Synthesis, Cytotoxicity, and Photophysical Investigations of 2-Amino-4,6-diphenylnicotinonitriles: An Experimental and Theoretical Study
Abstract
:1. Introduction
2. Results and Discussion
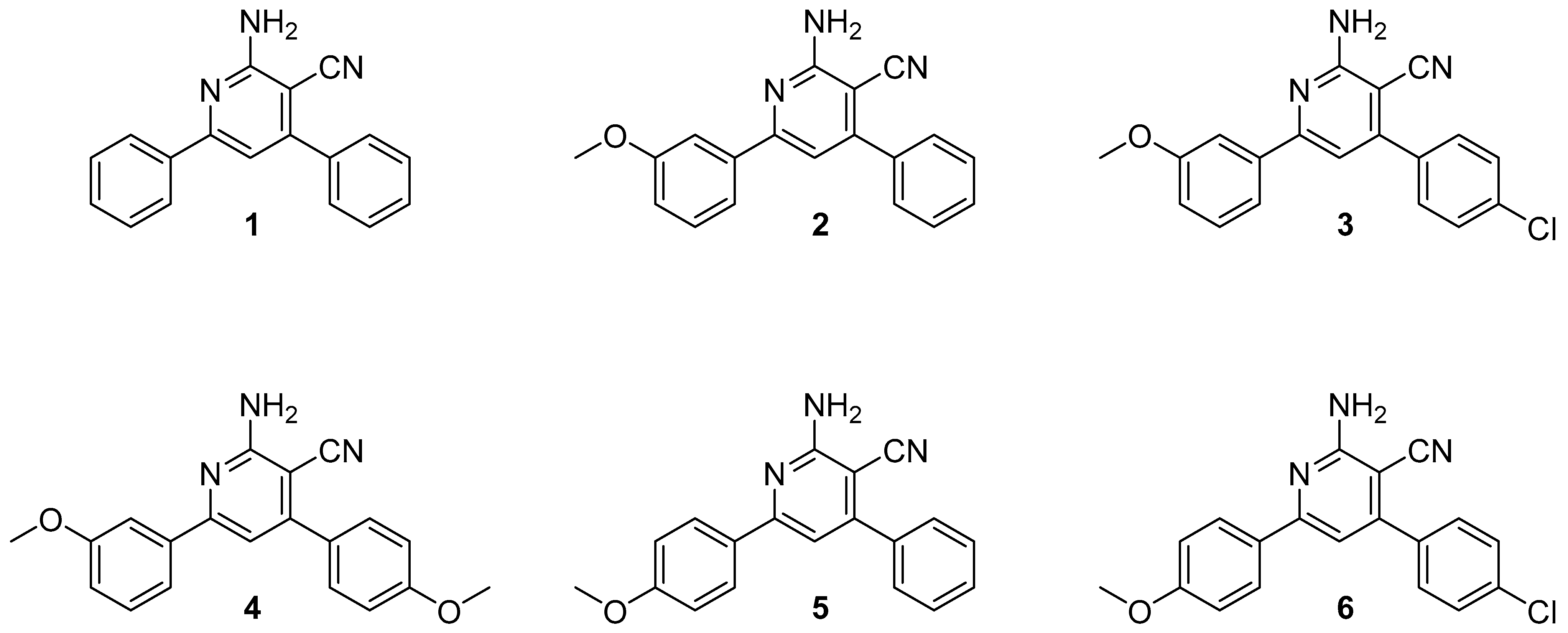
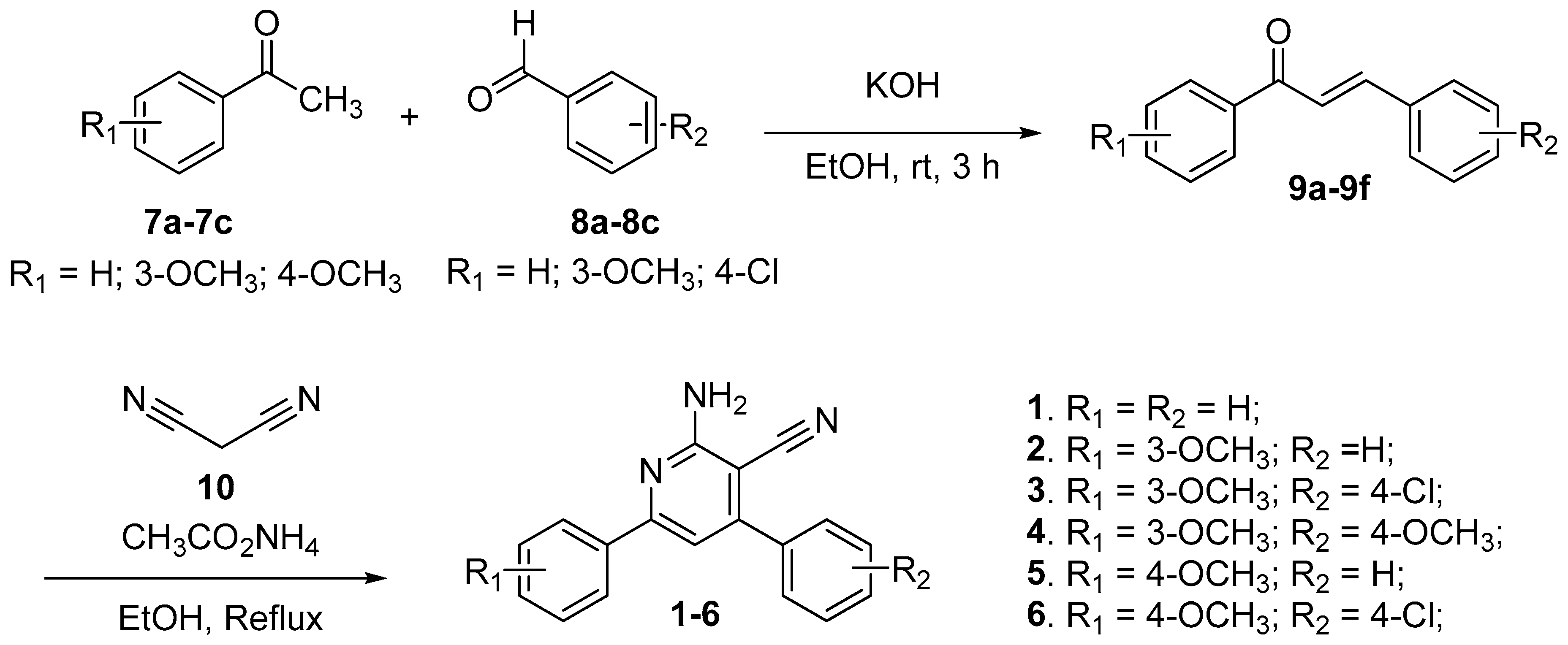
2.1. Synthesis of 2-Amino-4,6-diphenylnicotinonitriles (APNs, 1–6)
2.2. Cytotoxicity of the Synthesized APNs (1–6)
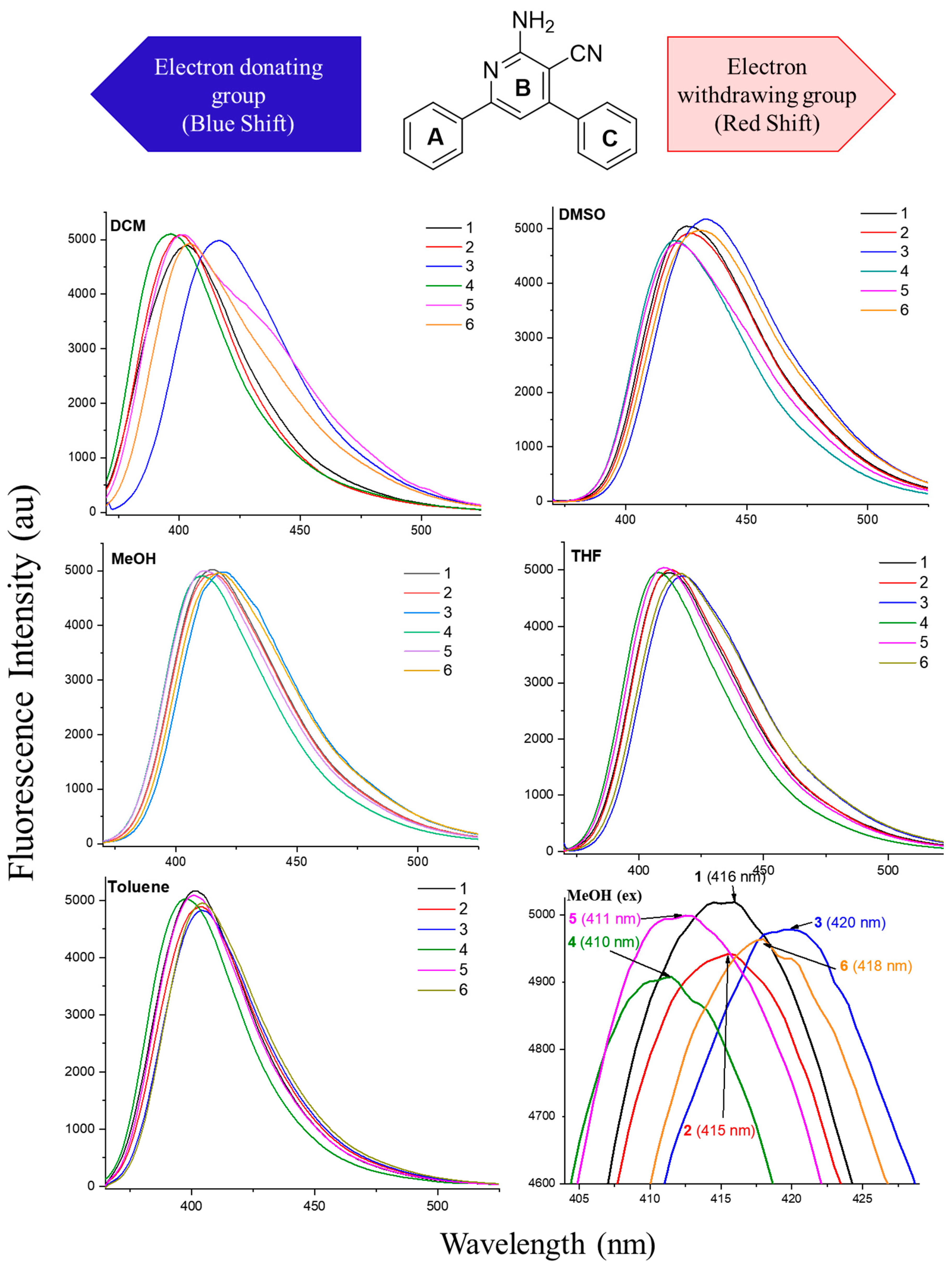
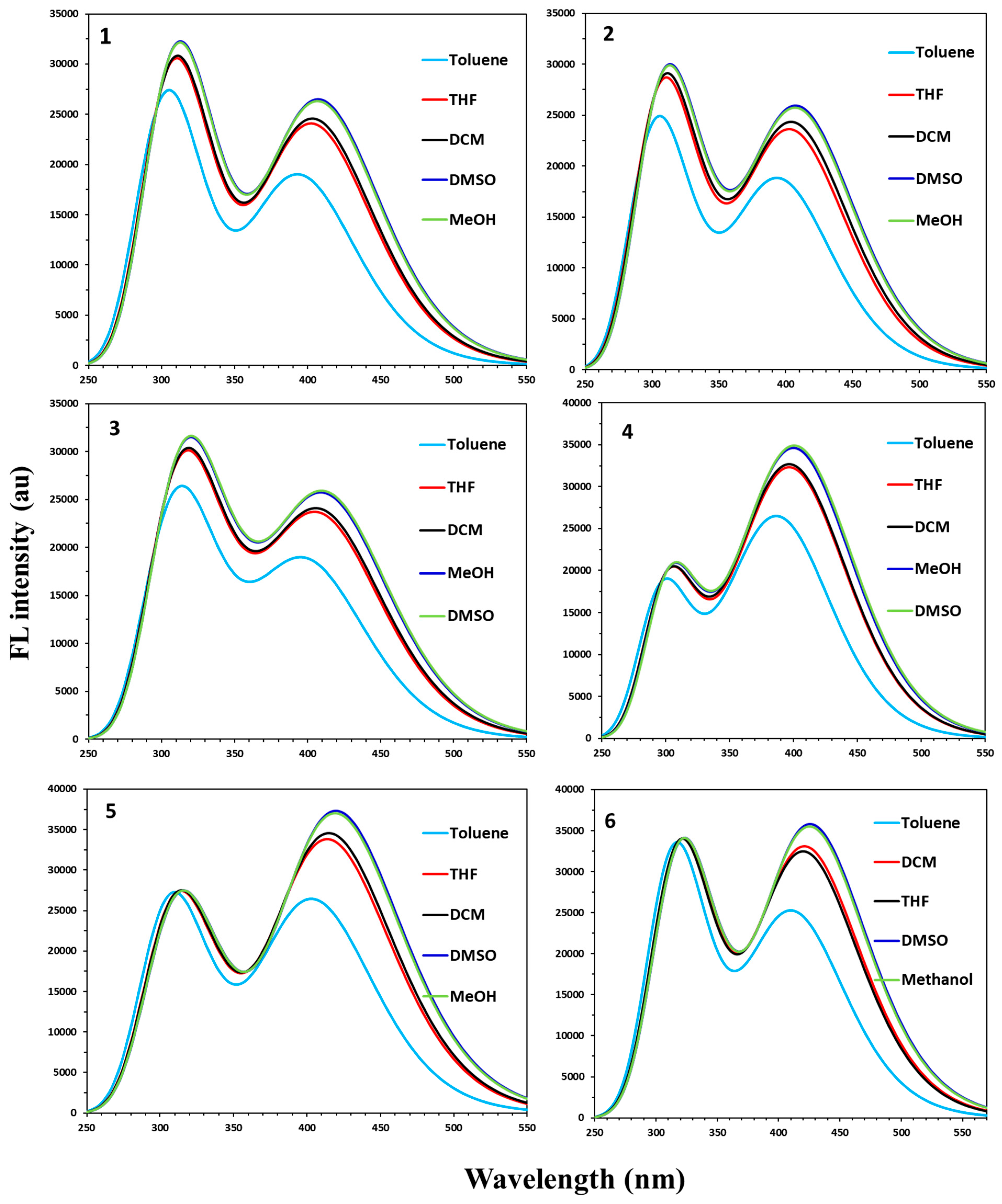
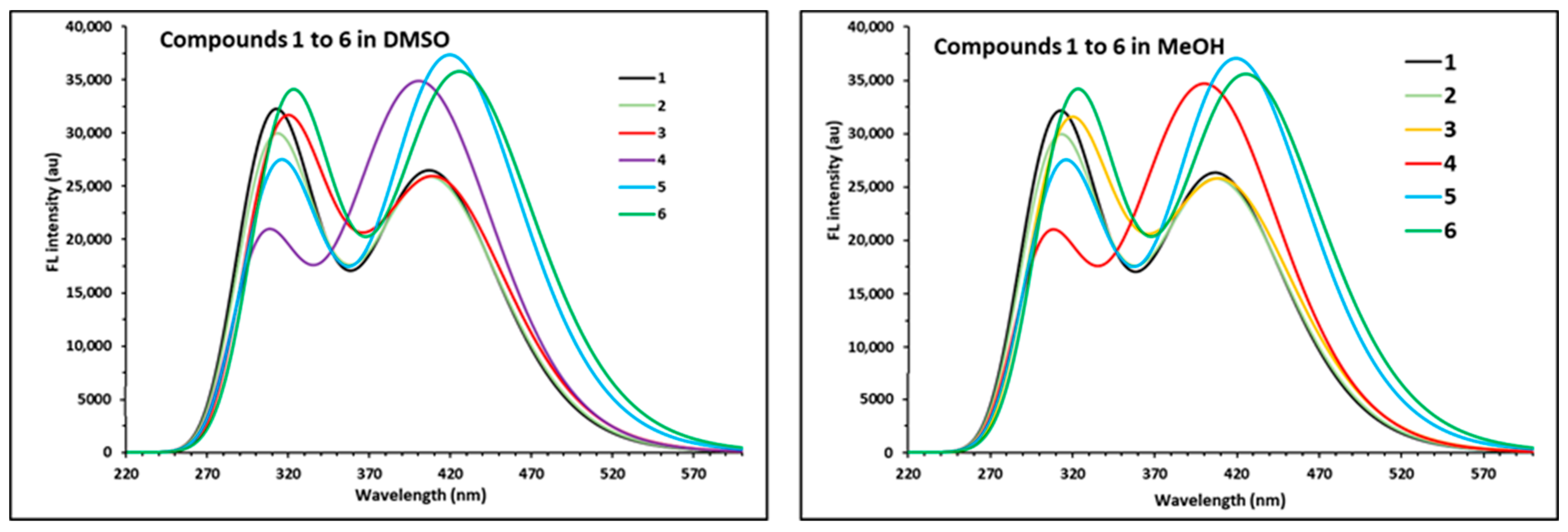
2.3. Photophysical Properties of APNs (1–6)
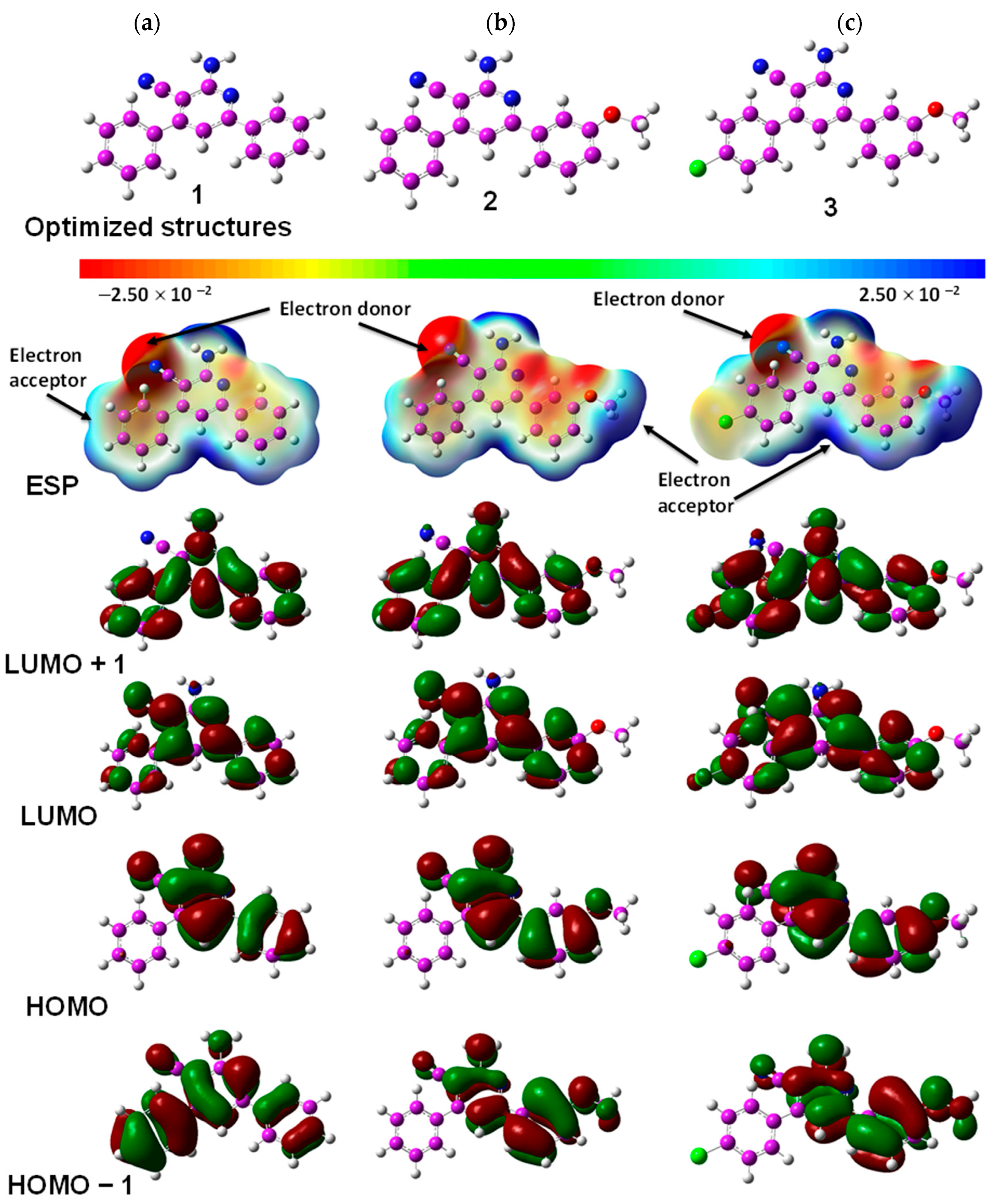
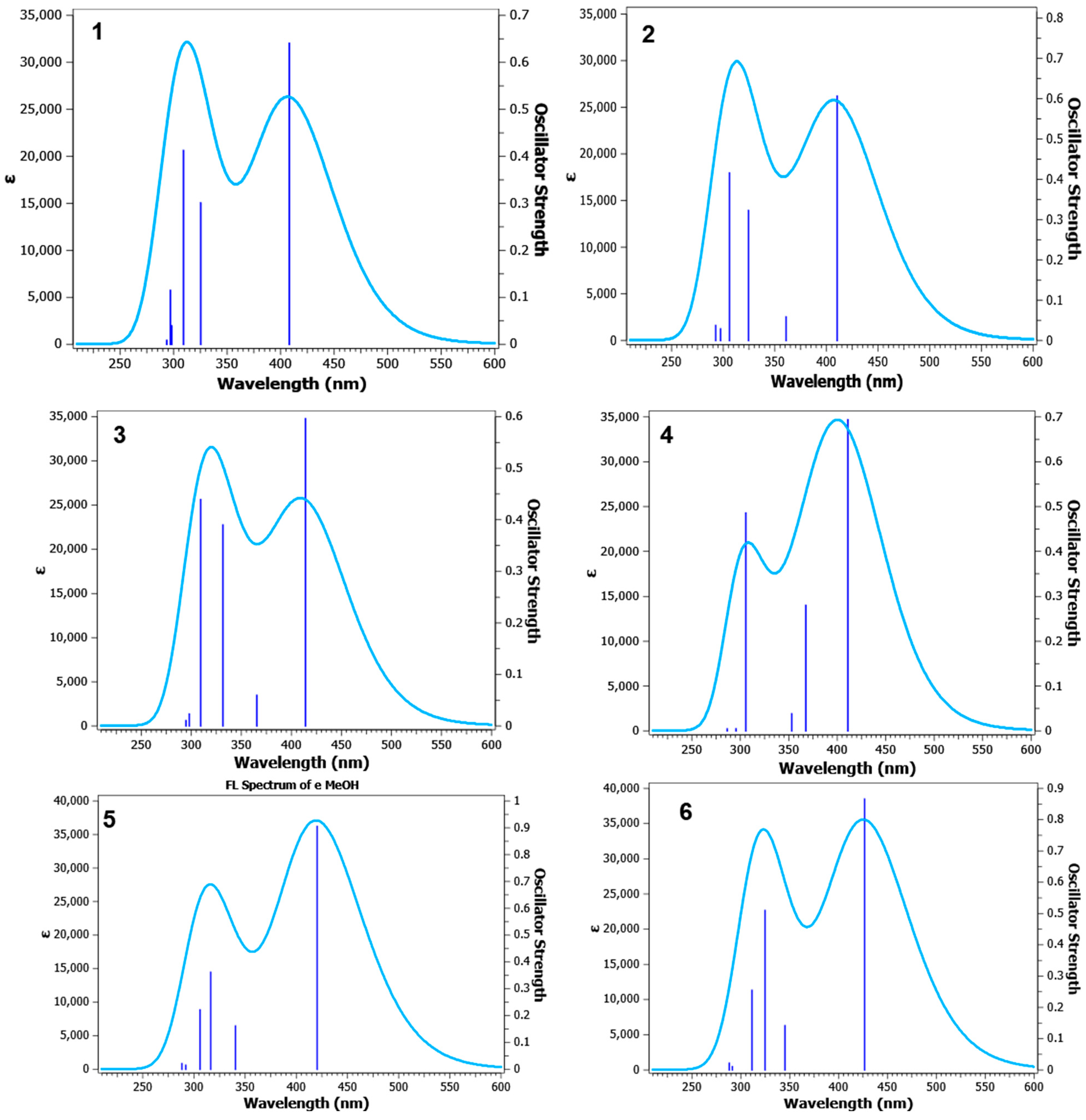
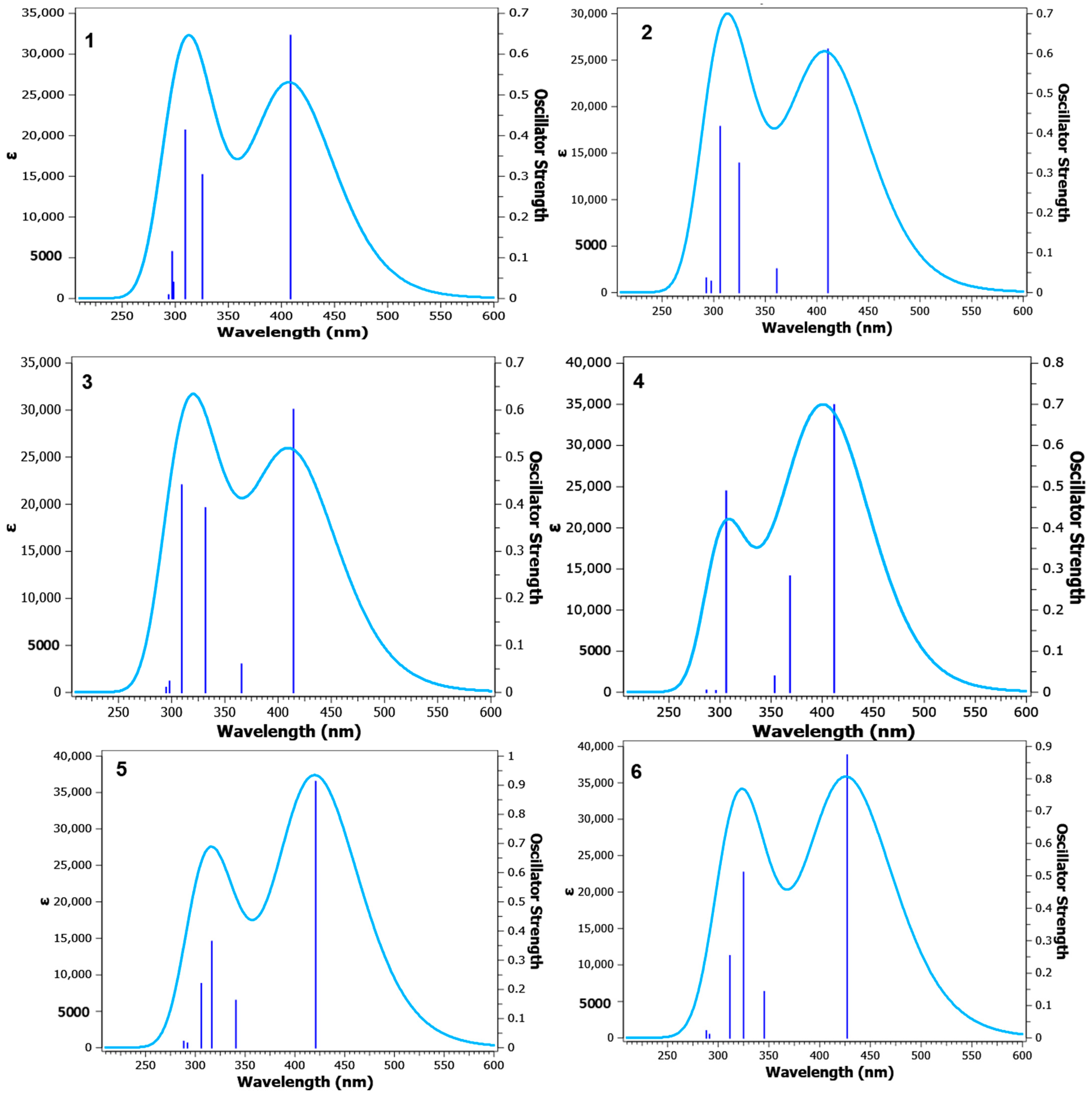
2.4. Quantum Chemical TD-DFT Analysis of APNs (1–6)
3. Materials and Methods
3.1. General
3.2. General Synthetic Method for the Synthesis of 1–6
3.2.1. 2-Amino-4,6-diphenylnicotinonitrile (1)
- Yellow powder (92%). Mp. 189 °C (lit. mp. 187–188 °C [35]; 179–181 °C [36]). FT-IR (KBr): ν (cm−1) = 3463, 3299, 3170, 2205, 1637, 1572, 1548, 1495, 1451, 1369, 1257, 1075, 848, 756, 699, and 525 cm−1. 1H-NMR (700 MHz, CDCl3) δ 8.03 (dd, J = 7.9, 1.8 Hz, 2H), 7.67 (dd, J = 7.9, 1.7 Hz, 2H), 7.55 (q, J = 7.7, 7.0 Hz, 3H), 7.52–7.49 (m, 3H), 7.25 (s, 1H), and 5.38 (s, 2H) ppm. 13C-NMR (176 MHz, CDCl3) δ 160.23, 159.85, 155.15, 137.96, 136.95, 130.22, 129.85, 128.97, 128.83, 128.19, 127.34, 117.15, 111.32, and 88.33 ppm. Mass (ESI): m/z 271.9693 [M + H]+; 293.9424 [M + Na]+.
3.2.2. 2-Amino-6-(3-methoxyphenyl)-4-phenylnicotinonitrile (2)
- White powder (76.4%). Mp. 163 °C (lit. mp. 163–164 °C [37]). FT-IR (KBr): ν (cm−1) = 3417, 3322, 3202, 3019, 2210, 1651, 1569, 1556, 1494, 1288, 1258, 1051, 904, 754, 779, 523, and 478 cm−1. 1H-NMR (700 MHz, CDCl3) δ 7.62 (d, J = 5.9 Hz, 2H), 7.56 (s, 1H), 7.54 (d, J = 7.7 Hz, 1H), 7.50 (d, J = 8.3 Hz, 3H), 7.36 (t, J = 7.9 Hz, 1H), 7.18 (s, 1H), 6.99 (d, J = 8.0 Hz, 1H), 5.33 (s, 2H), and 3.87 (s, 3H) ppm. 13C-NMR (176 MHz, CDCl3) δ 161.60, 160.19, 159.57, 153.63, 136.04, 135.50, 130.17, 129.53, 129.23, 128.88, 117.15, 114.22, 110.19, 87.14, and 55.45 ppm. Mass (ESI): m/z 302.0671 [M + H]+; 324.0705 [M + Na]+.
3.2.3. 2-Amino-4-(4-chlorophenyl)-6-(3-methoxyphenyl)nicotinonitrile (3)
- White powder (80.6%). Mp. 195 °C. FT-IR (KBr): ν (cm−1) = 3412, 3327, 3211, 2946, 2836, 2209, 1652, 1571, 1552, 1494, 1289, 1256, 1093, 1053, 1015, 844, 816, 815, 768, 523, and 488 cm−1. 1H-NMR (700 MHz, CDCl3) δ 7.55 (d, J = 8.3 Hz, 3H), 7.53 (d, J = 7.7 Hz, 1H), 7.49 (d, J = 6.6 Hz, 2H), 7.37 (t, J = 7.8 Hz, 1H), 7.14 (s, 1H), 7.00 (d, J = 8.0 Hz, 1H), 5.34 (s, 2H), and 3.87 (s, 3H) ppm. 13C-NMR (176 MHz, CDCl3) δ 160.16, 160.07, 159.84, 153.83, 139.21, 136.18, 135.29, 129.86, 129.54, 129.27, 119.71, 116.90, 116.20, 112.66, 111.16, 88.15, 55.45, and 55.45 ppm. Mass (ESI): m/z 336.0031 [M(35Cl) + H]+; 338.0375 [M(37Cl) + H]+.
3.2.4. 2-Amino-6-(3-methoxyphenyl)-4-(4-methoxyphenyl)nicotinonitrile (4)
- Green powder (72.5%). Mp. 165 °C. FT-IR (KBr): ν (cm−1) = 3447, 3414, 3329, 3216, 2938, 2835, 2208, 1654, 1643, 1612, 1516, 1295, 1254, 1178, 1052, 1034, 816, 767, and 505 cm−1. 1H-NMR (700 MHz, CDCl3) δ 7.59 (d, J = 8.8 Hz, 2H), 7.55 (s, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.36 (t, J = 7.9 Hz, 1H), 7.16 (s, 1H), 7.02 (d, J = 7.3 Hz, 2H), 6.98 (d, J = 8.1 Hz, 1H), 5.30 (s, 2H), and 3.86 (s, 6H) ppm. 13C-NMR (176 MHz, CDCl3) δ 161.01, 160.26, 160.04, 159.48, 154.72, 139.55, 129.80, 129.65, 129.16, 119.71, 117.47, 116.03, 114.40, 112.59, 111.21, 88.14, 55.45, and 55.44 ppm. Mass (ESI): m/z 332.1401 [M + H]+; 354.0757 [M + Na]+; 370.0165 [M + K]+.
3.2.5. 2-Amino-6-(4-methoxyphenyl)-4-phenylnicotinonitrile (5)
- Yellow powder (66.4%). Mp. 178 °C (lit. mp. 176–178 °C [38]). FT-IR (KBr): ν (cm−1) = 3487, 3368, 3200, 3066, 2970, 2204, 1897, 1617, 1606, 1572, 1543, 1238, 1172, 1027, 829, 766, 700, and 516 cm−1. 1H-NMR (700 MHz, CDCl3) δ 8.02 (d, J = 8.8 Hz, 2H), 7.66 (d, J = 6.9 Hz, 2H), 7.57–7.51 (m, 3H), 7.19 (s, 1H), 7.03–7.00 (m, 2H), 5.34 (s, 2H), and 3.90 (s, 4H) ppm. 13C-NMR (176 MHz, CDCl3) δ 161.49, 160.19, 159.34, 154.94, 137.11, 130.36, 129.76, 128.93, 128.86, 128.17, 117.35, 114.18, 110.49, 87.45, and 55.44 ppm. Mass (ESI): m/z 302.0650 [M + H]+; 324.0657 [M + Na]+.
3.2.6. 2-Amino-4-(4-chlorophenyl)-6-(4-methoxyphenyl)nicotinonitrile (6)
- Pink powder (90.1%). Mp. 195 °C (lit. mp. 194–197 °C [38]). FT-IR (KBr): ν (cm−1) = 3458, 3367, 3230, 2833, 2207, 1638, 1607, 1575, 1546, 1493, 1235, 1173, 1091, 1013, 822, 808, and 495 cm−1. 1H-NMR (700 MHz, CDCl3) δ 7.96 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.2 Hz, 2H), 7.48 (d, J = 8.6 Hz, 2H), 7.09 (s, 1H), 6.97 (d, J = 8.4 Hz, 2H), 5.30 (s, 2H), and 3.85 (s, 3H) ppm. 13C-NMR (176 MHz, CDCl3) δ 161.60, 160.19, 159.57, 153.63, 136.04, 135.50, 130.17, 129.53, 129.23, 128.88, 117.15, 114.22, 110.19, 87.14, and 55.45 ppm. Mass (ESI): m/z 336.116 [M(35Cl) + H]+; 338.0885 [M(37Cl) + H]+; 358.0559 [M(35Cl) + Na]+; 359.9924 [M(37Cl) + Na]+.
3.3. Cytotoxicity Assay of APNs 1–6
3.4. Computational Studies of APNs 1–6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mirjalili, B.B.F.; Khabnadideh, S.; Gholami, A.; Zamani, L.; Nezamalhosseini, S.M.; Kalantari, N. Cu(OAc)2 as a green promoter for one-pot synthesis of 2-amino-4,6-diarylpyridine- 3-carbonitrile as antibacterial agents. Bull. Chem. Soc. Ethiop. 2020, 34, 149–156. [Google Scholar] [CrossRef]
- Ismail, M.M.; Farrag, A.M.; Harras, M.F.; Ibrahim, M.H.; Mehany, A.B. Apoptosis: A target for anticancer therapy with novel cyanopyridines. Bioorganic Chem. 2020, 94, 103481. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, Z.; Razzaghi-Asl, N.; Ramazani, A.; Aghahosseini, H. Synthesis, cytotoxic assessment, and molecular docking studies of 2,6-diaryl-substituted pyridine and 3,4- dihydropyrimidine-2(1H)-one scaffolds. Turk. J. Chem. 2020, 44, 194–213. [Google Scholar] [CrossRef] [PubMed]
- Maurya, H.K.; Verma, R.; Alam, S.; Pandey, S.; Pathak, V.; Sharma, S.; Srivastava, K.K.; Negi, A.S.; Gupta, A. Studies on substituted benzo[h]quinazolines, benzo[g]indazoles, pyrazoles, 2,6-diarylpyridines as anti-tubercular agents. Bioorganic Med. Chem. Lett. 2013, 23, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Challa, C.S.; Katari, N.K.; Nallanchakravarthula, V.; Nayakanti, D.; Kapavarapu, R.; Pal, M. Amberlyst-15 catalysed sonochemical synthesis of 2-amino-4,6-disubstituted nicotinonitrile derivatives and their biological evaluation. J. Mol. Struct. 2021, 1240, 130541. [Google Scholar] [CrossRef]
- Mantri, M.; de Graaf, O.; van Veldhoven, J.; Göblyös, A.; von Frijtag Drabbe Künzel, J.K.; Mulder-Krieger, T.; Link, R.; de Vries, H.; Beukers, M.W.; Brussee, J.; et al. 2-Amino-6-furan-2-yl-4-substituted Nicotinonitriles as A2A Adenosine Receptor Antagonists. J. Med. Chem. 2008, 51, 4449–4455. [Google Scholar] [CrossRef]
- Ortyl, J.; Fiedor, P.; Chachaj-Brekiesz, A.; Pilch, M.; Hola, E.; Galek, M. The Applicability of 2-amino-4,6-diphenyl-pyridine-3-carbonitrile Sensors for Monitoring Different Types of Photopolymerization Processes and Acceleration of Cationic and Free-Radical Photopolymerization under Near UV Light. Sensors 2019, 19, 1668. [Google Scholar] [CrossRef] [PubMed]
- Belhadj, F.; Kibou, Z.; Cheikh, N.; Choukchou-Braham, N.; Villemin, D. Convenient access to new 4-substituted aminopyrido[2,3-d]pyrimidine derivatives. Tetrahedron Lett. 2015, 56, 5999–6002. [Google Scholar] [CrossRef]
- Sayahi, M.H.; Saghanezhad, S.J.; Mahdavi, M. Catalyst-free three-component synthesis of 2-amino-4,6-diarylpyridine-3-carbonitriles under solvent-free conditions. Chem. Heterocycl. Compd. 2019, 55, 725–728. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Abbaspour-Gilandeh, E.; Aghaei-Hashjin, M. Four-Component Synthesis of 2-Amino-3-Cyanopyridine Derivatives Catalyzed by Cu@imineZCMNPs as a Novel, Efficient and Simple Nanocatalyst under Solvent-Free Conditions. Catal. Lett. 2018, 148, 1254–1262. [Google Scholar] [CrossRef]
- Khalili, D. Graphene oxide: A reusable and metal-free carbocatalyst for the one-pot synthesis of 2-amino-3-cyanopyridines in water. Tetrahedron Lett. 2016, 57, 1721–1723. [Google Scholar] [CrossRef]
- Xin, X.; Huang, P.; Xiang, D.; Zhang, R.; Zhao, F.; Zhang, N.; Dong, D. [5C + 1N] Annulation of 2,4-pentadienenitriles with hydroxylamine: A synthetic route to multi-substituted 2-aminopyridines. Org. Biomol. Chem. 2012, 11, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Balaswamy, P.; Aravind, S.; Satyanarayana, B. Polyethylene Glycol-400 Used as Phase Transfer Catalyst for One-Pot Synthesis Of 2-Amino-3-Cyanopyridine Derivatives under Aqueous Conditions. Rasayan J. Chem. 2017, 10, 1334–1339. [Google Scholar] [CrossRef]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, P.N.; Malik, S. Aqueous media preparation of 2-amino-4,6-diphenylnicotinonitriles using cellulose sulfuric acid as an efficient catalyst. Res. Chem. Intermed. 2014, 40, 871–885. [Google Scholar] [CrossRef]
- Tamaddon, F.; Tadayonfar, S.-E. Facile microwave-assisted preparation of an ester-based cationic gemini surfactant for the improved micellar synthesis of aminocyanopyridines. J. Mol. Struct. 2020, 1207, 127728. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N.; Slepokura, K.; Lis, T. Boric acid as an efficient and green catalyst for the synthesis of2-amino-4,6-diarylnicotinonitrile under microwave irradiation in solvent-freeconditions. Turk. J. Chem. 2019, 43, 464–474. [Google Scholar] [CrossRef]
- Afradi, M.; Pour, S.A.; Dolat, M.; Yazdani-Elah-Abadi, A. Nanomagnetically modified vitamin B3 (Fe3O4@Niacin): An efficient and reusable green biocatalyst for microwave-assisted rapid synthesis of 2-amino-3-cyanopyridines in aqueous medium. Appl. Organomet. Chem. 2017, 32, e4103. [Google Scholar] [CrossRef]
- Gupta, R.; Jain, A.; Jain, M.; Joshi, R. ‘One Pot’ Synthesis of 2-Amino-3-cyano-4,6-diarylpyridines under Ultrasonic Irradiation and Grindstone Technology. Bull. Korean Chem. Soc. 2010, 31, 3180–3182. [Google Scholar] [CrossRef]
- Safari, J.; Banitaba, S.H.; Khalili, S.D. Ultrasound-promoted an efficient method for one-pot synthesis of 2-amino-4,6-diphenylnicotinonitriles in water: A rapid procedure without catalyst. Ultrason. Sonochemistry 2012, 19, 1061–1069. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Kiafar, M.; Yarie, M.; Taherpour, A.; Fellowes, T.; Hancok, A.N.; Yari, A. A convenient method for preparation of 2-amino-4,6-diphenylnicotinonitrile using HBF4 as an efficient catalyst via an anomeric based oxidation: A joint experimental and theoretical study. J. Mol. Struct. 2017, 1137, 674–680. [Google Scholar] [CrossRef]
- Ma’Mani, L.; Hajihosseini, E.; Saeedi, M.; Mahdavi, M.; Asadipour, A.; Firoozpour, L.; Shafiee, A.; Foroumadi, A. Sulfonic Acid Supported Phosphonium Based Ionic Liquid Functionalized SBA-15 for the Synthesis of 2-Amino-3-cyano-4,6-diarylpyridines. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2015, 46, 306–310. [Google Scholar] [CrossRef]
- Niya, H.F.; Hazeri, N.; Maghsoodlou, M.T.; Fatahpour, M. Synthesis, characterization, and application of CoFe2O4@TRIS@sulfated boric acid nanocatalyst for the synthesis of 2-amino-3-cyanopyridine derivatives. Res. Chem. Intermed. 2021, 47, 1315–1330. [Google Scholar] [CrossRef]
- Abdollahi-Alibeik, M.; Sadeghi-Vasafi, N.; Moaddeli, A.; Rezaeipoor-Anari, A. ClO−4/Al-MCM-41 nanoparticles as a solid acid catalyst for the synthesis of 2-amino-3-cyanopyridines. Res. Chem. Intermed. 2016, 42, 2867–2881. [Google Scholar] [CrossRef]
- Kheilkordi, Z.; Ziarani, G.M.; Bahar, S.; Badiei, A. The green synthesis of 2-amino-3-cyanopyridines using SrFe12O19 magnetic nanoparticles as efficient catalyst and their application in complexation with Hg2+ ions. J. Iran. Chem. Soc. 2018, 16, 365–372. [Google Scholar] [CrossRef]
- Behrouz, S. Highly efficient four-component synthesis of 2-amino-3-cyanopyridines using doped nano-sized copper(I) oxide (Cu2O) on melamine–formaldehyde resin. J. Chem. Res. 2016, 40, 540–544. [Google Scholar] [CrossRef]
- Mansoor, S.S.; Aswin, K.; Logaiya, K.; Sudhan, S.P.N. [Bmim]BF 4 ionic liquid: An efficient reaction medium for the one-pot multi-component synthesis of 2-amino-4,6-diphenylpyridine-3-carbonitrile derivatives. J. Saudi Chem. Soc. 2016, 20, 517–522. [Google Scholar] [CrossRef]
- Kurumurthy, C.; Kumar, R.N.; Yakaiah, T.; Rao, P.S.; Narsaiah, B. Novel Bu4N+Br− catalyzed one-pot multi-component synthesis of 2-amino nicotinonitriles in aqueous medium. Res. Chem. Intermed. 2013, 41, 3193–3199. [Google Scholar] [CrossRef]
- Yelwande, A.A.; Navgire, M.E.; Tayde, D.T.; Arbad, B.R.; Lande, M.K. SnO2/SiO2 nanocomposite catalyzed one-pot, four-component synthesis of 2-amino-3-cyanopyridines. S. Afr. J. Chem. 2012, 65, 131–137. [Google Scholar]
- Tang, J.; Wang, L.; Yao, Y.; Zhang, L.; Wang, W. One-pot synthesis of 2-amino-3-cyanopyridine derivatives catalyzed by ytterbium perfluorooctanoate [Yb(PFO)3]. Tetrahedron Lett. 2011, 52, 509–511. [Google Scholar] [CrossRef]
- Asadbegi, S.; Bodaghifard, M.A.; Mobinikhaledi, A. Poly N,N-dimethylaniline-formaldehyde supported on silica-coated magnetic nanoparticles: A novel and retrievable catalyst for green synthesis of 2-amino-3-cyanopyridines. Res. Chem. Intermed. 2017, 46, 1629–1643. [Google Scholar] [CrossRef]
- Medjdoub, A.; Belhadj, F.; Merzouk, A.S.; Hamed, Y.B.; Kibou, Z.; Choukchou-Braham, N.; Merzouk, H. In vitro peripheral blood mononuclear cell proliferation, cytokine secretion and oxidative stress modulation by pyrido[2,3-d] pyrimidines. Chem. Pap. 2019, 74, 903–913. [Google Scholar] [CrossRef]
- Edrisi, M.; Azizi, N. Sulfonic acid-functionalized graphitic carbon nitride composite: A novel and reusable catalyst for the one-pot synthesis of polysubstituted pyridine in water under sonication. J. Iran. Chem. Soc. 2019, 17, 901–910. [Google Scholar] [CrossRef]
- Khaksar, S.; Yaghoobi, M. A concise and versatile synthesis of 2-amino-3-cyanopyridine derivatives in 2,2,2-trifluoroethanol. J. Fluor. Chem. 2012, 142, 41–44. [Google Scholar] [CrossRef]
- Rahman, A.M.; Attwa, M.W.; Ahmad, P.; Baseeruddin, M.; Kadi, A.A. Fragmentation behavior studies of chalcones employing direct analysis in real time (DART). Mass Spectrom. Lett. 2013, 4, 30–33. [Google Scholar] [CrossRef]
- Shah, H.C.; Shah, V.H.; Desai, N.D. A novel strategy for the synthesis of 2-amino-4,6-diarylnicotinonitrile. Arkivoc 2009, 2009, 76–87. [Google Scholar] [CrossRef]
- Jalali-Mola, S.; Torabi, M.; Yarie, M.; Zolfigol, M.A. Acidic tributyl phosphonium-based ionic liquid: An efficient catalyst for preparation of diverse pyridine systems via a cooperative vinylogous anomeric-based oxidation. RSC Adv. 2022, 12, 34730–34739. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Cui, S. Divergent syntheses of 2-aminonicotinonitriles and pyrazolines by copper-catalyzed cyclization of oxime ester. Org. Lett. 2014, 16, 1350–1353. [Google Scholar] [CrossRef]
- Solgi, M.; Khazaei, A.; Akbarpour, T. Synthesis of magnetic nanoparticles Fe3O4@CQD@Si(OEt)(CH2)3@melamine@TC@Ni(NO3) with application in the synthesis of 2-amino-3-cyanopyridine and pyrano[2,3-c]pyrazole derivatives. Res. Chem. Intermed. 2022, 48, 2443–2468. [Google Scholar] [CrossRef]
- Alotaibi, A.A.; Asiri, H.H.; Rahman, A.F.M.M.; Alanazi, M.M. Novel pyrrolo[2,3-d]pyridine derivatives as multi-kinase inhibitors with VEGFR2 selectivity. J. Saudi Chem. Soc. 2023, 27, 101712. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, version 6.0.16; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.V.; Barone, V.G.A.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Luo, J.; Xue, Z.Q.; Liu, W.M.; Wu, J.L.; Yang, Z.Q. Koopmans’ theorem for large molecular systems within density functional theory. J. Phys. Chem. A 2006, 110, 12005–12009. [Google Scholar] [CrossRef] [PubMed]


| Cell Lines | IC50 * of APNs (1–6) | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | Dox ** | |
| MDA-MB-231 | 78.28 ± 3.9 | 8.01 ± 0.5 | 1.81 ± 0.1 | 6.93 ± 0.4 | 15.52 ± 1.2 | 10.23 ± 0.8 | 3.18 ± 0.1 |
| MCF-7 | >100 | 16.20 ± 1.3 | 2.85 ± 0.1 | 5.59 ± 0.3 | 20.07 ± 1.5 | 9.47 ± 0.7 | 4.17 ± 0.2 |
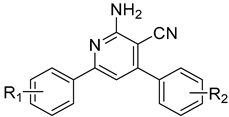 | Emission Maxima (λem, nm) at a Conc. of 1.45 × 10−8 M | ||||
|---|---|---|---|---|---|
| DCM | DMSO | MeOH | THF | Toluene | |
| 1: R1 = R2 = H | 403 | 425 | 415 | 412 | 401 |
| 2: R1 = 3-OCH3; R2 = H | 401 | 427 | 414 | 412 | 404 |
| 3: R1 = 3-OCH3; R2 = 4Cl | 416 | 433 | 419 | 418 | 405 |
| 4: R1 = 3-OCH3; R2 = 4-OCH3 | 396 | 420 | 410 | 407 | 397 |
| 5: R1 = 4-OCH3; R2 = H | 401 | 422 | 411 | 410 | 401 |
| 6: R1 = 4-OCH3; R2 = 4Cl | 404 | 431 | 418 | 417 | 404 |
| Compounds | Solvents | HOMO Energy | LUMO Energy | HOMO-LUMO Gap (ΔE) | Ionization Potential (IP) | Electron Affinity (EA) | Electro Negativity (c) | Chemical Potential (μ) | Hardness (h) | Softness (S) | Electrophilicity Index (ω) | Dipole Moment (Debye) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DMSO | −6.226 | −2.610 | 3.617 | 2.226 | 2.610 | 4.418 | −4.418 | 1.808 | 0.553 | 5.397 | 5.086 |
| MeOH | −6.226 | −2.608 | 3.617 | 6.226 | 2.608 | 4.417 | −4.417 | 1.809 | 0.553 | 5.393 | 5.064 | |
| 2 | DMSO | −6.194 | −2.604 | 3.590 | 6.194 | 2.604 | 4.399 | −4.399 | 1.795 | 0.557 | 5.390 | 6.854 |
| MeOH | −6.192 | −2.602 | 3.591 | 6.192 | 2.602 | 4.397 | −4.397 | 1.795 | 0.557 | 5.384 | 6.826 | |
| 3 | DMSO | −6.216 | −2.655 | 3.561 | 6.216 | 2.655 | 4.436 | −4.436 | 1.780 | 0.562 | 5.526 | 11.835 |
| MeOH | −6.216 | −2.655 | 3.561 | 6.216 | 2.655 | 4.435 | −4.435 | 1.781 | 0.562 | 5.524 | 11.787 | |
| 4 | DMSO | −6.147 | −2.531 | 3.616 | 6.147 | 2.531 | 4.339 | −4.339 | 1.808 | 0.553 | 5.206 | 5.471 |
| MeOH | −6.145 | −2.528 | 3.618 | 6.145 | 2.528 | 4.337 | −4.337 | 1.809 | 0.553 | 5.198 | 5.449 | |
| 5 | DMSO | −5.981 | −2.493 | 3.488 | 5.981 | 2.493 | 4.237 | −4.237 | 1.744 | 0.573 | 5.147 | 6.761 |
| MeOH | −5.980 | −2.491 | 3.489 | 5.980 | 2.491 | 4.235 | −4.235 | 1.745 | 0.573 | 5.141 | 6.726 | |
| 6 | DMSO | −6.000 | −2.558 | 3.442 | 6.000 | 2.558 | 4.279 | −4.279 | 1.721 | 0.581 | 5.321 | 8.135 |
| MeOH | −6.001 | −2.558 | 3.443 | 6.001 | 2.558 | 4.279 | −4.279 | 1.722 | 0.581 | 5.318 | 8.098 |
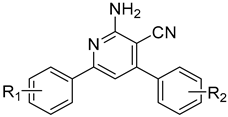 | TD-DFT Emission Maxima (λem, nm) Calculated by Using the TD-SCF-B3LYP/6311++G(d,p) Level of Theory in Different Solvent Systems | ||||
|---|---|---|---|---|---|
| DCM | DMSO | MeOH | THF | Toluene | |
| 1: R1 = R2 = H | 307, 404 | 309, 408 | 304, 408 | 307, 403 | 303, 394 |
| 2: R1 = 3-OCH3; R2 = H | 304, 410 | 306, 410 | 306, 410 | 304, 405 | 300, 396 |
| 3: R1 = 3-OCH3; R2 = 4Cl | 308, 414 | 309, 414 | 309, 414 | 307, 405 | 304, 401 |
| 4: R1 = 3-OCH3; R2 = 4-OCH3 | 304, 407 | 306, 411 | 305, 411 | 303, 406 | 299, 396 |
| 5: R1 = 4-OCH3; R2 = H | 315, 416 | 316, 420 | 316, 420 | 315, 415 | 311, 404 |
| 6: R1 = 4-OCH3; R2 = 4Cl | 323, 422 | 325, 427 | 324, 426 | 323, 421 | 320, 412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghamdi, A.R.; Rahman, S.; Al-Wabli, R.I.; Al-Mutairi, M.S.; Rahman, A.F.M.M. Synthesis, Cytotoxicity, and Photophysical Investigations of 2-Amino-4,6-diphenylnicotinonitriles: An Experimental and Theoretical Study. Molecules 2024, 29, 1808. https://doi.org/10.3390/molecules29081808
Al-Ghamdi AR, Rahman S, Al-Wabli RI, Al-Mutairi MS, Rahman AFMM. Synthesis, Cytotoxicity, and Photophysical Investigations of 2-Amino-4,6-diphenylnicotinonitriles: An Experimental and Theoretical Study. Molecules. 2024; 29(8):1808. https://doi.org/10.3390/molecules29081808
Chicago/Turabian StyleAl-Ghamdi, Alwah R., Shofiur Rahman, Reem I. Al-Wabli, Maha S. Al-Mutairi, and A. F. M. Motiur Rahman. 2024. "Synthesis, Cytotoxicity, and Photophysical Investigations of 2-Amino-4,6-diphenylnicotinonitriles: An Experimental and Theoretical Study" Molecules 29, no. 8: 1808. https://doi.org/10.3390/molecules29081808






