Predictive and Prognostic Biomarkers and Tumor Antigens for Targeted Therapy in Urothelial Carcinoma
Abstract
1. Introduction
2. Programmed Death Ligand-1
3. Tumor Mutational Burden—TMB
4. DNA Damage Repair Pathway Mutations
5. ERCC1 and ERCC2
6. Fibroblast Growth Factor Receptor—FGFR
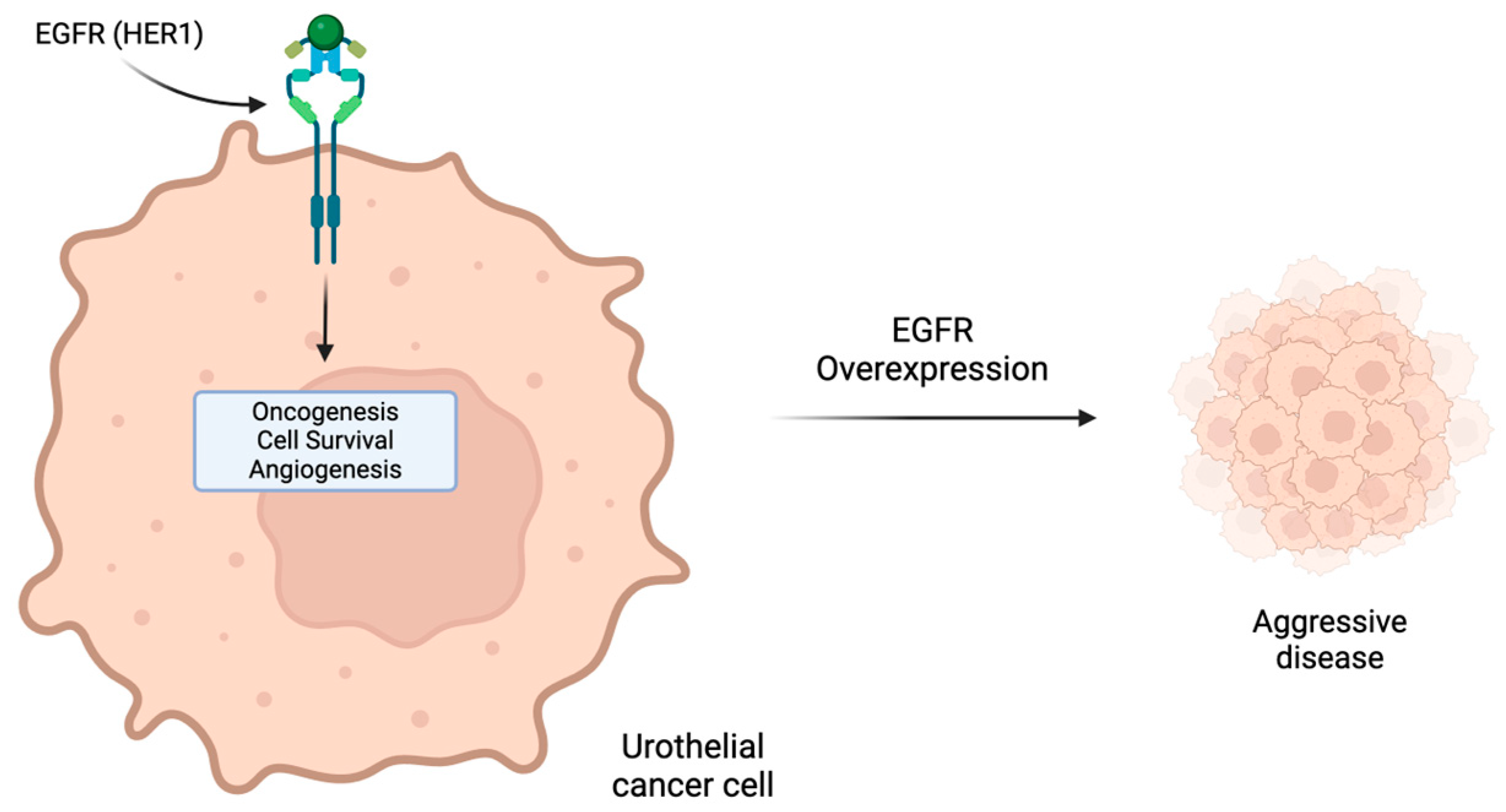
7. Human Epidermal Growth Factor Receptors Inhibitors: HER2 and EGFR (HER1)
8. Poly (ADP-Ribose) Polymerase (PARP)
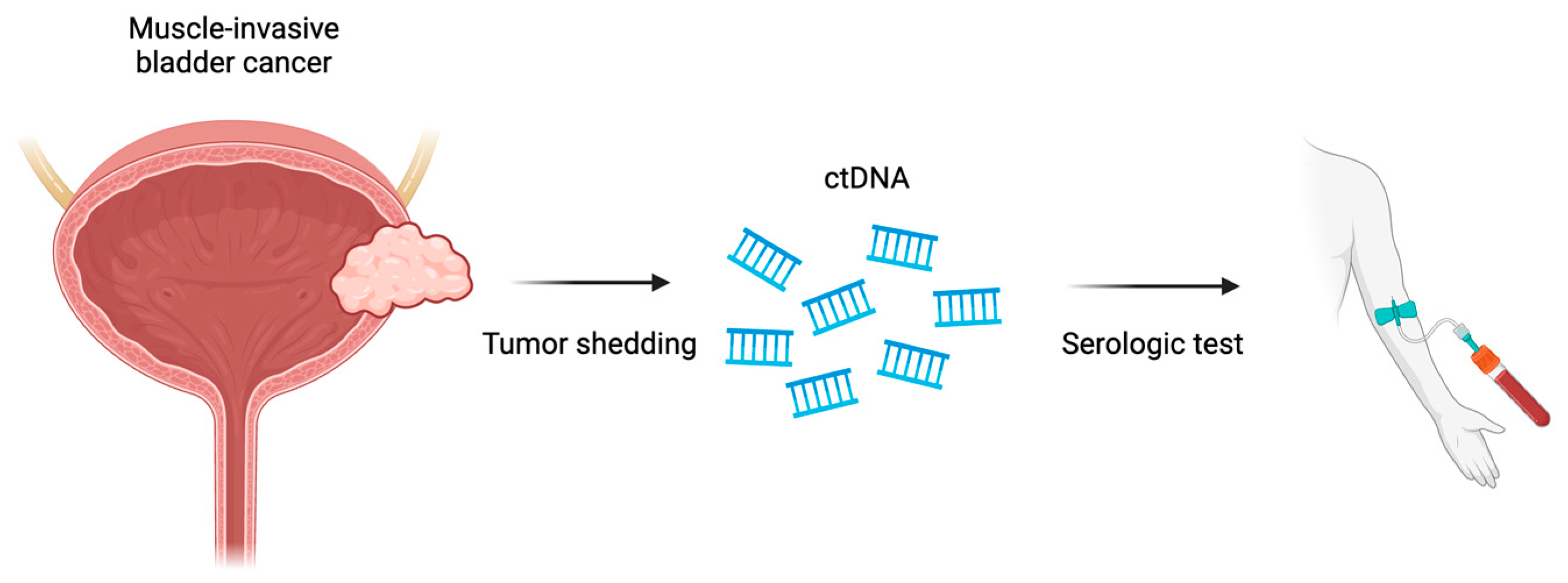
9. Circulating Tumor DNA (ctDNA)
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendiratta, P.; Grivas, P. Emerging biomarkers and targeted therapies in urothelial carcinoma. Ann. Transl. Med. 2018, 6, 250. [Google Scholar] [CrossRef] [PubMed]
- Abbosh, P.H.; Rosenberg, J.E.; Plimack, E.R. Circulating biomarkers to guide systemic therapy for urothelial carcinoma. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2016; Volume 34, pp. 502–509. [Google Scholar]
- Galsky, M.D.; Saci, A.; Szabo, P.M.; Han, G.C.; Grossfeld, G.; Collette, S.; Siefker-Radtke, A.; Necchi, A.; Sharma, P. Nivolumab in patients with advanced platinum-resistant urothelial carcinoma: Efficacy, safety, and biomarker analyses with extended follow-up from CheckMate 275. Clin. Cancer Res. 2020, 26, 5120–5128. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, M.; Cimadamore, A.; Hartmann, A.; Lopez-Beltran, A.; Cheng, L.; Scarpelli, M.; Montironi, R.; Gevaert, T. PD-L1 assessment in urothelial carcinoma: A practical approach. Ann. Transl. Med. 2019, 7, 690. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Ballman, K.V.; Sonpavde, G.P.; Berg, S.A.; Kim, W.Y.; Parikh, R.A.; Teo, M.Y.; Sweis, R.F.; Geynisman, D.M.; Grivas, P. AMBASSADOR Alliance A031501: Phase III Randomized Adjuvant Study of Pembrolizumab in Muscle-Invasive and Locally Advanced Urothelial Carcinoma (MIUC) vs Observation; American Society of Clinical Oncology: Alexandria, VA, USA, 2024. [Google Scholar]
- Apolo, A.B.; Ellerton, J.A.; Infante, J.R.; Agrawal, M.; Gordon, M.S.; Aljumaily, R.; Gourdin, T.; Dirix, L.; Lee, K.-W.; Taylor, M.H. Avelumab as second-line therapy for metastatic, platinum-treated urothelial carcinoma in the phase Ib JAVELIN Solid Tumor study: 2-year updated efficacy and safety analysis. J. Immunother. Cancer 2020, 8, e001246. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor mutational burden and response rate to PD-1 inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Jardim, D.L.; Goodman, A.; de Melo Gagliato, D.; Kurzrock, R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell 2021, 39, 154–173. [Google Scholar] [CrossRef]
- Scobie, M.R.; Zhou, K.I.; Ahmed, S.; Kelley, M.J. Utility of tumor mutational burden as a biomarker for response to immune checkpoint inhibition in the VA population. JCO Precis. Oncol. 2023, 7, e2300176. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Anichini, A.; Raggi, D.; Briganti, A.; Massa, S.; Lucianò, R.; Colecchia, M.; Giannatempo, P.; Mortarini, R.; Bianchi, M. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J. Clin. Oncol. 2018, 36, 3353–3360. [Google Scholar] [CrossRef] [PubMed]
- Vranic, S.; Gatalica, Z. PD-L1 testing by immunohistochemistry in immuno-oncology. Biomol. Biomed. 2023, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Grivas, P.; Wang, Q.E.; Mortazavi, A.; Emamekhoo, H.; Holder, S.L.; Drabick, J.J.; Woo, M.S.A.; Pal, S.; Vasekar, M. Prognostic value of DNA damage response genomic alterations in relapsed/advanced urothelial cancer. Oncologist 2020, 25, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Bambury, R.M.; Zabor, E.C.; Jordan, E.; Al-Ahmadie, H.; Boyd, M.E.; Bouvier, N.; Mullane, S.A.; Cha, E.K.; Roper, N. DNA damage response and repair gene alterations are associated with improved survival in patients with platinum-treated advanced urothelial carcinoma. Clin. Cancer Res. 2017, 23, 3610–3618. [Google Scholar] [CrossRef] [PubMed]
- Miron, B.; Hoffman-Censits, J.H.; Anari, F.; O’Neill, J.; Geynisman, D.M.; Zibelman, M.R.; Kutikov, A.; Viterbo, R.; Greenberg, R.E.; Chen, D. Defects in DNA repair genes confer improved long-term survival after cisplatin-based neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur. Urol. Oncol. 2020, 3, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Geynisman, D.M.; Abbosh, P.; Ross, E.A.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Ansel, K.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H. A Phase II Trial of Risk Enabled Therapy after Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN BLADDER): Interim ANALYSIS; American Society of Clinical Oncology: Alexandria, VA, USA, 2021. [Google Scholar]
- Geynisman, D.M.; Abbosh, P.; Ross, E.A.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Ansel, K.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H. A Phase II Trial of Risk-Enabled Therapy after Initiating Neoadjuvant Chemotherapy for Bladder Cancer (RETAIN); American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Galsky, M.D.; Daneshmand, S.; Chan, K.G.; Dorff, T.B.; Cetnar, J.P.; O Neil, B.; D’souza, A.; Mamtani, R.; Kyriakopoulos, C.; Garcia, P. Phase 2 Trial of Gemcitabine, Cisplatin, Plus Nivolumab with Selective Bladder Sparing in Patients with Muscle-Invasive Bladder Cancer (MIBC): HCRN GU 16-257; Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Galsky, M.D.; Daneshmand, S.; Lewis, S.C.; Chan, K.G.; Dorff, T.B.; Cetnar, J.P.; Mamtani, R.; Kyriakopoulos, C.; Gogerly-Moragoda, M.; Izadmehr, S. Co-Primary Endpoint Analysis of HCRN GU 16-257: Phase 2 Trial of Gemcitabine, Cisplatin, plus Nivolumab with Selective Bladder Sparing in Patients with Muscle-Invasive Bladder Cancer (MIBC); American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Choudhury, A.; Nelson, L.D.; Teo, M.T.; Chilka, S.; Bhattarai, S.; Johnston, C.F.; Elliott, F.; Lowery, J.; Taylor, C.F.; Churchman, M. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010, 70, 7017–7026. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, D.T.; Mouw, K.W.; Feng, F.Y.; Shipley, W.U.; Efstathiou, J.A. Molecular biomarkers in bladder preservation therapy for muscle-invasive bladder cancer. Lancet Oncol. 2018, 19, e683–e695. [Google Scholar] [CrossRef]
- Magliocco, A.M.; Moughan, J.; Miyamoto, D.T.; Simko, J.; Shipley, W.U.; Gray, P.J.; Hagan, M.P.; Parliament, M.; Tester, W.J.; Zietman, A.L. Analysis of MRE11 and Mortality Among Adults With Muscle-Invasive Bladder Cancer Managed With Trimodality Therapy. JAMA Netw. Open 2022, 5, e2242378. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H. Alterations in DNA damage response and repair genes as potential marker of clinical benefit from PD-1/PD-L1 blockade in advanced urothelial cancers. J. Clin. Oncol. 2018, 36, 1685. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Paz-Ares, L.; Cuello, M.; Cecere, F.; Albiol, S.; Guillem, V.; Gallardo, E.; Carles, J.; Mendez, P.; De la Cruz, J. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann. Oncol. 2007, 18, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Leow, J.J.; Martin-Doyle, W.; Rajagopal, P.S.; Patel, C.G.; Anderson, E.M.; Rothman, A.T.; Cote, R.J.; Urun, Y.; Chang, S.L.; Choueiri, T.K. Adjuvant chemotherapy for invasive bladder cancer: A 2013 updated systematic review and meta-analysis of randomized trials. Eur. Urol. 2014, 66, 42–54. [Google Scholar] [CrossRef]
- Sun, J.-M.; Sung, J.-Y.; Park, S.H.; Kwon, G.Y.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Jo, J.; Choi, H.Y. ERCC1 as a biomarker for bladder cancer patients likely to benefit from adjuvant chemotherapy. BMC Cancer 2012, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Obarzanowski, M.; Kopczynski, J.; Jaskulski, J.; Domagala, A.; Macek, P.; Gozdz, S.; Salagierski, M. Is ERCC1 a prognostic biomarker for urothelial cancer following radical cystectomy? A long-term analysis. Cent. Eur. J. Urol. 2021, 74, 348. [Google Scholar]
- Furuta, T.; Ueda, T.; Aune, G.; Sarasin, A.; Kraemer, K.H.; Pommier, Y. Transcription-coupled nucleotide excision repair as a determinant of cisplatin sensitivity of human cells. Cancer Res. 2002, 62, 4899–4902. [Google Scholar] [PubMed]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Gil-Jimenez, A.; van Dorp, J.; Contreras-Sanz, A.; van der Vos, K.; Vis, D.J.; Braaf, L.; Broeks, A.; Kerkhoven, R.; van Kessel, K.E.; Ribal, M.J. Assessment of predictive genomic biomarkers for response to cisplatin-based neoadjuvant chemotherapy in bladder cancer. Eur. Urol. 2023, 83, 313–317. [Google Scholar] [CrossRef]
- Katoh, M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat. Rev. Clin. Oncol. 2019, 16, 105–122. [Google Scholar] [CrossRef]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef]
- van Rhijn, B.W.; Lurkin, I.; Radvanyi, F.; Kirkels, W.J.; van der Kwast, T.H.; Zwarthoff, E.C. The fibroblast growth factor receptor 3 (FGFR3) mutation is a strong indicator of superficial bladder cancer with low recurrence rate. Cancer Res. 2001, 61, 1265–1268. [Google Scholar] [PubMed]
- Nimgaonkar, V.; Hubbard, R.A.; Carpenter, E.L.; Mamtani, R. Biomarker Testing, Treatment Uptake, and Survival Among Patients With Urothelial Cancer Receiving Gene-Targeted Therapy. JAMA Oncol. 2022, 8, 1070–1072. [Google Scholar] [CrossRef] [PubMed]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.H.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A. Genomic characterization of upper tract urothelial carcinoma. Eur. Urol. 2015, 68, 970–977. [Google Scholar] [CrossRef]
- Grivas, P.; Daneshmand, S.; Makarov, V.; Bellmunt, J.; Sridhar, S.S.; Sonpavde, G.P.; Cole, S.; Tripathi, A.; Faltas, B.M.; Lerner, S.P. Fibroblast Growth Factor Receptor 3 (FGFR3) Alterations in PROOF 302: A Phase III Trial of Infigratinib (BGJ398) as Adjuvant Therapy in Patients (pts) with Invasive Urothelial Carcinoma (UC); American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Scholtes, M.P.; Alberts, A.R.; Iflé, I.G.; Verhagen, P.C.; van der Veldt, A.A.; Zuiverloon, T.C. Biomarker-oriented therapy in bladder and renal cancer. Int. J. Mol. Sci. 2021, 22, 2832. [Google Scholar] [CrossRef]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef]
- Benjamin, D.J.; Mar, N.; Rezazadeh Kalebasty, A. Immunotherapy With Checkpoint Inhibitors in FGFR-Altered Urothelial Carcinoma. Clin. Med. Insights Oncol. 2022, 16, 11795549221126252. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Gizzi, M.; Bahleda, R.; Loriot, Y. Clinical development of FGFR3 inhibitors for the treatment of urothelial cancer. Bladder Cancer 2019, 5, 87–102. [Google Scholar] [CrossRef]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Laguerre, B.; Guadalupi, V.; Ku, J.H. Phase 3 THOR Study: Results of Erdafitinib (Erda) versus Chemotherapy (Chemo) in Patients (Pts) with Advanced or Metastatic Urothelial Cancer (mUC) with Select Fibroblast Growth Factor Receptor Alterations (FGFRalt); American Society of Clinical Oncology: Alexandria, VA, USA, 2023. [Google Scholar]
- Sternberg, C.N.; Petrylak, D.P.; Bellmunt, J.; Nishiyama, H.; Necchi, A.; Gurney, H.; Lee, J.-L.; Van Der Heijden, M.S.; Rosenbaum, E.; Penel, N. FORT-1: Phase II/III study of rogaratinib versus chemotherapy in patients with locally advanced or metastatic urothelial carcinoma selected based on FGFR1/3 mRNA expression. J. Clin. Oncol. 2023, 41, 629. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Lau, D.K.; Hodgson-Garms, M.; Lavis, A.; Jenkins, L.J.; Vukelic, N.; Ioannidis, P.; Luk, I.Y.; Mariadason, J.M. Dual targeting of FGFR3 and ERBB3 enhances the efficacy of FGFR inhibitors in FGFR3 fusion-driven bladder cancer. BMC Cancer 2022, 22, 478. [Google Scholar] [CrossRef]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, S21–S26. [Google Scholar] [CrossRef]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Pellat, A.; Vaquero, J.; Fouassier, L. Role of ErbB/HER family of receptor tyrosine kinases in cholangiocyte biology. Hepatology 2018, 67, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Wang, K.; Gay, L.M.; Al-Rohil, R.N.; Nazeer, T.; Sheehan, C.E.; Jennings, T.A.; Otto, G.A.; Donahue, A.; He, J. A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin. Cancer Res. 2014, 20, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Werner, L.; Bamias, A.; Fay, A.P.; Park, R.S.; Riester, M.; Selvarajah, S.; Barletta, J.A.; Berman, D.M.; de Muga, S. HER2 as a target in invasive urothelial carcinoma. Cancer Med. 2015, 4, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Caner, V.; Turk, N.S.; Duzcan, F.; Tufan, N.L.S.; Kelten, E.C.; Zencir, S.; Dodurga, Y.; Bagci, H.; Duzcan, S.E. No strong association between HER-2/neu protein overexpression and gene amplification in high-grade invasive urothelial carcinomas. Pathol. Oncol. Res. 2008, 14, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, P.; Sjödahl, G.; Chebil, G.; Liedberg, F.; Höglund, M. HER2 and EGFR amplification and expression in urothelial carcinoma occurs in distinct biological and molecular contexts. Oncotarget 2017, 8, 48905. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur. Urol. 2011, 60, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kolla, S.B.; Seth, A.; Singh, M.K.; Gupta, N.P.; Hemal, A.K.; Dogra, P.N.; Kumar, R. Prognostic significance of Her2/neu overexpression in patients with muscle invasive urinary bladder cancer treated with radical cystectomy. Int. Urol. Nephrol. 2008, 40, 321–327. [Google Scholar] [CrossRef]
- Skagias, L.; Politi, E.; Karameris, A.; Sambaziotis, D.; Archondakis, A.; Vasou, O.; Ntinis, A.; Michalopoulou, F.; Moreas, I.; Koutselini, H. Prognostic impact of HER2/neu protein in urothelial bladder cancer. Survival analysis of 80 cases and an overview of almost 20 years’ research. J. BUON 2009, 14, 457–462. [Google Scholar]
- Li, W.; Wang, Y.; Tan, S.; Rao, Q.; Zhu, T.; Huang, G.; Li, Z.; Liu, G. Overexpression of epidermal growth factor receptor (EGFR) and HER-2 in bladder carcinoma and its association with patients’ clinical features. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 7178. [Google Scholar] [CrossRef]
- Albarrán, V.; Rosero, D.I.; Chamorro, J.; Pozas, J.; San Román, M.; Barrill, A.M.; Alía, V.; Sotoca, P.; Guerrero, P.; Calvo, J.C. Her-2 targeted therapy in advanced urothelial cancer: From monoclonal antibodies to antibody-drug conjugates. Int. J. Mol. Sci. 2022, 23, 12659. [Google Scholar] [CrossRef] [PubMed]
- Oudard, S.; Culine, S.; Vano, Y.; Goldwasser, F.; Théodore, C.; Nguyen, T.; Voog, E.; Banu, E.; Vieillefond, A.; Priou, F. Multicentre randomised phase II trial of gemcitabine+ platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing Her2. Eur. J. Cancer 2015, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Daignault, S.; Agarwal, N.; Grivas, P.D.; Siefker-Radtke, A.O.; Puzanov, I.; MacVicar, G.R.; Levine, E.G.; Srinivas, S.; Twardowski, P. A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 2014, 120, 2684–2693. [Google Scholar] [CrossRef]
- Wülfing, C.; Machiels, J.P.H.; Richel, D.J.; Grimm, M.O.; Treiber, U.; De Groot, M.R.; Beuzeboc, P.; Parikh, R.; Pétavy, F.; El-Hariry, I.A. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 2009, 115, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Huddart, R.A.; Elliott, T.; Sarker, S.-J.; Ackerman, C.; Jones, R.; Hussain, S.; Crabb, S.; Jagdev, S.; Chester, J. Phase III, double-blind, randomized trial that compared maintenance lapatinib versus placebo after first-line chemotherapy in patients with human epidermal growth factor receptor 1/2–positive metastatic bladder cancer. J. Clin. Oncol. 2017, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Campanile, A.; Antic, T.; Yap, K.L.; Fitzpatrick, C.A.; Wade III, J.L.; Karrison, T.; Stadler, W.M.; Nakamura, Y.; O’Donnell, P.H. Afatinib activity in platinum-refractory metastatic urothelial carcinoma in patients with ERBB alterations. J. Clin. Oncol. 2016, 34, 2165. [Google Scholar] [CrossRef]
- Bedard, P.L.; Li, S.; Wisinski, K.B.; Yang, E.S.; Limaye, S.A.; Mitchell, E.P.; Zwiebel, J.A.; Moscow, J.A.; Gray, R.J.; Wang, V. Phase II Study of Afatinib in Patients With Tumors With Human Epidermal Growth Factor Receptor 2–Activating Mutations: Results From the National Cancer Institute–Molecular Analysis for Therapy Choice ECOG-ACRIN Trial (EAY131) Subprotocol EAY131-B. JCO Precis. Oncol. 2022, 6, e2200165. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.M.; Piha-Paul, S.A.; Won, H.; Rodon, J.; Saura, C.; Shapiro, G.I.; Juric, D.; Quinn, D.I.; Moreno, V.; Doger, B. HER kinase inhibition in patients with HER2-and HER3-mutant cancers. Nature 2018, 554, 189–194. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody–drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin. Cancer Res. 2021, 27, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, H.; Yan, X.; Chi, Z.; Cui, C.; Si, L.; Tang, B.; Mao, L.; Lian, B.; Wang, X. RC48-ADC Combined with Toripalimab, an Anti-PD-1 Monoclonal Antibody (Ab), in Patients with Locally Advanced or Metastatic Urothelial Carcinoma (UC): Preliminary Results of a Phase Ib/II Study; Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Drake, P.M.; Rabuka, D. An emerging playbook for antibody–drug conjugates: Lessons from the laboratory and clinic suggest a strategy for improving efficacy and safety. Curr. Opin. Chem. Biol. 2015, 28, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Xu, H.-G.; Chen, J.; Xu, Z.-X.; Levitt, J.M.; Stanley, J.A.; Yang, E.S.; Lerner, S.P.; Sonpavde, G. Activity of CEP-9722, a poly (ADP-ribose) polymerase inhibitor, in urothelial carcinoma correlates inversely with homologous recombination repair response to DNA damage. Anti-Cancer Drugs 2014, 25, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Kim, H.S.; Jung, E.-J.; Suh, J.H.; Min, H.; Chi, K.-C.; Kim, J.W.; Park, J.-M.; Hwang, I.G. Low PARP-1 expression level is an indicator of poor prognosis in patients with stage II and III gastric cancer. J. Cancer 2022, 13, 869. [Google Scholar] [CrossRef]
- Aiad, H.A.; Kandil, M.A.; El-Tahmody, M.A.; Abulkheir, I.L.; Abulkasem, F.M.; Elmansori, A.A.; Aleskandarany, M.A. The prognostic and predictive significance of PARP-1 in locally advanced breast cancer of Egyptian patients receiving neoadjuvant chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Klauschen, F.; von Winterfeld, M.; Stenzinger, A.; Sinn, B.V.; Budczies, J.; Kamphues, C.; Bahra, M.; Wittschieber, D.; Weichert, W.; Striefler, J. High nuclear poly-(ADP-ribose)-polymerase expression is prognostic of improved survival in pancreatic cancer. Histopathology 2012, 61, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Molnár, S.; Beke, L.; Méhes, G.; Póka, R. The prognostic value of PARP expression in high-grade epithelial ovarian cancer. Pathol. Oncol. Res. 2020, 26, 2549–2555. [Google Scholar] [CrossRef] [PubMed]
- Plimack, E.R.; Dunbrack, R.L.; Brennan, T.A.; Andrake, M.D.; Zhou, Y.; Serebriiskii, I.G.; Slifker, M.; Alpaugh, K.; Dulaimi, E.; Palma, N. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur. Urol. 2015, 68, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Mullane, S.A.; Werner, L.; Guancial, E.A.; Lis, R.T.; Stack, E.C.; Loda, M.; Kantoff, P.W.; Choueiri, T.K.; Rosenberg, J.; Bellmunt, J. Expression levels of DNA damage repair proteins are associated with overall survival in platinum-treated advanced urothelial carcinoma. Clin. Genitourin. Cancer 2016, 14, 352–359. [Google Scholar] [CrossRef]
- Crabb, S.J.; Hussain, S.; Soulis, E.; Hinsley, S.; Dempsey, L.; Trevethan, A.; Song, Y.; Barber, J.; Frew, J.; Gale, J. A randomized, double-blind, biomarker-selected, phase II clinical trial of maintenance poly ADP-ribose polymerase inhibition with rucaparib following chemotherapy for metastatic urothelial carcinoma. J. Clin. Oncol. 2023, 41, 54–64. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Park, S.H.; Kozlov, V.; Dao, T.V.; Castellano, D.; Li, J.-R.; Mukherjee, S.D.; Howells, K.; Dry, H.; Lanasa, M.C. Durvalumab plus olaparib in previously untreated, platinum-ineligible patients with metastatic urothelial carcinoma: A multicenter, randomized, phase II trial (BAYOU). J. Clin. Oncol. 2023, 41, 43. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; O’Donnell, P.H.; Hoffman-Censits, J.H.; Gupta, S.V.; Vaishampayan, U.; Heath, E.I.; Garcia, P.; Zhao, Q.; Yu, M.; Milowsky, M.I. Phase II trial of olaparib in patients with metastatic urothelial cancer harboring DNA damage response gene alterations. JCO Precis. Oncol. 2023, 7, e2300095. [Google Scholar] [CrossRef] [PubMed]
- Garje, R.; Vaddepally, R.K.; Zakharia, Y. PARP inhibitors in prostate and urothelial cancers. Front. Oncol. 2020, 10, 114. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Soave, A.; Chun, F.K.-H.; Hillebrand, T.; Rink, M.; Weisbach, L.; Steinbach, B.; Fisch, M.; Pantel, K.; Schwarzenbach, H. Copy number variations of circulating, cell-free DNA in urothelial carcinoma of the bladder patients treated with radical cystectomy: A prospective study. Oncotarget 2017, 8, 56398. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Lalani, A.-K.A.; Pond, G.R.; Nagy, R.J.; Faltas, B.; Agarwal, N.; Gupta, S.V.; Drakaki, A.; Vaishampayan, U.N.; Wang, J. Circulating tumor DNA alterations in advanced urothelial carcinoma and association with clinical outcomes: A pilot study. Eur. Urol. Oncol. 2020, 3, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA A Cancer J. Clin. 2021, 71, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; La Civita, E.; Liotti, A.; Cennamo, M.; Tortora, F.; Buonerba, C.; Crocetto, F.; Lucarelli, G.; Busetto, G.M.; Del Giudice, F. Liquid biopsy biomarkers in urine: A route towards molecular diagnosis and personalized medicine of bladder cancer. J. Pers. Med. 2021, 11, 237. [Google Scholar] [CrossRef]
- Li, S.; Xin, K.; Pan, S.; Wang, Y.; Zheng, J.; Li, Z.; Liu, X.; Liu, B.; Xu, Z.; Chen, X. Blood-based liquid biopsy: Insights into early detection, prediction, and treatment monitoring of bladder cancer. Cell. Mol. Biol. Lett. 2023, 28, 28. [Google Scholar] [CrossRef]
- Green, E.A.; Li, R.; Albiges, L.; Choueiri, T.K.; Freedman, M.; Pal, S.; Dyrskjøt, L.; Kamat, A.M. Clinical utility of cell-free and circulating tumor DNA in kidney and bladder cancer: A critical review of current literature. Eur. Urol. Oncol. 2021, 4, 893–903. [Google Scholar] [CrossRef]
- Agarwal, N.; Pal, S.K.; Hahn, A.W.; Nussenzveig, R.H.; Pond, G.R.; Gupta, S.V.; Wang, J.; Bilen, M.A.; Naik, G.; Ghatalia, P. Characterization of metastatic urothelial carcinoma via comprehensive genomic profiling of circulating tumor DNA. Cancer 2018, 124, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Vandekerkhove, G.; Todenhöfer, T.; Annala, M.; Struss, W.J.; Wong, A.; Beja, K.; Ritch, E.; Brahmbhatt, S.; Volik, S.V.; Hennenlotter, J. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin. Cancer Res. 2017, 23, 6487–6497. [Google Scholar] [CrossRef]
- Barata, P.; Koshkin, V.; Funchain, P.; Sohal, D.; Pritchard, A.; Klek, S.; Adamowicz, T.; Gopalakrishnan, D.; Garcia, J.; Rini, B. Next-generation sequencing (NGS) of cell-free circulating tumor DNA and tumor tissue in patients with advanced urothelial cancer: A pilot assessment of concordance. Ann. Oncol. 2017, 28, 2458–2463. [Google Scholar] [CrossRef] [PubMed]
- Chalfin, H.J.; Glavaris, S.A.; Gorin, M.A.; Kates, M.R.; Fong, M.H.; Dong, L.; Matoso, A.; Bivalacqua, T.J.; Johnson, M.H.; Pienta, K.J. Circulating tumor cell and circulating tumor DNA assays reveal complementary information for patients with metastatic urothelial cancer. Eur. Urol. Oncol. 2021, 4, 310–314. [Google Scholar] [CrossRef]
- Botezatu, I.; Serdyuk, O.g.; Potapova, G.; Shelepov, V.; Alechina, R.; Molyaka, Y.; Anan’ev, V.; Bazin, I.; Garin, A.; Narimanov, M. Genetic analysis of DNA excreted in urine: A new approach for detecting specific genomic DNA sequences from cells dying in an organism. Clin. Chem. 2000, 46, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.C.; Schroers-Martin, J.; Lazzareschi, D.V.; Shi, W.Y.; Chen, S.B.; Esfahani, M.S.; Trivedi, D.; Chabon, J.J.; Chaudhuri, A.A.; Stehr, H. Detection and surveillance of bladder cancer using urine tumor DNA. Cancer Discov. 2019, 9, 500–509. [Google Scholar] [CrossRef]
- Patel, K.; Van Der Vos, K.; Smith, C.G.; Mouliere, F.; Tsui, D.; Morris, J.; Chandrananda, D.; Marass, F.; Van Den Broek, D.; Neal, D. Association of plasma and urinary mutant DNA with clinical outcomes in muscle invasive bladder cancer. Sci. Rep. 2017, 7, 5554. [Google Scholar] [CrossRef]
- Christensen, E.; Birkenkamp-Demtröder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.-T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. Cancer Res. 2019, 79 (Suppl. 13), 913. [Google Scholar] [CrossRef]
- Christensen, E.; Nordentoft, I.; Birkenkamp-Demtröder, K.; Elbæk, S.K.; Lindskrog, S.V.; Taber, A.; Andreasen, T.G.; Strandgaard, T.; Knudsen, M.; Lamy, P. Cell-Free Urine and Plasma DNA Mutational Analysis Predicts Neoadjuvant Chemotherapy Response and Outcome in Patients with Muscle-Invasive Bladder Cancer. Clin. Cancer Res. 2023, 29, 1582–1591. [Google Scholar] [CrossRef]
- Birkenkamp-Demtröder, K.; Christensen, E.; Nordentoft, I.; Knudsen, M.; Taber, A.; Høyer, S.; Lamy, P.; Agerbæk, M.; Jensen, J.B.; Dyrskjøt, L. Monitoring treatment response and metastatic relapse in advanced bladder cancer by liquid biopsy analysis. Eur. Urol. 2018, 73, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtröder, K.; Nordentoft, I.; Christensen, E.; Høyer, S.; Reinert, T.; Vang, S.; Borre, M.; Agerbæk, M.; Jensen, J.B.; Ørntoft, T.F. Genomic alterations in liquid biopsies from patients with bladder cancer. Eur. Urol. 2016, 70, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sundahl, N.; Vandekerkhove, G.; Decaestecker, K.; Meireson, A.; De Visschere, P.; Fonteyne, V.; De Maeseneer, D.; Reynders, D.; Goetghebeur, E.; Van Dorpe, J. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur. Urol. 2019, 75, 707–711. [Google Scholar] [CrossRef]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef] [PubMed]


| Marker | Marker Value | Comment |
|---|---|---|
| PD-L1 | Prognostic Predictive | Inconsistent correlation with worse OS Weak predictive value for response to CPI |
| ERCC1 | Prognostic Predictive | Lower levels associated with longer OS Inconsistent correlation with response to cisplatin |
| ERCC2 | Prognostic Predictive | Inconsistent correlation with OS Lower levels associated with response to cisplatin |
| TMB | Prognostic Predictive | No demonstrated correlation with OS Correlation with response to CPI |
| DDR genes (ATM, RB1, FANCC, MRE11) | Prognostic Predictive | Trend toward longer OS Correlation with ORR to cisplatin +/− radiotherapy |
| FGFR3 | Predictive | Predictive of response to FGFR inhibitors |
| HER2/neu | Prognostic Predictive | Limited data—does not appear to have prognostic or predictive value |
| EGFR (HER1) | Prognostic Predictive | Limited data—does not appear to have prognostic or predictive value |
| PARP | Prognostic Predictive | Not yet shown to be predictive for OS or response |
| ctDNA | Prognostic Predictive | Elevated ctDNA levels predict clinical recurrence in patients treated by cystectomy Not shown to be predictive for response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eturi, A.; Bhasin, A.; Zarrabi, K.K.; Tester, W.J. Predictive and Prognostic Biomarkers and Tumor Antigens for Targeted Therapy in Urothelial Carcinoma. Molecules 2024, 29, 1896. https://doi.org/10.3390/molecules29081896
Eturi A, Bhasin A, Zarrabi KK, Tester WJ. Predictive and Prognostic Biomarkers and Tumor Antigens for Targeted Therapy in Urothelial Carcinoma. Molecules. 2024; 29(8):1896. https://doi.org/10.3390/molecules29081896
Chicago/Turabian StyleEturi, Aditya, Amman Bhasin, Kevin K. Zarrabi, and William J. Tester. 2024. "Predictive and Prognostic Biomarkers and Tumor Antigens for Targeted Therapy in Urothelial Carcinoma" Molecules 29, no. 8: 1896. https://doi.org/10.3390/molecules29081896
APA StyleEturi, A., Bhasin, A., Zarrabi, K. K., & Tester, W. J. (2024). Predictive and Prognostic Biomarkers and Tumor Antigens for Targeted Therapy in Urothelial Carcinoma. Molecules, 29(8), 1896. https://doi.org/10.3390/molecules29081896






