Fractionation of Carlina acaulis L. Root Methanolic Extract as a Promising Path towards New Formulations against Bacillus cereus and Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Fractions of Carlina acaulis Methanol Root Extract Contain Chlorogenic Acids
2.2. Fractions of Carlina acaulis Methanol Root Extract Exert Antimicrobial Activity
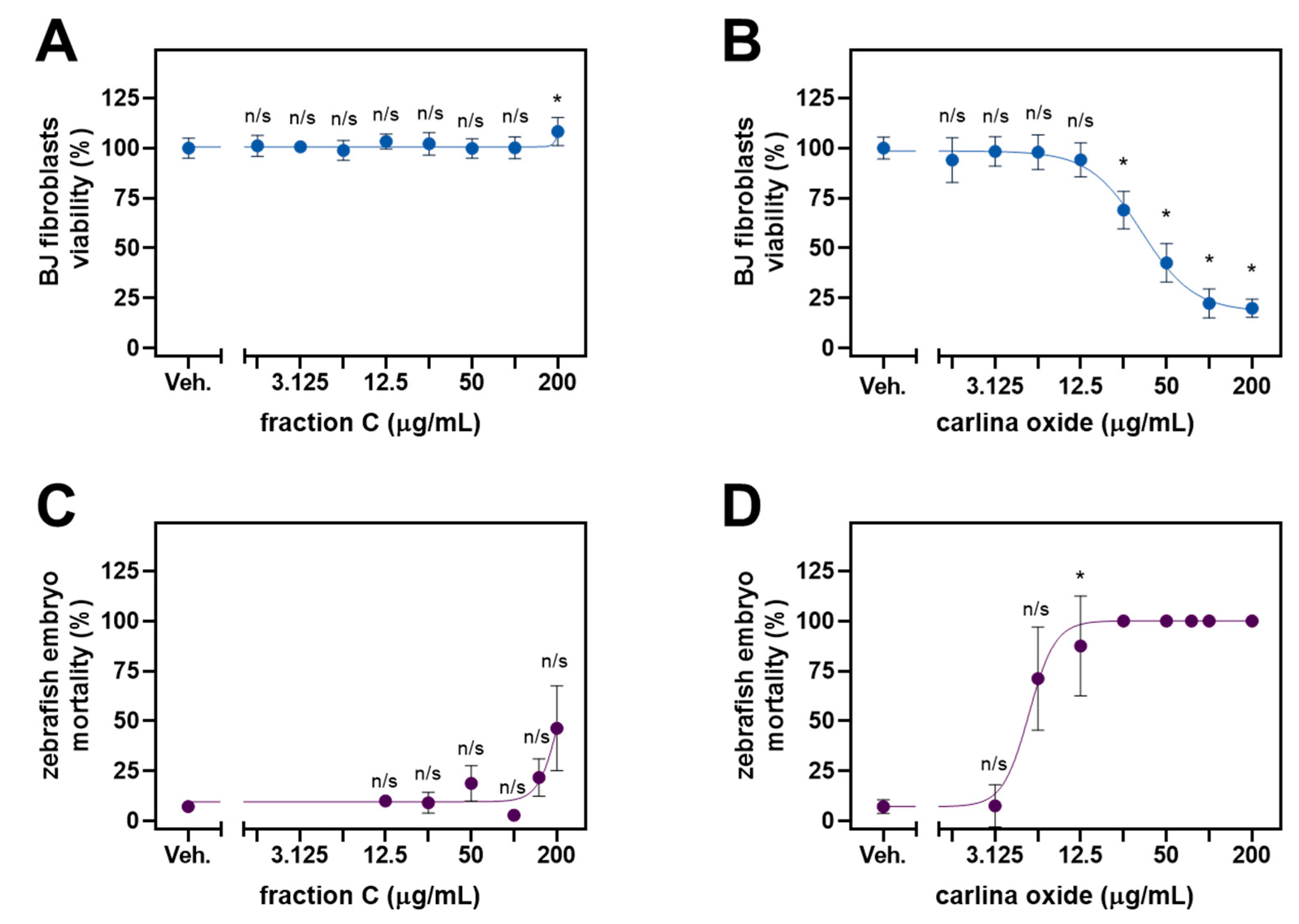
2.3. Cytotoxic Profile and Ecotoxicology
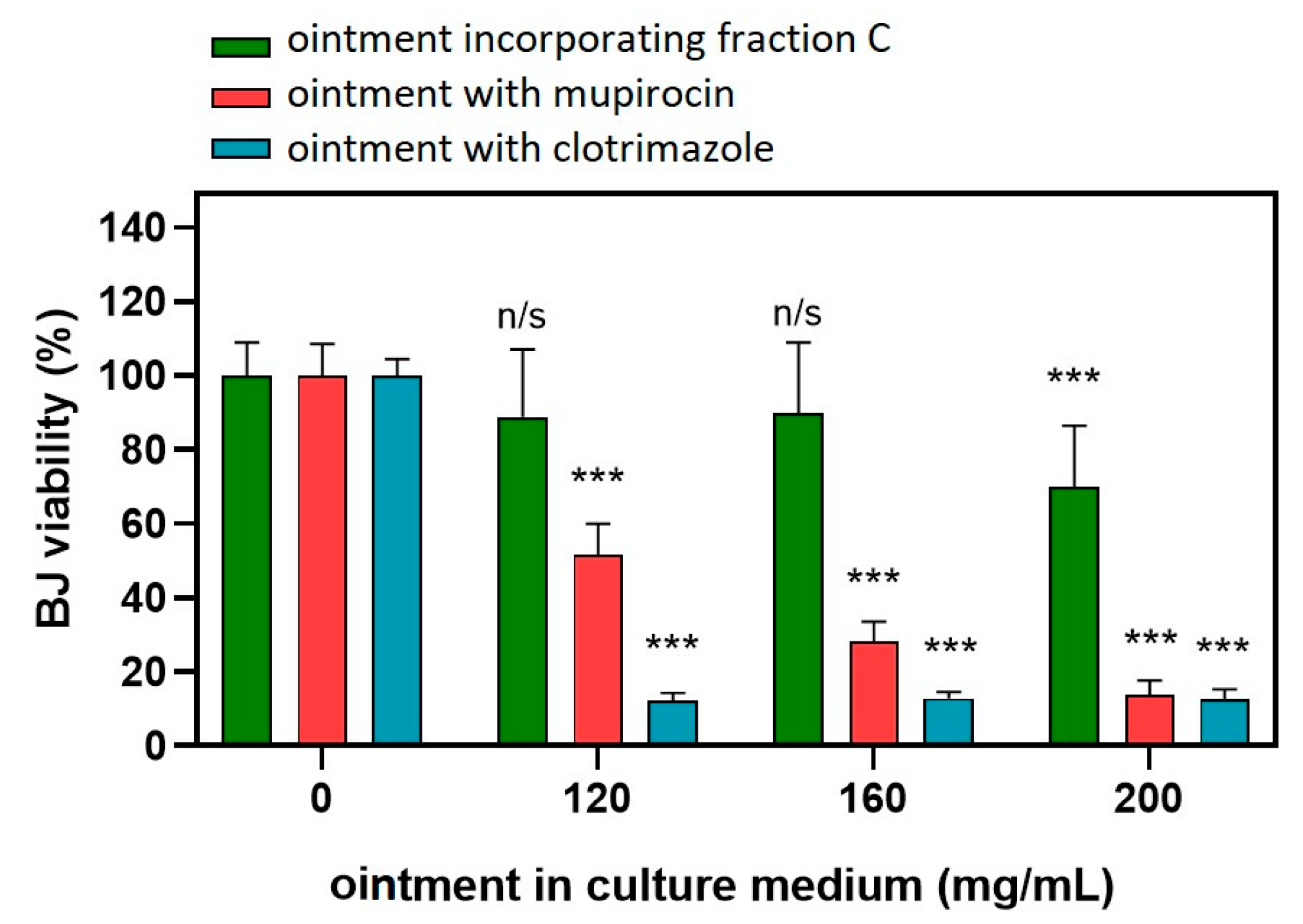
2.4. Application of C. acaulis Extract in Ointment Formulation
3. Discussion
4. Materials and Methods
4.1. Reference Standards and Chemicals
4.2. Plant Material, Preparation and Fractionation of the Extract
4.3. HPLC-PDA Analysis of Chlorogenic Acids
4.4. Antimicrobial Activity Assessment
4.5. Cell Culture
4.6. Cytotoxicity Assay
4.7. Ecotoxicity Assessment
4.8. Preparation of the Ointment
4.9. Data Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-Resistant Staphylococcus Aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Van Dyck, K.; Van Dijck, P. Candida Albicans and Staphylococcus Species: A Threatening Twosome. Front. Microbiol. 2019, 10, 486895. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vargas, F.M.; Abu-El-Haija, M.A.; Gómez-Duarte, O.G. Salmonella Infections: An Update on Epidemiology, Management, and Prevention. Travel. Med. Infect. Dis. 2011, 9, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Kotiranta, A.; Lounatmaa, K.; Haapasalo, M. Epidemiology and Pathogenesis of Bacillus Cereus Infections. Microbes Infect. 2000, 2, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Yagihara, Y.; Tatsuno, K.; Okazaki, M.; Okugawa, S.; Moriya, K. Clinical Characteristics and Antimicrobial Susceptibility of Bacillus Cereus Blood Stream Infections. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Liberti, A.; Loiacono, L. Ciprofloxacin versus Chloramphenicol in the Treatment of Salmonella Infection. Int. J. Antimicrob. Agents 2000, 16, 347–348. [Google Scholar] [CrossRef]
- Fiedler, G.; Schneider, C.; Igbinosa, E.O.; Kabisch, J.; Brinks, E.; Becker, B.; Stoll, D.A.; Cho, G.S.; Huch, M.; Franz, C.M.A.P. Antibiotics Resistance and Toxin Profiles of Bacillus Cereus-Group Isolates from Fresh Vegetables from German Retail Markets. BMC Microbiol. 2019, 19, 250. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 21 November 2023).
- Ventola, C.L. The Antibiotic Resistance Crisis: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A Systematic Review of Plants with Antibacterial Activities: A Taxonomic and Phylogenetic Perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Załuski, D.; Verpoorte, R. Historical and Traditional Medical Applications of Carlina acaulis L.—A Critical Ethnopharmacological Review. J. Ethnopharmacol. 2019, 239, 111842. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Tyszczuk-Rotko, K. Methodological Approach to Determine Carlina Oxide—A Main Volatile Constituent of Carlina acaulis L. Essential Oil. Talanta 2019, 191, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Dzida, K.; Dresler, S.; Sowa, I.; Kurzepa, J.; Szymczak, G.; Wójciak, M. Nitrogen Fertilisation Decreases the Yield of Bioactive Compounds in Carlina acaulis L. Grown in the Field. Ind. Crops Prod. 2021, 170, 113698. [Google Scholar] [CrossRef]
- Wnorowski, A.; Wnorowska, S.; Wojas-Krawczyk, K.; Grenda, A.; Staniak, M.; Michalak, A.; Woźniak, S.; Matosiuk, D.; Biała, G.; Wójciak, M.; et al. Toxicity of Carlina Oxide—A Natural Polyacetylene from the Carlina acaulis Roots—In Vitro and In Vivo Study. Toxins 2020, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Rosato, A.; Barbarossa, A.; Mustafa, A.M.; Bonacucina, G.; Perinelli, D.R.; Petrelli, R.; Maggi, F.; Spinozzi, E. Comprehensive Evaluation of the Antibacterial and Antifungal Activities of Carlina acaulis L. Essential Oil and Its Nanoemulsion. Antibiotics 2021, 10, 1451. [Google Scholar] [CrossRef] [PubMed]
- Spinozzi, E.; Ferrati, M.; Cappellacci, L.; Caselli, A.; Perinelli, D.R.; Bonacucina, G.; Maggi, F.; Strzemski, M.; Petrelli, R.; Pavela, R.; et al. Carlina acaulis L. (Asteraceae): Biology, Phytochemistry, and Application as a Promising Source of Effective Green Insecticides and Acaricides. Ind. Crops Prod. 2023, 192, 116076. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Nzekoue, F.K.; Cappellacci, L.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Sut, S.; Dall’Acqua, S.; et al. Carlina Oxide from Carlina acaulis Root Essential Oil Acts as a Potent Mosquito Larvicide. Ind. Crops Prod. 2019, 137, 356–366. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinozzi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a Highly Stable Carlina acaulis Essential Oil Nanoemulsion for Managing Lobesia Botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Benelli, G.; Rizzo, R.; Zeni, V.; Govigli, A.; Samková, A.; Sinacori, M.; Lo Verde, G.; Pavela, R.; Cappellacci, L.; Petrelli, R.; et al. Carlina acaulis and Trachyspermum Ammi Essential Oils Formulated in Protein Baits Are Highly Toxic and Reduce Aggressiveness in the Medfly, Ceratitis Capitata. Ind. Crops Prod. 2021, 161, 113191. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Petrelli, R.; Cappellacci, L.; Buccioni, M.; Palmieri, A.; Canale, A.; Benelli, G. Outstanding Insecticidal Activity and Sublethal Effects of Carlina acaulis Root Essential Oil on the Housefly, Musca Domestica, with Insights on Its Toxicity on Human Cells. Food Chem. Toxicol. 2020, 136, 111037. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Pavoni, L.; Bonacucina, G.; Cespi, M.; Cappellacci, L.; Petrelli, R.; Spinozzi, E.; Aguzzi, C.; Zeppa, L.; Ubaldi, M.; et al. Encapsulation of Carlina acaulis Essential Oil and Carlina Oxide to Develop Long-Lasting Mosquito Larvicides: Microemulsions versus Nanoemulsions. J. Pest Sci. 2021, 94, 899–915. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Hanaka, A.; Gołoś, I.; Skalska-Kamińska, A.; Cieślak, M.; Kováčik, J. Metabolic Changes Induced by Silver Ions in Carlina acaulis. Plants 2019, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Dresler, S.; Sowa, I.; Czubacka, A.; Agacka-Mołdoch, M.; Płachno, B.J.; Granica, S.; Feldo, M.; Wójciak-Kosior, M. The Impact of Different Cultivation Systems on the Content of Selected Secondary Metabolites and Antioxidant Activity of Carlina acaulis Plant Material. Molecules 2019, 25, 146. [Google Scholar] [CrossRef] [PubMed]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Blagojević, P.; Mihajilov-Krstev, T.; Rajković, J. Commercial Carlinae Radix Herbal Drug: Botanical Identity, Chemical Composition and Antimicrobial Properties. Pharm. Biol. 2012, 50, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Hamoud, R.; Sporer, F.; Tahrani, A.; Wink, M. Carlina Oxide—A Natural Polyacetylene from Carlina acaulis (Asteraceae) with Potent Antitrypanosomal and Antimicrobial Properties. Planta Med. 2011, 77, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, S.; Targowska-Duda, K.; Kurzepa, J.; Wnorowski, A.; Strzemski, M. Carlina Oxide Inhibits the Interaction of SARS-CoV-2 S Glycoprotein with Angiotensin-Converting Enzyme 2. Ind. Crops Prod. 2022, 187, 115338. [Google Scholar] [CrossRef] [PubMed]
- Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Agacka-Mołdoch, M.; Drączkowski, P.; Matosiuk, D.; Kurach, Ł.; Kocjan, R.; Dresler, S. Application of Raman Spectroscopy for Direct Analysis of Carlina Acanthifolia Subsp. Utzka Root Essential Oil. Talanta 2017, 174, 633–637. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Wu, H.; Wang, H.; Bian, H.; Zhu, Y.; Xu, W.; Liu, F.; Wang, D.; Fu, L. Antibacterial Activity and Action Mode of Chlorogenic Acid against Salmonella Enteritidis, a Foodborne Pathogen in Chilled Fresh Chicken. World J. Microbiol. Biotechnol. 2020, 36, 24. [Google Scholar] [CrossRef]
- Le, Y.J.; He, L.Y.; Li, S.; Xiong, C.J.; Lu, C.H.; Yang, X.Y. Chlorogenic Acid Exerts Antibacterial Effects by Affecting Lipid Metabolism and Scavenging ROS in Streptococcus Pyogenes. FEMS Microbiol. Lett. 2022, 369, fnac061. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic Acid Is a Fungicide Active against Phytopathogenic Fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Aneke, C.I.; Annoscia, G.; Otranto, D.; Boekhout, T.; Cafarchia, C. Effect of Chlorogenic and Gallic Acids Combined with Azoles on Antifungal Susceptibility and Virulence of Multidrug-Resistant Candida Spp. And Malassezia Furfur Isolates. Med. Mycol. 2020, 58, 1091–1101. [Google Scholar] [CrossRef]
- Strzemski, M.; Wojnicki, K.; Sowa, I.; Wojas-Krawczyk, K.; Krawczyk, P.; Kocjan, R.; Such, J.; Latalski, M.; Wnorowski, A.; Wójciak-Kosior, M. In Vitro Antiproliferative Activity of Extracts of Carlina acaulis Subsp. Caulescens and Carlina Acanthifolia Subsp. Utzka. Front. Pharmacol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Allambergenova, Z.; Kasela, M.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Świątek, Ł.; Boguszewska, A.; Rajtar, B.; Józefczyk, A.; Baj, T.; et al. Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus Alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan. Molecules 2022, 27, 3468. [Google Scholar] [CrossRef] [PubMed]
- Mamatova, A.S.; Korona-Glowniak, I.; Skalicka-Woźniak, K.; Józefczyk, A.; Wojtanowski, K.K.; Baj, T.; Sakipova, Z.B.; Malm, A. Phytochemical Composition of Wormwood (Artemisia Gmelinii) Extracts in Respect of Their Antimicrobial Activity. BMC Complement. Altern. Med. 2019, 19, 288. [Google Scholar] [CrossRef]
- Malm, A.; Grzegorczyk, A.; Biernasiuk, A.; Baj, T.; Rój, E.; Tyśkiewicz, K.; Dębczak, A.; Stolarski, M.J.; Krzyżaniak, M.; Olba-Zięty, E. Could Supercritical Extracts from the Aerial Parts of Helianthus Salicifolius a. Dietr. and Helianthus Tuberosus L. Be Regarded as Potential Raw Materials for Biocidal Purposes? Agriculture 2020, 11, 10. [Google Scholar] [CrossRef]
- Harahap, D.; Niaci, S.; Mardina, V.; Zaura, B.; Qanita, I.; Purnama, A.; Puspita, K.; Rizki, D.R.; Iqhrammullah, M. Antibacterial Activities of Seven Ethnomedicinal Plants from Family Annonaceae. J. Adv. Pharm. Technol. Res. 2022, 13, 148–153. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Deng, Z.-P.; Zhu, X.-J.; Zhang, Z.-G.; Zhang, X.; Tian, J.-L.; Li, W.; Zhao, P. Traditional Uses, Phytochemistry, Pharmacology and Clinical Applications of Cortex Juglandis Mandshuricae: A Comprehensive Review. J. Ethnopharmacol. 2022, 285, 114887. [Google Scholar] [CrossRef]
- Kha, T.C.; Le, L.T.P. Plant Extracts: Antimicrobial Properties, Mechanisms of Action and Applications. In Advanced Antimicrobial Materials and Applications. Environmental and Microbial Biotechnology; Springer: Singapore, 2021; pp. 257–283. [Google Scholar]
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.A.A.; Al-Omar, M.S.; Al Rugaie, O.; Abdellatif, A.A.H.; et al. Comparative Phytochemical Profile and Biological Activity of Four Major Medicinal Halophytes from Qassim Flora. Plants 2021, 10, 2208. [Google Scholar] [CrossRef] [PubMed]
- Fakruddin, M.; Bin Mannan, K.S.; Mazumdar, R.M.; Afroz, H. Antibacterial, Antifungal and Antioxidant Activities of the Ethanol Extract of the Stem Bark of Clausena Heptaphylla. BMC Complement. Altern. Med. 2012, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Ouknin, M.; Romane, A.; Costa, J.; Majidi, L. Comparative Study of the Chemical Profiling, Antioxidant and Antimicrobial Activities of Essential Oils of Different Parts of Thymus Willdenowii Boiss & Reut. Nat. Prod. Res. 2019, 33, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Talla, R.M.; Jouda, J.-B.; Mawabo, I.K.; Tegasne, C.; Happi, G.M.; Kapche, G.D.W.F.; Lenta, B.N.; Sewald, N.; Wandji, J. One New Constituent from the Stem Bark of Chrysophyllum Lacourtianum De Wild. (Sapotaceae). Nat. Prod. Res. 2023, 37, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadid, K.J.; Al-Karablieh, N.; Sharab, A.; Mutlak, I. Phytochemical Analyses and Antibacterial Activities of Erodium, Euphorbia, Logoecia and Tamarix Species. J. Infect. Dev. Ctries. 2019, 13, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, R.F.; Duarte Figueiredo, I.A.; Dantas Ferreira, S.R.; Lima de Farias Cavalcanti Silva, A.R.; Marinho Paiva, R.L.; Cordeiro, L.V.; de Oliveira Lima, E.; Cabrera, S.P.; Sarmento Silva, T.M.; de Andrade Cavalcante, F. Investigation of Ethnomedicinal Use of Commiphora leptophloeos (Mart.) J. B. Gillett (Burseraceae) in Treatment of Diarrhea. J. Ethnopharmacol. 2021, 268, 113564. [Google Scholar] [CrossRef]
- Irshad, M.; Shreaz, S.; Manzoor, N.; Khan, L.A.; Rizvi, M.M.A. Anticandidal Activity of Cassia Fistula and Its Effect on Ergosterol Biosynthesis. Pharm. Biol. 2011, 49, 727–733. [Google Scholar] [CrossRef]
- Han, J.; Lv, Q.-Y.; Jin, S.-Y.; Zhang, T.-T.; Jin, S.-X.; Li, X.-Y.; Yuan, H.-L. Comparison of Anti-Bacterial Activity of Three Types of Di-O-Caffeoylquinic Acids in Lonicera Japonica Flowers Based on Microcalorimetry. Chin. J. Nat. Med. 2014, 12, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial Effect and Mode of Action of Chlorogenic Acid on Staphylococcus Aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, S.; Zhang, M.; Wang, Z.; Wang, Z.; Ren, X. The Effect of Chlorogenic Acid on Bacillus Subtilis Based on Metabolomics. Molecules 2020, 25, 4038. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mannerström, M.; Toimela, T.; Sarkanen, J.-R.; Heinonen, T. Human BJ Fibroblasts Is an Alternative to Mouse BALB/c 3T3 Cells in In Vitro Neutral Red Uptake Assay. Basic. Clin. Pharmacol. Toxicol. 2017, 121 (Suppl. S3), 109–115. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; ISBN 9783319157917. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 Cell Line as a Model of the Intestinal Barrier: Influence of Cell and Culture-Related Factors on Caco-2 Cell Functional Characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 2827. [Google Scholar] [CrossRef]
- OECD Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013; pp. 1–22.
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using Zebrafish in Systems Toxicology for Developmental Toxicity Testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef]



| Species | Extract Type | Activity | Reference |
|---|---|---|---|
| Escherichia coli ATCC 8739 | Essential oil | MIC 0.39 µL/mL | [27] |
| E. coli ATCC 25922 | Essential oil | MIC 0.78 µL/mL | [27] |
| Staphylococcus aureus ATCC 6538 | Essential oil | MIC 0.02 µL/mL | [27] |
| Klebsiella pneumoniae ATCC 10031 | Essential oil | MIC 0.78 µL/mL | [27] |
| Proteus vulgaris ATCC 8427 | Essential oil | MIC 0.09 µL/mL | [27] |
| Pseudomonas aeruginosa ATCC 9027 | Essential oil | MIC 0.09 µL/mL | [27] |
| Candida albicans ATCC 10231 | Essential oil | MIC 0.19 µL/mL | [27] |
| S. aureus ATCC 6538 | Water decoction | MIC 3.1% | [27] |
| S. aureus ATCC 6538 | Apple cider vinegar decoction | MIC 0.7% | [27] |
| S. aureus ATCC 6538 | Wine decoction | MIC 1.5% | [27] |
| MRSA 1000/93 | Hexane extract | MIC 0.5 mg/mL | [28] |
| MRSA ATCC 10442 | Hexane extract | MIC 2.0 mg/mL | [28] |
| VRE ATCC 31299 | Hexane extract | MIC 0.002 mg/mL | [28] |
| C. albicans ATCC 90028 | Hexane extract | MIC 0.25 mg/mL | [28] |
| C. glabrata ATCC MYA 2950 | Hexane extract | MIC 0.5 mg/mL | [28] |
| C. albicans ATCC 10231 | Essential oil | 0.68 mg/mL | [28] |
| C. albicans ATCC 10231 | COx | 0.04 mg/mL | [28] |
| C. albicans ATCC 10231 | COx nanoemulsion | 1.9 mg/mL | [28] |
| SARS-CoV-2 | COx | IC50 = 234.2 µg/mL in binding to host cell receptor | [29] |
| Fraction | 5-CQA | 3-CQA | 4-CQA | 3,5-CQA | Total Content |
|---|---|---|---|---|---|
| A | n.d. | 2.30 ± 0.09 | 0.40 ± 0.02 | n.d. | 2.69 |
| B | n.d. | n.d. | n.d. | n.d. | 0.00 |
| C | 0.12 ± 0.04 | 13.15 ± 0.39 | 0.68 ± 0.07 | 37.89 ± 1.75 | 51.84 |
| D | 0.25 ± 0.03 | 6.94 ± 0.04 | 0.81 ± 0.01 | n.d. | 8.00 |
| Fraction B | Fraction C | Fraction D | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| mg/mL | mg/mL | mg/mL | |||||||
| Staphylococcus aureus ATCC 25923 (MSSA) | 0.6 | 2.5 | 4 bactericidal | 2.5 | 20 | 8 bacteriostatic | 20 | >20 | none |
| Staphylococcus aureus ATCC 6538 (MSSA) | 0.16 | 0.6 | 4 bactericidal | 2.5 | 20 | 8 bacteriostatic | 20 | >20 | none |
| Staphylococcus aureus ATCC 29213 (MSSA) | 0.6 | 5 | 8 bacteriostatic | 5 | 10 | 2 bactericidal | 20 | >20 | none |
| Staphylococcus aureus ATCC BAA 1707 (MRSA) | 0.3 | 2.5 | 8 bacteriostatic | 10 | 10 | 1 bactericidal | >20 | >20 | none |
| Staphylococcus aureus ATCC 43300 (MRSA) | 2.5 | 5 | 2 bactericidal | 5 | 5 | 1 bactericidal | 20 | >20 | none |
| Staphylococcus epidermidis ATCC 12228 (MSSE) | 10 | 10 | 1 bactericidal | 5 | 20 | 4 bactericidal | 20 | >20 | none |
| Bacillus cereus ATCC 10876 | 0.08 | 0.08 | 1 bactericidal | 0.16 | 0.16 | 1 bactericidal | 20 | 20 | 1 bactericidal |
| Salmonella Typhimurium ATCC 14028 | 10 | >20 | bactericidal | 10 | 20 | 2 bactericidal | 20 | >20 | none |
| Escherichia coli ATCC 25922 | 20 | 20 | 1 bactericidal | 10 | 20 | 2 bactericidal | 20 | >20 | none |
| Pseudomonas aeruginosa ATCC 27853 | 5 | 20 | 4 bactericidal | 10 | 20 | 2 bactericidal | 20 | >20 | none |
| Fraction A | Fraction B | Fraction C | Fraction D | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | |
| mg/mL | mg/mL | mg/mL | mg/mL | |||||||||
| Candida albicans ATCC 10231 | 10 | 20 | 2 fungicidal | 0.6 | 10 | 16 fungistatic | 2.5 | 10 | 4 fungicidal | >20 | >20 | - none |
| Candida glabrata ATCC 90030 | 20 | 20 | 1 fungicidal | 1.25 | 5 | 4 fungicidal | 5 | 10 | 2 fungicidal | >20 | >20 | - none |
| Candida krusei ATCC 14243 | 10 | 20 | 2 fungicidal | 1.25 | 10 | 8 fungistatic | 5 | 10 | 2 fungicidal | >20 | >20 | - none |
| Ointment Base (Hascobaza) | Ointment with Fraction C | Ointment with Mupirocin (2%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| mg/mL | mg/mL | mg/mL | |||||||
| Staphylococcus aureus ATCC 25923 (MSSA) | 80 | >320 | none | 40 | 40 | 1 | 0.78 | 12.5 | 16 |
| Staphylococcus aureus ATCC 6538 (MSSA) | 40 | 320 | 8 | 20 | 80 | 4 | 0.39 | 12.5 | 32 |
| Staphylococcus aureus ATCC 29213 (MSSA) | 80 | 320 | 4 | 20 | 80 | 4 | 0.39 | 25 | 64 |
| Staphylococcus aureus ATCC BAA 1707 (MRSA) | 80 | >320 | none | 40 | 80 | 2 | 0.78 | 12.5 | 16 |
| Staphylococcus aureus ATCC 43300 (MRSA) | 160 | >320 | none | 80 | 80 | 1 | 0.39 | 12.5 | 32 |
| Staphylococcus epidermidis ATCC 12228 (MSSE) | 20 | 160 | 8 | 10 | 40 | 4 | 0.39 | 12.5 | 32 |
| Ointment Base (Hascobaza) | Ointment with Fraction C | Ointment with Clotrimazole (1%) | |||||||
| MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | MIC | MFC | MFC/MIC | |
| mg/mL | mg/mL | mg/mL | |||||||
| Candida albicans ATCC 10231 | 40 | 80 | 2 | 20 | 40 | 2 | 0.03 | 0.5 | 16 |
| Candida glabrata ATCC 90030 | 80 | 160 | 2 | 40 | 40 | 1 | 0.03 | 0.5 | 16 |
| Candida krusei ATCC 14243 | 40 | 320 | 8 | 20 | 40 | 2 | 0.002 | 0.03 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wnorowska, S.; Grzegorczyk, A.; Kurzepa, J.; Maggi, F.; Strzemski, M. Fractionation of Carlina acaulis L. Root Methanolic Extract as a Promising Path towards New Formulations against Bacillus cereus and Methicillin-Resistant Staphylococcus aureus. Molecules 2024, 29, 1939. https://doi.org/10.3390/molecules29091939
Wnorowska S, Grzegorczyk A, Kurzepa J, Maggi F, Strzemski M. Fractionation of Carlina acaulis L. Root Methanolic Extract as a Promising Path towards New Formulations against Bacillus cereus and Methicillin-Resistant Staphylococcus aureus. Molecules. 2024; 29(9):1939. https://doi.org/10.3390/molecules29091939
Chicago/Turabian StyleWnorowska, Sylwia, Agnieszka Grzegorczyk, Jacek Kurzepa, Filippo Maggi, and Maciej Strzemski. 2024. "Fractionation of Carlina acaulis L. Root Methanolic Extract as a Promising Path towards New Formulations against Bacillus cereus and Methicillin-Resistant Staphylococcus aureus" Molecules 29, no. 9: 1939. https://doi.org/10.3390/molecules29091939
APA StyleWnorowska, S., Grzegorczyk, A., Kurzepa, J., Maggi, F., & Strzemski, M. (2024). Fractionation of Carlina acaulis L. Root Methanolic Extract as a Promising Path towards New Formulations against Bacillus cereus and Methicillin-Resistant Staphylococcus aureus. Molecules, 29(9), 1939. https://doi.org/10.3390/molecules29091939










