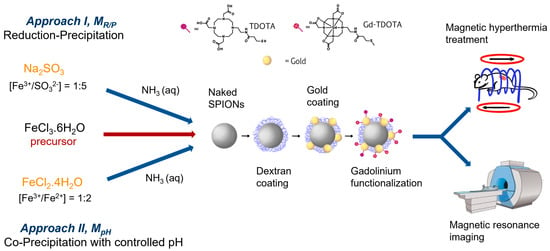
Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile
Abstract
:1. Introduction
2. Results and Discussion
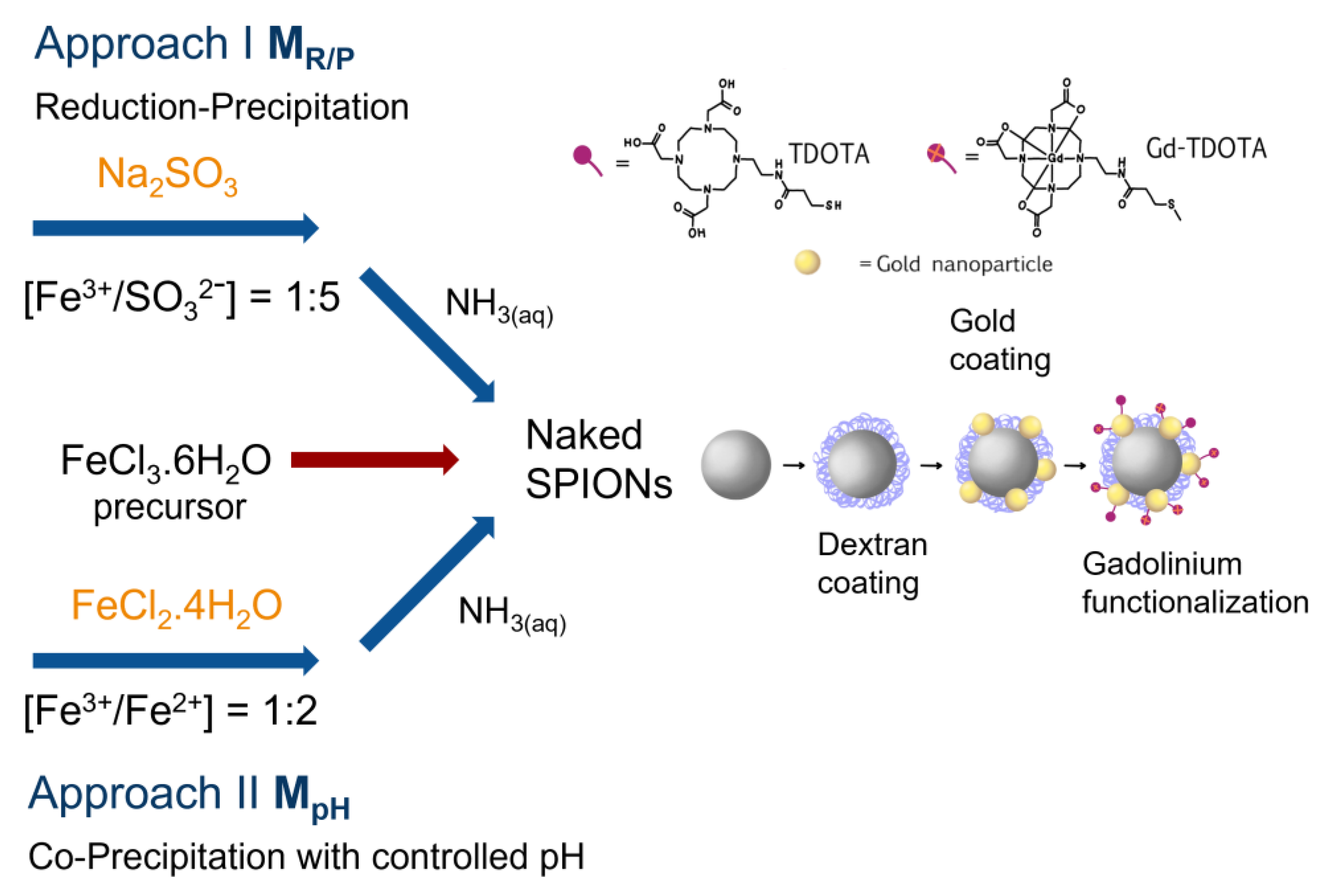
2.1. UV-Vis and ATR-FTIR
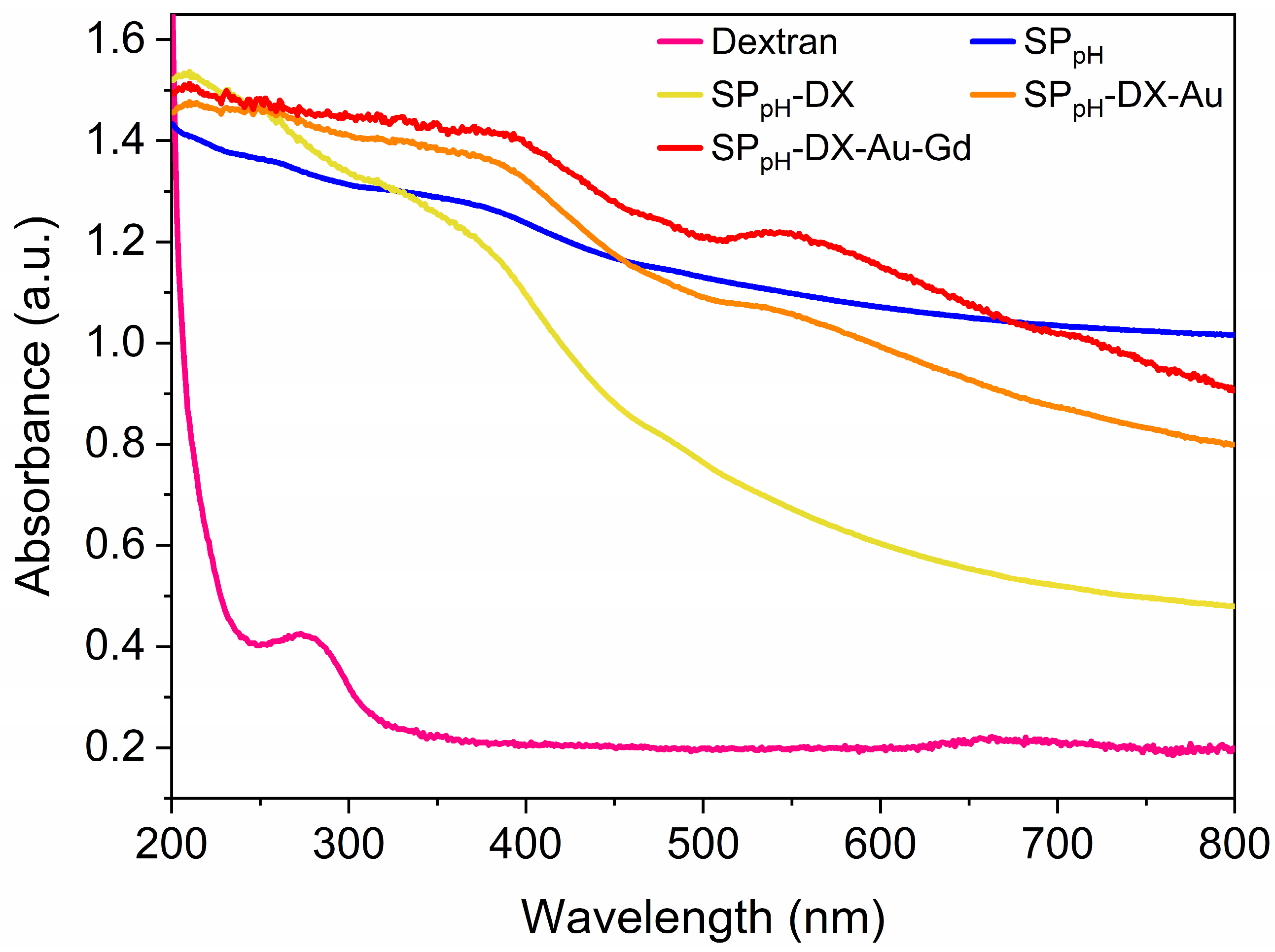
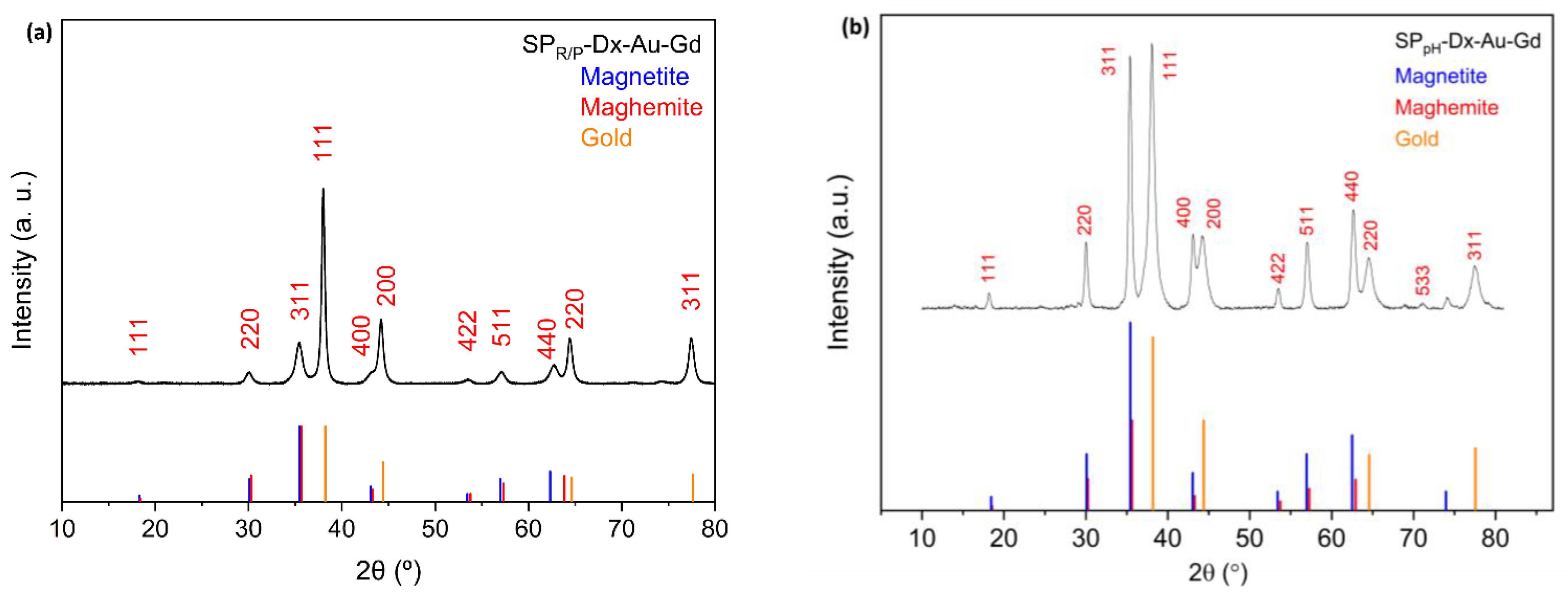
2.2. Powder X-ray Diffraction (PXRD)
2.3. Transmission Electron Microscopy (TEM) and Dynamic Light Scattering (DLS)
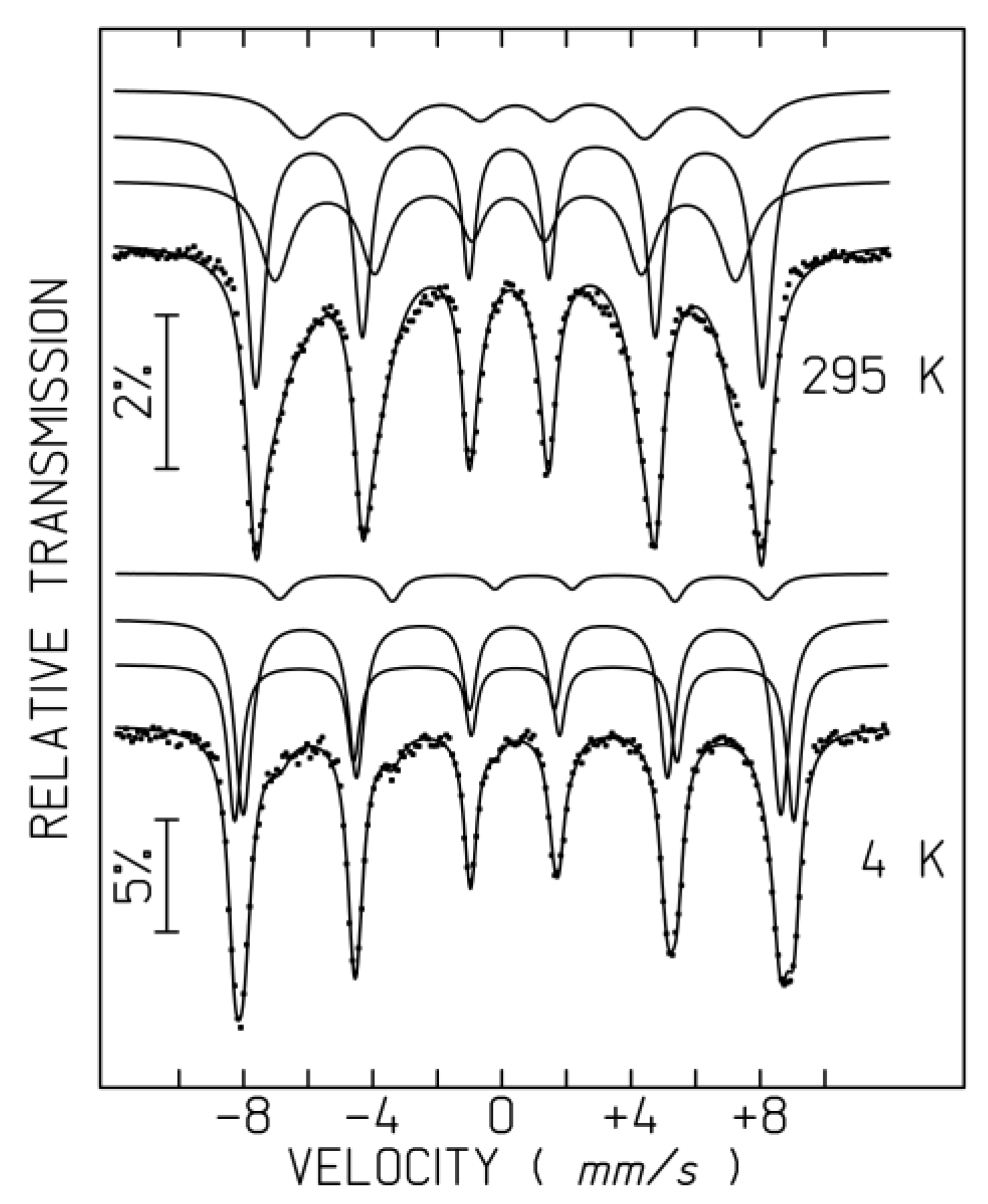
2.4. 57Fe Mössbauer Spectroscopy
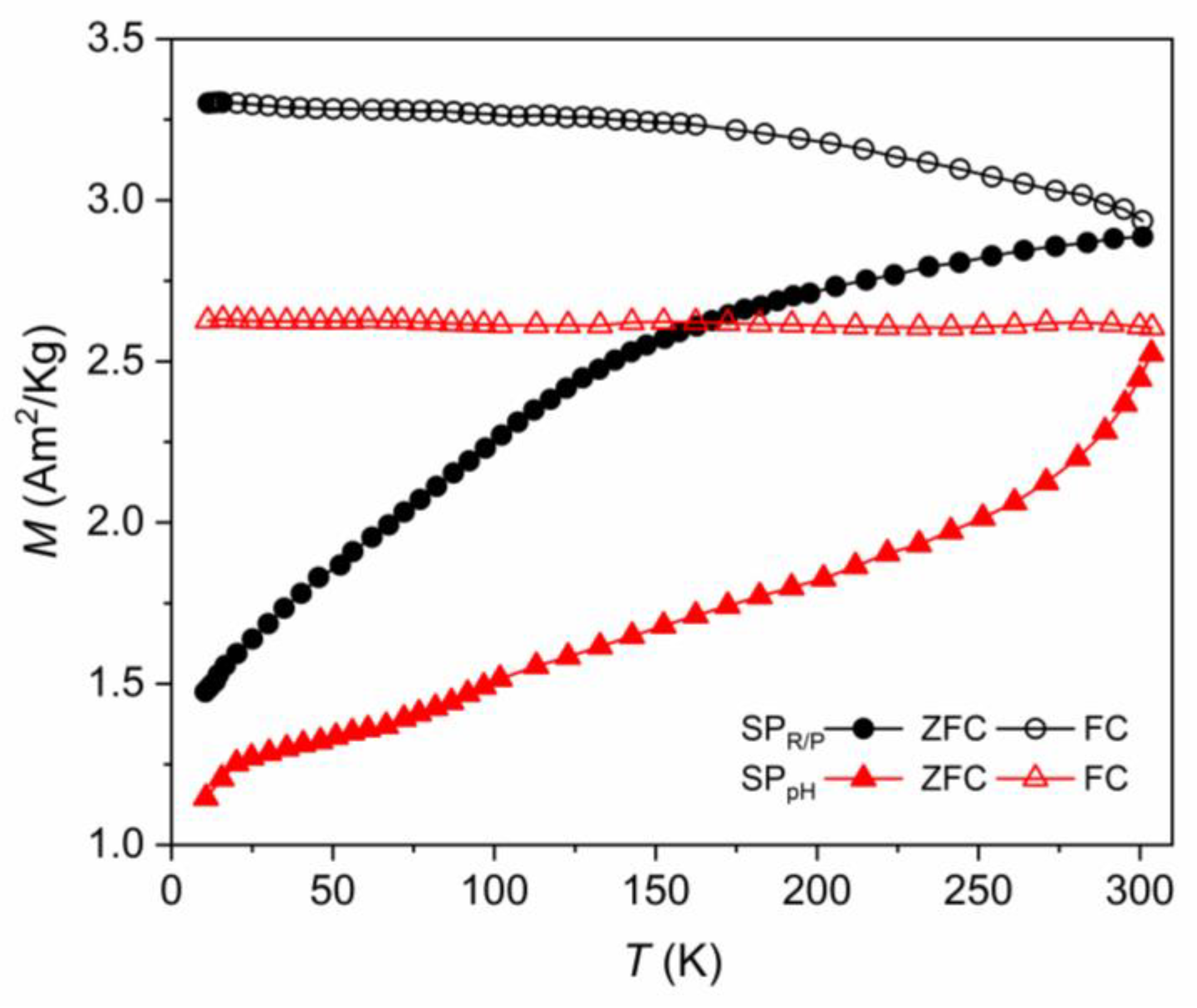
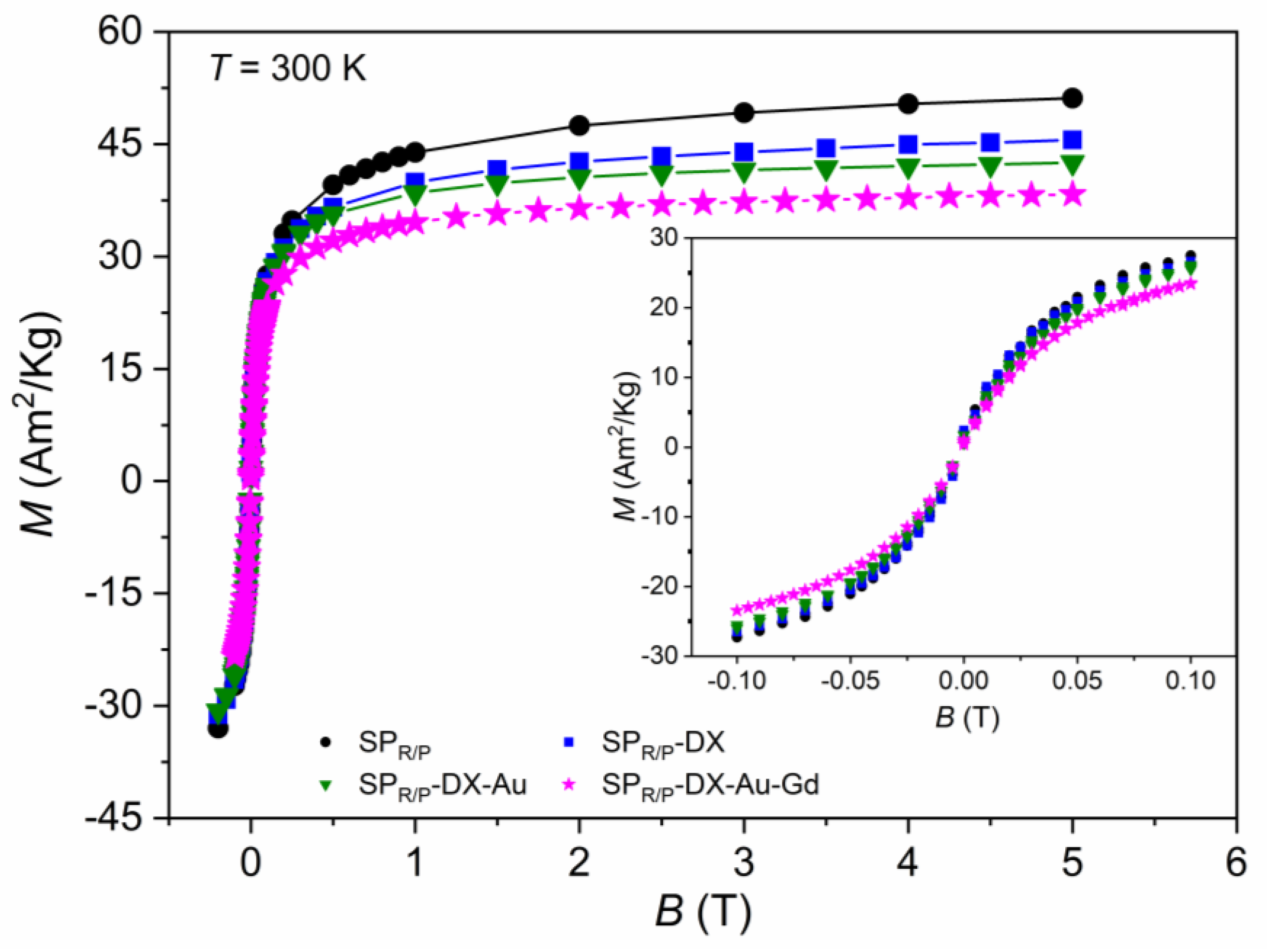
2.5. Magnetic Characterization by Magnetometry
2.5.1. Samples Obtained with the Reduction–Precipitation Process (SPR/P)
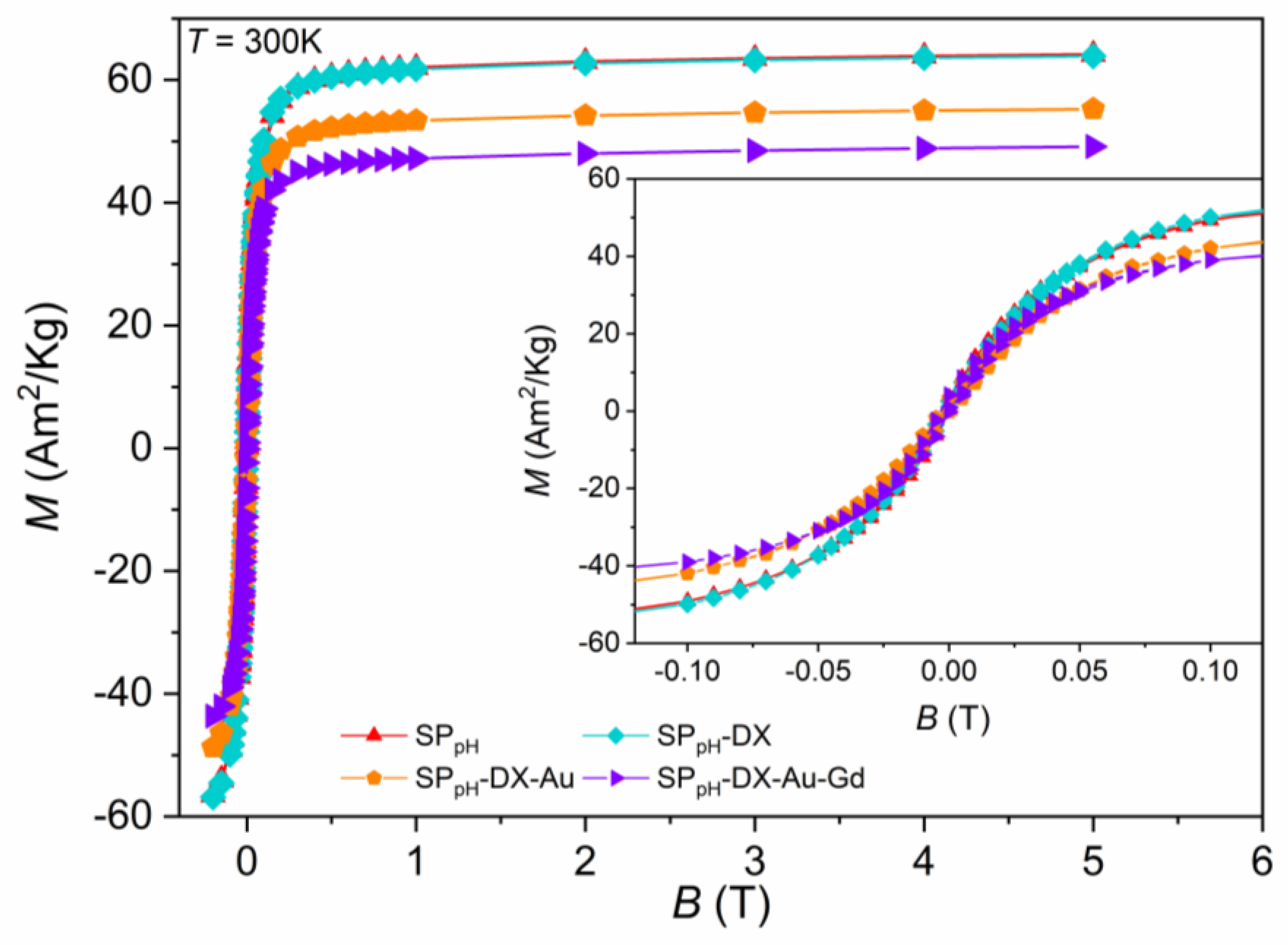
2.5.2. Samples Obtained with the Co-Precipitation with Controlled pH (SPpH)
2.6. Magnetic Hyperthermia
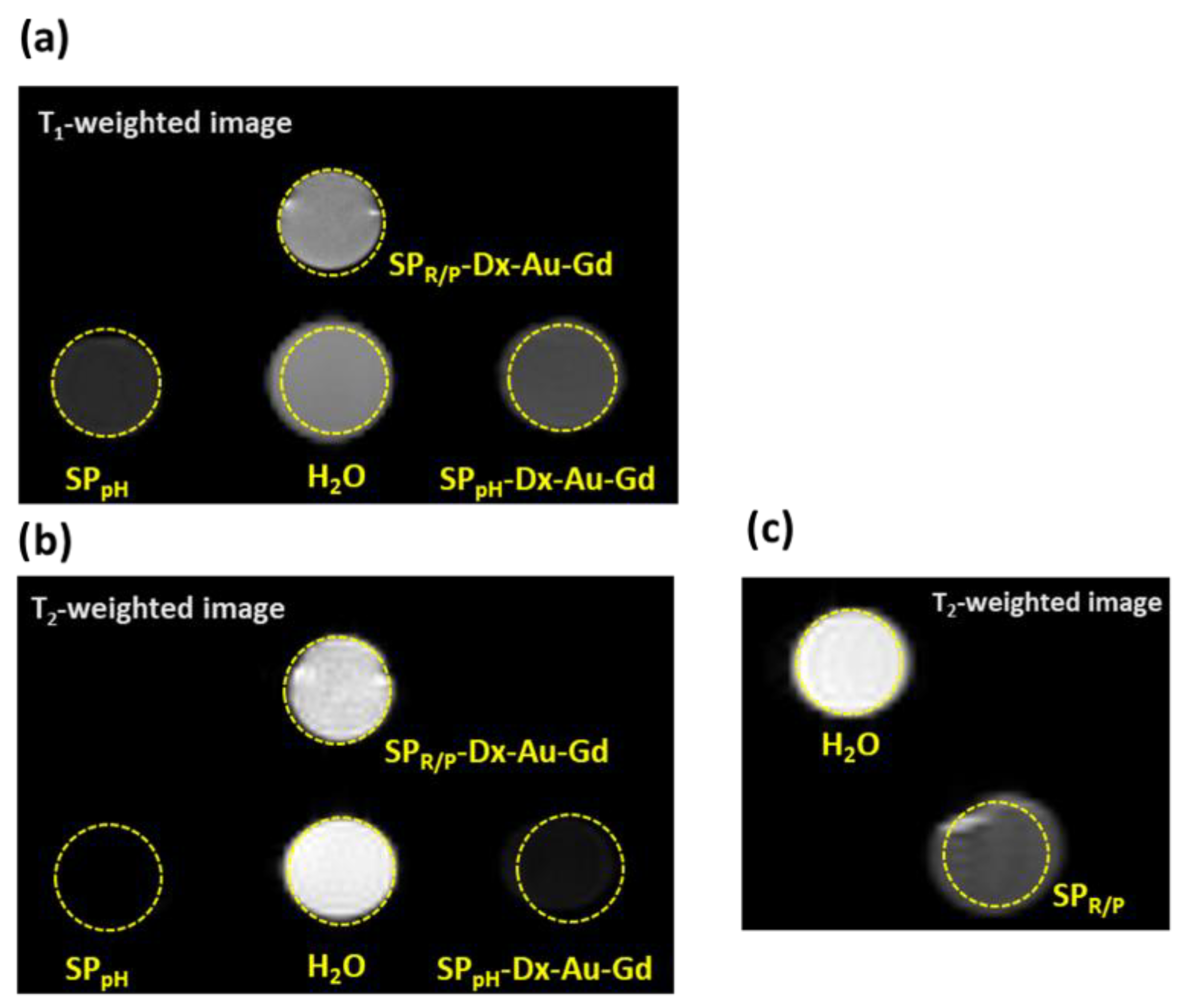
2.7. Relaxitivity Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Naked SPIONs
3.2.1. Reduction–Precipitation Process (MR/P)
3.2.2. Co-Precipitation Process with Controlled pH (MpH)
3.3. Post-Synthesis of SPIONs Coating
3.3.1. Dextran Coating
3.3.2. Gold Coating
3.3.3. Gadolinium Functionalization
3.4. Characterization of the SPIONs
3.4.1. UV-Vis Spectrophotometry (UV-Vis)
3.4.2. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
3.4.3. Powder X-ray Diffraction (PXRD)
3.4.4. Transmission Electron Microscopy (TEM)
3.4.5. Dynamic Light Scattering (DLS) and Zeta-Potential
3.4.6. Determination of the Iron, Gold, and Gadolinium Content
3.4.7. Mössbauer Spectroscopy
3.4.8. SQUID Magnetometry
3.4.9. Magnetic Hyperthermia
3.4.10. MRI Phantoms and Relaxometric Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterson, D.I.; Wiebe, N.; Cheung, W.; Mackey, J.R.; Pituskin, E.; Reiman, A.; Tonelli, M. Incident cardiovascular disease among adults with cancer: A population-based cohort study. J. Am. Coll. Cardiol. Cardio Oncol. 2022, 4, 85–94. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Abed, A.; Derakhshan, M.; Karimi, M.; Shirazinia, M.; Mahjoubin-Tehran, M.; Homayonfal, M.; Hamblin, M.R.; Mirzaei, S.A.; Soleimanpour, H.; Dehghani, S.; et al. Cutting-Edge Applications of Nanomaterials in Biomedical Sciences. Front. Pharmacol. 2022, 13, 797804. [Google Scholar] [CrossRef]
- Khursheed, R.; Dua, K.; Vishwas, S.; Gulati, M.; Jha, N.K.; Aldhafeeri, G.M.; Alanazi, F.G.; Goh, B.H.; Gupta, G.; Paudel, K.R.; et al. Biomedical applications of metallic nanoparticles in cancer: Current status and future perspectives. Biomed. Pharmacother. 2022, 150, 112951. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.V.; Ghanouni, P.; Campbell, J.L.; Mittra, E.; Gambhir, S.S. Clinically Approved Nanoparticle Imaging Agents. J. Nucl. Med. 2016, 57, 1833–1837. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Analysis of Experimental Data on Changes in Various Structures and Functions of the Rat Brain following Intranasal Administration of Fe2O3 Nanoparticles. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Soetaert, F.; Korangath, P.; Serantes, D.; Fiering, S.; Ivkov, R. Cancer therapy with iron oxide nanoparticles: Agents of thermal and immune therapies. Adv. Drug Delivery Rev. 2020, 163–164, 65–83. [Google Scholar] [CrossRef]
- Aziz, O.A.A.; Arafa, K.; Dena, A.S.A.; El-Sherbiny, I.M. Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Preparation and Recent Applications. J. Nanotech. Adv. Mat. 2020, 8, 21–29. [Google Scholar]
- Gaeta, M.; Cavallaro, M.; Vinci, S.L.; Mormina, E.; Blandino, A.; Marino, M.A.; Granata, F.; Tessitore, A.; Galletta, K.; D’Angelo, T.; et al. Magnetism of materials: Theory and practice in magnetic resonance imaging. Insights Imaging 2021, 12, 179. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Camozzi, D.; Darguzyte, M.; Roemhild, K.; Varvarà, P.; Metselaar, J.; Banala, S.; Straub, M.; Güvener, N.; Engelmann, U.; et al. Size-isolation of superparamagnetic iron oxide nanoparticles improves MRI, MPI and hyperthermia performance. J. Nanobiotechnol. 2020, 18, 22. [Google Scholar] [CrossRef]
- Liu, G.; Gao, J.; Ai, H.; Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 2013, 9, 1533–1545. [Google Scholar] [CrossRef]
- Dizaji, A.N.; Yilmaz, M.; Piskin, E. Silver or gold deposition onto magnetite nanoparticles by using plant extracts as reducing and stabilizing agents. Artifi. Cells Nanomed. Biotechnol. 2016, 44, 1109–1115. [Google Scholar]
- Yang, Q.; Li, Y.; Zhao, X.; Zhang, J.; Cheng, X.; Zhu, N. Nanoenzyme-Based Electrodes in Biomolecular Screening and Analysis. Appl. Nanosci. 2023, 13, 5489–5500. [Google Scholar] [CrossRef]
- Matos, J.C.; Gonçalves, M.C.; Pereira, L.C.J.; Vieira, B.J.C.; Waerenborgh, J.C. SPIONs Prepared in Air through Improved Synthesis Methodology: The Influence of γ-Fe2O3/Fe3O4 Ratio and Coating Composition on Magnetic Properties. Nanomaterials 2019, 9, 943. [Google Scholar] [CrossRef]
- Hoffman, A.S.; Stayton, P.S. Applications of “Smart Polymers” as Biomaterials. In Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2020; pp. 191–203. [Google Scholar]
- Silva, F.; Zambre, A.; Campello, M.P.C.; Gano, L.; Santos, I.; Ferraria, A.M.; Ferreira, M.J.; Singh, A.; Upendran, A.; Paulo, A.; et al. Interrogating the Role of Receptor-Mediated Mechanisms: Biological Fate of Peptide-Functionalized Radiolabeled Gold Nanoparticles in Tumor Mice. Bioconjugate Chem. 2016, 27, 1153–1164. [Google Scholar] [CrossRef]
- Silva, F.; Paulo, A.; Pallier, A.; Même, É.; Tóth, S.; Gano, L.; Marques, F.; Geraldes, C.F.G.C.; Castro, M.M.C.A.; Cardoso, A.M.; et al. Dual Imaging Gold Nanoplatforms for Targeted Radiotheranostics. Materials 2020, 13, 00513. [Google Scholar] [CrossRef]
- Marques, A.; Belchior, A.; Silva, F.; Marques, F.; Campello, M.P.C.; Pinheiro, T.; Santos, P.; Santos, L.; Matos, A.P.A.; Paulo, A. Dose rate effects on the selective radiosensitization of prostate cells by GRPR-targeted gold nanoparticles. Int. J. Mol. Sci. 2022, 23, 5279. [Google Scholar] [CrossRef]
- Silva, F.; Mendes, C.; D’Onofrio, A.; Campello, M.P.C.; Marques, F.; Pinheiro, T.; Gonçalves, K.; Figueiredo, S.; Gano, L.; Ravera, M.; et al. Image-guided nanodelivery of Pt (IV) prodrugs to GRP-receptor positive tumors. Nanotheranostics 2023, 7, 22–40. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Ribeiro, I.A.C.; Fernandes, M.H.; Cerdeira, A.C.; Vieira, B.J.C.; Waerenborgh, J.C.; Pereira, L.C.J.; Cláudio, R.; Carmezim, M.J.; Gomes, P.; et al. 3D-printed platform multi-loaded with bioactive, magnetic nanoparticles and an antibiotic for re-growing bone tissue. Int. J. Pharm. 2021, 593, 120097. [Google Scholar] [CrossRef]
- Zschiesche, L.; Janko, C.; Friedrich, B.; Frey, B.; Band, J.; Lyer, S.; Alexiou, C.; Unterweger, H. Biocompatibility of dextran-coated 30 nm and 80 nm sized spions towards monocytes, dendritic cells and lymphocytes. Nanomaterials 2023, 13, 14. [Google Scholar] [CrossRef]
- Devi, S.M.; Nivetha, A.; Prabha, I. Role of citric acid/glycine-reinforced nanometal oxide for the enhancement of physio-chemical specifications in catalytic properties. J. Supercond. Nov. Magn. 2020, 33, 3893–3901. [Google Scholar] [CrossRef]
- Hernández, G.L.A. Light scattering and plasmonic response of Au–Fe3O4 nanoparticles. SN Appl. Sci. 2020, 2, 1844. [Google Scholar] [CrossRef]
- Samrot, A.V.; Justin, C.; Padmanaban, S.; Burman, U. A study on the effect of chemically synthesized magnetite nanoparticles on earthworm: Eudrilus eugeniae. Appl. Nanosci. 2017, 7, 17–23. [Google Scholar] [CrossRef]
- Uvarov, V.; Popov, I. Metrological characterization of X-ray diffraction methods at different acquisition geometries for determination of crystallite size in nano-scale materials. Mater. Charact. 2013, 85, 111–123. [Google Scholar] [CrossRef]
- Farhana, A.; Selvarani, A.J.; Samrot, A.V.; Alsrhani, A.; Raji, P.; Sahitya, C.S.; Cypriyana, P.J.J.; Senthikumar, P.; Ling, M.P.; Yishak, S. Utilization of superparamagnetic iron oxide nanoparticles (SPIONs) impregnated activated carbon for removal of hexavalent chromium. J. Nanomater. 2022, 2022, 4326939. [Google Scholar] [CrossRef]
- Battisha, I.K.; Afify, H.H.; Ibrahim, M. Synthesis of Fe2O3 concentrations and sintering temperature on FTIR and magnetic susceptibility measured from 4 to 300 K of monolith silica gel prepared by sol–gel technique. J. Magn. Magn. Mater. 2006, 306, 211–217. [Google Scholar] [CrossRef]
- Singh, B.P.; Kumar, A.; Areizaga-Martinez, H.I.; Vega-Olivencia, C.A.; Tomar, M.S. Synthesis, characterization, and electrocatalytic ability of γ-Fe2O3 nanoparticles for sensing acetaminophen. Indian J. Pure Appl. Phys. 2017, 55, 722–728. [Google Scholar]
- Ra’ad, Z.; Al-Karam, L.Q.; Abid Alsahib, N.K. Synthesis and Characterization of Dextran coated Iron Oxide Nanoparticles as a T1 Contrast Agent. J. Phys. Conf. Ser. 2021, 2114, 012037. [Google Scholar] [CrossRef]
- Mahadevan, S.; Gnanaprakash, G.; Philip, J.; Rao, B.P.C.; Jayakumar, T. X-ray diffraction-based characterization of magnetite nanoparticles in presence of goethite and correlation with magnetic properties. Phys. E Low-Dimens. Syst. Nanostructures 2007, 39, 20–25. [Google Scholar] [CrossRef]
- Bala, H.; Guo, Y.; Zhao, X.; Zhao, J.; Fu, W.; Ding, X.; Jiang, Y.; Yu, K.; Lv, X.; Wang, Z. Controlling the particle size of nanocrystalline titania via a thermal dissociation of substrates with ammonium chloride. Mater. Lett. 2006, 60, 494–498. [Google Scholar] [CrossRef]
- Dorsey, J.F.; Sun, L.; Joh, D.Y.; Witztum, A.; Al Zaki, A.; Kao, G.D.; Alonso-Basanta, M.; Avery, S.; Tsourkas, A.; Hahn, S.M. Gold nanoparticles in radiation research: Potential applications for imaging and radiosensitization. Transl. Cancer Res. 2013, 2, 280–291. [Google Scholar]
- Ferreira, L.F.P.; Oliveira, T.M.; Toma, S.H.; Toyama, M.M.; Araki, K.; Avanzi, L.H. Superparamagnetic iron oxide nanoparticles (SPIONs) conjugated with lipase Candida antarctica A for biodiesel synthesis. RSC Adv. 2020, 10, 38490–38496. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Sahu, S.K.; Banerjee, I.; Das, M.; Mishra, D.; Maiti, T.K.; Pramanik, P. Synthesis, characterization, and in vitro biological evaluation of highly stable diversely functionalized superparamagnetic iron oxide nanoparticles. J. Nanopart. Res. 2011, 13, 4173–4188. [Google Scholar] [CrossRef]
- Rajendran, S.; Wanale, S.G.; Gacem, A.; Yadav, V.K.; Ahmed, I.A.; Algethami, J.S.; Kakodiya, S.D.; Modi, T.; Alsuhaibani, A.M.; Yadav, K.K.; et al. Nanostructured iron oxides: Structural, optical, magnetic, and adsorption characteristics for cleaning industrial effluents. Crystals 2023, 13, 472. [Google Scholar] [CrossRef]
- Kolen’ko, Y.V.; Bañobre-López, M.; Rodríguez-Abreu, C.; Carbó-Argibay, E.; Sailsman, A.; Piñeiro-Redondo, Y.; Cerqueira, M.F.; Petrovykh, D.Y.; Kovnir, K.; Lebedev, O.I.; et al. Large-Scale Synthesis of Colloidal Fe3O4 Nanoparticles Exhibiting High Heating Efficiency in Magnetic Hyperthermia. J. Phys. Chem. C 2014, 118, 8691–8701. [Google Scholar] [CrossRef]
- Ngo, T.H.; Tran, D.L.; Do, H.M.; Tran, V.H.; Le, V.H.; Nguyen, X.P. Facile and solvent-free routes for the synthesis of size-controllable Fe3O4 nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 035001. [Google Scholar] [CrossRef]
- Yousuf, S.; Enoch, I.V.M.V.; Selvakumar, P.M.; Premnath, D. Loading of chromenones on superparamagnetic iron oxide-modified dextran core–shell nanoparticles: Openness to bind to β-cyclodextrin and DNA. New J. Chem. 2015, 39, 7879–7888. [Google Scholar] [CrossRef]
- Khalkhali, M.; Rostamizadeh, K.; Sadighian, S.S.; Khoeini, F.; Naghibi, M.; Hamidi, M. The impact of polymer coatings on magnetite nanoparticles performance as MRI contrast agents: A comparative study. DARU J. Pharm. Sci. 2015, 23, 45. [Google Scholar] [CrossRef]
- Bakhtari, A.; Nazari, S.; Alaee, S.; Kargar-Abarghouei, E.; Mesbah, F.; Mirzaei, E.; Molaei, M.J. Toxic Effects of Dextran-Coated Superparamagnetic Iron Oxide Nanoparticles on Mouse Embryo Development, Genes Expression of Antioxidant Enzymes and Apoptosis, and Ultrastructure of Sperm, Oocytes and Granulosa Cells. Int. J. Fertil. Steril. 2020, 14, 161–170. [Google Scholar]
- Unterweger, H.; Dézsi, L.; Matuszak, J.C.; Poettler, M.; Jordan, J.; Bäuerle, T.; Szebeni, J.; Fey, T.; Boccaccini, A.R.; Alexiou, C.; et al. Dextran-coated superparamagnetic iron oxide nanoparticles for magnetic resonance imaging: Evaluation of size-dependent imaging properties, storage stability and safety. Int. J. Nanomed. 2018, 13, 1899. [Google Scholar] [CrossRef]
- Nurdin, I.; Johan, M.R.; Yaacob, I.I.; Ang, B.C. Effect of nitric acid concentrations on synthesis and stability of maghemite nanoparticles suspension. Sci. World J. 2014, 2014, 589479. [Google Scholar] [CrossRef]
- Chen, B.-W.; Hatamie, S.; Garu, P.; Heravi, P.; Chen, J.-Y.; Liu, B.-T.; Wei, Z.-H.; Yao, D.-J. Synthesis of iron-oxide magnetic nanoparticles coated with dextran of varied molecular mass using a facile ball-milling method. Micro Nano Lett. 2020, 15, 645–650. [Google Scholar] [CrossRef]
- Osborne, E.A.; Atkins, T.M.; Gilbert, D.A.; Kauzlarich, S.M.; Liu, K.; Louie, A.Y. Rapid microwave-assisted synthesis of dextran-coated iron oxide nanoparticles for magnetic resonance imaging. Nanotechnology 2012, 23, 215602. [Google Scholar] [CrossRef]
- Maleki, H.; Simchi, A.; Imani, M.; Costa, B.F.O. Size-controlled synthesis of superparamagnetic iron oxide nanoparticles and their surface coating by gold for biomedical applications. J. Magn. Magn. Mat. 2012, 324, 3997. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution–a perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Manescu, V.; Paltanea, G.; Antoniac, I.; Vasilescu, M. Magnetic nanoparticles used in oncology. Materials 2021, 14, 5948. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Pachiyappan, S.; Kumar, S.S. Surface-engineered super-paramagnetic iron oxide nanoparticles for chromium removal. Int. J. Nanomed. 2019, 14, 8105. [Google Scholar] [CrossRef]
- Silva, G.B.; Marciello, M.; Puerto Morales, M.; Serna, C.J.; Vargas, M.D.; Ronconi, C.M.; Costo, R. Studies of the Colloidal Properties of Superparamagnetic Iron Oxide Nanoparticles Functionalized with Platinum Complexes in Aqueous and PBS Buffer Media. J. Braz. Chem. Soc. 2016, 28, 731–739. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of Nanoparticle Concentration on Zeta-Potential Measurement Results and Reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Wang, W.; Ding, X.; Xu, Q.; Wang, J.; Wang, L.; Lou, X. Zeta-Potential Data Reliability of Gold Nanoparticle Biomolecular Conjugates and Its Application in Sensitive Quantification of Surface Absorbed Protein. Colloids Surf. B Biointerfaces 2016, 148, 541–548. [Google Scholar] [CrossRef]
- Mørup, S. Mössbauer Effect Studies of Microcrystalline Materials. In Mössbauer Spectroscopy Applied to Inorganic Chemistry; Long, G.J., Ed.; Plenum Press: New York, NY, USA; London, UK, 1987; Volume 2, pp. 89–124. [Google Scholar]
- Murad, E. Clays and clay minerals: What can Mössbauer spectroscopy do to help understand them? Hyperfine Interact. 1998, 117, 39. [Google Scholar] [CrossRef]
- Roca, A.G.; Marco, J.F.; Morales, M.D.P.; Serna, C.J. Uniform, and water stable magnetite nanoparticles with diameters around the monodomain–multidomain limit. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Predoi, D.; Kuncser, V.; Tronc, E.; Nogues, M.; Russo, U.; Principi, G.; Filoti, G. Magnetic relaxation phenomena and inter-particle interactions in nanosized γ-Fe2O3 systems. J. Phys. Condens. Matter 2003, 15, 1797. [Google Scholar] [CrossRef]
- Vandenberghe, R.E.; Barrero, C.A.; Da Costa, G.M.; Van San, E.; De Grave, E. Mössbauer characterization of iron oxides and (oxy) hydroxides: The present state of the art. Hyperfine Interact. 2000, 126, 247. [Google Scholar] [CrossRef]
- Singh, M.; Ulbrich, P.; Prokopec, V.; Svoboda, P.; Šantavá, E.; Štěpánek, F. Vapour phase approach for iron oxide nanoparticle synthesis from solid precursors. J. Solid. State Chem. 2013, 200, 150–156. [Google Scholar] [CrossRef]
- Box, G.E.P.; Lucas, H.L. Design of experiments in non-linear situations. Biometrika 1959, 46, 77–90. [Google Scholar] [CrossRef]
- Freitas, S.C.; Belo, J.H.; Granja, A.; Canhota, M.; Silva, A.S.; Reis, S.; Crespo, H.; Araújo, J.P.; Sousa, C.T. Key parameters in phototherapy with gold nanorods using continuous near infrared radiation. Adv. Mater. Interfaces 2023, 10, 2202214. [Google Scholar] [CrossRef]
- Liu, N.N.; Pyatakov, A.P.; Saletsky, A.M.; Zharkov, M.N.; Pyataev, N.A.; Sukhorukov, G.B.; Gun’ko, Y.K.; Tishin, A.M. The “field or frequency” dilemma in magnetic hyperthermia: The case of ZnMn ferrite nanoparticles. J. Magn. Magn. Mat. 2022, 555, 169379. [Google Scholar] [CrossRef]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mat. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Cabrera, D.; Yoshida, T.; Rincón-Domínguez, T.; Cuñado, J.L.F.; Salas, G.; Bollero, A.; Puerto-Morales, M.; Camarero, J.; Teran, F.J. Superparamagnetic-blocked state transition under alternating magnetic fields: Towards determining the magnetic anisotropy in magnetic suspensions. Nanoscale 2022, 14, 8789–8796. [Google Scholar] [CrossRef]
- Blasiak, B.; van Veggel, F.C.J.M.; Tomanek, B. Applications of nanoparticles for MRI cancer diagnosis and therapy. J. Nanomater. 2013, 2013, 148578. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–relaxivity relationships of magnetic nanoparticles for magnetic resonance imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wang, J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar]
- Na, H.B.; Song, I.C.; Hyeon, T. Inorganic nanoparticles for MRI contrast agents. Adv. Mater. 2009, 21, 2133–2148. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, Y.B.; Lee, Y.; Hwang, J.Y.; Park, H.W.; Park, T.G. Bioinspired Synthesis and Characterization of Gadolinium-Labeled Magnetite Nanoparticles for Dual Contrast T1- and T2-Weighted Magnetic Resonance Imaging. Bioconj. Chem. 2010, 21, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; Diaz-Diestra, D.; Beltran-Huarac, J.; Weiner, B.R.; Morell, G. Enhanced MRI T 2 Relaxivity in Contrast-Probed Anchor-Free PEGylated Iron Oxide Nanoparticles. Nanoscale Res. Lett. 2017, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI contrast agents: Current challenges and new frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Tóth, E.; Helm, L.; Merbach, A. Relaxivity of gadolinium (III) complexes: Theory and mechanism. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Merbach, A., Helm, L., Tóth, É., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 2; pp. 25–81. [Google Scholar]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-Dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef]
- Cheng, K.; Yang, M.; Zhang, R.; Qin, C.; Su, X.; Chen, Z. Hybrid Nanotrimers for Dual T1 and T2-Weighted Magnetic Resonance Imaging. ACS Nano 2014, 8, 9884–9896. [Google Scholar] [CrossRef]
- Roch, A.; Muller, R.N.; Gillis, P. Theory of proton relaxation induced by superparamagnetic particles. J. Chem. Phys. 1999, 110, 5403–5411. [Google Scholar] [CrossRef]
- Ta, H.T.; Li, Z.; Wu, Y.; Cowin, G.; Zhang, S.; Yago, A.; Whittaker, A.K.; Xu, Z.P. Effects of magnetic field strength and particle aggregation on relaxivity of ultra-small dual contrast iron oxide nanoparticles. Mater. Res. Express 2017, 4, 116105. [Google Scholar] [CrossRef]
- Girardet, T.; Bianchi, E.; Henrionnet, C.; Pinzano, A.; Bouguet-Bonnet, S.; Boulogne, C.; Leclerc, S.; Cleymand, F.; Fleutot, S. SPIONs magnetic nanoparticles for MRI applications: Microwave synthesis and physicochemical, magnetic and biological characterizations. Mater. Today Commun. 2023, 36, 106819. [Google Scholar] [CrossRef]
- Zhang, W.; Peters, J.A.; Mayer, F.; Helm, L.; Djanashvili, K. Prototropic Exchange Governs T1 and T2 Relaxivities of a Potential MRI Contrast Agent Nanozeolite Gd−LTL with a High pH Responsiveness. J. Phys. Chem. C 2015, 119, 5080. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef]
- Hesse, J.; Rübartsch, A. Model independent evaluation of overlapped Mossbauer spectra. J. Phys. E 1974, 7, 526. [Google Scholar] [CrossRef]




| Sample | Description | Precursor |
|---|---|---|
| SPR/P | SPIONs obtained by reduction–precipitation | FeCl3·6H2O/Na2SO3 |
| SPR/P-Dx | SPR/P coated with dextran | SPR/P |
| SPR/P-Dx-Au | SPR/P-Dx coated with gold | SPR/P-Dx |
| SPR/P-Dx-Au-Gd | SPR/P-Dx-Au functionalized with Gd3+ | SPR/P-Dx-Au |
| SPpH | SPIONs obtained by co-precipitation with controlled pH | FeCl3·6H2O/FeCl2·4H2O |
| SPpH-Dx | SPpH coated with dextran | SPpH |
| SPpH-Dx-Au | SPpH-Dx coated with gold | SPpH-Dx |
| SPpH-Dx-Au-Gd | SPpH-Dx-Au functionalized with Gd3+ | SPpH-Dx-Au |
| Sample | Crystallite Size—DXRD (nm) | Particle Size—DTEM (nm) | Size Dispersion—σ (%) | Particle Size—Dmag (nm) | Hydrodynamic Size—DH (nm) (PDI) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| SPR/P | 9.0 ± 0.6 | 10.0 ± 2.1 | 20.94 | 10.51 | 52.5 (0.6) | 40.3 ± 8.3 |
| SPR/P-Dx | 7.4 ± 0.9 | 9.6 ± 1.5 | 27.58 | 11.00 | 352.4 (0.4) | −6.2 ± 7.6 |
| SPR/P-Dx-Au | 9.8 ± 0.8 | 10.9 ± 2.7 | 24.84 | 11.68 | 47.4 (0.6) | −19.1 ± 6.0 |
| SPR/P-Dx-Au-Gd | 9.4 ± 0.7 | 11.0 ± 2.8 | 24.22 | 12.28 | 127.9 (0.3) | 35.4 ± 8.5 |
| SPpH | 10.0 ± 0.5 | 12.2 ± 2.5 | 20.84 | 10.09 | 25.4 (0.5) | 43.8 ± 10.0 |
| SPpH-Dx | 12.0 ± 0.6 | 16.5 ± 4.1 | 24.80 | 9.46 | 136.1 (0.6) | −15.1 ± 1.3 |
| SPpH-Dx-Au | 18.2 ± 0.9 | 19.4 ± 5.6 | 28.62 | 9.65 | 28.2 (0.7) | −21.5 ± 0.7 |
| SPpH-Dx-Au-Gd | 18.3 ± 1.3 | 20.0 ± 5.0 | 25.18 | 10.85 | 42.5 (0.5) | −16.7 ± 0.7 |
| TB | T = 300 K | T = 10 K | |||
|---|---|---|---|---|---|
| Samples | K | ||||
| SPR/P | 85 | 51.00 | 60.59 | 10.12 | 0.018 |
| SPR/P-Dx | 72 | 45.50 | 53.60 | 10.90 | 0.018 |
| SPR/P-Dx-Au | 87 | 42.54 | 48.32 | 8.83 | 0.017 |
| SPR/P-Dx-Au-Gd | 90 | 38.30 | 45.20 | 7.76 | 0.016 |
| SPpH | 91 | 64.20 | 74.90 | 16.00 | 0.013 |
| SPpH-Dx | 82 | 63.90 | 73.68 | 14.00 | 0.014 |
| SPpH-Dx-Au | 132 | 55.26 | 64.47 | 12.50 | 0.018 |
| SPpH-Dx-Au-Gd | 140 | 49.16 | 64.02 | 14.00 | 0.012 |
| H (kA/m) | 4 | 12 | 16 | 16 | 16 | 12 | |
| Sample | F (kHz) | 140 | 140 | 140 | 90 | 45 | 180 |
| SPR/P | 424 | 794 | 934 | 872 | 513 | 997 | |
| SPR/P-Dx | - | 0 | 0 | - | - | 247 | |
| SPR/P-Dx-Au-Gd | - | 375 | 4726 | - | - | 3588 | |
| SPpH | 0 | 607 | 730 | - | 2023 | 1860 | |
| SPpH-Dx-Au | - | 2970 | 57,082 | - | - | 32,143 | |
| SPpH-Dx-Au-Gd | - | 8970 | 97,673 | - | - | 12,991 |
| Sample | 7 T (300 MHz, RT)/mM−1s−1 | 1.41 T (60 MHz, 25 °C)/mM−1s−1 | ||||||
|---|---|---|---|---|---|---|---|---|
| r1 | r2 | r2/r1 | r2/r1 (Gd + Fe) | r1 | r2 | r2/r1 | r2/r1 (Gd + Fe) | |
| SPR/P | --- | 122 | --- | 340 | --- | 61.8 | --- | 35 |
| SPR/P-Dx-Au-Gd | 5 | 10 | 2 | 23 | 5.5 | 7.7 | 1.4 | 29 |
| SPpH | --- | 186 | --- | 240 | --- | 169.7 | --- | 22 |
| SPpH-Dx-Au-Gd | 55 | 56 | 1 | 282 | 23.2 | 11.7 | 0.5 | 123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Filho, V.C.; Morais, B.; Vieira, B.J.C.; Waerenborgh, J.C.; Carmezim, M.J.; Tóth, C.N.; Même, S.; Lacerda, S.; Jaque, D.; Sousa, C.T.; et al. Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile. Molecules 2024, 29, 1824. https://doi.org/10.3390/molecules29081824
Ferreira-Filho VC, Morais B, Vieira BJC, Waerenborgh JC, Carmezim MJ, Tóth CN, Même S, Lacerda S, Jaque D, Sousa CT, et al. Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile. Molecules. 2024; 29(8):1824. https://doi.org/10.3390/molecules29081824
Chicago/Turabian StyleFerreira-Filho, Vital Cruvinel, Beatriz Morais, Bruno J. C. Vieira, João Carlos Waerenborgh, Maria João Carmezim, Csilla Noémi Tóth, Sandra Même, Sara Lacerda, Daniel Jaque, Célia T. Sousa, and et al. 2024. "Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile" Molecules 29, no. 8: 1824. https://doi.org/10.3390/molecules29081824
APA StyleFerreira-Filho, V. C., Morais, B., Vieira, B. J. C., Waerenborgh, J. C., Carmezim, M. J., Tóth, C. N., Même, S., Lacerda, S., Jaque, D., Sousa, C. T., Campello, M. P. C., & Pereira, L. C. J. (2024). Influence of SPION Surface Coating on Magnetic Properties and Theranostic Profile. Molecules, 29(8), 1824. https://doi.org/10.3390/molecules29081824