Dereplication of Natural Product Antifungals via Liquid Chromatography–Tandem Mass Spectrometry and Chemical Genomics
Abstract
1. Introduction
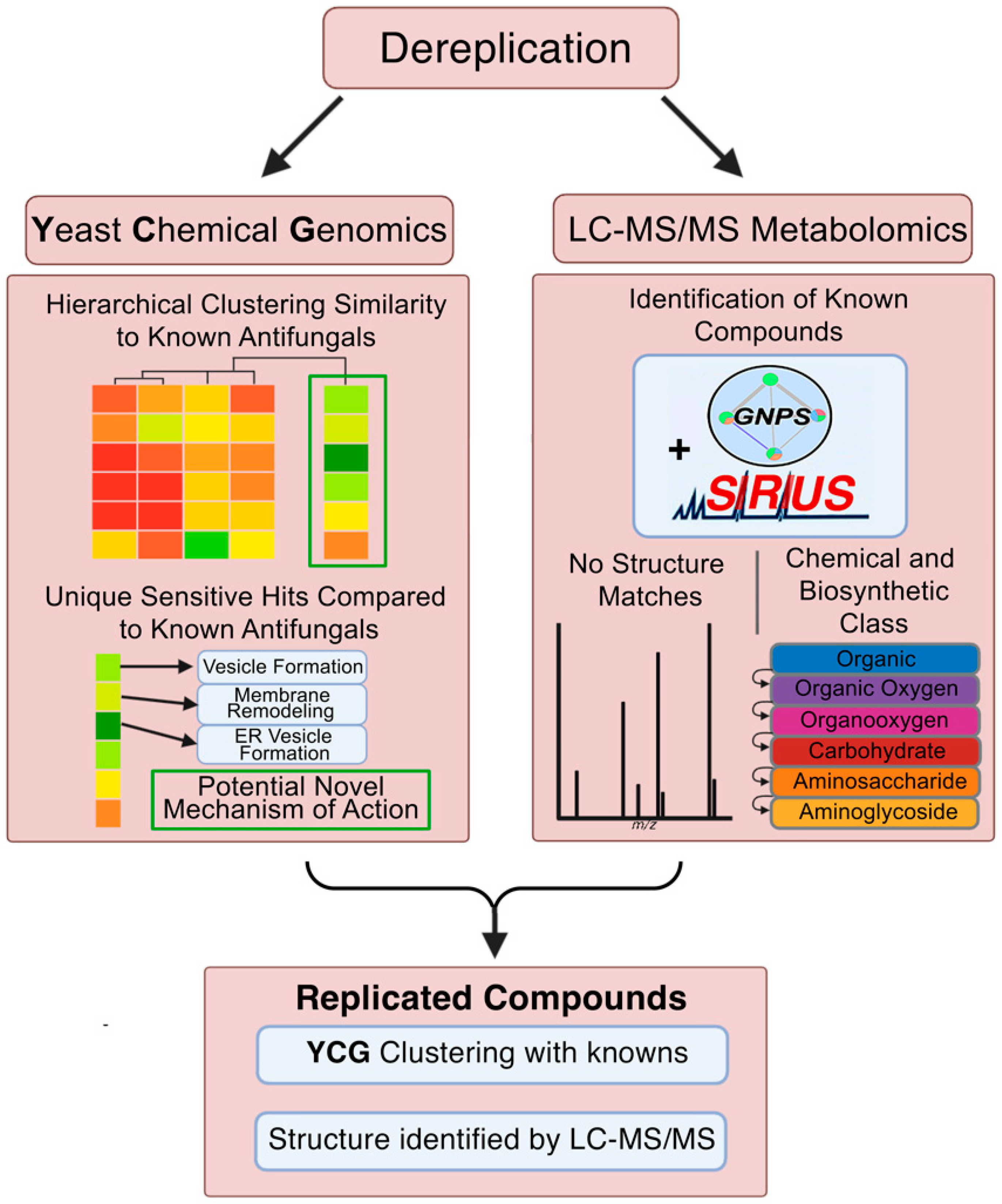
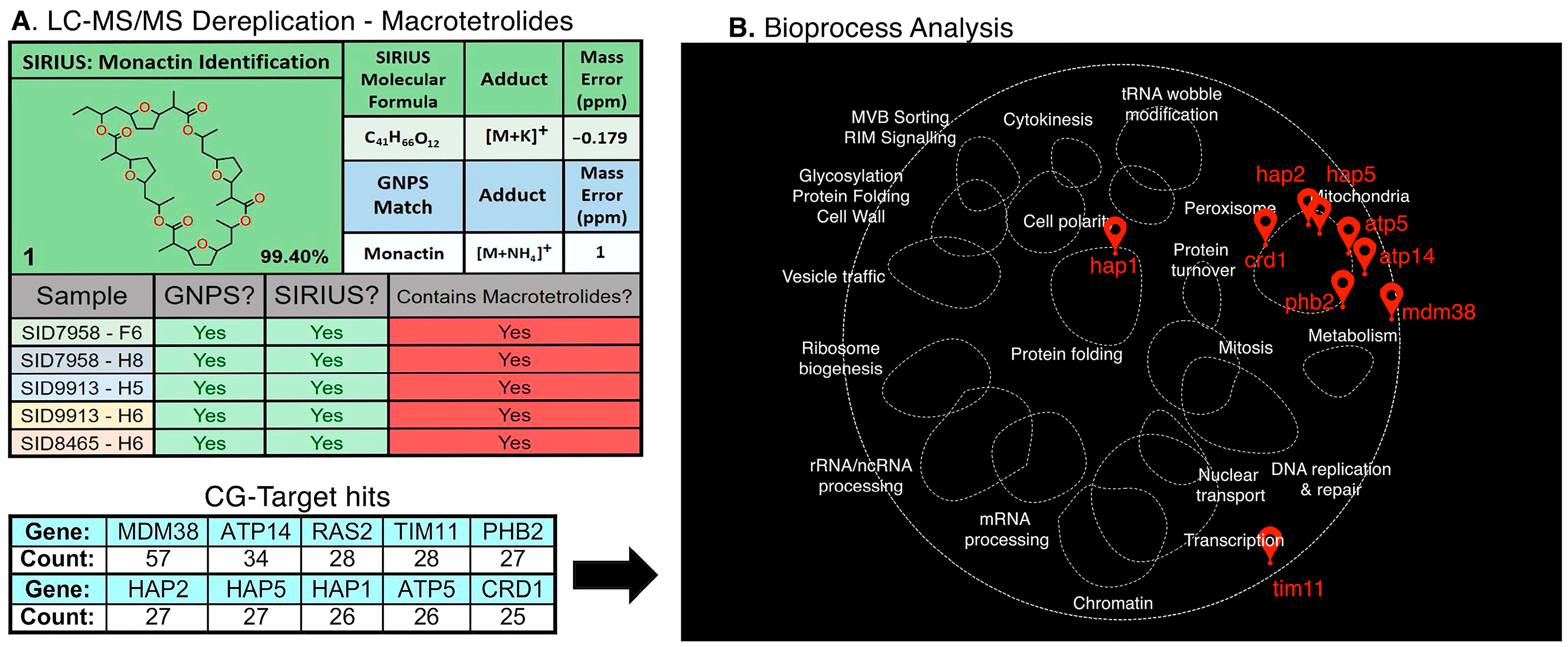
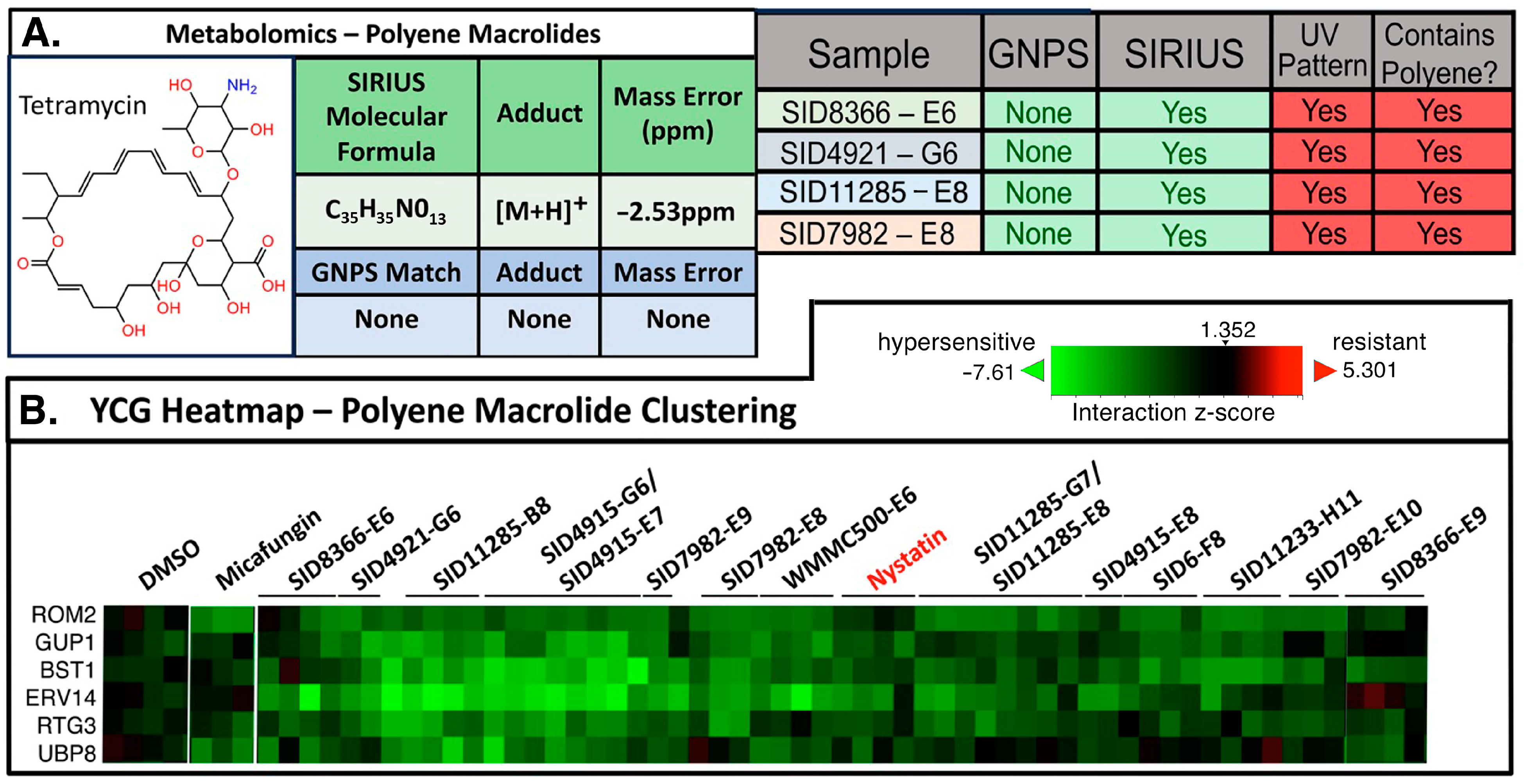
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Alastruey-Izquierdo, A.; Berman, J.; Bicanic, T.; Bignell, E.M.; Bowyer, P.; Bromley, M.; Brüggemann, R.; Garber, G.; Cornely, O.A.; et al. Tackling the Emerging Threat of Antifungal Resistance to Human Health. Nat. Rev. Microbiol. 2022, 20, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4, 165rv13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A Global View on Fungal Infections in Humans and Animals: Opportunistic Infections and Microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113. [Google Scholar] [CrossRef]
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- De Oliveira, H.C.; Bezerra, B.T.; Rodrigues, M.L. Antifungal Development and the Urgency of Minimizing the Impact of Fungal Diseases on Public Health. ACS Bio Med Chem Au 2023, 3, 137–146. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the Epidemiological Landscape of Invasive Candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Sulaiman, T.; Al-Ahmed, S.H.; Buhaliqah, Z.A.; Buhaliqah, A.A.; AlYuosof, B.; Alfaresi, M.; Al Fares, M.A.; Alwarthan, S.; Alkathlan, M.S.; et al. Potential Strategies to Control the Risk of Antifungal Resistance in Humans: A Comprehensive Review. Antibiotics 2023, 12, 608. [Google Scholar] [CrossRef]
- Hou, Y.; Braun, D.R.; Michel, C.R.; Klassen, J.L.; Adnani, N.; Wyche, T.P.; Bugni, T.S. Microbial Strain Prioritization Using Metabolomics Tools for the Discovery of Natural Products. Anal. Chem. 2012, 84, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Chanana, S.; Thomas, C.S.; Zhang, F.; Rajski, S.R.; Bugni, T.S. HCAPCA: Automated Hierarchical Clustering and Principal Component Analysis of Large Metabolomic Datasets in R. Metabolites 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The Antimicrobial Potential of Streptomyces from Insect Microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Månsson, M.; Rank, C.; Frisvad, J.C.; Larsen, T.O. Dereplication of Microbial Natural Products by LC-DAD-TOFMS. J. Nat. Prod. 2011, 74, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Jouaneh, T.M.M.; Rosario, M.E.; Li, Y.; Leibovitz, E.; Bertin, M.J. Incorporating LC-MS/MS Analysis and the Dereplication of Natural Product Samples into an Upper-Division Undergraduate Laboratory Course. J. Chem. Ed. 2022, 99, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Van Moll, L.; De Smet, J.; Cos, P.; Van Campenhout, L. Microbial Symbionts of Insects as a Source of New Antimicrobials: A Review. Crit. Rev. Microbiol. 2021, 47, 562–579. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, M.; Braun, D.R.; Ericksen, S.S.; Piotrowski, J.S.; Nelson, J.; Peng, J.; Ananiev, G.E.; Chanana, S.; Barns, K.; et al. A Marine Microbiome Antifungal Targets Urgent-Threat 1 Drug-Resistant Fungi. Science 2020, 370, 974–978. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated Chemical Classification with a Comprehensive, Computable Taxonomy. J. Cheminform. 2016, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Nothias, L.F.; Fleischauer, M.; Reher, R.; Ludwig, M.; Hoffmann, M.A.; Petras, D.; Gerwick, W.H.; Rousu, J.; Dorrestein, P.C.; et al. Systematic Classification of Unknown Metabolites Using High-Resolution Fragmentation Mass Spectra. Nat. Biotechnol. 2021, 39, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.A.; Nothias, L.F.; Ludwig, M.; Fleischauer, M.; Gentry, E.C.; Witting, M.; Dorrestein, P.C.; Dührkop, K.; Böcker, S. High-Confidence Structural Annotation of Metabolites Absent from Spectral Libraries. Nat. Biotechnol. 2022, 40, 411–421. [Google Scholar] [CrossRef]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Giaever, G.; Chu, A.M.; Ni, L.; Connelly, C.; Riles, L.; Veronneau, S.; Dow, S.; Lucau-Danila, A.; Anderson, K.; Andre, B.; et al. Functional Profiling of the Saccharomyces cerevisiae Genome. Nature 2002, 418, 387–391. [Google Scholar] [CrossRef]
- Parsons, A.B.; Lopez, A.; Givoni, I.E.; Williams, D.E.; Gray, C.A.; Porter, J.; Chua, G.; Sopko, R.; Brost, R.L.; Ho, C.H.; et al. Exploring the Mode-of-Action of Bioactive Compounds by Chemical-Genetic Profiling in Yeast. Cell 2006, 126, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.S.; Li, S.C.; Deshpande, R.; Simpkins, S.W.; Nelson, J.; Yashiroda, Y.; Barber, J.M.; Safizadeh, H.; Wilson, E.; Okada, H.; et al. Functional Annotation of Chemical Libraries across Diverse Biologic Processes. Nat. Chem. Biol. 2017, 13, 982–993. [Google Scholar] [CrossRef]
- Simpkins, S.W.; Deshpande, R.; Nelson, J.; Li, S.C.; Piotrowski, J.S.; Ward, H.N.; Yashiroda, Y.; Osada, H.; Yoshida, M.; Boone, C.; et al. Using BEAN-Counter to Quantify Genetic Interactions from Multiplexed Barcode Sequencing Experiments. Nat. Protoc. 2019, 14, 415–440. [Google Scholar] [CrossRef] [PubMed]
- Keil, C.; Leach, R.W.; Faizaan, S.M.; Bezawada, S.; Parsons, L. Treeview 3.0 (beta 1)—Visualization and analysis of large data matrices. Zenodo 2016, 10, 5281. [Google Scholar] [CrossRef]
- Simpkins, S.W.; Nelson, J.; Deshpande, R.; Li, S.C.; Piotrowski, J.S.; Wilson, E.H.; Gebre, A.A.; Safizadeh, H.; Okamoto, R.; Yoshimura, M.; et al. Predicting Bioprocess Targets of Chemical Compounds through Integration of Chemical-Genetic and Genetic Interactions. PLoS Comput. Biol. 2018, 14, e1006532. [Google Scholar] [CrossRef]
- Usaj, M.; Tan, Y.; Wang, W.; VanderSluis, B.; Zou, A.; Myers, C.L.; Costanzo, M.; Andrews, B.; Boone, C. TheCellMap.Org: A Web-Accessible Database for Visualizing and Mining the Global Yeast Genetic Interaction Network. G3 Genes Genomes Genet. 2017, 7, 1539–1549. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin Forms an Extramembranous and Fungicidal Sterol Sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Szomek, M.; Reinholdt, P.; Petersen, D.; Caci, A.; Kongsted, J.; Wüstner, D. Direct Observation of Nystatin Binding to the Plasma Membrane of Living Cells. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183528. [Google Scholar] [CrossRef] [PubMed]
- Salamzade, R.; Cheong, J.Z.A.; Sandstrom, S.; Swaney, M.H.; Stubbendieck, R.M.; Starr, N.L.; Currie, C.R.; Singh, A.M.; Kalan, L.R. Evolutionary Investigations of the Biosynthetic Diversity in the Skin Microbiome Using IsaBGC. Microb. Genom. 2023, 9, 000988. [Google Scholar] [CrossRef]
- Adnani, N.; Michel, C.R.; Bugni, T.S. Universal Quantification of Structurally Diverse Natural Products Using an Evaporative Light Scattering Detector. J. Nat. Prod. 2012, 75, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.S.; Simpkins, S.W.; Li, S.C.; Deshpande, R.; McIlwain, S.J.; Ong, I.M.; Myers, C.L.; Boone, C.; Andersen, R.J. Chemical Genomic Profiling via Barcode Sequencing to Predict Compound Mode of Action. In Chemical Biology: Methods and Protocols; Hempel, J.E., Williams, C.H., Hong, C.C., Eds.; Humana Press: Totowa, NJ, USA, 2015; pp. 299–318. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brittin, N.J.; Aceti, D.J.; Braun, D.R.; Anderson, J.M.; Ericksen, S.S.; Rajski, S.R.; Currie, C.R.; Andes, D.R.; Bugni, T.S. Dereplication of Natural Product Antifungals via Liquid Chromatography–Tandem Mass Spectrometry and Chemical Genomics. Molecules 2025, 30, 77. https://doi.org/10.3390/molecules30010077
Brittin NJ, Aceti DJ, Braun DR, Anderson JM, Ericksen SS, Rajski SR, Currie CR, Andes DR, Bugni TS. Dereplication of Natural Product Antifungals via Liquid Chromatography–Tandem Mass Spectrometry and Chemical Genomics. Molecules. 2025; 30(1):77. https://doi.org/10.3390/molecules30010077
Chicago/Turabian StyleBrittin, Nathaniel J., David J. Aceti, Doug R. Braun, Josephine M. Anderson, Spencer S. Ericksen, Scott R. Rajski, Cameron R. Currie, David R. Andes, and Tim S. Bugni. 2025. "Dereplication of Natural Product Antifungals via Liquid Chromatography–Tandem Mass Spectrometry and Chemical Genomics" Molecules 30, no. 1: 77. https://doi.org/10.3390/molecules30010077
APA StyleBrittin, N. J., Aceti, D. J., Braun, D. R., Anderson, J. M., Ericksen, S. S., Rajski, S. R., Currie, C. R., Andes, D. R., & Bugni, T. S. (2025). Dereplication of Natural Product Antifungals via Liquid Chromatography–Tandem Mass Spectrometry and Chemical Genomics. Molecules, 30(1), 77. https://doi.org/10.3390/molecules30010077






