Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of the Hydrogels
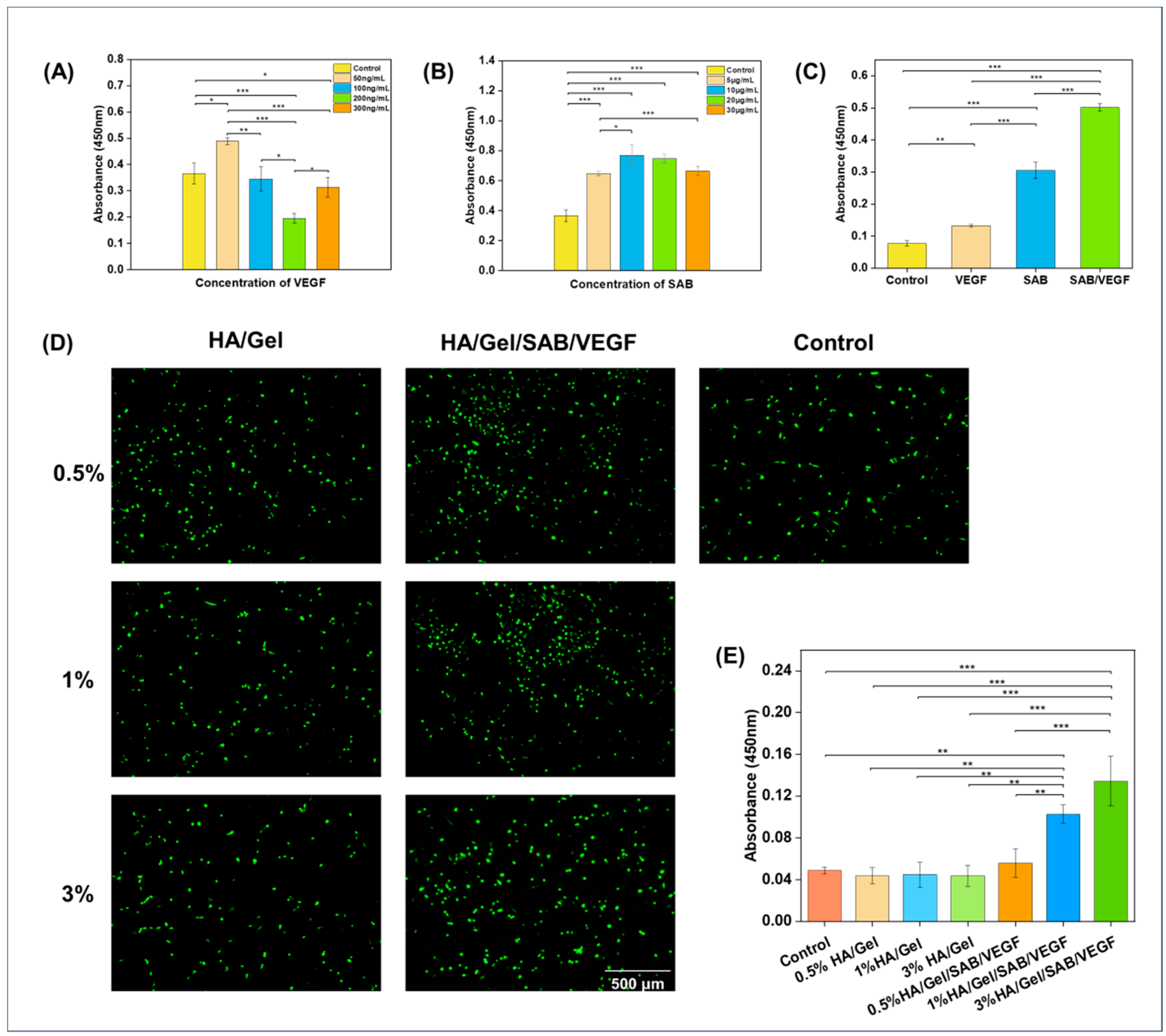
2.2. Cytocompatibility Evaluation of the Hydrogels
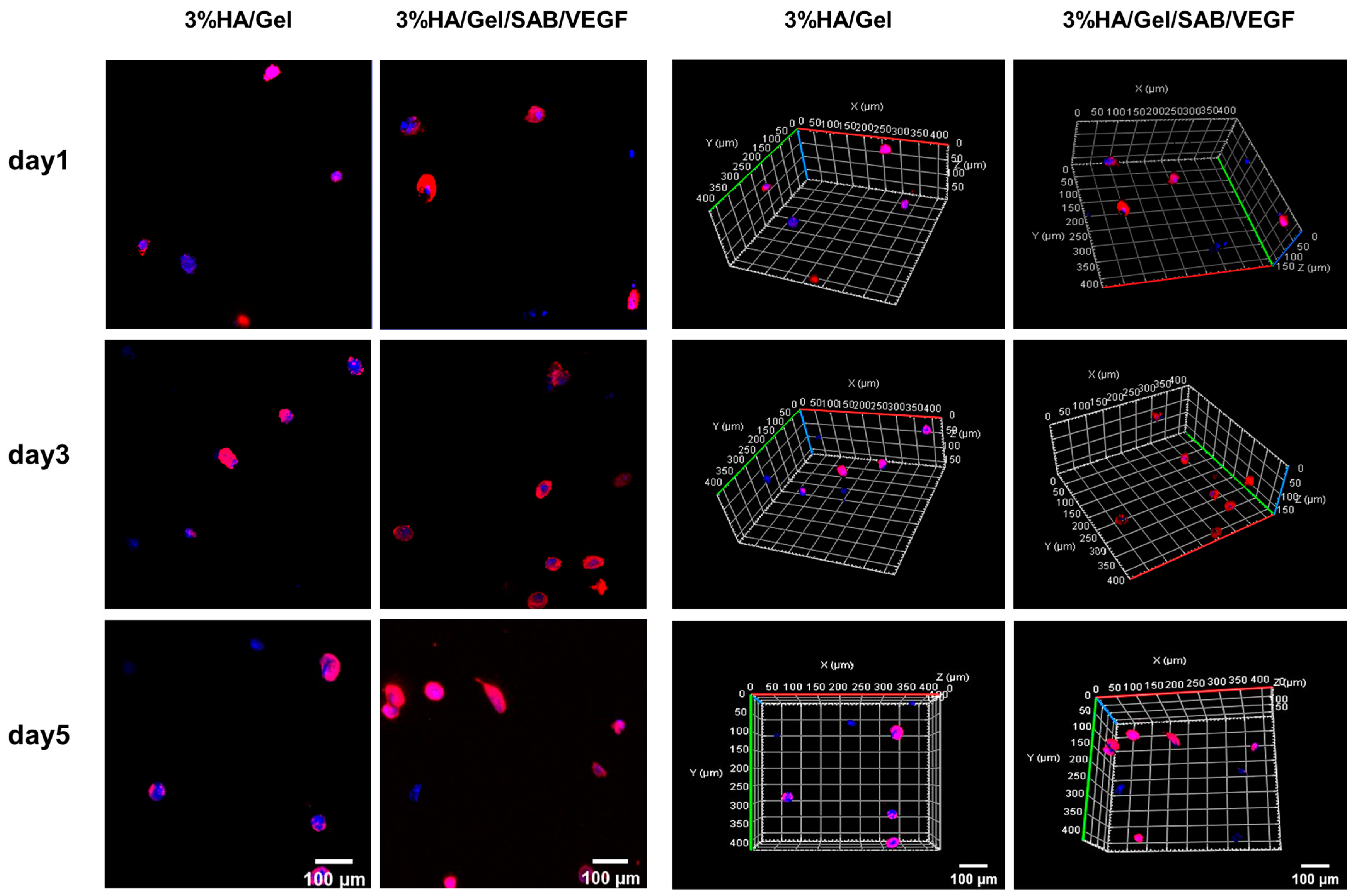
2.3. Three-Dimensional (3D) Culture of BMSCs in HA/Gel/SAB/VEGF Hydrogels
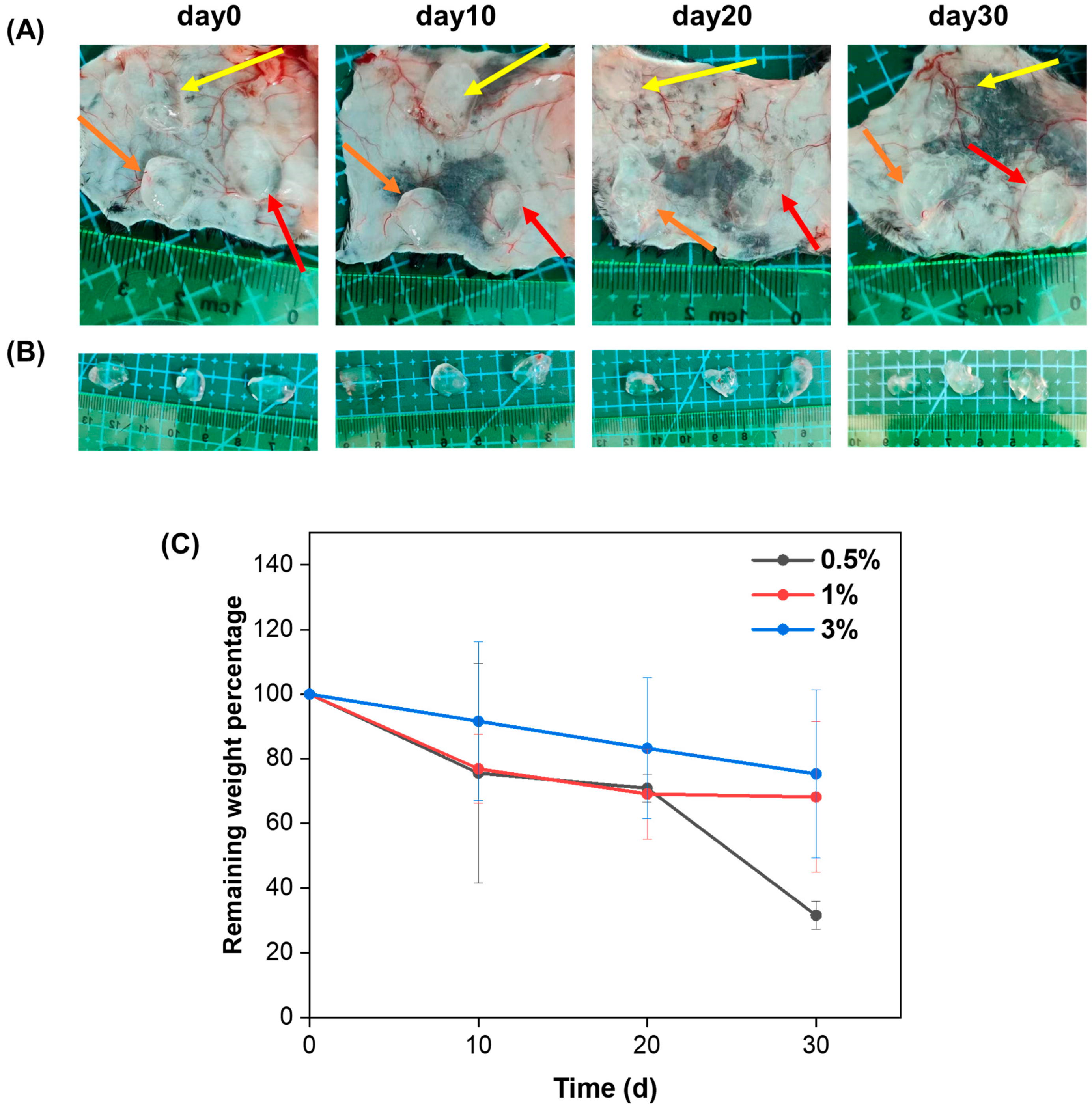
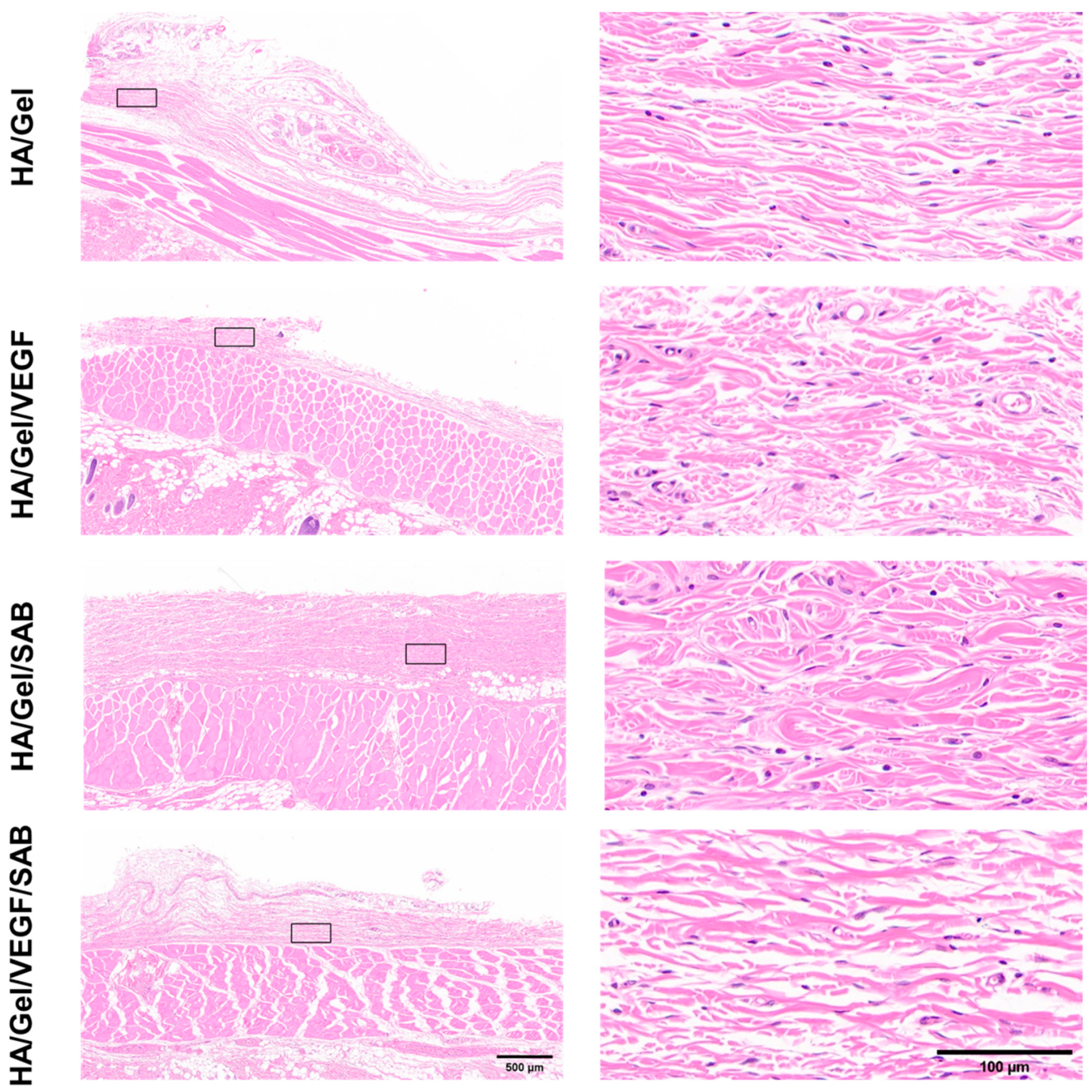
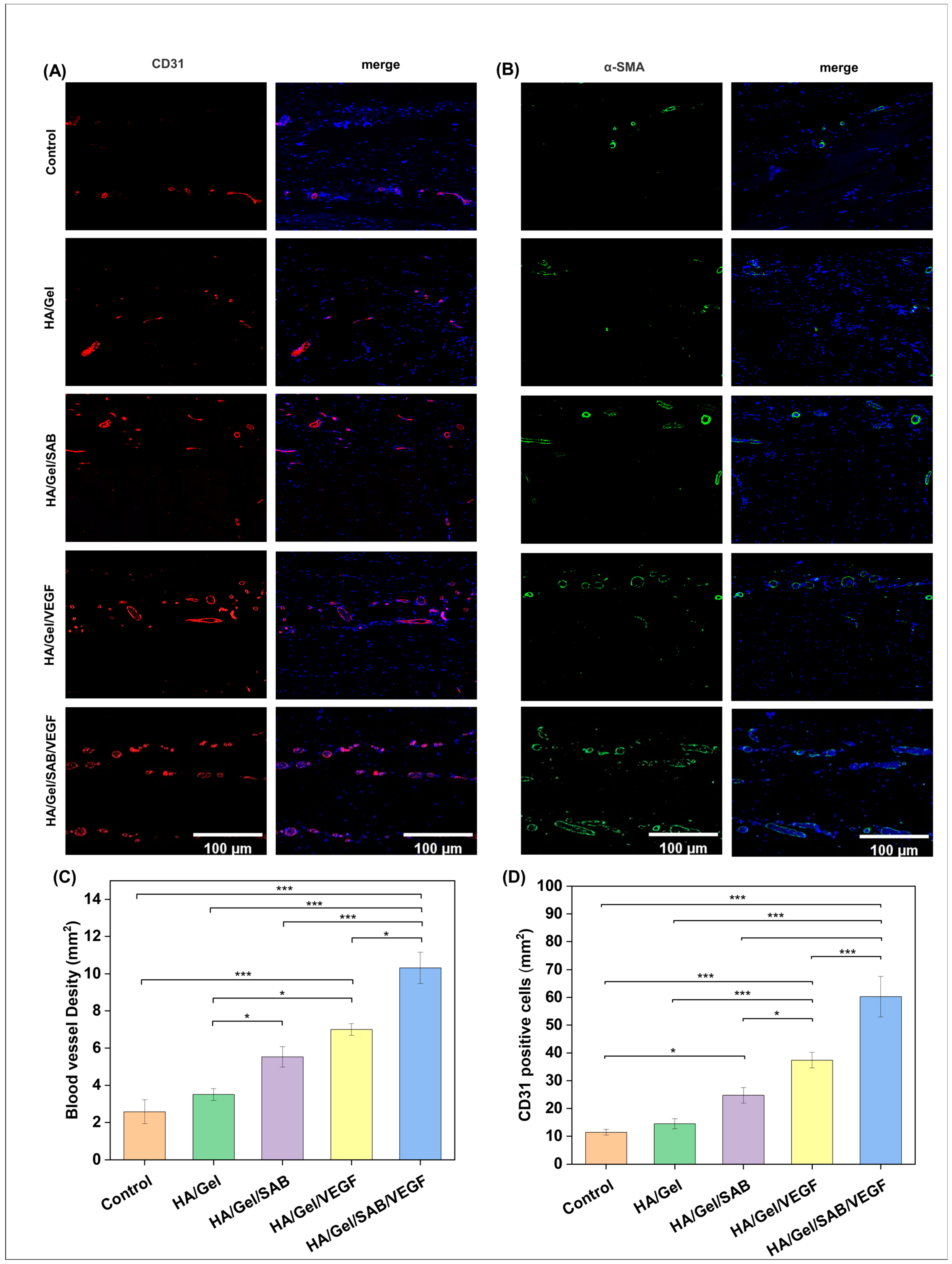
2.4. Subcutaneous Degradation, Histology and Immunofluorescence Staining
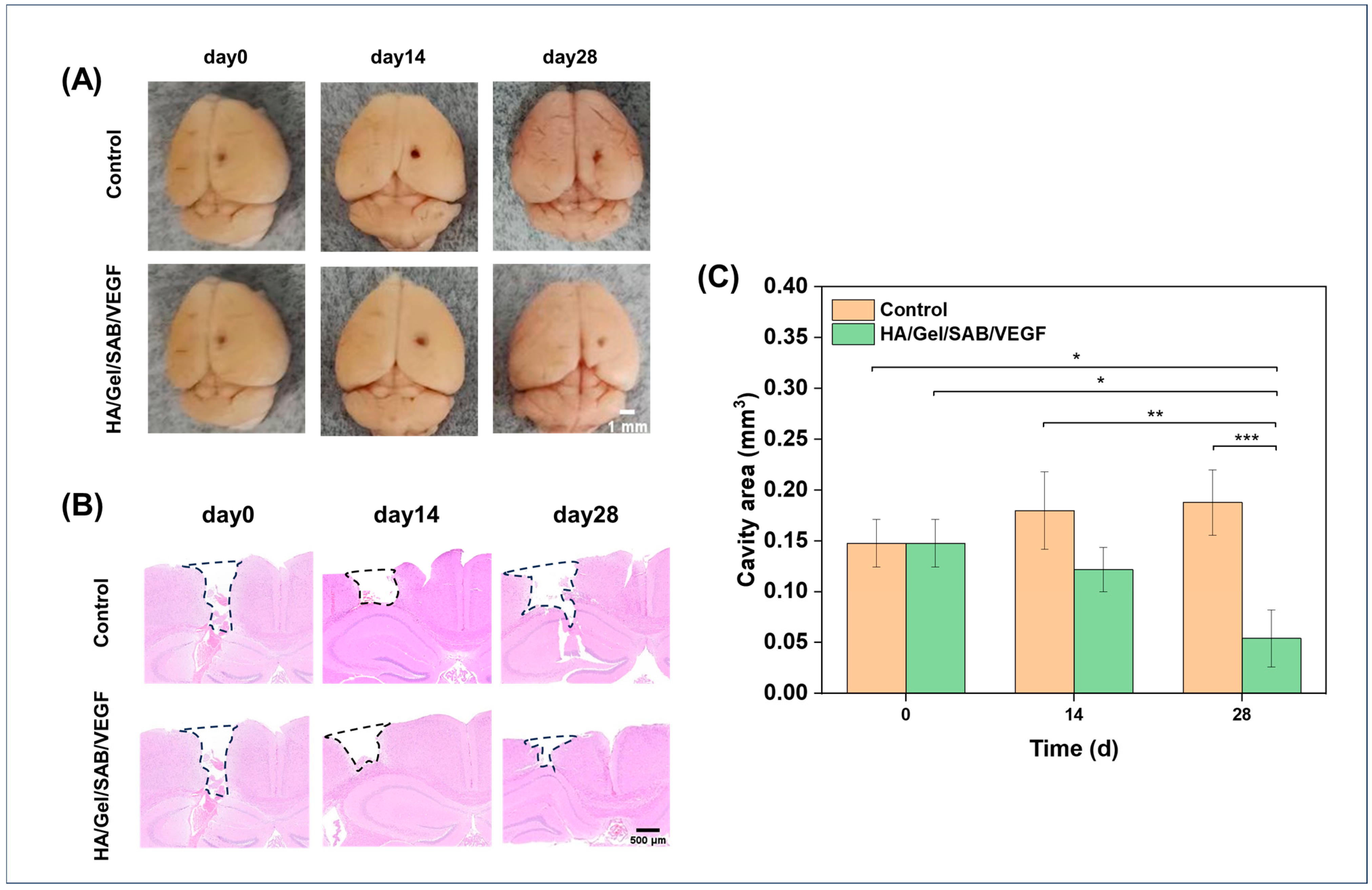
2.5. HA/Gel/SAB/VEGF Hydrogel Promotes the Repair of Brain Injury in Mice
3. Discussion
4. Materials and Methods
4.1. Materials, Cells, and Animals
4.2. Synthesis and Characterization of HA-Tyramine (HA-TA) and Gel-Tyramine (Gel-TA)
4.3. Hydrogel Fabrication and Physical Characterization
4.4. The Cytocompatibility of the HA/Gel Hydrogels
4.5. Three-Dimensional (3D) Culture of BMSCs in HA/Gel/SAB/VEGF Hydrogels
4.6. In Vivo Biocompatibility of the HA/Gel Hydrogels
4.7. Establishment of TBI Model and Hydrogel Implantation
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ladak, A.A.; Enam, S.A.; Ibrahim, M.T. A Review of the Molecular Mechanisms of Traumatic Brain Injury. World Neurosurg. 2019, 131, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Aertker, B.M.; Bedi, S.; Cox, C.S. Strategies for CNS repair following TBI. Exp. Neurol. 2016, 275, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Fehily, B.; Fitzgerald, M. Repeated Mild Traumatic Brain Injury: Potential Mechanisms of Damage. Cell Transplant. 2017, 26, 1131–1155. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Wang, W.; Lin, F.; Wang, S.; Zhao, J. Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J. Neurol. 2021, 268, 4095–4107. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Zhang, W.; He, Y.; Wang, J.; Li, S.; Chen, S.; Ye, Q.; Li, M. Classification and Characteristics of Mesenchymal Stem Cells and Its Potential Therapeutic Mechanisms and Applications against Ischemic Stroke. Stem Cells Int. 2021, 2021, 2602871. [Google Scholar] [CrossRef]
- Greenberg, D.A.; Jin, K. Vascular endothelial growth factors (VEGFs) and stroke. Cell. Mol. Life Sci. CMLS 2013, 70, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, C.; Wang, J.; Lin, X.; Zhang, L.; Yang, Y.; Wang, Y.; Zhang, Z.; Bulte, J.W.M.; Yang, G.-Y. MRI/SPECT/Fluorescent Tri-Modal Probe for Evaluating the Homing and Therapeutic Efficacy of Transplanted Mesenchymal Stem Cells in a Rat Ischemic Stroke Model. Adv. Funct. Mater. 2015, 25, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.U.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular Endothelial Growth Factor-Capturing Aligned Electrospun Polycaprolactone/Gelatin Nanofibers Promote Patellar Ligament Regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Lu, J.; Guan, F.; Cui, F.; Sun, X.; Zhao, L.; Wang, Y.; Wang, X. Enhanced angiogenesis by the hyaluronic acid hydrogels immobilized with a VEGF mimetic peptide in a traumatic brain injury model in rats. Regen. Biomater. 2019, 6, 325–334. [Google Scholar] [CrossRef]
- Lee, T.-H.; Yen, C.-T.; Hsu, S.-H. Preparation of Polyurethane-Graphene Nanocomposite and Evaluation of Neurovascular Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 597–609. [Google Scholar] [CrossRef]
- Li, Y.; Men, Y.; Wang, B.; Chen, X.; Yu, Z. Co-transplantation of Schwann cells and neural stem cells in the laminin-chitosan-PLGA nerve conduit to repair the injured recurrent laryngeal nerve in SD rats. J. Mater. Sci. Mater. Med. 2020, 31, 99. [Google Scholar] [CrossRef]
- Liu, F.-D.; Duan, H.-M.; Hao, F.; Zhao, W.; Gao, Y.-D.; Hao, P.; Yang, Z.-Y.; Li, X.-G. Biomimetic chitosan scaffolds with long-term controlled release of nerve growth factor repairs 20-mm-long sciatic nerve defects in rats. Neural Regen. Res. 2022, 17, 1146–1155. [Google Scholar]
- Maclean, F.L.; Lau, C.L.; Ozergun, S.; O’Shea, R.D.; Cederfur, C.; Wang, J.; Healy, K.E.; Walker, F.R.; Tomas, D.; Horne, M.K.; et al. Galactose-functionalised PCL nanofibre scaffolds to attenuate inflammatory action of astrocytes in vitro and in vivo. J. Mater. Chem. B 2017, 5, 4073–4083. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.Z.; Spector, M. Treatment of penetrating brain injury in a rat model using collagen scaffolds incorporating soluble Nogo receptor. J. Tissue Eng. Regen. Med. 2015, 9, 137–150. [Google Scholar] [CrossRef]
- Yao, M.; Chen, Y.; Zhang, J.; Gao, F.; Ma, S.; Guan, F. Chitosan-based thermosensitive composite hydrogel enhances the therapeutic efficacy of human umbilical cord MSC in TBI rat model. Mater. Today Chem. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Chen, T.; Xia, Y.; Zhang, L.; Xu, T.; Yi, Y.; Chen, J.; Liu, Z.; Yang, L.; Chen, S.; Zhou, X.; et al. Loading neural stem cells on hydrogel scaffold improves cell retention rate and promotes functional recovery in traumatic brain injury. Mater. Today Bio 2023, 19, 100606. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Yang, Z.; Liu, C.; Chen, X.; Zhang, Y.; Zhang, F.; Shi, H.; Chen, X.; Tao, L.; et al. Implantation of injectable SF hydrogel with sustained hydrogen sulfide delivery reduces neuronal pyroptosis and enhances functional recovery after severe intracerebral hemorrhage. Biomater. Adv. 2022, 135, 212743. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wei, Z.; Yang, K.; Lu, Y.; Lu, P.; Zhao, J.; Du, Y.; Zhang, H.; Li, R.; Lei, S.; et al. Neural Stem Cell-Laden Self-Healing Polysaccharide Hydrogel Transplantation Promotes Neurogenesis and Functional Recovery after Cerebral Ischemia in Rats. ACS Appl. Bio Mater. 2021, 4, 3046–3054. [Google Scholar] [CrossRef]
- de la Cruz, R.; Díaz, D.D. Self-Healing Collagen-Based Hydrogel for Brain Injury Therapy. In Self-Healing and Self-Recovering Hydrogels; Creton, C., Okay, O., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 355–378. [Google Scholar]
- Li, J.; Zhang, D.; Guo, S.; Zhao, C.; Wang, L.; Ma, S.; Guan, F.; Yao, M. Dual-enzymatically cross-linked gelatin hydrogel promotes neural differentiation and neurotrophin secretion of bone marrow-derived mesenchymal stem cells for treatment of moderate traumatic brain injury. Int. J. Biol. Macromol. 2021, 187, 200–213. [Google Scholar] [CrossRef]
- Ravina, K.; Briggs, D.I.; Kislal, S.; Warraich, Z.; Nguyen, T.; Lam, R.K.; Zarembinski, T.I.; Shamloo, M. Intracerebral Delivery of Brain-Derived Neurotrophic Factor Using HyStem(®)-C Hydrogel Implants Improves Functional Recovery and Reduces Neuroinflammation in a Rat Model of Ischemic Stroke. Int. J. Mol. Sci. 2018, 19, 3782. [Google Scholar] [CrossRef]
- Cook, D.J.; Nguyen, C.; Chun, H.N.; L Llorente, I.; Chiu, A.S.; Machnicki, M.; Zarembinski, T.I.; Carmichael, S.T. Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J. Cereb. Blood Flow Metab. 2016, 37, 1030–1045. [Google Scholar] [CrossRef]
- Wang, C.; Chen, H.; Wang, W.; Yan, G.; Zheng, S.; Wang, C.; Li, N.; Tang, H. Facile strategy for gelatin-based hydrogel with multifunctionalities to remodel wound microenvironment and accelerate healing of acute and diabetic wounds. Int. J. Biol. Macromol. 2024, 256, 128372. [Google Scholar] [CrossRef]
- Zaviskova, K.; Tukmachev, D.; Dubisova, J.; Vackova, I.; Hejcl, A.; Bystronova, J.; Pravda, M.; Scigalkova, I.; Sulakova, R.; Velebny, V.; et al. Injectable hydroxyphenyl derivative of hyaluronic acid hydrogel modified with RGD as scaffold for spinal cord injury repair. J. Biomed. Mater. Res. Part A 2018, 106, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, J.; Guan, S.; Zhang, K.; Zhang, K.; Li, J. Injectable multifunctional CMC/HA-DA hydrogel for repairing skin injury. Mater. Today. Bio. 2022, 14, 100257. [Google Scholar] [CrossRef]
- Lee, C. Injectable glucose oxidase-immobilized gelatin hydrogel prevents tumor recurrence via oxidation therapy. Colloids Surf. B Biointerfaces 2023, 232, 113581. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, D.M.; Chang, W.H.; Huang, R.N.; Hsu, J.C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Yao, M.; Gao, F.; Xu, R.; Zhang, J.; Chen, Y.; Guan, F. A dual-enzymatically cross-linked injectable gelatin hydrogel loaded with BMSC improves neurological function recovery of traumatic brain injury in rats. Biomater. Sci. 2019, 7, 4088–4098. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, G.; Chen, L.; Zhang, Y.; Luo, Y.; Zheng, Y.; Hu, F.; Forouzanfar, T.; Lin, H.; Liu, B. Neuro-regenerative imidazole-functionalized GelMA hydrogel loaded with hAMSC and SDF-1α promote stem cell differentiation and repair focal brain injury. Bioact. Mater. 2021, 6, 627–637. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Zhang, K.; Shi, J.; Liu, Z.; Wang, X.; Guo, M.; Lv, J.; Ding, X. Inhibition of ASC enhances the protective role of salvianolic acid A in traumatic brain injury via inhibition of inflammation and recovery of mitochondrial function. Folia Neuropathol. 2021, 59, 50–66. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.-T.; Chen, T.-B.; Niu, R.-Z.; Chen, J.-L.; Chen, Y.; Huang, J. Lu Tong Ke Li protects neurons from injury by regulating inflammation in rats with brain trauma. Ibrain 2022, 8, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, K.; Lei, L.; Bai, L.; Liang, R.; Qiao, Y.; Duan, J.; Gao, K.; Cao, S.; Zhao, C.; et al. Astragaloside and/or Hydroxysafflor Yellow A Attenuates Oxygen-Glucose Deprivation-Induced Cultured Brain Microvessel Endothelial Cell Death through Downregulation of PHLPP-1. Evid.-Based Complement. Altern. Med. 2020, 2020, 3597527. [Google Scholar] [CrossRef]
- Xu, S.; Zhong, A.; Ma, H.; Li, D.; Hu, Y.; Xu, Y.; Zhang, J. Neuroprotective effect of salvianolic acid B against cerebral ischemic injury in rats via the CD40/NF-κB pathway associated with suppression of platelets activation and neuroinflammation. Brain Res. 2017, 1661, 37–48. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Yu, X.; Li, Y.; Zhang, L.; He, Z.; Zhang, N.; Yang, X.; Zhao, Y.; Li, N.; et al. Salvianolic Acid B Ameliorates Cerebral Ischemia/Reperfusion Injury Through Inhibiting TLR4/MyD88 Signaling Pathway. Inflammation 2016, 39, 1503–1513. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Gu, T.; Yu, D.; Shi, Y.; Gao, Z.; Wang, Z.; Liu, W.; Fan, Z.; Hou, W.; et al. Astrocytic glycogen mobilization participates in salvianolic acid B-mediated neuroprotection against reperfusion injury after ischemic stroke. Exp. Neurol. 2022, 349, 113966. [Google Scholar] [CrossRef]
- Huang, X.; Li, T.; Jiang, X.; Wang, Z.; Wang, M.; Wu, X.; Li, J.; Shi, J. Co-assembled Supramolecular Hydrogel of Salvianolic Acid B and a Phosphopeptide for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 45606–45615. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, J.; Jin, L.; Chen, J.; Xu, R.; Zhao, Y.; Yan, T.; Wan, H. Salvianolic-Acid-B-Loaded HA Self-Healing Hydrogel Promotes Diabetic Wound Healing through Promotion of Anti-Inflammation and Angiogenesis. Int. J. Mol. Sci. 2023, 24, 6844. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Zheng, C.; Hu, C.; Yang, L.; Kong, Q.; Zhang, H.; Wang, Y. A Versatile Glycopeptide Hydrogel Promotes Chronic Refractory Wound Healing Through Bacterial Elimination, Sustained Oxygenation, Immunoregulation, and Neovascularization. Adv. Funct. Mater. 2023, 33, 2305992. [Google Scholar] [CrossRef]
- Chen, R.; Zhu, C.; Xu, L.; Gu, Y.; Ren, S.; Bai, H.; Zhou, Q.; Liu, X.; Lu, S.; Bi, X.; et al. An injectable peptide hydrogel with excellent self-healing ability to continuously release salvianolic acid B for myocardial infarction. Biomaterials 2021, 274, 120855. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Jin, S.; Ye, Y.; Fang, Y.; Xu, P.; Zhang, C. Salvianolic acid B combined with bone marrow mesenchymal stem cells piggybacked on HAMA hydrogel re-transplantation improves intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2022, 10, 950625. [Google Scholar] [CrossRef]
- Loebel, C.; D’Este, M.; Alini, M.; Zenobi-Wong, M.; Eglin, D. Precise tailoring of tyramine-based hyaluronan hydrogel properties using DMTMM conjugation. Carbohydr. Polym. 2015, 115, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Abinzano, F.; Bernal, P.N.; Albillos Sanchez, A.; Atienza-Roca, P.; Otto, I.A.; Peiffer, Q.C.; Matsusaki, M.; Woodfield, T.B.F.; Malda, J.; et al. One-Step Photoactivation of a Dual-Functionalized Bioink as Cell Carrier and Cartilage-Binding Glue for Chondral Regeneration. Adv. Healthc. Mater. 2020, 9, 1901792. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Lee, F.; Lim, J.; Du, C.; Wan, A.C.A.; Lee, S.S.; Kurisawa, M. Enzymatic conjugation of a bioactive peptide into an injectable hyaluronic acid–tyramine hydrogel system to promote the formation of functional vasculature. Acta Biomater. 2014, 10, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.B.; Hsu, C.-C.; Ye, H.; Cui, Z. Development of an in situ injectable hydrogel containing hyaluronic acid for neural regeneration. Biomed. Mater. 2020, 15, 055005. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, S.; Ebisu, Y.; Sedlačík, T.; Semba, S.; Nonoyama, T.; Kurokawa, T.; Hirota, A.; Takahashi, T.; Yamaguchi, K.; Imajo, M.; et al. Engineering of an electrically charged hydrogel implanted into a traumatic brain injury model for stepwise neuronal tissue reconstruction. Sci. Rep. 2023, 13, 2233. [Google Scholar] [CrossRef]
- Caplan, H.W.; Prabhakara, K.S.; Toledano Furman, N.E.; Zorofchian, S.; Kumar, A.; Martin, C.; Xue, H.; Olson, S.D.; Cox, C.S., Jr. Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cells 2020, 39, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2020, 37, 14–26. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Xu, D.; Zhang, Q.; Cui, T. Growth Differentiation Factor 5 Improves Neurogenesis and Functional Recovery in Adult Mouse Hippocampus Following Traumatic Brain Injury. Front. Neurol. 2018, 9, 592. [Google Scholar] [CrossRef]
- Lin, Y.; Wan, J.-Q.; Gao, G.-Y.; Pan, Y.-H.; Ding, S.-H.; Fan, Y.-L.; Wang, Y.; Jiang, J.-Y. Direct hippocampal injection of pseudo lentivirus-delivered nerve growth factor gene rescues the damaged cognitive function after traumatic brain injury in the rat. Biomaterials 2015, 69, 148–157. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, L.; Li, L.; Li, H.; Li, Y.; Jin, W.; Chen, C.; Zhang, J. Relationship between HIF-1α and apoptosis in rats with traumatic brain injury and the influence of traditional Chinese medicine Sanqi. Saudi J. Biol. Sci. 2019, 26, 1995–1999. [Google Scholar] [CrossRef]
- Jiang, T.; Zhou, X.; Jiang, H.; Ying, R.; Zhang, Z.; Cai, D.; Wu, Y.; Fang, H.; Wang, L. Efficacy of Sanqi (Radix Notoginseng) in treating cerebral hemorrhage in rats with traumatic brain injury. J. Tradit. Chin. Med. 2021, 41, 262–269. [Google Scholar] [PubMed]
- Yang, F.; Li, G.; Lin, B.; Zhang, K. Gastrodin suppresses pyroptosis and exerts neuroprotective effect in traumatic brain injury model by inhibiting NLRP3 inflammasome signaling pathway. J. Integr. Neurosci. 2022, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Green, M.A.; Bilston, L.E.; Sinkus, R. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 2008, 21, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.M.; Kim, H.C.; Jeong, J.E.; Park, S.A.; Park, W.H. Visible-light-induced hyaluronate hydrogel for soft tissue fillers. Int. J. Biol. Macromol. 2020, 165 Pt B, 2834–2844. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, W.; Zhang, M.; Ma, Z.; Zhao, T.; Zhang, Y. Preparation and characterization of Panax notoginseng saponins loaded hyaluronic acid/carboxymethyl chitosan hydrogel for type o diabetic wound healing. Mater. Today Commun. 2023, 34, 105284. [Google Scholar] [CrossRef]
- Ren, P.; Wei, D.; Liang, M.; Xu, L.; Zhang, T.; Zhang, Q. Alginate/gelatin-based hybrid hydrogels with function of injecting and encapsulating cells in situ. Int. J. Biol. Macromol. 2022, 212, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Ho, C.K.; Gao, Y.; Chong, C.H.; Zheng, D.N.; Zhang, Y.F.; Yu, L. Salvianolic acid-B improves fat graft survival by promoting proliferation and adipogenesis. Stem Cell Res. Ther. 2021, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wu, X.; Zhu, J. VEGF promotes proliferation of human glioblastoma multiforme stem-like cells through VEGF receptor 2. Sci. World J. 2013, 2013, 417413. [Google Scholar] [CrossRef] [PubMed]
- Gwon, K.; Kim, E.; Tae, G. Heparin-hyaluronic acid hydrogel in support of cellular activities of 3D encapsulated adipose derived stem cells. Acta Biomater. 2017, 49, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Fitch, M.T.; Silver, J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008, 209, 294–301. [Google Scholar] [CrossRef]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Vallon, M.; Chang, J.; Zhang, H.; Kuo, C.J. Developmental and pathological angiogenesis in the central nervous system. Cell. Mol. Life Sci. CMLS 2014, 71, 3489–3506. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Mahmood, A.; Chopp, M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr. Opin. Investig. Drugs 2010, 11, 298–308. [Google Scholar] [PubMed]

| Sample | HA-TA (mL) | Gel-TA (mL) | SAB (mg) | VEGF (ng) |
|---|---|---|---|---|
| HA/Gel | 0.5 | 0.5 | 0 | 0 |
| HA/Gel/SAB | 0.5 | 0.5 | 1 | 0 |
| HA/Gel/VEGF | 0.5 | 0.5 | 0 | 50 |
| HA/Gel/SAB/VEGF | 0.5 | 0.5 | 1 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Cao, Y.; Yan, Y.; Xu, H.; Zhang, X.; Yan, T.; Wan, H. Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice. Molecules 2024, 29, 1705. https://doi.org/10.3390/molecules29081705
Zhou G, Cao Y, Yan Y, Xu H, Zhang X, Yan T, Wan H. Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice. Molecules. 2024; 29(8):1705. https://doi.org/10.3390/molecules29081705
Chicago/Turabian StyleZhou, Guoying, Yajie Cao, Yujia Yan, Haibo Xu, Xiao Zhang, Tingzi Yan, and Haitong Wan. 2024. "Injectable Hydrogels Based on Hyaluronic Acid and Gelatin Combined with Salvianolic Acid B and Vascular Endothelial Growth Factor for Treatment of Traumatic Brain Injury in Mice" Molecules 29, no. 8: 1705. https://doi.org/10.3390/molecules29081705





