Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products
Abstract
1. Introduction
2. Resveratrol
Resveratrol in Gastric Cancer and Inflammation
3. Piceatannol
Piceatannol and Gastric Cancer
4. Curcumin
4.1. Curcumin and Gastric Cancer
4.2. Clinical Trials Based on Curcumin in Gastric Cancer Therapy
5. Quercetin
Quercetin and Gastric Cancer
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Poniewierska-Baran, A.; Warias, P.; Zgutka, K. Sirtuins (SIRTs) As a Novel Target in Gastric Cancer. Int. J. Mol. Sci. 2022, 23, 15119. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunie, R.P.; Peyron, D.; Trollat, P. Effect of enological practices on the resveratrol isomer content of wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of Resveratrol. Rev. Mutat. Rese. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Rojo, D.; Madrid, A.; Martín, S.S.; Párraga, M.; Silva Pinhal, M.A.; Villena, J.; Valenzuela-Valderrama, M. Resveratrol Decreases the Invasion Potential of Gastric Cancer Cells. Molecules 2022, 27, 3047. [Google Scholar] [CrossRef]
- Dai, H.; Deng, H.B.; Wang, Y.H.; Guo, J.J. Resveratrol inhibits the growth of gastric cancer via the Wnt/β-catenin pathway. Oncol. Lett. 2018, 16, 1579–1583. [Google Scholar] [CrossRef]
- Mieszala, K.; Rudewicz, M.; Gomulkiewicz, A.; Ratajczak-Wielgomas, K.; Grzegrzolka, J.; Dziegiel, P.; Borska, S. Expression of genes and proteins of multidrug resistance in gastric cancer cells treated with resveratrol. Oncol. Lett. 2018, 15, 5825–5832. [Google Scholar] [CrossRef]
- Huangfu, L.; Wang, X.; Tian, S.; Chen, J.; Wang, X.; Fan, B.; Yao, Q.; Wang, G.; Chen, C.; Han, J.; et al. Piceatannol enhances Beclin-1 activity to suppress tumor progression and its combination therapy strategy with everolimus in gastric cancer. Sci. China Life Sci. 2023, 66, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. Profiles of Drug Substances, Excipients and Related Methodology. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M.C. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.S. Quercetin. Monograph. Altern. Med. Rev. 2011, 16, 172–194. [Google Scholar] [PubMed]
- Boots, A.W.; Haenen, G.R.M.M.; Bas, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. Sci. Direct 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Gautheron, B. The production of resveratrol (3,5,4′-trihydroxystilbene) by grape berries in different developmental stages. Am. J. Enol. Vitic. 1991, 42, 41–46. [Google Scholar] [CrossRef]
- Raal, A.; Pokk, P.; Arend, A.; Aunapuu, M.; Jõgi, J.; Okva, K.; Püssa, T. Trans-resveratrol alone and hydroxystilbenes of rhubarb (Rheum rhaponticum L.) root reduce liver damage induced by chronic ethanol administration: A comparative study in mice. Phytother. Res. 2009, 23, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.M.; Yu, C.; Toma, R.B.; Cho, S.Y.; Reiboldt, W.; Lee, J.; Van Breemen, R.B. Resveratrol in raw and baked blueberries and bilberries. J. Agric. Food Chem. 2003, 51, 5867–5870. [Google Scholar] [CrossRef]
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M. Resveratrol, a new phenolic compound from Vera-trum grandiflorum. Nippon Kagaku Kaishi 1939, 60, 1090–1100. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gi-meno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Caruso, F.; Tanski, J.; Villegas-Estrada, A.; Rossi, M. Structural basis for antioxidant activity of trans-resveratrol: Ab initio calculations and crystal and molecular structure. J. Agric. Food Chem. 2004, 52, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yao, W.; Xiao, Y.; Dong, Z.; Huang, W.; Zhang, F.; Zhou, X.; Liang, M. Resveratrol-modified mesoporous silica nanoparticle for tumor-targeted therapy of gastric cancer. Bioengineered 2021, 12, 6343–6353. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Li, L.; Zhou, E.; Li, H.; Wu, S.; Cao, Z. Resveratrol Downregulates miR-155-5p to Block the Malignant Behavior of Gastric Cancer Cells. Biomed. Res. Int. 2022, 2022, 6968641. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Y.; Zhu, B.; Liu, Q.; Yao, Q.; Zhao, G. Resveratrol induces apoptosis in SGC-7901 gastric cancer cells. Oncol. Lett. 2018, 16, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, Q.; Chen, Z.; Ni, H.; Xia, L.; Zhao, Q.; Chen, Z.; Chen, P. Resveratrol suppresses the invasion and migration of human gastric cancer cells via inhibition of MALAT1-mediated epithelial-to-mesenchymal transition. Exp. Ther. Med. 2019, 17, 1569–1578. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, L. Resveratrol inhibits the proliferation, invasion, and migration, and induces the apoptosis of human gastric cancer cells through the MALAT1/miR-383-5p/DDIT4 signaling pathway. J. Gastrointest. Oncol. 2022, 13, 985–996. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cisplatin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Baeeri, M.; Mousavi, T.; Azarnezhad, A.; Haghi-Aminjan, H.; Abdollahi, M. Combination therapy of cisplatin and resveratrol to induce cellular aging in gastric cancer cells: Focusing on oxidative stress, and cell cycle arrest. Front. Pharmacol. 2023, 13, 1068863. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, P.; Munoz-Olaya, J.M.; Gagliostro, V.; Casas, J.; Ghidoni, R.; Fabriàs, G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009, 282, 238–243. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, R.; Hu, Y.; Li, W.; Wang, M.; Liang, Z.; Sun, Z.; Ji, R.; Xu, W.; Qian, H. Gastric-cancer-derived mesenchymal stem cells: A promising target for resveratrol in the suppression of gastric cancer metastasis. Hum. Cell 2020, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, D.; Niu, H.; Zhu, G.; Xu, Y.; Ye, D.; Li, J.; Zhang, Q. Resveratrol reverses Doxorubicin resistance by inhibiting epithelial-mesenchymal transition (EMT) through modulating PTEN/Akt signaling pathway in gastric cancer. J. Exp. Clin. Cancer Res. 2023, 42, 23. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, Y.; Dong, Y.; Lin, S.; Guan, W.; Song, J. Resveratrol as a cardioprotective adjuvant for 5-fluorouracil in the treatment of gastric cancer cells. Braz. J. Med. Biol. Res. 2024, 6, e13537. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Cai, L.; Li, G.; Ren, Y.; Li, E.; Deng, K.; Zhu, M.; Han, S.; Che, X.; Li, X.; et al. Res@ZIF-90 suppress gastric cancer progression by disturbing mitochondrial homeostasis. Transl. Oncol. 2024, 51, 102179. [Google Scholar] [CrossRef]
- Bhat, K.P.L.; Kosmeder, J.W.; Pezzuto, J.M. Biological effects of resveratrol. Antioxid. Redox. Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.; Karima, N.; Rezaul Islam Shishira, M.; Xie, L.; Chen, W. Dietary polyphenols to combat the metabolic diseases via altering gut microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Points of control in inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Liu, J.; Cao, X. Cellular and molecular regulation of innate inflammatory responses. Cell Mol. Immunol. 2016, 13, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Yau, Y.F.; Leung, K.S.; El-Nezami, H.; Lee, J.C.-Y. Interaction of Polyphenols as Antioxidant and Anti-Inflammatory Compounds in Brain–Liver–Gut Axis. Antioxidants 2020, 9, 669. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharmacol. 2001, 62, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Fuggetta, M.P.; Bordignon, V.; Cottarelli, A.; Macchi, B.; Frezza, C.; Cordiali-Fei, P.; Ensoli, F.; Ciafre, S.; Marino-Merlo, F.; Mastino, A.; et al. Downregulation of proinflammatory cytokines in HTLV-1-infected T cells by Resveratrol. J. Exp. Clin. Cancer Res. 2016, 35, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Chen, Y.; Ji, S.; Jia, P.; Li, Y.; Wang, T. Comparison of the protective effects of resveratrol and pterostilbene against intestinal damage and redox imbalance in weanling piglets. J. Anim. Sci. Biotechnol. 2020, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Gao, Y.; Lv, W.; Ma, X.; Hu, J.; Chi, J.; Wang, W.; Wang, Y. Resveratrol prevents diabetic nephropathy by reducing chronic inflammation and improving the blood glucose memory effect in non-obese diabetic mice. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2009–2017. [Google Scholar] [CrossRef]
- Zou, M.; Yang, W.; Niu, L.; Sun, Y.; Luo, R.; Wang, Y.; Peng, X. Polydatin attenuates Mycoplasma gallisepticum (HS strain)-induced inflammation injury via inhibiting the TLR6/ MyD88/NF-kappaB pathway. Microb. Pathog. 2020, 149, 104552. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Simao, F.; Matte, A.; Pagnussat, A.S.; Netto, C.A.; Salbego, C.G. Resveratrol preconditioning modulates inflammatory response in the rat hippocampus following global cerebral ischemia. Neurochem. Inter. 2012, 61, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Duan, J.; Xu, K.; Zhang, W. Resveratrol protects against asthma-induced airway inflammation and remodeling by inhibiting the HMGB1/TLR4/NF-kappa B pathway. Exp. Ther. Med. 2019, 18, 459–466. [Google Scholar] [PubMed]
- Liu, L.L.; He, J.H.; Xie, H.B.; Yang, Y.S.; Li, J.C.; Zou, Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014, 93, 54–62. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, L.; He, Y.; Chen, F.; Ma, Y.; Xiao, D.; He, J. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, barrier integrity and inflammation in yellow-feather broilers. Anim. Prod. Sci. 2020, 60, 1547. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, K.; Zhao, X.; Geng, Z. Protective effects of resveratrol against high ambient temperature-induced spleen dysplasia in broilers through modulating splenic redox status and apoptosis. J. Sci. Food Agric. 2018, 98, 5409–5417. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of nitric oxide synthase and the down- regulation of the activation of NF-kappa B in macrophages by resveratrol. Br. J. Pharmacol. 1999, 126, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Das, A. Heat stress-induced hepatotoxicity and its prevention by resveratrol in rats. Toxicol. Mech. Methods 2011, 21, 393–399. [Google Scholar] [CrossRef]
- Babu, D.; Leclercq, G.; Goossens, V.; Remijsen, Q.; Vandenabeele, P.; Motterlini, R.; Lefebvre, R.A. Antioxidant potential of CORM-A1 and resveratrol during TNF-alpha/cycloheximide-induced oxidative stress and apoptosis in murine intestinal epithelial MODE-K cells. Toxicol. Appl. Pharmacol. 2015, 288, 161–178. [Google Scholar] [CrossRef]
- Kortam, M.A.; Ali, B.M.; Fathy, N. The deleterious effect of stress-induced depression on rat liver: Protective role of resveratrol and dimethyl fumarate via inhibiting the MAPK/ERK/JNK pathway. J. Biochem. Mol. Toxicol. 2020, 35, e22627. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, Y.; Dong, L.; Li, M.; Cai, W. Anti-inflammatory effect of resveratrol through the suppression of NF-kappa B and JAK/STAT signaling pathways. Acta Biochim. Biophys. Sin. 2015, 47, 207–213. [Google Scholar] [CrossRef]
- Wahdan, S.A.; Azab, S.S.; Elsherbiny, D.A.; El-Demerdash, E. Piceatannol protects against cisplatin nephrotoxicity via activation of Nrf2/HO-1 pathway and hindering NF-κB inflammatory cascade. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1331–1345. [Google Scholar] [CrossRef] [PubMed]
- Geahlen, R.L.; Mclaughlin, J.L. Piceatannol (3,4,3′,5′-Tetrahydroxy-Trans-Stilbene) Is a Naturally-Occurring Protein-Tyrosine Kinase Inhibitor. Biochem. Biophys. Res. Commun. 1989, 165, 241–245. [Google Scholar] [CrossRef]
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Sugiyama, K.; Ito, T.; Katano, Y.; Ishihata, A. Identification of the strong vasorelaxing substance scirpusin B, a dimer of piceatannol, from passion fruit (Passiflora edulis) seeds. J. Agric. Food Chem. 2011, 59, 6209–6213. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Wang, C. IGF-1-induced MMP-11 expression promotes the proliferation and invasion of gastric cancer cells through the JAK1/STAT3 signaling pathway. Oncol. Lett. 2018, 15, 7000–7006. [Google Scholar] [CrossRef]
- Ahmad, R.S.; Hussain, M.B.; Sultan, M.T.; Arshad, M.S.; Waheed, M.; Shariati, M.A.; Plygun, S.; Hashempur, M.H. Biochemistry, Safety, Pharmacological Activities, and Clinical Applications of Turmeric: A Mechanistic Review. Evid.-Based Complement. Altern. Med. 2020; 7656919. [Google Scholar]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I.; Gandhi, V.V.; Kunwar, A. Important chemical structural features of curcumin and its derivatives: How do they influence their anticancer activity? IJBB 2020, 57, 228–235. [Google Scholar]
- Sun, C.; Zhang, S.; Liu, C.; Liu, X. Curcumin Promoted miR-34a Expression and Suppressed Proliferation of Gastric Cancer Cells. Cancer Biother. Radiopharm. 2019, 34, 634–641. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Li, S.; Zhao, H.; Chen, Y. Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis. Open Life Sci. 2021, 16, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell. Psychol. 2017, 233, 4634–4642. [Google Scholar] [CrossRef]
- Xi, G.; Dong, Q.; Yang, B.; Jiao, D.; Khan, S. Curcumin’s Dose-Dependent Attenuation of Gastric Cancer Cell Progression Via the PI3K Pathway Blockade. Dose-Response 2023, 21, 15593258231203585. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Hu, W.; Jiang, B.; Zhou, X.; Zhang, M.; Song, M. Curcumin Induces Autophagy-mediated Ferroptosis by Targeting the PI3K/AKT/mTOR Signaling Pathway in Gastric Cancer. Turk. J. Gastroenterol. 2024, 35, 625–633. [Google Scholar] [PubMed]
- Tong, R.; Wu, X.; Liu, Y.; Liu, Y.; Zhou, J.; Jiang, X.; Zhang, L.; He, X.; Ma, L. Curcumin-Induced DNA Demethylation in Human Gastric Cancer Cells Is Mediated by the DNA-Damage Response Pathway. Oxidative Med. Cell. Longev. 2020, 2543504. [Google Scholar] [CrossRef]
- Huang, F.; Yao, Y.; Wu, J.; Liu, Q.; Zhang, J.; Pu, X.; Zhang, Q.; Xia, L. Curcumin inhibits gastric cancer-derived mesenchymal stem cells mediated angiogenesis by regulating NF-κB/VEGF signaling. Am. J. Transl. Res. 2017, 9, 5538–5547. [Google Scholar] [PubMed]
- Zhou, X.; Wang, W.; Li, P.; Zheng, Z.; Tu, Y.; Zhang, Y.; You, T. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Inducing Gastric Cancer Cell Apoptosis Both In Vitro and In Vivo. Oncol. Res. 2016, 23, 29–34. [Google Scholar] [CrossRef]
- Zheng, R.; Deng, Q.; Liu, Y.; Zhao, P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med. Sci. Monit. 2017, 12, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sierżant, K.; Pyrkosz-Biardzka, K.; Gabrielska, J. Właściwości przeciwutleniające naturalnych ekstraktów polifenolowych z wybranych roślin w układach modelowych. Żywność Nauka Technologia Jakość 2012, 6, 41–53. [Google Scholar]
- Mieszkowski, J.; Pałys, A.; Budzisz, E. Kwercetyna-struktura, funkcje i zastosowanie kliniczne. Farm. Pol. 2011, 67, 18–24. [Google Scholar]
- Gheribi, E. Związki polifenolowe w owocach i warzywach. Med. Rodz. 2011, 4, 111–115. [Google Scholar]
- Materska, M. Quercetin and its derivatives: Chemical structure and bioactivity—A review. Pol. J. Food Nutr. Sci. 2008, 58, 407–413. [Google Scholar]
- Valentová, K.; Káňová, K.; Di Meo, F.; Pelantová, H.; Chambers, C.; Rydlová, L.; Petrásková, L.; Křenková, A.; Cvačka, J.; Trouillas, P.; et al. Chemoenzymatic Preparation and Bio-physical Properties of Sulfated Quercetin Metabolites. Int. J. Mol. Sci. 2017, 18, 2231. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, K.D. Quercetin—A versatile flavonoid. Int. J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Gliszczyńska-Świgło, A.; Szymusiak, H. In-terakcje między składnikami suplementów diety na przykładzie kwercetyny i witaminy C. Żywność Nauka Technol. Jakość 2009, 4, 278–285. [Google Scholar]
- Zhang, S.; Huang, J.; Xie, X.; He, Y.; Mo, F.; Luo, Z. Quercetin from Polygonum capitatum Protects against Gastric Inflammation and Apoptosis Associated with Helicobacter pylori Infection by Affecting the Levels of p38MAPK, BCL-2 and BAX. Molecules 2017, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Kawała, K. Właściwości antyoksydacyjne flawonoidów oraz ich wpływ na zdrowie człowieka. Kosmos 2019, 68, 153–159. [Google Scholar] [CrossRef]
- McCullough, M.L.; Peterson, J.J.; Patel, R.; Jacques, P.F.; Shah, R.; Dwyer, J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutrit. 2012, 95, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Russo, G.; Dagali, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Liu, S.H.; Tang, M.Z.; Yang, X.J. Quercetin inhibits the proliferative effect of gastric cancer cells by activating the pyroptosis pathway. Asian J. Surg. 2023, 46, 5286–5288. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, H.; Ge, J.; Yang, B.; Wang, Y.; Wang, W.; Wen, Y.; Zeng, S.; Chen, Q.; Huang, J.; et al. A study related to the treatment of gastric cancer with Xiang-Sha-Liu-Jun-Zi-Tang based on network analysis. Heliyon 2023, 9, e19546. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Jiang, Y.; Zheng, X.; Wang, T.; Li, J.; Zhang, B.; Zhu, J.; Wei, X.; Huang, R.; et al. Quercetin Promotes Apoptosis of Gastric Cancer Cells through the EGFR-ERK Signaling Pathway. J. Food Biochem. 2024, 1, 9945178. [Google Scholar] [CrossRef]
- Zhao, G.; Xue, S. Mechanism of Quercetin as a Multidrug-resistant Reversing Compound in Oxaliplatin-resistant Gastric-cancer Cell Lines. Altern. Ther. Health Med. 2023, 29, 54–59. [Google Scholar]
- Du, F.; Feng, Y.; Fang, J.; Yang, M. MicroRNA-143 enhances chemosensitivity of Quercetin through autophagy inhibition via target GABARAPL1 in gastric cancer cells. Biomed. Pharmacother. 2015, 74, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, J.; Li, J. Quercetin promotes ATG5-mediating autophagy-dependent ferroptosis in gastric cancer. J. Mol. Histol. 2024, 55, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Gao, Y.; An, N. The inhibitory effect of quercetin on chemotherapeutic drug resistance of gastric cancer through forkhead box D3 signaling pathway. Mater. Express 2023, 13, 1982–1989. [Google Scholar] [CrossRef]
- Lei, C.S.; Hou, Y.C.; Pai, M.H.; Lin, M.T.; Yeh, S.L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: In vitro and in vivo studies. J. Nutr. Biochem. 2018, 51, 105–113. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, S.; Shin, Y.S.; Cho, M.; Kang, H.; Cho, H. Anti-Cancer Effect of Quercetin in Xenograft Models with EBV-Associated Human Gastric Carcinoma. Molecules 2016, 21, 1286. [Google Scholar] [CrossRef]
- Maddineni, G.; Xie, J.J.; Brahmbhatt, B.; Mutha, P. Diet and carcinogenesis of gastric cancer. Curr. Opin. Gastroenterol. 2022, 38, 588–591. [Google Scholar] [CrossRef]
- Hu, Q.; Li, Z.; Li, Y.; Deng, X.; Chen, Y.; Ma, X.; Zeng, J.; Zhao, Y. Natural products targeting signaling pathways associated with regulated cell death in gastric cancer: Recent advances and perspectives. Phytother. Res. 2023, 37, 2661–2692. [Google Scholar] [CrossRef]
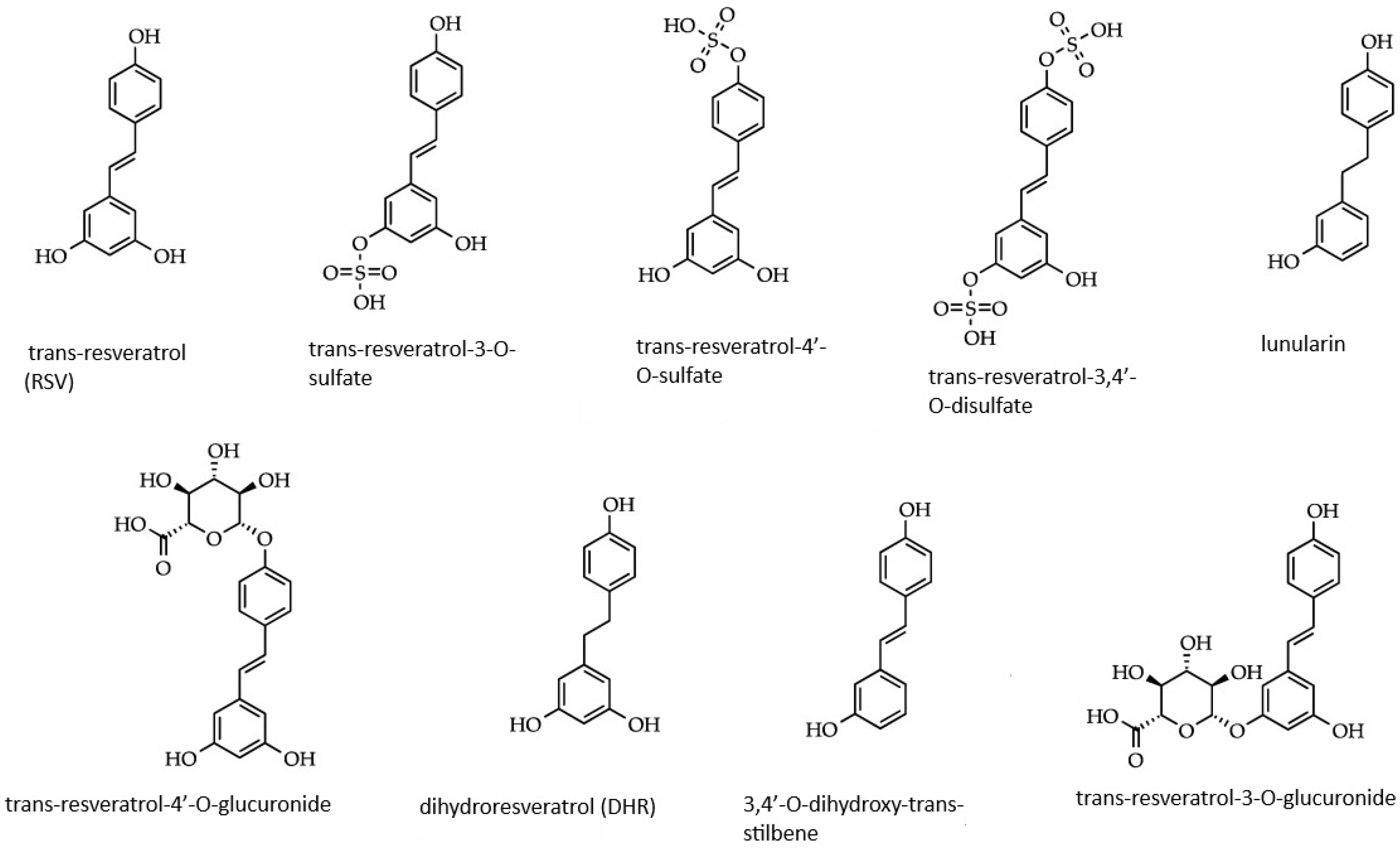


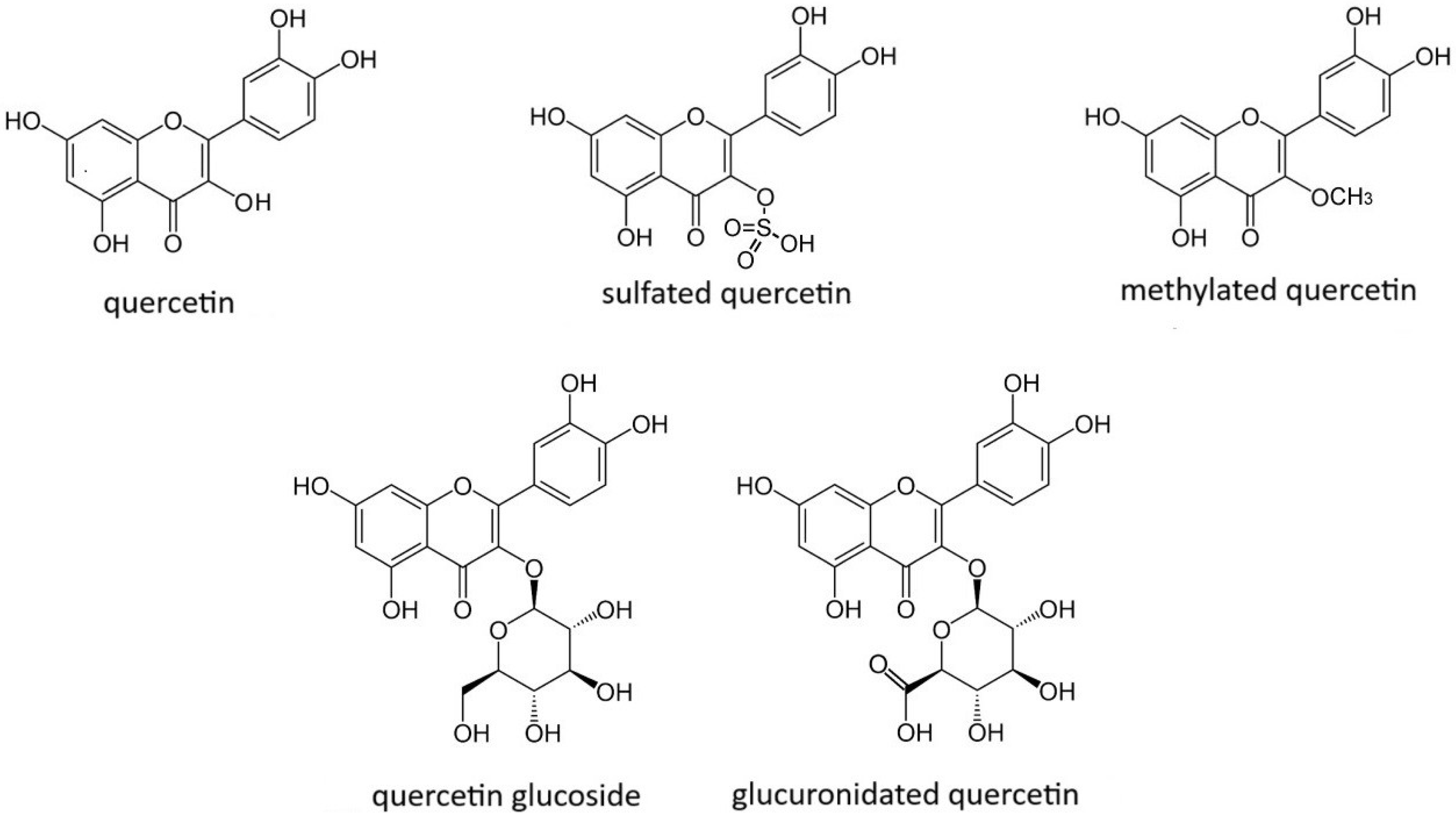
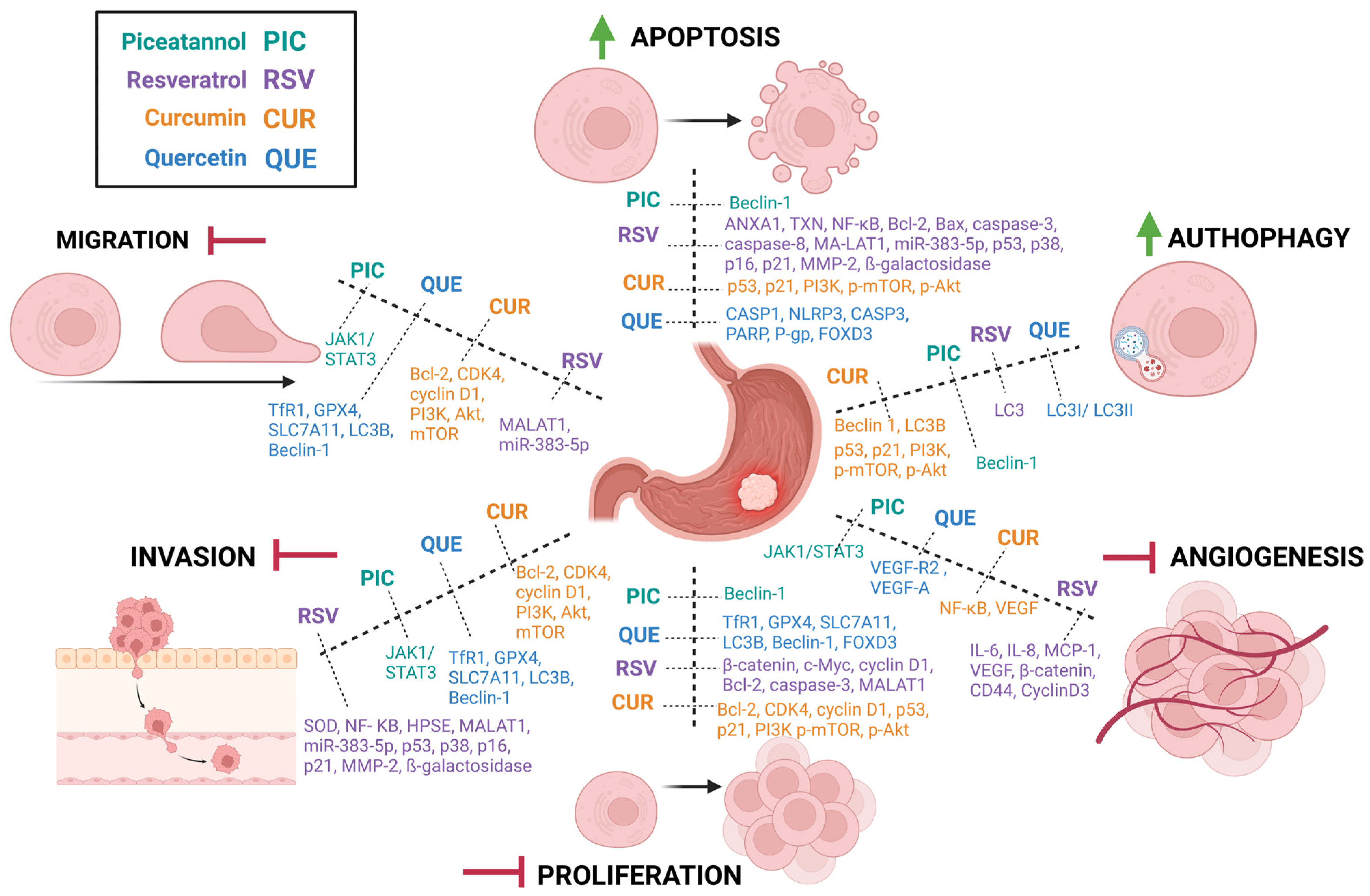
| Process of Carcinogenesis | Model | Dose | Influence on GC | Molecular Target of Interest | References |
|---|---|---|---|---|---|
| Apoptosis | HGC-27, AGS EPG85-257 (RDB), EPG85-257 (RNOV), SGC-7901 | 100 μg/mL 10,20,30,40,50,200, 400 µM | ↑ | ↓↑ANXA1,↓TXN, ↓NF-κB, ↓Bcl-2, Bax, ↑caspase-3, ↑caspase-8, ↓MALAT1/miR-383-5p, ↑p53, ↑p38, ↑p16, ↑p21, ↑MMP-2, ↑ß-galactosidase | [7,22,24,26,27,28] |
| Autophagy | HGC-27 | 50 µM | ↓ | ↓LC3 | [29] |
| Angiogenesis | GC–MSCs, HGC-27, AGS | 20 µM | ↓ | ↓IL-6, ↓IL-8, ↓MCP-1, ↓VEGF, ↓β-catenin, ↓CD44, ↓CyclinD3 | [30] |
| Proliferation | SGC7901, GES-1, MGC803, AGS | 25, 50, 75, 100, 200 (μM) | ↓ | ↓miR-155-5p, ↓claudin-1, ↓Wnt/β-catenin, ↓c-Myc, ↓cyclin D1, ↓Bcl-2, ↑caspase-3, ↓MALAT1/miR-383-5p | [6,23,26] |
| Invasion | AGS, MKN45, BGC823 | 25–200 μM | ↓ | ↑SOD, ↓NF-KB, ↓HPSE, ↓MALAT1/ miR-383-5p, ↑p53, ↑p38, ↑p16, ↑p21, ↑MMP-2, ↑ß-galactosidase | [5,25,26] |
| Migration | BGC823 | 200 µM | ↓ | ↓MALAT1/miR-383-5p | [25,26] |
| ROS | AGS | 100 µM | ↑ | - | [28] |
| Process of Carcinogenesis | Model | Dose | Influence on GC | Molecular Target of Interest | References |
|---|---|---|---|---|---|
| Apoptosis | SGC7901, BGC823, MKN28, MGC803, HGC27, and AGS | 5 μmol L−1 | ↑ | ↑Beclin-1, ↑Cacspase-9-Caspase-3, ↑PARP, ↓Bcl-2, | [8] |
| Autophagy | SGC7901, BGC823, MKN28, MGC803, HGC27, and AGS | 5 μmol L−1 | ↑ | ↑Beclin-1, ↑LC3, ↓SQSTM1/p62 | [8] |
| Angiogenesis | SGC-7901 | 10, 20 µM | ↓ | ↓JAK1/STAT3 | [67] |
| Proliferation | SGC7901, BGC823, MKN28, MGC803, HGC27, and AGS | 5 μmol L−1 | ↓ | ↑Beclin-1, ↑LC3B, ↑PARP | [8] |
| Invasion | SGC-7901 | 10, 20 µM | ↓ | ↓JAK1/STAT3 | [67] |
| Migration | SGC-7901 | 10, 20 µM | ↓ | ↓JAK1/STAT3 | [67] |
| Process of Carcinogenesis | Model | Dose | Influence on GC | Molecular Target of Interest | References |
|---|---|---|---|---|---|
| Apoptosis | SGC-7901, hGCC, BGC-823 | 10,20,40 μM | ↑ | ↑p53, ↑p21, ↓PI3K, ↓mTOR, ↓Akt β-catenin | [73,79] |
| Autophagy | SGC-7901, AGS, BGC-823, HGC-27 | 10,20,40 μM | ↑ | ↑p53, ↑p21, ↓PI3K, ↓mTOR, ↓Akt, ↑ATG5, ↑ATG7, ↑Beclin 1, ↑LC3B | [73,75] |
| Angiogenesis | GC-MSC | 30 μM | ↓ | ↓NF-κB, ↓VEGF | [77] |
| Proliferation | SGC-7901, hGCC, BGC-823, | 10,20,40 μM | ↓ | ↓Bcl-2, ↓CDK4, ↓cyclin D1, ↑p53, ↑p21, ↓PI3K, ↓mTOR, ↓Akt | [71,73] |
| Invasion | SGC-7901, AGS HGC-37 | 20, 30, 50, 75, 100 μM | ↓ | ↓Bcl-2, ↓CDK4, ↓cyclin D1, ↓circ_0056618, ↓PI3K, ↓Akt, ↓ mTOR | [71,72,74,76] |
| Migration | SGC-790, HGC-37, AGS, hGCC | 20, 30, 50, 75, 100 μM | ↓ | ↓Bcl-2, ↓CDK4, ↓cyclin D1, ↓circ_0056618, ↓PI3K, ↓Akt, ↓mTOR | [71,72,74,76] |
| ROS | hGCC | 20 μM | ↑ | - | [76] |
| Trial Number | Conditions | Status/Phase | Age (Years) | Locations |
|---|---|---|---|---|
| NCT05856500 | Stage IIIA Gastric Cancer, Stage IIIB Gastric Cancer, Stage IV Gastric Cancer | not yet recruiting | 18–80 | location not provided |
| NCT04871412 | Gastric Cancer | recruiting | ≥18 | Canada |
| NCT02782949 | Chronic Atrophic Gastritis | active, not recruiting | ≥21 | Honduras, Puerto Rico |
| Process of Carcinogenesis | Model | Dose | Influence on GC | Molecular Target of Interest | References |
|---|---|---|---|---|---|
| Apoptosis | AGS, KATOIII/OxR SGC-7901 | 20,40,80 μM | ↑ | ↑GSDMD, ↑GSDME, ↑CASP1, ↑NLRP3, ↑CASP3, ↑PARP, ↑P-gp, ↑FOXD3, ↓TP53, ↓TIMP1, ↓MYC, ↑Cyt-C, ↓Bcl-2 | [91,92,93,94,97] |
| Autophagy | AGS, MKN28 | 40, 150 µM | ↑ | ↑LC3I/LC3II | [95] |
| Angiogenesis | xenograft model nude mice/AGS | 20 mg/kg | ↓ | ↓VEGF-R2, ↓VEGF-A | [98] |
| Proliferation | AGS, MKN45 SGC-7901 | 20, 40, 80, 160, 320, 640 μM | ↓ | ↓TfR1, ↓GPX4, ↓SLC7A11, ↑LC3B, ↑Beclin-1, ↑FOXD3, ↓TP53, ↓TIMP1, ↓MYC | [92,96,97] |
| Invasion | AGS, MKN45 | 20, 40, 80, 160, 320, 640 μM | ↓ | ↓TfR1, ↓GPX4, ↓SLC7A11, ↑LC3B, ↑Beclin-1 | [96] |
| Migration | AGS, MKN45 | 20, 40, 80, 160, 320, 640 μM | ↓ | ↓TfR1, ↓GPX4, ↓SLC7A11, ↑LC3B, ↑Beclin-1 | [96] |
| ROS | AGS, MKN45 | 20, 40, 80, 160, 320, 640 μM | ↑ | - | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warias, P.; Plewa, P.; Poniewierska-Baran, A. Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products. Molecules 2025, 30, 3. https://doi.org/10.3390/molecules30010003
Warias P, Plewa P, Poniewierska-Baran A. Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products. Molecules. 2025; 30(1):3. https://doi.org/10.3390/molecules30010003
Chicago/Turabian StyleWarias, Paulina, Paulina Plewa, and Agata Poniewierska-Baran. 2025. "Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products" Molecules 30, no. 1: 3. https://doi.org/10.3390/molecules30010003
APA StyleWarias, P., Plewa, P., & Poniewierska-Baran, A. (2025). Resveratrol, Piceatannol, Curcumin, and Quercetin as Therapeutic Targets in Gastric Cancer—Mechanisms and Clinical Implications for Natural Products. Molecules, 30(1), 3. https://doi.org/10.3390/molecules30010003







