Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process
Abstract
1. Introduction
2. Results and Discussion
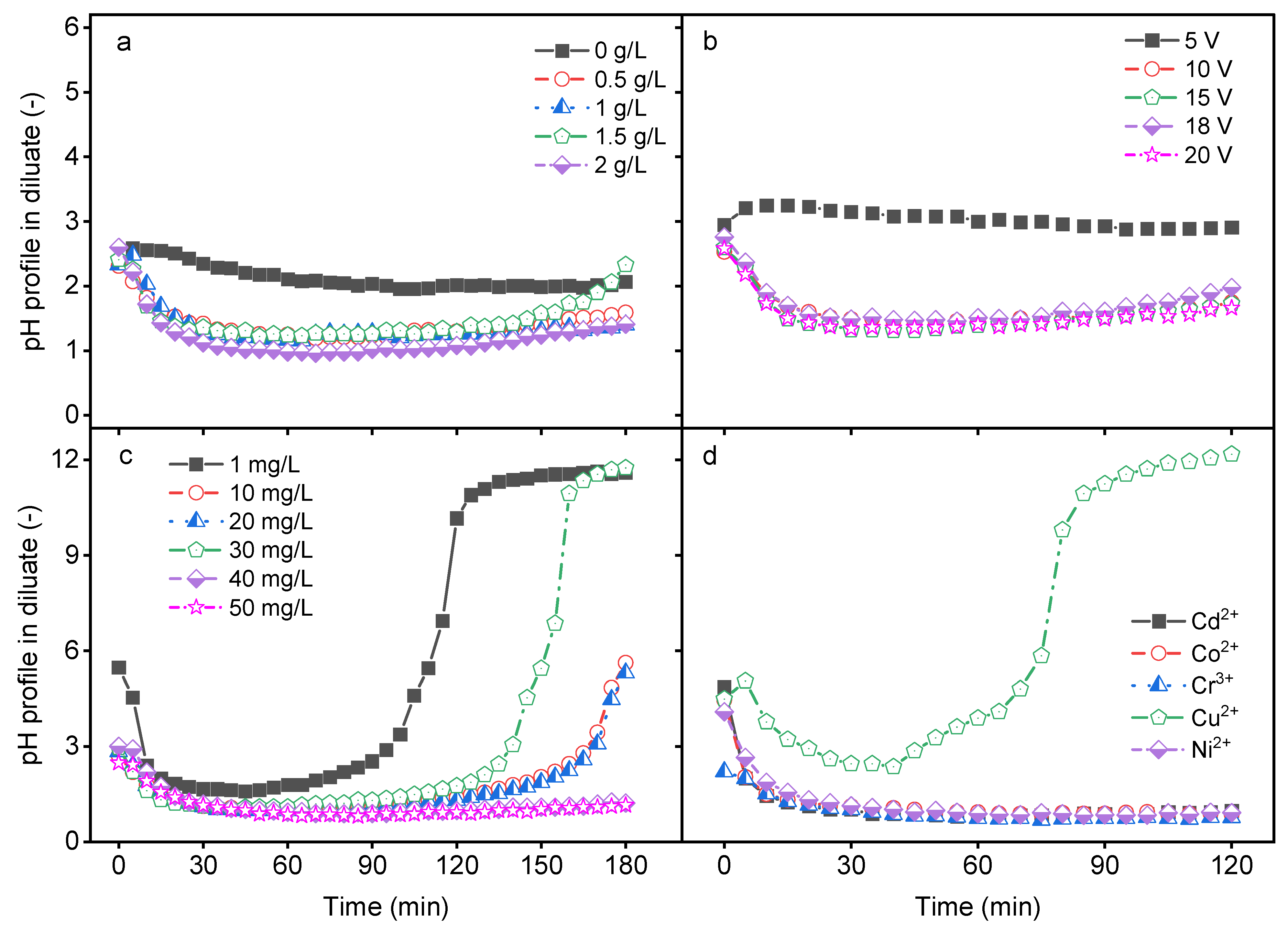
2.1. Impact of Process Parameters on pH Profile in Diluate Compartment
2.2. Impact of pH on HM Species Distribution
2.3. Impact of Initial Salt Concentration
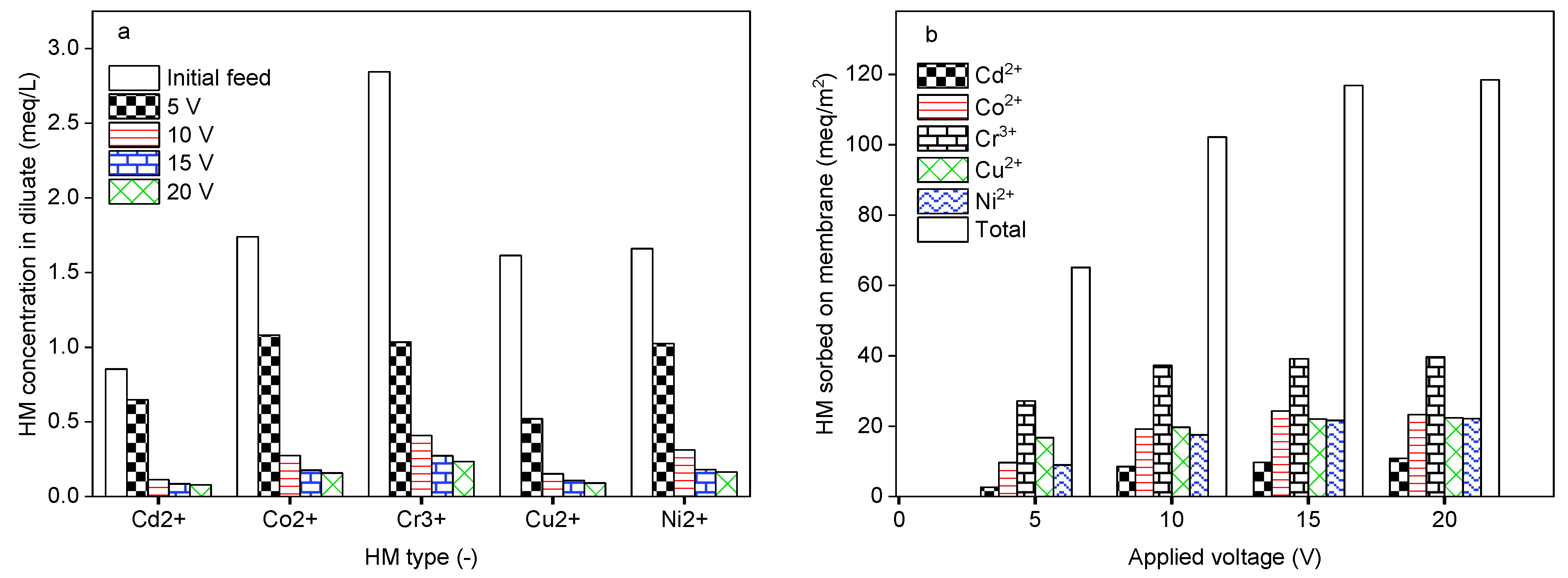
2.4. Impact of Applied Electrical Potential
2.5. Impact of the Type of HM
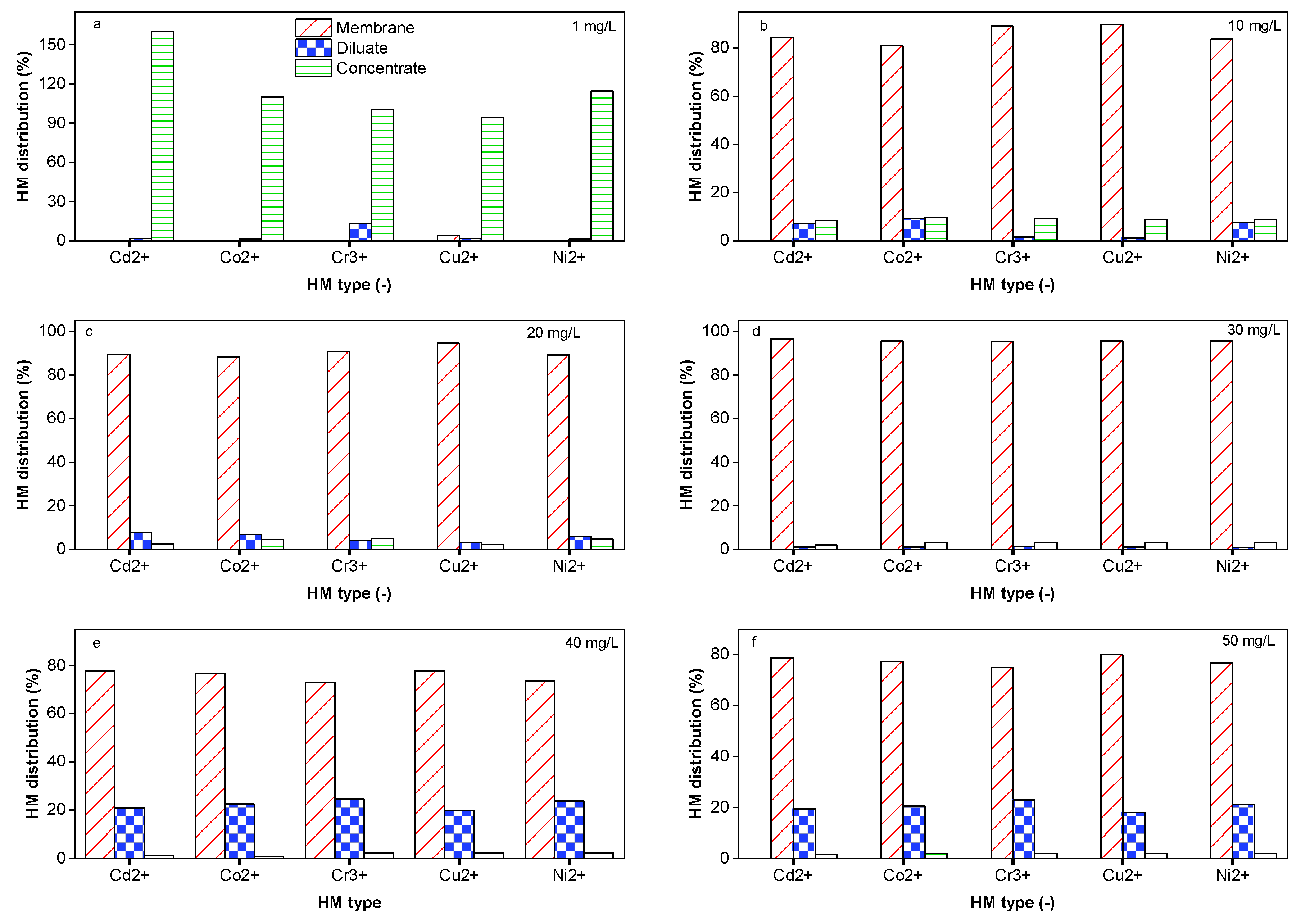
2.6. Impact of the HM Feed Concentration
3. Methods and Materials
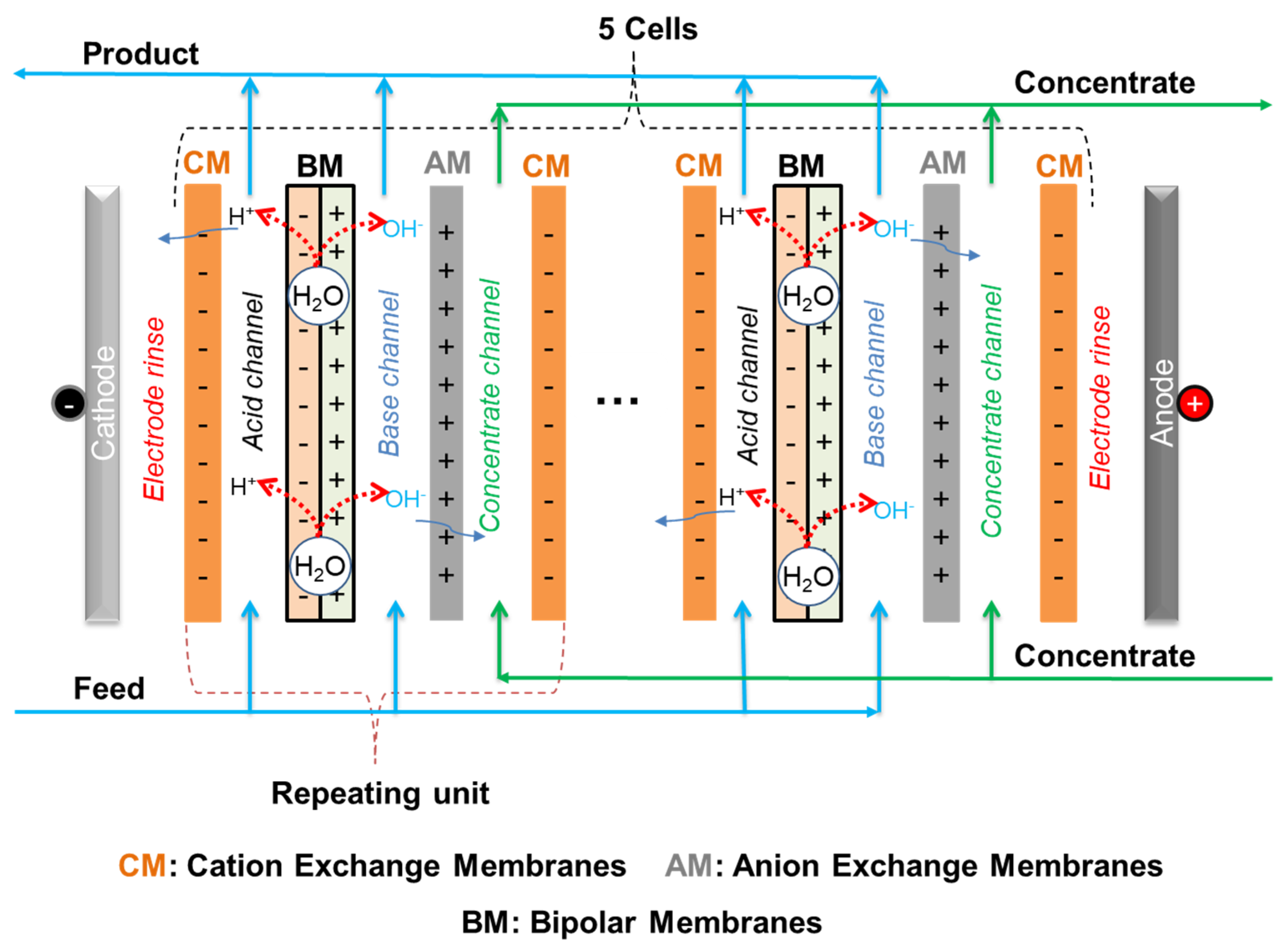
3.1. DS-BMED Process Set Up
3.2. DS-BMED Evaluation Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urbaniec, K.; Mikulčić, H.; Duić, N.; Lozano, R. SDEWES 2014—Sustainable Development of Energy, Water and Environment Systems. J. Clean. Prod. 2016, 130, 1–11. [Google Scholar] [CrossRef]
- KUrbaniec, K.; Mikulčić, H.; Rosen, M.A.; Duić, N. A holistic approach to sustainable development of energy, water and environment systems. J. Clean. Prod. 2017, 155, 1–11. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Scarazzato, T.; Panossian, Z.; Tenório, J.; Pérez-Herranz, V.; Espinosa, D. A review of cleaner production in electroplating industries using electrodialysis. J. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Malik, D.S.; Jain, C.K.; Yadav, A.K. Removal of heavy metals from emerging cellulosic low-cost adsorbents: A review. Appl. Water Sci. 2017, 7, 2113–2136. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of nitrate from aqueous solution by nitrate selective ion exchange resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Rengaraj, S.; Joo, C.K.; Kim, Y.; Yi, J. Kinetics of removal of chromium from water and electronic process wastewater by ion exchange resins: 1200H, 1500H and IRN97H. J. Hazard. Mater. 2003, 102, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Thaçi, B.S.; Gashi, S.T. Reverse Osmosis Removal of Heavy Metals from Wastewater Effluents Using Biowaste Materials Pretreatment. Pol. J. Environ. Stud. 2019, 28, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Mikulášek, P.; Cuhorka, J. Removal of heavy metal ions from aqueous solutions by nanofiltration. Chem. Eng. Trans. 2016, 47, 379–384. [Google Scholar] [CrossRef]
- Al-Rashdi, B.; Johnson, D.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseini, S.M.; Parvizian, F.; Rafiei, N.; Van der Bruggen, B. Desalination and heavy metal ion removal from water by new ion exchange membrane modified by synthesized NiFe2O4/HAMPS nanocomposite. Ionics 2019, 25, 3847–3857. [Google Scholar] [CrossRef]
- Moura, R.C.A.; Bertuol, D.A.; Ferreira, C.A.; Amado, F.D.R. Study of Chromium Removal by the Electrodialysis of Tannery and Metal-Finishing Effluents. Int. J. Chem. Eng. 2012, 2012, 179312. [Google Scholar] [CrossRef]
- Aytac, E.; Altin, S. Influence of initial feed concentration and pH on the removal of copper, lead and nickel ions from solutions in electrodialysis process. Int. J. Sci. Res. Sci. Technol. 2018, 5, 654–660. [Google Scholar]
- İpekçi, D.; Altıok, E.; Bunani, S.; Yoshizuka, K.; Nishihama, S.; Arda, M.; Kabay, N. Effect of acid-base solutions used in acid-base compartments for simultaneous recovery of lithium and boron from aqueous solution using bipolar membrane electrodialysis (BMED). Desalination 2018, 448, 69–75. [Google Scholar] [CrossRef]
- Bunani, S.; Yoshizuka, K.; Nishihama, S.; Arda, M.; Kabay, N. Application of bipolar membrane electrodialysis (BMED) for simultaneous separation and recovery of boron and lithium from aqueous solutions. Desalination 2017, 424, 37–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Liba, D.; Alvarado, L.; Chen, A. Separation and recovery of Cr(III) and Cr(VI) using electrodeionization as an efficient approach. Sep. Purif. Technol. 2014, 137, 86–93. [Google Scholar] [CrossRef]
- Bunani, S.; Arda, M.; Kabay, N. Effect of operational conditions on post-treatment of RO permeate of geothermal water by using electrodeionization (EDI) method. Desalination 2018, 431, 100–105. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Xu, F.; Innocent, C.; Pourcelly, G. Electrodialysis with ion exchange membranes in organic media. Sep. Purif. Technol. 2005, 43, 17–24. [Google Scholar] [CrossRef]
- YTanaka, Y. Ion-Exchange Membrane Electrodialysis for Saline Water Desalination and Its Application to Seawater Concentration. Ind. Eng. Chem. Res. 2011, 50, 7494–7503. [Google Scholar] [CrossRef]
- Reig, M.; Casas, S.; Aladjem, C.; Valderrama, C.; Gibert, O.; Valero, F.; Centeno, C.M.; Larrotcha, E.; Cortina, J.L. Concentration of NaCl from seawater reverse osmosis brines for the chlor-alkali industry by electrodialysis. Desalination 2014, 342, 107–117. [Google Scholar] [CrossRef]
- İpekçi, D.; Kabay, N.; Bunani, S.; Altıok, E.; Arda, M.; Yoshizuka, K.; Nishihama, S. Application of heterogeneous ion exchange membranes for simultaneous separation and recovery of lithium and boron from aqueous solution with bipolar membrane electrodialysis (EDBM). Desalination 2020, 479, 114313. [Google Scholar] [CrossRef]
- Bunani, S.; Arda, M.; Kabay, N.; Yoshizuka, K.; Nishihama, S. Effect of process conditions on recovery of lithium and boron from water using bipolar membrane electrodialysis (BMED). Desalination 2017, 416, 10–15. [Google Scholar] [CrossRef]
- Melnikov, S.; Mugtamov, O.; Zabolotsky, V. Study of electrodialysis concentration process of inorganic acids and salts for the two-stage conversion of salts into acids utilizing bipolar electrodialysis. Sep. Purif. Technol. 2020, 235, 116198. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.D.M.; Villen-Guzman, M.; Rodriguez-Maroto, J.M.; Paz-Garcia, J.M. Metal recovery from wastewater using electrodialysis separation. Metals 2024, 14, 38. [Google Scholar] [CrossRef]
- Alvarado, L.; Chen, A. Electrodeionization: Principles, Strategies and Applications. Electrochim. Acta 2014, 132, 583–597. [Google Scholar] [CrossRef]
- Alvarado, L.; Ramírez, A.; Rodríguez-Torres, I. Cr(VI) removal by continuous electrodeionization: Study of its basic technologies. Desalination 2009, 249, 423–428. [Google Scholar] [CrossRef]
- Tanaka, Y. Mass transport in a boundary layer and in an ion exchange membrane: Mechanism of concentration polarization and water dissociation. Russ. J. Electrochem. 2012, 48, 665–681. [Google Scholar] [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V.; Pismenskaya, N.D. On the role of gravitational convection in the transfer enhancement of salt ions in the course of dilute solution electrodialysis. J. Membr. Sci. 1996, 119, 171–181. [Google Scholar] [CrossRef]
- Gavish, B.; Lifson, S. Membrane polarisation at high current densities. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1979, 75, 463–472. [Google Scholar] [CrossRef]
- Frilette, V.J. Electrogravitational Transport at Synthetic Ion Exchange Membrane Surfaces. J. Phys. Chem. 1957, 61, 168–174. [Google Scholar] [CrossRef]
- Rubinstein, I.; Staude, E.; Kedem, O. Role of the membrane surface in concentration polarization at ion-exchange membrane. Desalination 1988, 69, 101–114. [Google Scholar] [CrossRef]
- Nikonenko, V.V.; Pismenskaya, N.D.; Belova, E.I.; Sistat, P.; Huguet, P.; Pourcelly, G.; Larchet, C. Intensive current transfer in membrane systems: Modelling, mechanisms and application in electrodialysis. Adv. Colloid Interface Sci. 2010, 160, 101–123. [Google Scholar] [CrossRef]
- Caprarescu, S.; Corobea, M.C.; Purcar, V.; Spataru, C.I.; Ianchis, R.; Vasilievici, G.; Vuluga, Z. San copolymer membranes with ion exchangers for Cu(II) removal from synthetic wastewater by electrodialysis. J. Environ. Sci. 2015, 35, 27–37. [Google Scholar] [CrossRef] [PubMed]


| Type of HM | pH | 1 | 2 | 3 | 7 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|
| HM Species | ||||||||
| Cd2+ | Cd2+ | 51.3 | 44.2 | 42.9 | 41.9 | |||
| CdCl+ | 45.0 | 49.2 | 49.3 | 49.5 | ||||
| CdOH+ | 11.1 | |||||||
| Cd(OH)2 (aq) | 80.3 | 88.1 | 39.7 | |||||
| Cd(OH)3− | 10.4 | 50.0 | ||||||
| Cd(OH)42− | 10.2 | |||||||
| Co2+ | Co2+ | 98.3 | 94.1 | 92.0 | 90.5 | |||
| CoSO4 (aq) | 5.2 | 7.2 | 8.6 | |||||
| Co(OH)2 (aq) | 96.1 | 80.5 | 28.0 | |||||
| Co(OH)3− | 19.4 | 72.0 | ||||||
| Cr3+ | Cr3+ | 97.0 | 84.4 | 74.2 | ||||
| CrSO4+ | 14.7 | 19.1 | ||||||
| Cr2(OH)24+ | 7.30 | |||||||
| Cr3(OH)45+ | 69.0 | |||||||
| Cr(OH)2+ | 18.5 | |||||||
| Cr(OH)3 (aq) | 70.8 | 19.4 | 2.20 | |||||
| Cr(OH)4− | 29.2 | 80.6 | 97.8 | |||||
| Cu2+ | Cu2+ | 96.4 | 91.6 | 89.4 | 38.7 | |||
| CuSO4 (aq) | 5.7 | 7.9 | ||||||
| Cu2(OH)22+ | 25.3 | |||||||
| Cu3(OH)42+ | 24.6 | |||||||
| CuOH+ | 6.0 | |||||||
| Cu(OH)3− | 87.1 | 94.0 | 62.2 | |||||
| Cu(OH)42− | 37.7 | |||||||
| Cu(OH)2 (aq) | 11.3 | |||||||
| Ni2+ | Ni2+ | 98.2 | 94.0 | 91.9 | 90.5 | |||
| NiSO4 (aq) | 5.2 | 7.2 | 8.6 | |||||
| Ni(OH)2 (aq) | 45.3 | 7.6 | ||||||
| Ni(OH)3− | 54.1 | 92.3 | 99.2 |
| Membrane | PC SK | PC Acid 60 |
|---|---|---|
| Ion exchange form | Cation exchange membrane (CM), sodium | Anion exchange membrane (AM), chloride |
| Membrane type | Strongly acidic (sulfonic acid) | Strongly alkaline (ammonium) |
| Transference number: | >0.95 | |
| KCl (0.1/0.5 M) a | >0.95 | |
| Acid (0.7/3 M) b | 0.55 | |
| Thickness (mm) | 0.16–0.20 | 0.16–0.20 |
| Water content (wt%) | ~9 | ~17 |
| Ion exchange capacity: | n/a | |
| Strong basic (meq/g) | ca 1.14 | |
| Weak basic (meq/g) | ca 0.45 | |
| Electrical resistance (ohm cm2) | ~2.5 | ~2 |
| Burst strength (kg/cm2) | 4–5 | 4–5 |
| Operating maximum temperature (°C) | 50 | 60 |
| PCcell bipolar type: PC bip | ||
| Water splitting voltage (V) | 1.2–2.2 | |
| Water splitting efficiency (%) | >95 | |
| Operating maximum temperature (°C) | 40 | |
| Thickness (mm) a | 0.2–0.35 | |
| Operational Conditions | |||||||
|---|---|---|---|---|---|---|---|
| Sample | |||||||
| Parameters | NaCl (g/L) | Voltage (V) | Type of HM | Concentration of HM (mg/L) | Volume (L) | Feed Flow Rate (L/h) | Electrode Solution |
| Ionic strength | 0 | 15 | Cd2+, Co2+, Cr3+, Cu2+ and Ni2+ | 50 | 1 | 20 | Na2SO4 (50 g/L) |
| 0.5 | |||||||
| 1 | |||||||
| 1.5 | |||||||
| 2 | |||||||
| Electrical potential | 1 | 5 | |||||
| 10 | |||||||
| 15 | |||||||
| 20 | |||||||
| Type of HM | 1.5 | 15 | Cd2+ | ||||
| Co2+ | |||||||
| Cr3+ | |||||||
| Cu2+ | |||||||
| Ni2+ | |||||||
| Concentration of HM | Cd2+, Co2+, Cr3+, Cu2+ and Ni2+ | 1 | |||||
| 10 | |||||||
| 20 | |||||||
| 30 | |||||||
| 40 | |||||||
| 50 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunani, S.; Abbt-Braun, G.; Horn, H. Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process. Molecules 2024, 29, 1754. https://doi.org/10.3390/molecules29081754
Bunani S, Abbt-Braun G, Horn H. Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process. Molecules. 2024; 29(8):1754. https://doi.org/10.3390/molecules29081754
Chicago/Turabian StyleBunani, Samuel, Gudrun Abbt-Braun, and Harald Horn. 2024. "Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process" Molecules 29, no. 8: 1754. https://doi.org/10.3390/molecules29081754
APA StyleBunani, S., Abbt-Braun, G., & Horn, H. (2024). Heavy Metal Removal from Aqueous Solutions Using a Customized Bipolar Membrane Electrodialysis Process. Molecules, 29(8), 1754. https://doi.org/10.3390/molecules29081754





