Impact of Backbone Substitution on Organocatalytic Activity of Sterically Encumbered NHC in Benzoin Condensation
Abstract
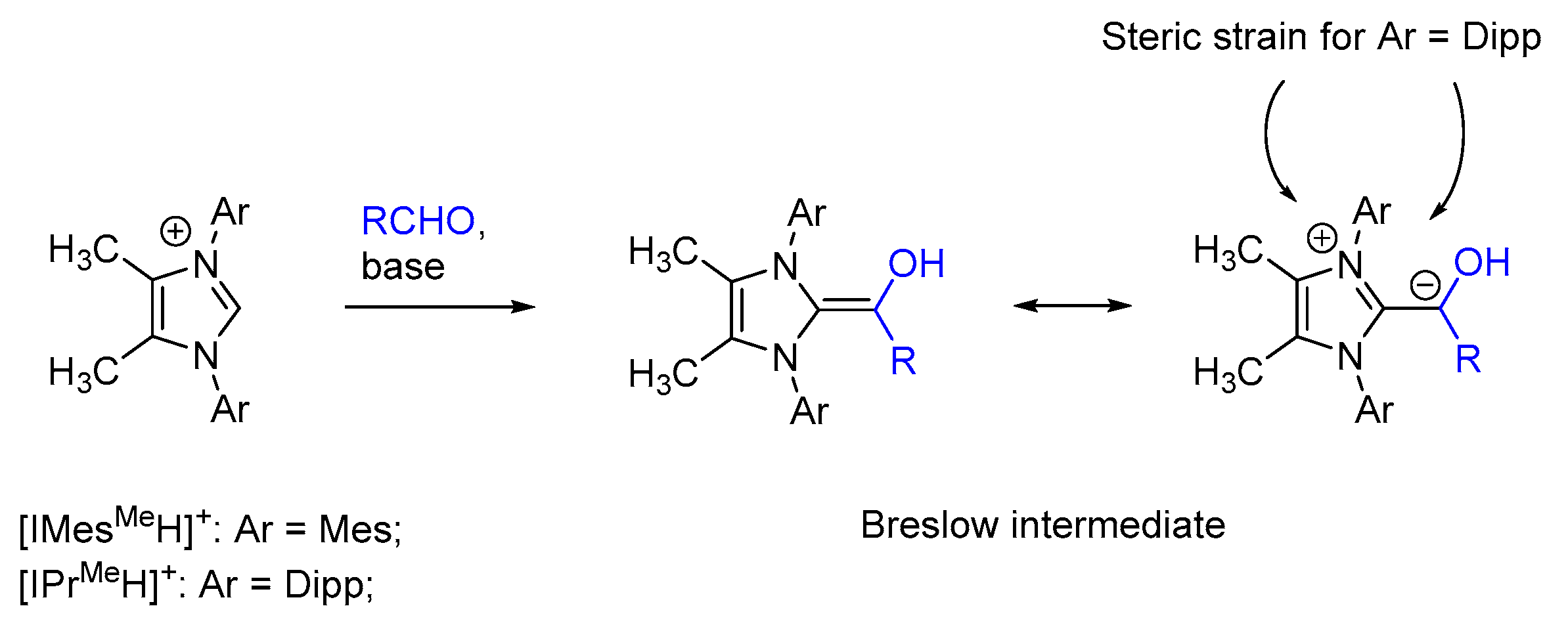
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Procedure for Benzoin Condensation
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biju, A.T.; Kuhl, N.; Glorius, F. Extending NHC-catalysis: Coupling aldehydes with unconventional reaction partners. Acc. Chem. Res. 2011, 44, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, G.; Guo, D.; Wang, J. Recent developments on NHC-driven dual catalytic approaches. Org. Chem. Front. 2022, 9, 5016–5040. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, E.Y.X. Integrated Catalytic Process for Biomass Conversion and Upgrading to C12 Furoin and Alkane Fuel. ACS Catal. 2014, 4, 1302–1310. [Google Scholar] [CrossRef]
- Gaggero, N.; Pandini, S. Advances in chemoselective intermolecular cross-benzoin-type condensation reactions. Org. Biomol. Chem. 2017, 15, 6867–6887. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.S.; Biju, A.T.; Nair, V. Recent advances in N-heterocyclic carbene (NHC)-catalysed benzoin reactions. Beilstein J. Org. Chem. 2016, 12, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.; Hsieh, M.H.; Yu, J.K. Formation of Breslow Intermediates under Aprotic Conditions: A Computational Study. J. Org. Chem. 2022, 87, 2501–2507. [Google Scholar] [CrossRef]
- Nandi, A.; Alassad, Z.; Milo, A.; Kozuch, S. Quantum Tunneling on Carbene Organocatalysis: Breslow Intermediate Formation via Water-Bridges. ACS Catal. 2021, 11, 14836–14841. [Google Scholar] [CrossRef]
- Pareek, M.; Reddi, Y.; Sunoj, R.B. Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: Then and now. Chem. Sci. 2021, 12, 7973–7992. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Klussmann, M.; Breugst, M.; Schlorer, N.E.; Berkessel, A. Formation of Breslow Intermediates from N-Heterocyclic Carbenes and Aldehydes Involves Autocatalysis by the Breslow Intermediate, and a Hemiacetal. Angew. Chem. Int. Ed. 2022, 61, e202117682. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. On the Mechanism of Thiamine Action. IV.1 Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, L.-L.; Melaimi, M.; Cao, L.; Xu, X.; Bouffard, J.; Bertrand, G.; Yan, X. Mesoionic Carbene (MIC)-Catalyzed H/D Exchange at Formyl Groups. Chem 2019, 5, 2484–2494. [Google Scholar] [CrossRef]
- Urban, S.; Tursky, M.; Frohlich, R.; Glorius, F. Investigation of the properties of 4,5-dialkylated N-heterocyclic carbenes. Dalton Trans. 2009, 35, 6934–6940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cesar, V.; Storch, G.; Lugan, N.; Lavigne, G. Skeleton decoration of NHCs by amino groups and its sequential booster effect on the palladium-catalyzed Buchwald-Hartwig amination. Angew. Chem. Int. Ed. 2014, 53, 6482–6486. [Google Scholar] [CrossRef]
- Nelson, D.J.; Nolan, S.P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 2013, 42, 6723–6753. [Google Scholar] [CrossRef] [PubMed]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ota, S.; Fukuta, Y.; Ueda, Y.; Sato, M. N-heterocyclic carbene-catalyzed nucleophilic aroylation of fluorobenzenes. J. Org. Chem. 2008, 73, 2420–2423. [Google Scholar] [CrossRef]
- Dove, A.P.; Li, H.; Pratt, R.C.; Lohmeijer, B.G.; Culkin, D.A.; Waymouth, R.M.; Hedrick, J.L. Stereoselective polymerization of rac- and meso-lactide catalyzed by sterically encumbered N-heterocyclic carbenes. Chem. Commun. 2006, 27, 2881–2883. [Google Scholar] [CrossRef]
- Burstein, C.; Glorius, F. Organocatalyzed conjugate umpolung of alpha,beta-unsaturated aldehydes for the synthesis of gamma-butyrolactones. Angew. Chem. Int. Ed. 2004, 43, 6205–6208. [Google Scholar] [CrossRef]
- Pesch, J.; Harms, K.; Bach, T. Preparation of Axially Chiral N,N′-Diarylimidazolium and N-Arylthiazolium Salts and Evaluation of Their Catalytic Potential in the Benzoin and in the Intramolecular Stetter Reactions. Eur. J. Org. Chem. 2004, 2004, 2025–2035. [Google Scholar] [CrossRef]
- Aysin, R.R.; Galkin, K.I. Adaptive carbonyl umpolung involving a carbanionic carbene Breslow intermediate: An alternative mechanism for NHC-mediated organocatalysis. Org. Biomol. Chem. 2023, 21, 8702–8707. [Google Scholar] [CrossRef]
- Galkin, K.I.; Karlinskii, B.Y.; Kostyukovich, A.Y.; Gordeev, E.G.; Ananikov, V.P. Ambident Reactivity of Imidazolium Cations as Evidence of the Dynamic Nature of N-Heterocyclic Carbene-Mediated Organocatalysis. Chem. Eur. J. 2020, 26, 8567–8571. [Google Scholar] [CrossRef]
- Galkin, K.I.; Gordeev, E.G.; Ananikov, V.P. Organocatalytic Deuteration Induced by the Dynamic Covalent Interaction of Imidazolium Cations with Ketones. Adv. Synth. Catal. 2021, 363, 1368–1378. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. Towards Improved Biorefinery Technologies: 5-Methylfurfural as a Versatile C(6) Platform for Biofuels Development. ChemSusChem 2019, 12, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Melancon, K.M.; Cundari, T.R. Computational investigations of NHC-backbone configurations for applications in organocatalytic umpolung reactions. Org. Biomol. Chem. 2020, 18, 7437–7447. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Klamt, A.; Schüürmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2 1993, 5, 799–805. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Neese, F.; Izsak, R. Speeding up equation of motion coupled cluster theory with the chain of spheres approximation. J. Chem. Phys. 2016, 144, 034102. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A ‘chain-of-spheres’ algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]


| № | Imidazolium Salt, Base | Aldehyde | T (°C) | Conversion/Yield of Benzoin (%) |
|---|---|---|---|---|
| 1 | [IMesH]Cl, DBU | Furfural | 22 °C | 98/51 |
| 2 | [IMesH]Cl, tBuONa | Furfural | 22 °C | 99/32 |
| 3 | [IMesMeH]Cl, DBU | Furfural | 22 °C | 99/42 |
| 4 | [IPrH]Cl, DBU | Furfural | 22 °C | 99/40 |
| 5 | [IPrH]Cl, DBU | Furfural | 80 °C | 99/38 |
| 6 | [IPrH]Cl, tBuONa | Furfural | 22 °C | 99/34 |
| 7 | [IMesH]Cl, DBU | 5-Methylfurfural | 22 °C | 93/63 |
| 8 | [IMesMeH]Cl, DBU | 5-Methylfurfural | 22 °C | 93/67 |
| 9 | [IPrH]Cl, DBU | 5-Methylfurfural | 22 °C | 93/53 |
| 10 | [IMesH]Cl, DBU | Benzaldehyde | 80 °C | 77/24 |
| 11 | [IMesMeH]Cl, DBU | Benzaldehyde | 80 °C | 29/6 |
| 12 | [IPrH]Cl, DBU | Benzaldehyde | 80 °C | 53/5 |
| 13 | [IMesH]Cl, DBU | m-Anisaldehyde | 80 °C | 84/41 |
| 14 | [IMesMeH]Cl, DBU | m-Anisaldehyde | 80 °C | 23/9 |
| 15 | [IPrH]Cl, DBU | m-Anisaldehyde | 80 °C | 31/14 |
| 16 | [IPrMeH]Cl, DBU | Furfural | 80 °C | 29/0 |
| 17 | [IPrMeH]Cl, tBuONa | Furfural | 80 °C | 48/0 |
| 18 | [IPrMeH]Cl, DBU | 5-Methylfurfural | 80 °C | 2/0 |
| 19 | [IPrMeH]Cl, DBU | Benzaldehyde | 80 °C | 9/0 |
| 20 | [IPrMeH]Cl, DBU | m-Anisaldehyde | 80 °C | 16/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aysin, R.R.; Galkin, K.I. Impact of Backbone Substitution on Organocatalytic Activity of Sterically Encumbered NHC in Benzoin Condensation. Molecules 2024, 29, 1704. https://doi.org/10.3390/molecules29081704
Aysin RR, Galkin KI. Impact of Backbone Substitution on Organocatalytic Activity of Sterically Encumbered NHC in Benzoin Condensation. Molecules. 2024; 29(8):1704. https://doi.org/10.3390/molecules29081704
Chicago/Turabian StyleAysin, Rinat R., and Konstantin I. Galkin. 2024. "Impact of Backbone Substitution on Organocatalytic Activity of Sterically Encumbered NHC in Benzoin Condensation" Molecules 29, no. 8: 1704. https://doi.org/10.3390/molecules29081704
APA StyleAysin, R. R., & Galkin, K. I. (2024). Impact of Backbone Substitution on Organocatalytic Activity of Sterically Encumbered NHC in Benzoin Condensation. Molecules, 29(8), 1704. https://doi.org/10.3390/molecules29081704






