Nanoscale Evaluation of the Degradation Stability of Black Phosphorus Nanosheets Functionalized with PEG and Glutathione-Stabilized Doxorubicin Drug-Loaded Gold Nanoparticles in Real Functionalized System
Abstract
:1. Introduction
2. Results and Discussion
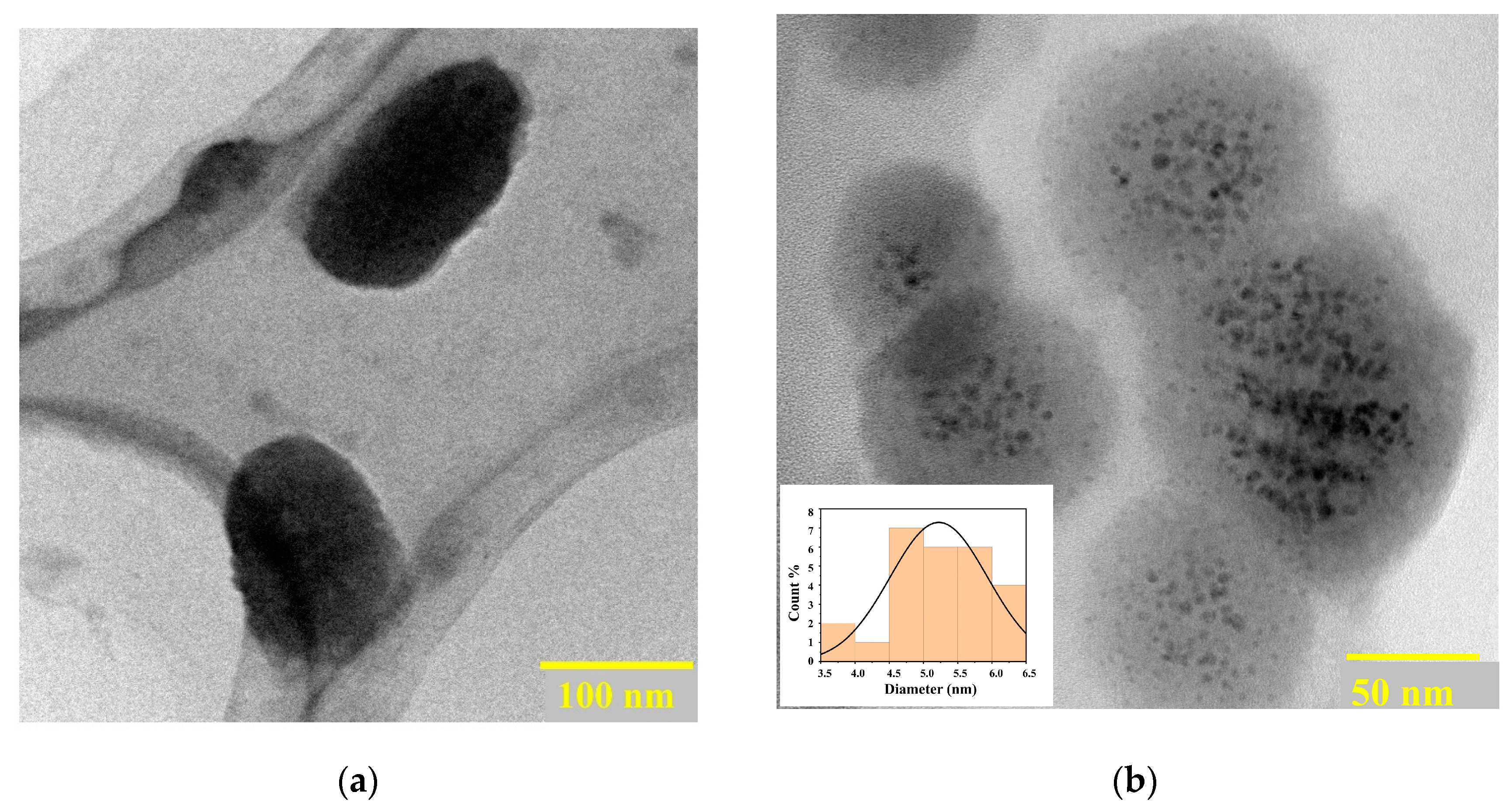
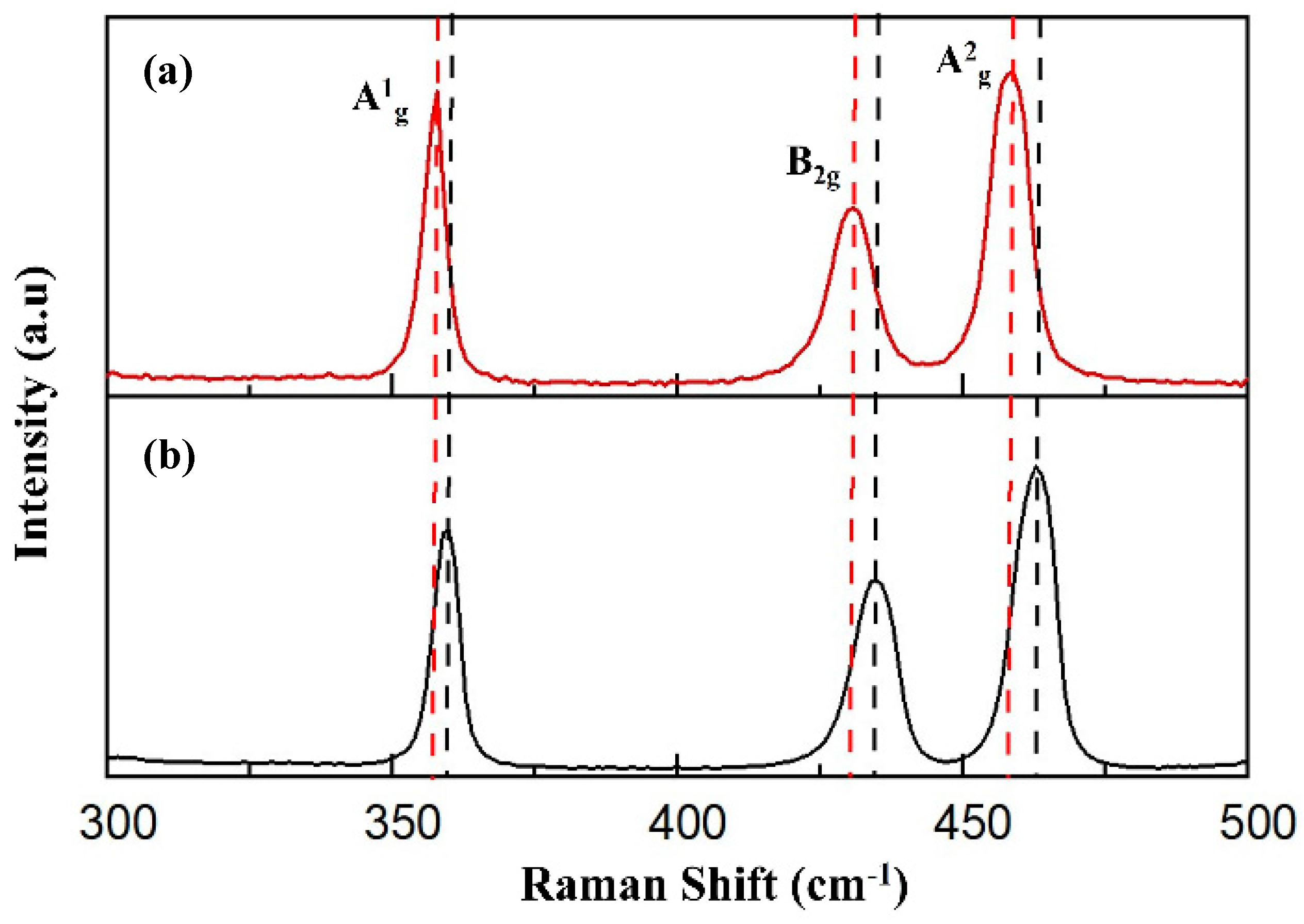
2.1. Morphology and Characterization of Synthesized Nanomaterials
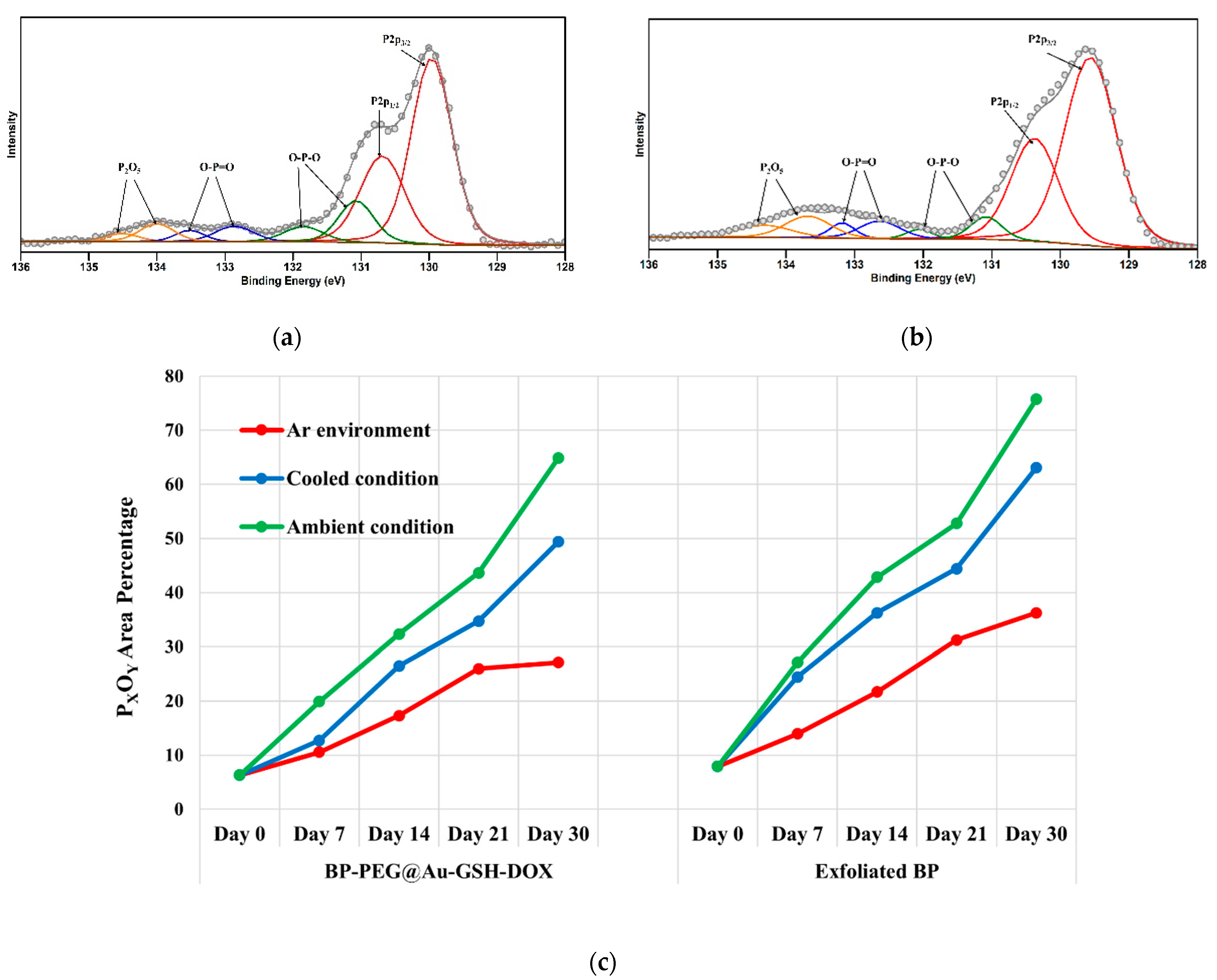
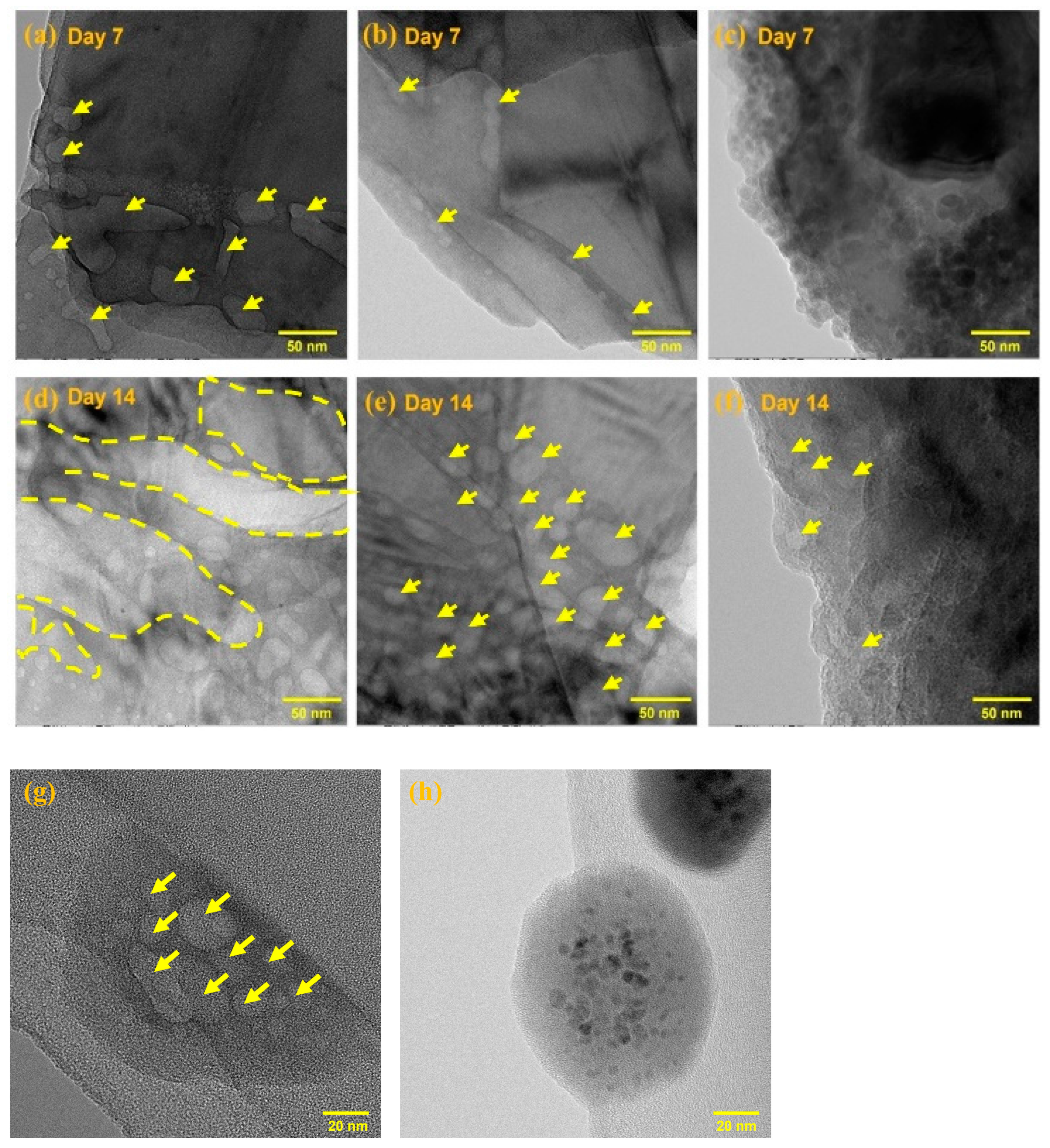
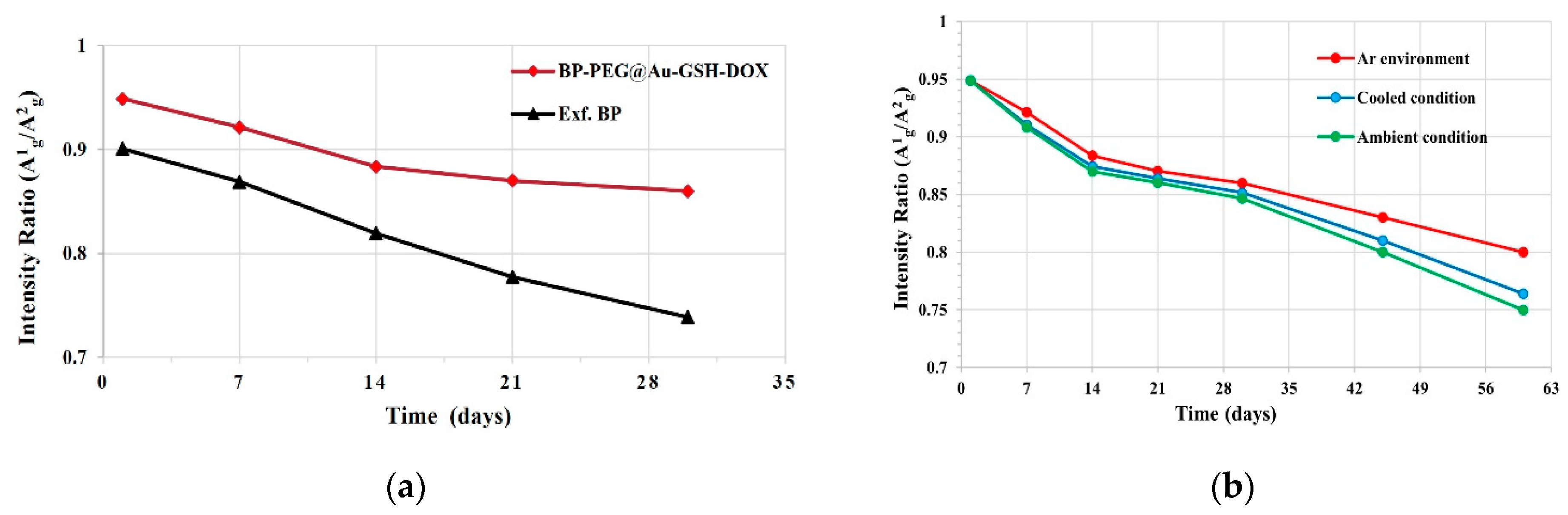
2.2. Stability Evaluation of Exf. BP and BP-PEG@Au-GSH-DOX NSs
3. Materials and Methods
3.1. Synthesis of Exf. BP NSs and BP-PEG@Au-GSH-DOX NSs
3.2. Stability Evaluation of Exf. BP and BP-PEG@Au-GSH-DOX NSs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Chen, Y.; Zhang, Y.H.; Liu, S.F. Recent advance in black phosphorus: Properties and applications. Mater. Chem. Phys. 2017, 189, 215–229. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, Z. Synthesis and stabilization of black phosphorus and phosphorene: Recent progress and perspectives. IScience 2021, 24, 103116–103141. [Google Scholar] [CrossRef]
- Aidarkhanov, D.; Yelzhanova, Z.; Ren, Z.; Nigmetova, G.; Lau, S.P.; Balanay, M.P. Synergic effects of incorporating black phosphorus for interfacial engineering in perovskite solar cells. Surf. Interfaces 2023, 43, 230–245. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, E.; Gao, Y.; Zhou, J.; Sun, Z. Construction of 0D Ti3C2O2/2D black phosphorus S-scheme heterostructure for photocatalytic CO2 reduction. Surf. Interfaces 2024, 44, 103741–103749. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, X.; Liu, Y.; Fan, T.; Wang, Q.; Zhang, H. Black phosphorus as a versatile nanoplatform: From unique properties to biomedical applications. J. Innov. Opt. Health Sci. 2020, 13, 2030008–2030048. [Google Scholar] [CrossRef]
- Appalakondaiah, S.; Vaitheeswaran, G.; Lebègue, S.; Christensen, N.E.; Svane, A. Effect of van der Waals interactions on the structural and elastic properties of black phosphorus. Phys. Rev. B Condens. Matter. Mater. Phys. 2012, 86, 35105–35114. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, G.; Fan, F.; Song, C.; Wang, F.; Xing, Q. Strain-tunable van der Waals interactions in few-layer black phosphorus. Nat. Commun. 2019, 10, 2447–2456. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Nilghaz, A.; Lin, Y.; Xu, J. Black phosphorus and its biomedical applications. Theranostics 2018, 8, 1005–1026. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2D Black Phosphorus–Based Biomedical Applications. Adv. Funct. Mater. 2019, 29, 1808306–1808325. [Google Scholar] [CrossRef]
- Anju, S.; Ashtami, J.; Mohanan, P.V. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater. Sci. Eng. C 2019, 97, 978–993. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Y.; Sun, C.; Yun, B.; Sun, Q.; Zhao, C. Functionalization of small black phosphorus nanoparticles for targeted imaging and photothermal therapy of cancer. Sci. Bull. 2018, 63, 917–924. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; Shi, Y.; Huang, W.; Shao, J.; Dong, X. Nano-black phosphorus for combined cancer phototherapy: Recent advances and prospects. Nanotechnology 2018, 29, 222001–222017. [Google Scholar] [CrossRef]
- Ying-Yan, M.; Meng, L.; Jin-Da, W.; Kai-Jie, W.; Jing-Shang, Z.; Shu-Ying, C. NIR-triggered drug delivery system for chemo-photothermal therapy of posterior capsule opacification. J. Control. Release 2021, 339, 391–402. [Google Scholar] [CrossRef]
- Liu, W.; Dong, A.; Wang, B.; Zhang, H. Current Advances in Black Phosphorus-Based Drug Delivery Systems for Cancer Therapy. Adv. Sci. 2021, 8, 2003033. [Google Scholar] [CrossRef]
- Zong, S.; Wang, L.; Yang, Z.; Wang, H.; Wang, Z.; Cui, Y. Black Phosphorus-Based Drug Nanocarrier for Targeted and Synergetic Chemophotothermal Therapy of Acute Lymphoblastic Leukemia. ACS Appl. Mater. Interfaces 2019, 11, 5896–5902. [Google Scholar] [CrossRef]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Keizer, H.G.; Pinedo, H.M.; Schuurhuis, G.J.; Joenje, H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol. Ther. 1990, 47, 219–231. [Google Scholar] [CrossRef]
- Chen, T.; Zeng, W.; Tie, C.; Yu, M.; Hao, H.; Deng, Y. Engineered gold/black phosphorus nanoplatforms with remodeling tumor microenvironment for sonoactivated catalytic tumor theranostics. Bioact. Mater. 2022, 10, 515–525. [Google Scholar] [CrossRef]
- Lim, Z.Z.J.; Li, J.E.J.; Ng, C.T.; Yung, L.Y.L.; Bay, B.H. Gold nanoparticles in cancer therapy. Acta. Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Wang, Y.; Sheng, Q.; Yue, T.; Zheng, J. Electrostatic assembly of gold nanoparticles on black phosphorus nanosheets for electrochemical aptasensing of patulin. Microchim. Acta 2019, 186, 238. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, J.; Jiang, X.; Li, X.; Yang, M. Gold nanoparticle–modified black phosphorus nanosheets with improved stability for detection of circulating tumor cells. Microchim. Acta 2020, 187, 397. [Google Scholar] [CrossRef] [PubMed]
- Abellán, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U. Fundamental Insights into the Degradation and Stabilization of Thin Layer Black Phosphorus. J. Am. Chem. Soc. 2017, 139, 10432–10440. [Google Scholar] [CrossRef] [PubMed]
- Hyun, C.; Kim, J.H.; Lee, J.Y.; Lee, G.H.; Kim, K.S. Atomic scale study of black phosphorus degradation. RSC Adv. 2019, 10, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, Q.; Shi, L.; Chen, Q.; Wang, J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J. Mater. Chem. A Mater. 2019, 7, 4291–4312. [Google Scholar] [CrossRef]
- Shao, J.; Xie, H.; Huang, H.; Li, Z.; Sun, Z.; Xu, Y. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016, 7, 12967–12976. [Google Scholar] [CrossRef]
- Ryder, C.; Wood, J.D.; Wells, S.A.; Yang, Y.; Jariwala, D.; Marks, T.Y.; Schatz, G.C.; Hersam, M.C. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, D.; Backes, C.; Doherty, E.; Cucinotta, C.S.; Berner, N.C.; Boland, C. Liquid exfoliation of solvent-stabilized few-layer black phosphorus for applications beyond electronics. Nat. Commun. 2015, 6, 8563–8574. [Google Scholar] [CrossRef] [PubMed]
- Passaglia, E.; Cicogna, F.; Costantino, F.; Coiai, S.; Legnaioli, S.; Lorenzetti, G. Polymer-Based Black Phosphorus (bP) Hybrid Materials by in Situ Radical Polymerization: An Effective Tool to Exfoliate bP and Stabilize bP Nanoflakes. Chem. Mater. 2018, 30, 2036–2048. [Google Scholar] [CrossRef]
- Island, J.O.; Steele, G.A.; Van Der Zant, H.S.J.; Castellanos-Gomez, A. Environmental instability of few-layer black phosphorus. 2D Mater. 2015, 2, 11002–11008. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef]
- Thurakkal, S.; Zhang, X. Recent Advances in Chemical Functionalization of 2D Black Phosphorous Nanosheets. Adv. Sci. 2020, 7, 1902359–1902379. [Google Scholar] [CrossRef]
- van Druenen, M. Degradation of Black Phosphorus and Strategies to Enhance Its Ambient Lifetime. Adv. Mater Interfaces 2020, 7, 2001102–2001116. [Google Scholar] [CrossRef]
- Zeng, X.; Luo, M.; Liu, G.; Wang, X.; Tao, W.; Lin, Y. Polydopamine-Modified Black Phosphorous Nanocapsule with Enhanced Stability and Photothermal Performance for Tumor Multimodal Treatments. Adv. Sci. 2018, 5, 1800510–1800518. [Google Scholar] [CrossRef]
- Ni, H.; Liu, X.; Cheng, Q. A new strategy for air-stable black phosphorus reinforced PVA nanocomposites. J. Mater. Chem. A Mater. 2018, 6, 7142–7147. [Google Scholar] [CrossRef]
- Alsaffar, F.; Alodan, S.; Alrasheed, A.; Alhussain, A.; Alrubaiq, N.; Abbas, A. Raman Sensitive Degradation and Etching Dynamics of Exfoliated Black Phosphorus. Sci. Rep. 2017, 7, 44540–44549. [Google Scholar] [CrossRef]
- An, C.J.; Kang, Y.H.; Lee, C.; Cho, S.Y. Preparation of Highly Stable Black Phosphorus by Gold Decoration for High-Performance Thermoelectric Generators. Adv. Funct. Mater. 2018, 28, 1800532–1800539. [Google Scholar] [CrossRef]
- Yang, B.; Wan, B.; Zhou, Q.; Wang, Y.; Hu, W.; Lv, W. Te-Doped Black Phosphorus Field-Effect Transistors. Adv. Mat. 2016, 28, 9408–9415. [Google Scholar] [CrossRef]
- Valt, M.; Caporali, M.; Fabbri, B.; Gaiardo, A.; Krik, S.; Iacob, E. Air Stable Nickel-Decorated Black Phosphorus and Its Room-Temperature Chemiresistive Gas Sensor Capabilities. ACS Appl. Mater. Interfaces 2021, 13, 44711–44722. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Zhao, S.; Bi, F.; Song, L.; Liu, N. Stable Black Phosphorus Encapsulation in Porous Mesh-like UiO-66 Promoted Charge Transfer for Photocatalytic Oxidation of Toluene and o-Dichlorobenzene: Performance, Degradation Pathway, and Mechanism. ACS Catal. 2022, 12, 8069–8081. [Google Scholar] [CrossRef]
- Zhu, H.; McDonnell, S.; Qin, X.; Azcatl, A.; Cheng, L.; Addou, R. Al2O3 on Black Phosphorus by Atomic Layer Deposition: An in Situ Interface Study. ACS Appl. Mater. Interfaces 2015, 7, 13038–13043. [Google Scholar] [CrossRef]
- Wu, S.; He, F.; Xie, G.; Bian, Z.; Ren, Y.; Liu, X. Super-Slippery Degraded Black Phosphorus/Silicon Dioxide Interface. ACS Appl. Mater. Interfaces 2020, 12, 7717–7726. [Google Scholar] [CrossRef]
- Wang, G.; Slough, W.J.; Pandey, R.; Karna, S.P. Degradation of phosphorene in air: Understanding at atomic level. 2D Mater. 2016, 3, 25011–25018. [Google Scholar] [CrossRef]
- Zhou, T.; Ni, H.; Wang, Y.; Wu, C.; Zhang, H.; Zhang, J. Ultratough graphene–black phosphorus films. Proc. Natl. Acad. Sci. USA 2020, 117, 8727–8735. [Google Scholar] [CrossRef]
- Gunathilaka, T.M.; Shimomura, M. Chemical and morphological study on functionalized black phosphorus nanosheets with PEG and glutathione stabilized doxorubicin drug-loaded Au nanoparticles. J. Mater. Sci. Mater. Electron. 2023, 34, 2062–2072. [Google Scholar] [CrossRef]
- Tiouitchi, G.; Ali, M.A.; Benyoussef, A.; Hamedoun, M.; Lachgar, A.; Kara, A. Efficient production of few-layer black phosphorus by liquid-phase exfoliation: Black phosphorus layers using LPE. R. Soc. Open. Sci. 2020, 7, 201210–201219. [Google Scholar] [CrossRef]
- Kang, J.; Wood, J.D.; Wells, S.A.; Lee, J.H.; Liu, X.; Chen, K.S. Solvent exfoliation of electronic-grade, two-dimensional black phosphorus. ACS Nano 2015, 9, 3596–3604. [Google Scholar] [CrossRef]
- Sun, C.; Wen, L.; Zeng, J.; Wang, Y.; Sun, Q.; Deng, L. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials 2016, 91, 81–89. [Google Scholar] [CrossRef]
- Wójcik, M.; Lewandowski, W.; Król, M.; Pawłowski, K.; Mieczkowski, J.; Lechowski, R. Enhancing anti-tumor efficacy of doxorubicin by non-covalent conjugation to gold nanoparticles—In Vitro studies on Feline fibrosarcoma cell lines. PLoS ONE 2015, 10, e0124955. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; What is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Dong, W.; Wang, H.; Liu, H.; Zhou, C.; Zhang, X.; Wang, S. Potential of Black Phosphorus in Immune-Based Therapeutic Strategies. Bioinorg. Chem. Appl. 2022, 20, 2122–2129. [Google Scholar] [CrossRef]
- Sugai, S.; Shirotani, I. Raman and infrared reflection spectroscopy in black phosphorus. Solid State Commun. 1985, 53, 753–755. [Google Scholar] [CrossRef]
- Wu, J.; Mao, N.; Xie, L.; Xu, H.; Zhang, J. Identifying the crystalline orientation of black phosphorus using angle-resolved polarized Raman spectroscopy. Angew. Chem. Int. Ed. 2015, 54, 2366–2369. [Google Scholar] [CrossRef]
- Li, R.; Shang, Y.; Xing, H.; Wang, X.; Sun, M.; Qiu, W. Orientation identification of the black phosphorus with different thickness based on B2g mode using a micro-raman spectroscope under a nonanalyzer configuration. Materials 2020, 13, 5572–5584. [Google Scholar] [CrossRef]
- Moschetto, S.; Ienco, A.; Manca, G.; Serrano-Ruiz, M.; Peruzzini, M.; Mezzi, A. Easy and fast in situ functionalization of exfoliated 2D black phosphorus with gold nanoparticles. Dalton Trans. 2021, 50, 11610–11618. [Google Scholar] [CrossRef]
- Qu, G.; Xia, T.; Zhou, W.; Zhang, X.; Zhang, H.; Hu, L. Property-Activity Relationship of Black Phosphorus at the Nano-Bio Interface: From Molecules to Organisms. Chem. Rev. 2020, 120, 2288–2346. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Kuntz, K.L.; Wells, R.A.; Hu, J.; Yang, T.; Dong, B.; Guo, H. Control of Surface and Edge Oxidation on Phosphorene. ACS Appl. Mater. Interfaces 2017, 9, 9126–9935. [Google Scholar] [CrossRef]
- Wood, J.D.; Wells, S.A.; Jariwala, D.; Chen, K.S.; Cho, E.; Sangwan, V.K. Effective passivation of exfoliated black phosphorus transistors against ambient degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef] [PubMed]
- Thurakkal, S.; Zhang, X. Covalent functionalization of two-dimensional black phosphorus nanosheets with porphyrins and their photophysical characterization. Mater. Chem. Front. 2021, 5, 2824–2831. [Google Scholar] [CrossRef]
- Tian, B.; Tian, B.; Smith, B.; Scott, M.C.; Lei, Q.; Hua, R. Facile bottom-up synthesis of partially oxidized black phosphorus nanosheets as metal-free photocatalyst for hydrogen evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 4345–4350. [Google Scholar] [CrossRef] [PubMed]
- Van Druenen, M.; Davitt, F.; Collins, T.; Glynn, C.; O’Dwyer, C.; Holmes, J.D. Evaluating the Surface Chemistry of Black Phosphorus during Ambient Degradation. Langmuir 2019, 35, 2172–2178. [Google Scholar] [CrossRef] [PubMed]
- Bartus Pravda, C.; Hegedűs, T.; Oliveira, E.F.; Berkesi, D.; Szamosvölgyi, Á.; Kónya, Z. Hexagonal Boron Nitride Nanosheets Protect Exfoliated Black Phosphorus Layers from Ambient Oxidation. Adv. Mater. Interfaces 2022, 9, 2200857–2200870. [Google Scholar] [CrossRef]
- Ge, X.; Xia, Z.; Guo, S. Recent Advances on Black Phosphorus for Biomedicine and Biosensing. Adv. Funct. Mater. 2019, 29, 1900318–1900350. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Huang, H.; Yang, N.; Yu, B.; Wen, M. In-Plane Black Phosphorus/Dicobalt Phosphide Heterostructure for Efficient Electrocatalysis. Angew. Chem. Int. Ed. 2018, 57, 2630–2634. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, C.; Xie, J.; Ågren, H.; Zhang, H. Black Phosphorus/Polymers: Status and Challenges. Adv. Mater. 2021, 33, 2100113–2100150. [Google Scholar] [CrossRef]
- Tao, W.; Zhu, X.; Yu, X.; Zeng, X.; Xiao, Q.; Zhang, X. Black Phosphorus Nanosheets as a Robust Delivery Platform for Cancer Theranostics. Adv. Mater. 2017, 29, 1603276–1603285. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zhang, B.; Li, S.; He, B.; Pu, Y. Correction: Combination of PEG-decorated black phosphorus nanosheets and immunoadjuvant for photoimmunotherapy of melanoma. J. Mater. Chem. B 2020, 8, 2805–2813. [Google Scholar] [CrossRef]
- Sun, Z.; Xie, H.; Tang, S.; Yu, X.F.; Guo, Z.; Shao, J. Ultrasmall Black Phosphorus Quantum Dots: Synthesis and Use as Photothermal Agents. Angew. Chem. Int. Ed. 2015, 54, 11526–11530. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Li, Y.; Hou, Y.; Fei, X.; Su, C. Facile synthesis of black phosphorus-Au nanocomposites for enhanced photothermal cancer therapy and surface-enhanced Raman scattering analysis. Biomater. Sci. 2017, 5, 2048–2055. [Google Scholar] [CrossRef]
- Zabielska-Koczywas, K.; Dolka, I.; Król, M.; Zbikowski, A.; Lewandowski, W.; Mieczkowski, J. Doxorubicin conjugated to glutathione stabilized gold nanoparticles (Au-GSH-Dox) as an effective therapeutic agent for feline injection-site sarcomas—Chick embryo chorioallantoic membrane study. Molecules 2017, 22, 253–267. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilaka, T.M.; Shimomura, M. Nanoscale Evaluation of the Degradation Stability of Black Phosphorus Nanosheets Functionalized with PEG and Glutathione-Stabilized Doxorubicin Drug-Loaded Gold Nanoparticles in Real Functionalized System. Molecules 2024, 29, 1746. https://doi.org/10.3390/molecules29081746
Gunathilaka TM, Shimomura M. Nanoscale Evaluation of the Degradation Stability of Black Phosphorus Nanosheets Functionalized with PEG and Glutathione-Stabilized Doxorubicin Drug-Loaded Gold Nanoparticles in Real Functionalized System. Molecules. 2024; 29(8):1746. https://doi.org/10.3390/molecules29081746
Chicago/Turabian StyleGunathilaka, Thisari Maleesha, and Masaru Shimomura. 2024. "Nanoscale Evaluation of the Degradation Stability of Black Phosphorus Nanosheets Functionalized with PEG and Glutathione-Stabilized Doxorubicin Drug-Loaded Gold Nanoparticles in Real Functionalized System" Molecules 29, no. 8: 1746. https://doi.org/10.3390/molecules29081746





