Photoinduced Site-Selective Aryl C-H Borylation with Electron-Donor-Acceptor Complex Derived from B2Pin2 and Isoquinoline
Abstract
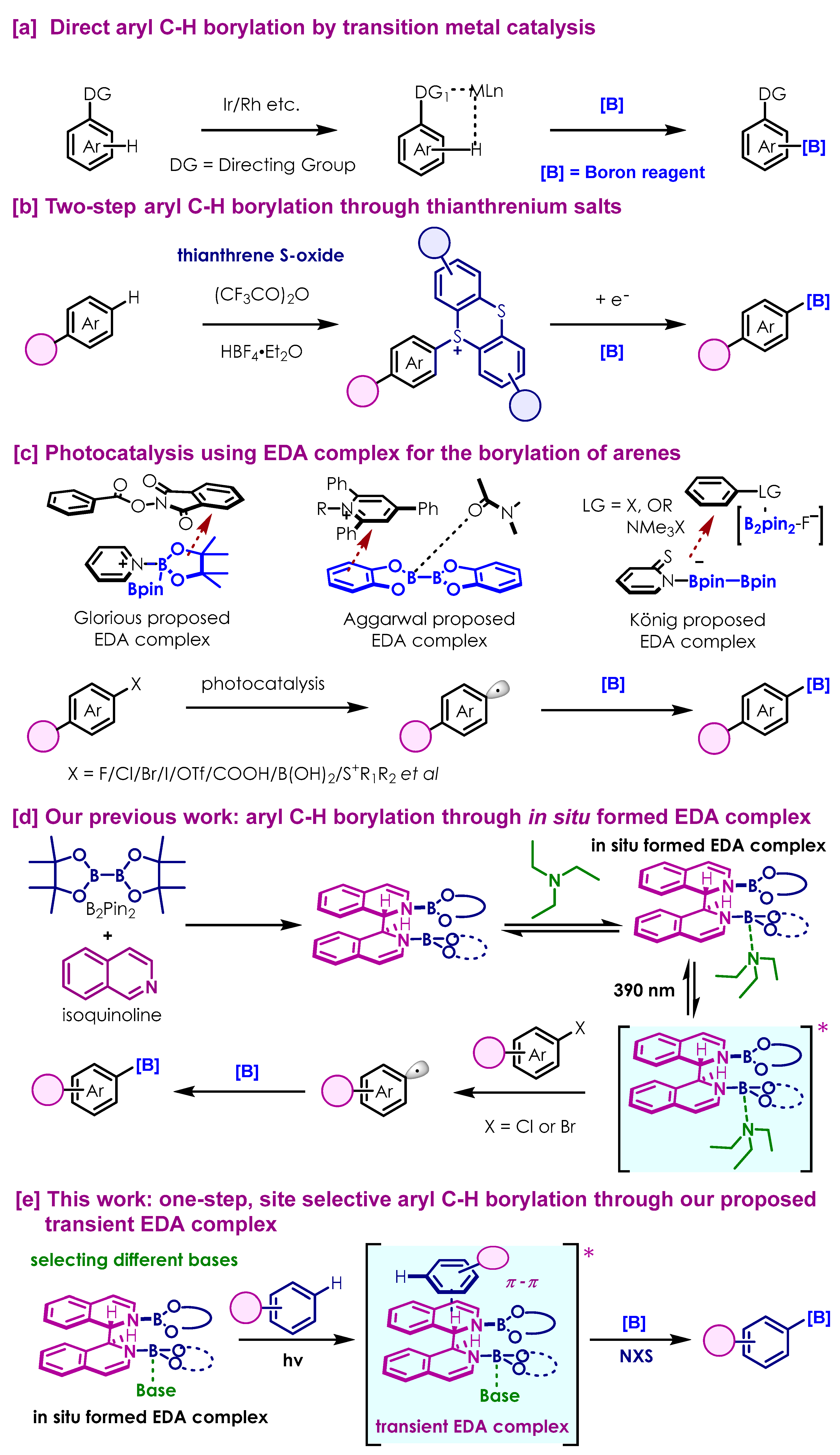
:1. Introduction
2. Results
2.1. Optimization of Reaction Conditions for the Aryl C-H Bond Borylation
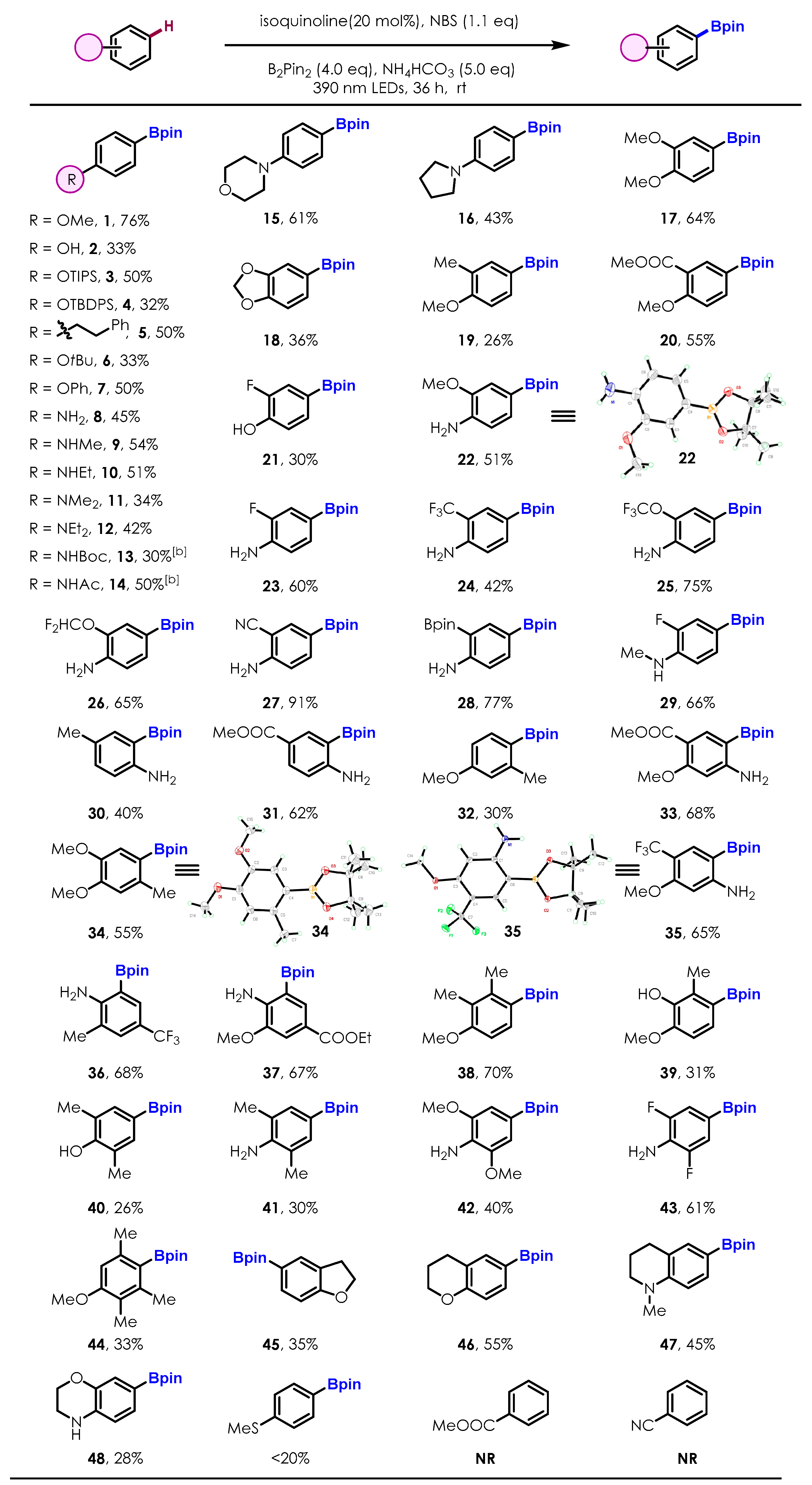
2.2. Substrate Scope of the Photoinduced Aryl C-H Borylation
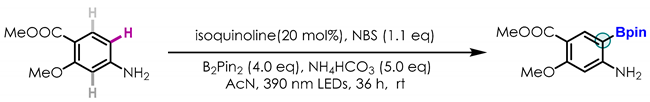
2.3. Late-Stage Functionalization and Novel Cascade Transformation
3. Discussion
4. Methods and Materials
4.1. Photo Reaction Setup
4.2. Thin-Layer Chromatography (TLC)
4.3. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.4. Materials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Chemler, S.R.; Trauner, D.; Danishefsky, S.J. The B-Alkyl Suzuki–Miyaura Cross-Coupling Reaction: Development, Mechanistic Study, and Applications in Natural Product Synthesis. Angew. Chem. Int. Ed. 2001, 40, 4544–4568. [Google Scholar] [CrossRef]
- Lam, P.Y.S.; Clark, C.G.; Saubern, S.; Adams, J.; Winters, M.P.; Chan, D.M.T.; Combs, A. New aryl/heteroaryl C-N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2949. [Google Scholar] [CrossRef]
- Antilla, J.C.; Buchwald, S.L. Copper-Catalyzed Coupling of Arylboronic Acids and Amines. Org. Lett. 2001, 3, 2077–2079. [Google Scholar] [CrossRef]
- West, M.J.; Fyfe, J.W.B.; Vantourout, J.C.; Watson, A.J.B. Mechanistic Development and Recent Applications of the Chan–Lam Amination. Chem. Rev. 2019, 119, 12491–12523. [Google Scholar] [CrossRef]
- Zou, Y.-Q.; Chen, J.-R.; Liu, X.-P.; Lu, L.-Q.; Davis, R.L.; Jørgensen, K.A.; Xiao, W.-J. Highly Efficient Aerobic Oxidative Hydroxylation of Arylboronic Acids: Photoredox Catalysis Using Visible Light. Angew. Chem. Int. Ed. 2012, 51, 784–788. [Google Scholar] [CrossRef]
- Wu, H.; Hynes, J. Copper-catalyzed chlorination of functionalized arylboronic acids. Org. Lett. 2010, 12, 1192–1195. [Google Scholar] [CrossRef]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern Transition-Metal-Catalyzed Carbon-Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- Fawcett, A.; Murtaza, A.; Gregson, C.H.U.; Aggarwal, V.K. Strain-Release-Driven Homologation of Boronic Esters: Application to the Modular Synthesis of Azetidines. J. Am. Chem. Soc. 2019, 141, 4573–4578. [Google Scholar] [CrossRef]
- Letsinger, R.L.; Skoog, I.H. The preparation and some properties of 2-methyl-1-propene-1-boronic acid. J. Org. Chem. 1953, 18, 895–897. [Google Scholar] [CrossRef]
- Légaré, M.; Rang, M.; Bélanger-Chabot, G.; Schweizer, J.I.; Krummenacher, I.; Bertermann, R.; Arrowsmith, M.; Holthausen, M.C.; Braunschweig, H. The reductive coupling of dinitrogen. Science 2019, 363, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- Partyka, D.V. Transmetalation of Unsaturated Carbon Nucleophiles from Boron-Containing Species to the Mid to Late d-Block Metals of Relevance to Catalytic C–X Coupling Reactions (X = C, F, N, O, Pb, S, Se, Te). Chem. Rev. 2011, 111, 1529–1595. [Google Scholar] [CrossRef] [PubMed]
- Trouvé, J.; Gramage-Doria, R. Beyond hydrogen bonding: Recent trends of outer sphere interactions in transition metal catalysis. Chem. Soc. Rev. 2021, 50, 3565–3584. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Perry, I.B.; Bissonnette, N.B.; Buksh, B.F.; Edwards, G.A.; Frye, L.I.; Garry, O.L.; Lavagnino, M.N.; Li, B.-X.; Liang, Y.; et al. Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis. Chem. Rev. 2022, 122, 1485–1542. [Google Scholar] [CrossRef]
- Lv, J.; Chen, X.; Xue, X.-S.; Zhao, B.; Liang, Y.; Wang, M.; Jin, L.; Yuan, Y.; Han, Y.; Zhao, Y.; et al. Metal-free directed sp2-C–H borylation. Nature 2019, 575, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Haldar, C.; Hoque, M.E.; Chaturvedi, J.; Hassan, M.M.M.; Chattopadhyay, B. Ir-catalyzed proximal and distal C-H borylation of arenes. Chem. Commun. 2021, 57, 13059–13074. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ferger, M.; Shi, Z.-Z.; Marder, T.B. Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev. 2021, 50, 13129–13188. [Google Scholar] [CrossRef] [PubMed]
- Bisht, R.; Haldar, C.; Hassan, M.M.M.; Hoque, M.E.; Chaturvedi, J.; Chattopadhyay, B. Metal-catalysed C–H bond activation and borylation. Chem. Soc. Rev. 2022, 51, 5042–5100. [Google Scholar] [CrossRef]
- Börgel, J.; Ritter, T. Late-Stage Functionalization. Chem 2020, 6, 1877–1887. [Google Scholar] [CrossRef]
- Zhang, L.; Ritter, T. A Perspective on Late-Stage Aromatic C–H Bond Functionalization. J. Am. Chem. Soc. 2022, 144, 2399–2414. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Ritter, T. Site-Selective Late-Stage C–H Functionalization via Thianthrenium Salts. Synlett 2022, 33, 339–345. [Google Scholar]
- Mo, F.; Qiu, D.; Zhang, L.; Wang, J. Recent Development of Aryl Diazonium Chemistry for the Derivatization of Aromatic Compounds. Chem. Rev. 2021, 121, 5741–5829. [Google Scholar] [CrossRef] [PubMed]
- Kvasovsa, N.; Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 2021, 50, 2244–2259. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Plutschack, M.B.; Riegger, J.; Yu, W.; Speicher, S.; Ho, M.; Frank, N.; Ritter, T. Site-selective and versatile aromatic C-H functionalization by thianthrenation. Nature 2019, 567, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Chen, J.-R.; Xiao, W.-J. Visible Light-Driven Radical-Mediated C–C Bond Cleavage/Functionalization in Organic Synthesis. Chem. Rev. 2021, 121, 506–561. [Google Scholar] [CrossRef] [PubMed]
- Bryden, M.A.; Zysman-Colman, E. Organic thermally activated delayed fluorescence (TADF) compounds used in photocatalysis. Chem. Soc. Rev. 2021, 50, 7587–7680. [Google Scholar] [CrossRef] [PubMed]
- Holmberg-Douglas, N.; Nicewicz, D.A. Photoredox-Catalyzed C-H Functionalization Reactions. Chem. Rev. 2022, 122, 1925–2016. [Google Scholar] [CrossRef] [PubMed]
- Beil, S.B.; Chen, T.Q.; Intermaggio, N.E.; MacMillan, D.W.C. Carboxylic Acids as Adaptive Functional Groups in Metallaphotoredox Catalysis. Acc. Chem. Res. 2022, 55, 3481–3494. [Google Scholar] [CrossRef]
- Li, Q.; Liu, M.; Jiang, M.; Wan, L.; Ning, Y.; Chen, F.-E. Photo-induced 1,2-aryl migration of 2-chloro-1-arylpropanone under batch and flow conditions: Rapid, scalable and sustainable approach to 2-arylpropionic acids. Chin. Chem. Lett. 2024, 35, 108576. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, L.; Wang, C.; Feng, S.; Ma, R.; Wan, J.-P. Recent advances in visible light-mediated chemical transformations of enaminones. Chin. Chem. Lett. 2024, 35, 108977. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Mazzarella, D.; Melchiorre, P. Synthetic Methods Driven by the Photoactivity of Electron Donor-Acceptor Complexes. J. Am. Chem. Soc. 2020, 142, 5461–5476. [Google Scholar] [CrossRef]
- Yuan, Y.-Q.; Majumder, S.; Yang, M.-H.; Guo, S.-R. Recent advances in catalyst-free photochemical reactions via electron-donor-acceptor (EDA) complex process. Tetrahedron Lett. 2021, 61, 151506. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Cao, K.; Zhang, X.; Jiang, H.; Li, J. Synthetic reactions driven by electron-donor–acceptor (EDA) complexes. Beilstein J. Org. Chem. 2021, 17, 771–799. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, L.J.C.; Pahar, S.; Richards, E.; Melen, R.L.; Chris Slootweg, J. Insights into Single-Electron-Transfer Processes in Frustrated Lewis Pair Chemistry and Related Donor–Acceptor Systems in Main Group Chemistry. Chem. Rev. 2023, 123, 9653–9675. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, F.; Strieth-Kalthoff, F.; Klauck, F.J.R.; James, M.J.; Glorius, F. Deaminative Borylation of Aliphatic Amines Enabled by Visible Light Excitation of an Electron Donor–Acceptor Complex. Chem. Eur. J. 2018, 24, 17210–17214. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, L.; Noble, A.; Aggarwal, V.K. Photoinduced Deaminative Borylation of Alkylamines. J. Am. Chem. Soc. 2018, 140, 10700–10704. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; König, B. Photo-induced thiolate catalytic activation of inert Caryl-hetero bonds for radical borylation. Chem 2021, 7, 1653–1665. [Google Scholar] [CrossRef]
- MacKenzie, I.A.; Wang, L.F.; Onuska, N.P.R.; Williams, O.F.; Begam, K.; Moran, A.M.; Dunietz, B.D.; Nicewicz, D.A. Discovery and characterization of an acridine radical photoreductant. Nature 2020, 580, 76–80. [Google Scholar] [CrossRef]
- Li, M.; Liu, S.; Bao, H.; Li, Q.; Deng, Y.-H.; Sun, T.-Y.; Wang, L.F. Photoinduced metal-free borylation of aryl halides catalysed by an in situ formed donor–acceptor complex. Chem. Sci. 2022, 3, 4909–4914. [Google Scholar] [CrossRef]
- Deng, Y.-H.; Li, Q.; Li, M.; Wang, L.F.; Sun, T.-Y. Rational design of super reductive EDA photocatalyst for challenging reactions: A theoretical and experimental study. RSC Adv. 2024, 14, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiao, L. Visible-Light-Induced Organocatalytic Borylation of Aryl Chlorides. J. Am. Chem. Soc. 2019, 141, 9124–9128. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, J.; Huang, C.-Y.; Li, C.-J. Aromatic Chemistry in the Excited State: Facilitating Metal-Free Substitutions and Cross-Couplings. Angew. Chem. Int. Ed. 2020, 59, 1786–1796. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Chen, S.; Zhou, Z.; He, Y.; Liu, Z.; Liu, Y.; Feng, Z. Iron/B2pin2 catalytic system enables the generation of alkyl radicals from inert alkyl C-O bonds for amien synthesis. Chin. Chem. Lett. 2024, 35, 108303. [Google Scholar] [CrossRef]
- Liao, L.; Lin, D.; Histand, G. Visible light induced oxidative coupling of purines with arenes. Chin. Chem. Lett. 2023, 34, 107467. [Google Scholar] [CrossRef]
- Nishii, Y.; Ikeda, M.; Hayashi, Y.; Kawauchi, S.; Miura, M. Triptycenyl Sulfide: A Practical and Active Catalyst for Electrophilic Aromatic Halogenation Using N-Halosuccinimides. J. Am. Chem. Soc. 2020, 142, 1621–1629. [Google Scholar] [CrossRef]
- Song, S.; Li, X.; Wei, J.; Wang, W.; Zhang, Y.; Ai, L.; Zhu, Y.; Shi, X.; Zhang, X.; Jiao, N. DMSO-catalysed late-stage chlorination of (hetero)arenes. Nat. Catal. 2020, 3, 107–115. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Jin, S.-F.; Dang, H.T.; Haug, G.C.; He, R.; Nguyen, V.D.; Nguyen, V.T.; Arman, H.D.; Schanze, K.S.; Larionov, O.V. Visible Light-Induced Borylation of C–O, C–N, and C–X Bonds. J. Am. Chem. Soc. 2020, 142, 1603–1613. [Google Scholar] [CrossRef]
- Chen, K.; Cheung, M.S.; Lin, Z.-Y.; Li, P.-F. Metal-free borylation of electron-rich aryl (pseudo)halides under continuous-flow photolytic conditions. Org. Chem. Front. 2016, 3, 875–879. [Google Scholar] [CrossRef]
- McManus, J.B.; Nicewicz, D.A. Direct C–H Cyanation of Arenes via Organic Photoredox Catalysis. J Am Chem Soc. 2017, 139, 2880–2883. [Google Scholar] [CrossRef]
- Uetake, Y.; Niwa, T.; Hosoya, T. Rhodium-Catalyzed ipso-Borylation of Alkylthioarenes via C–S Bond Cleavage. Org. Lett. 2016, 18, 2758–2761. [Google Scholar] [CrossRef] [PubMed]
- Mir, R.; Dudding, T. Phase-Transfer Catalyzed O-Silyl Ether Deprotection Mediated by a Cyclopropenium Cation. J. Org. Chem. 2017, 82, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Nitelet, A.; Thevenet; Schiavi, B.; Hardouin, C.; Fournier, J.; Tamion, R.; Pannecoucke, X.; Jubault, P.; Poisson, T. Copper-Photocatalyzed Borylation of Organic Halides under Batch and Continuous-Flow Conditions. Chem. -Eur. J. 2019, 25, 3262–3266. [Google Scholar]
- Gong, Y.-X.; Zhu, Z.-D.; Qian, Q.; Tong, W.-Q.; Gong, H.-G. Miyaura Borylation and One-Pot Two-Step Homocoupling of Aryl Chlorides and Bromides under Solvent-Free Conditions. Org. Lett. 2021, 23, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Dzhevakov, P.B.; Topchiy, M.A.; Zharkova, D.A.; Morozov, O.S.; Asachenko, A.F.; Nechaev, M.S. Miyaura Borylation and One-Pot Two-Step Homocoupling of Aryl Chlorides and Bromides under Solvent-Free Conditions. Adv. Synth. Catal. 2016, 358, 977–983. [Google Scholar] [CrossRef]
- Wei, D.; Sadek, O.; Dorcet, V.; Roisnel, T.; Darcel, C.; Gras, E.; Clot, E.; Sortais, J.-B. Selective mono N-methylation of anilines with methanol catalyzed by rhenium complexes: An experimental and theoretical study. J. Catal. 2018, 366, 300–309. [Google Scholar] [CrossRef]
- Firth, J.D.; Hammarback, L.A.; Burden, T.J.; Eastwood, J.B.; Donald, J.R.; Horbaczewskyj, C.S.; McRobie, T.; Tramaseur, A.; Clark, I.P.; Towrie, M.; et al. Light- and Manganese-Initiated Borylation of Aryl Diazonium Salts: Mechanistic Insight on the Ultrafast Time-Scale Revealed by Time-Resolved Spectroscopic Analysis. Chem. Eur. J. 2020, 27, 3979–3985. [Google Scholar]
- Yamamoto, T.; Morita, T.; Takagi, J.; Yamakawa, T. NiCl2(PMe3)2-Catalyzed Borylation of Aryl Chlorides. Org. Lett. 2011, 12, 5766–5769. [Google Scholar]
- Gisbertz, S.; Reischauer, S.; Pieber, B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 2020, 3, 611–620. [Google Scholar]
- Guerrand, H.D.S.; Marciasini, L.D.; Jousseaume, M.; Vaultier, M.; Pucheault, M. Borylation of Unactivated Aryl Chlorides under Mild Conditions by Using Diisopropylaminoborane as a Borylating Reagent. Chem. Eur. J. 2014, 20, 5573–5579. [Google Scholar] [CrossRef]
- Dong, J.; Guo, H.; Hu, Q.-S. Room Temperature Ni0/PCy3-Catalyzed Coupling Reactions of Aryl Arenesulfonates with Bis(pinacolato)diboron. Eur. J. Org. Chem. 2017, 2017, 7087–7090. [Google Scholar] [CrossRef]
- Waring, M.J.; Birch, A.M.; Birtles, S.; Buckett, L.K.; Butlin, R.J.; Campbell, L.; Gutierrez, P.M.; Kemmitt, P.D.; Leach, A.G.; MacFaul, P.A.; et al. Optimisation of biphenyl acetic acid inhibitors of diacylglycerol acetyl transferase 1—the discovery of AZD2353. Med. Chem. Commun. 2013, 4, 159–164. [Google Scholar] [CrossRef]
- Preshlock, S.M.; Plattner, D.L.; Maligres, P.E.; Krska, S.W.; Maleczka J, R.E.; Smith, M.R., II. A Traceless Directing Group for C-H Borylation. Angew. Chem. Int. Ed. 2013, 52, 12915–12919. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, S.; Tang, S.-B.; Meng, H.; Jin, L.; Mo, F.-Y.; Zhang, Y.; Wang, J.-B. Synthesis of Trimethylstannyl Arylboronate Compounds by Sandmeyer-Type Transformations and Their Applications in Chemoselective Cross-Coupling Reactions. J. Org. Chem. 2014, 79, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.T.; Williams, B.D.; Phipps, R.J. Para-Selective C–H Borylation of Common Arene Building Blocks Enabled by Ion-Pairing with a Bulky Countercation. J. Am. Chem. Soc. 2019, 141, 15477–15482. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.J.; Emer, E.; Preshlock, S.; Schedler, M.; Tredwell, M.; Verhoog, S.; Mercier, J.; Genicot, C.; Gouverneur, V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Jana, N.; Jones, C.; Driver, T.G. Control of the Chemoselectivity of Metal N-Aryl Nitrene Reactivity: C–H Bond Amination versus Electrocyclization. J. Am. Chem. Soc. 2016, 138, 13271–13280. [Google Scholar] [CrossRef]
- Inglis, S.R.; Woon, E.C.Y.; Thompson, A.L.; Schofield, C.J. Observations on the Deprotection of Pinanediol and Pinacol Boronate Esters via Fluorinated Intermediates. J. Org. Chem. 2010, 75, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Clary, J.W.; Rettenmaier, T.J.; Snelling, R.; Bryks, W.; Banwell, J.; Wipke, W.T.; Singaram, B. Hydride as a Leaving Group in the Reaction of Pinacolborane with Halides under Ambient Grignard and Barbier Conditions. One-Pot Synthesis of Alkyl, Aryl, Heteroaryl, Vinyl, and Allyl Pinacolboronic Esters. J. Org, Chem. 2011, 76, 9602–9610. [Google Scholar] [CrossRef] [PubMed]
- Zernickel, A.; Du, W.-Y.; Ghorpade, S.A.; Sawant, D.N.; Makki, A.A.; Sekar, N.; Eppinger, J. Bedford-Type Palladacycle-Catalyzed Miyaura Borylation of Aryl Halides with Tetrahydroxydiboron in Water. J. Org. Chem. 2018, 83, 1842–1851. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.R.; Sylvester, E.; Diver, S.T. Macrocyclic N-Heterocyclic Carbenes: Synthesis and Catalytic Applications. Organometallics. 2019, 38, 2338–2346. [Google Scholar] [CrossRef]
- Chapman, T.M.; Osborne, S.A.; Wallace, C.; Birchall, K.; Bouloc, N.; Jones, H.M.; Ansell, K.H.; Taylor, D.L.; Clough, B.; Green, J.L.; et al. Optimization of an Imidazopyridazine Series of Inhibitors of Plasmodium falciparum Calcium-Dependent Protein Kinase 1 (PfCDPK1). J. Med. Chem. 2014, 57, 3570–3587. [Google Scholar] [CrossRef]
- Lyu, H.-R.; Kevlishvili, I.; Yu, X.; Liu, P.; Dong, G.-B. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 2021, 372, 175–182. [Google Scholar] [CrossRef]
- Yin, Q.; Klare, H.F.T.; Oestreich, M. Catalytic Friedel–Crafts C−H Borylation of Electron-Rich Arenes: Dramatic Rate Acceleration by Added Alkenes. Angew. Chem. Int. Ed. 2017, 56, 3712–3717. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-C.; Li, M.-Y.; Liu, T.; Tong, L.-J.; Peng, T.; Wei, L.-X.; Ding, J.; Xie, H.; Duan, W.-H. Discovery of a New Series of Naphthamides as Potent VEGFR-2 Kinase Inhibitors. ACS Med. Chem. Lett. 2014, 5, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-P.; Xu, G.-Q.; Zhou, Q.-H.; Chung, L.-W.; Tang, W.-J. Practical and Asymmetric Reductive Coupling of Isoquinolines Templated by Chiral Diborons. J. Am. Chem. Soc. 2017, 139, 9767–9770. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V. Efficient diffuse function-augmented basis sets for anion calculations. III.† The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]




 | ||
| Entry | Deviations from Standard Condition | Yield (%) [b] |
| 1 | none | 72(68) [c] |
| 2 | NEt3 instead of NH4HCO3 | 31 |
| 3 | KOH instead of NH4HCO3 | trace |
| 4 | Cs2CO3 instead of NH4HCO3 | 54 |
| 5 | NaHCO3 instead of NH4HCO3 | 19 |
| 6 | 2.0 eq NBS instead of 1.1 eq NBS | 54 |
| 7 | NO NBS instead of 1.1 eq NBS | 0 |
| 8 | No light | 0 |
| 9 | No isoquinoline | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Deng, Y.-H.; Chang, Q.; Li, J.; Wang, C.; Wang, L.; Sun, T.-Y. Photoinduced Site-Selective Aryl C-H Borylation with Electron-Donor-Acceptor Complex Derived from B2Pin2 and Isoquinoline. Molecules 2024, 29, 1783. https://doi.org/10.3390/molecules29081783
Li M, Deng Y-H, Chang Q, Li J, Wang C, Wang L, Sun T-Y. Photoinduced Site-Selective Aryl C-H Borylation with Electron-Donor-Acceptor Complex Derived from B2Pin2 and Isoquinoline. Molecules. 2024; 29(8):1783. https://doi.org/10.3390/molecules29081783
Chicago/Turabian StyleLi, Manhong, Yi-Hui Deng, Qianqian Chang, Jinyuan Li, Chao Wang, Leifeng Wang, and Tian-Yu Sun. 2024. "Photoinduced Site-Selective Aryl C-H Borylation with Electron-Donor-Acceptor Complex Derived from B2Pin2 and Isoquinoline" Molecules 29, no. 8: 1783. https://doi.org/10.3390/molecules29081783






