Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities
Abstract
:1. Introduction
2. Classes of Terpenoidal Alkaloids
2.1. Monoterpenoid Alkaloids
2.1.1. Iridoid-Type Alkaloids (1–24)
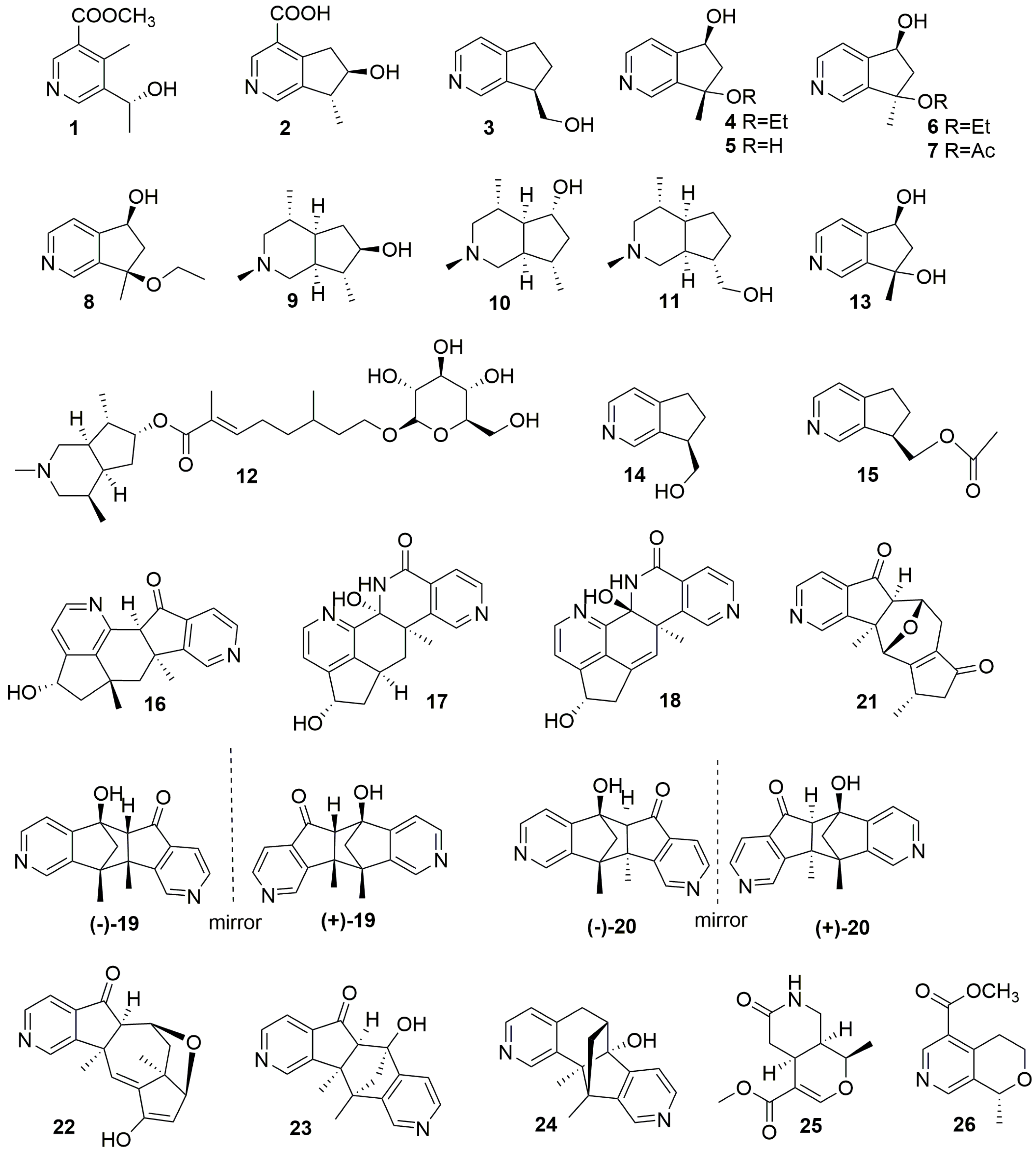
| No. | Compound Names | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 1 | Alstochonine A | Alstonia scholaris | branch | [16] |
| 2 | Alstochonine B | Alstonia scholaris | branch | [16] |
| 3 | (R)-10-hydroxyl-4-noractinidine | Rauvolfia vomitoria | trunk | [17] |
| 4 | Caryopterisine F | Caryopteris glutinosa | whole plant | [23] |
| 5 | Caryopterisine G | Caryopteris glutinosa | whole plant | [23] |
| 6 | Caryopterisine H | Caryopteris glutinosa | whole plant | [23] |
| 7 | Caryopterisine I | Caryopteris glutinosa | whole plant | [23] |
| 8 | (5S*,7R*)-7-Ethoxy-6,7-dihydro-7-methyl-5H-cyclopenta[c]pyridin-5-ol. | Caryopteris mongolica Bunge | aerial part | [24] |
| 9 | Delavatine C | Incarvillea delavayi | whole plant | [18] |
| 10 | Delavatine D | Incarvillea delavayi | whole plant | [18] |
| 11 | Delavatine E | Incarvillea delavayi | whole plant | [18] |
| 12 | Incarvine G | Incarvillea sinensis | whole herb | [19] |
| 13 | Isoxerine | Scrophularia ningpoensis | root | [20] |
| 14 | Forsyqinlingine C | Forsythia suspensa | fruit | [21] |
| 15 | Forsyqinlingine D | Forsythia suspensa | fruit | [21] |
| 16 | Caryopterisine C | Caryopteris glutinosa | whole plant | [23] |
| 17 | Caryopterisine D | Caryopteris glutinosa | whole plant | [23] |
| 18 | Caryopterisine E | Caryopteris glutinosa | whole plant | [23] |
| 19 | (±)-Caryopterisine A | Caryopteris glutinosa | whole plant | [22] |
| 20 | (±)-Caryopterisine B | Caryopteris glutinosa | whole plant | [22] |
| 21 | (5aR*,6S*,10S*,11R*,11aR*)-10,11a-Dimethyl-6,7,9,10,11,11a-hexahydro-5H-6,11-epoxycyclopenta [6,7]azuleno [1,2-c]pyridin-5,8(5aH)-dione. | Caryopteris mongolica Bunge | aerial part | [24] |
| 22 | (5aR*,6S*,7aR*,8S*,11aR*)-10-Hydroxy-7a,11a-dimethyl-5a,6,7,7a,8,11a-hexahydro-5H-6,8-epoxycyclopenta [6,7]azuleno [1,2-c] pyridin-5-one. | Caryopteris mongolica Bunge | aerial part | [24] |
| 23 | (5R*,5aR*,10bS*,11R*)-5-Hydroxy-10b,11-dimethyl-5,5a,10b,11-tetrahydro-6H-5,11-methanopyrido [3′,4′:3,4]cyclopenta [1,2-g]isoquinolin-6-one. | Caryopteris mongolica Bunge | aerial part | [24] |
| 24 | (6S*,6aR*,11R*,11aS*)-6a-Hydroxy-11,11a-dimethyl-6,6a,11,11a-tetrahydro-5H-6,11-methanopyrido [3′,4′:4,5]cyclopenta [1,2-h]isoquinolin-5-one | Caryopteris mongolica Bunge | aerial part | [24] |
| 25 | Longiflorine | Uncaria longiflora var. pteropoda | leaf | [25] |
| 26 | Lomatogonin C | Lomatogonium carinthiacum | whole plant | [26] |
2.1.2. Secoiridoid-Type Alkaloids (25–26)
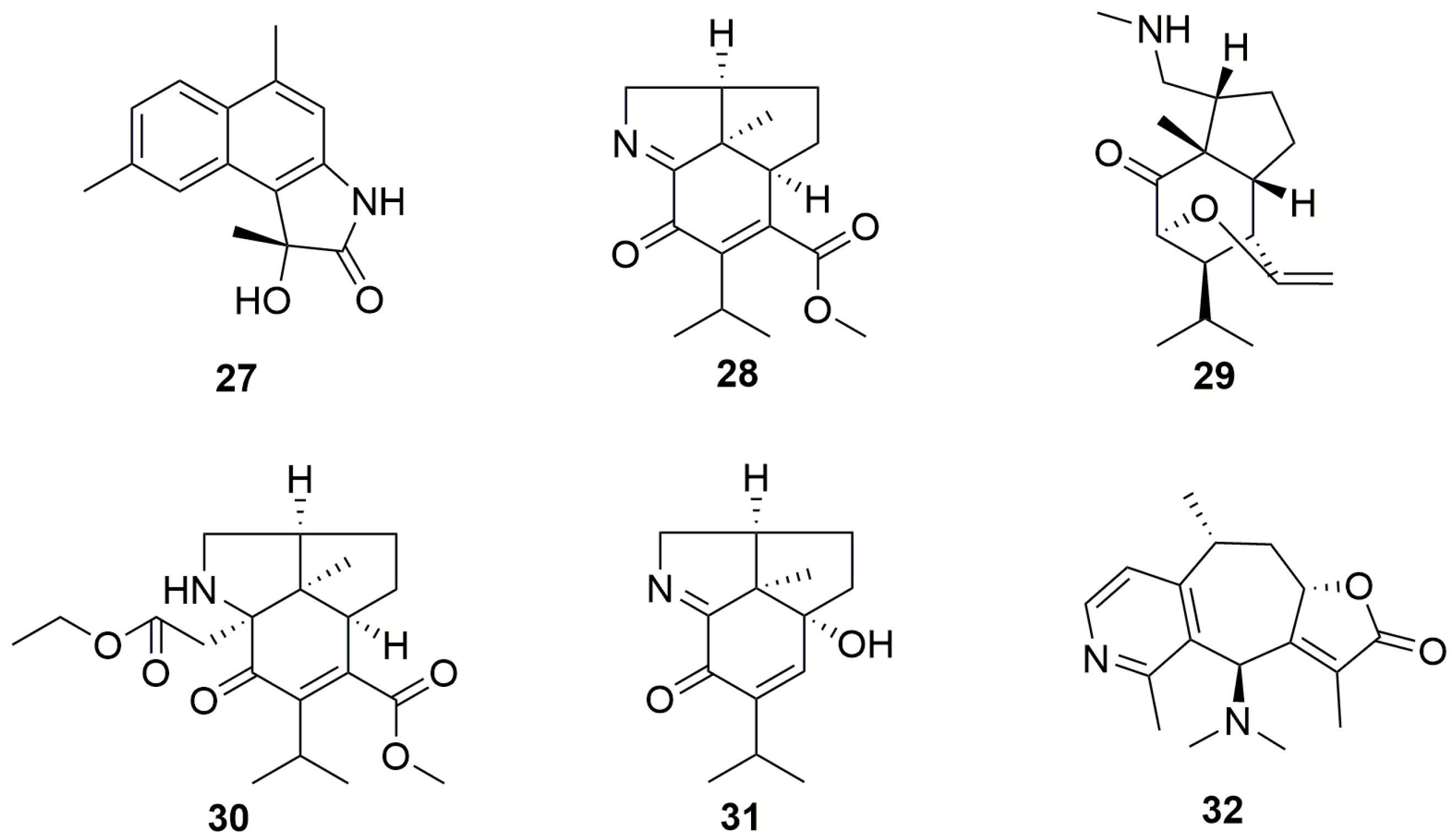
2.2. Sesquiterpene Alkaloids (27–32)
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 27 | Commipholactam A | Resina Commiphora | myrrh | [28] |
| 28 | Dendrofindline A | Dendrobium findlayanum | stem | [29] |
| 29 | Dendrofindline B | Dendrobium findlayanum | stem | [29] |
| 30 | Findlayine D | Dendrobium findlayanum | stem | [30] |
| 31 | Findlayine F | Dendrobium findlayanum | stem | [30] |
| 32 | Echinoflorine | gorgonian Echinogorgia flora | / | [31] |
2.3. Diterpenoid Alkaloids (DAs) (33–289)
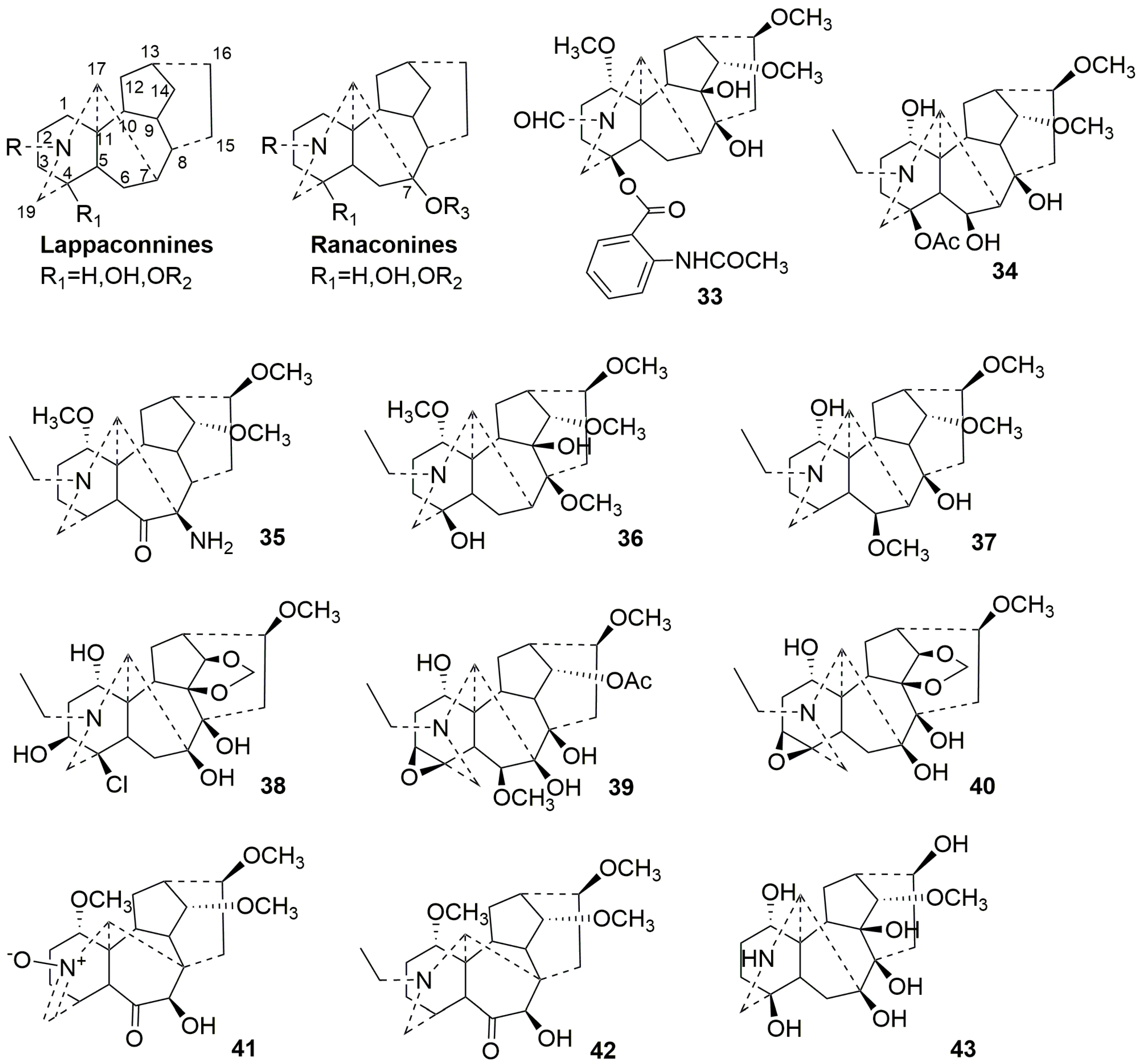
2.3.1. C18-DAs (33–43)
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 33 | Naviconine | Delphinium naviculare var. lasiocarpum | whole plant | [34] |
| 34 | Kirisine E | Aconitum kirinense Nakai | root | [35] |
| 35 | Leucostosine C | Aconitum leucostomum Worosch | root | [36] |
| 36 | Leucostosine D | Aconitum leucostomum Worosch | root | [36] |
| 37 | Kirisine D | Aconitum kirinense Nakai | root | [35] |
| 38 | Kirisine B | Aconitum kirinense Nakai | root | [35] |
| 39 | Kirisine C | Aconitum kirinense Nakai | root | [35] |
| 40 | Kirisine A | Aconitum kirinense Nakai | root | [35] |
| 41 | Barpubenine A | Aconitum barbatum var. puberulum Ledeb. | whole plant | [37] |
| 42 | Barpubenine B | Aconitum barbatum var. puberulum Ledeb. | whole plant | [37] |
| 43 | 1-N-deethyl-1,16-demethoxy-1,16-dihydroxyranaconidine | Aconitum iochanicumone | root | [38] |
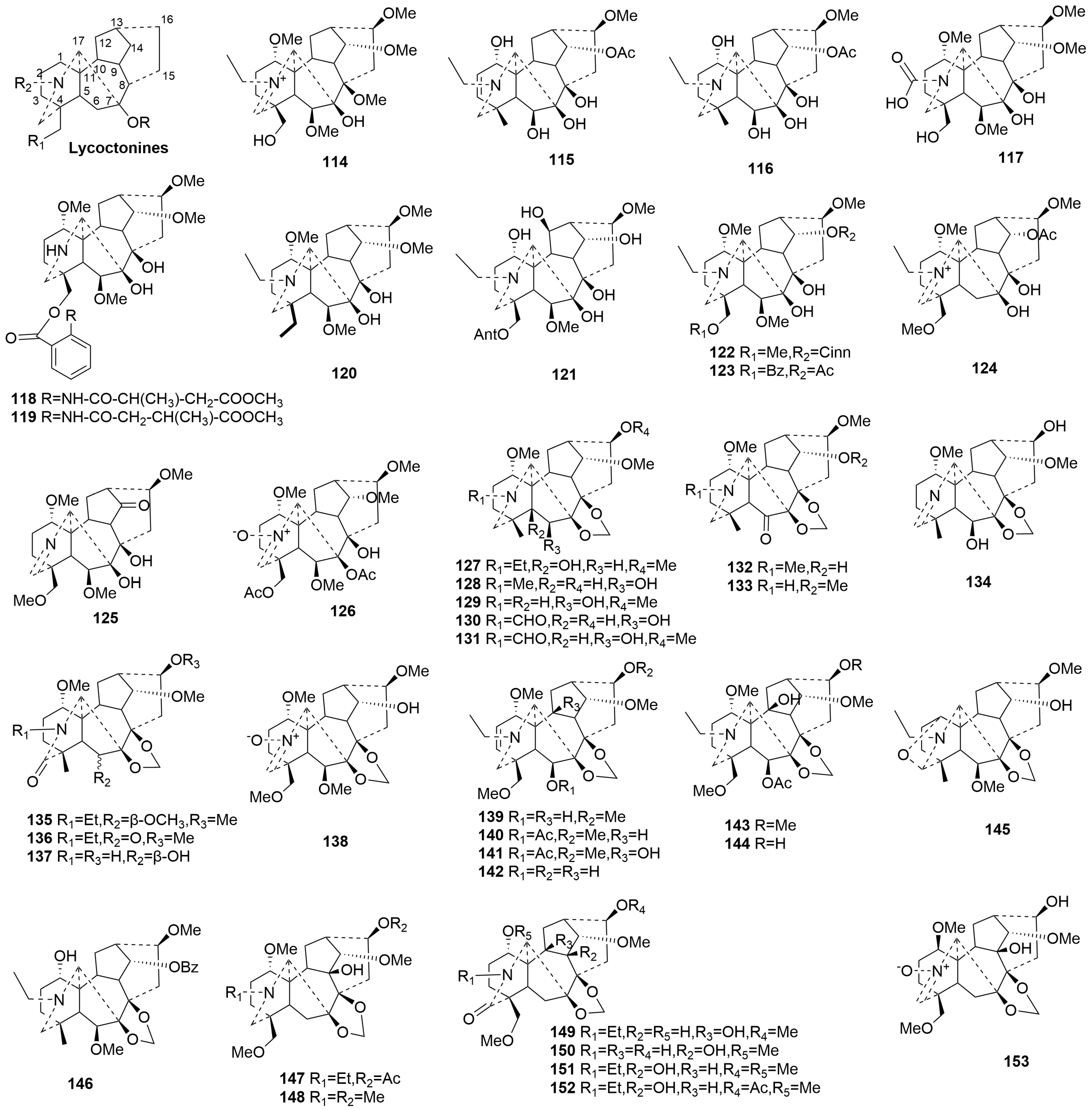
2.3.2. C19-DAs (44–182)
- Aconitine-type C19-DAs (44–113)
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 44 | Naviconitine | Delphinium naviculare var. lasiocarpum | whole plant | [34] |
| 45 | Acoapetaludine D | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 46 | Acoapetaludine E | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 47 | Acoapetaludine F | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 48 | Acoapetaludine G | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 49 | Forrestline D | Delphinium forrestii var. viride | whole herb | [53] |
| 50 | Episcopaline C | Aconitum episcopale | root | [50] |
| 51 | Acoapetaludine H | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 52 | Acoapetaludine I | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 53 | Acoapetaludine J | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 54 | Novolunine C | Aconitum novoluridum | root | [49] |
| 55 | Austroyunnanine C | Aconitum austroyunnanense | root | [45] |
| 56 | 1-N-deethyl-1,16-demethoxy-1,16 dihydroxy-N(19)-en-austroconitine A | Aconitum iochanicumone | root | [38] |
| 57 | 1-N-deethyl-1,16-demethoxy-1,16-dihydroxy-18-methoxy-N(19)-en-austroconitine A | Aconitum iochanicumone | root | [38] |
| 58 | Brevicanine A | Aconitum brevicalcaratum | root | [54] |
| 59 | Novolunine A | Aconitum novoluridum | root | [49] |
| 60 | Novolunine B | Aconitum novoluridum | root | [49] |
| 61 | Brevicanine B | Aconitum brevicalcaratum | root | [54] |
| 62 | Brevicanine C | Aconitum brevicalcaratum | root | [54] |
| 63 | Brevicanine D | Aconitum brevicalcaratum | root | [54] |
| 64 | Forrestline B | Delphinium forrestii var. viride | whole herb | [53] |
| 65 | Refractine A | Aconitum refractum var. circinatum | whole plant | [41] |
| 66 | Richardsonine B | Aconitum richardsonianum Lauener | root | [44] |
| 67 | Richardsonine C | Aconitum richardsonianum Lauener | root | [44] |
| 68 | Richardsonine A | Aconitum richardsonianum Lauener | root | [44] |
| 69 | Acoapetaludine K | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 70 | Acoapetaludine B | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 71 | Acoapetaludine C | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| 72 | Forrestline E | Delphinium forrestii var. viride | whole herb | [53] |
| 73 | Brevicalcarine B | Aconitum brevicalcaratum | root | [60] |
| 74 | Brevicalcarine C | Aconitum brevicalcaratum | root | [60] |
| 75 | Rockidine A | Aconitum genera | root | [43] |
| 76 | Pseudostapine A | Aconitum pseudostapfianum | root | [51] |
| 77 | Refractine B | Aconitum refractum var. circinatum | whole plant | [41] |
| 78 | Austroyunnanine A | Aconitum austroyunnanense | root | [45] |
| 79 | Apetalrine A | Aconitum apetalum | aerial part | [55] |
| 80 | Apetalrine B | Aconitum apetalum | aerial part | [55] |
| 81 | Apetalrine C | Aconitum apetalum | aerial part | [55] |
| 82 | Apetalrine D | Aconitum apetalum | aerial part | [55] |
| 83 | Apetalrine E | Aconitum apetalum | aerial part | [55] |
| 84 | Brevicalcarine A | Aconitum brevicalcaratum | root | [60] |
| 85 | Nagarumine A | Aconitum nagarum | root | [46] |
| 86 | Nagarutine A | Aconitum nagarum Stapf | root | [42] |
| 87 | Episcopaline A | Aconitum episcopale | root | [50] |
| 88 | Pseudostapine B | Aconitum pseudostapfianum | root | [51] |
| 89 | Pseudostapine C | Aconitum pseudostapfianum | root | [51] |
| 90 | Nagarumine B | Aconitum nagarum | root | [46] |
| 91 | Austroyunnanine B | Aconitum austroyunnanense | root | [45] |
| 92 | Smirnotine A | Aconitum smirnovii Steinb | aerial part | [58] |
| 93 | Pendulumine A | Aconitum pendulum | rhizome | [48] |
| 94 | Lipojesaconitine | Aconitum japonicum subsp. subcuneatum | rhizoma | [57] |
| 95 | 6-demethoxyhypaconine | Aconitum carmichaelii Debx. | lateral root | [47] |
| 96 | Carmichaeline K | Aconitum carmichaelii Debx. | lateral root | [47] |
| 97 | 10-hydroxychasmanine | Aconitum japonicum subsp. subcuneatum | rhizoma | [57] |
| 98 | Rockidine B | Aconitum genera | root | [43] |
| 99 | Geordine | Aconitum georgei Comber | root | [61] |
| 100 | 3-hydroxykaracoline | Aconitum japonicum subsp. subcuneatum | rhizoma | [57] |
| 101 | Episcopine B | Aconitum episcopale | root | [52] |
| 102 | Acotarine F | Aconitum taronense | root | [56] |
| 103 | Acotarine G | Aconitum taronense | root | [56] |
| 104 | Smirnotine B | Aconitum smirnovii Steinb | aerial part | [58] |
| 105 | Pendulumine E | Aconitum pendulum | rhizome | [48] |
| 106 | Nagarutine C | Aconitum nagarum Stapf | root | [42] |
| 107 | 8-O-ethyl-benzoyldeoxyaconine | Aconitum carmichaelii Debx. | lateral root | [47] |
| 108 | Pendulumine C | Aconitum pendulum | rhizome | [48] |
| 109 | Pendulumine D | Aconitum pendulum | rhizome | [48] |
| 110 | Nagarutine B | Aconitum nagarum Stapf | root | [42] |
| 111 | Nagarutine D | Aconitum nagarum Stapf | root | [42] |
| 112 | Pendulumine F | Aconitum pendulum | rhizome | [48] |
| 113 | Delcarpum | Delphinium peregrinum L. var. eriocarpum Boiss | aerial part | [59] |
- Lycoctonine-type C19-DAs (114–153)
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 114 | Naviculine | Delphinium naviculare var. lasiocarpum | whole plant | [34] |
| 115 | Sczukiniline D | Aconitum sczukinii Turcz | root | [65] |
| 116 | Sczukiniline E | Aconitum sczukinii Turcz | root | [65] |
| 117 | Grandifline C | Delphinium grandiflorum | aerial parts | [73] |
| 118 | Shawurenine C | Delphinium shawurense W. T. Wang | aerial parts | [62] |
| 119 | Shawurenine D | Delphinium shawurense W. T. Wang | aerial parts | [62] |
| 120 | Uncinatine-A | Delphinium uncinatum | whole plant | [66] |
| 121 | Ajacisine G | Delphinium ajacis | seed | [70] |
| 122 | Grandiflonine F | Delphinium grandiflorum L. | whole plant | [68] |
| 123 | Ajacisine F | Delphinium ajacis | seed | [70] |
| 124 | Grandiflonine E | Delphinium grandiflorum L. | whole plant | [68] |
| 125 | Grandiflonine G | Delphinium grandiflorum L. | whole plant | [68] |
| 126 | Chrysotrichumine A | Delphinium chrysotrichum | aerial parts | [69] |
| 127 | Elapaciline | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 128 | Meladine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 129 | N-deethyldelpheline | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 130 | N-deethyl-N-formyleladine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 131 | N-deethyl-N-formyldelpheline | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 132 | Melapacitine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 133 | N-deethylpacinine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 134 | Iminoeladine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 135 | 19-oxopaciline | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 136 | 19-oxopacinine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 137 | N-deethyl-19-oxoeladine | Delphinium elatum cv. Pacific Giant | seed | [67] |
| 138 | Brunodelphinine C | Delphinium brunonianum Royle | aerial parts | [64] |
| 139 | Grandifloline A | Delphinium grandiflorum L. | whole herb | [63] |
| 140 | Grandifloline B | Delphinium grandiflorum L. | whole herb | [63] |
| 141 | Grandifloline C | Delphinium grandiflorum L. | whole herb | [63] |
| 142 | Grandifloline E | Delphinium grandiflorum L. | whole herb | [63] |
| 143 | Grandifloline D | Delphinium grandiflorum L. | whole herb | [63] |
| 144 | Grandifloline F | Delphinium grandiflorum L. | whole herb | [63] |
| 145 | Liangshanine A | Delphinium liangshanense W. T. Wang | whole plant | [71] |
| 146 | Liangshanine B | Delphinium liangshanense W. T. Wang | whole plant | [71] |
| 147 | Kamaonensine A | Delphinium kamaonense Huth | whole plant | [72] |
| 148 | Kamaonensine B | Delphinium kamaonense Huth | whole plant | [72] |
| 149 | Kamaonensine C | Delphinium kamaonense Huth | whole plant | [72] |
| 150 | Kamaonensine D | Delphinium kamaonense Huth | whole plant | [72] |
| 151 | Kamaonensine E | Delphinium kamaonense Huth | whole plant | [72] |
| 152 | Kamaonensine G | Delphinium kamaonense Huth | whole plant | [72] |
| 153 | Kamaonensine F | Delphinium kamaonense Huth | whole plant | [72] |
- Lactone-type C19-DAs (154–158)
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 154 | Kusnezosine A | Aconitum kusnezoffii Reichb. var. gibbiferum | root | [75] |
| 155 | Kusnezosine B | Aconitum kusnezoffii Reichb. var. gibbiferum | root | [75] |
| 156 | Kusnezosine C | Aconitum kusnezoffii Reichb. var. gibbiferum | root | [75] |
| 157 | Stylosine A | Aconitum stylosum | root | [74] |
| 158 | Stylosine B | Aconitum stylosum | root | [74] |
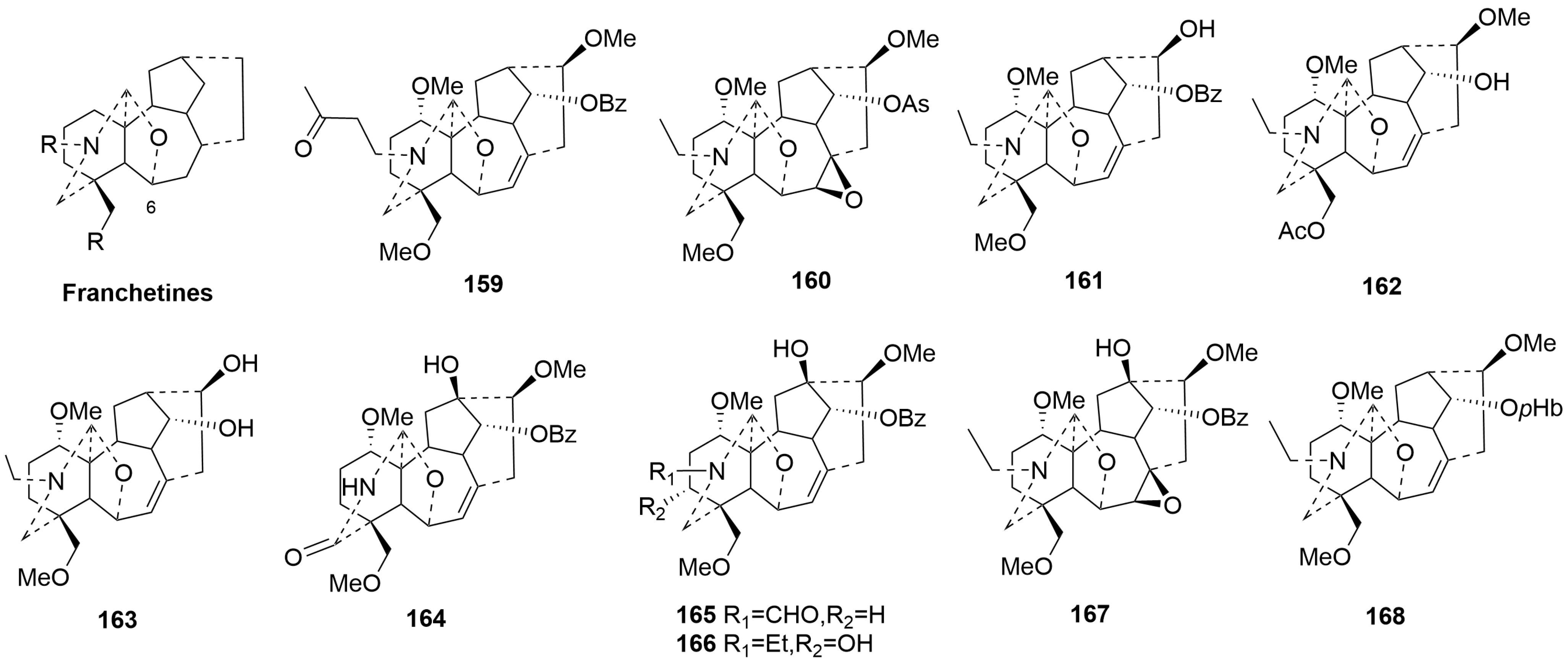
- Franchetine-type C19-DAs (159–168)
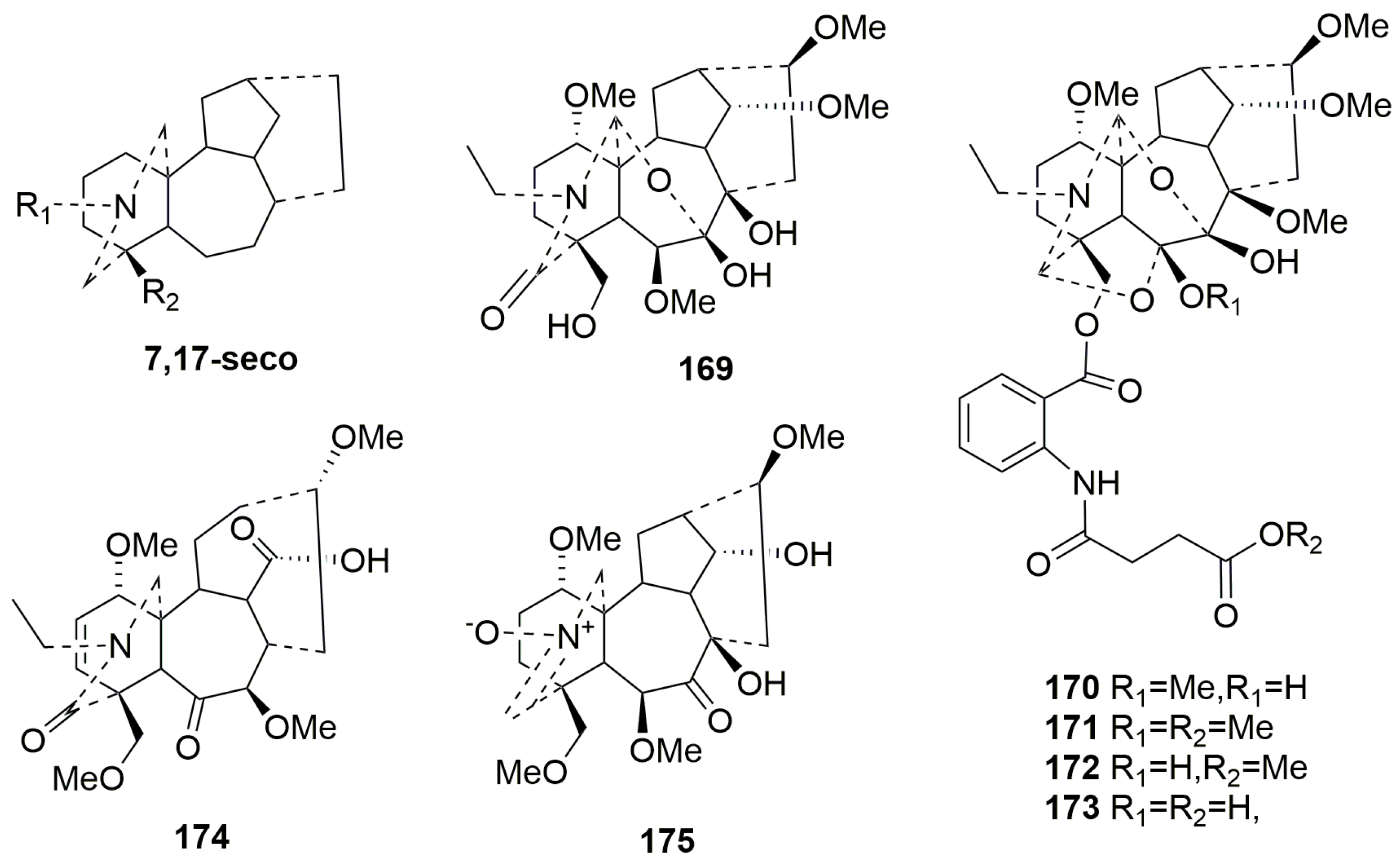
- 7,17-seco-type C19-DAs (169–175)
- Rearranged C19-DAs (176–182)
2.3.3. C20-DAs (183–266)
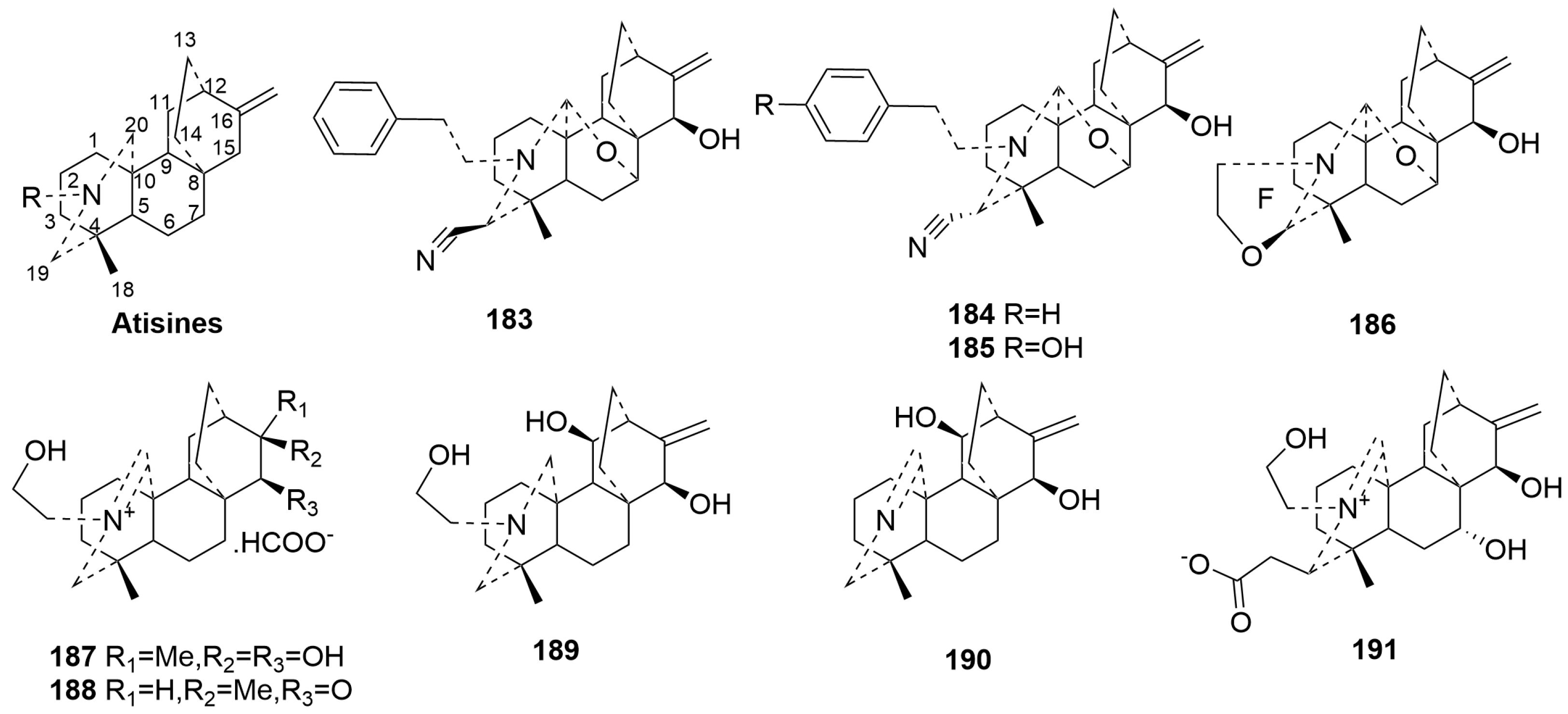
- Atisine-type C20-DA (183–191)
- Hetisine-type C20-DAs (192–217)
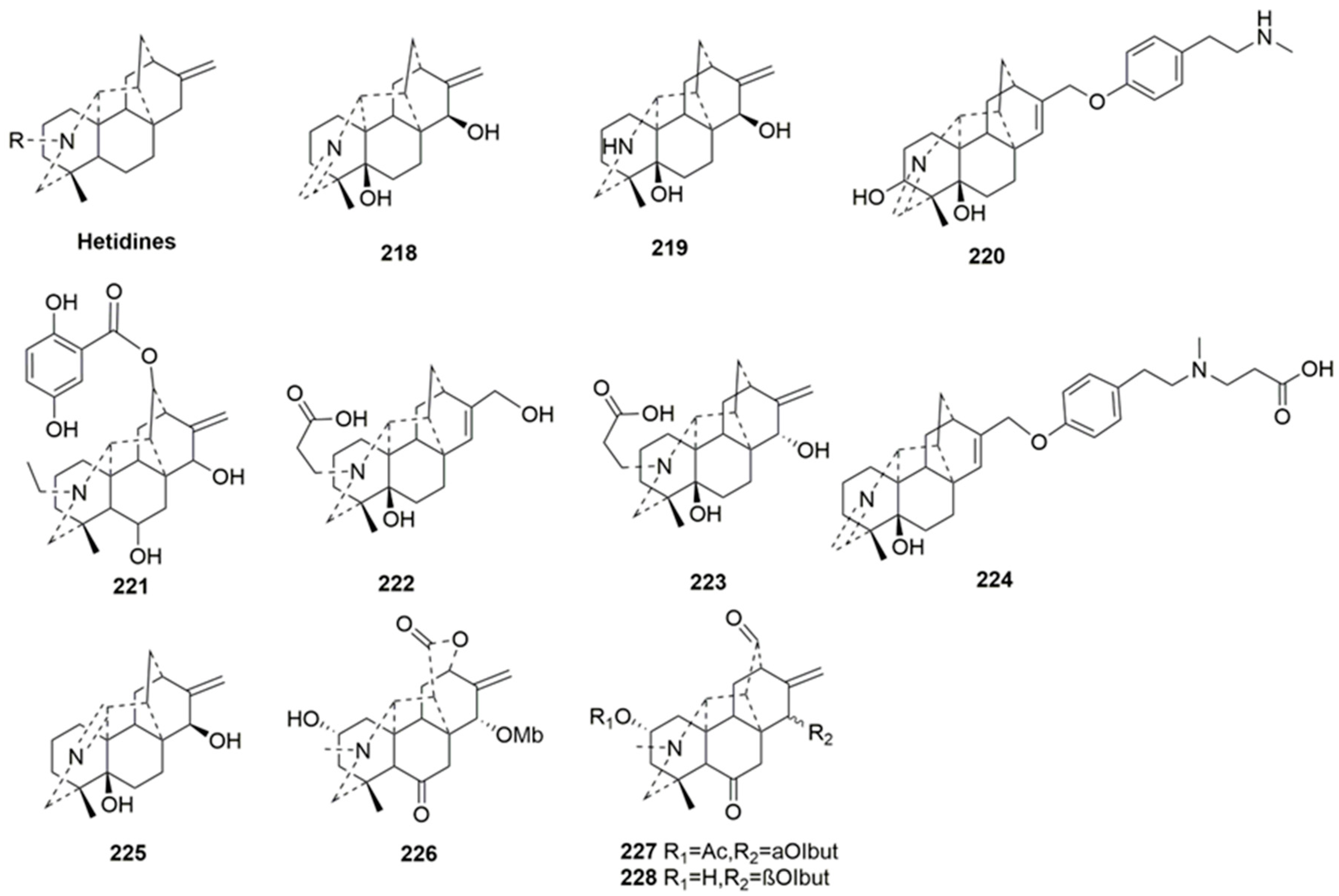
- Hetidine-type C20-DAs (218–228)
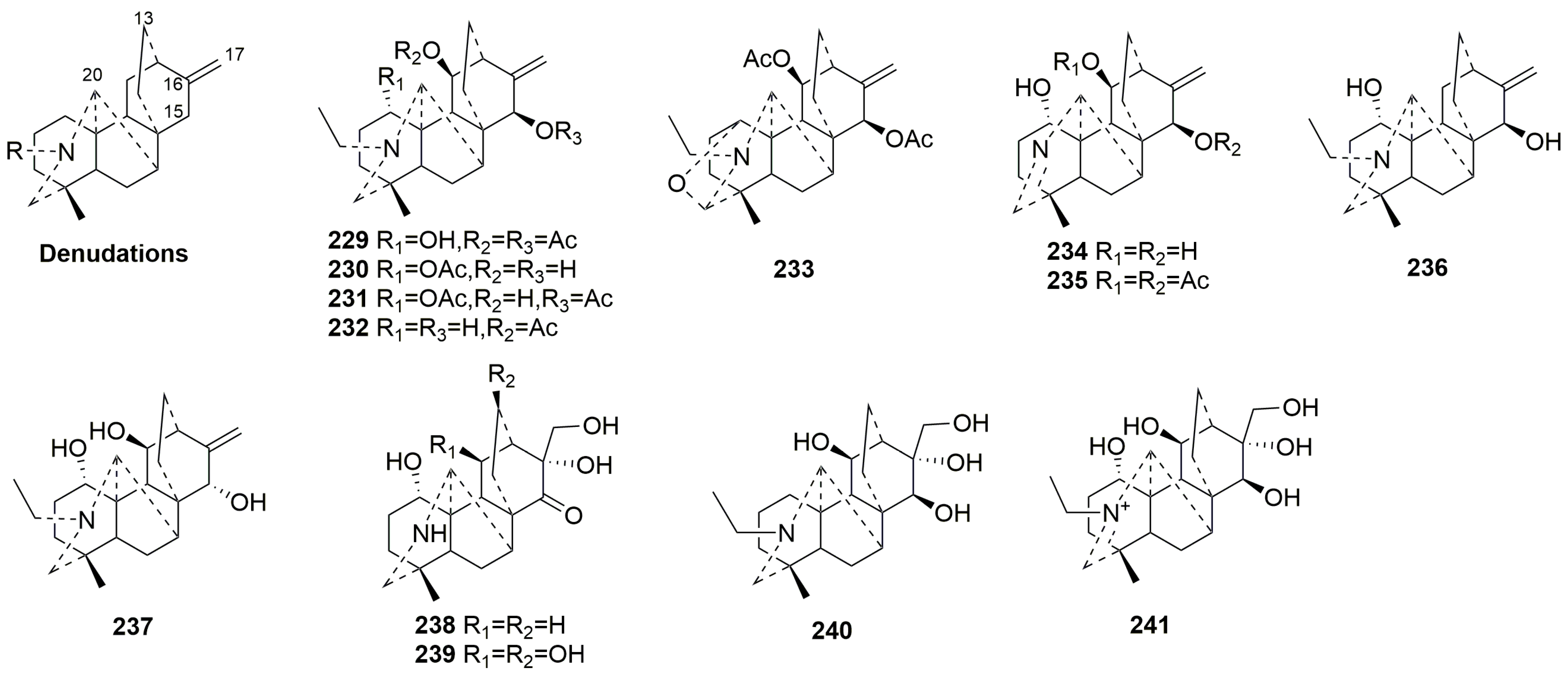
- Denudatine-type C20-DAs (229–241)
- Napelline-type C20-DAs (242–249)
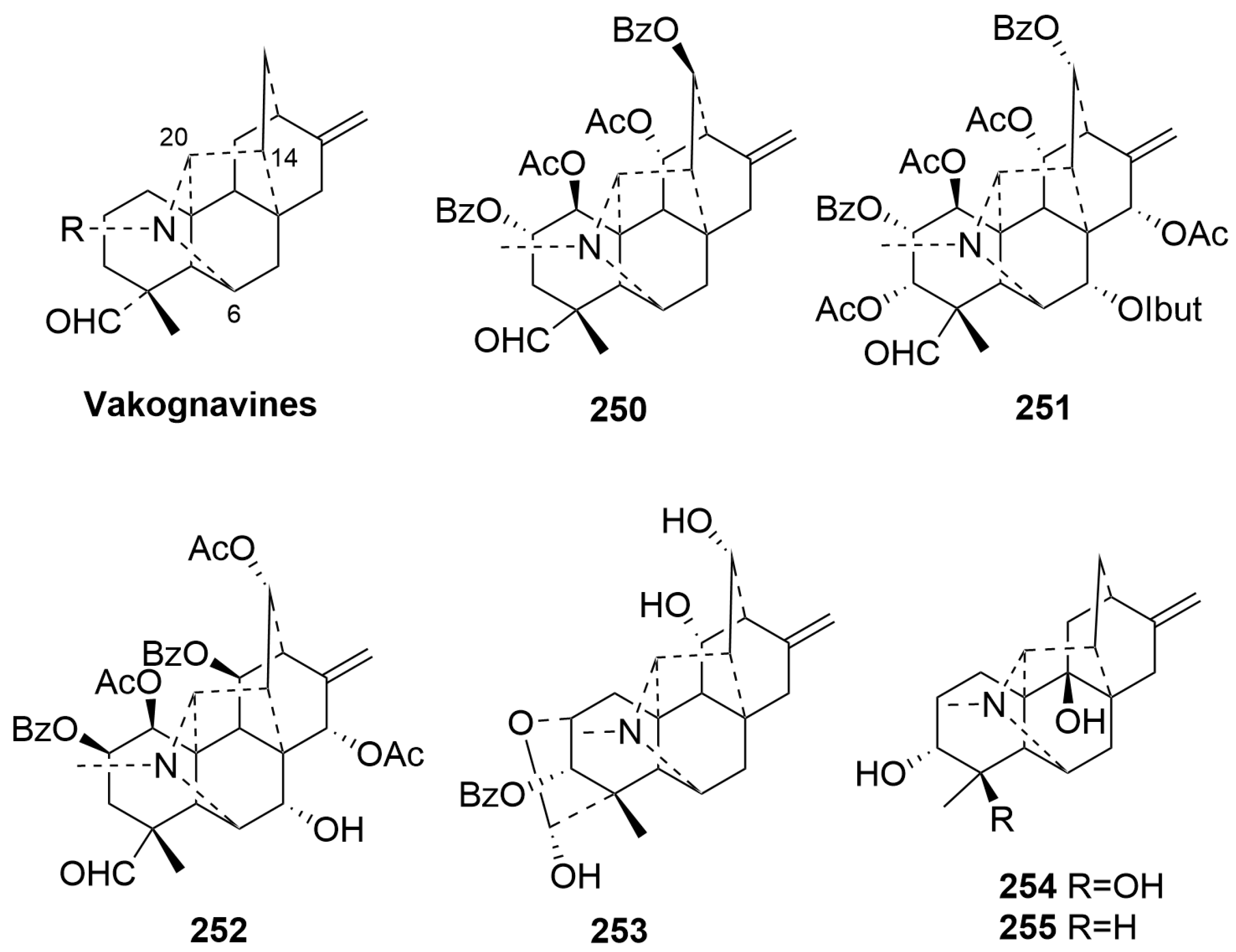
- Vakognavine-type C20-DAs (250–255)
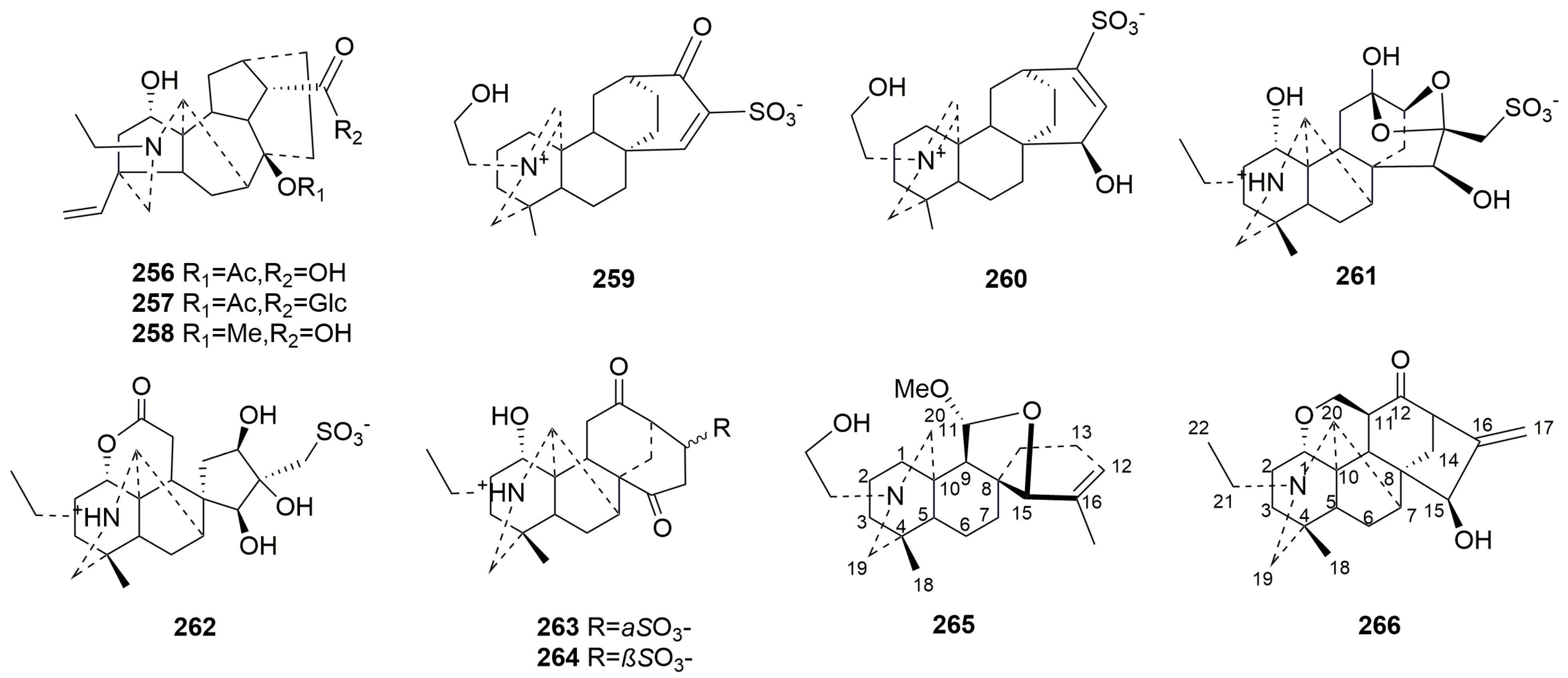
- Rearranged C20-DAs (256–266)
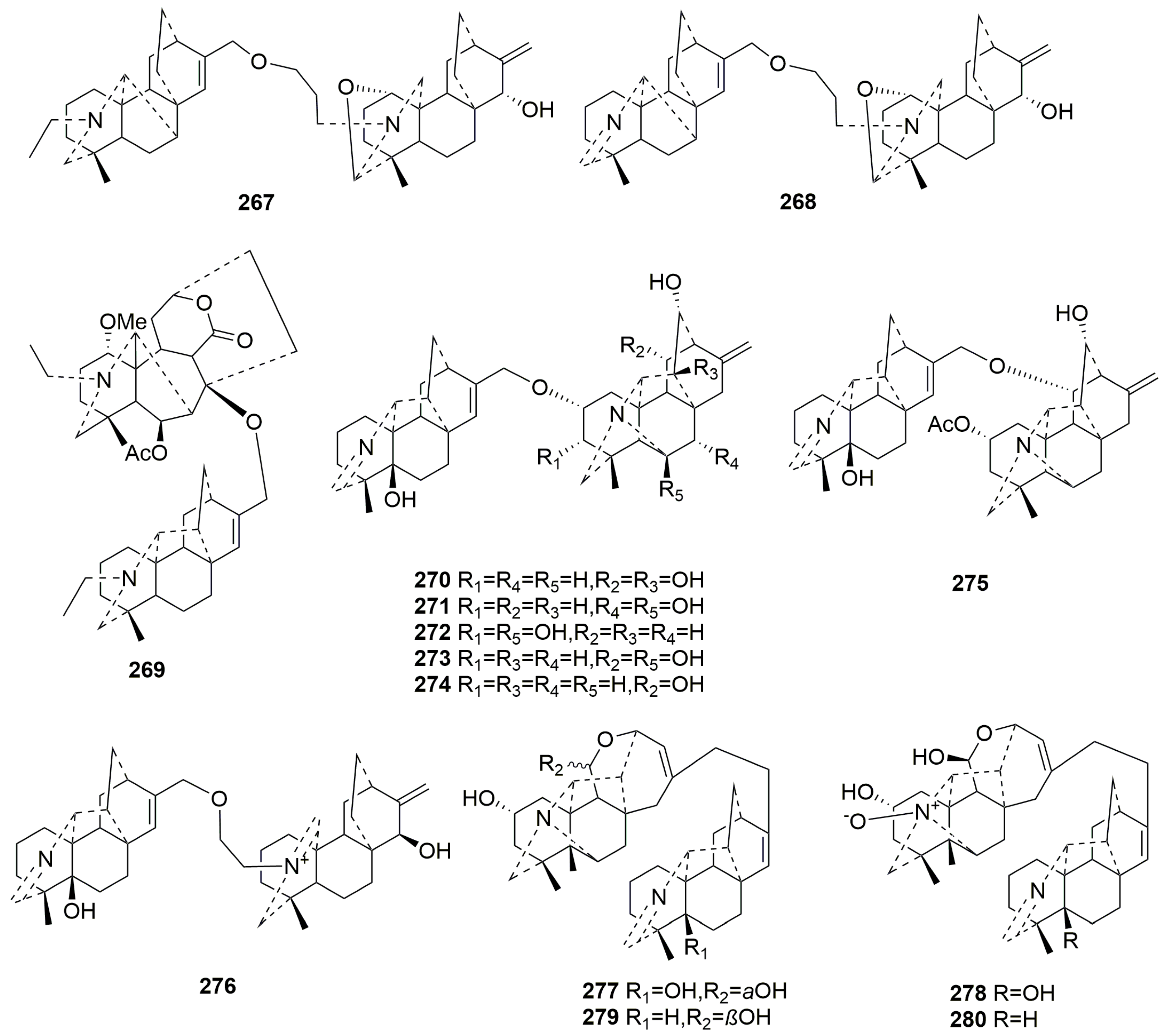
2.3.4. Bis-DAs (267–280)
2.3.5. Other DAs (281–289)
3. Biological Activity
3.1. Anti-Inflammatory Activity
3.2. Analgesic Activity
3.3. Antitumor Activity
3.4. Cardioprotective Activity
3.5. Antimicrobial Activity
3.5.1. Antiviral Activity
3.5.2. Antibacterial Activity
3.5.3. Antiplasmodial Activity
3.6. Other Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TeAs | Terpenoid alkaloids |
| MEP | Methylerythritol phosphate |
| NMR | Nuclear magnetic resonance |
| HR-ESI | High-resolution mass spectrometry |
| ECD | Electron capture detector |
| DAs | Diterpenoid alkaloids |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| NF-κB | Nuclear factor-κB |
| TRPV1 | Transient receptor vanilloid 1 |
| MEC | Minimum effective concentration |
| HEK-293 | Human Embryonic Kidney 293 Cells |
| LPS | Lipopolysaccharide |
| COX-2 | Cyclooxygenase-2 |
| PMNs | Polymorphonuclear leukocytes |
| PAF | Platelet-activating factor |
| MAPK | Mitogen-activated protein kinase |
| AChE | Acetylcholinesterase |
| MICs | Minimum inhibitory concentrations |
| IC50 | Half maximal inhibitory concentration |
| ID50 | Half infectious dose |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| ROS | Reactive oxygen species |
| Nrf2 | NF-E2-related factor 2 |
| Kyn | Kynurenine |
| IDO | Indoleamine 2,3-dioxygenase |
| P-gp | P-glycoprotein |
| HCPT | Hydroxy camptothecin |
| RSV | Respiratory syncytial virus |
| H1N1 | Influenza A virus |
| IFN–γ | Interferon-γ |
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| P38 | p38 mitogen-activated protein kinase (MAPK) |
| SMAD 2/3 | Mothers against decapentaplegic homolog 2/3 |
| CMCC 63501 | Bacillus subtilis |
| Me | Methyl |
| Et | Ethylic |
| Ibut | Isobutyryl |
| Mb | 2-metylbutyryl |
| Bz | Benzoyl |
| As | Anisoyl |
| Vr | Veratroyl |
| Cinn | Cinnamoyl |
| Ant | Anthranoyl |
| Glc | Glucose |
| Ac | Acetyl |
| pHb | p-hydroxybenzoyl |
| lipo | Stearoyl,oleoyl,linolenoyl,linoleoyl,palmitoly |
References
- Pereira, A.G.; Cassani, L.; Garcia-Oliveira, P.; Otero, P.; Mansoor, S.; Echave, J.; Xiao, J.; Simal-Gándara, J.; Prieto, M.A. Plant Alkaloids: Production, Extraction, and Potential Therapeutic Properties. In Natural Secondary Metabolites; Springer International Publishing: Cham, Switzerland, 2023; pp. 157–200. [Google Scholar]
- Wei, X.; Khan, A.; Song, D.; Dai, Z.; Liu, Y.P.; Yu, H.F.; Wang, B.; Zhu, P.F.; Ding, C.F.; Zhao, X.D.; et al. Three New Pyridine Alkaloids from Vinca major Cultivated in Pakistan. Nat. Prod. Bioprospecting 2017, 7, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Cherney, E.C.; Baran, P.S. Terpenoid-Alkaloids: Their Biosynthetic Twist of Fate and Total Synthesis. Isr. J. Chem. 2011, 51, 391–405. [Google Scholar] [CrossRef]
- Kukula-Koch, W.A.; Widelski, J. Chapter 9-Alkaloids. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 163–198. [Google Scholar]
- Zhang, X.; Tao, F.; Cui, T.; Luo, C.; Zhou, Z.; Huang, Y.; Tan, L.; Peng, W.; Wu, C. Sources, Transformations, Syntheses, and Bioactivities of Monoterpene Pyridine Alkaloids and Cyclopenta[c]pyridine Derivatives. Molecules 2022, 27, 7187. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, W.-J.; Shi, Y.-N.; Kennelly, E.J.; Zhao, D.-K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat. Prod. Rep. 2020, 37, 763–796. [Google Scholar] [CrossRef]
- Wang, M.L.; Yu, G.; Yi, S.P.; Zhang, F.Y.; Wang, Z.T.; Huang, B.; Su, R.B.; Jia, Y.X.; Gong, Z.H. Antinociceptive effects of incarvillateine, a monoterpene alkaloid from Incarvillea sinensis and possible involvement of the adenosine system. Sci. Rep. 2015, 5, 16107. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-P.; Liang, X.-T. C20-diterpenoid alkaloids. In The Alkaloids: Chemistry and Biology; Academic Press: Boston, MA, USA, 2002; Volume 59, pp. 1–280. [Google Scholar]
- Chodoeva, A.; Bosc, J.-J.; Robert, J. Aconitum Alkaloids and Biological Activities. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1503–1523. [Google Scholar]
- Tao, P.; Wang, Y.; Wang, Y. Attenuation and Structural Transformation of Crassicauline A During Sand Frying Process and Antiarrhythmic Effects of its Transformed Products. Front. Pharmacol. 2021, 12, 734671. [Google Scholar] [CrossRef]
- He, X.L.; Wang, S.B.; Du, G.H. Cyclovirobuxine. In Natural Small Molecule Drugs from Plants; Du, G.H., Ed.; Springer: Singapore, 2018; pp. 25–29. [Google Scholar]
- Munikishore, R.; Liu, R.; Zhang, S.; Zhao, Q.S.; Nian, Y.; Zuo, Z. Structurally modified Cyclovirobuxine-D Buxus alkaloids as effective analgesic agents through Cav3.2 T-Type calcium channel inhibition. Bioorganic Chem. 2023, 135, 106493. [Google Scholar] [CrossRef]
- Wang, F.P. Alkaloid Chemistry; Natural Products Chemistry Series; Chemical Industry Press: Beijing, China, 2008; pp. 378–431. [Google Scholar]
- Zhang, F.X.; Liu, H.H.; Yang, K.L.; Yang, T.; Zhou, R.X.; Miao, R.K.; Zhan, G.Q.; Guo, Z.J. New phenylpropanoids and monoterpene alkaloids with vasorelaxant activities from the branches of Alstonia scholaris. Fitoterapia 2022, 158, 105143. [Google Scholar] [CrossRef]
- Zhan, G.; Yang, K.; Yang, T.; Li, X.; Zhou, R.; Miao, R.; Guan, Y.; Teng, Y.; Guo, Z. A New Monoterpene Alkaloid From the Stems of Rauvolfia vomitoria. Rec. Nat. Prod. 2023, 17, 232–240. [Google Scholar] [CrossRef]
- Shen, X.P.; Chen, H.; Li, S.S.; Li, J.Y.; Li, X.; Zu, X.P.; Xu, X.K.; Li, X.; Shen, Y.H. Monoterpene Alkaloids from Incarvillea delavayi Bureau et Franchet and Their Inhibition against LPS Induced NO Production in BV2 Cells. Chem. Biodivers. 2022, 19, e202101013. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, J.; Zhao, P.; Huang, Y.; Zhang, S. A New Monoterpene Alkaloid from Incarvillea sinensis with Migration Inhibitory Activity on Cancer Cell. Chem. Biodivers. 2023, 20, e202201203. [Google Scholar] [CrossRef]
- Huo, Y.-F.; Wang, H.-L.; Wei, E.-H.; Jia, P.-S.; Liu, H.-Q.; Fan, X.-L.; Yana, K.-L. Two new compounds from the roots of Scrophularia ningpoensis and their anti-inflammatory activities. J. Asian Nat. Prod. Res. 2019, 21, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, L.T.; Zhao, L.; Yue, X.D.; Dai, S.J. New C9-Monoterpenoid Alkaloids Featuring a Rare Skeleton with Anti-Inflammatory and Anti-viral Activities from Forsythia suspensa. Chem. Biodivers. 2021, 19, e202100668. [Google Scholar]
- Zhang, X.; Luo, G.; Cao, Y.; Mishig, D.; Lodonjav, M.; Pan, D.; Yao, X.; Wang, F.; Zhang, G.; Luo, Y. (±)-Caryopterisines A and B, dimeric monoterpene alkaloids with unprecedented 6/5/5/5/6 pentacyclic rings scaffold from Caryopteris Glutinosa. Bioorganic Chem. 2021, 116, 105364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, Y.; Pan, D.; Yao, X.; Wang, F.; Zhang, G.; Luo, Y. Anti-fibrotic pyridine-containing monoterpene alkaloids from Caryopteris glutinosa. Phytochemistry 2022, 203, 113378. [Google Scholar] [CrossRef] [PubMed]
- Mishig, D.; Gruner, M.; Lübken, T.; Ganbaatar, C.; Regdel, D.; Knölker, H.J. Isolation and structure elucidation of pyridine alkaloids from the aerial parts of the Mongolian medicinal plant Caryopteris mongolica Bunge. Sci. Rep. 2021, 11, 13740. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Ren, X.; Zhou, T.T.; Li, T.; Liu, Y.C.; Tao, Y.; Hu, H.; Li, D.; Liu, Y.; Li, S.H. Secondary metabolites from the Mongolian medicine Lomatogonium carinthiacum. Fitoterapia 2023, 165, 105402. [Google Scholar] [CrossRef]
- Salim, F.; Yunus, Y.M.; Anouar, E.H.; Awang, K.; Langat, M.; Cordell, G.A.; Ahmad, R. Absolute Configuration of Alkaloids from Uncaria longiflora through Experimental and Computational Approaches. J. Nat. Prod. 2019, 82, 2933–2940. [Google Scholar] [CrossRef]
- Brocksom, T.; de Oliveira, K.; Desiderá, A. The Chemistry of the Sesquiterpene Alkaloids. J. Braz. Chem. Soc. 2017, 28, 933–942. [Google Scholar] [CrossRef]
- Zhu, S.S.; Qin, D.P.; Wang, S.X.; Yang, C.; Li, G.P.; Cheng, Y.X. Commipholactam A, a cytotoxic sesquiterpenoidal lactam from Resina Commiphora. Fitoter. 2019, 134, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Tan, L.; Cheng, L.; Ding, L.S.; Zhou, Y.; Deng, Y.; He, Y.Q.; Guo, D.L.; Xiao, S.J. Dendrobine-type alkaloids and bibenzyl derivatives from Dendrobium findlayanum. Fitoterapia 2020, 142, 104497. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cheng, Z.Q.; Hou, B.; Yang, L.; Zi, C.T.; Dong, F.W.; Hu, J.M.; Zhou, J. Two unusual dendrobine-type alkaloids from Dendrobium findlayanum. Fitoterapia 2020, 144, 104607. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, R.; Han, X.; Tang, X.; Wang, Q.; Li, P.; Li, G. Guaiane sesquiterpenes from the gorgonian Echinogorgia flora collected in the South China Sea. RSC Adv. 2022, 12, 2662–2667. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Thawabteh, A.; Lelario, F.; Bufo, S.A.; Scrano, L. Classification, Toxicity and Bioactivity of Natural Diterpenoid Alkaloids. Molecules 2021, 26, 4103. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Chen, Q.H.; Liu, X.Y. Diterpenoid alkaloids. Nat. Prod. Rep. 2010, 27, 529–570. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.J.; Zhao, B.; Zhao, J.Y.; Sagdullaev, S.S.; Akber Aisa, H. Three new diterpenoid alkaloids from Delphinium naviculare var. lasiocarpum W. T. Wang. Phytochem. Lett. 2019, 33, 12–16. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Qin, L.L.; Gao, F.; Huang, S.; Zhou, X.L. Fifteen new diterpenoid alkaloids from the roots of Aconitum kirinense Nakai. Fitoterapia 2020, 141, 104477. [Google Scholar] [CrossRef]
- Yang, H.B.; Luo, Y.Y.; Xu, J.B.; Liu, Y.; Gao, F.; Huang, S.; Chen, L.; Zhou, X.L. Two New C18-Diterpenoid Alkaloids from Aconitum leucostomum Worosch. Chem. Biodivers. 2022, 19, e202200483. [Google Scholar] [CrossRef]
- Ablajan, N.; Zhao, B.; Zhao, J.Y.; Wang, B.L.; Sagdullaev, S.S.; Aisa, H.A. Diterpenoid alkaloids from Aconitum barbatum var. puberulum Ledeb. Phytochemistry 2021, 181, 112567. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lv, Y.; Li, Q.; Mao, X.; Peng, T.; Gao, X.; Jin, N.; Yin, S.; Shi, X.; Wang, W. Diterpenoid Alkaloids from the Roots of Aconitum iochanicum. Chem. Nat. Compd. 2020, 56, 492–495. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ke, B.W.; Qin, Y.; Wang, F.P. Chapter One-The diterpenoid alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.J., Ed.; Academic Press: Boston, MA, USA, 2022; Volume 87, pp. 1–360. [Google Scholar]
- Hu, Z.X.; An, Q.; Tang, H.Y.; Chen, Z.H.; Aisa, H.A.; Zhang, Y.; Hao, X.J. Acoapetaludines A-K, C20 and C19-diterpenoid alkaloids from the whole plants of Aconitum apetalum (Huth) B. Fedtsch. Phytochemistry 2019, 167, 112111. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.L.; Zhang, M.; Xiao, Y.; Chen, L.; Xie, J.; Huang, S.; Zhou, X.L. C19-diterpenoid alkaloids from Aconitum refractum var. circinatum. Nat. Prod. Res. 2023, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Wang, S.; Xiao, Y.; Huang, S.; Chen, L.; Xie, J.; Zhou, X.L. Four New C19-Diterpenoid Alkaloids from Aconitum nagarum Stapf. Chem. Biodivers. 2023, 20, e202300058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, L.; Yang, C.; Ren, J.; Feng, Y.; Huang, S. Diterpenoid Alkaloids from the Roots of Aconitum rockii and Their Antifeedant Activity. Chin. J. Org. Chem. 2022, 42, 1856. [Google Scholar]
- Xing, F.; Xie, J.; Luo, Y.Y.; Huang, S.; Gao, F.; Chen, L.; Zhou, X.L. C19-Diterpenoid alkaloids from Aconitum richardsonianum. Phytochem. Lett. 2022, 51, 149–154. [Google Scholar] [CrossRef]
- Hu, J.; Wu, Q.; Li, Q.; Lv, T.; Peng, T.-F.; Yin, S.; Jin, H.-Z. Antinociceptive diterpenoid alkaloids from the roots of Aconitum Austroyunnanense. J. Asian Nat. Prod. Res. 2022, 25, 132–138. [Google Scholar] [CrossRef]
- Hu, J.; Li, S.F.; Li, Q.; Lv, T.; Peng, T.F.; Yin, S.; Min, Y. Antinociceptive C19–diterpenoid alkaloids from the roots of Aconitum nagarum. J. Asian Nat. Prod. Res. 2022, 25, 540–546. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Zhang, J.; Li, J.; Yao, C.; Wu, W.; Wang, Y.; Ji, H.; Wei, W.; Gao, M.; et al. Structurally diverse diterpenoid alkaloids from the lateral roots of Aconitum carmichaelii Debx. and their anti-tumor activities based on in vitro systematic evaluation and network pharmacology analysis. RSC Adv. 2021, 11, 26594–26606. [Google Scholar] [CrossRef]
- Wang, J.; Lou, H.; Li, J.; Liu, Y.; Han, H.; Yang, Z.; Pan, W.; Chen, Z. C19-diterpenoid alkaloids from the rhizomes of Aconitum pendulum. Fitoterapia 2021, 151, 104887. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xu, J.B.; Li, X.; Zhou, X.L.; Zhang, C.; Gao, F. Three New C19-Diterpenoid Alkaloids from Aconitum novoluridum. Chem. Pharm. Bull. 2021, 69, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.X.; Li, Q.; Mao, X.; Peng, T.F.; Liu, H.Q.; Yin, S.; Yuan, H.J. Antinociceptive C19–diterpenoid alkaloids from the root of Aconitum episcopale. J. Asian Nat. Prod. Res. 2021, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.X.; Li, Q.; Mao, X.; Peng, T.F.; Jin, N.H.; Yin, S.; Tang, Y. Antinociceptive C19–diterpenoid alkaloids isolated from Aconitum pseudostapfianum. J. Asian Nat. Prod. Res. 2021, 23, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, J.; Li, Q.; Mao, X.; Peng, T.; Jin, N.; Yin, S.; Shi, X.; Li, Y. Antinociceptive C19-Diterpenoid Alkaloids from Aconitum episcopale. Chem. Nat. Compd. 2021, 57, 503–506. [Google Scholar] [CrossRef]
- Song, Z.; Gao, C.; Jiang, Q.; Xu, J.; Xiong, L.; Liu, K.; Sun, D.; Li, H.; Chen, L. Diterpenoid alkaloids from Delphinium forrestii var. viride and their anti-inflammation activity. Phytochemistry 2021, 192, 112971. [Google Scholar]
- Wang, Z.S.; Chen, W.; Jiang, H.Y.; Gao, F.; Zhou, X.L. Semi-synthesis and structural elucidation of brevicanines A–D, four new C19-diterpenoid alkaloids with rotameric phenomenon from Aconitum brevicalcaratum. Fitoterapia 2019, 134, 404–410. [Google Scholar] [CrossRef]
- Wan, L.X.; Zhang, J.F.; Zhen, Y.Q.; Zhang, L.; Li, X.; Gao, F.; Zhou, X.L. Isolation, Structure Elucidation, Semi-Synthesis, and Structural Modification of C19-Diterpenoid Alkaloids from Aconitum apetalum and Their Neuroprotective Activities. J. Nat. Prod. 2021, 84, 1067–1077. [Google Scholar] [CrossRef]
- Si, Y.; Ding, X.; Adelakuna, T.A.; Zhang, Y.; Hao, X.J. Acotarines A-G, new diterpenoid alkaloids from Aconitum taronense induce lysosomal biogenesis. Fitoterapia 2020, 147, 104738. [Google Scholar] [CrossRef]
- Yamashita, H.; Miyao, M.; Hiramori, K.; Kobayashi, D.; Suzuki, Y.; Mizukami, M.; Goto, M.; Lee, K.H.; Wada, K. Cytotoxic diterpenoid alkaloid from Aconitum japonicum subsp. subcuneatum. J. Nat. Med. 2019, 74, 83–89. [Google Scholar] [CrossRef]
- Zhao, B.; Ablajan, N.; Zhao, J.Y.; Kodirova, D.R.; Sagdullaev, S.S.; Aisa, H.A. Two new C19-diterpenoid alkaloids from Aconitum smirnovii. Phytochem. Lett. 2020, 38, 96–100. [Google Scholar] [CrossRef]
- Alhilal, M.; Sulaiman, Y.A.M.; Alhilal, S.; Gomha, S.M.; Ouf, S.A. Antifungal Activity of New Diterpenoid Alkaloids Isolated by Different Chromatographic Methods from Delphinium peregrinum L. var. eriocarpum Boiss. Molecules 2021, 26, 1375. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, L.; Xie, J.; Ding, H.; Wan, D.; Li, Q.; Zeng, H.; Xing, F. Three New C19-Diterpenoid Alkaloids from Aconitum brevicalcaratum. Chin. J. Org. Chem. 2023, 43, 2245. [Google Scholar]
- Yao, L.L.; Zhang, S.Q.; Guo, C.; Li, B.X.; Yang, H.J.; Yin, T.P.; Cai, L. A new C19-diterpenoid alkaloid in Aconitum georgei Comber. Nat. Prod. Res. 2022, 38, 85–90. [Google Scholar] [CrossRef]
- Ablajan, N.; Xue, W.J.; Zhao, J.Y.; Sardorbek, A.; Boymirzayevich Begmatov, N.; Sagdullaev, S.; Zhao, B.; Akber Aisa, H. Two New C19-Diterpenoid Alkaloids from Delphinium shawurense. Chem. Biodivers. 2023, 20, e202200936. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, H.; Yang, X.; Ding, Z.; Yin, T. Grandiflolines A–F, new anti-inflammatory diterpenoid alkaloids isolated from Delphinium grandiflorum. Front. Chem. 2022, 10, 1012874. [Google Scholar] [CrossRef]
- Ma, H.; Ma, Y.; Dawa, Z.; Yao, Y.; Wang, M.; Zhang, K.; Zhu, C.; Liu, F.; Lin, C. Diterpenoid Alkaloids Isolated from Delphinium brunonianum and Their Inhibitory Effects on Hepatocytes Lipid Accumulation. Molecules 2022, 27, 2257. [Google Scholar] [CrossRef]
- Li, J.M.; Li, X.; Gao, F.; Cai, L.; Liang, X.X.; Chen, L.; Zhou, X.L. Five new diterpenoid alkaloids from Aconitum sczukinii Turcz. Phytochem. Lett. 2022, 47, 120–124. [Google Scholar] [CrossRef]
- Ahmad, S.; Gul, N.; Ahmad, M.; Almehmadi, M.; Shafie, A.; Shah, S.A.A.; Rahman, N.U.; Ahmad, H. Isolation, crystal structure, DFT calculation and molecular docking of uncinatine-A isolated from Delphinium uncinatum. Fitoterapia 2022, 162, 105268. [Google Scholar] [CrossRef]
- Yamashita, H.; Doi, N.; Hanawa, N.; Itoh, M.; Nakano, W.; Takahashi, E.; Mizukami, M.; Kaneda, K.; Suzuki, Y.; Goto, M.; et al. Eleven new C19-diterpenoid alkaloids from Delphinium elatum cv. Pacific Giant. J. Nat. Med. 2021, 76, 161–170. [Google Scholar]
- Xu, J.B.; Li, Y.Z.; Huang, S.; Chen, L.; Luo, Y.Y.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from the whole herb of Delphinium grandiflorum L. Phytochemistry 2021, 190, 112866. [Google Scholar] [CrossRef]
- He, Y.; Zhang, D.; West, L.M. Delphatisine D and Chrysotrichumine A, two new diterpenoid alkaloids from Delphinium chrysotrichum. Fitoterapia 2019, 139, 104407. [Google Scholar] [CrossRef]
- Tang, Q.; Shen, X.; Hao, Y.K.; Yang, S.Y.; Fu, J.T.; Wu, T.Y.; Zhao, H.Y.; Qin, B.; Li, Y.L.; Zhang, Y.B.; et al. Diterpenoid Alkaloids from Delphinium ajacis and Their Anti-inflammatory Activity. Chem. Biodivers. 2024, 21, e202301958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, L.; Liu, Y.; Zhao, Y.D.; Zhong, H.Y.; Chen, S.Y.; Huang, S. Diterpenoid Alkaloids from Delphinium liangshanense. Chem. Biodivers. 2024, e202301923. [Google Scholar] [CrossRef]
- Jing, D.; Zhang, Y.; Gong, C.; Du, K.; Wang, Y.; Lai, L.; Meng, D. Kamaonensine A-G: Lycaconitine-type C19-diterpenoid alkaloids with anti-inflammatory activities from Delphinium kamaonense Huth. Phytochemistry 2023, 215, 113822. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, D.; Chen, Y.; Xu, J.; Xu, Y.; Yue, X.; Jia, J.; Li, H.; Chen, L. Alkaloids of Delphinium grandiflorum and their implication to H2O2-induced cardiomyocytes injury. Bioorganic Med. Chem. 2021, 37, 116113. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, J.P.; Cai, X.Y.; Li, X.L.; Hu, J.T.; Sun, C.T.; Cai, L.; Ding, Z.T. Stylosines A and B, anti-inflammatory diterpenoid alkaloids from Aconitum stylosum. Tetrahedron 2020, 76, 131520. [Google Scholar] [CrossRef]
- Li, Y.Z.; Qin, L.L.; Gao, F.; Shan, L.H.; Zhou, X.L. Kusnezosines A-C, three C19-diterpenoid alkaloids with a new skeleton from Aconitum kusnezoffii Reichb. var. gibbiferum. Fitoterapia 2020, 144, 104609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xia, F.; Li, S.Y.; Nian, Y.; Wei, L.X.; Xu, G. Diterpenoid Alkaloids from the Aerial Parts of Aconitum flavum Hand.-Mazz. Nat. Prod. Bioprospecting 2021, 11, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.B.; Liang, Y.N.; Wang, Z.; Shi, L.K.; Zhang, Z.; Tang, Z.S.; Huang, L.Q. Aconicumines A–D, an advanced class of norditerpenoid alkaloids with an unprecedented N,O-diacetal motif from Aconitum taipeicum Hand.-Mazz., exhibit anti-inflammatory properties in vitro. Phytochemistry 2023, 210, 113675. [Google Scholar] [CrossRef]
- Li, X.; Ye, M.; Gao, F.; Zhou, X.; Chen, L.; Huang, S. A new diterpenoid alkaloid from Delphinium gyalanum C. Marquand & Airy Shaw. Nat. Prod. Res. 2021, 37, 130–135. [Google Scholar] [PubMed]
- Li, Y.; Zeng, J.; Tian, Y.H.; Hou, Y.; Da, H.; Fang, J.; Gao, K. Isolation, identification, and activity evaluation of diterpenoid alkaloids from Aconitum sinomontanum. Phytochemistry 2021, 190, 112880. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z.W.; Wang, M.X.; Yu, H.L.; Chen, L.; Cai, Z.; Zhang, Y.; Gu, M.M.; Shao, Y.L.; Han, H.P.; et al. Brunonianines A-C, C20-diterpenoid alkaloids with cyano group from Delphinium brunonianum Royle. Phytochemistry 2024, 219, 113987. [Google Scholar] [CrossRef] [PubMed]
- Ahunova, H.; Bobakulov, K.M.; Turgunov, K.K.; Tashkhodzhaev, B.; Mukarramov, N.I.; Abdullaev, S.V. Alkaloids from Delphinium oreophilum. The New Diterpene Alkaloid 15-Epinaviculine B. Chem. Nat. Compd. 2023, 59, 1137–1141. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, H.; Zhang, W.; Jiang, Z. Chemistry and biological activities of hetisine-type diterpenoid alkaloids. RSC Adv. 2021, 11, 36023–36033. [Google Scholar] [CrossRef]
- Pu, Y.L.; Tian, L.F.; Chen, L.; Deng, M.Y.; Xie, J.; Huang, S.; Zhou, X.-L. Diterpenoid alkaloids from Delphinium trichophorum. J. Asian Nat. Prod. Res. 2023, 25, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, J.F.; Chen, L.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from Aconitum anthoroideum that offer protection against MPP±Induced apoptosis of SH-SY5Y cells and acetylcholinesterase inhibitory activity. Phytochemistry 2020, 178, 112459. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, L.; Liu, Z.; Lin, L.; Li, C.; Guo, S.; Wang, Z.; Wang, Z.; Sui, F. Diterpenoid alkaloids from the whole plant of Aconitum tanguticum (Maxim.) Stapf. Phytochemistry 2019, 160, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.F.; Wang, Y.R.; Jiang, H.J.; Ding, Z.B.; Yin, T.P. New diterpenoid alkaloids from Delphinium pachycentrum Hemsl. Nat. Prod. Res. 2022, 38, 1487–1493. [Google Scholar] [CrossRef]
- Xu, J.B.; Luo, Y.Y.; Huang, S.; Gao, F.; Zhou, X.L. Four New Diterpenoid Alkaloids from The Roots of Aconitum coreanum. Chem. Biodivers. 2020, 17, e1900600. [Google Scholar] [CrossRef]
- Anmol; Kumari, S.; Kumar, R.; Singh, R.; Aggarwal, G.; Agrawal, P.; Sahal, D.; Sharma, U. Antiplasmodial diterpenoid alkaloid from Aconitum heterophyllum Wall. ex Royle: Isolation, characterization, and UHPLC-DAD based quantification. J. Ethnopharmacol. 2022, 287, 114931. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.Z.; Li, X.Y.; Xie, J.; Chen, L.; Gao, F.; Zhou, X.L.; Huang, S. Gyalanunines A and B, two new C20-diterpenoid alkaloids from Delphinium gyalanum. Tetrahedron Lett. 2022, 108, 154153. [Google Scholar] [CrossRef]
- Li, H.Y.; Yan, B.C.; Wei, L.X.; Sun, H.D.; Puno, P.T. Tangutidines A-C, Three Amphoteric Diterpene Alkaloids from Aconitum tanguticum. Nat. Prod. Bioprospecting 2021, 11, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kurbanov, U.K.; Levkovich, M.G.; Mukarramov, N.I. New Diterpene Alkaloid from Delphinium paradoxum. Chem. Nat. Compd. 2024, 60, 115–118. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Chen, Q.; Feng, Q.; Liu, T.; Feng, W.; Liang, Y.; Liu, X.; Li, C.; Wang, Z. Anti-inflammatory diterpenoid alkaloids from Aconitum tanguticum (Maxim.) Stapf. Phytochemistry 2023, 206, 113524. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Shao, S.; Xia, H.; Wu, Y.Z.; Zhu, C.G.; Xu, C.B.; Zhang, T.T.; Guo, Q.L.; Shi, J.G. Denudatine-type diterpenoid alkaloids from an aqueous extract of the lateral root of Aconitum carmichaelii. J. Asian Nat. Prod. Res. 2021, 23, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.; Zhang, X.; Liu, C.; Chen, N.; Naruo, A.; Qin, Z.; Ao, M. New napelline-type diterpenoid alkaloids from Aconiti kusnezoffii roots: Structure elucidation, plausible biogenetic pathway and biological activities. Phytochem. Lett. 2021, 43, 53–59. [Google Scholar] [CrossRef]
- Guo, Q.; Xia, H.; Wu, Y.; Shao, S.; Xu, C.; Zhang, T.; Shi, J. Structure, property, biogenesis, and activity of diterpenoid alkaloids containing a sulfonic acid group from Aconitum carmichaelii. Acta Pharm. Sin. B 2020, 10, 1954–1965. [Google Scholar] [CrossRef]
- Wang, J.; Meng, X.H.; Chai, T.; Yang, J.L.; Shi, Y.P. Diterpenoid Alkaloids and One Lignan from the Roots of Aconitum pendulum Busch. Nat. Prod. Bioprospecting 2019, 9, 419–423. [Google Scholar] [CrossRef]
- Huang, S.; Wang, J.Z.; Guo, Q.J.; Xiao, Y.; Feng, Y.M.; Chen, L.; Xie, J.; Zhou, X.L. Diterpenoid alkaloids from two species of Delphinium. J. Asian Nat. Prod. Res. 2022, 25, 718–730. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, S.; Guo, Q.; Xu, C.; Xia, H.; Zhang, T.; Shi, J. Aconicatisulfonines A and B, Analgesic Zwitterionic C20-Diterpenoid Alkaloids with a Rearranged Atisane Skeleton from Aconitum Carmichaelii. Org. Lett. 2019, 21, 6850–6854. [Google Scholar] [CrossRef]
- Liu, H.; Wu, Y.; Guo, Q.; Shao, S.; Xu, C.; Zhang, T.; Shi, J. Aconapelsulfonines A and B, seco C20-diterpenoid alkaloids deriving via Criegee rearrangements of napelline skeleton from Aconitum carmichaelii. Chin. Chem. Lett. 2021, 32, 33–36. [Google Scholar] [CrossRef]
- He, J.R.; Zhang, L.M.; Lou, H.B.; Lv, D.; Zuo, A.X.; Shen, Y. Two new bis-diterpenoid alkaloids from Aconitum weixiense. J. Asian Nat. Prod. Res. 2022, 25, 842–848. [Google Scholar] [CrossRef]
- Jomori, T.; Setiawan, A.; Sasaoka, M.; Arai, M. Cytotoxicity of New Diterpene Alkaloids, Ceylonamides G-I, Isolated From Indonesian Marine Sponge of Spongia sp. Nat. Prod. Commun. 2019, 14, 1934578X19857294. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Chen, Y.; Li, S.; Xu, J.; Guo, H.; Liu, Z.; Zhu, S.; Liu, H.; Zhang, W. Three new diterpenes and two new sesquiterpenoids from the endophytic fungus Trichoderma koningiopsis A729. Bioorganic Chem. 2019, 86, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhao, L.; Sun, L.T.; Xie, Z.P.; Zhang, S.M.; Yue, X.D.; Dai, S.J. Trinorlabdane diterpenoid alkaloids featuring an unprecedented skeleton with anti-inflammatory and antiviral activities from Forsythia suspensa. RSC Adv. 2021, 11, 29684–29689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, X.; Wei, Y.; Liu, H.; Guo, Q.; Zhang, T.; Shi, J. Two unique C21-diterpenoid alkaloids from Aconitum carmichaelii. Chin. Chem. Lett. 2022, 33, 5047–5050. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Jia, C.; Gao, C.; Zheng, T.; Wu, M.; Zhang, Q.; Zhao, X.; Li, Z.; Chen, J.; et al. Machine learning enables discovery of Gentianine targeting TLR4/NF-κB pathway to repair ischemic stroke injury. Pharmacol. Res. 2021, 173, 105913. [Google Scholar] [CrossRef]
- Yu, H.H.; Li, M.; Li, Y.B.; Lei, B.B.; Yuan, X.; Xing, X.K.; Xie, Y.F.; Wang, M.; Wang, L.; Yang, H.J.; et al. Benzoylaconitine Inhibits Production of IL-6 and IL-8 via MAPK, Akt, NF-κB Signaling in IL-1β-Induced Human Synovial Cells. Biol. Pharm. Bull. 2020, 43, 334–339. [Google Scholar] [CrossRef]
- Sisignano, M.; Geisslinger, G. Rethinking the use of NSAIDs in early acute pain. Trends Pharmacol. Sci. 2023, 44, 193–195. [Google Scholar] [CrossRef]
- Basso, L.; Aboushousha, R.; Fan, C.Y.; Iftinca, M.; Melo, H.; Flynn, R.; Agosti, F.; Hollenberg, M.D.; Thompson, R.; Bourinet, E.; et al. TRPV1 promotes opioid analgesia during inflammation. Sci. Signal. 2019, 12, eaav0711. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Pimenta, M.; Estevinho, L.M.; Szopa, A.; Basit, M.; Khan, K.; Armaghan, M.; Ibrayeva, M.; Sönmez Gürer, E.; Calina, D.; Hano, C.; et al. Chemotherapeutic properties and side-effects associated with the clinical practice of terpene alkaloids: Paclitaxel, docetaxel, and cabazitaxel. Front. Pharmacol. 2023, 14, 1157306. [Google Scholar] [CrossRef] [PubMed]
- Le Naour, J.; Galluzzi, L.; Zitvogel, L.; Kroemer, G.; Vacchelli, E. Trial watch: IDO inhibitors in cancer therapy. OncoImmunology 2020, 9, 1777625. [Google Scholar] [CrossRef] [PubMed]





| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 159 | Acotarine A | Aconitum taronense | root | [56] |
| 160 | Acotarine B | Aconitum taronense | root | [56] |
| 161 | Acotarine C | Aconitum taronense | root | [56] |
| 162 | Acotarine D | Aconitum taronense | root | [56] |
| 163 | Acotarine E | Aconitum taronense | root | [56] |
| 164 | Flavumoline A | Aconitum flavum Hand.-Mazz | aerial parts | [76] |
| 165 | Flavumoline B | Aconitum flavum Hand.-Mazz | aerial parts | [76] |
| 166 | Flavumoline C | Aconitum flavum Hand.-Mazz | aerial parts | [76] |
| 167 | Flavumoline D | Aconitum flavum Hand.-Mazz | aerial parts | [76] |
| 168 | Rockidine C | Aconitum genera | root | [43] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 169 | Grandifline B | Delphinium grandiflorum | aerial part | [73] |
| 170 | Aconicumine A | Aconitum taipeicum Hand.-Mazz. | root | [77] |
| 171 | Aconicumine B | Aconitum taipeicum Hand.-Mazz. | root | [77] |
| 172 | Aconicumine C | Aconitum taipeicum Hand.-Mazz. | root | [77] |
| 173 | Aconicumine D | Aconitum taipeicum Hand.-Mazz. | root | [77] |
| 174 | Brunodelphinine B | Delphinium brunonianum Royle | aerial part | [64] |
| 175 | Brunodelphinine D | Delphinium brunonianum Royle | aerial part | [64] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 176 | Nagarumine C | Aconitum nagarum | root | [46] |
| 177 | Acosinomonine A | Aconitum sinomontanum | root | [79] |
| 178 | Acosinomonine B | Aconitum sinomontanum | root | [79] |
| 179 | Grandifline A | Delphinium grandiflorum L. | whole herb | [73] |
| 180 | Episcopine A | Aconitum episcopale | root | [52] |
| 181 | Gyalanutine A | Delphinium gyalanum C. Marquand & Airy Shaw | whole plant | [78] |
| 182 | Episcopaline B | Aconitum episcopale | root | [50] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 183 | Brunonianine A | Delphinium brunonianum. | whole plant | [80] |
| 184 | Brunonianine B | Delphinium brunonianum. | whole plant | [80] |
| 185 | Brunonianine C | Delphinium brunonianum. | whole plant | [80] |
| 186 | Delphatisine D | Delphinium chrysotrichum | aerial part | [69] |
| 187 | Barpubesine A | Aconitum barbatum var. puberulum Ledeb | whole plant | [37] |
| 188 | Barpubesine B | Aconitum barbatum var. puberulum Ledeb | whole plant | [37] |
| 189 | Barpubesine C | Aconitum barbatum var. puberulum Ledeb | whole plant | [37] |
| 190 | Forrestline F | Delphinium forrestii var. viride | whole plant | [53] |
| 191 | Brunodelphinine E | Delphinium brunonianum | aerial parts | [64] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 192 | Trichophorine A | Delphinium trichophorum Franch. | whole herb | [83] |
| 193 | Trichophorine B | Delphinium trichophorum Franch. | whole herb | [83] |
| 194 | Trichophorine C | Delphinium trichophorum Franch. | whole herb | [83] |
| 195 | Coreanine A | Aconitum coreanum | root | [87] |
| 196 | Coreanine B | Aconitum coreanum | root | [87] |
| 197 | Coreanine C | Aconitum coreanum | root | [87] |
| 198 | Coreanine D | Aconitum coreanum | root | [87] |
| 199 | Tanguticuline A | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 200 | Tanguticuline B | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 201 | Tanguticuline C | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 202 | Tanguticuline D | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 203 | Tanguticuline E | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 204 | Anthoroidine G | Aconitum anthoroideum DC. | whole plant | [84] |
| 205 | Anthoroidine H | Aconitum anthoroideum DC. | whole plant | [84] |
| 206 | Anthoroidine I | Aconitum anthoroideum DC. | whole plant | [84] |
| 207 | Grandiflonine C | Delphinium grandiflorum L. | whole plant | [68] |
| 208 | Grandiflonine D | Delphinium grandiflorum L. | whole plant | [68] |
| 209 | 2-O-cinnamoyl hetisine | Aconitum heterophyllum | root | [88] |
| 210 | hetisane-15β-O-β-d-glucoside | Aconitum carmichaelii Debx. | root | [47] |
| 211 | Hydrodavisine | Delphinium peregrinum L. var. eriocarpum Boiss | aerial part | [59] |
| 212 | Pachycentine | Delphinium pachycentrum Hemsl | whole herb | [86] |
| 213 | Anthoroidine A | Aconitum anthoroideum DC. | whole plant | [84] |
| 214 | Ajacisine H | Delphinium ajacis | seed | [70] |
| 215 | Tanguticuline F | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 216 | Tanguticuline G | Aconitum tanguticum (Maxim.) Stapf | whole plant | [85] |
| 217 | Gyalanunine B | Delphinium gyalanum C. Marquand & Airy Shaw | whole plant | [89] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 218 | 15-epinaviculine B | Delphinium oreophilum | aerial part | [81] |
| 219 | Tangutidine D | Aconitum tanguticum (Maxim.) Stapf | whole plant | [92] |
| 220 | Tangutidine E | Aconitum tanguticum (Maxim.) Stapf | whole plant | [92] |
| 221 | Paradoxine | Delphinium paradoxum Bunge | aerial part | [91] |
| 222 | Tangutidine A | Aconitum tanguticum | whole plant | [90] |
| 223 | Tangutidine B | Aconitum tanguticum | whole plant | [90] |
| 224 | Tangutidine C | Aconitum tanguticum | whole plant | [90] |
| 225 | Anthoroidine F | Aconitum anthoroideum DC. | whole plant | [84] |
| 226 | Sczukiniline A | Aconitum sczukinii Turcz | root | [65] |
| 227 | Sczukiniline B | Aconitum sczukinii Turcz | root | [65] |
| 228 | Sczukiniline C | Aconitum sczukinii Turcz | root | [65] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 229 | Kirisine F | Aconitum kirinense Nakai | root | [35] |
| 230 | Kirisine G | Aconitum kirinense Nakai | root | [35] |
| 231 | Kirisine H | Aconitum kirinense Nakai | root | [35] |
| 232 | Kirisine I | Aconitum kirinense Nakai | root | [35] |
| 233 | Kirisine J | Aconitum kirinense Nakai | root | [35] |
| 234 | Kirisine K | Aconitum kirinense Nakai | root | [35] |
| 235 | Kirisine L | Aconitum kirinense Nakai | root | [35] |
| 236 | Barpubesine D | Aconitum barbatum var. puberulum | whole plant | [37] |
| 237 | Aconicarnine C | Aconitum carmichaelii | lateral root | [93] |
| 238 | Aconicarnine A | Aconitum carmichaelii | lateral root | [93] |
| 239 | Aconicarnine B | Aconitum carmichaelii | lateral root | [93] |
| 240 | Aconicarnine D | Aconitum carmichaelii | lateral root | [93] |
| 241 | Aconicarnine E | Aconitum carmichaelii | lateral root | [93] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 242 | Kirisine M | Aconitum kirinense Nakai | root | [35] |
| 243 | Kirisine N | Aconitum kirinense Nakai | root | [35] |
| 244 | Kirisine O | Aconitum kirinense Nakai | root | [35] |
| 245 | 12-epi-aconicarmichinium A | Aconitum pendulum Busch | root | [96] |
| 246 | Napelline C | Aconiti kusnezoffii Radix | root | [94] |
| 247 | Napelline D | Aconiti kusnezoffii Radix | root | [94] |
| 248 | Napelline E | Aconiti kusnezoffii Radix | root | [94] |
| 249 | Chuanfusulfonine A | Aconitum carmichaelii | lateral root | [95] |
| No. | Compound name | Sources | Plant parts | Ref. |
|---|---|---|---|---|
| 250 | Rockisine A | Aconitum genera | root | [43] |
| 251 | Umbrodine A | Delphinium umbrosum Hand.-Mazz. | whole plant | [97] |
| 252 | Kingiadine | Delphinium kingianum Bruhl. ex Huth. | whole plant | [97] |
| 253 | Gyalanunine A | Delphinium gyalanum C. Marquand & Airy Shaw | whole plant | [89] |
| 254 | Grandiflonine A | Delphinium grandiflorum L. | whole plant | [68] |
| 255 | Grandiflonine B | Delphinium grandiflorum L. | whole plant | [68] |
| No. | Compound Name | Sources | Plant Parts | Ref. |
|---|---|---|---|---|
| 256 | Anthoroidine C | Aconitum anthoroideum DC. | whole plant | [84] |
| 257 | Anthoroidine D | Aconitum anthoroideum DC. | whole plant | [84] |
| 258 | Anthoroidine E | Aconitum anthoroideum DC. | whole plant | [84] |
| 259 | Aconicatisulfonine A | Aconitum carmichaelii | lateral root | [98] |
| 260 | Aconicatisulfonine B | Aconitum carmichaelii | lateral root | [98] |
| 261 | Aconapelsulfonine A | Aconitum carmichaelii | lateral root | [99] |
| 262 | Aconapelsulfonine B | Aconitum carmichaelii | lateral root | [99] |
| 263 | Aconicarmisulfonine B | Aconitum carmichaelii | lateral root | [95] |
| 264 | Aconicarmisulfonine C | Aconitum carmichaelii | lateral root | [95] |
| 265 | Barpuberudine | Aconitum barbatum var. puberulum Ledeb | whole plant | [37] |
| 266 | Acoapetaludine A | Aconitum apetalum (Huth) B.Fedtsch | whole plant | [40] |
| No. | Compound Name | Types | Sources | Plant Parts | Ref. |
|---|---|---|---|---|---|
| 267 | Weisaconitine E | denudatine-atisine | Aconitum weixiense | root | [100] |
| 268 | Weisaconitine F | denudatine-atisine | Aconitum weixiense | root | [100] |
| 269 | Tangirine A | heteratisine-hetidine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 270 | Tanguticinine A | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 271 | Tanguticinine B | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 272 | Tanguticinine C | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 273 | Tanguticinine D | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 274 | Tanguticinine E | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 275 | Tanguticinine F | hetidine-hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 276 | Tanguticinine G | hetidine-atisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 277 | Anthoroidine B | hetidine-rearranged hetisine | Aconitum anthoroideum DC. | whole plant | [84] |
| 278 | N-oxide anthoroidine B | hetidine-rearranged hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 279 | 5-deoxyanthoridine B | hetidine-rearranged hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| 280 | N-oxide 5-deoxyanthoroidine B | hetidine-rearranged hetisine | Aconitum tanguticum (Maxim.) Stapf. | whole plant | [92] |
| No. | Compound Name | Sources | Ref. |
|---|---|---|---|
| 281 | Ceylonamide G | Spongia sp. | [101] |
| 282 | Ceylonamide H | Spongia sp. | [101] |
| 283 | Ceylonamide I | Spongia sp. | [101] |
| 284 | Koninginol A | Trichoderma koningiopsis A729 | [102] |
| 285 | Koninginol B | Trichoderma koningiopsis A729 | [102] |
| 286 | Forsyqinlingine A | Forsythia suspensa | [103] |
| 287 | Forsyqinlingine B | Forsythia suspensa | [103] |
| 288 | Aconidenusulfonine A | Aconitum carmichaelii | [104] |
| 289 | 12,16-secoaconidenusulfonine A | Aconitum carmichaelii | [104] |
| TeAs Types | Activities | Research Method | Possible Mechanism | Ref. |
|---|---|---|---|---|
| Monoterpenoid Alkaloids | ||||
| Alstochonine A (1) | Vasorelaxant | In vitro | Vasorelaxant activity against phenylephrine-induced contraction of rat mesenteric arteries with rates of 73.6 ± 2.8% and 95.4 ± 3.7% (IC50 = 93.30 ± 10.81, 60.56 ± 3.66 μM) | [16] |
| Alstochonine B (2) | In vitro | [16] | ||
| Delavatine C (9) | Anti-inflammatory | In vitro | Inhibition of NO production in LPS-stimulated BV2 cells (IC50 = 25.62, 17.29 μM) | [18] |
| Delavatine E (11) | In vitro | [18] | ||
| Forsyqinlingine C (14) | Anti-inflammatory and antiviral | In vitro | Anti-inflammatory activities by inhibiting the release of β-glucuronidase in PMNs with inhibition rates of 45.2% and 40.1%, and antiviral activities against H1N1 virus (IC50 = 11.9, 15.1 μM) and RSV (EC50 = 13.5, 14.0 μM) | [21] |
| Forsyqinlingine D (15) | In vitro | [21] | ||
| Incarvine G (12) | Antitumor | In vitro | Cytotoxicity (IC50 = 60.29 μM) against MDA-MB-231 cells and inhibiting actin cytoskeleton formation | [19] |
| (±)-Caryopterisine A (19) | In vitro | Reduction of Kyn biosynthesis in HeLa cells by inhibiting IDO at 10 μM with inhibition ratios of 25.7% and 29.8%, respectively | [22] | |
| (±)-Caryopterisine B (20) | In vitro | [22] | ||
| Caryopterisine C (16) | Antifibrotic | In vitro | Inhibition of collagen accumulation (IC50 = 14.26 ± 1.46 μM) in NIH3T3 cells and phosphorylation of ERK1/2, P38, and SMAD2/3 | [23] |
| Lomatogonin C (26) | Immunosuppressive | In vitro | Inhibition of T cell proliferation (21.62 ± 3.06%) and IFN–γ secretion (37.59 ± 5.41%) at 20 μM | [26] |
| Sesquiterpene alkaloids | ||||
| Commipholactam A (27) | Antitumor | In vitro | Cytotoxicity against HepG2 (IC50 = 21.73 ± 2.86 μM) and A549 (IC50 = 128.50 ± 17.06 μM) cells | [28] |
| Diterpenoid alkaloids | ||||
| Geordine (103) | Anti-inflammatory | In vitro | Inhibition of NO production (29.75%) in LPS-induced RAW264.7 cells at 50 μM | [61] |
| Stylosine A (157) | In vitro | Inhibition of production of IL-1β, COX-2, and TNF-α in LPS-induced RAW264.7 cells in a dose-dependent manner | [74] | |
| Ajacisine F (121) | In vitro | Inhibition of NO production in LPS-induced BV-2 cells with inhibition rates of 80% at 50 μM | [70] | |
| Ajacisine G (123) | In vitro | [70] | ||
| Ajacisine H (214) | In vitro | [70] | ||
| Kamaonensine B (148) | In vitro | Inhibition of NO production in LPS-stimulated RAW264.7 cells (IC50 = 2.7 ± 0.5 and 0.9 ± 0.2 μM) and might be mediated by the regulation of some related proteins in the MAPK signaling pathways | [72] | |
| Kamaonensine F (153) | In vitro | [72] | ||
| Aconicumine A (170) | In vitro | Inhibition of LPS-activated NO production in RAW264.7 cells (IC50 = 19.7 ± 1.1 μM) | [77] | |
| Forrestline F (190) | In vitro | Inhibition of NO production in RAW264.7 cells (IC50 = 9.57 ± 1.34 μM) through inhibiting NF-κB, MAPK, and Nrf2 signaling pathways | [53] | |
| Anthoroidine B (277) | In vitro | Inhibition of the production of NO and TNF-α in LPS-stimulated RAW264.7 cells, with IC50 values of 357.68 and 67.56 μM | [84] | |
| Forsyqinlingine A (286) | Anti-inflammatory and antiviral | In vitro | Anti-inflammatory activities by inhibiting the release of β-glucuronidase in PMNs with inhibition rates of 56.7 and 58.6%, and antiviral activities against H1N1 virus (IC50 = 6.9 and 7.7 μM) and RSV (EC50 = 5.0 and 4.8 μM) | [103] |
| Forsyqinlingine B (287) | In vitro | [103] | ||
| Pseudostapine C (89) | Analgesic | In vivo | Reduction of acetic acid-induced abdominal contractions in mice in a dose-related manner, with the ID50 values of 66.1, 60.3, 48.0, and 55.0 μmol/kg, respectively | [51] |
| Austroyunnanine B (91) | In vivo | [45] | ||
| Episcopine A (180) | In vivo | [52] | ||
| Episcopaline B (182) | In vivo | [50] | ||
| Nagarumine C (176) | In vivo | Inhibition of acetic acid-induced writhing in mice (ED50 = 76.0 μmol/kg) | [46] | |
| Acosinomonine B (178) | In vitro | Strong inhibitory effect on the activation of the TRPV1 channel in HEK-293 cells mediated by capsaicin, with an inhibition rate of 31.78% at 10 μM | [79] | |
| Aconicatisulfonine A (259) | In vivo | Reduction in acetic acid-induced writhing in mice by 43.2%, 64.7%, 63.6%, and 19.3% at 0.3 mg/kg, respectively | [98] | |
| Aconicatisulfonine B (260) | In vivo | [98] | ||
| Aconapelsulfonine A (261) | In vivo | [99] | ||
| Aconapelsulfonine B (262) | In vivo | [99] | ||
| Aconicarmisulfonine B (263) | In vivo | Reduction in acetic acid-induced writhing in mice by 31.26%, 26.84%, and 43.8% at 1.0 mg/kg (i.p.) | [95] | |
| Aconicarmisulfonine C (264) | In vivo | [95] | ||
| Aconicarnine E (241) | In vivo | [93] | ||
| Aconidenusulfonine A (288) | In vivo | Reduction in acetic acid-induced writhing in mice by 26.35% at 2.0 mg/kg (i.p.) | [104] | |
| Lipojesaconitine (98) | Antitumor | In vitro | Cytotoxicity against A549, MDA-MB-231, MCF-7, and KB with IC50 values ranging from 6.0 to 7.3 μM | [57] |
| 8-O-ethyl-benzoyldeoxyaconine (107) | In vitro | Anticancer activity against A549 (IC50 = 12.58 ± 1.82 μM) and H460 (IC50 = 12.76 ± 2.10 μM) cells | [47] | |
| Brunonianine B (182) | In vitro | Cytotoxicity on Caco-2 (IC50 = 3.14 ± 0.37 μM) and Skov-3 (IC50 = 2.20 ± 0.21 μM) cells and activation of the Bax/Bcl-2/caspase-3 signaling pathway | [80] | |
| Brunonianine C (183) | In vitro | Cytotoxicity on Caco-2 (IC50 = 2.41 ± 0.35 μM) and Skov-3 (IC50 = 6.88 ± 0.81 μM) cells | [80] | |
| Ceylonamide G (281) | In vitro | Cytotoxic to DU145 cells (IC50 = 6.9 μM, MEC = 10 μM) | [101] | |
| Smirnotine A (94) | Cardioprotective | In vivo | Some preventive effects on aconitine-induced arrhythmia in mice | [58] |
| Gyalanunine A (253) | In vitro | Significant cardiotonic activity after perfusion in frog hearts and could be related to the β receptor | [89] | |
| Tanguticuline A (199) | Antiviral | In vitro | Inhibition of the cytopathic effect against H1N1 with IC50 values of 2.9 and 2.4 μg/mL, respectively | [85] |
| Tanguticuline E (203) | In vitro | [85] | ||
| Acoapetaludine D (45) | Antibacterial | In vitro | Anti-Helicobacter pylori (MIC = 100 μg/mL) | [40] |
| Acoapetaludine E (46) | In vitro | Anti-Helicobacter pylori (MIC = 50 μg/mL) | [40] | |
| Stylosine A (157) | In vitro | Anti-Staphylococcus aureus (MIC = 2.00 μg/mL) | [74] | |
| Stylosine B (158) | In vitro | Anti-Staphylococcus aureus (MIC = 32.00 μg/mL) | [74] | |
| Koninginol A (284) | In vitro | Anti-Bacillus subtilis (MIC = 10.00 μg/mL) | [102] | |
| Koninginol B (285) | In vitro | Anti-Bacillus subtilis (MIC = 2.00 μg/mL) | [102] | |
| 2-O-cinnamoyl hetisine (209) | Antiplasmodial | In vitro | Anti-Plasmodium falciparum strains Pf INDO (IC50 = 1.92 μM) and the Pf 3D7 (IC50 = 10.8 μM) | [88] |
| Apetalrine B (82) | Neuroprotective | In vitro | Neuroprotective activity (77.4%) on H2O2-induced SH-SY5Y cell | [55] |
| Uncinatine-A (120) | AChEI activity | In vitro | Acetyl-cholinesterase inhibitory activity (IC50 = 207.73 ± 0.3 μM | [66] |
| Anthoroidine G (204) | In vitro | Acetyl-cholinesterase inhibitory activity (IC50 = 6.3 ± 1.6 μM) | [84] | |
| Anthoroidine I (206) | In vitro | Acetyl-cholinesterase inhibitory activity (IC50 = 9.3 ± 3 μM) | [84] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Xin, J.; Sun, L.; Sun, Y.; Xu, Y.; Zhao, F.; Niu, C.; Liu, S. Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities. Molecules 2024, 29, 1968. https://doi.org/10.3390/molecules29091968
Wang X, Xin J, Sun L, Sun Y, Xu Y, Zhao F, Niu C, Liu S. Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities. Molecules. 2024; 29(9):1968. https://doi.org/10.3390/molecules29091968
Chicago/Turabian StyleWang, Xuyan, Jianzeng Xin, Lili Sun, Yupei Sun, Yaxi Xu, Feng Zhao, Changshan Niu, and Sheng Liu. 2024. "Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities" Molecules 29, no. 9: 1968. https://doi.org/10.3390/molecules29091968
APA StyleWang, X., Xin, J., Sun, L., Sun, Y., Xu, Y., Zhao, F., Niu, C., & Liu, S. (2024). Exploring the Biomedical Potential of Terpenoid Alkaloids: Sources, Structures, and Activities. Molecules, 29(9), 1968. https://doi.org/10.3390/molecules29091968







