The Repellent Capacity against Sitophilus zeamais (Coleoptera: Curculionidae) and In Vitro Inhibition of the Acetylcholinesterase Enzyme of 11 Essential Oils from Six Plants of the Caribbean Region of Colombia
Abstract
:1. Introduction
2. Results
2.1. Identity of the Plants
2.2. Chemical Composition of the Essential Oils
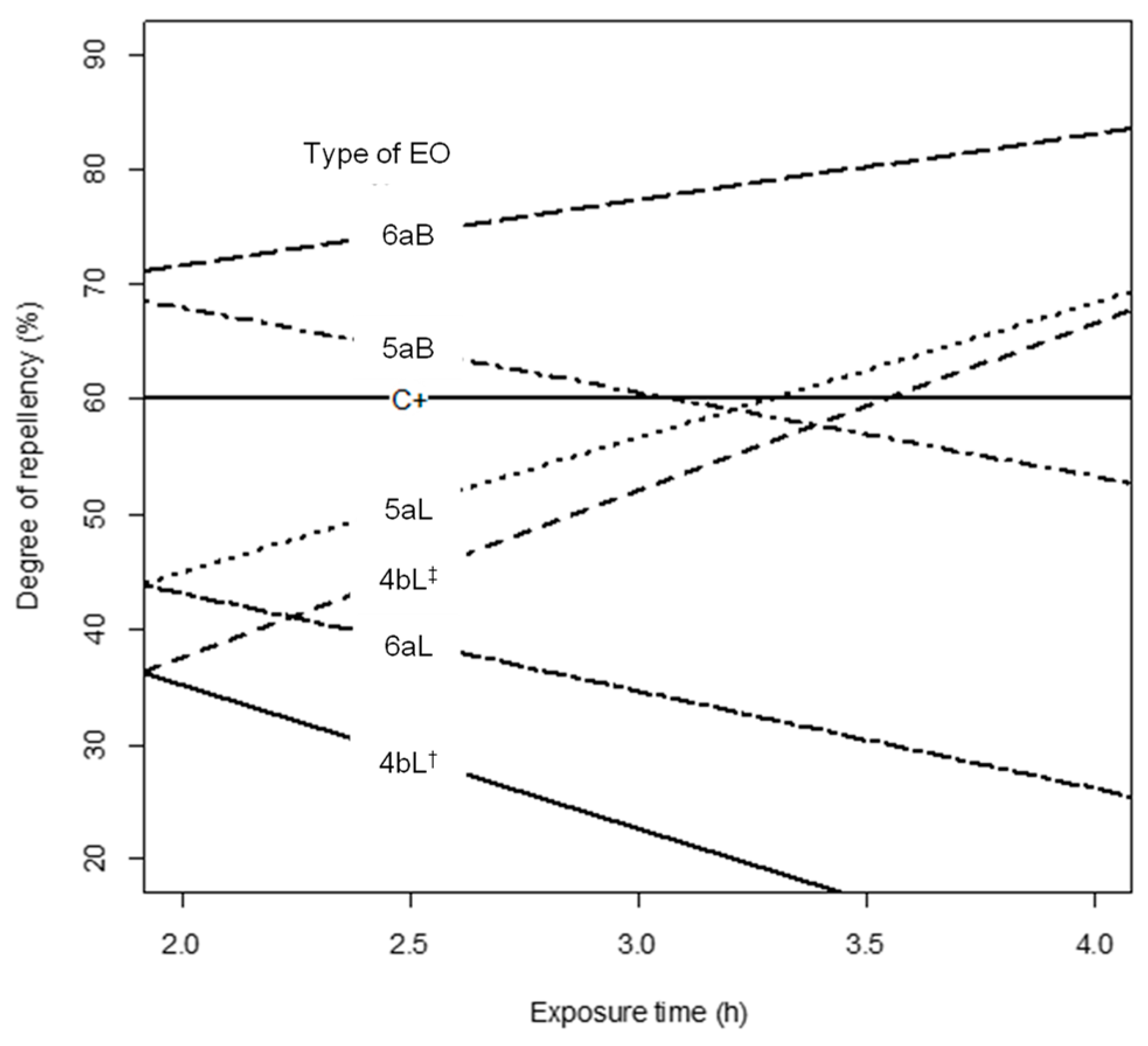
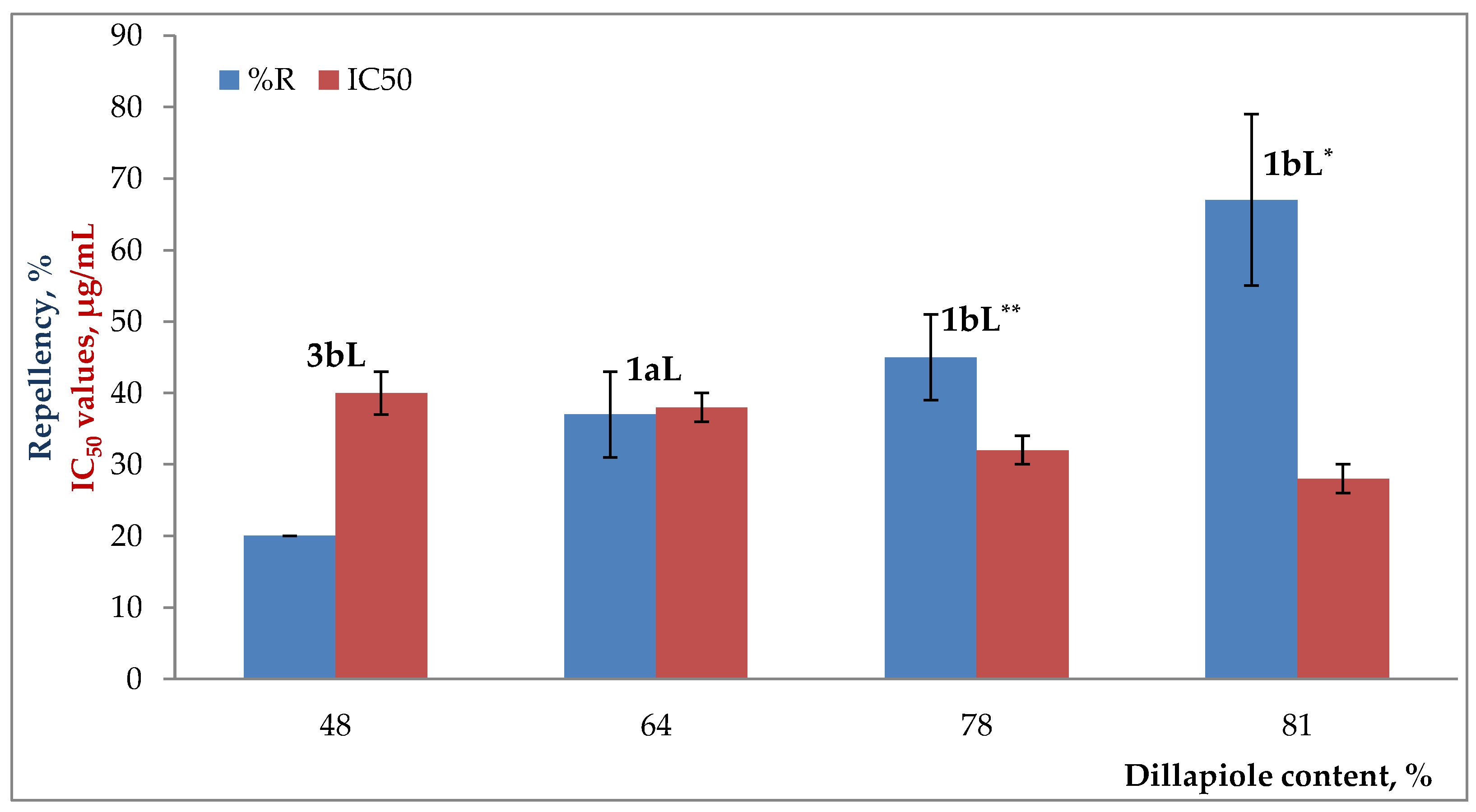
2.3. Degree of Repellency against Maize Weevils by Essential Oils
2.4. Inhibitory Effect on AChE Enzyme by Essential Oils
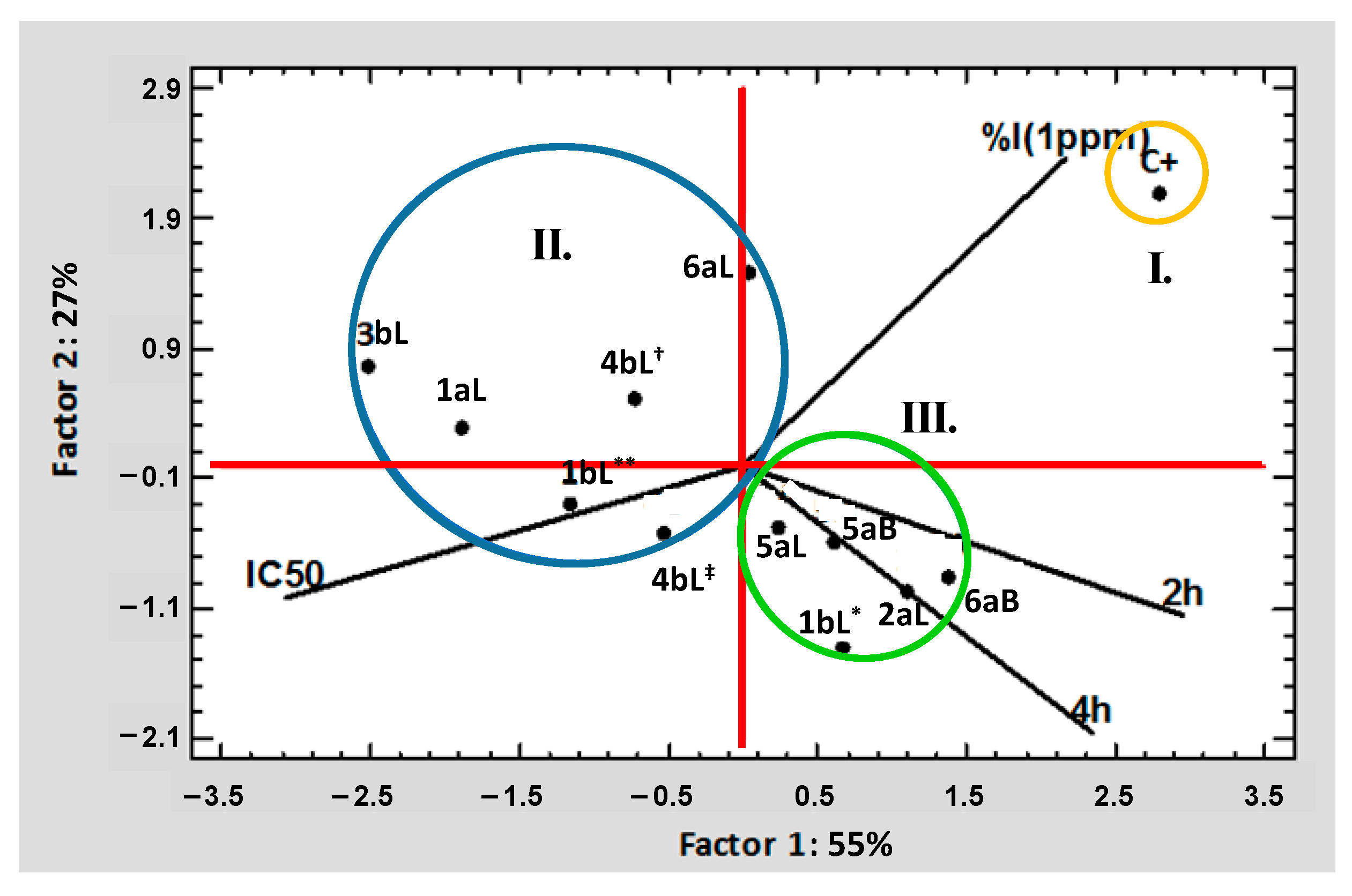
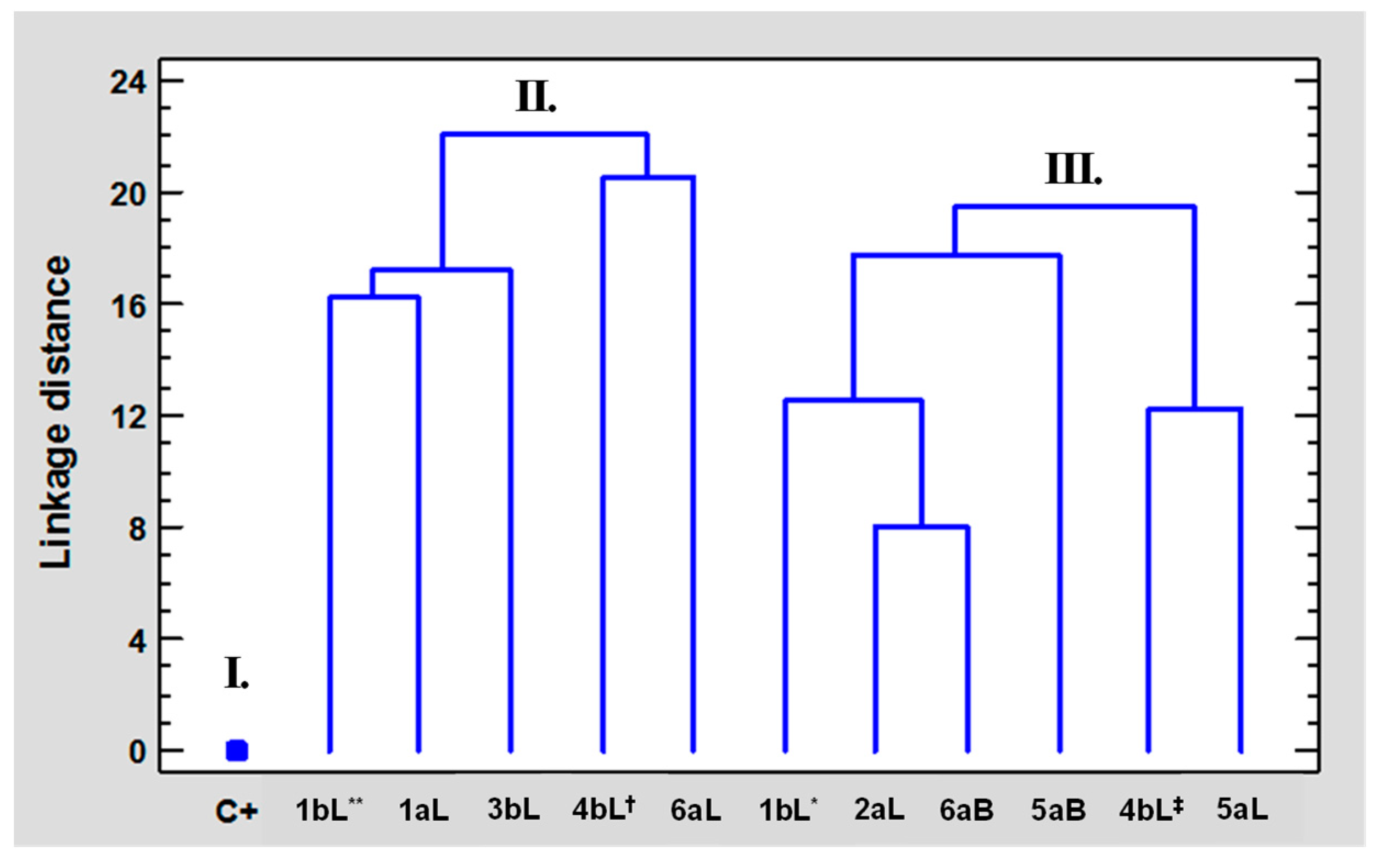
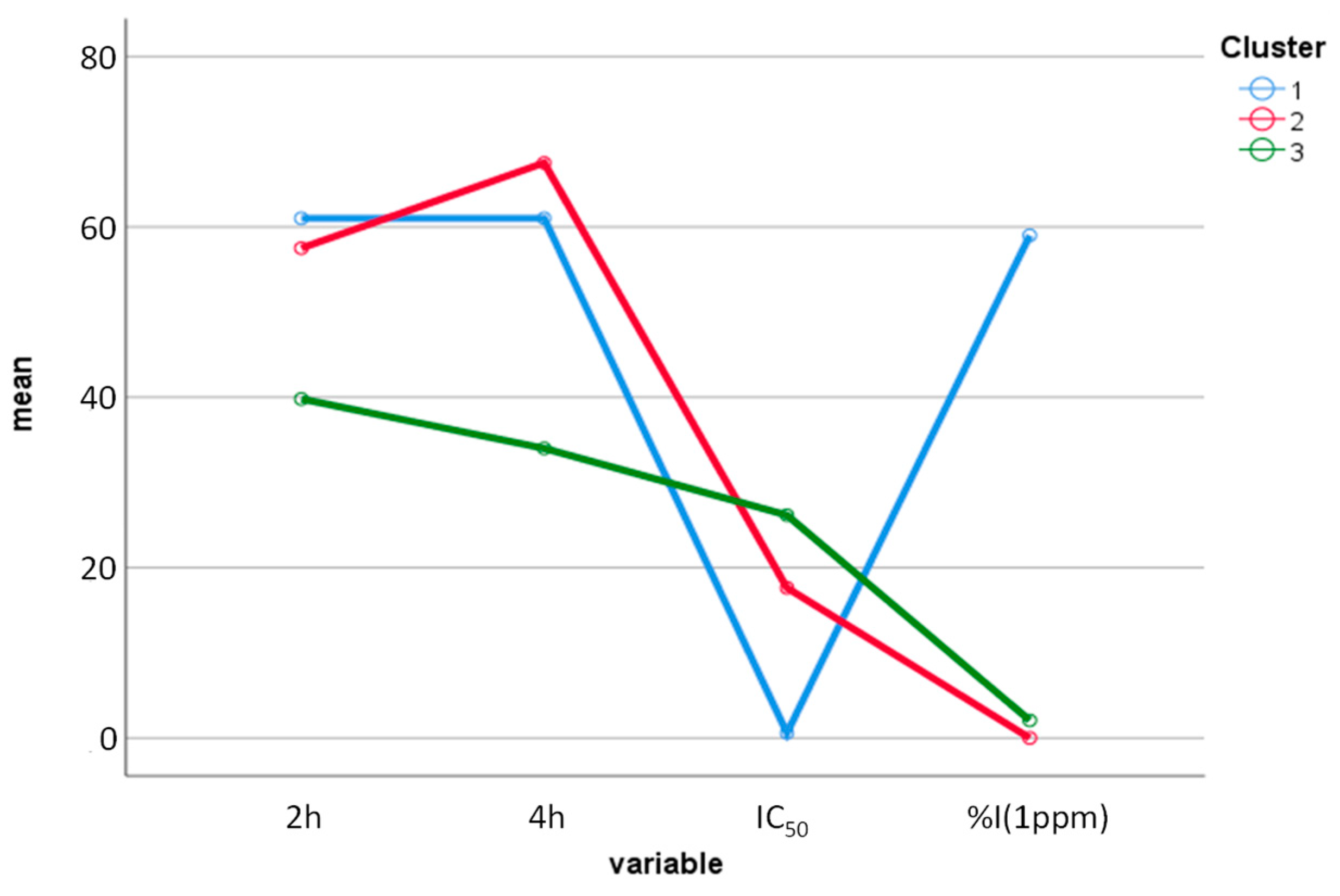
2.5. Multivariate Statistical Analysis Applied to the Results of Biological Tests of EOs
3. Discussion
4. Materials and Methods
4.1. Reagents and Standards
4.2. Plant Materials
4.3. Isolation of Essential Oils and GC-MS Analysis
4.4. NMR Analysis
4.5. Collection and Breeding of Maize Weevils
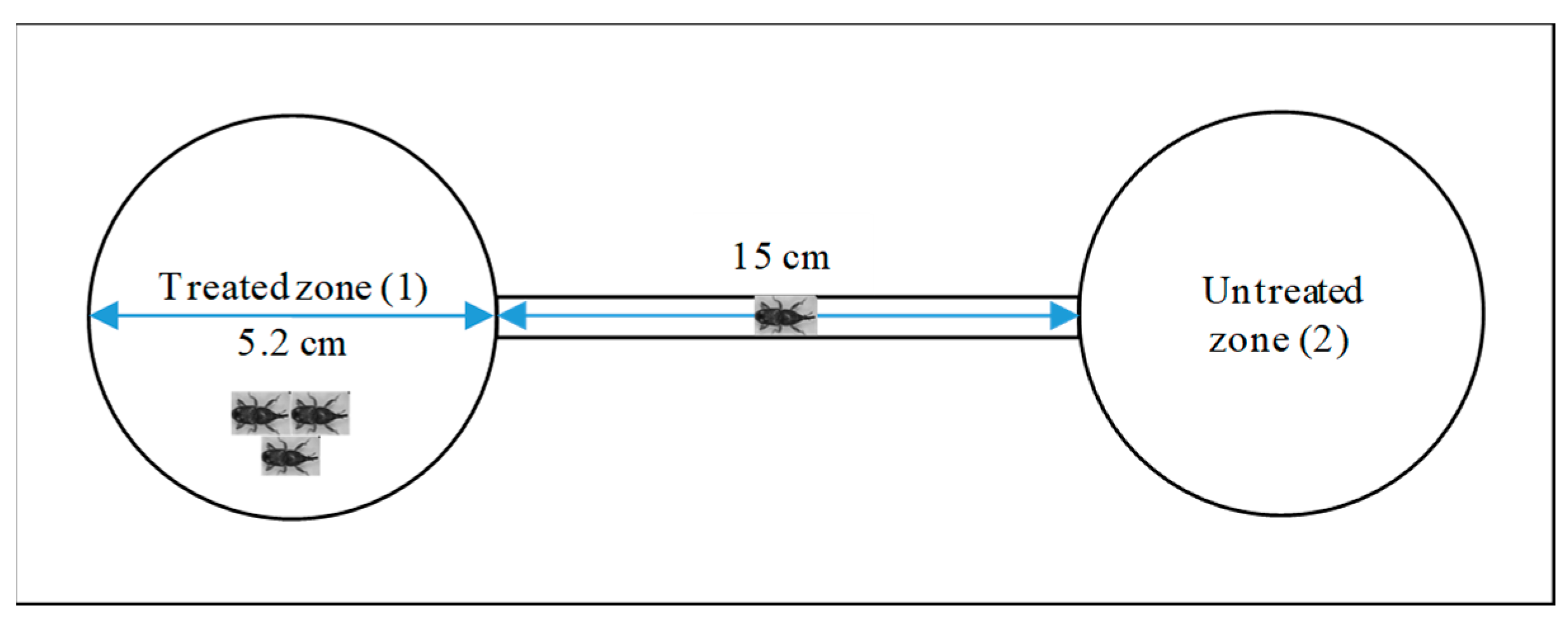
4.6. Implementation of Repellency Test
4.7. Acetylcholinesterase Inhibition Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serratos Hernández, J.A. El Origen y la Diversidad del Maíz en el Continente Americano; Greenpeace—UNAM: Ciudad de México, México, 2009. [Google Scholar]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Mejía, D. Maize Post-Harvest Operation. INPho—Post-Harvest Compendium; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Klopfenstein, T.J.; Erickson, G.E.; Berger, L.L. Maize is a critically important source of food, feed, energy, and forage in the USA. Field Crops Res. 2013, 153, 5–11. [Google Scholar] [CrossRef]
- Revilla, P.; Alves, M.L.; Andelković, V.; Balconi, C.; Dinis, I.; Mendes-Moreira, P.; Redaelli, R.; Ruiz de Galarreta, J.I.; Vaz Patto, M.C.; Žilić, S.; et al. Traditional foods from maize (Zea mays L.) in Europe. Front. Nutr. 2022, 7, 683399. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A.; McCulley, L.; Roh, R.; Lopez-Ridaura, S.; Palacios-Rojas, N.; Gunaratna, N.S. Maize agro-food systems to ensure food and nutrition security in reference to the sustainable development goals. Glob. Food Secur. 2020, 25, 100327. [Google Scholar] [CrossRef]
- Erenstein, O. The evolving maize sector in Asia: Challenges and opportunities. J. New Seeds 2010, 11, 1–15. [Google Scholar] [CrossRef]
- Grote, U.; Fasse, A.; Nguyen, T.T.; Erenstein, O. Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front. Sustain. Food Syst. 2021, 4, 617009. [Google Scholar] [CrossRef]
- Poole, N.; Donovan, J.; Erenstein, O. Agri-nutrition research: Revisiting the contribution of maize and wheat to human nutrition and health. Food Policy 2021, 100, 101976. [Google Scholar] [CrossRef] [PubMed]
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J.; Haddad, L.; Schneider, K.R.; Béné, C.; Covic, N.M.; Guarin, A.; Herforth, A.W.; Herrero, M.; Sumaila, U.R.; Aburto, N.J.; et al. Viewpoint: Rigorous monitoring is necessary to guide food system transformation in the countdown to the 2030 global goals. Food Policy 2021, 104, 102163. [Google Scholar] [CrossRef]
- Guzzon, F.; Arandia Rios, L.W.; Caviedes Cepeda, G.M.; Céspedes Polo, M.; Chavez Cabrera, A.; Muriel Figueroa, J.; Medina Hoyos, A.E.; Jara Calvo, T.W.; Molnar, T.L.; Narro León, L.A.; et al. Conservation and use of Latin American maize diversity: Pillar of nutrition security and cultural heritage of humanity. Agronomy 2021, 11, 172. [Google Scholar] [CrossRef]
- Carvajal-Larenas, F.E.; Caviedes Cepeda, G.M. Análisis comparativo de la eficiencia productiva del maíz en Ecuador, Sudamérica y el mundo en las dos últimas décadas y análisis prospectivo en el corto plazo. Av. Cienc. Ing. 2019, 11, 94–103. [Google Scholar]
- Kato, T.A.; Mapes, C.; Mera, L.M.; Serratos, J.A.; Bye, R.A. Origen y Diversificación del Maíz: Una Revisión Analítica; Universidad Nacional Autónoma de México, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Mexico City, Mexico, 2009. [Google Scholar]
- Federación Nacional de Cultivadores de Cereales y Leguminosas—FENALCE. Available online: http://www.agroinsumossa.com/cultivo-del-maiz-en-colombia/ (accessed on 25 February 2023).
- FENALCE—Federación Nacional de Cultivadores de Cereales y Leguminosas. Indicadores cerealistas; Departamento Económico y Apoyo a la Comercialización: Cundinamarca, Colombia, 2022; 79p. [Google Scholar]
- CIMMYT; CIAT. Maíz para Colombia. Visión para el 2030; CIMMYT: Texcoco, México, 2019; 110p. [Google Scholar]
- Paliwal, R.L.; Granados, G.; Lafitte, H.R.; Violic, A.D.; Marathée, J.P. El Maíz en los Trópicos: Mejoramiento y Producción; Organización de las Naciones Unidas para la Agricultura y la Alimentación: Rome, Italy, 2001; 392p. [Google Scholar]
- Salvadores, Y.; Silva, G.; Tapia, M.; Hepp, R. Spices powders for the control of maize weevil, Sitophilus zeamais Motschulsky, in stored wheat. Agric. Tec. 2007, 67, 147–154. [Google Scholar]
- Rees, D.P. Coleoptera. In Integrated Management of Insects in Stored Products; Subramanyan, B., Hagstrum, D.W., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 1–39. [Google Scholar]
- Subramanyan, B.; Hagstrum, D.W. Integrated Management of Insects in Stored Products; Marcel Dekker: New York, NY, USA, 1996; pp. 195–330; 399–408. [Google Scholar]
- Wiesner, J.; Kříž, Z.; Kuča, K.; Jun, D.; Koča, J. Acetylcholinesterases—The structural similarities and differences. J. Enzym. Inhib. Med. Chem. 2007, 22, 417–424. [Google Scholar] [CrossRef]
- Giesy, J.P.; Solomon, K.R.; Mackay, D.; Anderson, J. Evaluation of evidence that the organophosphorus insecticide chlorpyrifos is a potential persistent organic pollutant (POP) or persistent, bioaccumulative, and toxic (PBT). Environ. Sci. Eur. 2014, 26, 29. [Google Scholar] [CrossRef]
- Christensen, K.; Harper, B.; Luukinen, B.; Buhl, K.; Stone, D. Chlorpyrifos General Fact Sheet. National Pesticide Information Center, Oregon State University Extension Services. 2009. Available online: http://npic.orst.edu/factsheets/chlorpgen.html (accessed on 30 November 2023).
- Talukder, F. Pesticide resistance in stored-product insects and alternative biorational management: A brief review. J. Agric. Mar. Sci. 2009, 14, 9–15. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health. 2021, 18, 1112. [Google Scholar] [CrossRef]
- Crawford, S.E.; Hartung, T.; Hollert, H.; Mathes, B.; van Ravenzwaay, B.; Steger-Hartmann, T.; Studer, C.; Krug, H.F. Green toxicology: A strategy for sustainable chemical and material development. Environ. Sci. Eur. 2017, 29, 16. [Google Scholar] [CrossRef]
- Unsworth, J. Biopesticides. Agrochemicals. IUPAC—International Union Pure Applied Chemistry. 2010. Available online: http://agrochemicals.iupac.org/index.php?option=com_sobi2&sobi2Task=sobi2Details&catid=3&sobi2Id=7&Itemid=19 (accessed on 25 February 2023).
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trend Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of utilization vegetal extracts as natural plant protection products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticides in insect-pest management. J. Pharmacol. Phytother. 2009, 1, 52–63. [Google Scholar]
- López, M.D.; Pascual-Villalobos, M.J. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Kan, Y.; Sener, B. Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z. Für Naturforschung C J. Biosci. 2008, 63, 547–553. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2018, 23, 34. [Google Scholar] [CrossRef]
- Rangel-Ch, J.O. La biodiversidad de Colombia: Significado y distribución regional. Rev. Acad. Colomb. Cienc. Ex. Fis. Nat. 2015, 39, 176–200. [Google Scholar] [CrossRef]
- Devia, C.A.; Moncaleano, A.M.; Niño, L.M. Flora del Bosque Seco de los Archipiélagos Islas del Rosario y San Bernardo; Alpha, Ed.; Incoder—Universidad Jorge Tadeo Lozano: Cartagena, Colombia, 2014; 99p. [Google Scholar]
- López, C.R.; Sarmiento, C.; Espitia, L.; Barrero, A.M.; Consuegra, C.; Gallego Castillo, B. 100 Plantas del Caribe Colombiano. Usar Para Conservar: Aprendiendo de los Habitantes del Bosque Seco; Panamericana: Bogotá, Colombia, 2016; 240p. [Google Scholar]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- Cicció-Alberti, J.F.; Ballestero, C.M. Constituyentes volátiles de las hojas y espigas de Piper aduncum (Piperaceae) de Costa Rica. Rev. Biol. Trop. 1997, 45, 783–790. [Google Scholar]
- Rojas-Martínez, R.; Arrieta, J.; Cruz-Antonio, L.; Arrieta-Baez, D.; Velázquez-Méndez, A.M.; Sánchez-Mendoza, M.E. Dillapiole, isolated from Peperomia pellucida, shows gastroprotector activity against ethanol-induced gastric lesions in wistar rats. Molecules 2013, 18, 11327. [Google Scholar] [CrossRef]
- Pineda, R.; Vizcaíno, S.; García, C.M.; Gil, J.H.; Durango, D.L. Chemical composition and antifungal activity of Piper auritum Kunth and Piper holtonii C. DC. against phytopathogenic fungi. Chil. J. Agric. Res. 2012, 72, 507–515. [Google Scholar] [CrossRef]
- de Lira, P.N.B.; da Silva, J.K.R.; Andrade, E.H.A.; Sousa, P.J.C.; Silva, N.N.S.; Maia, J.G.S. Essential oil composition of three Peperomia species from the Amazon, Brazil. Nat. Prod. Commun. 2009, 4, 427–430. [Google Scholar] [PubMed]
- da Silva, M.H.L.; Zoghbi, M.G.B.; Andrade, E.H.A.; Maia, J.G.S. The essential oils of Peperomia pellucida Kunth and P. circinnata Link var. circinnata. Flavour Fragr. J. 1999, 14, 312–314. [Google Scholar] [CrossRef]
- Moreira, D.L.; de Souza, P.O.; Kaplan, M.A.C.; Guimarães, E.F. Essential oil analysis of four Peperomia species (Piperaceae). Acta Hortict. 1999, 500, 65–70. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Chauhan, A. Essential oil composition of Peperomia pellucida (L.) Kunth from India. J. Essent. Oil Res. 2014, 27, 89–95. [Google Scholar] [CrossRef]
- Santana, A.I.; Vila, R.; Cañigueral, S.; Gupta, M.P. Chemical composition and biological activity of essential oils from different species of Piper from Panama. Planta Med. 2016, 82, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Luz, A.I.R.; Zoghbi, M.G.B.; Maia, J.G.S. The essential oils of Piper reticulatum L. and P. crassinervium H.B.K. Acta Amaz. 2003, 33, 341–344. [Google Scholar] [CrossRef]
- Ruiz-Vásquez, L.; Ruiz Mesia, L.; Caballero Ceferino, H.D.; Ruiz Mesia, W.; Andrés, M.F.; Díaz, C.E.; Gonzalez-Coloma, A. Antifungal and herbicidal potential of Piper essential oils from the Peruvian Amazonia. Plants 2022, 11, 1793. [Google Scholar] [CrossRef]
- Carmona, R.; Quijano-Celís, C.E.; Pino, J.A. Leaf oil composition of Bursera graveolens (Kunth) Triana et Planch. J. Essent. Oil Res. 2009, 21, 387–389. [Google Scholar] [CrossRef]
- Monzote, L.; Hill, G.M.; Cuellar, A.; Scull, R.; Setzer, W.N. Chemical composition and anti-proliferative properties of Bursera graveolens essential oil. Nat. Prod. Commun. 2012, 7, 1531–1534. [Google Scholar] [CrossRef]
- Luján-Hidalgo, M.C.; Gutiérrez-Miceli, F.A.; Ventura-Canseco, L.M.C.; Dendooven, L.; Mendoza-López, M.R.; Cruz-Sánchez, R.; García-Barradas, O.; Abud-Archila, M. Composición química y actividad antimicrobiana de los aceites esenciales de hojas de Bursera graveolens y Taxodium mucronatum de Chiapas, México. Gayana Botánica 2012, 69, 7–14. [Google Scholar]
- Young, D.G.; Chao, S.; Casablanca, H.; Bertrand, M.-C.; Minga, D. Essential oil of Bursera graveolens (Kunth) Triana et Planch from Ecuador. J. Essent. Oil Res. 2007, 19, 525–526. [Google Scholar] [CrossRef]
- Fon-Fay, F.M.; Pino, J.A.; Hernández, I.; Rodeiro, I.; Fernández, M.D. Chemical composition and antioxidant activity of Bursera graveolens (Kunth) Triana et Planch essential oil from Manabí, Ecuador. J. Essent. Oil Res. 2019, 31, 211–216. [Google Scholar] [CrossRef]
- Manzano Santana, P.; Miranda, M.; Gutiérrez, Y.; García, G.; Orellana, T.; Orellana, A. Efecto antiinflamatorio y composición química del aceite de ramas de Bursera graveolens Triana & Planch. (palo santo) de Ecuador. Rev. Cuba. Plantas Med. 2009, 14, 45–53. [Google Scholar]
- Noel-Martinez, K.C.; Cruz, G.J.F.; Solis-Castro, R.L. Bursera graveolens essential oil: Physiochemical characterization and antimicrobial activity in pathogenic microorganisms found in Kajikia audax. Sci. Agropecu. 2021, 12, 303–309. [Google Scholar] [CrossRef]
- Sotelo-Méndez, A.H.; Figueroa Cornejo, C.G.; Césare Coral, M.F.; Alegría Arnedo, M.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Bursera graveolens (Burseraceae) from Perú. Indian J. Pharm. Educ. Res. 2017, 51, S429–S436. [Google Scholar] [CrossRef]
- Laurintino, T.N.S.; Tramontin, D.P.; Assreuy, J.; Cruz, A.B.; Cruz, C.C.B.; Marangoni, A.; Arauco Livia, M.; Bolzan, A. Evaluation of the biological activity and chemical profile of supercritical and subcritical extracts of Bursera graveolens from northern Peru. J. Supercrit. Fluids 2023, 198, 105934. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Suarez-López, S.; Marrugo-Santander, V. Volatile chemical composition of essential oil from Bursera graveolens (Kunth) Triana & Planch and their fumigant and repellent activities. Acta Sci. Biol. Sci. 2019, 41, 46822. [Google Scholar]
- Leyva, M.A.; Martínez, J.R.; Stashenko, E.E. Composición química del aceite esencial de hojas y tallos de Bursera graveolens (Burseraceae) de Colombia. Sci. Tech. 2007, 1, 201–202. [Google Scholar]
- Junor, G.A.O.; Porter, R.B.R.; Yee, T.H. The chemical composition of the essential oils from the leaves, bark, and fruits of Bursera simaruba (L. ) Sarg. from Jamaica. J. Essent. Oil Res. 2008, 20, 426–429. [Google Scholar] [CrossRef]
- Setzer, W.N. Leaf and bark essential oil compositions of Bursera simaruba from Monteverde, Costa Rica. American J. Essent. Oil Nat. Prod. 2014, 1, 34–36. [Google Scholar]
- Sylvestre, M.; Longtin, A.P.A.; Legault, J. Volatile leaf constituents and anticancer activity of Bursera simaruba (L.) Sarg. essential oil. Nat. Prod. Commun. 2007, 12, 1273–1276. [Google Scholar] [CrossRef]
- de Mohali, E.M.; Padilla-Baretic, A.; Rojas-Fermín, L. Aceite esencial extraído por hidrodestilación del tejido xilemático de ramas de Bursera simaruba (L.) Sarg. Rev. For. Latinoam. 2013, 28, 27–36. [Google Scholar]
- Abouelatta, A.M.; Keratum, A.Y.; Ahmed, S.I.; El-Zun, H.M. Repellent, contact and fumigant activities of geranium (Pelargonium graveolens L.’Hér) essential oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.). Int. J. Trop. Insect Sci. 2020, 40, 1021–1030. [Google Scholar] [CrossRef]
- Sahu, U.; Ibrahim, S.S.; Ezhil Vendan, S. Persistence and ingestion characteristics of phytochemical volatiles as bio-fumigants in Sitophilus oryzae adults. Ecotoxicol. Environ. Saf. 2021, 210, 111877. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, M.; Shin, E.; Kim, J.; Lee, S.H.; Park, C.G. Fumigant and contact toxicity of 22 wooden essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Pestic. Biochem. Physiol. 2016, 133, 35–43. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.A.; da Silva, L.; Macêdo, M.J.F.; Lacerda-Neto, L.J.; dos Santos, M.A.C.; Coutinho, H.D.M.; Cunha, F.A.B. Adulticide and repellent activity of essential oils against Aedes aegypti (Diptera: Culicidae)—A review. S. Afr. J. Bot. 2019, 124, 160–165. [Google Scholar] [CrossRef]
- Lima, B.; López, S.; Luna, L.; Agüero, M.B.; Aragón, L.; Tapia, A.; Zacchino, S.; López, M.L.; Zygadlo, J.; Feresin, G.E. Essential oils of medicinal plants from the Central Andes of Argentina: Chemical composition, and antifungal, antibacterial, and insect-repellent activities. Chem. Biodivers. 2011, 8, 924–936. [Google Scholar] [CrossRef]
- Islam, R.; Islam Khan, R.; Al-Reza, S.M.; Jeong, Y.T.; Song, C.H.; Khalequzzaman, M. Chemical composition and insecticidal properties of Cinnamomum aromaticum (Nees) essential oil against the stored product beetle Callosobruchus maculatus (F.). J. Sci. Food Agric. 2009, 89, 1241–1246. [Google Scholar] [CrossRef]
- Kim, S.-I.; Park, C.; Ohh, M.-H.; Cho, H.-C.; Ahn, Y.-J. Contact and fumigant activities of aromatic plant extracts and essential oils against Lasioderma serricorne (Coleoptera: Anobiidae). J. Stored Prod. Res. 2003, 39, 11–19. [Google Scholar] [CrossRef]
- Bett, P.K.; Deng, A.L.; Ogendo, J.O.; Kariuki, S.T.; Kamatenesi-Mugisha, M.; Mihalee, J.M.; Torto, B. Residual contact toxicity and repellence of Cupressus lusitanica Miller and Eucalyptus saligna Smith essential oils against major stored product insect pests. Ind. Crops Prod. 2017, 110, 65–74. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Yang, Y.-Y.; An, Y.; Shao, Y.-Z.; He, C.-Y.; Zhang, J.; Jia, L.-Y. Insecticidal and acetylcholine esterase inhibition activity of Rhododendron thymifolium essential oil and its main constituent against two stored product insects. J. Environ. Sci. Health Part B 2021, 42, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.-P.; Han, J.-X.; Li, X.-C.; Li, Y.-H.; Zhang, Y.; Chen, L.; Qu, Y.; Hao, C.-Y.; Li, H.-Z.; Yang, C.-R.; et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from Piper species. J. Agric. Food Chem. 2017, 65, 3702–3710. [Google Scholar] [CrossRef]
- Farag, M.A.; Ezzat, S.M.; Salama, M.M.; Tadros, M.G.; Serya, R.A.T. Anti-acetylcholinesterase activity of essential oils and their major constituents from four Ocimum species. Z. Naturforschung 2016, 71, 393–402. [Google Scholar] [CrossRef]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2015, 2, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Nakahashi, H.; Usami, A.; Matsuda, N. Chemical composition, aroma evaluation, and inhibitory activity towards acetylcholinesterase of essential oils from Gynura bicolor DC. J. Nat. Med. 2016, 70, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Nerio, L.S.; Stashenko, E.E. Bioactivity against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae) of Cymbopogon citratus and Eucalyptus citriodora essential oils grown in Colombia. Pest Manag. Sci. 2010, 66, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef]
- Ngassoum, M.B.; Tignkeu, L.S.N.; Ngatanko, I.; Tapondjou, L.A.; Lognay, G.; Malaisse, F.; Hance, T. Chemical composition, insecticidal effect and repellent activity of essential oils of three aromatic plants, alone and in combination, towards Sitophilus oryzae L. (Coleoptera: Curculionidae). Nat. Prod. Commun. 2007, 2, 1229–1232. [Google Scholar] [CrossRef]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef]
- Gvozdenac, S.; Kiprovski, B.; Aćimović, M.; Jeremić, J.S.; Cvetković, M.; Bursić, V.; Ovuka, J. Repellent activity of Cymbopogon citratus essential oil against four major stored product pests: Plodia interpunctella, Sitophilus oryzae, Acanthoscelides obtectus and Tribolium castaneum. Contemp. Agric. 2021, 70, 140–148. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Rolim, G.D.S.; Wilcken, C.F.; Zanuncio, J.C.; Serrão, J.E.; Martínez, L.C. Acute toxicity and sublethal effects of lemongrass essential oil and their components against the granary weevil, Sitophilus granarius. Insects 2020, 11, 379. [Google Scholar] [CrossRef]
- Wang, K.; Tang, L.; Zhang, N.; Zhou, Y.; Li, W.; Li, H.; Cheng, D.; Zhang, Z. Repellent and fumigant activities of Eucalyptus globulus and Artemisia carvifolia essential oils against Solenopsis invicta. Bull. Insectology 2014, 67, 207–211. [Google Scholar]
- Abdelgaleil, S.A.M.; Mohamed, M.I.E.; Badawy, M.E.I.; El-arami, S.A.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M.N.; Mejia, D.; Lewis, B. Insect Damage: Post-Harvest Operations; INPhO—Post-Harvest Compendium; AGSI/FAO: Rome, Italy, 2013; 37p. [Google Scholar]
- Chaubey, M.K. Acute, lethal, and synergistic effects of some terpenes against Tribolium castaneum Herbst (Coleoptera: Tenebrionidae). Ecol. Balk. 2012, 4, 53–62. [Google Scholar]
- Kim, S.-I.; Yoon, J.-S.; Jung, J.W.; Hong, K.-B.; Ahn, Y.-J.; Kwon, H.W. Toxicity and repellency of Origanum essential oil and its components against Tribolium castaneum (Coleoptera: Tenebrionidae) adults. J. Asia-Pac. Entomol. 2010, 13, 369–373. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Zhang, D.; Guo, S.-S.; Pang, X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Comparative evaluation of the chemical composition and bioactivities of essential oils from four spice plants (Lauraceae) against stored-product insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- Zaio, Y.P.; Gatti, G.; Ponce, A.A.; Saavedra Larralde, N.A.; Martinez, M.J.; Zunino, M.P.; Zygadlo, J.A. Cinnamaldehyde and related phenylpropanoids, natural repellents, and insecticides against Sitophilus zeamais (Motsch.). A chemical structure-bioactivity relationship. J. Sci. Food Agric. 2018, 98, 5822–5831. [Google Scholar] [CrossRef] [PubMed]
- Hematpoor, A.; Liew, S.Y.; Azirun, M.S.; Awang, K. Insecticidal activity and the mechanism of action of three phenylpropanoids isolated from the roots of Piper sarmentosum Rox. Sci. Rep. 2017, 7, 12576. [Google Scholar] [CrossRef]
- Bullangpoti, V. Essential oils and synthetic pesticides. In Green Pesticides Handbook—Essential Oils for Pest Control; Nollet, L.M.L., Rathore, H.S., Eds.; CRC Press: London, UK, 2017; pp. 441–478. [Google Scholar]
- Fernández-Ruiz, M.; Yepes-Fuentes, L.; Tirado-Ballestas, I.; Orozco, M. Actividad repelente del aceite esencial de Bursera graveolens Jacq. ex L., frente Tribolium castaneum Herbst, 1797 (Coleoptera: Tenebrionidae. Anal. Biol. 2018, 40, 87–93. [Google Scholar] [CrossRef]
- Patiño-Bayona, W.R.; Nagles Galeano, L.J.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Herrera Daza, E.; Cuca Suárez, L.E.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Effects of essential oils from 24 plant species on Sitophilus zeamais Motsch (Coleoptera, Curculionidae). Insects 2021, 12, 532. [Google Scholar] [CrossRef]
- Oviedo-Sarmiento, J.S.; Bustos Cortes, J.J.; Delgado Ávila, W.A.; Cuca Suárez, L.E.; Herrera Daza, E.; Patiño-Ladino, O.J.; Prieto-Rodríguez, J.A. Fumigant toxicity and biochemical effects of selected essential oils toward the red flour beetle, Tribolium castaneum (Coleoptera: Tenebrionidae). Pestic. Biochem. Physiol. 2021, 179, 104941. [Google Scholar] [CrossRef]
- Jaramillo-Colorado, B.E.; Duarte-Restrepo, E.; Pino-Benítez, N. Evaluación de la actividad repelente de aceites esenciales de plantas Piperáceas del departamento de Chocó, Colombia. Rev. Toxicol. 2015, 32, 112–116. [Google Scholar]
- Jaramillo-Colorado, B.E.; Palacio-Herrera, F.M.; Pino-Benitez, C.N. Volatile chemical composition of Colombian Piper gorgonillense Trel. & Yunck. essential oil and its repellent and fumigant activity against Tribolium castaneum Herbst. Rev. Colomb. Cienc. Hortícolas 2020, 14, 424–433. [Google Scholar]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stores Prod. Res. 2009, 45, 212–214. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Caballero-Gallardo, K.; Jaramillo-Colorado, B.; Stashenko, E. Actividad repelente de los aceites esenciales de Lippia origanoides, Citrus sinensis y Cymbopogon nardus cultivadas en Colombia frente a Tribolium castaneum, Herbst. Rev. Salud UIS 2009, 41, 244–250. [Google Scholar]
- Caballero, K.; Olivero-Verbel, J.; Stashenko, E. Repellent activity of essential oils and some of their constituents against Tribolium castaneum Herbst. J. Agric. Food Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef]
- Knaden, M.; Strutz, A.; Ahsan, J.; Sachse, S.; Hansson, B.S. Spatial representation of odorant valence in an insect brain. Cell Rep. 2012, 1, 392–399. [Google Scholar] [CrossRef]
- Cao, J.; Pang, X.; Guo, S.; Wang, Y.; Geng, Z.; Sang, Y.; Du, S. Pinene-rich essential oils from Haplophyllum dauricum (L.) G. Don display anti-insect activity on two stored-product insects. Int. Biodet. Biodegr. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Colares, H.C.; Campos, J.M.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Toxic effects of two essential oils and their constituents on the mealworm beetle, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 716–725. [Google Scholar] [CrossRef]
- Luo, C.; Li, D.-L.; Wang, Y.; Guo, S.-S.; Du, S.S. Bioactivities of 3-butylidenephthalide and n-butylbenzene from the essential oil of Ligusticum jeholense against stored-product insects. J. Oleo Sci. 2019, 68, 931–937. [Google Scholar] [CrossRef]
- Spencer, W.F.; Farmer, W.J.; Cliath, M.M. Pesticide volatilization. In Residue Reviews; Gunther, F.A., Ed.; Springer: New York, NY, USA, 1973; Volume 49, pp. 1–47. [Google Scholar]
- Sawicki, R.M.; Denholm, I. Adaptation of insects to insecticides. In Origins and Development of Adaptation; Evered, D., Collins, G.M., Eds.; Ciba Foundation: London, UK, 1984; Volume 102, pp. 152–166. [Google Scholar]
- Fazolin, M.; Bizzo, H.R.; Monteiro, A.F.M.; Lima, M.E.C.; Maisforte, N.S.; Gama, P.E. Synergism in two-component insecticides with dillapiole against fall armyworm. Plants 2023, 12, 3042. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.S.; Maheshwari, M.L.; Mukerjee, S.K. Synthesis and synergistic activity of dillapiole based pyrethrum synergists. J. Agric. Food Chem. 1979, 27, 547–550. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, J.; Choi, I.; Chang, Y.; Yoon, C.S.; Han, J. Isolation, screening and identification of key components having intense insect repellent activity against Plodia interpunctella from four different medicinal plant materials. J. Sci. Food Agric. 2022, 102, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.L.V.; Fazolin, M.; Catani, V.; Alécio, M.R.; de Lima, M.S. Toxicidade de óleos essenciais de Piper aduncum e Piper hispidinervum em Sitophilus zeamais. Pesqui. Agropecu. Bras. 2006, 41, 217–222. [Google Scholar] [CrossRef]
- Fazolin, M.; Monteiro, A.F.M.; Bizzo, H.R.; Gama, P.E.; Viana, L.O.; de Lima, M.E.C. Insecticidal activity of Piper aduncum oil: Variation in dillapiole content and chemical and toxicological stability during storage. Acta Amaz. 2022, 52, 179–188. [Google Scholar] [CrossRef]
- Ali, A.; Radwan, M.M.; Wanas, A.S.; Khan, I.A. Repellent activity of carrot seed essential oil and its pure compound, carotol, against mosquitoes. J. Am. Mosq. Control Assoc. 2018, 34, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Drenaggi, E.; Desneux, N.; Maggi, F. Phytol, (E)-nerolidol and spathulenol from Stevia rebaudiana leaf essential oil as effective and eco-friendly botanical insecticides against Metopolophium dirhodum. Ind. Crops Prod. 2020, 155, 112844. [Google Scholar] [CrossRef]
- Liu, J.; Hua, J.; Qu, B.; Guo, X.; Wang, Y.; Shao, M.; Luo, S. Insecticidal terpenes from the essential oils of Artemisia nakaii and their inhibitory effects on acetylcholinesterase. Front. Plant Sci. 2021, 12, 720816. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Espinoza, J.; Urzúa, A.; Bardehle, L.; Quiroz, A.; Echeverría, J.; González-Teuber, M. Antifeedant effects of essential oil, extracts, and isolated sesquiterpenes from Pilgerodendron uviferum (D. Don) Florin heartwood on red clover borer Hylastinus obscurus (Coleoptera: Curculionidae). Molecules 2018, 23, 1282. [Google Scholar] [CrossRef] [PubMed]
- Karr, L.L.; Coats, J.R. Insecticidal properties of d-limonene. J. Pestic. Sci. 1988, 13, 287–290. [Google Scholar] [CrossRef]
- Malacrinò, A.; Campolo, O.; Laudani, F.; Palmeri, V. Fumigant and repellent activity of limonene enantiomers against Tribolium confusum du Val. Neotrop. Entomol. 2016, 45, 597–603. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Guo, S.-S.; Zhang, W.-J.; Geng, Z.-F.; Deng, Z.-W.; Du, S.-S.; Zhang, J. Fumigant and repellent activities of essential oil extracted from Artemisia dubia and its main compounds against two stored product pests. Nat. Prod. Res. 2018, 32, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Feng, Y.-X.; Qi, X.-J.; Xi, C.; Du, S.-S. Acute toxicity and repellent activity of essential oil from Atalantia guillauminii Swingle fruits and its main monoterpenes against two stored product insects. Int. J. Food Prop. 2021, 24, 304–315. [Google Scholar] [CrossRef]
- Rosa, J.S.; Oliveira, L.; Sousa, R.M.O.F.; Escobar, C.B.; Fernandes-Ferreira, M. Bioactivity of some Apiaceae essential oils and their constituents against Sitophilus zeamais (Coleoptera: Curculionidae). Bull. Entomol. Res. 2020, 110, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Peterson, C.J.; Coats, J.R. Fumigation toxicity of monoterpenoids to several stored product insects. J. Stored Prod. Res. 2003, 39, 77–85. [Google Scholar] [CrossRef]
- Jumbo, L.O.V.; Corrêa, M.J.M.; Gomes, J.M.; González Armijos, M.J.; Valarezo, E.; Mantilla-Afanador, J.G.; Machado, F.P.; Rocha, L.; Aguiar, R.W.S.; Oliveira, E.E. Potential of Bursera graveolens essential oil for controlling bean weevil infestations: Toxicity, repellence, and action targets. Ind. Crops Prot. 2022, 178, 114611. [Google Scholar] [CrossRef]
- Humbert, M.; Lavoine-Hanneguelle, S. Extract of Euodia suaveolens Scheff, Repellent Compositions and Use Thereof. U.S. Patent 8481088 B2, 9 July 2013. [Google Scholar]
- Sousa, P.A.S.; Neto, J.; Bastos, M.M.S.M.; Aguiar, A.A.R.M. Eugenol and pulegone as potential biorational alternatives for Trioza erytreae (Hemiptera: Triozidae) control: Preliminary results on nymphal toxicity and applicability on Citrus limon. J. Nat. Pest. Res. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Scalerandi, E.; Flores, G.A.; Palacio, M.; Defagó, M.T.; Carpinella, M.C.; Valladares, G.; Bertoni, A.; Palacios, S.M. Understanding synergistic toxicity of terpenes as insecticides: Contribution of metabolic detoxification in Musca domestica. Front. Plant Sci. 2018, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.B.; Krishanmurty, H.G.; Chauret, D.; Durst, T.; Philogène, B.J.; Sánchez-Vindas, P.; Hasbun, C.; Poveda, L.; San Román, L.; Arnason, J.T. Insecticidal defenses of Piperaceae from the neotropics. J. Chem. Ecol. 1995, 21, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Ávila Murillo, M.C.; Cuca Suarez, L.E.; Cerón Salamanca, J.A. Chemical composition and insecticidal properties of essential oils of Piper septuplinervium and P. subtomentosum (Piperaceae). Nat. Prod. Commun. 2014, 9, 1527–1530. [Google Scholar] [CrossRef]
- Palacios, S.M.; Bertoni, A.; Rossi, Y.; Santander, R.; Urzúa, A. Efficacy of essential oils from edible plants as insecticides against the house fly, Musca domestica L. Molecules 2009, 14, 1938. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar]
- Eduarte-Saltos, R.; Bec, N.; Salinas-Rivera, M.; Ramírez-Robles, J.; Larroque, C.; Armijos-Riofrio, C. Composición química y actividad AChE-BuChE del aceite esencial de palo santo Bursera graveolens (Kunth) Triana & Planch de Jipijapa, Ecuador. Boletín Latinoam. Caribe Plantas Med. Aromáticas 2022, 21, 455–463. [Google Scholar]
- Seo, S.-M.; Jung, C.-S.; Kang, J.; Lee, H.-R.; Kim, S.-W.; Hyun, J.; Park, I.-K. Larvicidal and acetylcholinesterase inhibitory activities of Apiaceae plant essential oils and their constituents against Aedes albopictus and formulation development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef]
- Bettarini, F.; Borgonovi, G.E.; Fiorani, T.; Gagliardi, I.; Caprioli, V.; Massardo, P.; Ogoche, J.I.J.; Hassanali, A.; Nyandat, E.; Chapya, A. Antiparasitic compounds from East African plants: Isolation and biological activity of anonaine, matricarianol, canthin-6-one, and caryophyllene oxide. Insect Sci. Appl. 1993, 14, 93–99. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.C.; Dong, H.W.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and insecticidal activity of the essential oil of Illicium pachyphyllum fruits against two grain storage insects. Molecules 2012, 17, 14870. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts—Importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- El-Wakeil, N.E. Botanical pesticides and their mode of action. Gesunde Pflanz. 2013, 65, 125–149. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.H. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, V. Essential oils in stored product insect pest control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef]
- Muñoz-Acevedo, A.; González, M.C.; Rodríguez, J.D.; De Moya, Y.S. New chemovariety of Lippia alba from Colombia: Compositional analysis of the volatile secondary metabolites and some in vitro biological activities of the essential oil from plant leaves. Nat. Prod. Commun. 2019, 3, 563–566. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017; 804p. [Google Scholar]
- Joulian, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpenes Hydrocarbons; E.B.-Verlag: Hamburg, Germany, 1998; 658p. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20 M phases. J. Chromatogr. A. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg MD, 20899. Available online: http://webbook.nist.gov/chemistry/ (accessed on 1 November 2022).
- Throne, J.E. Life history of immature maize weevils (Coleoptera: Curculionidae) on corn stored at constant temperatures and relative humidities in the laboratory. Environ. Entomol. 1994, 23, 1459–1471. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Adler, C.; Fontem, D.A.; Bouda, H.; Reichmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]

| Constituents Ϯ | RI | Relative Amount, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal. | Lit. | 1bL** | 1aL | 1bL* | 2aL | 3bL | 4bL† | 4bL‡ | 5aL | 5aB | 6aB | 6aL | |
| β-Pinene | 969 | 970 | --- | --- | --- | --- | --- | 8.2 | --- | --- | --- | --- | --- |
| p-Cymene | 1010 | 1011 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 5.0 |
| Limonene | 1019 | 1020 | --- | --- | --- | --- | --- | --- | --- | 16.6 | --- | --- | --- |
| E-β-Ocimene | 1039 | 1036 | --- | --- | --- | --- | 4.0 | --- | --- | --- | --- | --- | --- |
| Linalool | 1081 | 1081 | --- | --- | --- | --- | --- | 6.9 | --- | --- | --- | --- | --- |
| Terpinen-4-ol | 1154 | 1160 | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- | 6.1 |
| trans-Carveol | 1196 | 1195 | --- | --- | --- | --- | --- | --- | --- | 5.4 | --- | --- | --- |
| Carvone | 1208 | 1213 | --- | --- | --- | --- | --- | --- | --- | 10.0 | 5.6 | --- | --- |
| Piperitone | 1218 | 1228 | --- | --- | --- | --- | 6.2 | --- | --- | --- | --- | --- | --- |
| Pulegone | 1223 | 1237 | --- | --- | --- | --- | --- | --- | --- | --- | 8.4 | --- | --- |
| Limonene-1,2-diol | 1310 | 1321 | --- | --- | --- | --- | --- | --- | --- | 7.7 | 6.2 | --- | --- |
| Mintlactone derivative | 1322 | ---- | --- | --- | --- | --- | --- | --- | --- | --- | 14.2 | --- | --- |
| β-Bourbonene | 1374 | 1386 | --- | --- | --- | --- | --- | --- | --- | 5.7 | --- | --- | --- |
| β-Elemene | 1380 | 1387 | --- | --- | --- | 4.7 | 7.9 | 8.6 | --- | --- | --- | --- | |
| β-Caryophyllene | 1407 | 1418 | --- | --- | --- | 7.3 | 4.8 | 6.7 | 4.4 | --- | --- | --- | --- |
| Mintlactone | 1461 | 1472 | --- | --- | --- | --- | --- | --- | --- | --- | 13.5 | --- | --- |
| Germacrene D | 1465 | 1470 | 5.2 | 5.2 | 3.7 | --- | --- | 7.8 | --- | --- | --- | --- | --- |
| β-Selinene | 1473 | 1483 | --- | --- | --- | --- | --- | --- | --- | --- | --- | 17.6 | --- |
| Bicyclogermacrene | 1490 | 1487 | 3.4 | 6.3 | 5.7 | --- | --- | --- | --- | --- | --- | --- | --- |
| Caryophyllene oxide | 1551 | 1558 | --- | --- | --- | --- | --- | --- | --- | --- | --- | 9.9 | 12.0 |
| Spathulenol | 1578 | 1577 | --- | --- | --- | --- | --- | --- | 4.3 | --- | --- | 24.3 | 10.9 |
| Carotol | 1579 | 1590 | --- | --- | --- | 43.7 | --- | --- | --- | --- | --- | --- | --- |
| Dillapiole | 1580 | 1593 | 78.5 | 64.4 | 80.9 | 20.9 | 48.2 | --- | --- | --- | --- | --- | --- |
| δ-Cadinol | 1633 | 1646 | --- | --- | --- | --- | --- | --- | 5.1 | --- | --- | --- | --- |
| α-Cadinol | 1638 | 1641 | --- | --- | --- | --- | --- | --- | 4.2 | --- | --- | --- | --- |
| Code | Sample Tested | Degree of Repellency (%) Ϯ | |
|---|---|---|---|
| 2 h | 4 h | ||
| C+ | Chlorpyrifos | 61 ± 8 | 61 ± 8 |
| ¥ 1bL** | P. holtonii | 45 ± 6 | 48 ± 5 |
| ¥ 1aL | P. holtonii | 37 ± 6 | 34 ± 6 |
| 1bL* | P. holtonii | 67 ± 12 | 73 ± 6 |
| 2aL | Pep. pellucida | 65 ± 6 | 70 ± 10 |
| ¥ 3bL | P. haugtii | 20 ± 0 | 32 ± 5 |
| ¥ 4bL† | P. reticulatum | 52 ± 5 | 28 ± 5 |
| ¥ 4bL‡ | P. reticulatum | 38 ± 5 | 67 ± 6 |
| 5aL | B. graveolens | 45 ± 6 | 68 ± 12 |
| 5aB | B. graveolens | 68 ± 8 | 53 ± 8 |
| 6aB | B. simaruba | 62 ± 5 | 74 ± 11 |
| ¥ 6aL | B. simaruba | 45 ± 6 | 28 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Acevedo, A.; González, M.C.; Alonso, J.E.; Flórez, K.C. The Repellent Capacity against Sitophilus zeamais (Coleoptera: Curculionidae) and In Vitro Inhibition of the Acetylcholinesterase Enzyme of 11 Essential Oils from Six Plants of the Caribbean Region of Colombia. Molecules 2024, 29, 1753. https://doi.org/10.3390/molecules29081753
Muñoz-Acevedo A, González MC, Alonso JE, Flórez KC. The Repellent Capacity against Sitophilus zeamais (Coleoptera: Curculionidae) and In Vitro Inhibition of the Acetylcholinesterase Enzyme of 11 Essential Oils from Six Plants of the Caribbean Region of Colombia. Molecules. 2024; 29(8):1753. https://doi.org/10.3390/molecules29081753
Chicago/Turabian StyleMuñoz-Acevedo, Amner, María C. González, Jesús E. Alonso, and Karen C. Flórez. 2024. "The Repellent Capacity against Sitophilus zeamais (Coleoptera: Curculionidae) and In Vitro Inhibition of the Acetylcholinesterase Enzyme of 11 Essential Oils from Six Plants of the Caribbean Region of Colombia" Molecules 29, no. 8: 1753. https://doi.org/10.3390/molecules29081753





