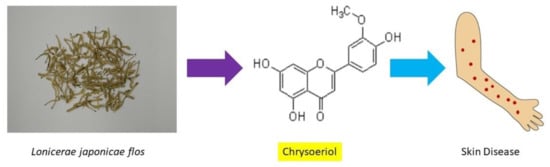
Pharmacological Activities of Lonicerae japonicae flos and Its Derivative—“Chrysoeriol” in Skin Diseases
Abstract
:1. Introduction
2. Lonicerae japonicae flos
2.1. Sources
2.2. Macroscopic Features
2.3. Identification of Lonicerae japonicae flos
3. Traditional Chinese Medicine Theory
| Chinese Medicinal Herb (CMH) | Metabolite (s) | Pharmacological Function (s) | Model/Dosage | Consequence | Reference |
|---|---|---|---|---|---|
| Lonicerae japonicae flos. | Flavonoids, phenolic compounds (polyphenolic). | Anti-inflammatory. | RAW264.7 cells; 2.5, 5 and 10 μg/mL (water extract). | Reduce the expression of proinflammatory mediators and inflammatory cytokines, such as cyclooxygenase inhibitors-2 and inducible nitric oxide synthase, through the suppression of the Janus kinase/signal transducers and activators of transcription-3-dependent Nuclear factor kappa-light-chain-enhancer of activated B cells pathway and the induction of Heme oxygenase-1 expression in Pseudorabies virus-infected RAW264.7 cells. | [27] |
| Chlorogenic acid. | Anti-inflammatory. | Human neutrophils; 3, 10, and 30 μg/mL (ethanol extract). | Attenuates inflammatory reactions in the activated neutrophils, including superoxide anion generation, release of elastase, CD11b expression, chemotactic migration, cell adhesion, and neutrophil extracellular trap formation. | [28] | |
| Flavonoid (Loniceralanside A). | Anti-inflammatory. | Rat; 3.05 µM (ethanol extract). | Inhibits the release of β-glucuronidase induced by platelet-activating factor in rat polymorphonuclear leukocytes. | [29] | |
| Flavonoids, iridoids, triterpenoids, organic acids. | Anti-inflammatory, antioxidant. | C57BL/6 mice; 12.5, 25, and 50 mg/mL (water extract). | Relieve pressure-overload-induced heart failure following transverse aortic constriction, through increased heart antioxidant defense systems. | [30] | |
| Flavonoids, iridoids, triterpenoids, organic acids. | Anti-inflammatory. | BV-2 microglial cells; 0.5, 5, 2.5, 5, and 10 μg/mL (water extract). | Prevent lipopolysaccharide-induced activation of Nuclear factor kappa-light-chain-enhancer of activated B cells localization, and consequently reduce lipopolysaccharide-induced DNA–protein-binding activity of Nuclear factor kappa-light-chain-enhancer of activated B cells, leading to downregulation of proinflammatory mediators. | [31] | |
| Chlorogenic acid. | Anti-inflammatory. | Male Wistar rats; 231 μg/mL (water extract). | Suppresses the induction of nitric oxide production and nitric oxide synthase expression, which may have therapeutic potential for inflammatory diseases, including liver injury. | [32] | |
| Flavonoids, phenolic compounds (polyphenolic). | Anti-inflammatory, antioxidant. | HaCaT cells; 0.1, 0.25, 0.5, 0.75, 1, 1.25, 2, 2.5, 5, 7.5, and 10 µg/mL (methanol extract). | Polyphenolic compounds with antioxidant and anti-inflammatory effects since their molecular structural binding or affinity are suggested for various inflammation pathways. | [33] | |
| Flavonoids, iridoids, triterpenoids, organic acids. | Anti-inflammatory, antioxidant. | HaCaT cells; 0.1, 0.25 or 0.5 mg/mL (ethanol extract). | Exhibit protective effects on HaCaT cells against H2O2-induced oxidative stress through reactive oxygen species release, and inhibit skin damage against oxidative stress. | [34] |
4. Extraction Techniques
4.1. Solvent (Liquid–Liquid) Extraction
4.2. Maceration
4.3. Reflux and Soxhlet
4.4. Ultrasonic-Assisted Extraction (UAE)
| Active Ingredient | Solvent/Temperature/ Time Duration | Advantages/Disadvantages | References | |
|---|---|---|---|---|
| Solvent (liquid–liquid) extraction. | Chrysoeriol. | Water or ethanol–water, 40 to 80 °C, 15 to 35 min. | Low equipment cost, wide extraction range, and simple operation. Time-consuming, compatibility issues, and potential contamination or cross-talk. | [41] |
| Maceration. | Chrysoeriol. | Methanol/ethanol, room temperature, several days or a few weeks at least. | Simple process, and no heat involved, suitability for thermal sensitive flavonoid. Low extraction yield, use of large volumes of solvents, long processing time, and further purification steps are required. | [42] |
| Reflux and Soxhlet. | Chrysoeriol. | Ethanol, boiling point of a solvent, 2 to 48 h. | High extraction efficiency. Long extraction time and consumption of large amounts of used solvents. | [43] |
| Ultrasonic-assisted extraction (UAE). | Chrysoeriol. | Ethanol, ultrasonic cleaning bath at 40 kHz, 40 to 60 °C, 10 to 60 min. | High efficiency and reduced extraction time. Energy and solvent consumption, present low extraction yields. | [44] |
4.5. Example for Multistep Extraction of Chrysoeriol from Lonicerae japonicae flos
- (i)
- Pulverize Lonicerae japonicae flos, and use the ultrasonic extraction for 0.5–3 h, then filter and concentrate to neutral with acid for adjusting pH. Suspension liquid is produced through precipitation by adding distilled water;
- (ii)
- Add ethyl acetate for extraction, and combine the extraction liquid after the suspension liquid is added to chloroform extraction;
- (iii)
- The extracted liquid then undergoes microfiltration, ultrafiltration, and nanofiltration successively in the multifunctional membrane separating device;
- (iv)
- Wash the extractant with deionized water until colorless, discard the water portion, and use the 30% aqueous ethanolic solution gradient elution again, from 10% and incremented to 90%, then collect elutriant;
- (v)
- Evaporate the ethanol, concentrate, and dry with 50% methanol. The extractant is cooled and stands overnight for crystallization to afford the chrysoeriol crude product;
- (vi)
- Repeat the extraction steps by using ethyl acetate, methanol, acetone, and chloroform or recrystallization again to obtain the pure product of chrysoeriol.
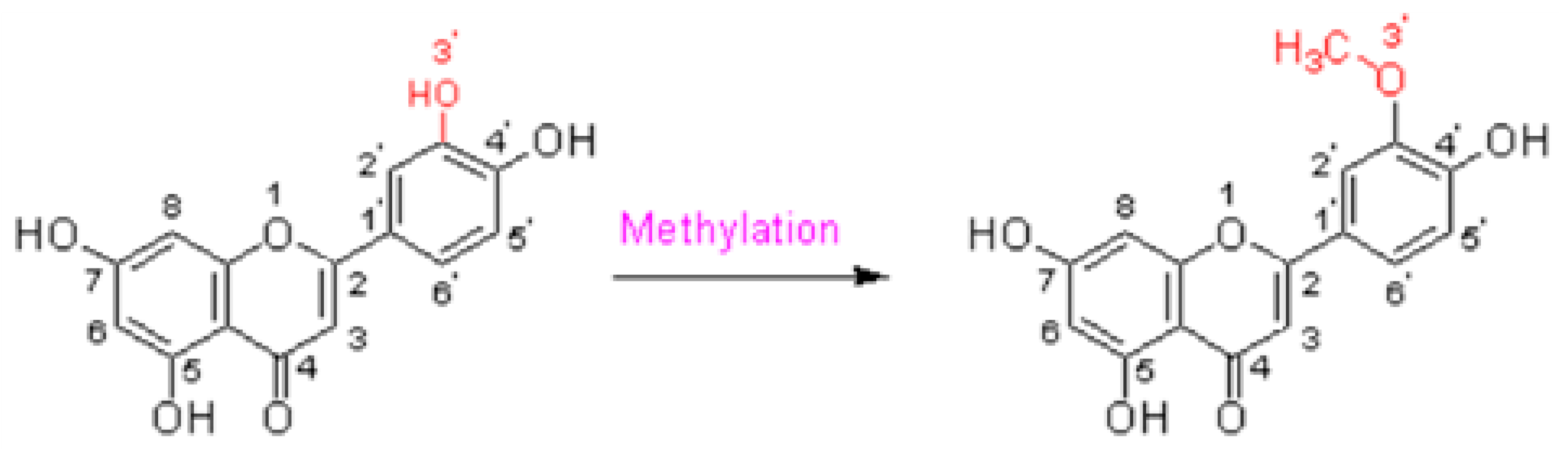
5. Chrysoeriol
5.1. Source
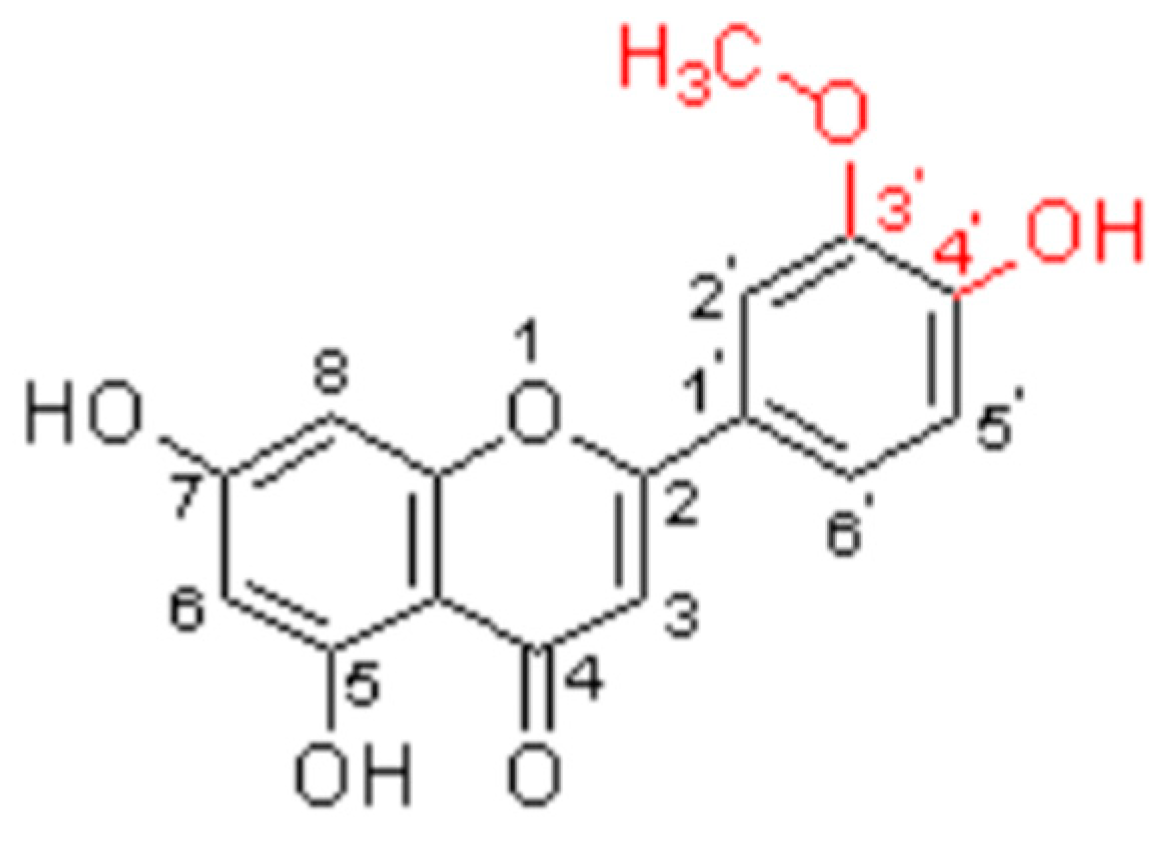
5.2. Structure
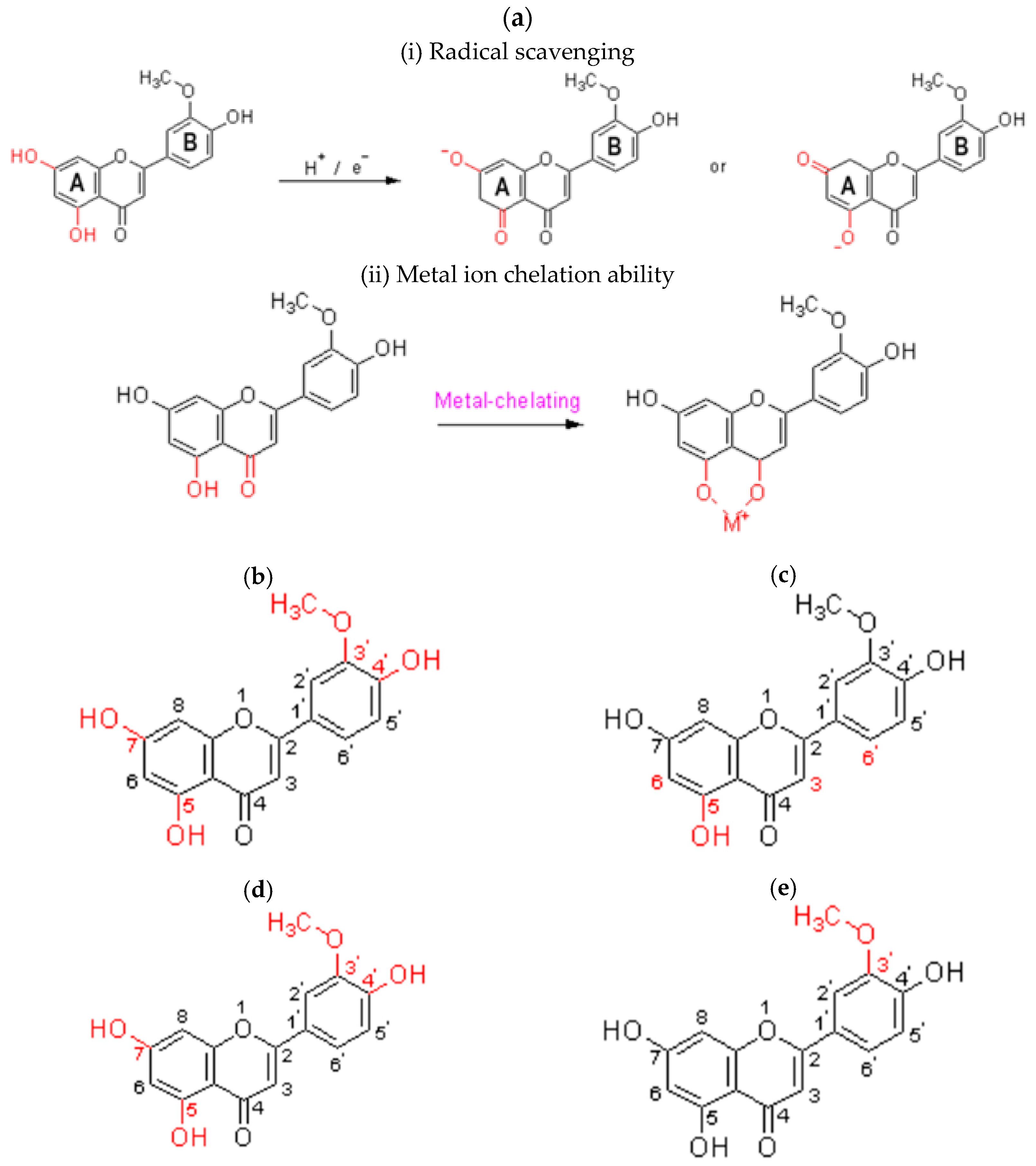
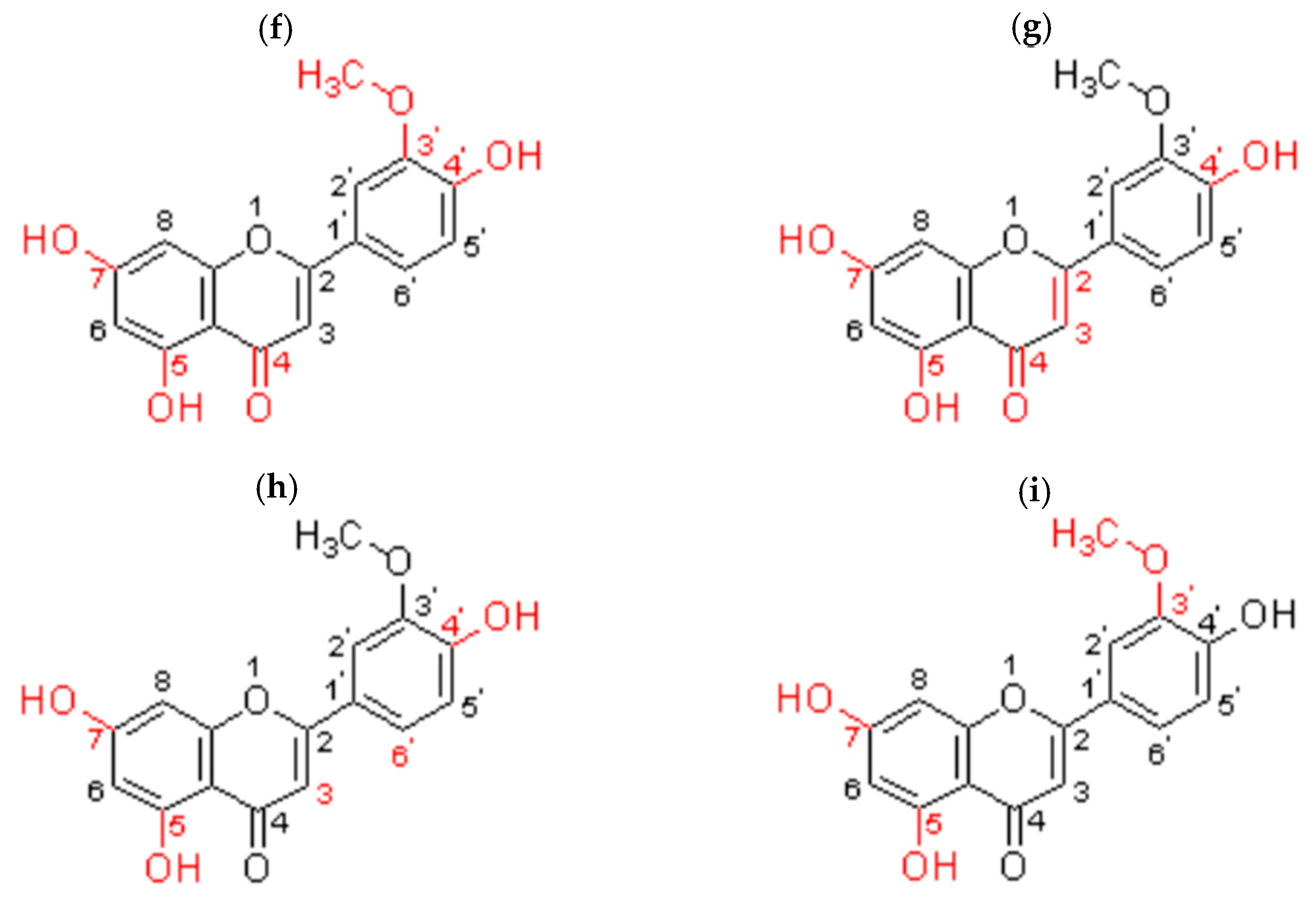
5.3. Structure–Activity Relationship
5.3.1. Antioxidant
5.3.2. Anti-Inflammatory
5.3.3. Anticancer
5.3.4. Antidiabetic
5.3.5. Antiarthritis
5.3.6. Antimicrobial
5.3.7. Antithrombotic
5.3.8. Antihyperlipidemic
5.3.9. Antinociceptive
5.4. Pharmacological Functions
5.5. Pharmacokinetic and Pharmacodynamic Effects
6. Cutaneous Delivery System
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADME | Absorption, distribution, metabolism, and excretion |
| CMH | Chinese medicinal herb |
| ChP | Chinese Pharmacopoeia |
| DPP-4 | Dipeptidyl peptidase IV |
| ERK1/2 | Extracellular signal-regulated protein kinases 1 and 2 |
| FCE | Flos chrysanthemi extract |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| HO-1 | Heme oxygenase-1 |
| IL-6 | Interleukin-6 |
| IL-7 | Interleukin-7 |
| IκB | IκB kinase |
| JAK2 | Janus kinase 2 |
| JAK/STAT/3 | Janus kinase/signal transducers and activators of transcription-3 |
| LC3II | LC3-phosphatidylethanolamine conjugate |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| 65 kDa | p65 |
| PI3K | Phosphatidylinositol 3-kinase |
| PAF | Platelet-activating factor |
| PDGFRß | Platelet-derived growth factor receptor beta |
| PMN | Polymorphonuclear leukocyte |
| PKA | Protein kinase A |
| AKT | Protein kinase B |
| PRV | Pseudorabies virus |
| ROS | Reactive oxygen species |
| STAT3 | Signal transducers and activators of transcription 3 |
| TPA | Tissue plasminogen activator |
| TCM | Traditional Chinese medicine |
References
- Li, Y.; Li, W.; Fu, C.; Song, Y.; Fu, Q. Lonicerae japonicae flos and Lonicerae flos: A systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2020, 19, 1–61. [Google Scholar] [CrossRef]
- Li, Y.; Cai, W.; Weng, X.; Li, Q.; Wang, Y.; Chen, Y.; Zhang, W.; Yang, Q.; Guo, Y.; Zhu, X.; et al. Lonicerae japonicae flos and Lonicerae Flos: A Systematic Pharmacology Review. Evid. Based Complement. Alternat. Med. 2015, 2015, 905063. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Q.; Hu, J.; Zhang, Y.; Li, J. Research Progress on Chemical Constituents of Lonicerae japonicae flos. BioMed Res. Int. 2016, 2016, 8968940. [Google Scholar]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. EADV burden of skin diseases project team. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.C. Acne and other facial eruptions. Medicine 1997, 25, 30–33. [Google Scholar]
- Ayer, J.; Burrows, N. Acne: More than skin deep. Postgrad. Med. J. 2006, 82, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.P.; Madke, B. The Current Advancement in Psoriosis. Cureus 2023, 15, e47006. [Google Scholar]
- Reid, C.; Griffiths, C.E.M. Psoriasis and Treatment: Past, Present and Future Aspects. Acta Derm. Venereol. 2020, 100, adv00032. [Google Scholar] [CrossRef]
- Guo, S.H.; Li, P. Research progress of eczema in the external treatment. J. Liaoning Univ. Tradit. Chin. Med. 2019, 21, 180–183. [Google Scholar]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar]
- Liu, L.; Zhao, H.; Sun, X.; Zheng, Q.; Luo, Y.; Ru, Y.; Zhang, Y.; Chen, X.; Zhu, B.; Yin, C.; et al. Efficacy and safety of Tripterygium wilfordii hook F for chronic urticaria: A systematic review and meta-analysis. BMC Complement. Altern. Med. 2018, 18, 243. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Arain, S. Traditional Chinese medicine for the treatment of dermatologic disorders. Arch. Dermatol. 1998, 134, 1388–1393. [Google Scholar] [CrossRef]
- Wang, Y.F.; Que, H.F.; Wang, Y.J.; Cui, X.J. Chinese herbal medicines for treating skin and soft-tissue infections. Cochrane Database Syst. Rev. 2014, 2014, CD010619. [Google Scholar] [CrossRef]
- Chen, H.Y.; Lin, Y.H.; Hu, S.; Yang, S.H.; Chen, J.L.; Chen, Y.C. Identifying chinese herbal medicine network for eczema: Implications from a nationwide prescription database. Evid. Based Complement. Alternat Med. 2015, 2015, 347164. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, L.; Zhong, T.; Mo, X.; Wang, D.; Gu, J.; Chen, D.; Zeng, X.; Yan, F. Gu-Ben-Hua-Shi (AESS) formula ameliorates atopic dermatitis via regulating NLRP3 signaling pathways. Saudi Pharm. J. 2023, 31, 101792. [Google Scholar] [CrossRef] [PubMed]
- Aboulaghras, S.; Sahib, N.; Bakrim, S.; Benali, T.; Charfi, S.; Guaouguaou, F.E.; Omari, N.E.; Gallo, M.; Montesano, D.; Zengin, G.; et al. Health Benefits and Pharmacological Aspects of Chrysoeriol. Pharmaceuticals 2022, 15, 973. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, L.Q.; Li, C.X.; Li, J.; Zhang, R.X. Literature study on species of honeysuckle flower. Zhongguo Zhong Yao Za Zhi 2014, 39, 2239–2245. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2020.
- Gao, H.; Lin, Y. The Yellow Emperor’s Classic of Internal Medicine; People’s Health Publisher: Beijing, China, 1963; p. 7. [Google Scholar]
- Cheung, T. The difference and similarity between traditional chinese and western medicine. Chin. J. Integr. Tradit. West. Med. 2000, 6, 68–70. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.L. On the concept of health in traditional Chinese medicine and its characteristics and advantages. Zhonghua Yi Shi Za Zhi 2010, 40, 13–14. [Google Scholar]
- Guo, Y.P.; Lin, L.G.; Wang, Y.T. Chemistry and pharmacology of the herb pair Flos Lonicerae japonicae-Forsythiae fructus. Chin. Med. 2015, 10, 16. [Google Scholar] [CrossRef]
- Lin, X.; Tu, C.; Yang, C. Study on treatment of eczema by Chinese herbal medicine with anti-type IV allergic activity. Zhongguo Zhong Xi Yi Jie He Za Zhi 2000, 20, 258–260. [Google Scholar]
- De Luna, S.L.; Ramírez-Garza, R.E.; Saldívar, S.O.S. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Kim, H.W.; Seong, J.K.; Ahn, M.; Park, K.I.; Kim, G.S. Antioxidant effects of phenolic compounds in through the distillation of Lonicera japonica & Chenpi extract and anti-inflammation on skin keratinocyte. Sci. Rep. 2023, 13, 20883. [Google Scholar] [PubMed]
- Lin, H.W.; Lee, Y.J.; Yang, D.J.; Hsieh, M.C.; Chen, C.C.; Hsu, W.L.; Chang, Y.Y.; Liu, C.W. Anti-inflammatory effects of Flos Lonicerae japonicae Water Extract are regulated by the STAT/NF-κB pathway and HO-1 expression in Virus-infected RAW264.7 cells. Int. J. Med. Sci. 2021, 18, 2285–2293. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.H.; Chen, Y.L.; Lin, M.F.; El-Shazly, M.; Chang, Y.C.; Chen, P.J.; Su, C.H.; Chiu, Y.C.; Illias, A.M.; Chen, C.C.; et al. Lonicerae japonicae flos Attenuates Neutrophilic Inflammation by Inhibiting Oxidative Stress. Antioxidants 2022, 11, 1781. [Google Scholar] [CrossRef]
- Yang, R.; Fang, L.; Li, J.; Zhang, Y.Q. A new anti-inflammatory lignan from Lonicerae japonicae flos. Nat. Prod. Res. 2021, 35, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Jang, W.S.; Baek, K.M. Protective Effect of Lonicerae Flos Aqueous Extracts on a Pressure Overload-induced Heart Failure Model. J. Int. Korean Med. 2017, 38, 877–890. [Google Scholar] [CrossRef]
- Kwon, S.H.; Ma, S.X.; Hong, S.I.; Lee, S.Y.; Jang, C.G. Lonicera japonica THUNB. Extract Inhibits Lipopolysaccharide-Stimulated Inflammatory Responses by Suppressing NF-κB Signaling in BV-2 Microglial Cells. J. Med. Food 2015, 18, 762–775. [Google Scholar] [CrossRef]
- Ohno, N.; Yoshigai, E.; Okuyama, T.; Yamamoto, Y.; Okumura, T.; Sato, K.; Ikeya, Y.; Nishizawa, M. Chlorogenic acid from the Japanese herbal medicine Kinginka (Flos Lonicerae japonicae) suppresses the expression of inducible nitric oxide synthase in rat hepatocytes. HOAJ Biol. 2012, 1, 2. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Won, C.K.; Park, K.L.L.; Kim, E.; Heo, J.D.; Kim, H.W.; Ahn, M.; et al. Potential Antioxidant and Anti-Inflammatory Effects of Lonicera japonica and Citri Reticulatae Pericarpium Polyphenolic Extract (LCPE). Free. Libr. 2023, 12, 1582. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Choi, M.O. Protectvie effects of Lonicerae japonicae flos against hydrogen peroxidase-induced oxidative stress on Human keratinocyte, HaCaT cells. Korean J. Med. Hist. 2013, 28, 57–62. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Marston, A.; Hostettmann, K. Separation and quantification of flavonoids. In Flavonoids: Chemistry, Biochemistry and Applications; Andersen, O.M., Markham, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–32. [Google Scholar]
- Damián-Reyna, A.A.; González-Hernández, J.C.; Chávez-Parga, M.C. Current procedures for extraction and purification of citrus flavonoids. Rev. Colomb. Biotecnol. 2016, 18, 135–147. [Google Scholar]
- Vongsak, B.; Sithisarn, P.; Mangmool, S.; Thongpraditchote, S.; Wongkrajang, Y.; Gritsanapan, W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera leaf extract by the appropriate extraction method. Ind. Crops Prod. 2013, 44, 566–571. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids from Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave-assisted extraction (MAE) of functional compounds from plant materials. Trends Analyt. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Pham, T.N.; Nguyen, T.N.P.; Lam, T.D.; Tran, T.H.; Nguyen, D.C.; Le, X.T.; Do, S.T.; Bach, L.G. Effects of various solvent concentration, liquidsolid ratio, temperatures and time values on the extraction yield of anthocyanin from Vietnam Hibiscus sabdariffa L. (Roselle). IOP Conf. Ser. Mater. Sci. Eng. 2019, 542, 012033. [Google Scholar]
- Subramanian, P.; Anandharamakrishnan, C. Extraction, Processing and Formulation of Bioactive Compounds. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals; Elsevier: New York, NY, USA, 2023; pp. 45–87. [Google Scholar]
- Ammar, I.; Ennouri, M.; Attia, H. Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind. Crops Prod. 2015, 64, 97–104. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of Ultrasonic-Assisted Extraction of Flavonoid Compounds and Antioxidants from Alfalfa Using Response Surface Method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef]
- Liu, D.F.; Guo, Q.; Yang, C.D. Preparation Method of Chrysoeriol. Nanjing Zelang Medical Technology Co., Ltd., 2010. Available online: https://patents.google.com/patent/CN101973974A/en (accessed on 8 March 2024).
- Yao, L.H.; Jiang, Y.M.; Shi, S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Blumenthal, D.K.; Terry, C.M.; He, Y.; Carlson, M.L.; Cheung, A.K. PDGF-induced proliferation in human arterial and venous smooth muscle cells: Molecular basis for differential effects of PDGF isoforms. J. Cell. Biochem. 2011, 112, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.Y.; Shi, W.L.; Yonezawa, T.; Teruya, T.; Nagai, K.; Woo, J.T. An inhibitory effect of chrysoeriol on platelet-derived growth factor (PDGF)-induced proliferation and PDGF receptor signaling in human aortic smooth muscle cells. J. Pharmacol. Sci. 2009, 110, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yuwen, M.; Pu, Z.; Zhao, Z.; Yu, H.; Zha, J. Engineering of flavonoid 3’-O-methyltransferase for improved biosynthesis of chrysoeriol in Escherichia coli. Appl. Microbiol. Biotechnol. 2023, 107, 1663–1672. [Google Scholar] [CrossRef]
- Kelly, E.H.; Anthony, R.T.; Dennis, J.B. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar]
- Pandey, A.K.; Mishra, A.K.; Mishra, A. Antifungal and antioxidative potential of oil and extracts derived from leaves of Indian spice plant Cinnamomum tamala. Cell. Mol. Biol. 2012, 58, 142–147. [Google Scholar] [PubMed]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; Tromp, M.N.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure-Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- López-Lázaro, M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anticancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef]
- Trovato, L.; Calvo, M.; De Pasquale, R.; Scalia, G.; Oliveri, S. Prevalence of Onychomycosis in Diabetic Patients: A Case-Control Study Performed at University Hospital Policlinico in Catania. J. Fungi 2022, 8, 922. [Google Scholar] [CrossRef] [PubMed]
- Mentlein, R. Dipeptidyl-peptidase IV (CD26)-role in the inactivation of regulatory peptides. Regul. Pept. 1999, 85, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Nauck, M.A.; Meier, J.; Hucking, K.; Holst, J.J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 2000, 85, 3575–3581. [Google Scholar] [PubMed]
- Semighini, E.P.; Resende, J.A.; Andrade, P.D. Using computer-aided drug design and medicinal chemistry strategies in the fight against diabetes. J. Biomol. Struct. Dyn. 2011, 28, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Jadav, P.; Bahekar, R.; Shah, S.R. Long-acting peptidomimetics based DPP-IV inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 3516–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, J.; Tu, Q.; Yan, F.; Hu, Z.; Zhang, Y.; Song, C. Antioxidant Capacities and Enzymatic Inhibitory Effects of Different Solvent Fractions and Major Flavones from Celery Seeds Produced in Different Geographic Areas in China. Antioxidants 2022, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, D.Y.; Chao, Y.H.; Chen, Y.M.; Wu, C.L.; Lai, K.L.; Lin, C.H.; Lin, C.C. Acarbose Decreases the Rheumatoid Arthritis Risk of Diabetic Patients and Attenuates the Incidence and Severity of Collagen-induced Arthritis in Mice. Sci. Rep. 2015, 5, 18288. [Google Scholar] [CrossRef] [PubMed]
- Bashyal, P.; Parajuli, P.; Pandey, R.P.; Sohng, J.K. Microbial Biosynthesis of Antibacterial Chrysoeriol in Recombinant Escherichia coli and Bioactivity Assessment. Catalysts 2019, 9, 112. [Google Scholar] [CrossRef]
- Tavares, L. d C.; Zanon, G.; Weber, A.D.; Neto, A.T.; Mostardeiro, C.P.; Da Cruz, I.B.; Oliveira, R.M.; Ilha, V.; Dalcol, I.I.; Morel, A.F. Structure-activity relationship of benzophenanthridine alkaloids from Zanthoxylum rhoifolium having antimicrobial activity. PLoS ONE 2014, 9, e97000. [Google Scholar] [CrossRef]
- Nastałek, M.; Wojas-Pelc, A.; Undas, A. Plasma fibrin clot properties in atopic dermatitis: Links between thrombosis and atopy. J. Thromb. Thrombolysis 2010, 30, 121–126. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Su, F.; Li, J.; Boadi, E.O.; Chang, Y.X.; Wang, H. Study on Structure Activity Relationship of Natural Flavonoids against Thrombin by Molecular Docking Virtual Screening Combined with Activity Evaluation In Vitro. Molecules 2020, 25, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Jiang, J.G. Active ingredients of traditional Chinese medicine in the treatment of diabetes and diabetic complications. Expert Opin. Investig. Drugs 2012, 21, 1625–1642. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Kou, X.; Wang, Y.; Yu, Y.; Zhen, N.; Jiang, J.; Zhaxi, P.; Xue, Z. Synergistic Hypolipidemic Effects and Mechanisms of Phytochemicals: A Review. Foods 2022, 11, 2774. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, V.; Fernandes, L.; Conde, T.; Zamith, H.; Silva, R.; Surrage, A.; Frutuoso, V.; Castro-Faria-Neto, H.; Amendoeira, F. Antinociceptive activity of Stephanolepis hispidus skin aqueous extract depends partly on opioid system activation. Mar. Drugs 2013, 11, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Tatli, I.I.; Akdemir, Z.S.; Yesilada, E.; Küpeli, E. Anti-inflammatory and antinociceptive potential of major phenolics from Verbascum salviifolium Boiss. Z. Naturforsch C J. Biosci. 2008, 63, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Chen, Y.J.; Bai, L.; Liu, Y.X.; Fu, X.Q.; Zhu, P.L.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Wang, Y.P.; et al. Chrysoeriol ameliorates TPA-induced acute skin inflammation in mice and inhibits NF-κB and STAT3 pathways. Phytomedicine 2020, 68, 153173. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Ruan, H.; Wang, Y. In vitro and in vivo cytotoxic effects of chrysoeriol in human lung carcinoma are facilitated through activation of autophagy, sub-G1/G0 cell cycle arrest, cell migration and invasion inhibition and modulation of MAPK/ERK signalling pathway. J. BUON 2019, 24, 936–942. [Google Scholar] [PubMed]
- Boro, A.; Shanmugam, R.; Latha, A.S.; Rajan, A.P.; Al-Dhabi, N.A.; Mariadhas, V.A.; Arumugam, V.A.; Balasubramanian, B. Chrysoeriol: Derivatives, Resources, Biosynthetic Pathway, Bioavailability, and Bioactivities. In Handbook of Dietary Flavonoids; Xiao, J., Ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Wu, J.Y.; Chen, Y.J.; Fu, X.Q.; Li, J.K.; Chou, J.Y.; Yin, C.L.; Bai, J.X.; Wu, Y.; Wang, X.Q.; Li, A.S.; et al. Chrysoeriol suppresses hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes and inhibits JAK2/STAT3 signaling. BMC Complement. Med. Ther. 2022, 22, 73. [Google Scholar] [CrossRef]
- Jang, Y.H.; Park, J.R.; Kim, K.M. Antimicrobial Activity of Chrysoeriol 7 and Chochlioquinone 9, White-Backed Planthopper-Resistant Compounds, Against Rice Pathogenic Strains. Biology 2020, 9, 382. [Google Scholar] [CrossRef]
- Zouheira, D.; Ngnokam, S.L.W.; Kamani, S.L.P.; Tchegnitegni, B.T.; Jouda, J.B.; Mba, J.R.; Nchouwet, M.L.; Nfor, N.G.; Nyirimigabo, A.K.; Kowa, T.K.; et al. In Vitro Antilipidic and Antithrombotic Activities of Plectranthus glandulosus (Lamiaceae) Leaves Extracts and Fractions. BioMed Res. Int. 2022, 2022, 4145659. [Google Scholar] [CrossRef]
- Shrivastava, A.K.; Shrestha, L.; Pokhrel, B.R.; Joshi, B.; Lamichhane, G.; Vidović, B.; Koirala, N. LC-MS based metabolite profiling, in-vitro antioxidant and in-vivo antihyperlipidemic activity of Nigella sativa extract. eFood 2023, 4, e107. [Google Scholar] [CrossRef]
- Jaffal, S.M.; Al-Najjar, B.O.; Abbas, M.A.; Oran, S.A. Antinociceptive Action of Moringa peregrina is Mediated by an Interaction with α2-Adrenergic Receptor. Balkan Med. J. 2020, 37, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Zia-Amirhosseini, P. Basic principles of pharmacokinetics. Toxicol. Pathol. 1995, 23, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Prasain, J.K.; Barnes, S. Metabolism and bioavailability of flavonoids in chemoprevention: Current analytical strategies and future prospectus. Mol. Pharm. 2007, 4, 846–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kong, S.; Song, F.; Li, L.; Jiang, H. Pharmacokinetic study of luteolin, apigenin, chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract in rats. Fitoterapia 2012, 83, 1616–1622. [Google Scholar] [CrossRef]
- Liu, Y.; Han, C.; Lu, T.; Liu, Y.; Chen, H.; Yang, C.; Tu, Y.; Li, Y. Investigation of the interaction between Chrysoeriol and xanthine oxidase using computational and in vitro approaches. Int. J. Biol. Macromol. 2021, 190, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Jamal, Z.; Zito, P.M. Pharmacodynamics. 2023 Jan 29. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Salahudeen, M.S.; Nishtala, P.S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharm. J. 2017, 25, 165–175. [Google Scholar] [CrossRef]
- Domaszewska-Szostek, A.; Puzianowska-Kuźnicka, M.; Kuryłowicz, A. Flavonoids in Skin Senescence Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 6814. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Costa Lima, S.A.; Gameiro, P.; Reis, S. On the Development of a Cutaneous Flavonoid Delivery System: Advances and Limitations. Antioxidants 2021, 10, 1376. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S.J. Recent advances in topical delivery of flavonoids: A review. Control. Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Lai, S.; Chen, J.N.; Huang, H.W.; Zhang, X.Y.; Jiang, H.L.; Li, W.; Wang, P.L.; Wang, J.; Liu, F.N. Structure activity relationships of chrysoeriol and analogs as dual c-Met and VEGFR2 tyrosine kinase inhibitors. Oncol. Rep. 2018, 40, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Rikala, M.S.; Rys, J.; Lasota, J.; Wang, Z.F. Vascular endothelial growth factor receptor 2 as a marker for malignant vascular tumors and mesothelioma: An immunohistochemical study of 262 vascular endothelial and 1640 nonvascular tumors. Am. J. Surg. Pathol. 2012, 36, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Hyun, C.G. Chrysoeriol Enhances Melanogenesis in B16F10 Cells Through the Modulation of the MAPK, AKT, PKA, and Wnt/β-Catenin Signaling Pathways. Nat. Prod. Commun. 2022, 17, 1934578X211069204. [Google Scholar] [CrossRef]

| Flavonoids | (1) quercetin, (2) rutin, (3) luteolin-7-O-β-d-glucopyranoside, (4) kaempferol-3-O-β-d-glucopyranoside, (5) apigenin-7-O-α-l-rhamnopyranoside, (6) chrysoeriol-7-O-β-d-glucopyranosyl, (7) luteolin-3′-l-rhamnoside, (8) luteolin, (9) flavoyadorinin-B, (10) rhoifolin, (11) quercetin-3-O-β-d-glucopyranoside, (12) 3′-methoxy luteolin, (13) 5,3′-dimethoxy luteolin, (14) luteolin-5-O-β-d-glucopyranoside, (15) apigenin, (16) isorhamnetin-3-O-β-d-glucopyranoside, (17) hyperoside, (18) quercetin-7-O-β-d-glucopyranoside, (19) kaempferol-3-O-β-d-rutinoside, (20) isorhamnetin-3-O-β-d-rutinoside, (21) 5-hydroxyl-3′,4′,7-trimethoxy flavone, (22) 5-hydroxyl-6,7,8,4′-tetramethoxy flavone, (23) corymbosin, (24) 5-hydroxyl-7,4′-dimethoxy flavone, (25) lonicerin, (26) 5,7,3′,4′,5′-pentamethoxy flavone, and (27) 5,4′-dihydroxy-3′,5′-dimethoxy-7-β-d-glucoxy-flavone |
| Iridoids | Consist of iridoid glucosides, secoiridoid glycosides, and N-contained iridoid glycosides.iridoid glucosides: (1) loganin, (2) 8-epiloganin, (3) loganic acid, (4) 8-epiloganic acid, and (5) ketologanin. secoiridoid glycosides: (6) secologanin, (7) secologanoside, (8) secoxyloganin, (9) secologanin dimethyl acetal, (10) secologanoside-7-methyl ester, (11) secologanic acid, (12) sweroside, (13) 7-O-ethylsweroside, vogeloside, (14) 7-epi-vogeloside, secoxyloganin-7-butyl ester, (15) kingiside, (16) 8-epikingiside, (17) 7α-morroniside, (18) 7β-morroniside, (19) dehydromorroniside, (20) 7-hydroxy-methyl-vogeloside, (21) (Z)-aldosecologanin, (22) (E)-aldosecologanin, (23) loniaceticiridoside, (24) lonimalondialiridoside, (25) 6′-O-acetylvogeloside, (26) 6′-O-acetylsecoxyloganin, (27) loniceracetalide A, (28) loniceracetalide B, (29) adinoside A, (30) stryspinoside, (31) secologanoside A, (32) dimethyl secologanoside, (33–36) loniphenyruviridoside A~D, (37) centauroside, (38) loniceranan A, (39) loniceranan B, (40) loniceranan C, (41) ethyl secologanoside, (42) demethylsecologanol, (43) harpagide, (44) harpagoside, (45) 6′′-O-β-glucopyranosylharpagoside, (46) (7β)-7-O-methyl morroniside, (47) lonicerjaponin A, and (48) lonicerjaponin B. N-contained iridoid glycosides: (49) serinosecologanin, (50) threoninosecologanin, (51) lonijaponinicotinosides A, (52) lonijaponinicotinosides B, (53) lonijapospiroside A, (54) L-phenylalaninosecologanin B, (55) L-phenylalaninosecologanin C, (56) dehydroprolinoylloganin A, (57–59) lonijaposides A-C, (60–70) lonijaposides D-N, and (71–83) lonijaposides O-W. |
| Triterpenoids | (1) limonin, (2) ursolic acid, (3) oleanolic acid triterpenoid saponins, (4) hederagenin triterpenoid saponins, (5) oleanolic acid, 3-O-β-d-glucopyranosyl-(12)-α-l-arabinopyranosyl oleanolic acid-28-O-β-d-glucopyranosyl-(16)-β-d-glucopyranoside, (6) oleanolic acid 28-O-α-l-rhamnopyranosyl-(12)-[β-D-xylopyranosyl(16)]-β-d-glucopyranosyl ester, (7) loniceroside E, hederagenin 3-O-α-l-arabinopyranoside, (8) loniceroside D, (9) loniceroside A, (10) loniceroside B, (11) loniceroside C, (12) 3-O-β-d-glucopyranosyl(14)-β-d-glucopyranosyl(13)-α-l-rhamnopyranosyl(12)-α-l-arabinopyranosyl-hederagenin-28-O-β-d-glucopyranosyl(16)-β-d-glucopyranosyl ester, (13) hederagenin-3-O-α-l-rhamnopyranosyl(12)-α-l-arabinopyranoside, (14) 3-O-α-l-rhamnopyranosyl(12)-α-l-arabinopyranosyl-hederagenin-28-O-β-d-xylopyranosyl(16)-β-d-glucopyranosyl ester, (15) 3-O-α-l-rhamnopyranosyl(12)-α-l-arabinopyranosyl-hederagenin-28-O-β-d-glucopyranosyl(16)-β-d-glucopyranosyl ester, (16) 3-O-α-l-rhamnopyranosyl(12)-α-l-arabinopyranosyl-hederagenin-28-O-β-d-rhamnopyranosyl(12)-[β-D-xylopyranosyl(16)]-β-d-glucopyranosyl ester, and (17) 3-O-β-d-glucopyranosyl(13)-α-l-rhamnopyranosyl(12)-α-l-arabinopyranosyl-hederagenin-28-O-β-d-glucopyranosyl(16)-β-d-glucopyranosyl ester. |
| Organic acids | (1) myristic acid, (2) palmitic acid, (3) 2(E)-3-ethoxy acrylic acid, (4) ethyl laurate, (5) protocatechuic acid, (6) abscisic acid, (7) 3-(3, 4-dihydroxyphenyl) propionic acid, (8) caffeic acid, (9) ferulic acid, (10) caffeic acid methyl ester, (11) methyl 4-O-β-d-glucopyranosyl caffeate, (12) caffeic acid ethyl ester, (13) cinnamic acid, (14) 4-hydroxycinnamic acid, (15) methyl 4-hydroxycinnamate, (16) 1-O-caffeoylquinic acid, (17) 3-O-caffeoylquinic acid, (18) 4-O-caffeoylquinic acid, (19) 5-O-caffeoylquinic acid, (20) 3-O-caffeoylquinic acid methyl ester, (21) 3-O-caffeoylquinic acid ethyl ester, (22) 3-O-caffeoylquinic acid butyl ester, (23) 4-O-caffeoylquinic acid methyl ester, (24) 5-O-caffeoylquinic acid butyl ester, (25) 5-O-caffeoylquinic acid methyl ester, (26) 3,5-O-dicaffeoylquinic acid, (27) 3,4-O-dicaffeoylquinic acid, (28) 4,5-O-dicaffeoylquinic acid, (29) 3,5-O-dicaffeoylquinic acid methyl ester, (30) 3,5-O-dicaffeoylquinic acid butyl ester, (31) 3,5-O-dicaffeoylquinic acid ethyl ester, (32) 3,4-O-dicaffeoylquinic acid methyl ester, (33) 3,4-O-dicaffeoylquinic acid ethyl ester, (34) 4,5-O-dicaffeoylquinic acid methyl ester, (35) 3,4,5-O-tricaffeoylquinic acid, (36) vanillic acid, (37) 4-O-β-d-(6-O-benzoylglucopyranoside), (38) (−)-4-O-(4-O-β-d-glucopyranosylcaffeoyl) quinic acid, (39) (−)-3-O-(4-O-β-d-glucopyranosylcaffeoyl) quinic acid, (40) (−)-5-O-(4-O-β-d-glucopyranosylcaffeoyl) quinic acid, and (41) dichlorogelignate. |
| Pharmacological Function (s) | Model/Dosage | Consequence | Reference |
|---|---|---|---|
| Antioxidant. | Human aortic smooth muscle cells; 5 and 10 μM. | The downstream signal transduction pathways of platelet-derived growth factor receptor beta, including extracellular signal-regulated protein kinases 1 and 2, p38, and Protein kinase B phosphorylation for preventing and treating vascular diseases. | [48] |
| Anti-inflammatory. | RAW264.7 cell, and TPA (12-O-tetradecanoylphorbol-13-acetate)-induced ear edema mouse; 0, 10, 20 μM. | Chrysoeriol ameliorated TPA-induced ear edema in mice and inhibition of JAK2/STAT3 and IκB/p65 NF-κB pathways. | [71] |
| Anticancer. | A549 cells and xenografted mice; 7.5, 15, and 30 μM. | The expression of LC3-phosphatidylethanolamine conjugate and Beclin-1 are significantly upregulated, and also induce sub-G1/G0 cell cycle arrest, as well as inhibit the migration and invasion of the A549 cells. | [72] |
| Antidiabetic. | Diabetic rats; 20 mg/kg. | The level of glucose reduced with the decreased in the enzyme HbA1 in diabetic rats. | [73] |
| Antiarthritis. | Rheumatoid arthritis-fibroblast-like synoviocytes; 5, 10, 20, 40, and 80 μM. | Suppress hyperproliferation of, and evoke apoptosis in, Interleukin-6/receptor-stimulated rheumatoid arthritis-fibroblast-like synoviocytes by its ability to cleave caspase-3 and caspase-9. | [74] |
| Antimicrobial. | Fusarium graminearum and Pythium graminicola; 0.1, 0.5, 1 μM. | High inhibition rate and limiting the growth of pathogens of Fusarium graminearum and Pythium graminicola. | [75] |
| Antithrombotic. | SW872 Human liposarcoma cell; 25, 50, 100, and 200 μM. | Inhibition of pancreatic lipase, cholesterol esterase, adipocytes lipid uptake, and antithrombotic activity, which act as a potential source for future antiatherosclerotic drug discovery. | [76] |
| Antioxidant, antihyperlipidemic. | Wistar rats; 800 mg/kg. | Reduce triglyceride, low-density lipoprotein, cholesterol, and total cholesterol, as well as increase the high-density lipoprotein cholesterol level for improving lipid metabolism. | [77] |
| Antinociceptive. | Male BALB/c mice; 200 mg/kg or 400 mg/kg. | Based on the molecular docking simulations, chrysoeriol interacts with the α2-adrenergic receptor to exert its analgesic. | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Law, S.K.; Wu, X.X.; Jiang, Z.; Tong, C.W.S.; Chow, W.Y.L.; Au, D.C.T. Pharmacological Activities of Lonicerae japonicae flos and Its Derivative—“Chrysoeriol” in Skin Diseases. Molecules 2024, 29, 1972. https://doi.org/10.3390/molecules29091972
Law SK, Wu XX, Jiang Z, Tong CWS, Chow WYL, Au DCT. Pharmacological Activities of Lonicerae japonicae flos and Its Derivative—“Chrysoeriol” in Skin Diseases. Molecules. 2024; 29(9):1972. https://doi.org/10.3390/molecules29091972
Chicago/Turabian StyleLaw, Siu Kan, Xiao Xiao Wu, Zhou Jiang, Christy Wing Sum Tong, Wesley Yeuk Lung Chow, and Dawn Ching Tung Au. 2024. "Pharmacological Activities of Lonicerae japonicae flos and Its Derivative—“Chrysoeriol” in Skin Diseases" Molecules 29, no. 9: 1972. https://doi.org/10.3390/molecules29091972
APA StyleLaw, S. K., Wu, X. X., Jiang, Z., Tong, C. W. S., Chow, W. Y. L., & Au, D. C. T. (2024). Pharmacological Activities of Lonicerae japonicae flos and Its Derivative—“Chrysoeriol” in Skin Diseases. Molecules, 29(9), 1972. https://doi.org/10.3390/molecules29091972