Zanthoxylum bungeanum Waste-Derived High-Nitrogen Self-Doped Porous Carbons as Efficient Adsorbents for Methylene Blue
Abstract
1. Introduction
2. Results and Discussion
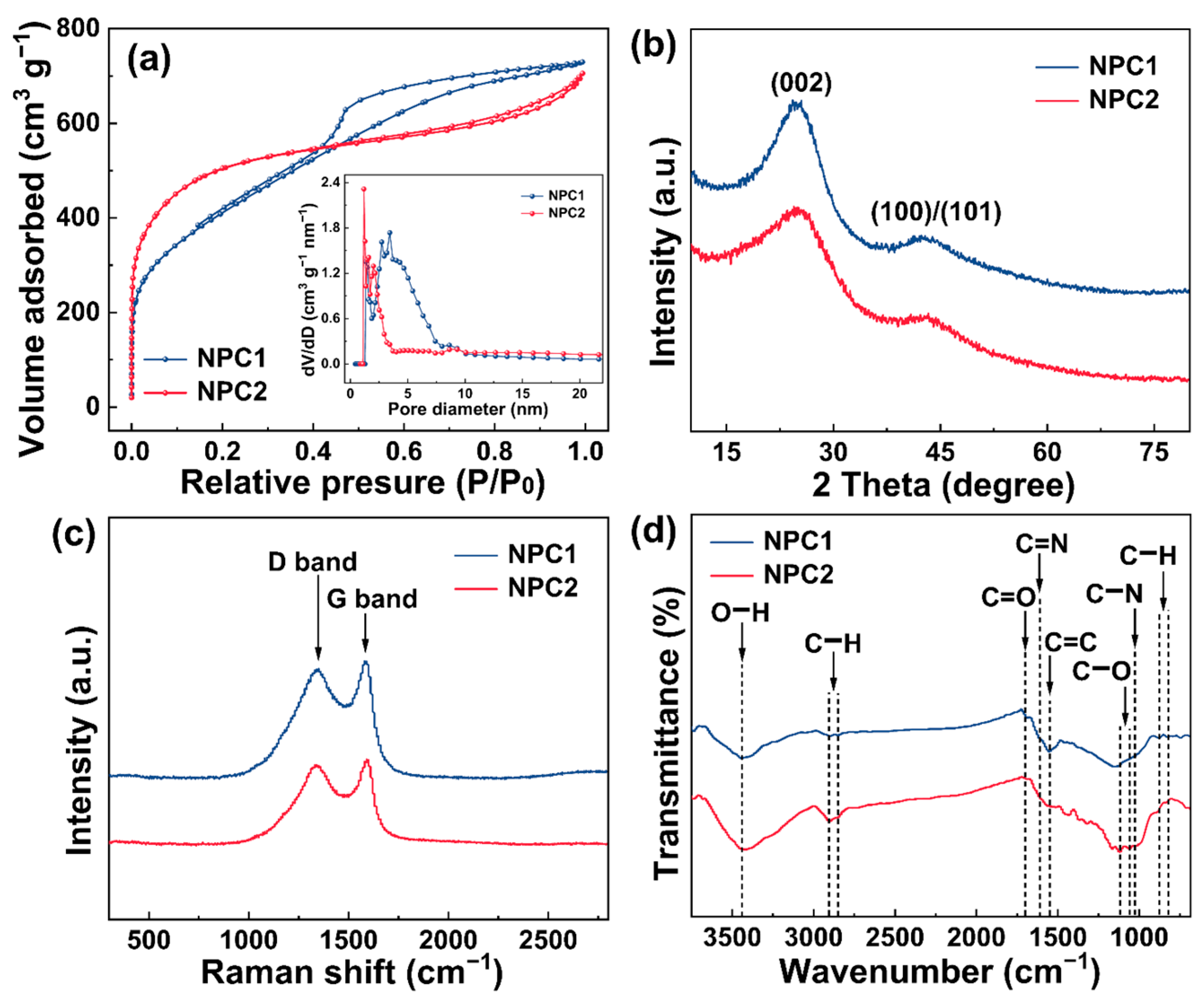
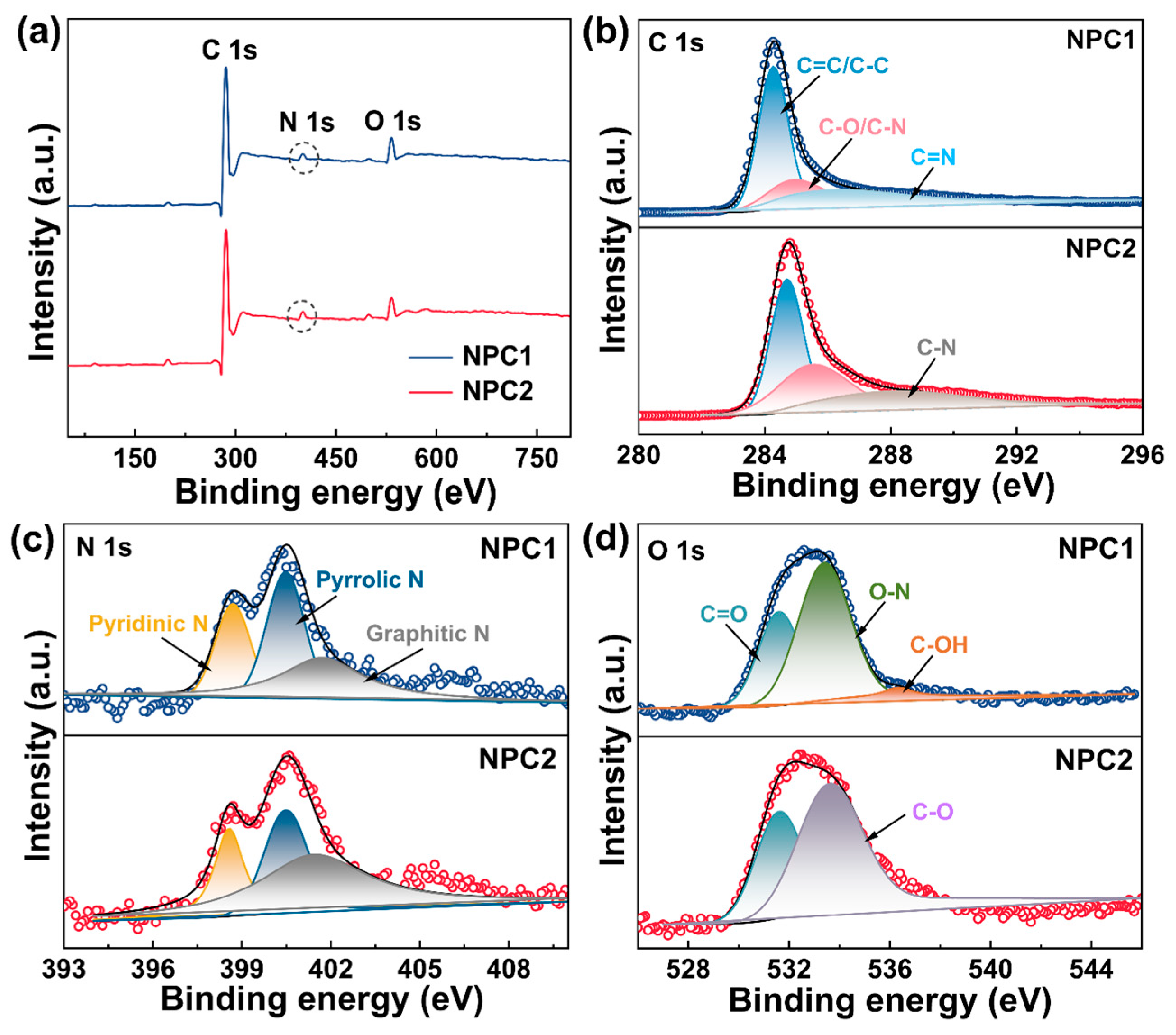
2.1. Morphology and Structural Characterizations of N Self-Doped Porous Carbons
2.2. Adsorption Performance of N Self-Doped Porous Carbons towards MB
2.3. Underlying Adsorption Mechanism of MB by N Self-Doped Porous Carbons
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of N Self-Doped Porous Carbons
3.3. Characterization of N Self-Doped Porous Carbons
3.4. Adsorption of MB by N Self-Doped Porous Carbons
3.5. Recyclability of N Self-Doped Porous Carbons
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.Y.; Fagbohun, E.O.; Zhu, H.K.; Hussain, A.; Wang, F.; Cui, Y.B. One-step synthesis of magnetic asphalt-based activated carbon with high specific surface area and adsorption performance for methylene blue. Sep. Purif. Technol. 2023, 321, 124205. [Google Scholar] [CrossRef]
- Do, J.Y.; Moradi, H.; Kim, D.S.; Yang, J.K.; Chang, Y.Y.; Choi, S.S. Mesoporous biobased carbonaceous adsorbent for dye removal from water: Eutectic molten salts effect and adsorption mechanisms. Diam. Relat. Mater. 2023, 136, 110018. [Google Scholar] [CrossRef]
- Dolas, H. Activated carbon synthesis and methylene blue adsorption from pepper stem using microwave assisted impregnation method: Isotherm and kinetics. J. King Saud Univ. Sci. 2023, 35, 102559. [Google Scholar] [CrossRef]
- Wu, T.T.; Yang, G.P.; Cao, J.X.; Xu, Z.W.; Jiang, X.H. Activation and adsorption mechanisms of methylene blue removal by porous biochar adsorbent derived from eggshell membrane. Chem. Eng. Res. Des. 2022, 188, 330–341. [Google Scholar] [CrossRef]
- Singh, J.; Basu, S.; Bhunia, H. CO2 capture by modified porous carbon adsorbents: Effect of various activating agents. J. Taiwan Inst. Chem. E 2019, 102, 438–447. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhao, Y.H.; Liao, Q.H.; Li, Z.X.; Jue, D.W.; Tang, J.M. Sweet-potato-vine-based high-performance porous carbon for methylene blue adsorption. Molecules 2023, 28, 819. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Liu, Y.H.; Zhang, L.; Xing, B.S. N self-doped hierarchically porous carbon derived from biomass as an efficient adsorbent for the removal of tetracycline antibiotics. Sci. Total Environ. 2022, 822, 153567. [Google Scholar] [CrossRef]
- Lin, F.L.; Liu, X.H.; Ma, M.N.; Qi, F.L.; Pan, Y.; Wang, L.; Ma, P.Y.; Zhang, Y. Real-time monitoring the carbonization and activation process of activated carbon prepared from Chinese parasol via zinc chloride activation. J. Anal. Appl. Pyrol. 2021, 155, 105089. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, H.F.; Jiang, M.L.; Zhang, Q.P. Preparation and characterization of activated carbon derived from deashing coal slime with ZnCl2 activation. Colloid. Surface A 2022, 641, 128124. [Google Scholar] [CrossRef]
- Rosli, N.A.; Ahmad, M.A.; Noh, T.U. Unleashing the potential of pineapple peel-based activated carbon: Response surface methodology optimization and regeneration for methylene blue and methyl red dyes adsorption. Inorg. Chem. Commun. 2023, 155, 111041. [Google Scholar] [CrossRef]
- Mariana, M.; Mistar, E.M.; Alfatah, T.; Supardan, M.D. High-porous activated carbon derived from Myristica fragrans shell using one-step KOH activation for methylene blue adsorption. Bioresour. Technol. Rep. 2021, 16, 100845. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhang, C.; Chen, W.X.; Liu, J.L.; Chen, D.L. Excellent electrochemical performance of porous carbon by in situ nitrogen-doped derived from polyurethane. Mater. Sci. Eng. B 2023, 296, 116610. [Google Scholar] [CrossRef]
- Ma, P.Y.; Yao, S.W.; Wang, Z.Q.; Qi, F.L.; Liu, X.H. Preparation of nitrogen-doped hierarchical porous carbon aerogels from agricultural wastes for efficient pollution adsorption. Sep. Purif. Technol. 2023, 311, 123250. [Google Scholar] [CrossRef]
- Sun, H.Z.; Xiao, M.J.; Zhu, F.L. Nitrogen doped porous carbon with high rate performance for lithium ion storage. J. Electroanal. Chem. 2023, 932, 117254. [Google Scholar] [CrossRef]
- Chung, J.; Sharma, N.; Kim, M.; Yun, K. Activated carbon derived from sucrose and melamine as low-cost adsorbent with fast adsorption rate for removal of methylene blue in wastewaters. J. Water Process Eng. 2022, 47, 102763. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.P.; Cheng, X.X.; Ling, Q.F.; Chen, H.; Barati, B.; Yao, Q.F.; Abomohra, A.; Hu, X.; Bartocci, P.; et al. A mechanism study of methylene blue adsorption on seaweed biomass derived carbon: From macroscopic to microscopic scale. Process Saf. Environ. 2023, 172, 1132–1143. [Google Scholar] [CrossRef]
- Li, Z.H.; Xing, B.; Ding, Y.; Li, Y.C.; Wang, S.R. A high-performance biochar produced from bamboo pyrolysis with in-situ nitrogen doping and activation for adsorption of phenol and methylene blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Lv, L.J.; Huang, Y.; Cao, D.P. Nitrogen-doped porous carbons with ultrahigh specific surface area as bifunctional materials for dye removal of wastewater and supercapacitors. Appl. Surf. Sci. 2018, 456, 184–194. [Google Scholar] [CrossRef]
- Hu, X.D.; Zhuo, K.L.; Sun, D.; Du, Q.Z.; Sun, L.; Chen, Y.J.; Bai, G.Y.; Wang, J.J. Nitrogen-doped agar-derived porous carbon with long cycle life for high-performance ionic liquid-based supercapacitors. Diam. Relat. Mater. 2023, 139, 110332. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, H.M.; Wang, X.H.; Kong, F.G.; Liu, Y.; Zhang, F.S. Fabrication of N-doped micro-mesoporous carbons from industrial alkali lignin with urea assistance for high-efficiency adsorption of methylene blue. Ind. Crop. Prod. 2023, 203, 117146. [Google Scholar] [CrossRef]
- Chang, B.B.; Shi, W.W.; Yin, H.; Zhang, S.R. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture. Chem. Eng. J. 2019, 358, 1507–1518. [Google Scholar] [CrossRef]
- Xie, L.L.; Jin, Z.H.; Dai, Z.D.; Zhou, T.X.; Zhang, X.G.; Chang, Y.L.; Jiang, X. Fabricating self-templated and N-doped hierarchical porous carbon spheres via microfluidic strategy for enhanced CO2 capture. Sep. Purif. Technol. 2023, 322, 124267. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.X.; Zhou, T.T.; Lai, Q.; Egabaierdi, G.; Chen, S.W.; Song, H.O.; Zhang, S.P.; Shi, C.F.; Yang, S.G.; et al. Platanus acerifolia (Aiton) Willd. fruit-derived nitrogen-doped porous carbon as an electrode material for the capacitive deionization of brackish water. J. Environ. Chem. Eng. 2023, 11, 109914. [Google Scholar] [CrossRef]
- Dong, K.M.; Liu, S.; Guo, F.Q.; Wang, J.J.; Tang, B.; Zhao, N.J.; Kong, L.W.; Zhang, Y.D. Facile synthesis of hierarchical porous carbon electrodes with 3D self-supporting structure and N/S self-doping for advanced energy storage device. J. Energy Storage 2023, 72, 108218. [Google Scholar] [CrossRef]
- Zhang, P.H.; Li, Y.Y.; Wang, M.Z.; Zhang, D.J.; Ouyang, W.Z.; Liu, L.; Wang, M.L.; Zhang, K.Y.; Wang, H.Y.; Chen, C. Self-doped (N/O/S) nanoarchitectonics of hierarchically porous carbon from palm flower for high-performance supercapacitors. Diam. Relat. Mater. 2023, 136, 109976. [Google Scholar] [CrossRef]
- Liu, S.; Dong, K.M.; Guo, F.Q.; Qiao, Q.X.; Xu, L.Y.; Wang, J.J.; Kong, L.W.; Zhang, Y.D.; Chang, J.F.; Yan, W.J. Green synthesis of nitrogen self-doped porous carbons from waste garlic peels for high-performance supercapacitor applications. J. Anal. Appl. Pyrol. 2023, 173, 106063. [Google Scholar] [CrossRef]
- Zhang, X.k.; Ma, X.Q.; Yu, Z.S.; Yi, Y.J.; Lu, C.X.; Lu, X.L. Microwave-assisted two-step pyrolysis of water hyacinth for the preparation of N-self-doped porous carbon. J. Anal. Appl. Pyrol. 2023, 173, 106061. [Google Scholar] [CrossRef]
- Xie, L.; Meng, Y.; Wang, Q.R.; Zhang, G.Z.; Xie, H.M.; Zhou, G.L. Zanthoxylum bungeanum branches activated carbons with rich micropore structure prepared by low temperature H3PO4 hydrothermal pretreatment method for toluene adsorption. Diam. Relat. Mater. 2022, 130, 109474. [Google Scholar] [CrossRef]
- Chen, Q.L.; Wang, Z.R.; Yang, B.; Yang, Q.Q.; Kan, J.Q. Determination of main alkylamides responsible for Zanthoxylum bungeanum pungency through quantitative analysis of multi-components by a single marker. Food Chem. 2022, 396, 133645. [Google Scholar] [CrossRef]
- Lei, B.M.; Xie, H.M.; Chen, S.M.; Liu, B.Y.; Zhou, G.L. Control of pore structure and surface chemistry of activated carbon derived from waste Zanthoxylum bungeanum branches for toluene removal in air. Environ. Sci. Pollut. R. 2020, 27, 27072–27092. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.T.; Yang, F.X.; Zhang, J.H. Evaluation of Soxhlet extractor for one-step biodiesel production from Zanthoxylum bungeanum seeds. Fuel Process. Technol. 2015, 131, 452–457. [Google Scholar] [CrossRef]
- Luo, Q.Y.; Li, X.M.; Zhang, Z.Q.; Chen, A.J.; Li, S.S.; Shen, G.H.; Li, M.L.; Liu, X.Y.; Yin, X.Q.; Cheng, L.; et al. Extraction of Zanthoxylum seed protein and identification of its simulated digestion products. LWT 2022, 161, 113412. [Google Scholar] [CrossRef]
- Gong, L.Z.; Bao, A.G.L. High-value utilization of lignin to prepare N, O-codoped porous carbon as a high-performance adsorbent for carbon dioxide capture. J. CO2 Util. 2023, 68, 102374. [Google Scholar] [CrossRef]
- Liao, Z.; Zhu, Y.H.; Sun, G.T.; Qiu, L.; Zhu, M.Q. Micromorphology control of the lignin-based activated carbon and the study on the pyrolysis and adsorption kinetics. Ind. Crop. Prod. 2022, 175, 114266. [Google Scholar] [CrossRef]
- Zaidi, Z.; Manchanda, A.; Sharma, A.; Shehnaz; Choudhry, M.S.; Khan, S.A.; Khan, A.; Chaudhry, S.A. Adsorptive removal of Methylene blue using fruit waste activated carbon and its binary metal oxide nanocomposite. Chem. Eng. J. Adv. 2023, 16, 100571. [Google Scholar] [CrossRef]
- Wu, W.J.; Wu, C.L.; Zhang, G.J.; Liu, J. Preparation of microporous carbonaceous CO2 adsorbents by activating bamboo shoot shells with different chlorides: Experimental and theoretical calculations. J. Anal. Appl. Pyrol. 2022, 168, 105742. [Google Scholar] [CrossRef]
- Xing, X.Y.; Zhang, Y.T.; Zhou, G.Y.; Zhang, Y.J.; Yue, J.P.; Wang, X.Y.; Yang, Z.W.; Chen, J.R.; Wang, Q.G.; Zhang, J. Mechanisms of polystyrene nanoplastics adsorption onto activated carbon modified by ZnCl2. Sci. Total Environ. 2023, 876, 162763. [Google Scholar] [CrossRef] [PubMed]
- Razzak, A.; Yılmaz, M.; Khiari, R.; Hedhili, F.; Alimi, F.; Mechi, L.; Moussaoui, Y. Bioadsorbent derived from Schinus molle for effective retention of aqueous methylene blue. J. Polym. Environ. 2023, 31, 1787–1799. [Google Scholar] [CrossRef]
- Beh, J.H.; Lim, T.H.; Lew, J.H.; Lai, J.C. Cellulose nanofibril-based aerogel derived from sago pith waste and its application on methylene blue removal. Int. J. Bio. Macromol. 2020, 160, 836–845. [Google Scholar] [CrossRef]
- Lu, J.; Zhou, Y.; Zhou, Y.B. Recent advance in enhanced adsorption of ionic dyes from aqueous solution: A review. Crit. Rev. Env. Sci. Technol. 2023, 53, 1709–1730. [Google Scholar] [CrossRef]
- Bao, Y.C.; Yang, L.; Fu, Q.W.; Fu, Y.; Tian, Q.Q.; Wang, C.; Huang, Q.W. The current situation of Zanthoxylum bungeanum industry and the research and application prospect. A review. Fitoterapia 2022, 164, 105380. [Google Scholar] [CrossRef]
- Yan, S.J.; Qu, J.H.; Bi, F.X.; Wei, S.Q.; Wang, S.Q.; Jiang, Z.; Wang, L.; Yu, H.W.; Zhang, Y. One-pot synthesis of porous N-doped hydrochar for atrazine removal from aqueous phase: Co-activation and adsorption mechanisms. Bioresour. Technol. 2022, 364, 128056. [Google Scholar] [CrossRef] [PubMed]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M. Low-cost activated carbon preparation from Corn stigmata fibers chemically activated using H3PO4, ZnCl2 and KOH: Study of methylene blue adsorption, stochastic isotherm and fractal kinetic. Ind. Crop. Prod. 2022, 178, 114546. [Google Scholar] [CrossRef]
- Huang, X.L.; Huang, Y.; Pan, Z.; Xu, W.T.; Zhang, W.H.; Zhang, X. Tailored high mesoporous activated carbons derived from Lotus seed shell using one-step ZnCl2-activated method with its high Pb (II) capturing capacity. Environ. Sci. Pollut. R. 2019, 26, 26517–26528. [Google Scholar] [CrossRef]
- Mao, Y.; Cai, B.; Huang, M.; Li, J.; Liu, X.H.; Zhang, B.W.; Ma, Z.Q. Pyrolysis polygeneration of the marine and terrestrial biomass and the chemical activation of bio-char as adsorbent to remove tetracycline hydrochloride from wastewater. J. Anal. Appl. Pyrol. 2024, 177, 106272. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhang, X.F.; Hou, L.M.; Zheng, H.; Niu, B.L.; Weng, K.R.; Liu, S.H.; Fu, J.W. Nitrogen-rich hierarchical porous polyphosphazene for rapid and efficient adsorption of anionic contaminants: Kinetics, isotherm, thermodynamics and mechanism. Appl. Surf. Sci. 2023, 616, 156538. [Google Scholar] [CrossRef]
- da Silva, A.I.C.; Paranha, G.; Maia, L.S.; Mulinari, D.R. Development of activated carbon from pineapple crown wastes and its potential use for removal of methylene blue. J. Nat. Fibers 2021, 19, 5211–5226. [Google Scholar] [CrossRef]
- Mbarki, F.; Kesraoui, A.; Seffen, M.; Ayrault, P. Kinetic, thermodynamic, and adsorption behavior of cationic and anionic dyes onto corn stigmata: Nonlinear and stochastic analyses. Water Air Soil Pollut. 2018, 229, 95. [Google Scholar] [CrossRef]
- Jung, K.W.; Jeong, T.U.; Choi, J.W.; Ahn, K.H.; Lee, S.H. Adsorption of phosphate from aqueous solution using electrochemically modified biochar calcium-alginate beads: Batch and fixed-bed column performance. Bioresour. Technol. 2017, 244, 23–32. [Google Scholar] [CrossRef]
- Valentini, F.; Cerza, E.; Campana, F.M. Efficient synthesis and investigation of waste-derived adsorbent for water purification. Exploring the impact of surface functionalization on methylene blue dye removal. Bioresour. Technol. 2023, 390, 129847. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.S.; Moradi, H.; Chang, Y.Y.; Yang, G.K. Highly porous biobased graphene-like carbon adsorbent for dye removal: Preparation, adsorption mechanisms and optimization. J. Environ. Chem. Eng. 2023, 11, 109278. [Google Scholar] [CrossRef]
- Zhang, W.L.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z.Q. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Mao, Y.T.; Cai, B.; Huang, M.; Liu, X.H.; Zhang, W.B.; Ma, Z.Q. A sustainable preparation strategy for the nitrogen-doped hierarchical biochar with high surface area for the enhanced removal of organic dye. Biochar 2023, 5, 70. [Google Scholar] [CrossRef]
- Waghmare, C.; Ghodmare, S.; Ansari, K.; Dehghani, M.H.; Khan, M.A.; Hasan, M.A.; Islam, S.; Khan, N.A.; Zahmatkesh, S. Experimental investigation of H3PO4 activated papaya peels for methylene blue dye removal from aqueous solution: Evaluation on optimization, kinetics, isotherm, thermodynamics, and reusability studies. J. Environ. Manag. 2023, 345, 118815. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.R.; Lima, E.C.; Umpierres, C.S.; Thue, P.S.; El-Chaghaby, G.A.; da Silva, R.S.; Pavan, F.A.; Dias, S.L.P.; Biron, C. Removal of amoxicillin from simulated hospital effluents by adsorption using activated carbons prepared from capsules of cashew of Para. Environ. Sci. Pollut. R. 2019, 26, 16396–16408. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; Mosbah, M.; Salem, R.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef]
- Khadhri, N.; Saad, M.E.K.; ben Mosbah, M.; Moussaoui, Y. Batch and continuous column adsorption of indigo carmine onto activated carbon derived from date palm petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Mani, D.; Elango, D.; Priyadharsan, A.; Al-Humaid, L.A.; Al-Dahmash, N.D.; Ragupathy, S.; Jayanthi, P.; Ahn, Y.H. Groundnut shell chemically treated with KOH to prepare inexpensive activated carbon: Methylene blue adsorption and equilibrium isotherm studies. Environ. Res. 2023, 231, 116026. [Google Scholar] [CrossRef]
- Hao, R.N.; Ma, R.N.; Cao, Y.L.; Zhou, W.Y.; Chai, H. Facile fabrication of N-self-doped porous carbons from green solid waste for supercapacitors with high cycling stability and flexibility. Mater. Today Commun. 2022, 33, 104911. [Google Scholar] [CrossRef]
- Yağmur, H.K.; Kaya, İ. Synthesis and characterization of magnetic ZnCl2-activated carbon produced from coconut shell for the adsorption of methylene blue. J. Mol. Struct. 2021, 1232, 130071. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, M.W.; Zhao, Y.H.; Liao, Q.H. Facile preparation of porous carbon derived from pomelo peel for efficient adsorption of methylene blue. Molecules 2022, 27, 3096. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wei, X.Y.; Shao, C.W.; Yin, F.; Sun, B.K.; Liu, F.J.; Li, J.H.; Liu, Z.Q.; Zong, Z.M. Honeycomb-like N/O self-doped hierarchical porous carbons derived from low-rank coal and its derivatives for high-performance supercapacitor. Fuel 2023, 331, 125658. [Google Scholar] [CrossRef]
- Yang, B.Y.; Gao, J.P.; Xie, M.H.; Zuo, S.S.; Kang, H.; Sun, Y.; Xu, X.Y.; Wang, W.; Gao, C.J.; Liu, Y.; et al. N-self-doped porous carbon derived from animal-heart as an electrocatalyst for efficient reduction of oxygen. J. Colloid Interf. Sci. 2020, 579, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Wang, Y.F.; Yan, L.; Wang, Q.; Li, J.; Zhong, X.; Liu, Q.Q.; Li, Q.C.; Cui, S.; Xie, G. From pollutant to high-performance supercapacitor: Semi-coking wastewater derived N–O–S self-doped porous carbon. Colloi. Surface. A 2023, 657, 130596. [Google Scholar] [CrossRef]
- Joshiba, G.J.; Kumar, P.S.; Rangasamy, G.; Ngueagni, P.T.; Pooja, G.; Balji, G.B.; Alagumalai, K.; El-Serehy, H.A. Iron doped activated carbon for effective removal of tartrazine and methylene blue dye from the aquatic systems: Kinetics, isotherms, thermodynamics and desorption studies. Environ. Res. 2022, 215, 114317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Peng, B.; Liu, Q.Y.; Wu, C.S.; Li, Z.W. Preparation of porous biochar from heavy bio-oil for adsorption of methylene blue in wastewater. Fuel Process. Technol. 2022, 238, 107485. [Google Scholar] [CrossRef]
- Li, L.X.; Wang, J.; Jia, C.; Lv, Y.; Liu, Y. Co-pyrolysis of cyanobacteria and plastics to synthesize porous carbon and its application in methylene blue adsorption. J. Water Process Eng. 2020, 39, 101753. [Google Scholar] [CrossRef]

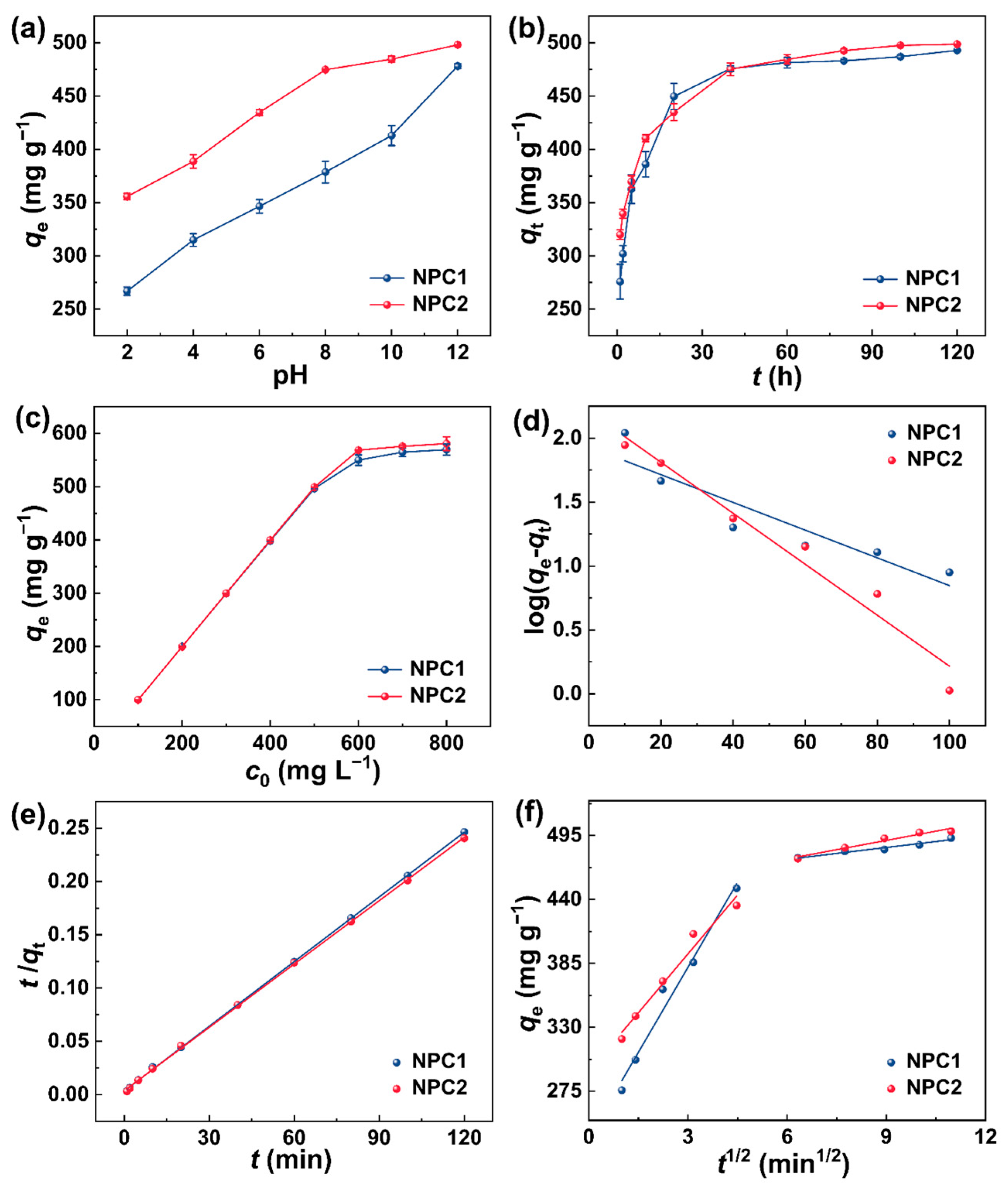
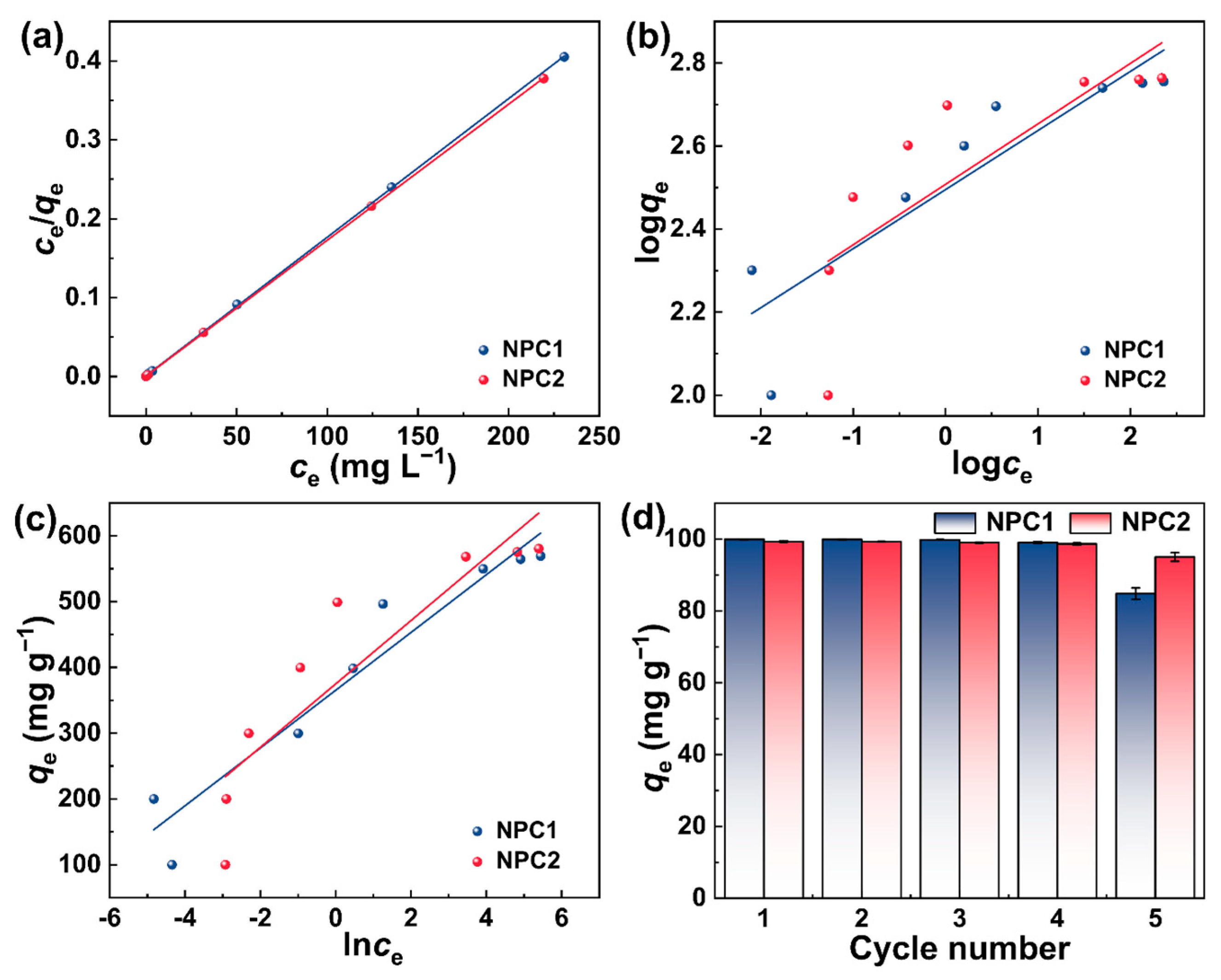
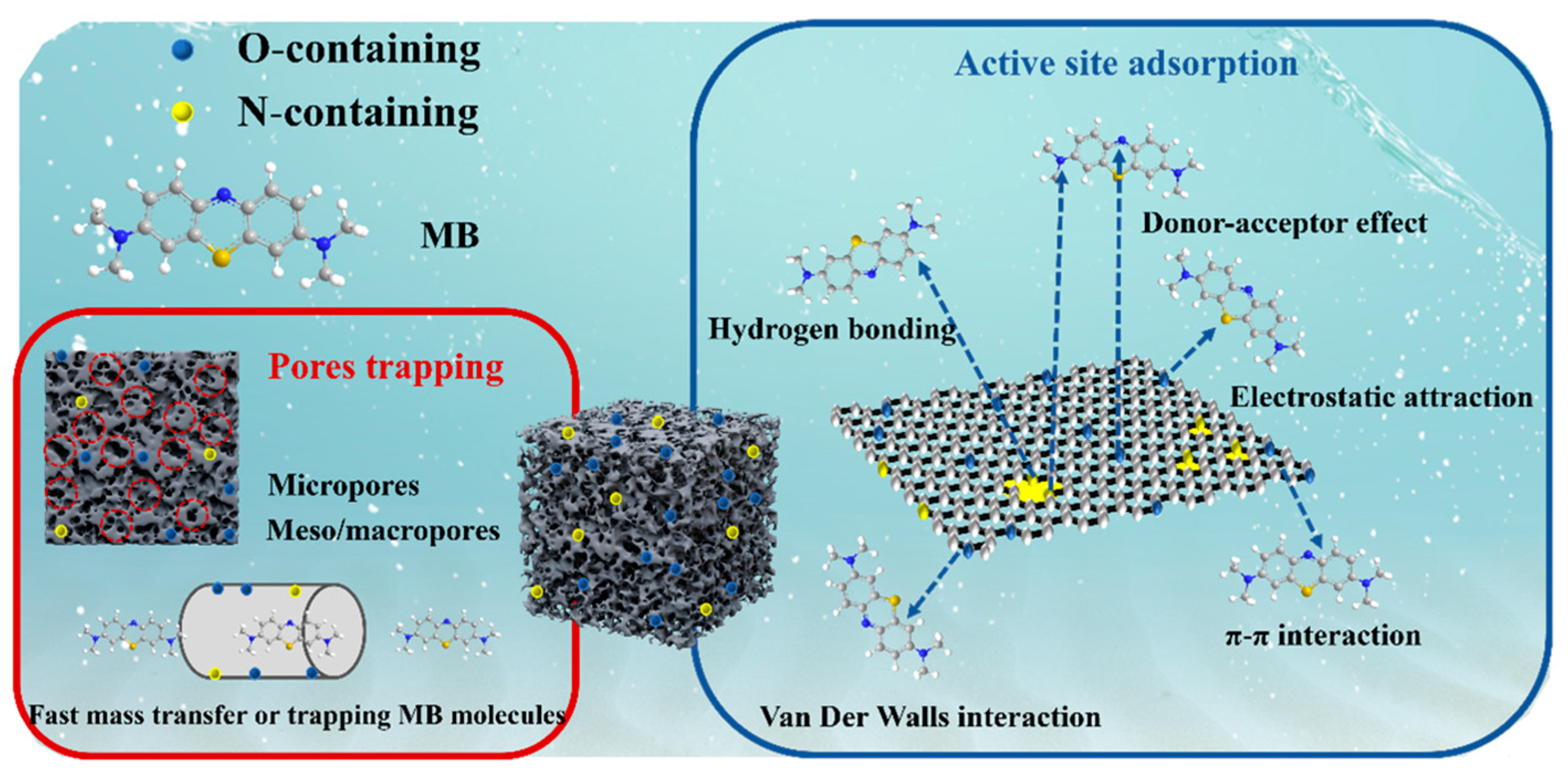
| Materials | NPC1 | NPC2 | |
|---|---|---|---|
| qe, exp (mg g−1) | 492.9 | 498.7 | |
| PFO | qe, cal (mg g−1) | 85.4 | 170.22 |
| k1 (min−1) | 0.02 | 0.05 | |
| R2 | 0.8264 | 0.9770 | |
| ∆q | 407.5 | 328.48 | |
| RMSE | 0.1387 | 0.1010 | |
| χ2 | 0.0289 | 0.0131 | |
| PSO | qe, cal (mg g−1) | 495.0 | 505.05 |
| k2 (g mg−1 min−1) | 0.0012 | 0.0011 | |
| R2 | 0.9998 | 0.9996 | |
| ∆q | −2.1 | −6.35 | |
| RMSE | 0.0001 | 0.0015 | |
| χ2 | 1.2403 × 10−6 | 2.7634 × 10−6 |
| Materials | NPC1 | NPC2 | |
|---|---|---|---|
| First stage | ki1 (mg g−1 h−1/2) | 48.80 | 33.76 |
| ci1 (mg g−1) | 235.30 | 291.92 | |
| R2 | 0.9695 | 0.9649 | |
| RMSE | 9.3446 | 6.9376 | |
| χ2 | 145.5373 | 80.2181 | |
| Second stage | ki2 (mg g−1 h−1/2) | 3.41 | 5.30 |
| ci2 (mg g−1) | 454.01 | 443.09 | |
| R2 | 0.9437 | 0.9476 | |
| RMSE | 1.1711 | 1.7522 | |
| χ2 | 2.2858 | 5.1168 |
| Materials | NPC1 | NPC2 | |
|---|---|---|---|
| Langmuir | qm (mg g−1) | 568.18 | 581.40 |
| b (L mg−1) | 1.79 | 3.49 | |
| R2 | 0.9999 | 0.9999 | |
| RMSE | 0.0010 | 0.0006 | |
| χ2 | 1.4298 × 10−6 | 4.6118 × 10−7 | |
| Freundlich | k (mg g−1(L mg−1)1/n) | 312.73 | 321.99 |
| 1/n | 0.14 | 0.15 | |
| R2 | 0.7878 | 0.5847 | |
| RMSE | 0.1079 | 0.1530 | |
| χ2 | 0.0155 | 0.0312 | |
| Temkin | KT (L g−1) | 316.83 | 264.42 |
| B (J mol−1) | 43.92 | 48.18 | |
| R2 | 0.9190 | 0.7836 | |
| RMSE | 44.4434 | 74.8569 | |
| χ2 | 2.6363 × 103 | 7.4714 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Q.; Gong, Z.; Zhang, W.; Ren, Y.; Li, Q.; Lu, H.; Liao, Q.; Chen, Z.; Tang, J. Zanthoxylum bungeanum Waste-Derived High-Nitrogen Self-Doped Porous Carbons as Efficient Adsorbents for Methylene Blue. Molecules 2024, 29, 1809. https://doi.org/10.3390/molecules29081809
Zhao Y, Zhang Q, Gong Z, Zhang W, Ren Y, Li Q, Lu H, Liao Q, Chen Z, Tang J. Zanthoxylum bungeanum Waste-Derived High-Nitrogen Self-Doped Porous Carbons as Efficient Adsorbents for Methylene Blue. Molecules. 2024; 29(8):1809. https://doi.org/10.3390/molecules29081809
Chicago/Turabian StyleZhao, Yuhong, Qi Zhang, Zhuhua Gong, Wenlin Zhang, Yun Ren, Qiang Li, Hongjia Lu, Qinhong Liao, Zexiong Chen, and Jianmin Tang. 2024. "Zanthoxylum bungeanum Waste-Derived High-Nitrogen Self-Doped Porous Carbons as Efficient Adsorbents for Methylene Blue" Molecules 29, no. 8: 1809. https://doi.org/10.3390/molecules29081809
APA StyleZhao, Y., Zhang, Q., Gong, Z., Zhang, W., Ren, Y., Li, Q., Lu, H., Liao, Q., Chen, Z., & Tang, J. (2024). Zanthoxylum bungeanum Waste-Derived High-Nitrogen Self-Doped Porous Carbons as Efficient Adsorbents for Methylene Blue. Molecules, 29(8), 1809. https://doi.org/10.3390/molecules29081809