Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC
Abstract
:1. Introduction
2. Results
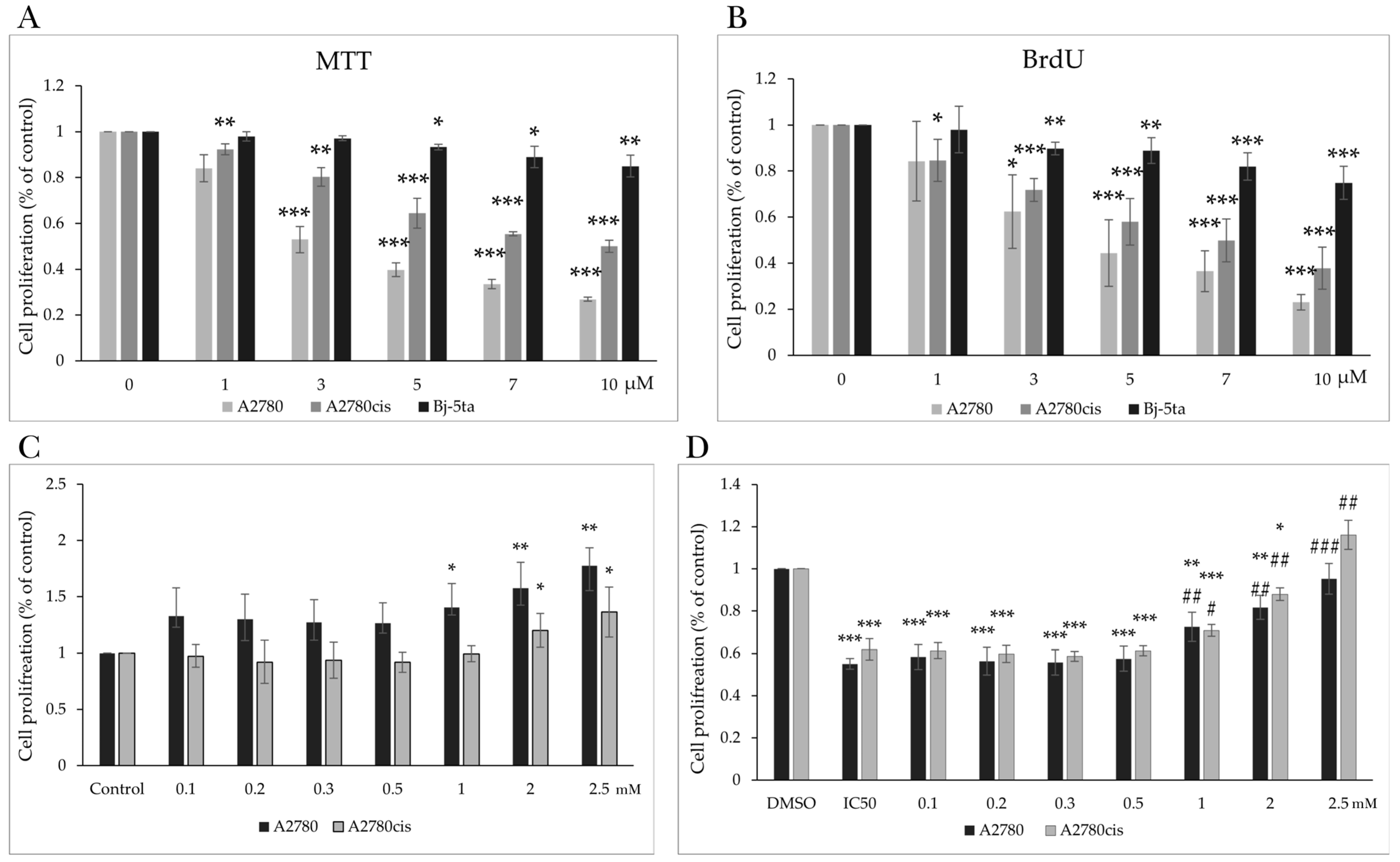
2.1. Effect of MB-591 on Metabolic Activity and Viability
2.2. Effect of MB-591 on Cell Proliferation
2.3. MB-591 Induces Cell Cycle Arrest
2.3.1. Cell Cycle Analysis
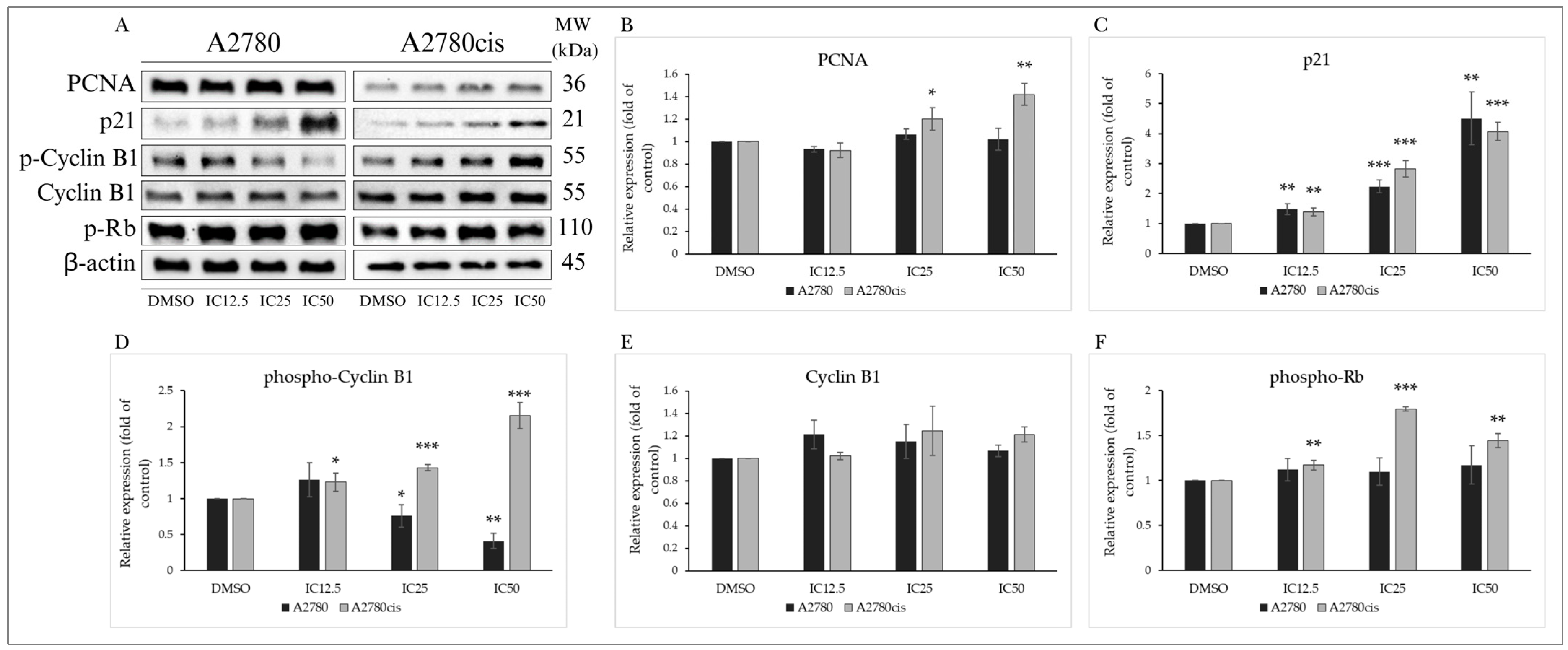
2.3.2. Analysis of Cell Cycle-Regulating Proteins
2.4. MB-591-Induced Mitochondrial Apoptotic Pathway
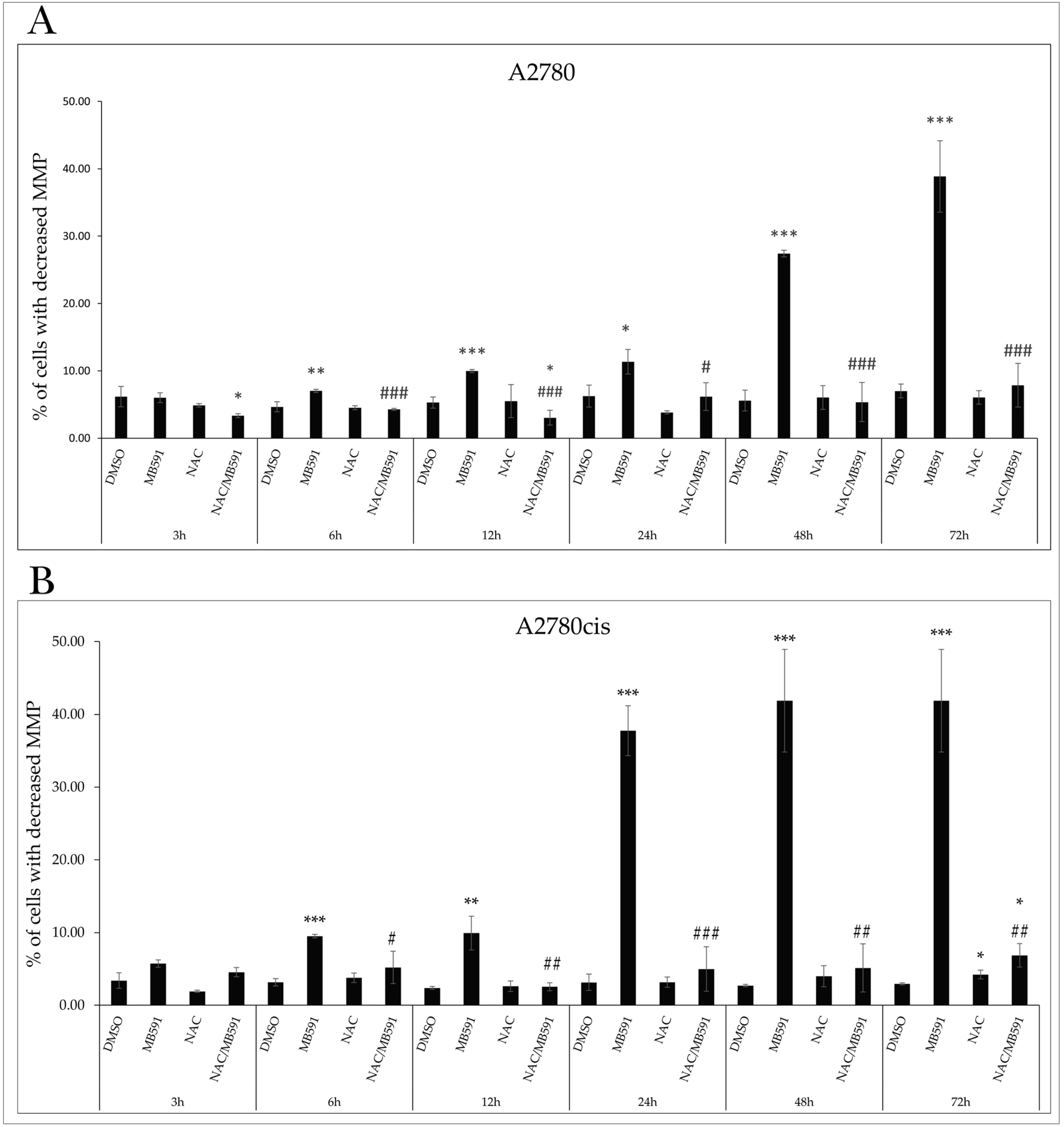
2.4.1. Mitochondrial Membrane Potential
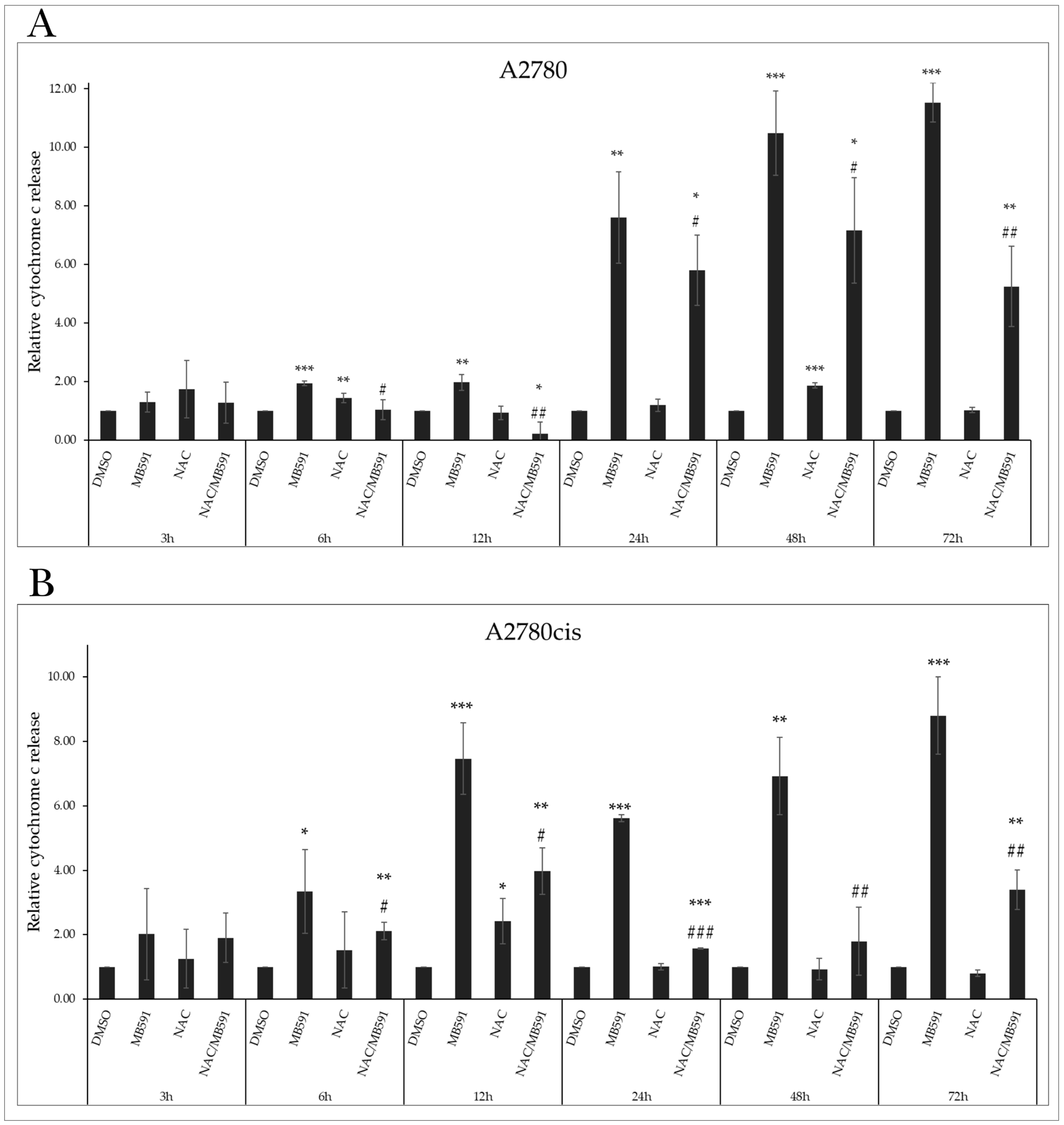
2.4.2. Cytochrome c Release
2.4.3. Phosphatidylserine Externalization
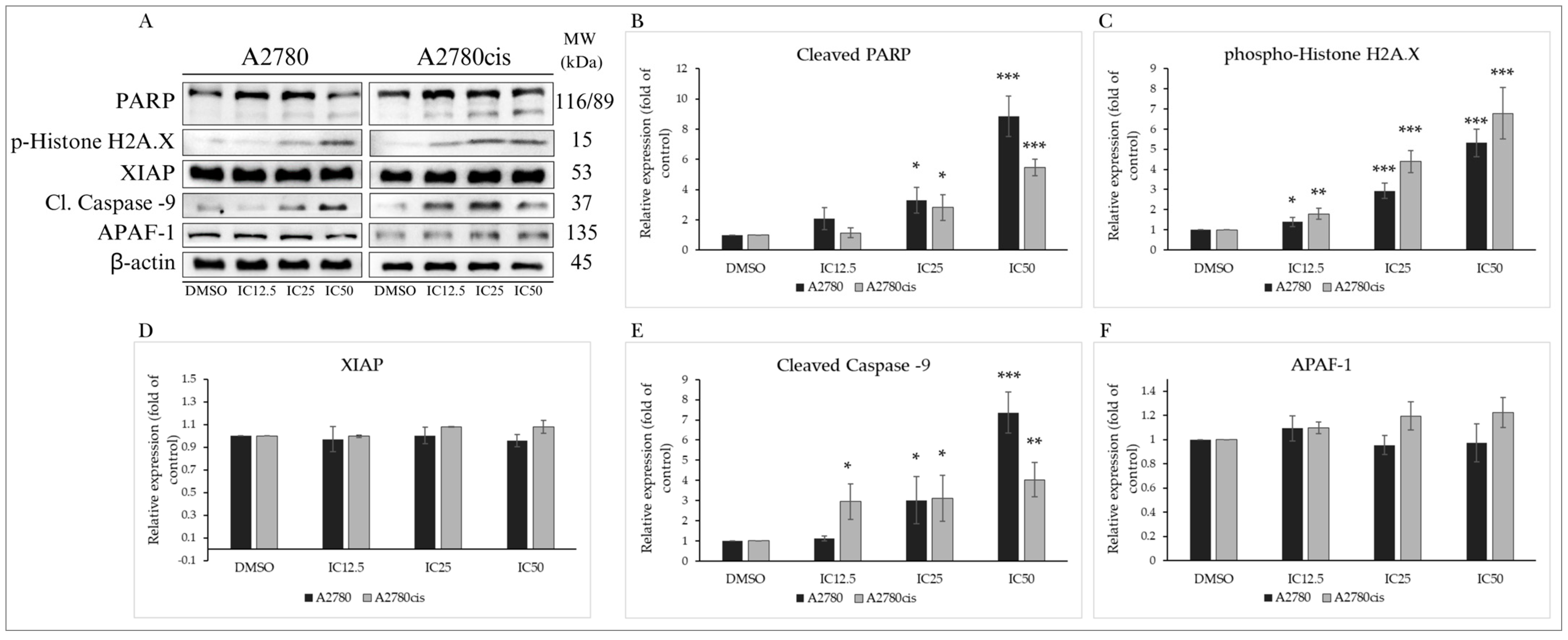
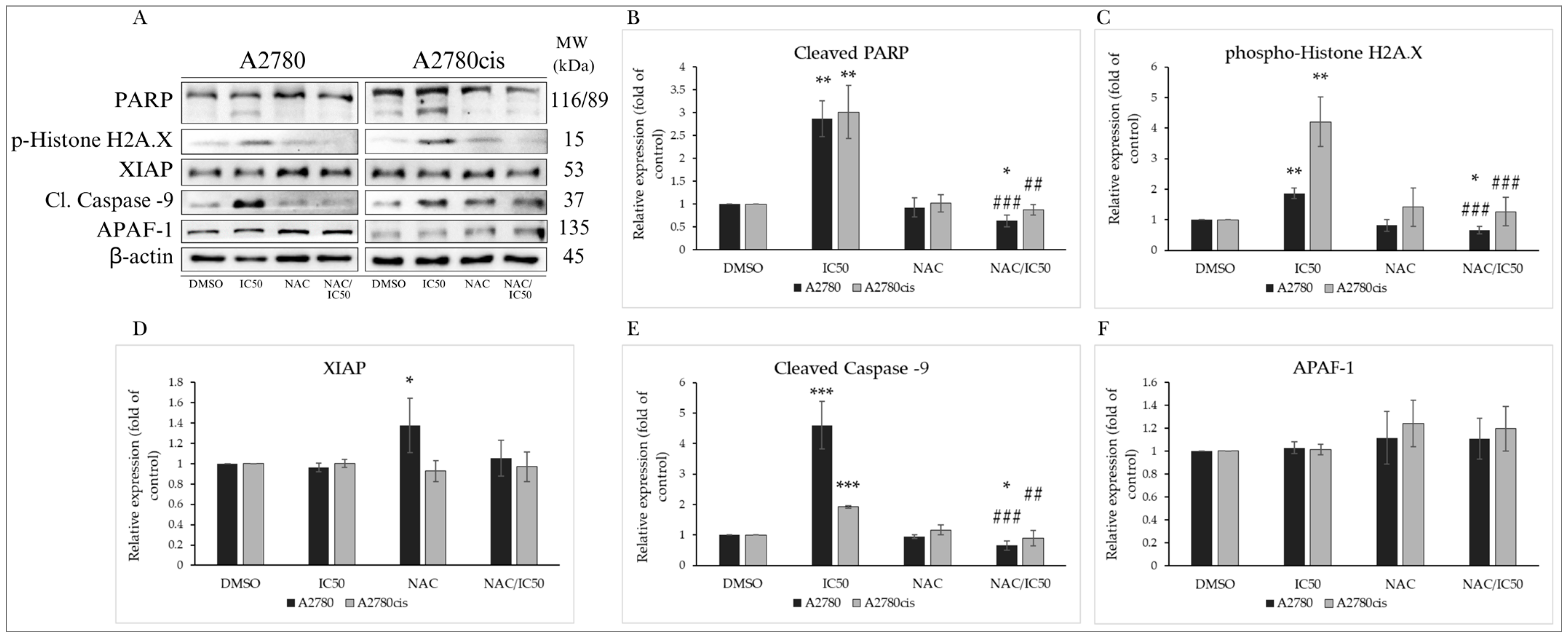
2.4.4. Changes in Levels of Proteins Associated with the Regulation of Apoptotic Cell Death
2.5. Effect of the Indole Phytoalexin MB-591 on Autophagy Induction
2.5.1. PTEN/Akt/mTOR Signaling Pathway
2.5.2. Effect on Autophagy-Associated Proteins
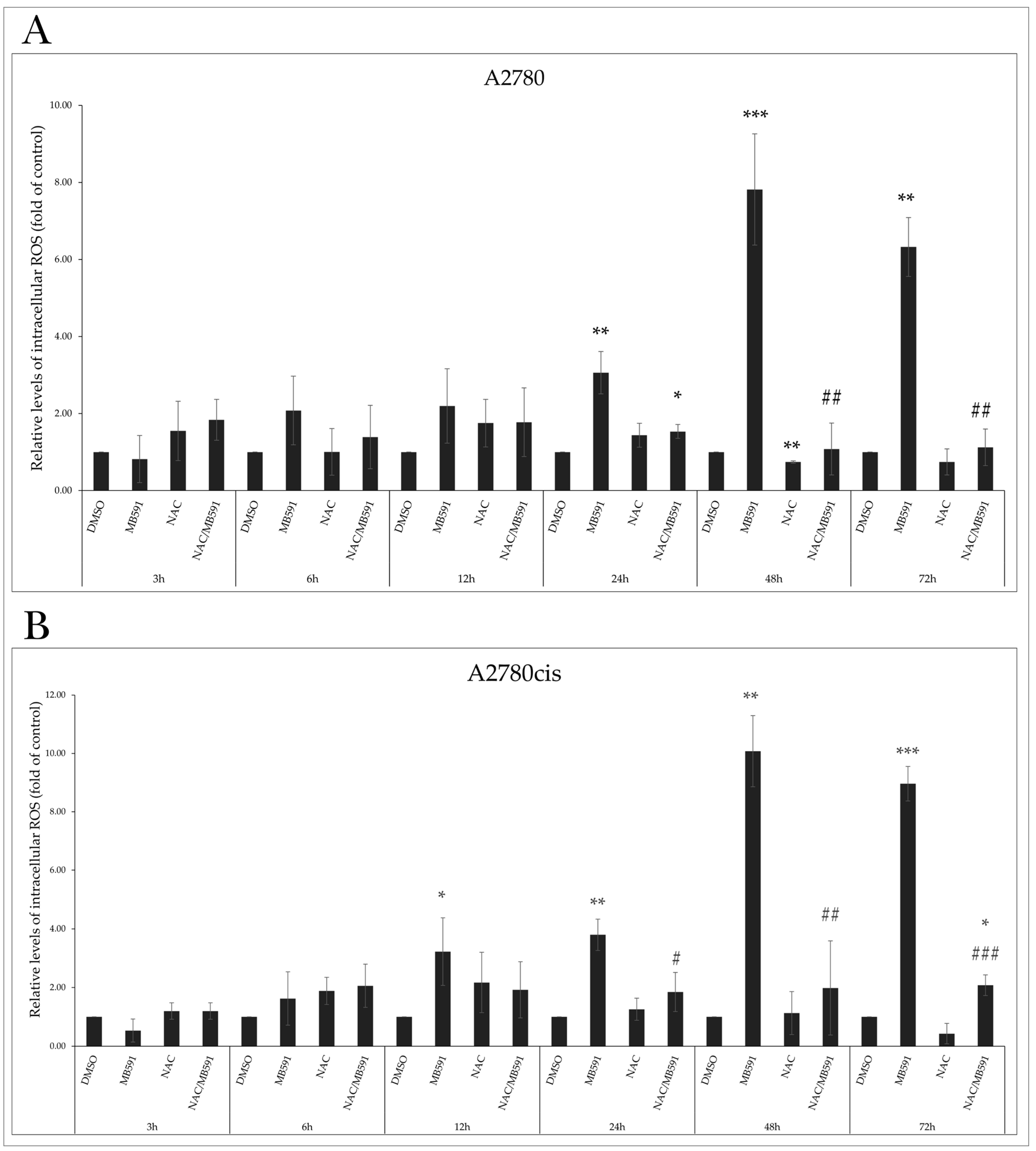
2.6. MB-591 Induces Oxidative Stress by ROS Production
2.7. Effect of NAC on MB-591-Induced Cell Cycle Arrest
Analysis of the Effect of NAC on Cell Cycle-Regulating Proteins
2.8. NAC Inhibits MB-591-Induced Apoptotic Cell Death
NAC Modulates the Expression of Proteins Involved in Apoptosis
2.9. Effect of NAC on MB-591-Induced Autophagy
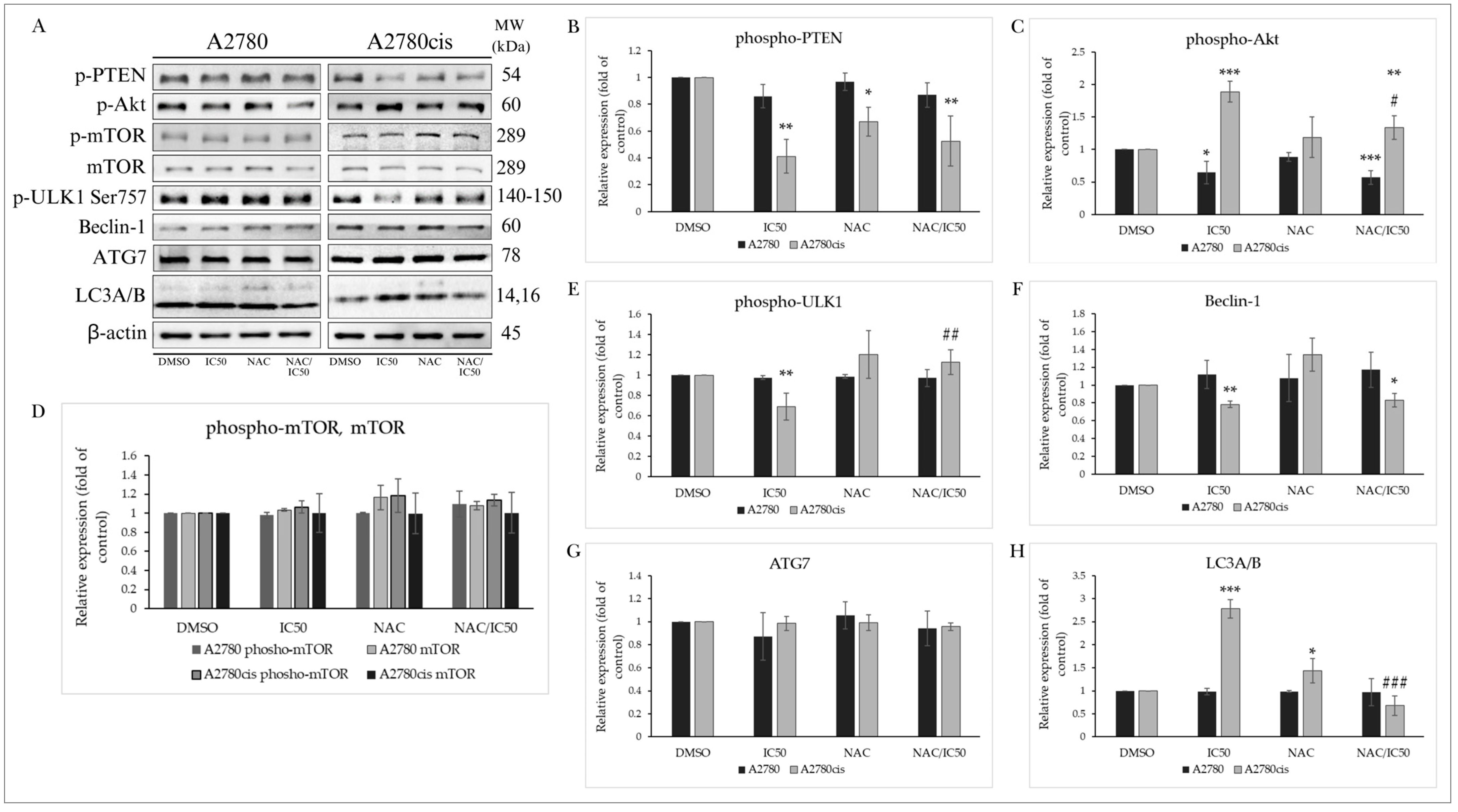
2.9.1. PTEN/Akt/mTOR Signaling Pathway
2.9.2. Effect of NAC on Autophagy-Associated Proteins
3. Discussion
4. Materials and Methods
4.1. Tested Compound
4.2. Cell Culture
4.3. MTT Viability Assay
4.4. 5-Bromo-2’-Deoxyuridine (BrdU) Cell Proliferation Assay
4.5. Flow Cytometric Analyses
4.5.1. Annexin V/PI Staining
4.5.2. Detection of Mitochondrial Membrane Potential Changes
4.5.3. Analysis of Cytochrome c Release
4.5.4. Cell Cycle Analysis
4.5.5. Flow Cytometry Analysis of ROS
4.6. Western Blot Analyses
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef]
- Bizuneh, G.K. The chemical diversity and biological activities of phytoalexins. Adv. Tradit. Med. 2021, 21, 31–43. [Google Scholar] [CrossRef]
- Kumar, S.; Chang, Y.C.; Lai, K.H.; Hwang, T.L. Resveratrol, a Molecule with Anti-Inflammatory and Anti-Cancer Activities: Natural Product to Chemical Synthesis. Curr. Med. Chem. 2021, 28, 3773–3786. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.; Yaya, E.E.; Glawischnig, E. The phytoalexins from cultivated and wild crucifers: Chemistry and biology. Nat. Prod. Rep. 2011, 28, 1381–1405. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Trotel-Aziz, P.; Clément, C.; Jeandet, P.; Baillieul, F.; Aziz, A. Camalexin accumulation as a component of plant immunity during interactions with pathogens and beneficial microbes. Planta 2022, 255, 116. [Google Scholar] [CrossRef] [PubMed]
- Angarita-Rodríguez, A.; Quiroga, D.; Coy-Barrera, E. Indole-Containing Phytoalexin-Based Bioisosteres as Antifungals: In Vitro and In Silico Evaluation against Fusarium oxysporum. Molecules 2020, 25, 45. [Google Scholar] [CrossRef]
- Xu, B.X.; Alarfaj, A.A.; Hirad, A.H.; Natarajan, N.; Iyappan, P.; Al Ali, S.H.H. Brassinin Exhibits Anti-Diabetic Activity against Streptozotocin-induced Diabetes Mellitus in Experimental Rats. Indian J. Pharm. Educ. 2023, 57, S701–S709. [Google Scholar] [CrossRef]
- Lee, M.K.; Ryu, H.; Jeong, H.H.; Lee, B. Brassinin Abundant in Brassicaceae Suppresses Melanogenesis through Dual Mechanisms of Tyrosinase Inhibition. Foods 2022, 12, 121. [Google Scholar] [CrossRef]
- Liao, A.; Li, L.; Wang, T.; Lu, A.; Wang, Z.; Wang, Q. Discovery of Phytoalexin Camalexin and Its Derivatives as Novel Antiviral and Antiphytopathogenic-Fungus Agents. J. Agric. Food Chem. 2022, 70, 2554–2563. [Google Scholar] [CrossRef]
- Manasa, K.; Chitra, V. Evaluation of in-vitro antioxidant activity of camalexin—A novel anti-parkinson’s agent. Res. J. Pharm. Technol. 2020, 13, 578–582. [Google Scholar] [CrossRef]
- Gu, Y.; Becker, V.; Qiu, M.; Tang, T.; Ampofo, E.; Menger, M.D.; Laschke, M.W. Brassinin Promotes the Degradation of Tie2 and FGFR1 in Endothelial Cells and Inhibits Triple-Negative Breast Cancer Angiogenesis. Cancers 2022, 14, 3540. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Ham, J.; Song, J.; Song, G.; Lim, W. Brassinin Inhibits Proliferation in Human Liver Cancer Cells via Mitochondrial Dysfunction. Cells 2021, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Taga, C.; Ozawa, M.; Kanno, Y.; Sanada, N.; Kizu, R. Camalexin, an indole phytoalexin, inhibits cell proliferation, migration, and mammosphere formation in breast cancer cells via the aryl hydrocarbon receptor. J. Nat. Med. 2022, 76, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pilatova, M.; Sarissky, M.; Kutschy, P.; Mirossay, A.; Mezencev, R.; Curillova, Z.; Suchy, M.; Monde, K.; Mirossay, L.; Mojzis, J. Cruciferous phytoalexins: Antiproliferative effects in T-Jurkat leukemic cells. Leuk. Res. 2005, 29, 415–421. [Google Scholar] [CrossRef]
- Monde, K.; Taniguchi, T.; Miura, N.; Kutschy, P.; Curillova, Z.; Pilatova, M.; Mojzis, J. Chiral cruciferous phytoalexins: Preparation, absolute configuration, and biological activity. Bioorg. Med. Chem. 2005, 13, 5206–5212. [Google Scholar] [CrossRef]
- Mezencev, R.; Kutschy, P.; Salayova, A.; Curillova, Z.; Mojzis, J.; Pilatova, M.; McDonald, J. Anticancer properties of 2-piperidyl analogues of the natural indole phytoalexin 1-methoxyspirobrassinol. Chemotherapy 2008, 54, 372–378. [Google Scholar] [CrossRef]
- Pilatova, M.; Ivanova, L.; Kutschy, P.; Varinska, L.; Saxunova, L.; Repovska, M.; Sarissky, M.; Seliga, R.; Mirossay, L.; Mojzis, J. In vitro toxicity of camalexin derivatives in human cancer and non-cancer cells. Toxicol. In Vitro 2013, 27, 939–944. [Google Scholar] [CrossRef]
- Chripkova, M.; Drutovic, D.; Pilatova, M.; Mikes, J.; Budovska, M.; Vaskova, J.; Broggini, M.; Mirossay, L.; Mojzis, J. Brassinin and its derivatives as potential anticancer agents. Toxicol. In Vitro 2014, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Drutovic, D.; Chripkova, M.; Pilatova, M.; Budovska, M.; Kulikova, L.; Urdzik, P.; Mojzis, J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules 2014, 19, 10877–10897. [Google Scholar] [CrossRef]
- Tischlerova, V.; Kello, M.; Budovska, M.; Mojzis, J. Indole phytoalexin derivatives induce mitochondrial-mediated apoptosis in human colorectal carcinoma cells. World J. Gastroenterol. 2017, 23, 4341–4353. [Google Scholar] [CrossRef]
- Budovská, M.; Selesová, I.; Tischlerová, V.; Michalková, R.; Mojzis, J. Design, synthesis, and biological evaluation of novel 5-bromo derivatives of indole phytoalexins. Monatsh. Chem. 2020, 151, 1737–1758. [Google Scholar] [CrossRef]
- Ocenásová, L.; Budovská, M.; Ocenás, P.; Tomásková, N.; Pilátová, M.B.; Mojzis, J. The first synthesis of natural alkaloid capparine A. Tetrahedron 2021, 77, 131772. [Google Scholar] [CrossRef]
- Budovská, M.; Krochtová, K.; Kuba, M.; Tischlerová, V.; Mojzis, J. Synthesis and cytotoxicity evaluation of novel 5-fluorinated indoles. J. Fluorine Chem. 2021, 250, 109879. [Google Scholar] [CrossRef]
- Marianna, B.; Radka, M.; Martin, K.; Janka, V.; Jan, M. Design, Synthesis and Antiproliferative Evaluation of Bis-Indole Derivatives with a Phenyl Linker: Focus on Autophagy. Molecules 2022, 28, 251. [Google Scholar] [CrossRef] [PubMed]
- Budovská, M.; Michalková, R.; Mojzis, J. Indole phytoalexins-derived bis-indoles: Design, synthesis and in vitro antiproliferative evaluation. Tetrahedron 2023, 143, 133573. [Google Scholar] [CrossRef]
- Budovská, M.; Krochtová, K.; Michalková, R.; Mojzis, J. Aminoanalogues of isobrassinin, erucalexin and isocyclobrassinin: Synthesis and evaluation of the antiproliferative and cytotoxic properties. Tetrahedron 2022, 120, 132898. [Google Scholar] [CrossRef]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef]
- Csomos, P.; Zupko, I.; Rethy, B.; Fodor, L.; Falkay, G.; Bernath, G. Isobrassinin and its analogues: Novel types of antiproliferative agents. Bioorg. Med. Chem. Lett. 2006, 16, 6273–6276. [Google Scholar] [CrossRef]
- Csomós, P.; Fodor, L.; Zupkó, I.; Csámpai, A.; Sohár, P. Synthesis and antiproliferative effect of isomeric analogs of cyclobrassinin phytoalexin possessing the 1,3-thiazino [5,6-]indole-4-one skeleton. Arkivoc 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Costantini, D. Understanding diversity in oxidative status and oxidative stress: The opportunities and challenges ahead. J. Exp. Biol. 2019, 222, jeb194688. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Du, J.; Jin, L.B.; Pan, L.Y.; Yan, X.F.; Lin, S. Iberverin exhibits antineoplastic activities against human hepatocellular carcinoma via DNA damage-mediated cell cycle arrest and mitochondrial-related apoptosis. Front. Pharmacol. 2023, 14, 1326346. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Cha, H.S.; Nam, M.J.; Park, K.; Yang, Y.H.; Lee, J.; Park, S.H. Broussochalcone A Induces Apoptosis in Human Renal Cancer Cells via ROS Level Elevation and Activation of FOXO3 Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 2800706. [Google Scholar] [CrossRef] [PubMed]
- Li, K.K.; Zhao, S.; Long, J.; Su, J.; Wu, L.H.; Tao, J.; Zhou, J.D.; Zhang, J.L.; Chen, X.; Peng, C. A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products. Cancer Cell Int. 2020, 20, 36. [Google Scholar] [CrossRef]
- Ren, Y.L.; Lei, J.T.; Zhang, T.R.; Lu, P.; Cui, D.D.; Yang, B.; Zhao, G.Y.; Peng, F.; Cao, Z.X.; Peng, C.; et al. Isobavachalcone, a natural sirtuin 2 inhibitor, exhibits anti-triple-negative breast cancer efficacy in vitro and in vivo. Phytother. Res. 2024, 38, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Vilková, M.; Michalková, R.; Kello, M.; Sabolová, D.; Takác, P.; Kudlicková, Z.; Garberová, M.; Tvrdonová, M.; Béres, T.; Mojzis, J. Discovery of novel acridine-chalcone hybrids with potent DNA binding and antiproliferative activity against MDA-MB-231 and MCF-7 cells. Med. Chem. Res. 2022, 31, 1323–1338. [Google Scholar] [CrossRef]
- Almalki, S.G. The pathophysiology of the cell cycle in cancer and treatment strategies using various cell cycle checkpoint inhibitors. Pathol. Res. Pract. 2023, 251, 154854. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Magana, A.; Blanco, F.J. Human PCNA Structure, Function and Interactions. Biomolecules 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Wang, Y.H.; Liu, Y.C. Overexpression of PCNA Attenuates Oxidative Stress-Caused Delay of Gap-Filling during Repair of UV-Induced DNA Damage. J. Nucleic Acids 2017, 2017, 8154646. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Xiao, B.D.; Zhao, Y.J.; Jia, X.Y.; Wu, J.; Wang, Y.G.; Huang, F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J. Stem Cells 2020, 12, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.J.; Kabeer, A.; Abbas, Z.; Siddiqui, H.A.; Calina, D.; Sharifi-Rad, J.; Cho, W.C. Interplay of oxidative stress, cellular communication and signaling pathways in cancer. Cell Commun. Signal. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, R.; Tomasello, B.; Di Giacomo, C.; Santangelo, R.; La Mantia, A.; Naletova, I.; Sarpietro, M.G.; Castelli, F.; Malfa, G.A. Protocatechuic Acid, a Simple Plant Secondary Metabolite, Induced Apoptosis by Promoting Oxidative Stress through HO-1 Downregulation and p21 Upregulation in Colon Cancer Cells. Biomolecules 2021, 11, 1485. [Google Scholar] [CrossRef] [PubMed]
- Dick, F.A.; Rubin, S.M. Molecular mechanisms underlying RB protein function. Nat. Rev. Mol. Cell Biol. 2013, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hilgendorf, K.I.; Leshchiner, E.S.; Nedelcu, S.; Maynard, M.A.; Calo, E.; Ianari, A.; Walensky, L.D.; Lees, J.A. The retinoblastoma protein induces apoptosis directly at the mitochondria. Gene Dev. 2013, 27, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Ianari, A.; Natale, T.; Calo, E.; Ferretti, E.; Alesse, E.; Screpanti, I.; Haigis, K.; Gulino, A.; Lees, J.A. Proapoptotic Function of the Retinoblastoma Tumor Suppressor Protein. Cancer Cell 2009, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Hayward, D.; Alfonso-Perez, T.; Gruneberg, U. Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1. FEBS Lett. 2019, 593, 2889–2907. [Google Scholar] [CrossRef]
- Yang, T.S.; Xiao, Y.; Liu, S.; Luo, F.Z.; Tang, D.Y.; Yu, Y.L.; Xie, Y. Isorhamnetin induces cell cycle arrest and apoptosis by triggering DNA damage and regulating the AMPK/mTOR/p70S6K signaling pathway in doxorubicin-resistant breast cancer. Phytomedicine 2023, 114, 154780. [Google Scholar] [CrossRef]
- Sabbaghi, M.A.; Gil-Gómez, G.; Guardia, C.; Servitja, S.; Arpí, O.; García-Alonso, S.; Menendez, S.; Arumi-Uria, M.; Serrano, L.; Salido, M.; et al. Defective Cyclin B1 Induction in Trastuzumab-emtansine (T-DM1) Acquired Resistance in HER2-positive Breast Cancer. Clin. Cancer Res. 2017, 23, 7006–7019. [Google Scholar] [CrossRef]
- Sousa, F.G.; Matuo, R.; Soares, D.G.; Escargueil, A.E.; Henriques, J.A.; Larsen, A.K.; Saffi, J. PARPs and the DNA damage response. Carcinogenesis 2012, 33, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.; Jubin, T.; Roychowdhury, R.; Begum, R. Role of PARP-1 in mitochondrial homeostasis. BBA-Gen. Subj. 2020, 1864, 129669. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, E.; Armour, S.M.; Harris, M.H.; Thompson, C.B. Mitochondrial membrane potential regulates matrix configuration and cytochrome release during apoptosis. Cell Death Differ. 2003, 10, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Updegrove, T.; Kutschy, P.; Repovská, M.; McDonald, J.F. Camalexin induces apoptosis in T-leukemia Jurkat cells by increased concentration of reactive oxygen species and activation of caspase-8 and caspase-9. J. Nat. Med. Tokyo 2011, 65, 488–499. [Google Scholar] [CrossRef]
- Kwon, H.H.; Ahn, C.H.; Lee, H.J.; Sim, D.Y.; Park, J.E.; Park, S.Y.; Kim, B.; Shim, B.S.; Kim, S.H. The Apoptotic and Anti-Warburg Effects of Brassinin in PC-3 Cells via Reactive Oxygen Species Production and the Inhibition of the c-Myc, SIRT1, and beta-Catenin Signaling Axis. Int. J. Mol. Sci. 2023, 24, 13912. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Ha, I.J.; Lee, S.G.; Lee, J.; Um, J.Y.; Sethi, G.; Ahn, K.S. Brassinin Induces Apoptosis, Autophagy, and Paraptosis via MAPK Signaling Pathway Activation in Chronic Myelogenous Leukemia Cells. Biology 2023, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Janku, F.; McConkey, D.J.; Hong, D.S.; Kurzrock, R. Autophagy as a target for anticancer therapy. Nat. Rev. Clin. Oncol. 2011, 8, 528–539. [Google Scholar] [CrossRef]
- Edinger, A.L.; Thompson, C.B. Defective autophagy leads to cancer. Cancer Cell 2003, 4, 422–424. [Google Scholar] [CrossRef]
- Tóth, S.; Nagy, K.; Pálfia, Z.; Réz, G. Cellular autophagic capacity changes during azaserine-induced tumour progression in the rat pancreas -: Up-regulation in all premalignant stages and down-regulation with loss of cycloheximide sensitivity of segregation along with malignant transformation. Cell Tissue Res. 2002, 309, 409–416. [Google Scholar]
- Vitto, V.A.M.; Bianchin, S.; Zolondick, A.A.; Pellielo, G.; Rimessi, A.; Chianese, D.; Yang, H.N.; Carbone, M.; Pinton, P.; Giorgi, C.; et al. Molecular Mechanisms of Autophagy in Cancer Development, Progression, and Therapy. Biomedicines 2022, 10, 1596. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, H.G. Apigenin Induces Autophagy and Cell Death by Targeting EZH2 under Hypoxia Conditions in Gastric Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13455. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A.; Ito, Y.; Ahmed, S.; Radwan, N.; Ahmed, H.S.; Eid, N. Targeting Autophagy with Natural Products as a Potential Therapeutic Approach for Cancer. Int. J. Mol. Sci. 2021, 22, 9807. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Liao, Y.; Qin, J.Y.; Li, W.H.; Tang, Z.Y. Adverse effects of chemoradiotherapy on invasion and metastasis of tumor cells. Genes. Dis. 2020, 7, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.P.; Lee, C.H.; Ying, T.H.; Lin, C.L.; Lin, C.L.; Hsueh, J.T.; Hsieh, Y.H. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget 2015, 6, 28851–28866. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Kello, M.; Kudlickova, Z.; Gazdova, M.; Mirossay, L.; Mojzisova, G.; Mojzis, J. Programmed Cell Death Alterations Mediated by Synthetic Indole Chalcone Resulted in Cell Cycle Arrest, DNA Damage, Apoptosis and Signaling Pathway Modulations in Breast Cancer Model. Pharmaceutics 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, M.A.A.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Wang, N.; Zhang, H. N-acetylcysteine induces apoptosis via the mitochondria-dependent pathway but not via endoplasmic reticulum stress in H9c2 cells. Mol. Med. Rep. 2017, 16, 6626–6633. [Google Scholar] [CrossRef]
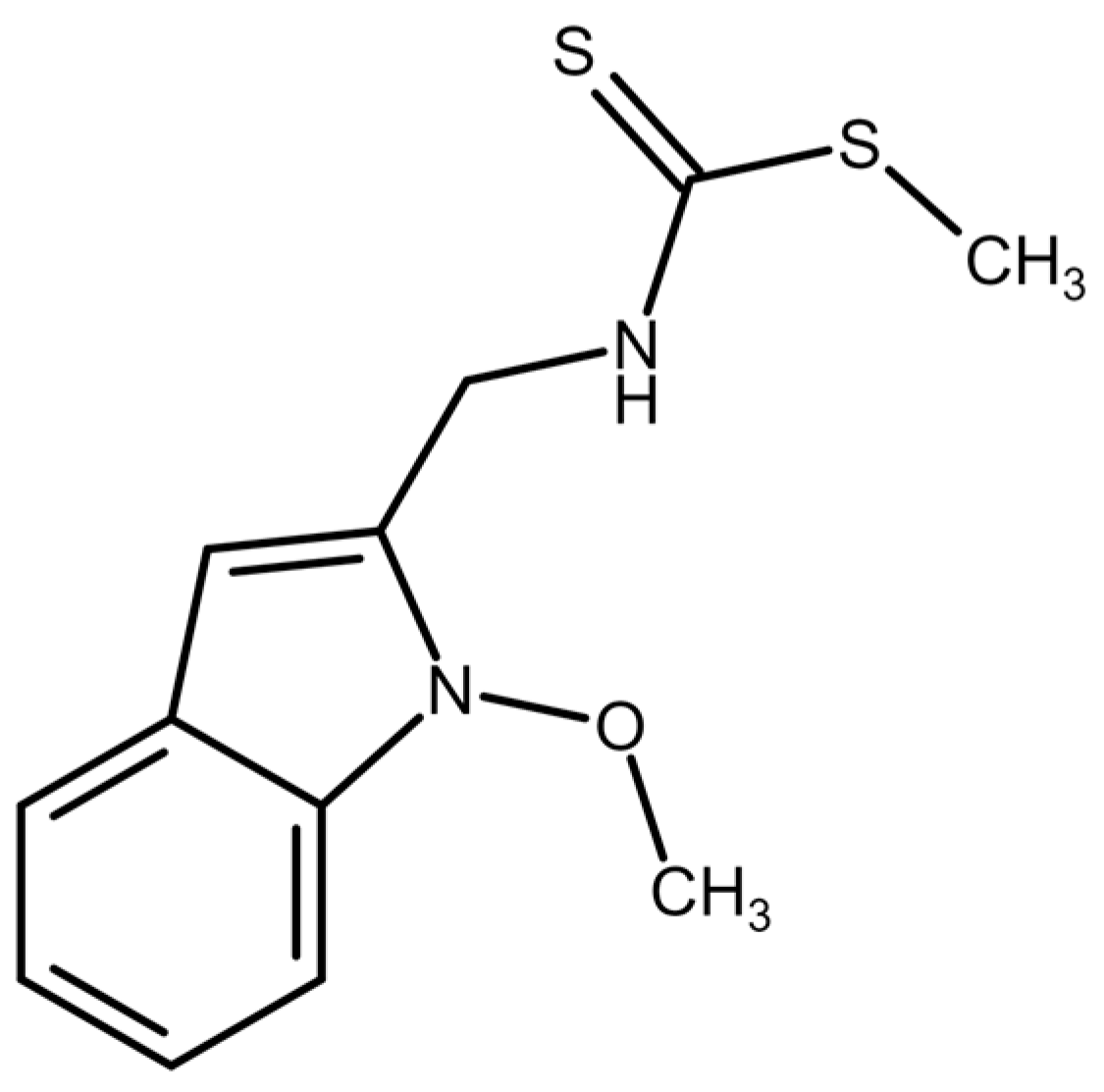




| Compound | Assay | Cell Line | ||
|---|---|---|---|---|
| A2780 | A2780cis | Bj-5ta | ||
| MB-591 | MTT | 3.62 ± 1.19 | 7.00 ± 0.82 | 27.43 ± 2.42 |
| BrdU | 5.41 ± 1.27 | 7.06 ± 1.09 | 18.11 ± 0.93 | |
| Selectivity index | 7.57/3.34 | 3.91/2.56 | ||
| CisPt | MTT | 1.64 ± 0.35 | 12.73 ± 2.55 | 31.3 ± 3.86 |
| Selectivity index | 19.08 | 2.45 |
| A | G1 | S | G2/M |
| DMSO | 61.7 ± 2.8 | 17.3 ± 2.6 | 22.4 ± 2.4 |
| IC12.5 | 59.7 ± 2.4 | 19.1 ± 2.4 | 22.5 ± 3.6 |
| IC25 | 61.9 ± 0.4 | 25.1 ± 0.2 * | 13.45 ± 0.7 * |
| IC50 | 56.1 ± 3.5 | 17.7 ± 2.9 | 26.3 ± 2.7 |
| B | G1 | S | G2/M |
| DMSO | 61.03 ± 2.57 | 22.53 ± 2.41 | 16.53 ± 2.67 |
| IC12.5 | 62.95 ± 4.17 | 11.08 ± 4.12 * | 26.66 ± 4.44 * |
| IC25 | 64.65 ± 0.21 | 25.3 ± 5.3 | 10.20 ± 4.8 |
| IC50 | 41.4 ± 3.55 ** | 29.5 ± 2.82 * | 28.2 ± 3.11 ** |
| A | G1 | S | G2/M | |
| 3 h | DMSO | 47.1 ± 0.1 | 31.7 ± 1.8 | 21.95 ± 3.6 |
| MB-591 | 50.65 ± 1.6 | 30.5 ± 4.2 | 19.25 ± 1.1 | |
| NAC | 47.9 ± 1.3 | 30.2 ± 5.8 | 21.1 ± 5.7 | |
| NAC/MB-591 | 53.85 ± 0.1 *** | 30.05 ± 4.3 | 15.1 ± 4.4 | |
| 6 h | DMSO | 52.05 ± 0.07 | 27.95 ± 0.63 | 19.05 ± 0.7 |
| MB-591 | 44.05 ± 4.2 | 40.05 ± 2.0 ** | 15.1 ± 4.0 | |
| NAC | 57.65 ± 1.48 * | 24.4 ± 6.5 | 18.1 ± 3.95 | |
| NAC/MB-591 | 54.95 ± 1.34 | 24.25 ± 3.6 ## | 19.9 ± 2.4 | |
| 12 h | DMSO | 53.55 ± 0.35 | 23.6 ± 0.14 | 18.1 ± 0.56 |
| MB-591 | 53.75 ± 1.9 | 6.20 ± 2.22 ** | 39.9 ± 0.42 *** | |
| NAC | 56.15 ± 1.48 | 24.25 ± 1.9 | 20.95 ± 2.61 | |
| NAC/MB-591 | 55.55 ± 0.21 * | 23.4 ± 3.95 # | 19.5 ± 3.99 # | |
| 24 h | DMSO | 49.1 ± 3.4 | 33.6 ± 2.8 | 17.3 ± 2.6 |
| MB-591 | 42.3 ± 4.2 | 22.3 ± 2.8 * | 35.4 ± 4.6 ** | |
| NAC | 52.1 ± 2.1 | 26.0 ± 2.0 | 21.1 ± 2.7 | |
| NAC/MB-591 | 52.45 ± 0.1 | 26.2 ± 3.4 | 21.15 ± 3.5 | |
| 48 h | DMSO | 58.86 ± 3.61 | 24.03 ± 5.7 | 16.76 ± 4.24 |
| MB-591 | 39.67 ± 3.05 ** | 24.8 ± 2.8 | 35.30 ± 1.83 * | |
| NAC | 56.26 ± 4.9 | 25.6 ± 5.7 | 18.58 ± 1.11 | |
| NAC/MB-591 | 57.7 ± 0.45 ### | 19.7 ± 3.9 | 22.67 ± 4.07 # | |
| 72 h | DMSO | 61.7 ± 2.81 | 17.36 ± 2.65 | 22.4 ± 2.98 |
| MB-591 | 56.1 ± 3.5 | 17.7 ± 2.9 | 26.3 ± 2.7 | |
| NAC | 60.0 ± 0.84 | 19.1 ± 2.55 | 21.87 ± 2.7 | |
| NAC/MB-591 | 62.1 ± 4.4 | 18.96 ± 4.3 | 18.26 ± 3.0 | |
| B | G1 | S | G2/M | |
| 3 h | DMSO | 51.2 ± 2.26 | 26.3 ± 3.25 | 21.65 ± 0.91 |
| MB-591 | 39.76 ± 5.19 | 50.15 ± 4.03 ** | 9.31 ± 3.8 ** | |
| NAC | 46.45 ± 0.21 | 29.35 ± 2.19 | 23.4 ± 0.42 | |
| NAC/MB-591 | 52.8 ± 0.34 | 24.86 ± 1.5 | 21.1 ± 1.9 | |
| 6 h | DMSO | 52.25 ± 0.07 | 22.3 ± 3.11 | 24.3 ± 3.81 |
| MB-591 | 46.35 ± 2.33 | 18.5 ± 3.52 | 34.9 ± 1.69 | |
| NAC | 52.1 ± 1.5 | 25.1 ± 3.2 | 21.36 ± 2.03 | |
| NAC/MB-591 | 53 ± 1.41 | 24.15 ± 5.44 | 23.0 ± 5.6 | |
| 12 h | DMSO | 54.93 ± 1.2 | 27.66 ± 3.06 | 16.76 ± 1.88 |
| MB-591 | 49.2 ± 1.69 * | 23.1 ± 1.27 | 28.6 ± 2.26 ** | |
| NAC | 60.46 ± 1.96 * | 16.06 ± 5.75 | 23.06 ± 4.65 | |
| NAC/MB-591 | 55.2 ± 1.55 | 29.4 ± 3.65 | 16.25 ± 1.76 # | |
| 24 h | DMSO | 51.63 ± 1.92 | 28.23 ± 2.95 | 19.07 ± 1.62 |
| MB-591 | 35.33 ± 4.85 ** | 36.06 ± 4.5 | 29.36 ± 3.17 ## | |
| NAC | 50.36 ± 3.26 | 28.0 ± 2.42 | 21.03 ± 1.7 | |
| NAC/MB-591 | 46.96 ± 2.9 # | 30.46 ± 5.7 | 22.8 ± 1.11 ## | |
| 48 h | DMSO | 56.36 ± 0.49 | 23.86 ± 0.28 | 19.83 ± 0.85 |
| MB-591 | 29.95 ± 0.21 *** | 29.86 ± 2.66 * | 38.3 ± 1.8 *** | |
| NAC | 51.63 ± 1.83 * | 24.16 ± 1.04 | 22.46 ± 3.59 | |
| NAC/MB-591 | 56.8 ± 1.21 ### | 19.76 ± 1.9 * | 24.46 ± 3.35 ## | |
| 72 h | DMSO | 61.03 ± 2.57 | 22.53 ± 2.41 | 16.53 ± 2.67 |
| MB-591 | 41.4 ± 3.55 ** | 29.5 ± 2.82 * | 28.2 ± 3.11 ** | |
| NAC | 57.56 ± 0.45 | 23.4 ± 0.52 | 17.8 ± 0.51 | |
| NAC/MB-591 | 41.4 ± 3.66 ** | 29.5 ± 2.82 * | 26.2 ± 3.92 * |
| Primary Antibody | MW (kDa) | Origin | Dilution | Catalogue No. | Manufacturer |
| PCNA (D3H8P) XP® Rabbit mAb | 36 | Rabbit | 1:1000 | #13110 | Cell Signaling Technology, Danvers, MA, USA |
| p21 Waf1/Cip1 (12D1) Rabbit mAb | 21 | Rabbit | 1:1000 | #2947 | |
| Phospho-Cyclin B1 (Ser133) (9E3) Rabbit mAb | 55 | Rabbit | 1:1000 | #4133 | |
| Cyclin B1 (D5C10) Rabbit mAb | 55 | Rabbit | 1:1000 | #12231 | |
| Phospho-Rb (Ser807/811) (D20B12) XP Rabbit mAb | 110 | Rabbit | 1:1000 | #8516 | |
| PARP (46D11) Rabbit mAb | 116/89 | Rabbit | 1:1000 | #9091 | |
| Phospho-Histone H2A.X (Ser139) (20E3) Rabbit mAb | 15 | Rabbit | 1:1000 | #9718 | |
| XIAP (3B6) Rabbit mAb | 53 | Rabbit | 1:1000 | #2045 | |
| Cleaved Caspase-9 (Asp315) (D8I9E) Rabbit mAb | 35 | Rabbit | 1:1000 | #20750 | |
| Phospho-PTEN (Ser380) Antibody | 54 | Rabbit | 1:1000 | #9551 | |
| Phospho-Akt (Thr308) (244F9) Rabbit mAb | 60 | Rabbit | 1:1000 | #4056 | |
| Phospho-mTOR (Ser2448) (D9C2) XP Rabbit mAb | 289 | Rabbit | 1:1000 | #5536 | |
| mTOR (7C10) Rabbit mAb | 289 | Rabbit | 1:1000 | #2983 | |
| Phospho-ULK1 (Ser757) (D7O6U) Rabbit mAb | 140–151 | Rabbit | 1:1000 | #14202 | |
| Beclin-1 (D40C5) | 60 | Rabbit | 1:1000 | #3495 | |
| Atg7 (D12B11) Rabbit mAb | 78 | Rabbit | 1:1000 | #8558 | |
| LC3A/B (D3U4C) XP® Rabbit mAb | 15–18 | Rabbit | 1:1000 | #12741 | |
| β-Actin (8H10D10) Mouse mAb | 45 | Mouse | 1:1000 | #3700 | |
| APAF1 Recombinant Rabbit Monoclonal Antibody (SY22-02) | 135 | Rabbit | 1:1000 | MA5-32082 | Thermo Scientific |
| Secondary Antibody | MW (kDa) | Origin | Dilution | Catalogue No. | Manufacturer |
| Anti-rabbit IgG, HRP-linkedAntibody | - | Goat | 1:1000 | #7074 | Cell Signaling Technology, Danvers, MA, USA |
| Anti-mouse IgG, HRP-linked Antibody | - | Goat | 1:1000 | #7076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zigová, M.; Miškufová, V.; Budovská, M.; Michalková, R.; Mojžiš, J. Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules 2024, 29, 1773. https://doi.org/10.3390/molecules29081773
Zigová M, Miškufová V, Budovská M, Michalková R, Mojžiš J. Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules. 2024; 29(8):1773. https://doi.org/10.3390/molecules29081773
Chicago/Turabian StyleZigová, Martina, Viktória Miškufová, Marianna Budovská, Radka Michalková, and Ján Mojžiš. 2024. "Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC" Molecules 29, no. 8: 1773. https://doi.org/10.3390/molecules29081773
APA StyleZigová, M., Miškufová, V., Budovská, M., Michalková, R., & Mojžiš, J. (2024). Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules, 29(8), 1773. https://doi.org/10.3390/molecules29081773






