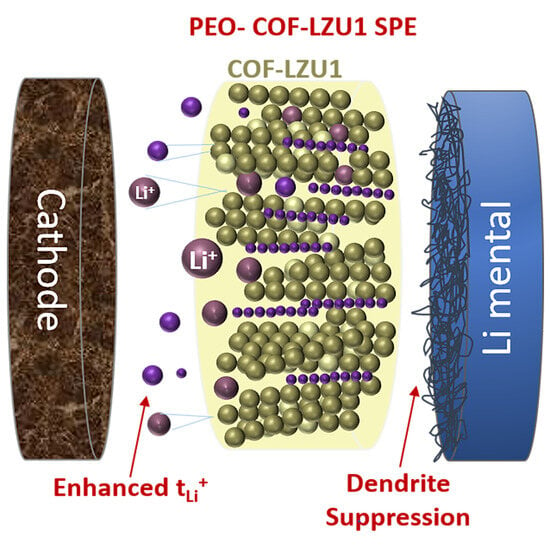
Covalent Organic Framework Enhanced Solid Polymer Electrolyte for Lithium Metal Batteries
Abstract
:1. Introduction
2. Results and Discussion
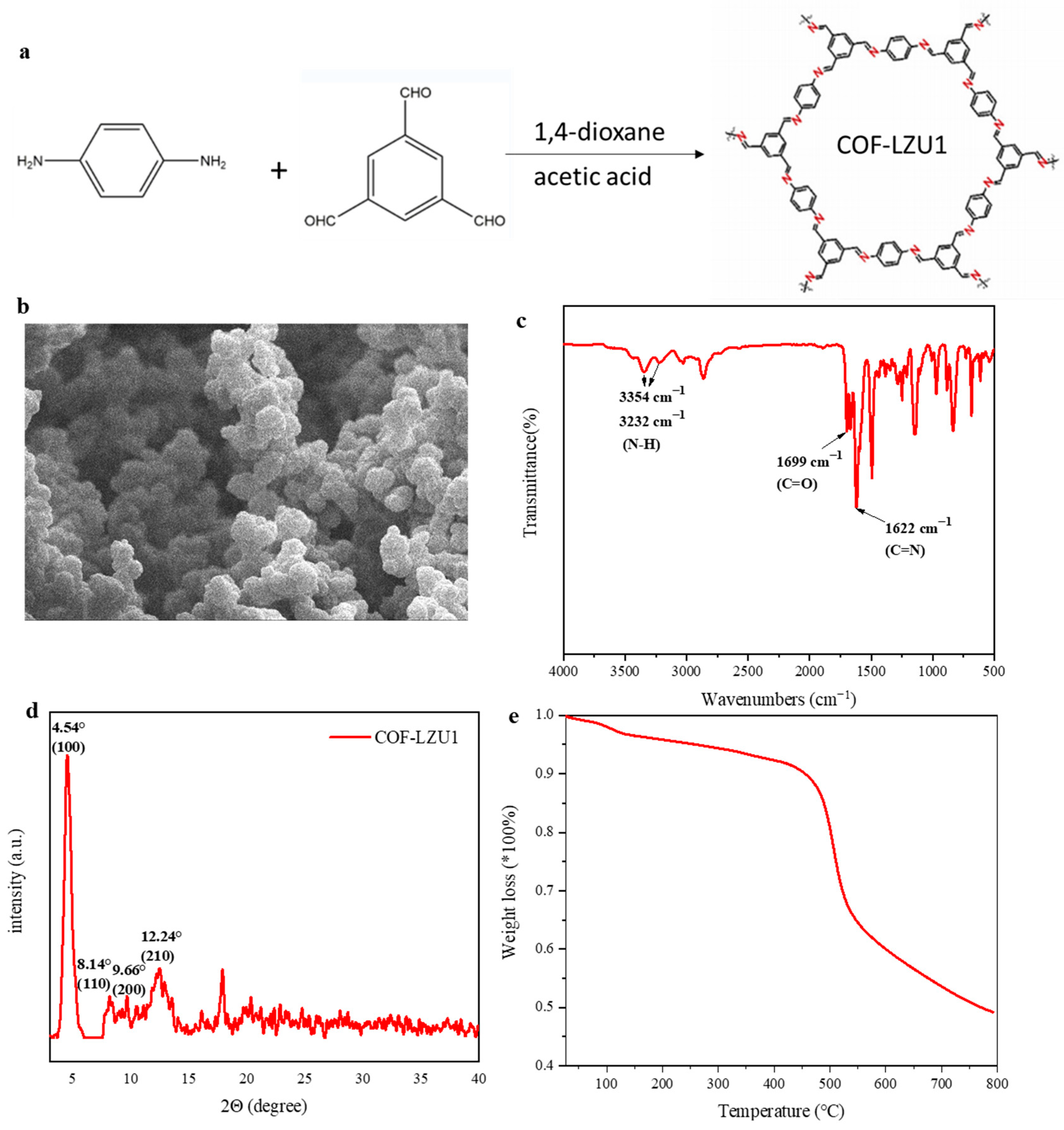
2.1. Physico-Chemical Characterization
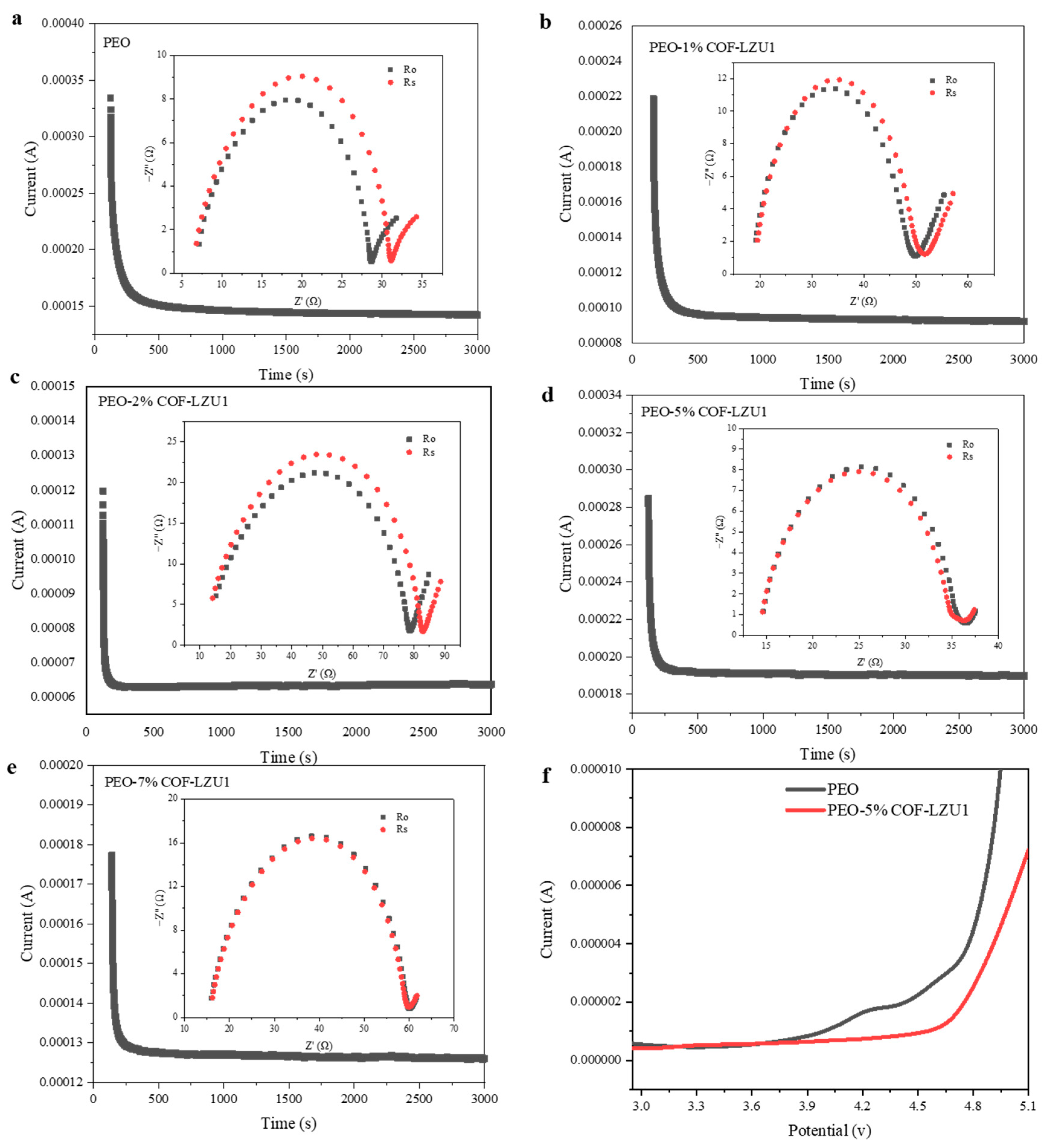
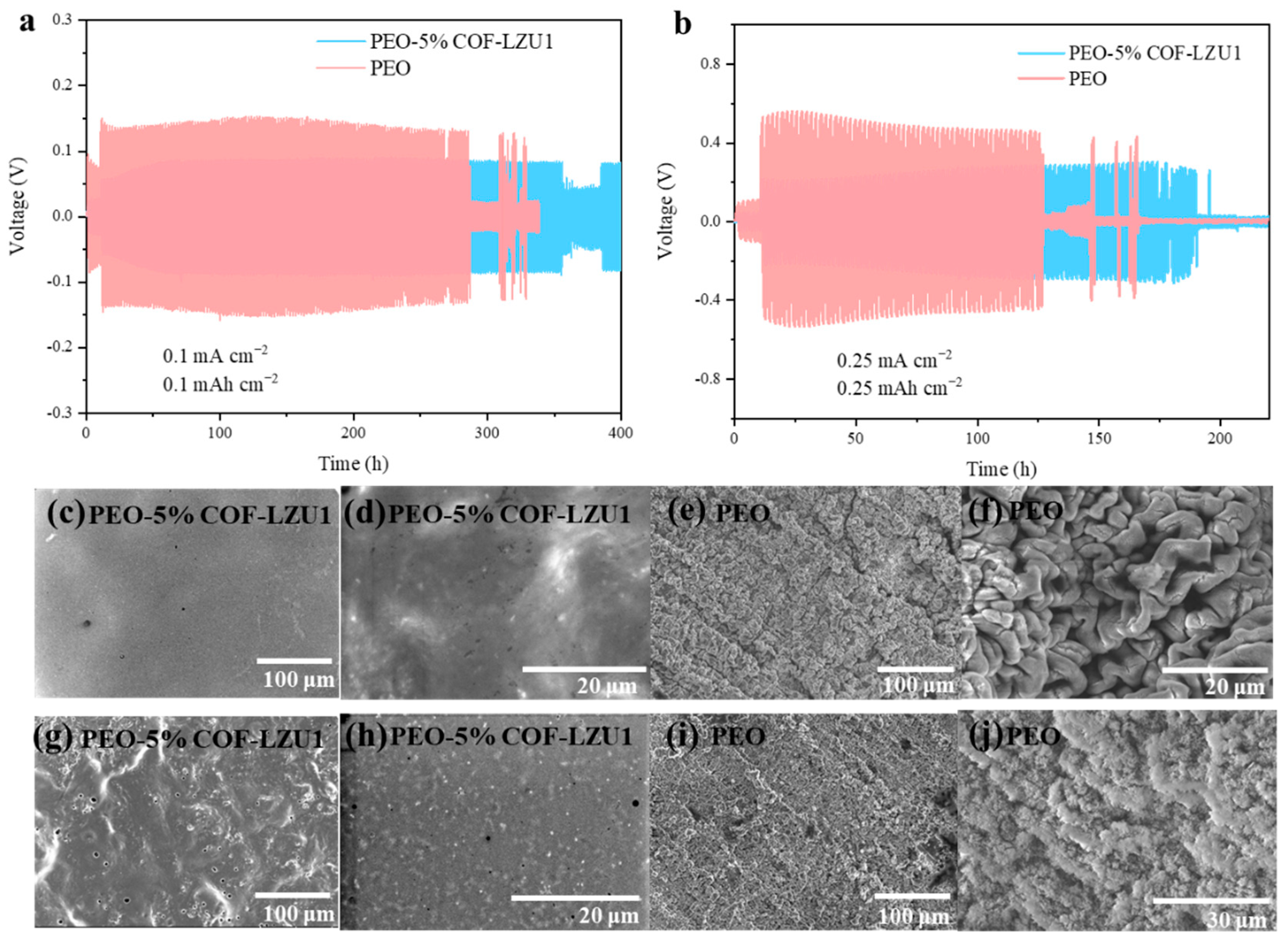
2.2. Electrochemical Characterization
3. Materials and Methods
3.1. Materials
3.2. Synthesis of COF-LZU1
3.3. Preparation of Solid Polymer Electrolyte Membrane
3.4. Preparation and Modification of LFP Electrodes
3.5. Characterizations
3.6. Cell Assembly and Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Lin, C.; Wang, Y.; Xu, Y.; Pham, D.T.; Meng, X.; Van Tran, K.; Cao, S.; Kardjilov, N.; Hilger, A.; et al. Enhancing ionic conductivity and suppressing Li dendrite formation in lithium batteries using a vinylene-linked covalent organic framework solid polymer electrolyte. J. Mater. Chem. A 2024, 12, 1694–1702. [Google Scholar] [CrossRef]
- Xing, J.L.; Bliznakov, S.; Bonville, L.; Oljaca, M.; Maric, R. A Review of Nonaqueous Electrolytes, Binders, and Separators for Lithium-Ion Batteries. Electrochem. Energy Rev. 2022, 5, 14. [Google Scholar] [CrossRef]
- Jian, S.; Cao, Y.; Feng, W.; Yin, G.; Zhao, Y.; Lai, Y.; Zhang, T.; Ling, X.; Wu, H.; Bi, H.; et al. Recent progress in solid polymer electrolytes with various dimensional fillers: A review. Mater. Today Sustain. 2022, 20, 100224. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Cao, X.; He, Y.; Wu, K.; Yang, J.; Zhou, H.; Liu, W.; Sun, X. Thiol-Branched Solid Polymer Electrolyte Featuring High Strength, Toughness, and Lithium Ionic Conductivity for Lithium-Metal Batteries. Adv. Mater. 2020, 32, 2001259. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, S.; Zhong, L.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. In Situ Preparation of Thin and Rigid COF Film on Li Anode as Artificial Solid Electrolyte Interphase Layer Resisting Li Dendrite Puncture. Adv. Funct. Mater. 2020, 30, 1907717. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Hao, Z.D.; Zhao, Q.; Tang, J.D.; Zhang, Q.Q.; Liu, J.B.; Jin, Y.H.; Wang, H. Functional separators towards the suppression of lithium dendrites for rechargeable high-energy batteries. Mater. Horiz. 2021, 8, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, J.; Zhang, K.; Fang, Q.; Zuo, Y.; Yang, T.; Yang, Y.; Gao, C.; Wang, X.; Pang, Q.; et al. A strongly complexed solid polymer electrolyte enables a stable solid state high-voltage lithium metal battery. Energy Environ. Sci. 2022, 15, 5149–5158. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, L.; Xiao, M.; Han, D.; Wang, S.; Huang, Z.; Huang, S.; Sun, L.; Meng, Y. Block copolymer electrolytes for lithium metal batteries: Strategies to boost both ionic conductivity and mechanical strength. Prog. Polym. Sci. 2023, 146, 101743. [Google Scholar] [CrossRef]
- Su, G.; Zhang, X.; Xiao, M.; Wang, S.; Huang, S.; Han, D.; Meng, Y. Polymeric Electrolytes for Solid-state Lithium Ion Batteries: Structure Design, Electrochemical Properties and Cell Performances. ChemSusChem 2024, 17, e202300293. [Google Scholar] [CrossRef]
- Geng, M.; Su, G.; Huang, S.; Wang, S.; Xiao, M.; Han, D.; Meng, Y. Practical challenges and future perspectives of solid polymer electrolytes: Microscopic structure and interface design. Mater. Chem. Front. 2023, 7, 5963–5988. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 5, 229–252. [Google Scholar] [CrossRef]
- Lu, G.L.; Zhang, Y.J.; Zhang, J.J.; Du, X.F.; Lv, Z.L.; Du, J.Z.; Zhao, Z.M.; Tang, Y.; Zhao, J.W.; Cui, G.L. Trade-offs between ion-conducting and mechanical properties: The case of polyacrylate electrolytes. Carbon Energy 2022, 5, e287. [Google Scholar] [CrossRef]
- Kang, J.B.; Yan, Z.R.; Gao, L.; Zhang, Y.F.; Liu, W.C.; Yang, Q.; Zhao, Y.X.; Deng, N.P.; Cheng, B.W.; Kang, W.M. Improved ionic conductivity and enhancedinterfacial stability of solid polymer electrolytes with porous ferroelectric ceramic nanofibers. Energy Storage Mater. 2022, 53, 192–203. [Google Scholar] [CrossRef]
- Wang, X.E.; Kerr, R.; Chen, F.F.; Goujon, N.; Pringle, J.M.; Mecerreyes, D.; Forsyth, M.; Howlett, P.C. Toward High-Energy-Density Lithium Metal Batteries: Opportunities and Challenges for Solid Organic Electrolytes. Adv. Mater. 2020, 32, 1905219. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Yu, X.W.; Wang, S.F. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Fu, F.; Zheng, Y.; Jiang, N.; Liu, Y.; Sun, C.; Zhang, A.T.; Teng, H.; Sun, L.Q.; Xie, H.M. A Dual-Salt PEO-based polymer electrolyte with Cross-Linked polymer network for High-Voltage lithium metal batteries. Chem. Eng. J. 2022, 450, 137776. [Google Scholar] [CrossRef]
- Yin, J.Y.; Xu, X.; Jiang, S.; Lei, Y.; Gao, Y.F. Bi-nanofillers integrated into PEO-based electrolyte for high-performance solid-state Li metal batteries. J. Power Sources 2022, 550, 232139. [Google Scholar] [CrossRef]
- Hsu, S.T.; Tran, B.T.; Subramani, R.; Nguyen, H.T.T.; Rajamani, A.; Lee, M.Y.; Hou, S.S.; Lee, Y.L.; Teng, H. Free-standing polymer electrolyte for all-solid-state lithium batteries operated at room temperature. J. Power Sources 2020, 449, 227518. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.T.; Li, S.P.; Fan, L.Z.; Nan, C.W.; Goodenough, J.B. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yu, T.; Yang, H.C.; Xu, S.J.; Li, H.C.; Chen, K.; Xu, R.G.; Zhou, T.Y.; Sun, Z.H.; Li, F. Ion coordination to improve ionic conductivity in polymer electrolytes for high performance solid-state batteries. Nano Energy 2022, 103, 107763. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Nie, J.H.; Li, F.; Wang, Z.L.; Sun, C.Q. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Y.; Gao, H.; Hang, J.X.; Li, C.; Liu, P.B. MOF-derived ionic conductor enhancing polymer electrolytes with superior electrochemical performances for all solid lithium metal batteries. J. Membr. Sci. 2020, 598, 117800. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Vu, D.; Warren, A.; Deng, Y.; Biswal, P.; Archer, L.A. Solid-state polymer electrolytes for high-performance lithium metal batteries. Nat. Commun. 2019, 10, 4398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, J.; Wei, D. Two-Dimensional Metal-Organic Frameworks and Covalent Organic Frameworks. Chin. J. Chem. 2022, 40, 1359–1385. [Google Scholar] [CrossRef]
- Sasmal, H.S.; Mahato, A.K.; Majumder, P.; Banerjee, R. Landscaping Covalent Organic Framework Nanomorphologies. J. Am. Chem. Soc. 2022, 144, 11482–11498. [Google Scholar] [CrossRef]
- Gao, Z.H.; Liu, Q.; Zhao, G.F.; Sun, Y.J.; Guo, H. Covalent organic frameworks for solid-state electrolytes of lithium metal batteries. J. Mater. Chem. A 2022, 10, 7497–7516. [Google Scholar] [CrossRef]
- Shan, Z.; Wu, M.M.; Du, Y.H.; Xu, B.Q.; He, B.Y.; Wu, X.W.; Zhang, G. Covalent Organic Framework-Based Electrolytes for Fast Li+ Conduction and High-Temperature Solid-State Lithium-Ion Batteries. Chem. Mater. 2021, 33, 5058–5066. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, M.D.; Wang, H.J.; Han, C.Y.; Pan, F.S.; Sun, J. Covalent Organic Framework for Rechargeable Batteries: Mechanisms and Properties of Ionic Conduction. Adv. Energy Mater. 2022, 12, 2200057. [Google Scholar] [CrossRef]
- Dong, D.R.; Zhang, H.; Zhou, B.; Sun, Y.F.; Zhang, H.L.; Cao, M.; Li, J.B.; Zhou, H.; Qian, H.; Lin, Z.Y.; et al. Porous covalent organic frameworks for high transference number polymer-based electrolytes. Chem. Commun. 2019, 55, 1458–1461. [Google Scholar] [CrossRef]
- Xuan, Y.F.; Wang, Y.X.; He, B.Y.; Bian, S.Y.; Liu, J.C.; Xu, B.Q.; Zhang, G. Covalent Organic Framework-Derived Quasi-Solid Electrolyte for Low-Temperature Lithium-Ion. Chem. Mater. 2022, 34, 9104–9110. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.H.; Ji, H.Q.; Zhang, J.; Zhou, P.X.; Cao, Y.F.; Zhou, J.Q.; Yan, C.L.; Qian, T. Cationic Covalent Organic Framework with Ultralow HOMO Energy Used as Scaffolds for 5.2 V Solid Polycarbonate Electrolytes. Adv. Sci. 2022, 9, 2200390. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.L.; Zhang, J.S.; Xie, M.L.; Lu, D.R.; Zhao, Z.; Li, Y.Q.; Cheng, Z.Y.; Zhang, S.J.; Chen, H.W. Ultrathin Aramid/COF Heterolayered Membrane for Solid-State Li-Metal Batteries. Nano Lett. 2020, 20, 8120–8126. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.Y.; Lee, M.S.; Hsu, H.C.; Lin, K.J. One-Pot Green Synthesis of a PEO/TCPP/LiClO4 Solid Polymer Electrolyte with Improvement of Ion Transport. J. Phys. Chem. C 2021, 125, 22960–22969. [Google Scholar] [CrossRef]
- Zhang, K.G.; Niu, C.Q.; Yu, C.B.; Zhang, L.; Xu, Y.X. Highly crystalline vinylene-linked covalent organic frameworks enhanced solid polycarbonate electrolyte for dendrite-free solid lithium metal batteries. Nano Res. 2022, 15, 8083–8090. [Google Scholar] [CrossRef]
- Polczyk, T.; Nagai, A. Covalent Organic Framework-Based Electrolytes for Lithium Solid-State Batteries-Recent Progress. Batteries 2023, 9, 469. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, J.; Rim, J.; Hong, D.; Kang, J.; Park, S. Vinyl-Integrated In Situ Cross-Linked Composite Gel Electrolytes for Stable Lithium Metal Anodes. ACS Appl. Energy Mater. 2021, 4, 2922–2931. [Google Scholar] [CrossRef]
- Ding, S.Y.; Cui, X.H.; Feng, J.; Lu, G.X.; Wang, W. Facile synthesis of -C=N-linked covalent organic frameworks under ambient conditions. Chem. Commun. 2017, 53, 11956–11959. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, Y.; Li, T.; Jiang, S.H.; Qian, X.; Yue, Q.; Kang, Y.J. Multifunctional covalent organic frameworks for high capacity and dendrite-free lithium metal batteries. Energy Storage Mater. 2020, 25, 334–341. [Google Scholar] [CrossRef]

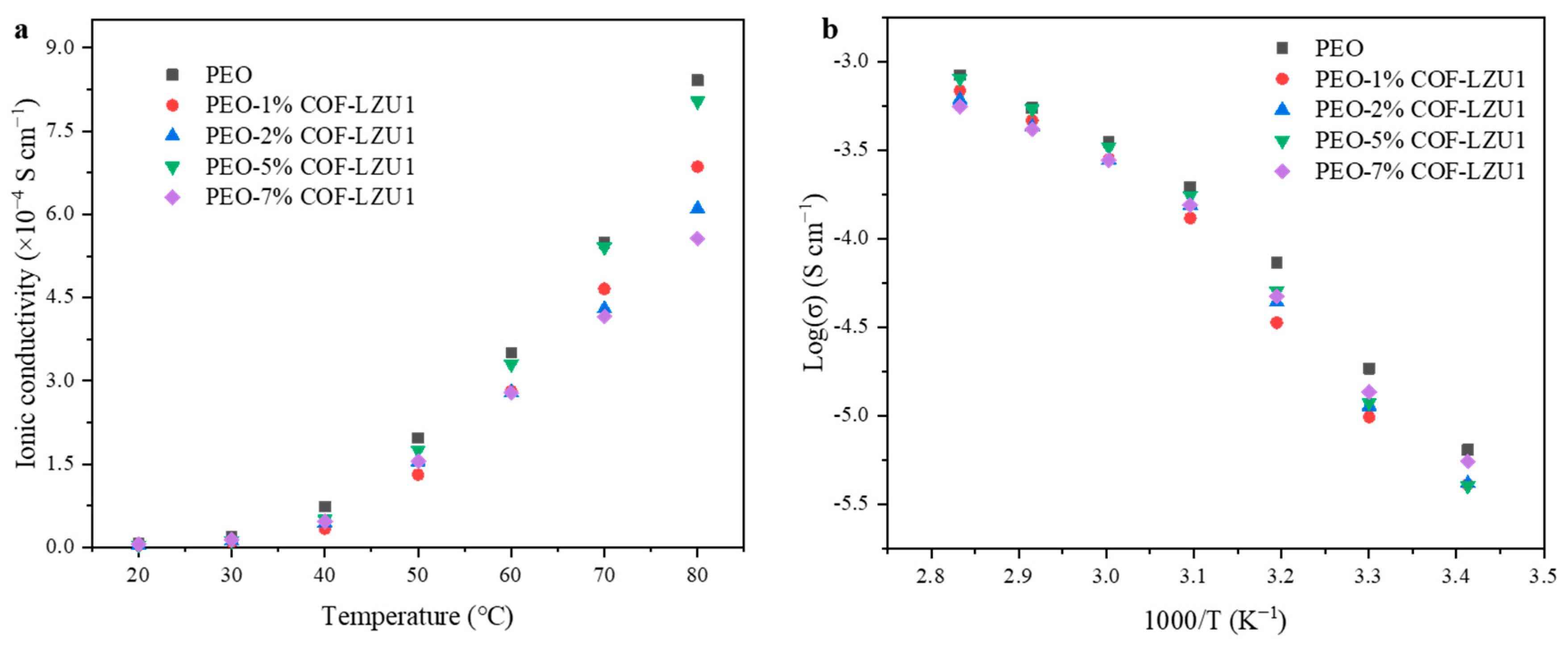

| 30 °C (S cm−1) | 40 °C (S cm−1) | 50 °C (S cm−1) | 60 °C (S cm−1) | 70 °C (S cm−1) | 80 °C (S cm−1) | |
|---|---|---|---|---|---|---|
| PEO | 1.85 × 10−5 | 7.73 × 10−5 | 1.97 × 10−4 | 3.50 × 10−4 | 5.49 × 10−4 | 8.41 × 10−4 |
| PEO-1% COF-LZU1 | 9.84 × 10−6 | 3.36 × 10−5 | 1.31 × 10−4 | 2.81 × 10−4 | 4.66 × 10−4 | 6.86 × 10−4 |
| PEO-2% COF-LZU1 | 1.14 × 10−5 | 4.39 × 10−5 | 1.54 × 10−4 | 2.79 × 10−4 | 4.30 × 10−4 | 6.10 × 10−4 |
| PEO-5% COF-LZU1 | 1.89 × 10−5 | 5.06 × 10−5 | 1.75 × 10−4 | 3.30 × 10−4 | 5.41 × 10−4 | 8.04 × 10−4 |
| PEO-7% COF-LZU1 | 1.36 × 10−5 | 4.74 × 10−5 | 1.55 × 10−4 | 2.78 × 10−4 | 4.16 × 10−4 | 5.56 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Zhong, L.; Huang, S.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Covalent Organic Framework Enhanced Solid Polymer Electrolyte for Lithium Metal Batteries. Molecules 2024, 29, 1759. https://doi.org/10.3390/molecules29081759
Ma B, Zhong L, Huang S, Xiao M, Wang S, Han D, Meng Y. Covalent Organic Framework Enhanced Solid Polymer Electrolyte for Lithium Metal Batteries. Molecules. 2024; 29(8):1759. https://doi.org/10.3390/molecules29081759
Chicago/Turabian StyleMa, Bingyi, Lei Zhong, Sheng Huang, Min Xiao, Shuanjin Wang, Dongmei Han, and Yuezhong Meng. 2024. "Covalent Organic Framework Enhanced Solid Polymer Electrolyte for Lithium Metal Batteries" Molecules 29, no. 8: 1759. https://doi.org/10.3390/molecules29081759