Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research
Abstract
:1. Introduction
2. Materials and Methods
3. Mechanisms of Anti-Inflammatory Action
4. Anti-UV Activity of Azulene Derivatives and Their Probable Mechanisms of Action
5. Activity of Azulene Derivatives against Inflammatory Skin Diseases
6. Potential Anticancer Activity of Azulene Derivatives
7. The Activity of Azulene Derivatives in Erectile Dysfunction
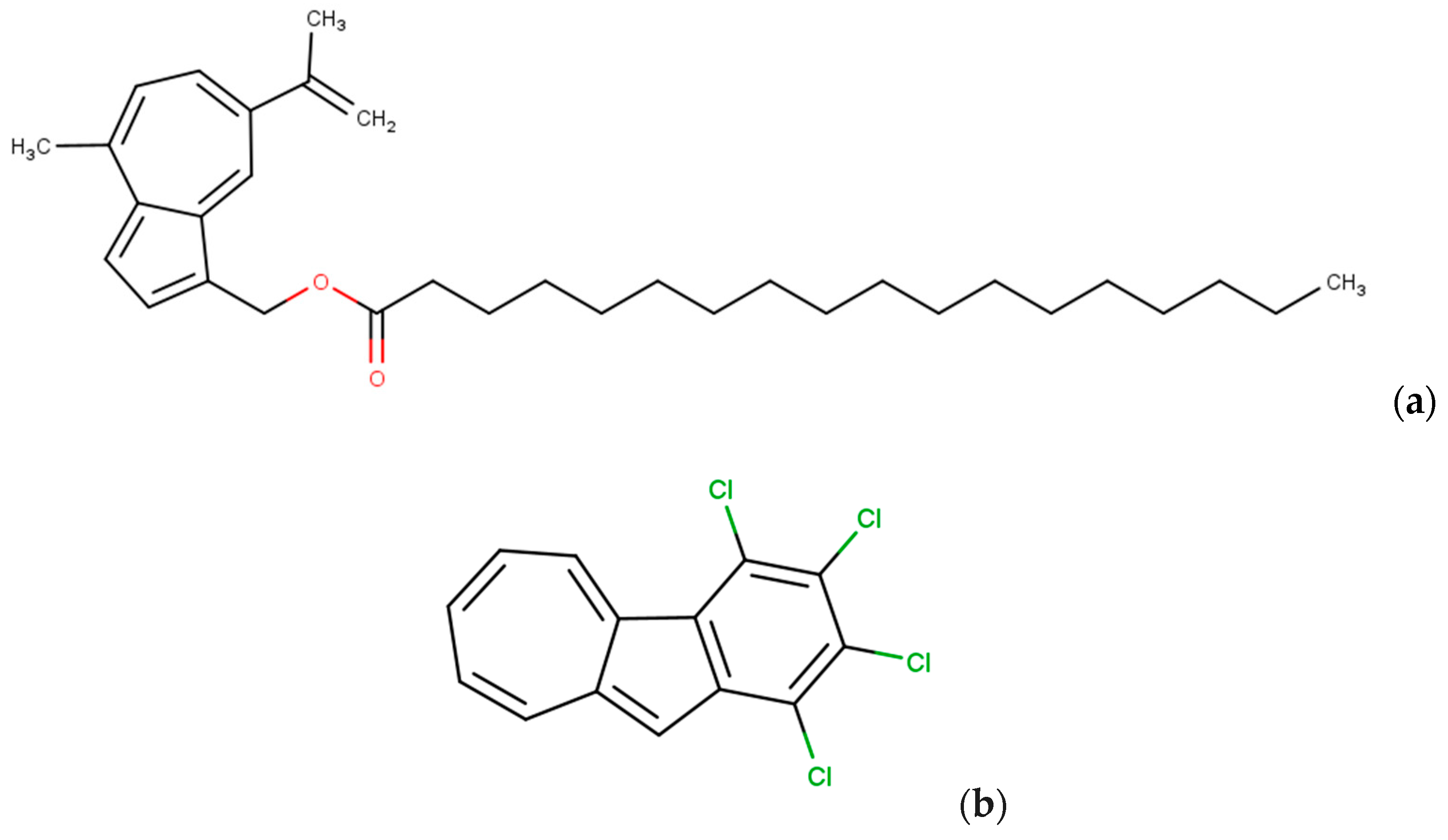
8. Limitations in the Use of Azulene and Its Derivatives on the Skin
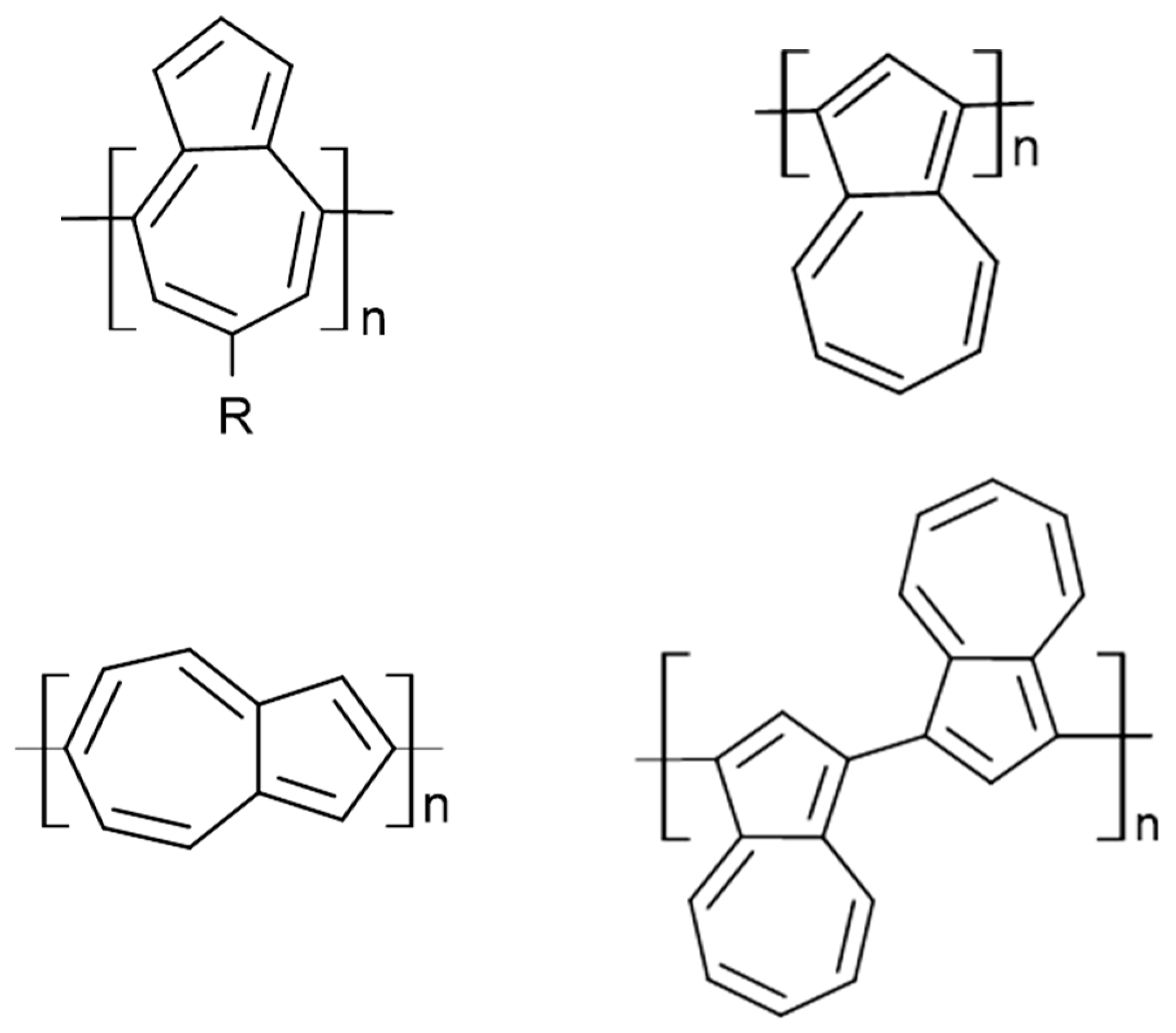
9. Azulene-Based Polymers
10. Perspective for Further Research
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, J.-J.; Shao, C.-L.; Chen, M.; Gan, L.-S.; Fang, Y.-C.; Wang, X.-H.; Wang, C.-Y. Ochracenoids A and B, Guaiazulene-Based Analogues from Gorgonian Anthogorgia Ochracea Collected from the South China Sea. Mar. Drugs 2014, 12, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, S.; van Ofwegen, L.; Proksch, P.; Lin, W. Anthogorgienes A–O, New Guaiazulene-Derived Terpenoids from a Chinese Gorgonian Anthogorgia Species, and Their Antifouling and Antibiotic Activities. J. Agric. Food Chem. 2012, 60, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–Medicinal Mushrooms: A Review of Organic Compounds and Bioelements with Antioxidant Activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Nicholas, G.M. Australasian Fungi: A Natural Product Study. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 1998. [Google Scholar]
- Akram, W.; Tagde, P.; Ahmed, S.; Arora, S.; Emran, T.B.; Babalghith, A.O.; Sweilam, S.H.; Simal-Gandara, J. Guaiazulene and Related Compounds: A Review of Current Perspective on Biomedical Applications. Life Sci. 2023, 316, 121389. [Google Scholar] [CrossRef] [PubMed]
- Höferl, M.; Wanner, J.; Tabanca, N.; Ali, A.; Gochev, V.; Schmidt, E.; Kaul, V.K.; Singh, V.; Jirovetz, L. Biological Activity of Matricaria Chamomilla Essential Oils of Various Chemotypes. Planta Medica Int. Open 2020, 7, e114–e121. [Google Scholar] [CrossRef]
- Veys, K.; Escudero, D. Computational Protocol To Predict Anti-Kasha Emissions: The Case of Azulene Derivatives. J. Phys. Chem. A 2020, 124, 7228–7237. [Google Scholar] [CrossRef]
- Patiño Cano, L.P.; Quintana Manfredi, R.; Pérez, M.; García, M.; Blustein, G.; Cordeiro, R.; Pérez, C.D.; Schejter, L.; Palermo, J.A. Isolation and Antifouling Activity of Azulene Derivatives from the Antarctic Gorgonian Acanthogorgia Laxa. Chem. Biodivers. 2018, 15, e1700425. [Google Scholar] [CrossRef]
- Zeng, H.N.; Png, Z.M.; Xu, J. Azulene in Polymers and Their Properties. Chem.–Asian J. 2020, 15, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Shetti, V.S. Chemical Syntheses and Salient Features of Azulene-Containing Homo- and Copolymers. Beilstein J. Org. Chem. 2021, 17, 2164–2185. [Google Scholar] [CrossRef]
- Razus, A.C. Dancing with Azulene. Symmetry 2022, 14, 297. [Google Scholar] [CrossRef]
- Liu, R.; Fu, Y.; Wu, F.; Liu, F.; Zhang, J.-J.; Yang, L.; Popov, A.A.; Ma, J.; Feng, X. Modular Synthesis of Structurally Diverse Azulene-Embedded Polycyclic Aromatic Hydrocarbons by Knoevenagel-Type Condensation. Angew. Chem. Int. Ed. 2023, 62, e202219091. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Zhou, Y.; Wu, B.; Zhu, L. The Unusual Physicochemical Properties of Azulene and Azulene-Based Compounds. Chin. Chem. Lett. 2019, 30, 1903–1907. [Google Scholar] [CrossRef]
- Phutim-Mangkhalthon, A.; Teerakapong, A.; Tippayawat, P.; Morales, N.P.; Morkmued, S.; Puasiri, S.; Priprem, A.; Damrongrungruang, T. Anti-Inflammatory Effect of Photodynamic Therapy Using Guaiazulene and Red Lasers on Peripheral Blood Mononuclear Cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101747. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a Novel and Selective COX-2 Inhibitor with Anti-Inflammatory Activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Yuzer, A.; Ince, T.; Ince, M. Anti-Cancer and Anti-Inflammatory Activities of Bromo- and Cyano-Substituted Azulene Derivatives. Inflammation 2020, 43, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Gao, X. Design, Synthesis and Anti-Inflammatory Activity of Azulene Derivatives Containing Benzimidazole Unit. Chin. J. Org. Chem. 2023, 43, 3246. [Google Scholar] [CrossRef]
- Yetkin, D.; Ince, T.; Ayaz, F. Photodynamic Anti-Inflammatory Activity of Azulene Derivatives on Mammalian Macrophages and Their Intracellular Mechanism of Action. Photodiagnosis Photodyn. Ther. 2022, 39, 102963. [Google Scholar] [CrossRef] [PubMed]
- Alim, F.G.; Hayuningtyas, R.A. Potensi Chamomile Sebagai Agen Antiinflamasi Oral. J. Kedokt. Gigi Terpadu 2023, 5, 1. [Google Scholar] [CrossRef]
- Uesugi, A.; Tsushima, F.; Miyamoto, Y.; Harada, H. Pollen Food Allergy Syndrome Caused by Japanese Radish: A Case Report. Indian J. Dermatol. 2023, 68, 123. [Google Scholar] [CrossRef]
- Ma, Z.; Han, X.; Ren, J.; Liu, K.; Zhang, W.; Li, G. Design, Synthesis, and Biological Activity of Guaiazulene Derivatives. Chem. Biodivers. 2023, 20, e202201174. [Google Scholar] [CrossRef]
- Ueki, J.-I.; Shimada, A.; Sakagami, H.; Wakabayashi, H. Hormetic and UV-Protective Effects of Azulene-Related Compounds. In Vivo 2011, 25, 41–48. [Google Scholar] [PubMed]
- Ueki, J.-I.; Sakagami, H.; Wakabayashi, H. Anti-UV Activity of Newly-Synthesized Water-Soluble Azulenes. In Vivo 2013, 27, 119–126. [Google Scholar] [PubMed]
- Sakagami, H.; Sheng, H.; Okudaira, N.; Yasui, T.; Wakabayashi, H.; Jia, J.; Natori, T.; Suguro-Kitajima, M.; Oizumi, H.; Oizumi, T. Prominent Anti-UV Activity and Possible Cosmetic Potential of Lignin-Carbohydrate Complex. In Vivo 2016, 30, 331–339. [Google Scholar] [PubMed]
- Rekka, E.; Chrysselis, M.; Siskou, I.; Kourounakis, A. Synthesis of New Azulene Derivatives and Study of Their Effect on Lipid Peroxidation and Lipoxygenase Activity. Chem. Pharm. Bull. (Tokyo) 2002, 50, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; He, L.; Wang, W.; Wei, G.; Ma, L.; Liu, H.; Yao, L. Artemisia Sieversiana Ehrhart Ex Willd. Essential Oil and Its Main Component, Chamazulene: Their Photoprotective Effect against UVB-Induced Cellular Damage and Potential as Novel Natural Sunscreen Additives. ACS Sustain. Chem. Eng. 2023, 11, 17675–17686. [Google Scholar] [CrossRef]
- Ma, L.; Xu, J.; Wang, W.; Lu, J.; Li, Y.; Yao, L. Thin Layer Chromatography-Direct Bioautography for Investigation of Antibacterial Activities of Artemisia Sieversiana Ehrhart Ex Willd. Essential Oil. Nat. Prod. Res. 2023, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Chen, Y.; Song, C.; Xu, S.; Lin, S.; Li, M.; Chen, X.; Gu, H. Possible Treatment for UVB-Induced Skin Injury: Anti-Inflammatory and Cytoprotective Role of Metformin in UVB-Irradiated Keratinocytes. J. Dermatol. Sci. 2021, 102, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Babaeian Jelodar, N.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of Chamazulene and α-Bisabolol Contents of the Essential Oil of German Chamomile (Matricaria chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nakano, Y.; Inayama, K.; Sakai, A.; Kamiya, T. Dietary Intake of the Flower Extracts of German Chamomile (Matricaria recutita L.) Inhibited Compound 48/80-Induced Itch-Scratch Responses in Mice. Phytomedicine 2003, 10, 657–664. [Google Scholar] [CrossRef]
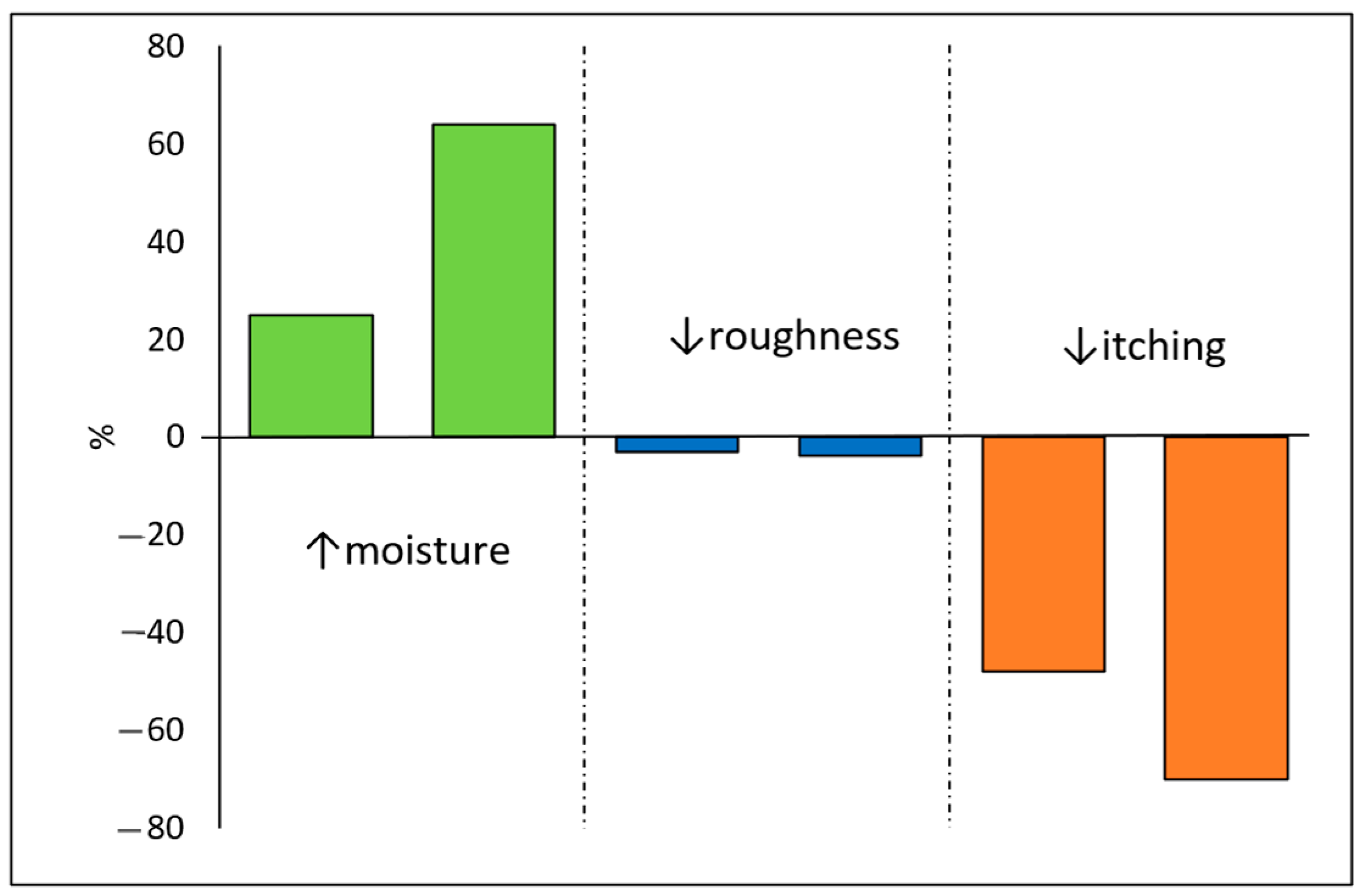
- Park, S.I.; Lee, K.W.; Park, S.; Shin, M.S. Itching and Atopy Relief Using Azulene Derivatives and High-Content Ceramide Skin Barrier Nano-Liposome Structures by Dry Skin. Int. J. Intell. Syst. Appl. Eng. 2022, 10, 151–157. [Google Scholar]
- Park, S.I.; Lee, J.; Shin, M.S. Clinical Study on Itching Relief Caused by Dry Skin of Cosmetics Containing Ceramide NP and Guaiazulene in Smart Healthcare Products. In Smart Healthcare Analytics: State of the Art; Pattnaik, P.K., Vaidya, A., Mohanty, S., Mohanty, S., Hol, A., Eds.; Intelligent Systems Reference Library; Springer: Singapore, 2022; pp. 65–74. ISBN 9789811653049. [Google Scholar]
- Mahato, R.K.; Singh, M.; Pathak, H.; Gogoi, N.R.; Kharbithai, R.; Chowrasia, P.; Bora, P.L.; Sarkar, T.; Jana, B.K.; Mazumder, B. Emerging Nanotechnology Backed Formulations for the Management of Atopic Dermatitis. Ther. Deliv. 2023, 14, 543–569. [Google Scholar] [CrossRef] [PubMed]
- Park, S.I.; Lee, K.W.; Park, S.; Shin, M.S. In Vitro Biological Activities of Azulene, Guaiazulene, and Sodium Guaiazulene Sulfonate and Its Application to Formulations through PEG-PCL Micelles. RIGEO 2021, 11, 8. [Google Scholar]
- Bakun, P.; Czarczynska-Goslinska, B.; Goslinski, T.; Lijewski, S. In Vitro and in Vivo Biological Activities of Azulene Derivatives with Potential Applications in Medicine. Med. Chem. Res. 2021, 30, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Gunes, T.; Akin, M.A.; Sarici, D.; Hallac, K.; Kurtoglu, S.; Hashimoto, T. Guaiazulene: A New Treatment Option for Recalcitrant Diaper Dermatitis in NICU Patients. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2013, 26, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Furau, C.; Grossmann, H.; Furau, G.; Vormann, J. Effect of a Guaiazulene-Containing Ointment on Nipple and Areola Area Health of Women. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 167–173. [Google Scholar] [CrossRef]
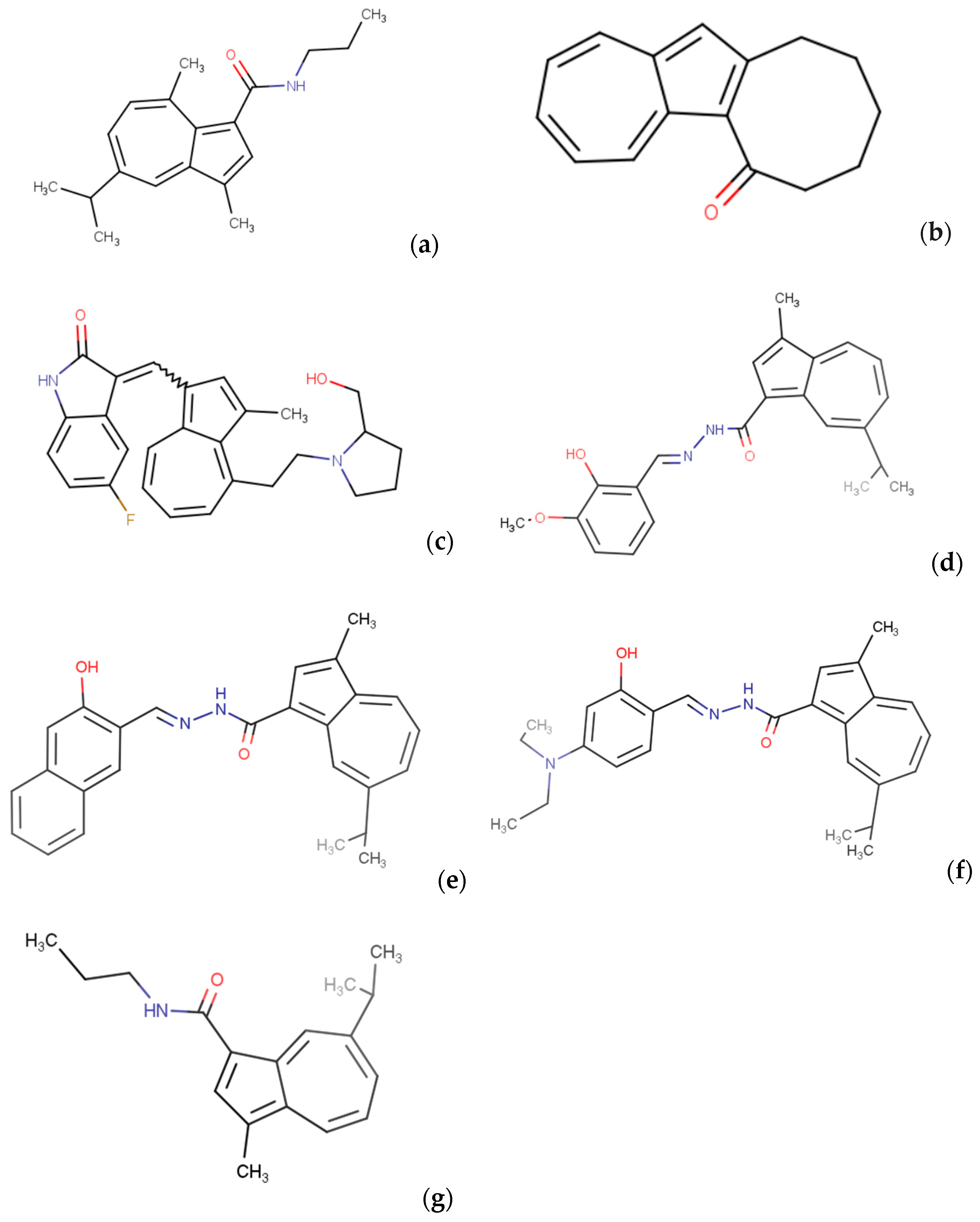
- Wada, T.; Maruyama, R.; Irie, Y.; Hashimoto, M.; Wakabayashi, H.; Okudaira, N.; Uesawa, Y.; Kagaya, H.; Sakagami, H. In Vitro Anti-Tumor Activity of Azulene Amide Derivatives. In Vivo Athens Greece 2018, 32, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kasami, C.; Yamaguchi, J.-I.; Inoue, H. Guaiazulene Derivative 1,2,3,4-Tetrahydroazuleno[1,2-b] Tropone Reduces the Production of ATP by Inhibiting Electron Transfer Complex II. FEBS Open Bio 2021, 11, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lee, O.; Yao, C.-N.; Chuang, M.-Y.; Chang, Y.-L.; Chang, M.-H.; Wen, Y.-F.; Yang, W.-H.; Ko, C.-H.; Chou, N.-T.; et al. Novel Azulene-Based Derivatives as Potent Multi-Receptor Tyrosine Kinase Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6129–6132. [Google Scholar] [CrossRef] [PubMed]
- Brogyányi, T.; Kaplánek, R.; Kejík, Z.; Hosnedlová, B.; Antonyová, V.; Abramenko, N.; Veselá, K.; Martásek, P.; Vokurka, M.; Richardson, D.R.; et al. Azulene Hydrazide-Hydrazones for Selective Targeting of Pancreatic Cancer Cells. Biomed. Pharmacother. 2022, 155, 113736. [Google Scholar] [CrossRef]
- Imanari, K.; Hashimoto, M.; Wakabayashi, H.; Okudaira, N.; Bandow, K.; Nagai, J.; Tomomura, M.; Tomomura, A.; Uesawa, Y.; Sakagami, H. Quantitative Structure–Cytotoxicity Relationship of Azulene Amide Derivatives. Anticancer. Res. 2019, 39, 3507–3518. [Google Scholar] [CrossRef]
- Löber, S.; Hübner, H.; Buschauer, A.; Sanna, F.; Argiolas, A.; Melis, M.R.; Gmeiner, P. Novel Azulene Derivatives for the Treatment of Erectile Dysfunction. Bioorg. Med. Chem. Lett. 2012, 22, 7151–7154. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-M.; Yin, J.-J.; Xia, Q.; Zhao, Y.; Fu, P.P.; Wen, K.-C.; Yu, H. Photoirradiation of Azulene and Guaiazulene—Formation of Reactive Oxygen Species and Induction of Lipid Peroxidation. J. Photochem. Photobiol. Chem. 2010, 211, 123–128. [Google Scholar] [CrossRef]
- Struwe, M.; Csato, M.; Singer, T.; Gocke, E. Comprehensive Assessment of the Photomutagenicity, Photogenotoxicity and Photo(Cyto)Toxicity of Azulene. Mutat. Res. Toxicol. Environ. Mutagen. 2011, 723, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, J.; Fu, P.P.; Parekh, K.A.; Yu, H. Photomutagenicity of Cosmetic Ingredient Chemicals Azulene and Guaiazulene. Mutat. Res. 2003, 530, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, J.; Wang, S.; Cohly, H.; Fu, P.P.; Hwang, H.-M.; Yu, H. Phototoxicity and DNA Damage Induced by the Cosmetic Ingredient Chemical Azulene in Human Jurkat T-Cells. Mutat. Res. 2004, 562, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yin, J.J.; Cherng, S.-H.; Wamer, W.G.; Boudreau, M.; Howard, P.C.; Fu, P.P. UVA Photoirradiation of Retinyl Palmitate--Formation of Singlet Oxygen and Superoxide, and Their Role in Induction of Lipid Peroxidation. Toxicol. Lett. 2006, 163, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Xia, Q.; Chen, L.; Moore, M.M.; Fu, P.P.; Chen, T. Photomutagenicity of Retinyl Palmitate by Ultraviolet A Irradiation in Mouse Lymphoma Cells. Toxicol. Sci. 2005, 88, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.; Rouby, M.N.E.; Al-Sherbini, E.-S.A.M.; Noury, A.H.E.; Morsy, M.E. Photodecomposition, Photomutagenicity and Photocytotoxicity of Retinyl Palmitate under He–Ne Laser Photoirradiation and Its Effects on Photodynamic Therapy of Cancer Cells in Vitro. Photodiagnosis Photodyn. Ther. 2016, 13, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xia, Q.; Cherng, S.-H.; Wamer, W.G.; Howard, P.C.; Yu, H.; Fu, P.P. Photo-Induced DNA Damage and Photocytotoxicity of Retinyl Palmitate and Its Photodecomposition Products. Toxicol. Ind. Health 2005, 21, 167–175. [Google Scholar] [CrossRef]
- Damrongrungruang, T.; Kitchindaopat, N.; Thanasothon, P.; Theeranut, K.; Tippayawat, P.; Ruangsuwan, C.; Suwannee, B. Effects of Photodynamic Therapy with Azulene on Peripheral Blood Mononuclear Cell Viability and Singlet Oxygen Formation. Photodiagnosis Photodyn. Ther. 2018, 24, 318–323. [Google Scholar] [CrossRef]
- Leino, T.O.; Sieger, P.; Yli-Kauhaluoma, J.; Wallén, E.A.A.; Kley, J.T. The Azulene Scaffold from a Medicinal Chemist’s Perspective: Physicochemical and in Vitro Parameters Relevant for Drug Discovery. Eur. J. Med. Chem. 2022, 237, 114374. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Noguchi, M.; Noguchi, T.; Wakabayashi, H.; Motohashi, N.; Sakagami, H. Relationship between Electronic Structure and Cytotoxic Activity of Azulenes. In Vivo 2006, 20, 385–389. [Google Scholar] [PubMed]
- Damrongrungruang, T.; Rattanayatikul, S.; Sontikan, N.; Wuttirak, B.; Teerakapong, A.; Kaewrawang, A. Effect of Different Irradiation Modes of Azulene-Mediated Photodynamic Therapy on Singlet Oxygen and PGE2 Formation. Photochem. Photobiol. 2021, 97, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, T.; Higashibeppu, M.; Mazaki, Y. Synthesis and Properties of Twisted and Helical Azulene Oligomers and Azulene-Based Polycyclic Hydrocarbons. ChemistryOpen 2023, 12, e202100298. [Google Scholar] [CrossRef] [PubMed]
- Maruoka, K.; Suzuki, R.; Kamishima, T.; Koseki, Y.; Ngoc Dao, A.T.; Murafuji, T.; Kasai, H. Total Synthesis of Azulene Derivative, a Blue Pigment Isolated from Lactarius Indigo, and Colorant Application of Its Aqueous Dispersion. J. Agric. Food Chem. 2023, 71, 11607–11614. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, P.; Bernardo, O.; Borge, J.; González, J.; López, L.A. Synthesis of Phenethylamine-Azulene Conjugates Enabled by Regioselective Ring Opening of Aziridines. Adv. Synth. Catal. 2024, 366, 1007–1012. [Google Scholar] [CrossRef]
- Razus, A.C. Syntheses of Azulene Embedded Polycyclic Compounds. Symmetry 2024, 16, 382. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slon, E.; Slon, B.; Kowalczuk, D. Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research. Molecules 2024, 29, 2020. https://doi.org/10.3390/molecules29092020
Slon E, Slon B, Kowalczuk D. Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research. Molecules. 2024; 29(9):2020. https://doi.org/10.3390/molecules29092020
Chicago/Turabian StyleSlon, Emilia, Bartosz Slon, and Dorota Kowalczuk. 2024. "Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research" Molecules 29, no. 9: 2020. https://doi.org/10.3390/molecules29092020
APA StyleSlon, E., Slon, B., & Kowalczuk, D. (2024). Azulene and Its Derivatives as Potential Compounds in the Therapy of Dermatological and Anticancer Diseases: New Perspectives against the Backdrop of Current Research. Molecules, 29(9), 2020. https://doi.org/10.3390/molecules29092020





