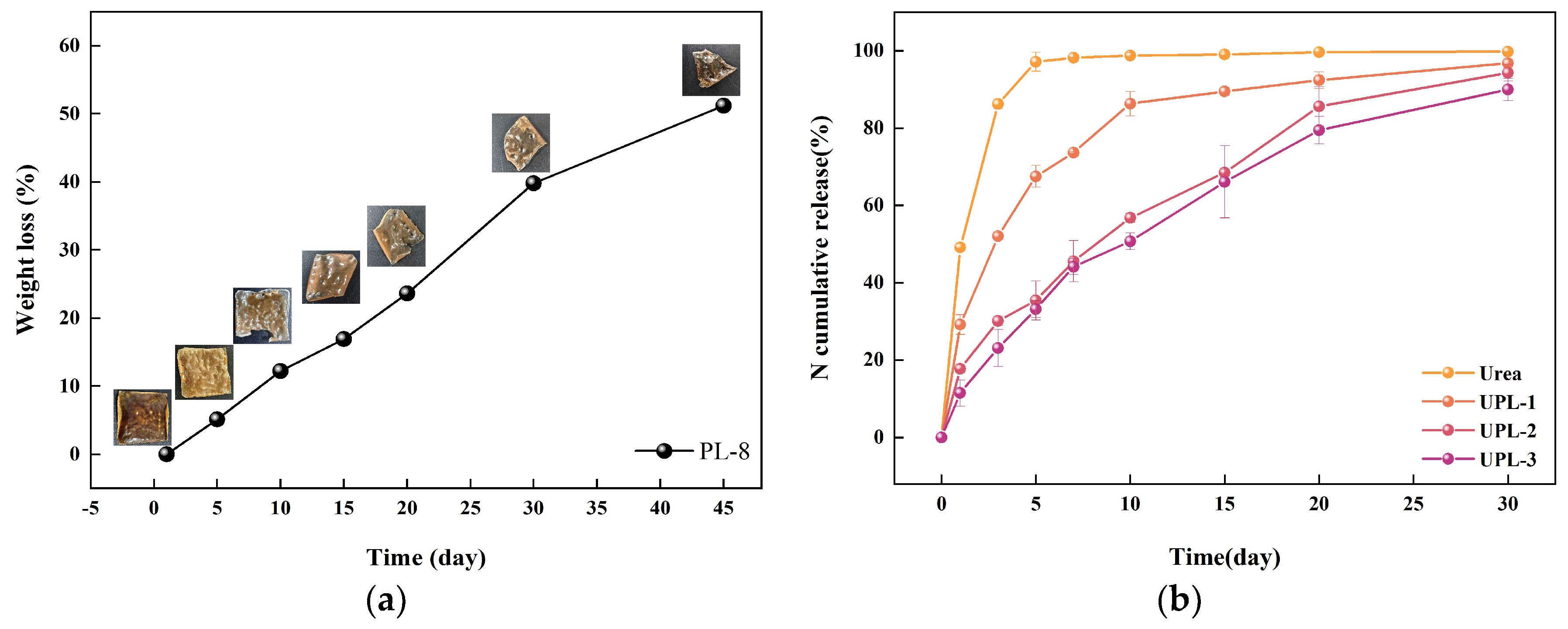
2.2.4. The Morphology Structure and Degradation Properties
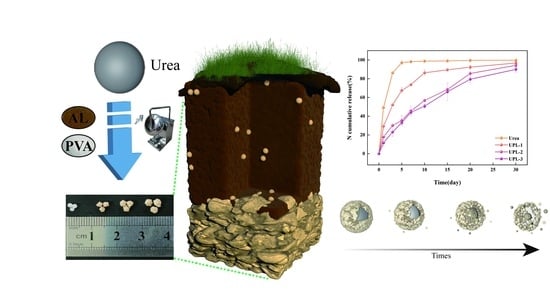
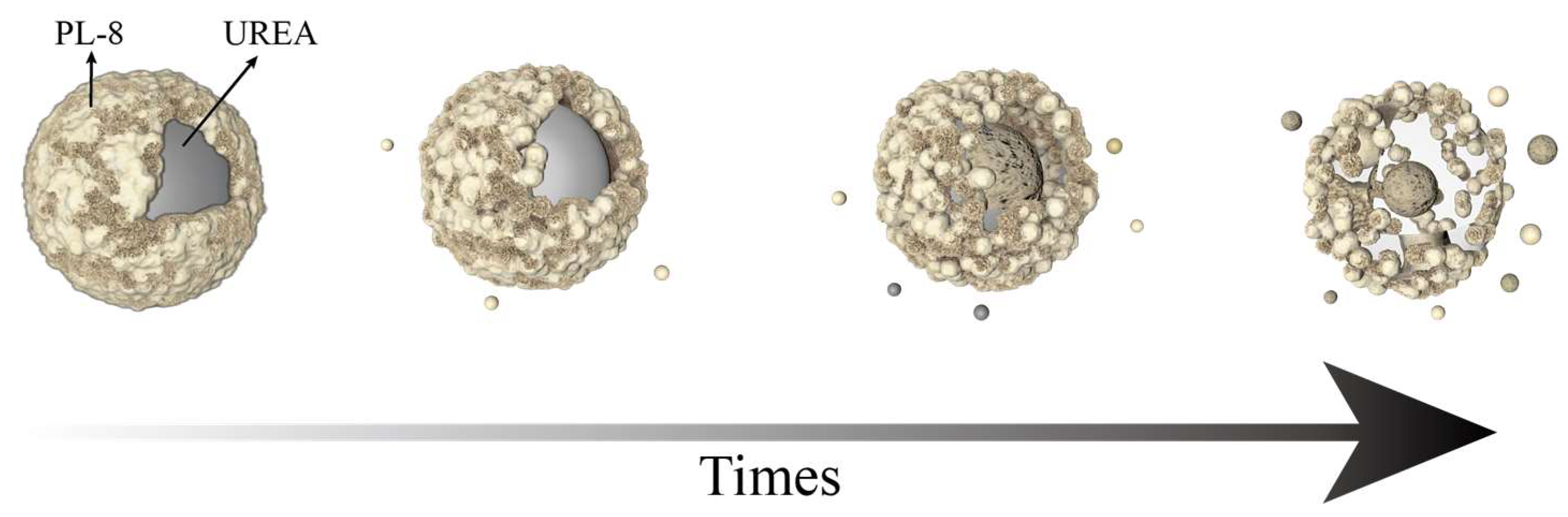
2.3. The Sustained-Release Properties of Coated Urea
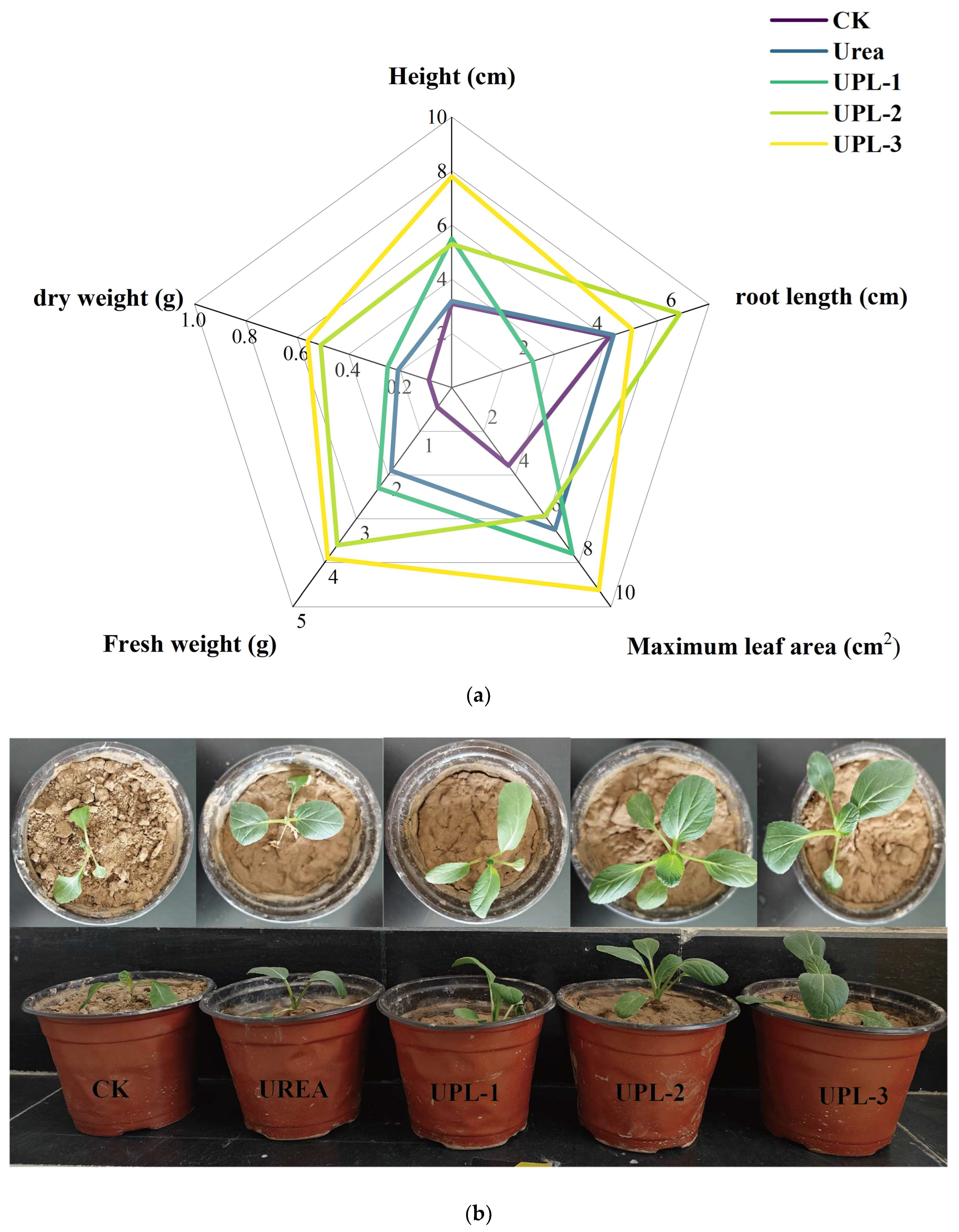
2.4. Potting Experiments
3. Methods
3.1. Materials
3.2. Liquid Chromatography (LC)–Electrospray Ionization (ESI) Tandem MS(MS/MS) Analysis
3.3. Preparation of Aminated Lignin
3.4. Preparation of PVA/AL Composite Membrane
3.5. Preparation of Coated Urea
3.6. Characterization
3.7. Properties and Degradation of Membranes
3.7.1. Mechanical Properties
3.7.2. Water Absorption Rate
3.7.3. Ultraviolet Shielding Performance
3.7.4. Degradation of Membranes
3.8. Slow-Release Kinetics of Coated Urea Particles
3.9. Potting Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, S. Effects of Nitrogen Levels on Anatomy, Growth, and Chlorophyll Content in Sunflower (Helianthus annuus L.) Leaves. J. Agric. Sci. 2017, 9, 208. [Google Scholar] [CrossRef]
- Ma, J.; Faqir, Y.; Chai, Y.; Wu, S.; Luo, T.; Liao, S.; Kaleri, A.R.; Tan, C.; Qing, Y.; Kalhoro, M.T.; et al. Chitosan Microspheres-Based Controlled Release Nitrogen Fertilizers Enhance the Growth, Antioxidant, and Metabolite Contents of Chinese Cabbage. Sci. Hortic. 2023, 308, 111542. [Google Scholar] [CrossRef]
- Qiong, W.U.; Wang, Y.H.; Ding, Y.F.; Tao, W.K.; Shen, G.A.O.; Li, Q.X.; Li, W.W.; Liu, Z.H.; Li, G.H. Effects of Different Types of Slow- and Controlled-Release Fertilizers on Rice Yield. J. Integr. Agric. 2021, 20, 1503–1514. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Bi, H.; Shang, Y.; Shao, Q. A Slow-Release Fertilizer of Urea Prepared via Biochar-Coating with Nano-SiO2-Starch-Polyvinyl Alcohol: Formulation and Release Simulation. Environ. Technol. Innov. 2023, 32, 103264. [Google Scholar] [CrossRef]
- Han, X.; Chen, S.; Hu, X. Controlled-Release Fertilizer Encapsulated by Starch/Polyvinyl Alcohol Coating. Desalination 2009, 240, 21–26. [Google Scholar] [CrossRef]
- Vo, P.T.; Nguyen, H.T.; Trinh, H.T.; Nguyen, V.M.; Le, A.T.; Tran, H.Q.; Nguyen, T.T.T. The Nitrogen Slow-Release Fertilizer Based on Urea Incorporating Chitosan and Poly(Vinyl Alcohol) Blend. Environ. Technol. Innov. 2021, 22, 101528. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, T.J.; Qin, L.; Jin, Y. Urea Particle Coating for Controlled Release by Using DCPD Modified Sulfur. Powder Technol. 2008, 183, 88–93. [Google Scholar] [CrossRef]
- Ke, J.; Xing, X.; Li, G.; Ding, Y.; Dou, F.; Wang, S.; Liu, Z.; Tang, S.; Ding, C.; Chen, L. Effects of Different Controlled-Release Nitrogen Fertilisers on Ammonia Volatilisation, Nitrogen Use Efficiency and Yield of Blanket-Seedling Machine-Transplanted Rice. Field Crop. Res. 2017, 205, 147–156. [Google Scholar] [CrossRef]
- Jintakanon, N.; Opaprakasit, P.; Petchsuk, A.; Opaprakasit, M. Controlled-Release Materials for Fertilizer Based on Lactic Acid Polymers. Adv. Mater. Res. 2008, 55–57, 905–908. [Google Scholar] [CrossRef]
- Mathews, A.S.; Narine, S. Poly[N-Isopropyl Acrylamide]-Co-Poryurethane Copolymers for Controlled Release of Urea. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3236–3243. [Google Scholar] [CrossRef]
- Beig, B.; Niazi, M.B.K.; Jahan, Z.; Hussain, A.; Zia, M.H.; Mehran, M.T. Coating Materials for Slow Release of Nitrogen from Urea Fertilizer: A Review. J. Plant Nutr. 2020, 43, 1510–1533. [Google Scholar] [CrossRef]
- Mittal, A.; Garg, S.; Kohli, D.; Maiti, M.; Jana, A.K.; Bajpai, S. Effect of Cross Linking of PVA/Starch and Reinforcement of Modified Barley Husk on the Properties of Composite Films. Carbohydr. Polym. 2016, 151, 926–938. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Luzi, F.; Hao, X.; Yang, W.; Torre, L.; Xiao, Z.; Xie, Y.; Puglia, D. Thermal, Antioxidant and Swelling Behaviour of Transparent Polyvinyl (Alcohol) Films in Presence of Hydrophobic Citric Acid-Modified Lignin Nanoparticles. Int. J. Biol. Macromol. 2019, 127, 665–676. [Google Scholar] [CrossRef]
- Espinosa, E.; Bascón-Villegas, I.; Rosal, A.; Pérez-Rodríguez, F.; Chinga-Carrasco, G.; Rodríguez, A. PVA/(Ligno)Nanocellulose Biocomposite Films. Effect of Residual Lignin Content on Structural, Mechanical, Barrier and Antioxidant Properties. Int. J. Biol. Macromol. 2019, 141, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Bandyopadhyay, K.K.; Saha, M.; Chawla, G.; Saha, J.K.; et al. Preparation of Novel Biodegradable Starch/Poly(Vinyl Alcohol)/Bentonite Grafted Polymeric Films for Fertilizer Encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Zhang, L.; Wei, Y.; Zhang, L.; Ma, Y.; Ruso, J.M.; Liu, Z. Engineering and Slow-Release Properties of Lignin-Based Double-Layer Coated Fertilizer. Polym. Adv. Technol. 2023, 34, 2029–2043. [Google Scholar] [CrossRef]
- Elhassani, C.E.; El Gharrak, A.; Essamlali, Y.; Elamiri, S.; Dânoun, K.; Aboulhrouz, S.; Zahouily, M. Lignin Nanoparticles Filled Chitosan/Polyvinyl Alcohol Polymer Blend as a Coating Material of Urea with a Slow-Release Property. J. Appl. Polym. Sci. 2023, 140, e53755. [Google Scholar] [CrossRef]
- Jiao, G.J.; Peng, P.; Sun, S.L.; Geng, Z.C.; She, D. Amination of Biorefinery Technical Lignin by Mannich Reaction for Preparing Highly Efficient Nitrogen Fertilizer. Int. J. Biol. Macromol. 2019, 127, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shen, T.; Li, M.; Shaoyu, J.; Zhuang, W.; Li, M.; Xu, H.; Zhu, C.; Ying, H.; Ouyang, P. Synthesis, Characterization, and Utilization of Poly-Amino Acid-Functionalized Lignin for Efficient and Selective Removal of Lead Ion from Aqueous Solution. J. Clean. Prod. 2022, 347, 131219. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and Characterization of Aminated Lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Pang, Y.; Chen, Z.; Zhao, R.; Yi, C.; Qiu, X.; Qian, Y.; Lou, H. Facile Synthesis of Easily Separated and Reusable Silver Nanoparticles/Aminated Alkaline Lignin Composite and Its Catalytic Ability. J. Colloid Interface Sci. 2021, 587, 334–346. [Google Scholar] [CrossRef]
- Fang, R.; Cheng, X.; Xu, X. Synthesis of Lignin-Base Cationic Flocculant and Its Application in Removinganionic Azo-Dyes from Simulated Wastewater. Bioresour. Technol. 2010, 101, 7323–7329. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Yu, H.; Yang, H.; Chen, T. Synthesis of Lignin-Derived Nitrogen-Doped Carbon as a Novel Catalyst for 4-NP Reduction Evaluation. Sci. Rep. 2020, 10, 20075. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Gao, C.; Fatehi, P.; Wang, S.; Jiang, C.; Kong, F. Influence of Structure and Functional Group of Modified Kraft Lignin on Adsorption Behavior of Dye. Int. J. Biol. Macromol. 2023, 240, 124368. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wu, H.; Zhou, M.; Yang, D. Preparation of a Novel High-Performance Lignin-Based Anionic Adsorption Resin for Efficient Removal of Cr(VI) in Aqueous Solutions. Ind. Crop. Prod. 2023, 199, 116720. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Q.; Zhu, R.; Liu, Y.; Xu, F.; Zhang, X. Facile Fabrication of Transparent Lignin Sphere/PVA Nanocomposite Films with Excellent UV-Shielding and High Strength Performance. Int. J. Biol. Macromol. 2021, 189, 635–640. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ren, X. Transparent and Ultra-Tough PVA/Alkaline Lignin Films with UV Shielding and Antibacterial Functions. Int. J. Biol. Macromol. 2022, 216, 86–94. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Yang, D.; Qiu, X. Biomimetic Supertough and Strong Biodegradable Polymeric Materials with Improved Thermal Properties and Excellent UV-Blocking Performance. Adv. Funct. Mater. 2019, 29, 1806912. [Google Scholar] [CrossRef]
- Korbag, I.; Mohamed Saleh, S. Studies on the Formation of Intermolecular Interactions and Structural Characterization of Polyvinyl Alcohol/Lignin Film. Int. J. Environ. Stud. 2016, 73, 226–235. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, J.; Wang, C.; Yang, G.; Janaswamy, S.; Xu, F.; Liu, Z. Preparation and Characterization of Lignin Nanoparticles and Chitin Nanofibers Reinforced PVA Films with UV Shielding Properties. Ind. Crop. Prod. 2022, 188, 115669. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, W.; Liu, W.; Qiu, X. High Performance PVA/Lignin Nanocomposite Films with Excellent Water Vapor Barrier and UV-Shielding Properties. Int. J. Biol. Macromol. 2020, 142, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Cuo, T.; Chen, Y. Molecular-Level Dispersion of Graphene into Poly(Vinyl Alcohol) and Effective Reinforcement of Their Nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Wang, H.; Tang, X.; Arvanitis, M.A.; Yang, V.; Stark, N.; Liu, C.; Considine, J.M.; Zhu, J.Y. Colloidal Lignin Nanoparticles from Acid Hydrotropic Fractionation for Producing Tough, Biodegradable, and UV Blocking PVA Nanocomposite. Ind. Crop. Prod. 2021, 168, 113584. [Google Scholar] [CrossRef]
- Xu, J.; Ren, L.; Song, W.; Wu, N.; Zeng, R. Homogeneous PVA/Sodium Lignosulfonate Mulch Films with Improved Mechanical and UV/Water Vapor Barrier Properties. Ind. Crop. Prod. 2024, 209, 117975. [Google Scholar] [CrossRef]
- Wu, L.; Liu, S.; Wang, Q.; Wang, Y.; Ji, X.; Yang, G.; Chen, J.; Li, C.; Fatehi, P. High Strength and Multifunctional Polyurethane Film Incorporated with Lignin Nanoparticles. Ind. Crop. Prod. 2022, 177, 114526. [Google Scholar] [CrossRef]
- Xin, Q.; Li, H.; Sun, W.; Li, X.; Lu, X.; Zhao, J. Lignin-Xylan Nanospheres Prepared by Green and Quick Method from Lignocellulose and Used as Additive in PVA Films. Int. J. Biol. Macromol. 2024, 264, 129762. [Google Scholar] [CrossRef]
- Korbag, I.; Mohamed Saleh, S. Studies on Mechanical and Biodegradability Properties of PVA/Lignin Blend Films. Int. J. Environ. Stud. 2016, 73, 18–24. [Google Scholar] [CrossRef]
- Zhou, Y.; Essawy, H.; Liu, A.; Yang, C.; Hou, D.; Zhou, X.; Du, G.; Zhang, J. Preparation of Film Based on Polyvinyl Alcohol Modified by Alkaline Starch and Lignin Fiber. J. Renew. Mater. 2023, 11, 837–852. [Google Scholar] [CrossRef]
- Tian, R.; Liu, Y.; Wang, C.; Jiang, W.; Janaswamy, S.; Yang, G.; Ji, X.; Lyu, G. Strong, UV-Blocking, and Hydrophobic PVA Composite Films Containing Tunable Lignin Nanoparticles. Ind. Crop. Prod. 2024, 208, 117842. [Google Scholar] [CrossRef]
- Aqlil, M.; Moussemba Nzenguet, A.; Essamlali, Y.; Snik, A.; Larzek, M.; Zahouily, M. Graphene Oxide Filled Lignin/Starch Polymer Bionanocomposite: Structural, Physical, and Mechanical Studies. J. Agric. Food Chem. 2017, 65, 10571–10581. [Google Scholar] [CrossRef]
- Xiong, F.; Wu, Y.; Li, G.; Han, Y.; Chu, F. Transparent Nanocomposite Films of Lignin Nanospheres and Poly(Vinyl Alcohol) for UV-Absorbing. Ind. Eng. Chem. Res. 2018, 57, 1207–1212. [Google Scholar] [CrossRef]
- Zhang, J.; Tian, Z.; Ji, X.; Zhang, F. Fabrication Mechanisms of Lignin Nanoparticles and Their Ultraviolet Protection Ability in PVA Composite Film. Polymers 2022, 14, 4196. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qian, Y.; Lou, H.; Yang, D.; Qiu, X. Enhancing the Broad-Spectrum Adsorption of Lignin through Methoxyl Activation, Grafting Modification, and Reverse Self-Assembly. ACS Sustain. Chem. Eng. 2019, 7, 15966–15973. [Google Scholar] [CrossRef]
- Wang, J.; Deng, Y.; Qian, Y.; Qiu, X.; Ren, Y.; Yang, D. Reduction of Lignin Color via One-Step UV Irradiation. Green Chem. 2016, 18, 695–699. [Google Scholar] [CrossRef]
- Zhou, Y.; Qian, Y.; Wang, J.; Qiu, X.; Zeng, H. Bioinspired Lignin-Polydopamine Nanocapsules with Strong Bioadhesion for Long-Acting and High-Performance Natural Sunscreens. Biomacromolecules 2020, 21, 3231–3241. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qian, Y.; Zhang, A.; Lou, H.; Yang, D.; Qiu, X. Light Color Dihydroxybenzophenone Grafted Lignin with High UVA/UVB Absorbance Ratio for Efficient and Safe Natural Sunscreen. Ind. Eng. Chem. Res. 2020, 59, 17057–17068. [Google Scholar] [CrossRef]
- Hararak, B.; Winotapun, C.; Inyai, J.; Wannid, P.; Prahsarn, C. Production of UV-Shielded Spherical Lignin Particles as Multifunctional Bio-Additives for Polyvinyl Alcohol Composite Films. J. Nanoparticle Res. 2021, 23, 193. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Suwanakood, P.; Saengsuwan, S. Biodegradable Hydrogels of Cassava Starch-g-Polyacrylic Acid/Natural Rubber/Polyvinyl Alcohol as Environmentally Friendly and Highly Efficient Coating Material for Slow-Release Urea Fertilizers. J. Ind. Eng. Chem. 2021, 101, 237–252. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, X.; Zhang, Y.; Zhang, X.; Zhu, W.; Huang, H.; Han, X.; Liu, Y.; Xu, C. Poly(Vinyl Alcohol)/Sodium Alginate Polymer Membranes as Eco-Friendly and Biodegradable Coatings for Slow Release Fertilizers. J. Sci. Food Agric. 2023, 103, 3592–3601. [Google Scholar] [CrossRef]
- Zou, S.L.; Xiao, L.P.; Yin, W.Z.; Gui, T.; Zhang, Y.; Li, Q.; Sun, R.C. Biodegradable, Recyclable, Waterproof, Antibacterial and High-Strength Supramolecular Plastic Fabricated from Lignin Derivatives. Sustain. Mater. Technol. 2024, 39, e00861. [Google Scholar] [CrossRef]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L.P.B.M. Water Absorption, Retention and the Swelling Characteristics of Cassava Starch Grafted with Polyacrylic Acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Uzoh, C.F.; Onukwuli, O.D.; Ozofor, I.H.; Odera, R.S. Encapsulation of Urea with Alkyd Resin-Starch Membranes for Controlled N2 Release: Synthesis, Characterization, Morphology and Optimum N2 Release. Process Saf. Environ. Prot. 2019, 121, 133–142. [Google Scholar] [CrossRef]
- Kassem, I.; Ablouh, E.H.; El Bouchtaoui, F.Z.; Kassab, Z.; Khouloud, M.; Sehaqui, H.; Ghalfi, H.; Alami, J.; El Achaby, M. Cellulose Nanocrystals-Filled Poly (Vinyl Alcohol) Nanocomposites as Waterborne Coating Materials of NPK Fertilizer with Slow Release and Water Retention Properties. Int. J. Biol. Macromol. 2021, 189, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Sofyane, A.; Ablouh, E.; Lahcini, M.; Elmeziane, A.; Khouloud, M.; Kaddami, H.; Raihane, M. Slow-Release Fertilizers Based on Starch Acetate/Glycerol/Polyvinyl Alcohol Biocomposites for Sustained Nutrient Release. Mater. Today Proc. 2020, 36, 74–81. [Google Scholar] [CrossRef]
- Azeem, B.; Kushaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhu, C.; Ma, L.; Liu, C.; Zhang, H.; Zhang, S. Preparation of an Aminated Lignin/Fe(III)/Polyvinyl Alcohol Film: A Packaging Material with UV Resistance and Slow-Release Function. Foods 2023, 12, 2794. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal Sample Preparation Method for Proteome Analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
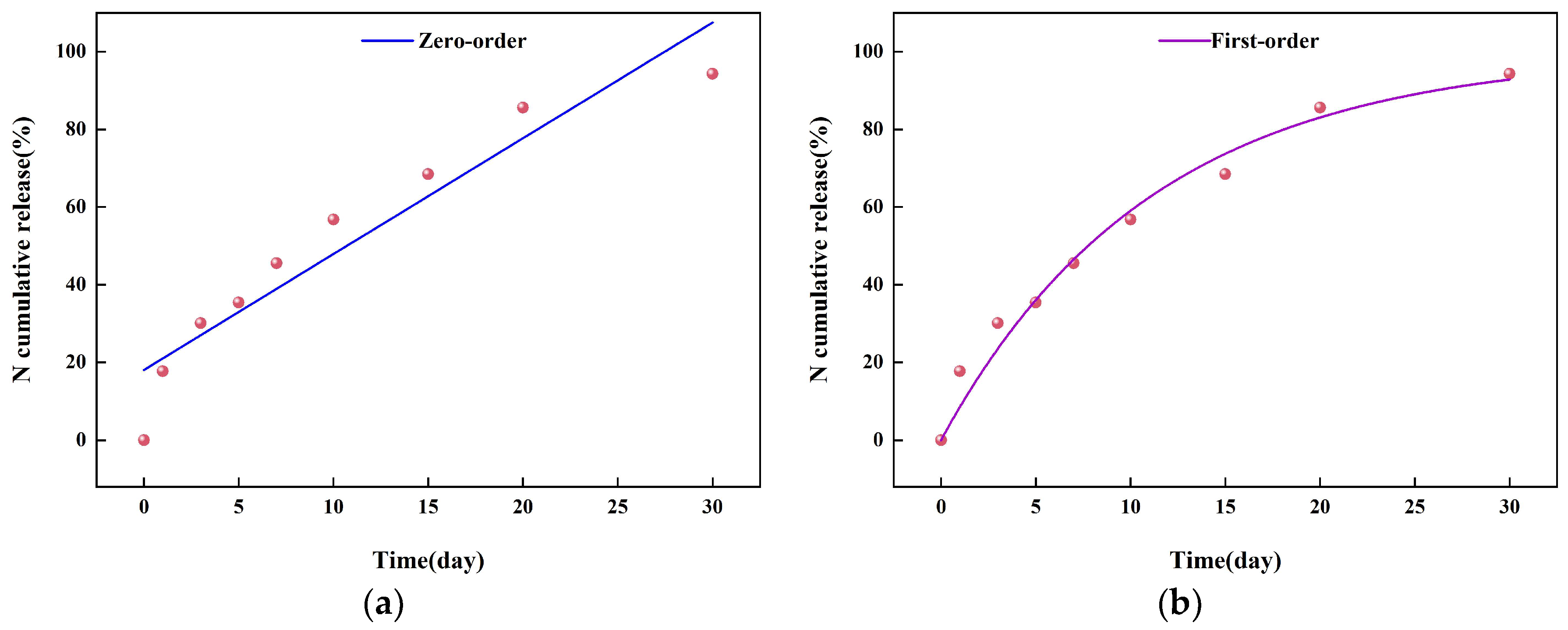
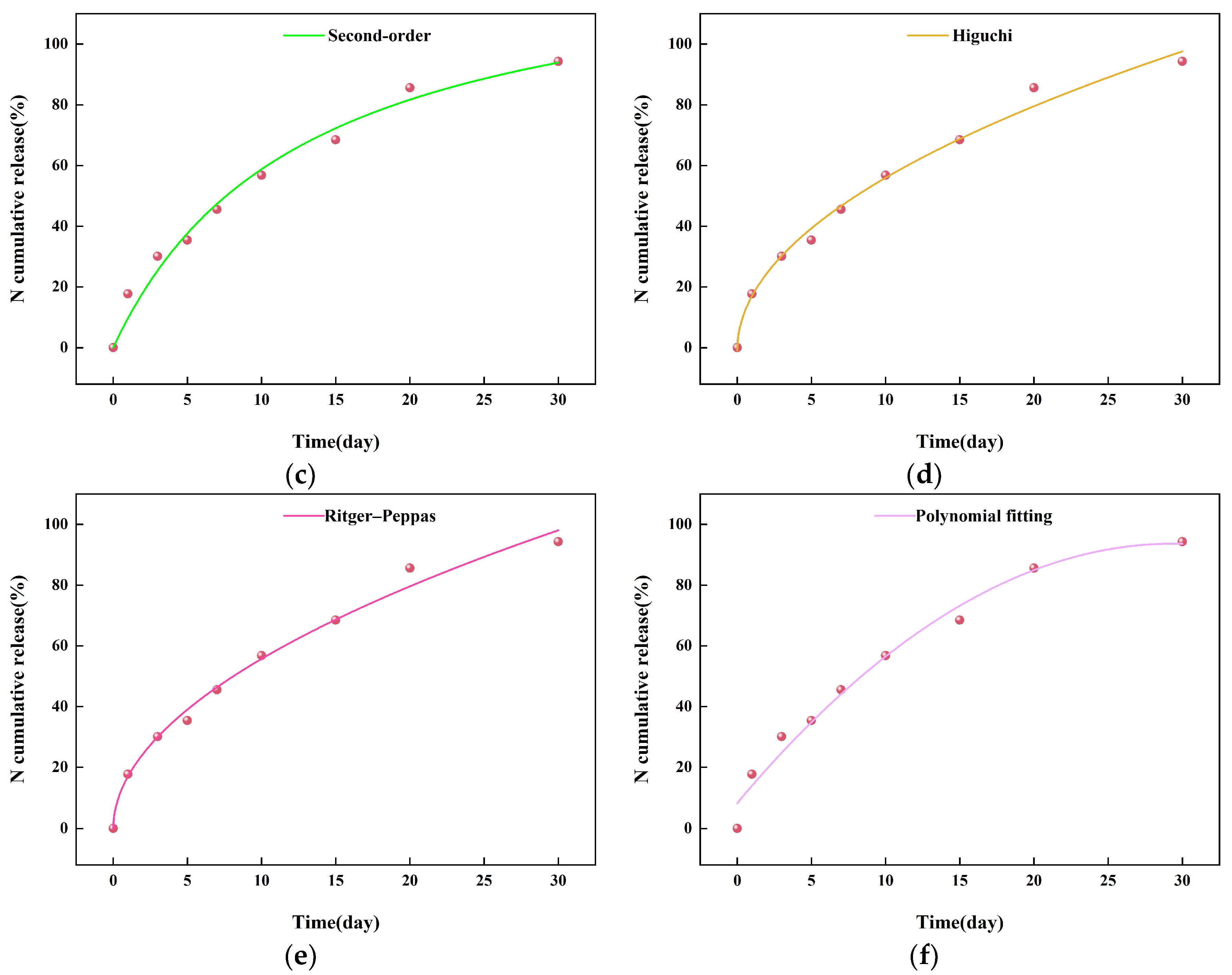
| Model | Fitting Equation | R2 |
|---|---|---|
| Zero-level dynamics model | CR = 2.98293t + 18.06885 | 0.89030 |
| First-order kinetic model | CR = 99.57789 × (1 − 2.71828−0.08986t) | 0.97505 |
| Second-order dynamic model | CR = 10.46328t ÷ (1 + 0.07808t) | 0.98112 |
| Higuchi model | CR = 17.99707t1/2 − 0.95934 | 0.99023 |
| Ritger-Peppas model | CR = 17.02172 × (t0.51495) | 0.99036 |
| Polynomial fitting model | CR = 5.81572 − 0.09892t2 + 8.21041 | 0.97667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cao, L.; Wang, L.; Qi, Y.; Zhao, Y.; Lu, H.; Lu, L.; Zhang, D.; Wang, Z.; Zhang, H. Preparation and Application of Degradable Lignin/Poly (Vinyl Alcohol) Polymers as Urea Slow-Release Coating Materials. Molecules 2024, 29, 1699. https://doi.org/10.3390/molecules29081699
Liu Y, Cao L, Wang L, Qi Y, Zhao Y, Lu H, Lu L, Zhang D, Wang Z, Zhang H. Preparation and Application of Degradable Lignin/Poly (Vinyl Alcohol) Polymers as Urea Slow-Release Coating Materials. Molecules. 2024; 29(8):1699. https://doi.org/10.3390/molecules29081699
Chicago/Turabian StyleLiu, Yue, Long Cao, Linshan Wang, Yanjiao Qi, Yamin Zhao, Huining Lu, Lina Lu, Derong Zhang, Zifan Wang, and Hong Zhang. 2024. "Preparation and Application of Degradable Lignin/Poly (Vinyl Alcohol) Polymers as Urea Slow-Release Coating Materials" Molecules 29, no. 8: 1699. https://doi.org/10.3390/molecules29081699
APA StyleLiu, Y., Cao, L., Wang, L., Qi, Y., Zhao, Y., Lu, H., Lu, L., Zhang, D., Wang, Z., & Zhang, H. (2024). Preparation and Application of Degradable Lignin/Poly (Vinyl Alcohol) Polymers as Urea Slow-Release Coating Materials. Molecules, 29(8), 1699. https://doi.org/10.3390/molecules29081699