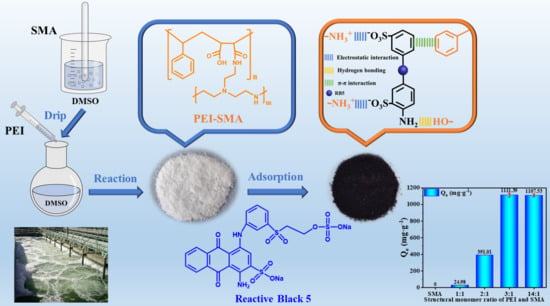
One-Step Synthesis of Polyethyleneimine-Grafted Styrene-Maleic Anhydride Copolymer Adsorbents for Effective Adsorption of Anionic Dyes
Abstract
:1. Introduction
2. Results and Discussion
2.1. PEI-SMA Characterization
2.2. Optimization of Synthesis Parameters
2.3. The Effect of Initial pH
2.4. The Effect of Adsorbent Dosage
2.5. The Effects of Contact Time and Adsorption Kinetics
2.6. Adsorption Isotherm Evaluation
2.7. Adsorption Thermodynamics
2.8. Selective Adsorption Behavior
2.9. Effects of Coexisting Ions
2.10. Regeneration of Adsorbent and Continuous Sorption Assessment
2.11. Adsorption Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of PEI-SMA
3.3. Characterization
3.4. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hynes, N.R.J.; Kumar, J.S.; Kamyab, H.; Sujana, J.A.J.; Al-Khashman, O.A.; Kuslu, Y.; Ene, A.; Kumar, B.S. Modern enabling techniques and adsorbents based dye removal with sustainability concerns in textile industrial sector–A comprehensive review. J. Clean. Prod. 2020, 272, 122636. [Google Scholar] [CrossRef]
- Teo, S.H.; Ng, C.H.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.G.; Janaun, J.; Taufiq-Yap, Y.H.; Khandaker, S.; Islam, G.J.; Znad, H.; et al. Sustainable toxic dyes removal with advanced materials for clean water production: A comprehensive review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.B.; Lu, J.; Zhou, Y.; Liu, Y.D. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2018, 16, 1193–1226. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.W.; Zhu, H.W.; Zhang, J.W.; Li, S.; Chen, Q.H.; Wang, C.X.; Wu, T.; Xu, M.X. Adsorptive removal of organic dyes via porous materials for wastewater treatment in recent decades: A review on species, mechanisms and perspectives. Chemosphere 2022, 293, 133464. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.H.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.X.; Fu, Y.Y.; Sun, J.Z. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Safe 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abba, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of the art. J. Environ. Manag. 2019, 236, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Vikrant, K.; Giri, B.S.; Raza, N.; Roy, K.; Kim, K.H.; Rai, B.N.; Singh, R.S. Recent advancements in bioremediation of dye: Current status and challenges. Bioresour. Technol. 2018, 253, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.M.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Nabizadeh, R.; Nasseri, S.; Mahvi, A.H.; Mesdaghinia, A.R. Comprehensive systematic review and meta-analysis of dyes adsorption by carbon-based adsorbent materials: Classification and analysis of last decade studies. Chemosphere 2020, 250, 126238. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Ali, I.; Karim, S.M.A.; Hossain Firoz, M.S.; Chowdhury, A.-N.; Morton, D.W.; Angove, M.J. Removal of dye from polluted water using novel nano manganese oxide-based materials. J. Water Process Eng. 2019, 32, 100911. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Bendre, A.; Mariappan, E.; Altalhi, T.; Kigga, M.; Ching, Y.C.; Jung, H.Y.; Bhaduri, B.; Kurkuri, M. Recent trends in the application of metal-organic frameworks (MOFs) for the removal of toxic dyes and their removal mechanism–A review. Sustain. Mater. Technol. 2022, 31, e00378. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Nasar, A.; Mashkoor, F. Application of polyaniline-based adsorbents for dye removal from water and wastewater–A review. Environ. Sci. Pollut. Res. 2019, 26, 5333–5356. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Aryee, A.A.; Dovi, E.; Li, Q.Y.; Han, R.P.; Li, Z.H.; Qu, L.B. Magnetic biocomposite based on peanut husk for adsorption of hexavalent chromium, Congo red and phosphate from solution: Characterization, kinetics, equilibrium, mechanism and antibacterial studies. Chemosphere 2022, 287, 132030. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.D.; Wang, X.M.; Yao, J.; Zhan, S.Y.; Li, H.; Zhang, J.; Qiu, Z.M. Synthesis of polyethyleneimine modified CoFe2O4-loaded porous biochar for selective adsorption properties towards dyes and exploration of interaction mechanisms. Sep. Purif. Technol. 2021, 277, 119474. [Google Scholar] [CrossRef]
- Hu, S.Z.; Huang, T.; Zhang, N.; Lei, Y.Z.; Wang, Y. Enhanced removal of lead ions and methyl orange from wastewater using polyethyleneimine grafted UiO-66-NH2 nanoparticles. Sep. Purif. Technol. 2022, 297, 121470. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, Y.; Kong, L.; Zhang, J.; Zuo, W.; Li, Y.; Cai, G. A novel 3D superelastic polyethyleneimine functionalized chitosan aerogels for selective removal of Cr(VI) from aqueous solution: Performance and mechanisms. Chem. Eng. J. 2021, 425, 131722. [Google Scholar] [CrossRef]
- Usman, M.A.; Khan, A.Y. Selective adsorption of anionic dye from wastewater using polyethyleneimine based macroporous sponge: Batch and continuous studies. J. Hazard Mater. 2022, 428, 128238. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Ghosh, S.; Paul, S.; Khan, M.E.H.; De, P.Y. Styrene-Maleimide/Maleic Anhydride Alternating Copolymers: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, 202100501. [Google Scholar] [CrossRef] [PubMed]
- Klumperman, B. Mechanistic considerations on styrene–maleic anhydride copolymerization reactions. Polym. Chem. 2010, 1, 558–562. [Google Scholar] [CrossRef]
- Park, J.J.; Kim, Y.; Lee, C.; Kook, J.-W.; Kim, D.; Kim, J.-H.; Hwang, K.-S.; Lee, J.-Y. Colorimetric Visualization Using Polymeric Core–Shell Nanoparticles: Enhanced Sensitivity for Formaldehyde Gas Sensors. Polymers 2020, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Xiao, J.; Yin, Q.; Zhang, Z.; Mao, S.; Li, Y. Amphiphilic graft copolymer based on poly(styrene-co-maleic anhydride) with low molecular weight polyethylenimine for efficient gene delivery. Int. J. Nanomed. 2012, 7, 4961–4972. [Google Scholar] [CrossRef]
- Han, S.Q.; Zhou, X.L.; Xie, H.H.; Wang, X.H.; Yang, L.Z.; Wang, H.L.; Hao, C. Chitosan-based composite microspheres for treatment of hexavalent chromium and EBBR from aqueous solution. Chemosphere 2022, 305, 135486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Ning, J.; Gao, M.; Jiang, W.; Zhou, Z.; Li, G. Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Anbazhagan, R.; Tsai, H.-C.; Wang, C.-F.; Lai, J.-Y. Preparation of caffeic acid-polyethyleneimine modified sponge for emulsion separation and dye adsorption. J. Taiwan Inst. Chem. Eng. 2021, 118, 325–333. [Google Scholar] [CrossRef]
- Fan, K.H.; Zhang, T.J.; Xiao, S.Y.; He, H.; Yang, J.S.; Qin, Z.Y. Preparation and adsorption performance of functionalization cellulose-based composite aerogel. Int. J. Biol. Macromol. 2022, 211, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.X.; Li, F.H.; Sun, B.; Wang, Y.B.; Liu, Q.Z.; Gao, T.T.; Zhou, G.W. Copolymerization of catechol and polyethyleneimine onto activated carbon for efficient removal of Congo red dye. J. Appl. Polym. Sci. 2022, 139, 52050. [Google Scholar] [CrossRef]
- Wong, S.; Ghafar, N.A.; Ngadi, N.; Razmi, F.A.; Inuwa, I.M.; Mat, R.; Amin, N.A.S. Effective removal of anionic textile dyes using adsorbent synthesized from coffee waste. Sci. Rep. 2020, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Tumari, H.H.; Ngadi, N.; Mohamed, N.B.; Hassan, O.; Mat, R.; Saidina Amin, N.A. Adsorption of anionic dyes on spent tea leaves modified with polyethyleneimine (PEI-STL). J. Clean. Prod. 2019, 206, 394–406. [Google Scholar] [CrossRef]
- Elsayed, I.; Madduri, S.; El-Giar, E.M.; Hassan, E. Effective removal of anionic dyes from aqueous solutions by novel polyethylenimine-ozone oxidized hydrochar (PEI-OzHC) adsorbent. Arab. J. Chem. 2022, 15, 103757. [Google Scholar] [CrossRef]
- Nordin, A.H.; Wong, S.; Ngadi, N.; Mohammad Zainol, M.; Abd Latif, N.A.F.; Nabgan, W. Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater. J. Environ. Chem. Eng. 2021, 9, 104639. [Google Scholar] [CrossRef]
- Kim, M.H.; Hwang, C.-H.; Kang, S.B.; Kim, S.; Park, S.W.; Yun, Y.-S.; Won, S.W. Removal of hydrolyzed Reactive Black 5 from aqueous solution using a polyethylenimine–polyvinyl chloride composite fiber. Chem. Eng. J. 2015, 280, 18–25. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, T.; Woo, S.H. Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for Reactive Black 5 from aqueous solutions. Chem. Eng. J. 2011, 166, 168–175. [Google Scholar] [CrossRef]
- Wang, Z.; Bin Kang, S.; Won, S.W. Polyethylenimine-aminated polyvinyl chloride fiber for adsorption of reactive dyes from single and binary component systems: Adsorption kinetics and isotherm studies. Colloid Surf. A 2022, 647, 128983. [Google Scholar] [CrossRef]
- Qiu, C.P.; Tang, Q.; Zhang, X.L.; Li, M.C.; Zhang, X.F.; Xie, J.L.; Zhang, S.B.; Su, Z.P.; Qi, J.Q.; Xiao, H.; et al. High-efficient double-cross-linked biohybrid aerogel biosorbent prepared from waste bamboo paper and chitosan for wastewater purification. J. Clean. Prod. 2022, 338, 130550. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef] [PubMed]
- Li, F.H.; Liu, M.; Yan, D.X.; Yin, Z.G.; Xiao, J.H.; Sun, B.; Shen, Y.H.; Gao, T.T.; Liu, Q.Z.; Zhou, G.W. A high-speed, salt-free, and dyebath-recyclable circular coloration technology inspired by mussel bionic. J. Appl. Polym. Sci. 2022, 139, e53178. [Google Scholar] [CrossRef]
- Cimino, R.T.; Rasmussen, C.J.; Brun, Y.; Neimark, A.V. Mechanisms of chain adsorption on porous substrates and critical conditions of polymer chromatography. J. Colloid Interface Sci. 2016, 481, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhu, G.; Yan, D.; Liu, Q.; Gao, T.; Zhou, G. Tannin cross-linked polyethyleneimine for highly efficient removal of hexavalent chromium. J. Taiwan Inst. Chem. Eng. 2021, 119, 52–59. [Google Scholar] [CrossRef]
- Wu, Q.H.; Ling, X.H.; Huang, W.G.; Zeng, X.H.; Fan, L.F.; Lin, J.Y.; Yu, W.H.; Yao, J.E.; Wen, W. Preparation of aminated porous polyacrylonitrile nanofibers as adsorbent for methyl orange removal. Rsc. Adv. 2022, 12, 15337–15347. [Google Scholar] [CrossRef] [PubMed]











| Kinetic Model | Parameter | RB5 |
|---|---|---|
| PFO | k1 (min−1) | 5.69 × 10−3 |
| qe (mg g−1) | 919.62 | |
| R2 | 0.974 | |
| PSO | k2 (min−1) | 2.06 × 10−5 |
| qe (mg g−1) | 1772.42 | |
| R2 | 0.997 | |
| IPD | ki1 (mg g−1 min−0.5) | 93.32 |
| R2 | 0.986 | |
| ki2 (mg g−1 min−0.5) | 31.40 | |
| R2 | 0.989 | |
| ki3 (mg g−1 min−0.5) | 0.94 | |
| R2 | 0.979 | |
| Experimental qe (mg g−1) | 1749.19 |
| Adsorbent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| KL | qm | RL | R2 | KF | 1/n | R2 | |
| PEI-SMA | 3.76 | 1809.30 | 0.00044 | 0.999 | 1666.79 | 0.020 | 0.754 |
| Adsorbent | qmax (mg g–1) | pH | Temperature | Equilibrium Time | Reuse Times | Reference |
|---|---|---|---|---|---|---|
| PEI-STL PEI-CW | 71.9 77.52 | 3 7 | 318 K 303 K | 200 m 180 m | / / | [41] [40] |
| PEI-OzHC MCPEI PEI–PVC fiber PEI-CSB PEI-CB PEI-PVCF fiber PEI-SMA | 182.7 330.0 314.4 413.23 709.27 1265.0 1809.3 | 2 7 2 6 6 7 2 | 318 K 300 K 298 K 303 K 303 K 298 K 308 K | 360 m 180 m 360 m 1440 m 1440 m 320 m 720 m | / 5 / / / 5 3 | [42] [43] [44] [45] [45] [46] This study |
| Adsorbent | ΔH (kJ/mol) | ΔS (J/mol K) | ΔG (kJ/mol) | Temperature (K) |
|---|---|---|---|---|
| −3.96 | 298 | |||
| PEI-SMA | 31.10 | 117.51 | −5.06 | 308 |
| −6.31 | 318 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Q.; Wang, Y.; Hu, F.; Sun, B.; Gao, T.; Zhou, G. One-Step Synthesis of Polyethyleneimine-Grafted Styrene-Maleic Anhydride Copolymer Adsorbents for Effective Adsorption of Anionic Dyes. Molecules 2024, 29, 1887. https://doi.org/10.3390/molecules29081887
Xu Y, Wang Q, Wang Y, Hu F, Sun B, Gao T, Zhou G. One-Step Synthesis of Polyethyleneimine-Grafted Styrene-Maleic Anhydride Copolymer Adsorbents for Effective Adsorption of Anionic Dyes. Molecules. 2024; 29(8):1887. https://doi.org/10.3390/molecules29081887
Chicago/Turabian StyleXu, Yao, Qinwen Wang, Yuanbo Wang, Falu Hu, Bin Sun, Tingting Gao, and Guowei Zhou. 2024. "One-Step Synthesis of Polyethyleneimine-Grafted Styrene-Maleic Anhydride Copolymer Adsorbents for Effective Adsorption of Anionic Dyes" Molecules 29, no. 8: 1887. https://doi.org/10.3390/molecules29081887
APA StyleXu, Y., Wang, Q., Wang, Y., Hu, F., Sun, B., Gao, T., & Zhou, G. (2024). One-Step Synthesis of Polyethyleneimine-Grafted Styrene-Maleic Anhydride Copolymer Adsorbents for Effective Adsorption of Anionic Dyes. Molecules, 29(8), 1887. https://doi.org/10.3390/molecules29081887