Advanced Spectroscopic Characterization of Synthetic Oil from Oil Sands via Pyrolysis: An FTIR, GC–MSD, and NMR Study
Abstract
1. Introduction
2. Results and Discussion
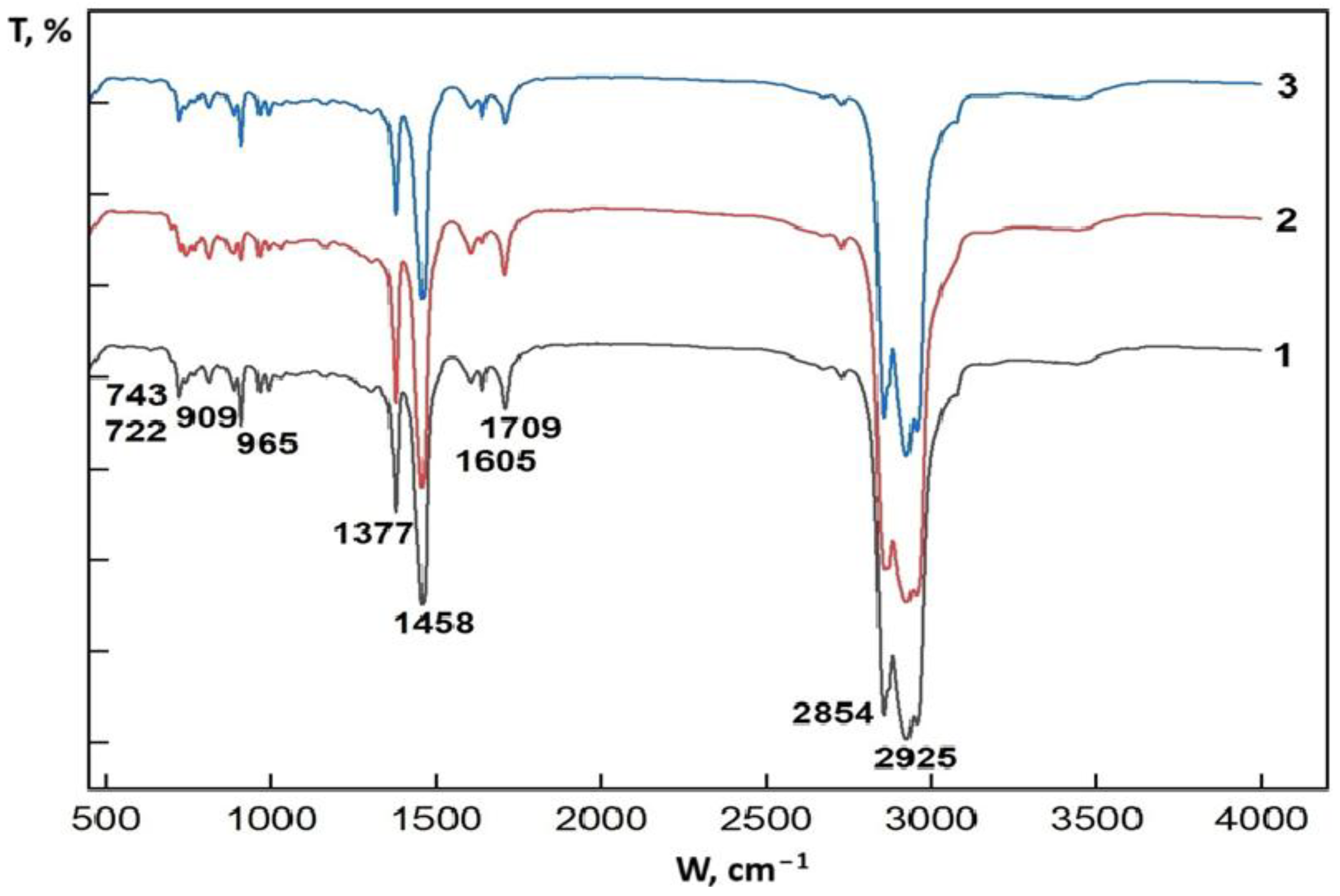
2.1. Infrared Spectroscopic Study of the Synthetic Oil
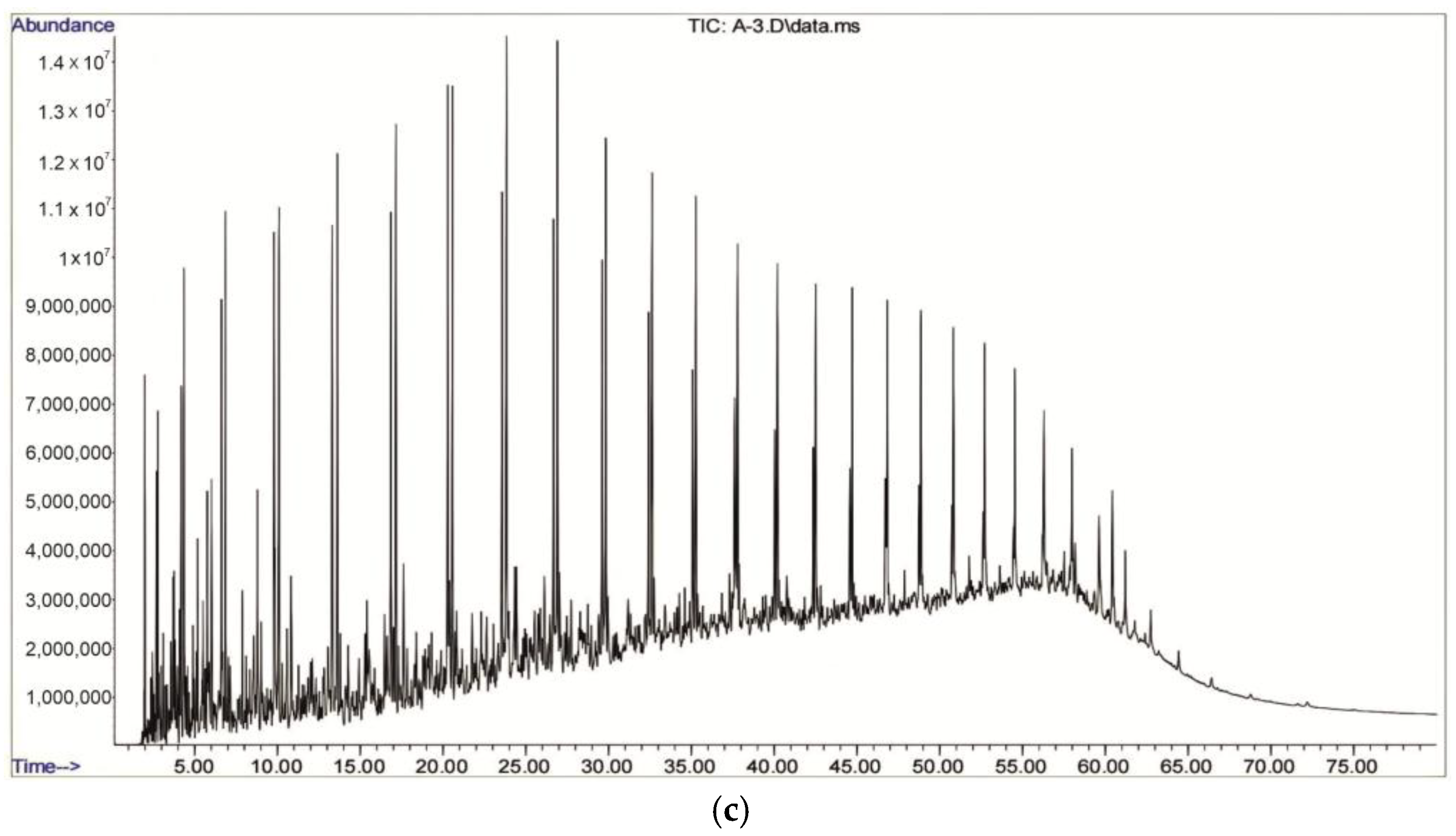
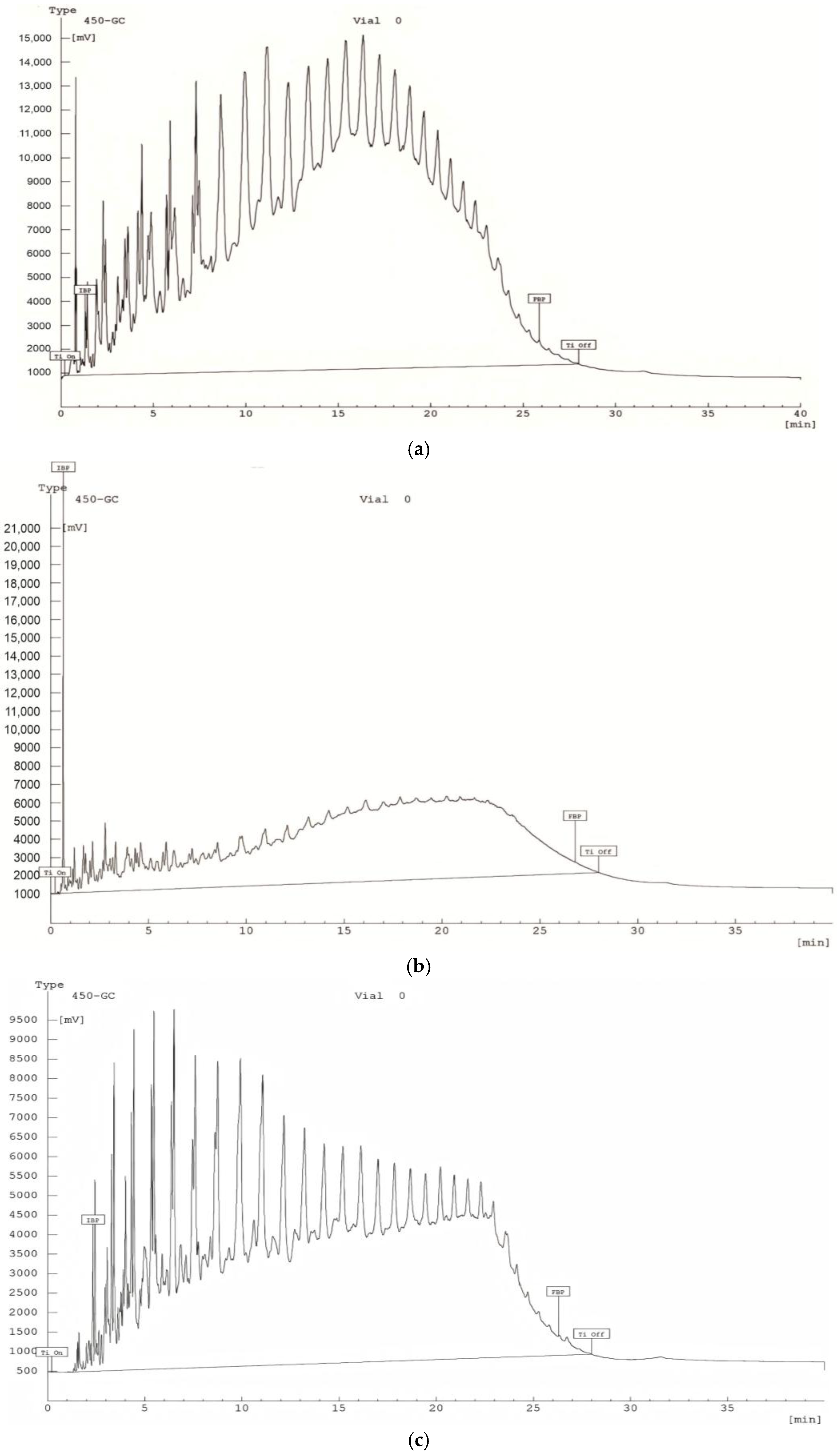
2.2. GC–MSD Analysis of the Composition of Synthetic Oil
- Effective removal of heavy and polar components (such as asphaltenes and resins) that may interfere with accurate analysis;
- Clear separation of the petroleum mixture into classes of hydrocarbons and other organic compounds;
- Enhanced sensitivity and reproducibility of chromatographic analysis;
- More comprehensive and reliable information about the chemical composition of synthetic oil from oil sand.
- Asphaltenes and resins are absent or present in extremely low concentrations, as these heavy, high-molecular-weight compounds are retained in the adsorption column. This method enables the separation of crude oil into lighter and medium-molecular-weight fractions.
- The composition is predominantly characterized by aliphatic alkanes (e.g., n-hexane, heptane, octane, nonane), aliphatic cycloalkanes (e.g., derivatives of cyclopentane and cyclohexane), alkenes (e.g., 2,4,4-trimethyl-1-hexene, 1-heptene, 2-octene), and aromatic compounds (e.g., toluene, p-xylene).
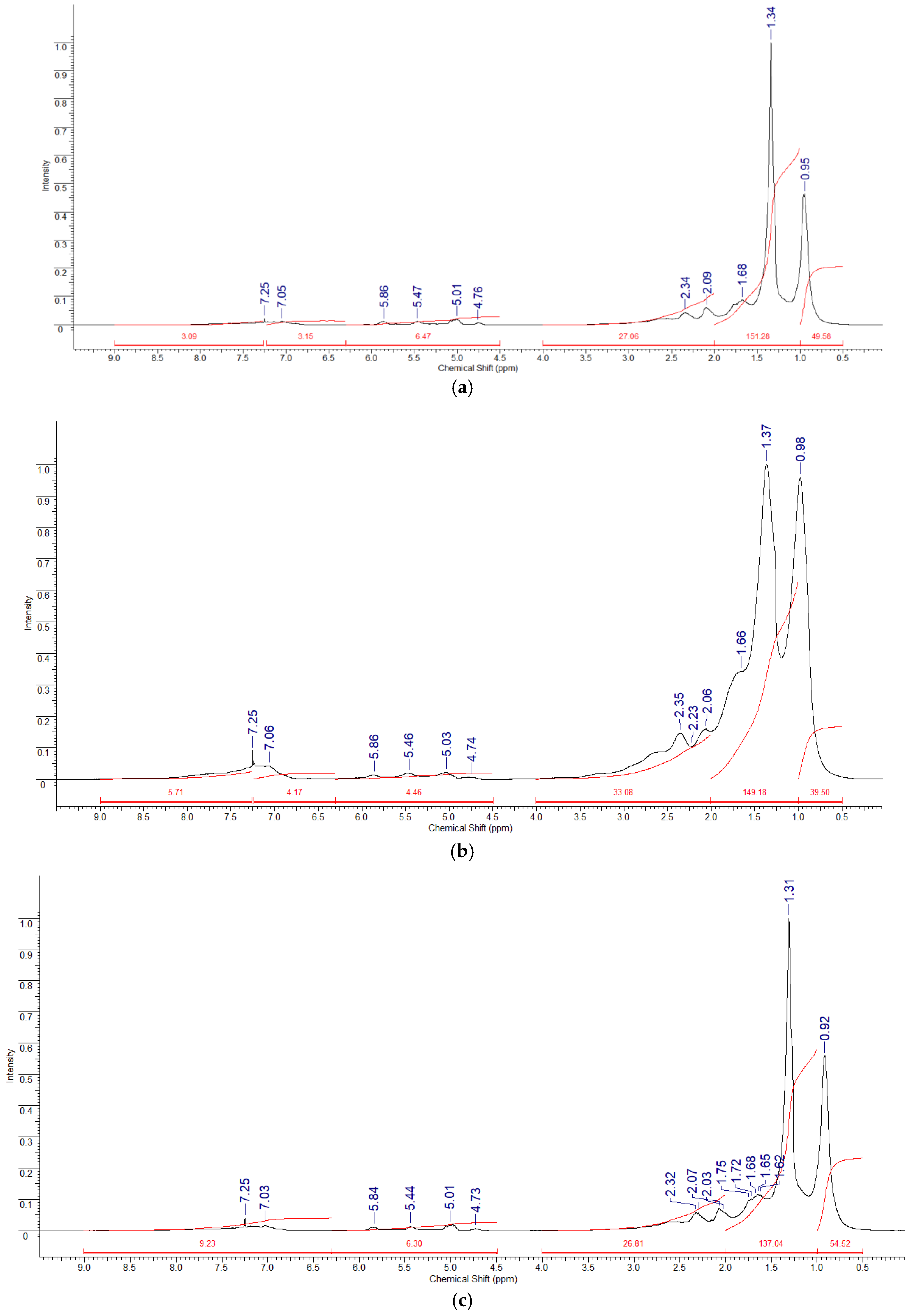
2.3. Analysis of the Structural Composition of the Synthetic Oil by NMR Spectroscopy
3. Materials and Methods
3.1. Raw Materials and Supplies
3.2. Pyrolysis Procedure
3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4. Gas Chromatography–Mass Spectrometry (GC–MSD)
3.5. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, V.; Haszeldine, R.S.; Tett, S.; Oschlies, A. Fossil fuels in a trillion-tonne world. Nat. Clim. Change 2015, 55, 419–423. [Google Scholar] [CrossRef]
- Gibson, J.J.; Peters, D.L. Water and environmental management in oil sands regions. J. Hydrol. Reg. Stud. 2022, 44, 101274. [Google Scholar] [CrossRef]
- Rosa, L.; Davis, K.F.; Rulli, M.C.; D’Odorico, P. Environmental consequences of oil production from oil sands. Earth’s Future 2017, 5, 158–170. [Google Scholar] [CrossRef]
- Tileuberdi, Y.; Mansurov, Z.; Ongarbayev, Y.; Tuleutaev, B. Structural study and upgrading of Kazakhstan oil sands. Eurasian Chem.-Technol. J. 2015, 17, 41–45. [Google Scholar] [CrossRef]
- Soroush, M.; Roostaei, M.; Hosseini, S.A.; Mohammadtabar, M.; Pourafshary, P.; Mahmoudi, M.; Ghalambor, A.; Fattahpour, V. Challenges and Potentials for Sand and Flow Control and Management in the Sandstone Oil Fields of Kazakhstan: A Literature Review. SPE Drill. Complet. 2021, 36, 208–231. [Google Scholar] [CrossRef]
- Bealessio, B.A.; Blánquez Alonso, N.A.; Mendes, N.J.; Sande, A.V.; Hascakir, B. A review of enhanced oil recovery (EOR) methods applied in Kazakhstan. Petroleum 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Hong, P.; Cha, Z.; Zhao, X.; Cheng, C.; Duyvesteyn, W. Extraction of bitumen from oil sands with hot water and pressure cycles. Fuel Process. Technol. 2013, 106, 460–467. [Google Scholar] [CrossRef]
- Abe, A.A.; Oliviero Rossi, C.; Caputo, P. Biomaterials and Their Potentialities as Additives in Bitumen Technology: A Review. Molecules 2022, 27, 8826. [Google Scholar] [CrossRef]
- Revuelta, M.B. Bituminous Materials. In Construction Materials; Springer: Cham, Switzerland, 2021; pp. 401–434. [Google Scholar] [CrossRef]
- Payzant, J.D.; Montgomery, D.S.; Strausz, O.P. Sulfides in petroleum. Org. Geochem. 1986, 9, 357–369. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Clifford, C.W.; Kwiatek, P.J.; Freund, H.; Afeworki, M.; Sansone, M.; Lamberti, W.A.; Pottorf, R.J.; Machel, H.G.; Peters, K.E.; et al. Characterization of solid bitumens originating from thermal chemical alteration and thermochemical sulfate reduction. Geochim. Cosmochim. Acta 2010, 74, 5305–5332. [Google Scholar] [CrossRef]
- Najib Razali, M.; Izatie Ismanni, N.; Mohd Zuhan, M.; Musa, M.; Hamid Nour, A. Formulation of modified bitumen emulsions from polymer wastes and used motor oil. Mater. Today Proc. 2021, 41, 14–18. [Google Scholar] [CrossRef]
- Zuodong, L.; Hongjun, W.; Blackbourn, G.; Feng, M.; Zhengjun, H.; Zhixing, W.; Zhaoming, W.; Zi, Y.; Tiansi, L.; Zhenzhen, W. Heavy Oils and Oil Sands: Global Distribution and Resource Assessment. Acta Geol. Sin. 2019, 93, 199–212. [Google Scholar] [CrossRef]
- Seidy-Esfahlan, M.; Tabatabaei-Nezhad, S.A.; Khodapanah, E. Comprehensive review of enhanced oil recovery strategies for heavy oil and bitumen reservoirs in various countries: Global perspectives, challenges, and solutions. Heliyon 2024, 10, e37826. [Google Scholar] [CrossRef] [PubMed]
- Alade, O.; Isah, M.; Abdel-Azeim, S.; Shakil Hussain, S.M.; Kamal, M.S.; Mahmoud, M.; Al Shehri, D.; Saibi, H.; Adewunmi, A.A.; Murtaza, M.; et al. Review on Applications of Ionic Liquids (ILs) for Bitumen Recovery: Mechanisms, Challenges, and Perspectives. Energy Fuels 2023, 37, 8718–8738. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Q. Bitumen Coating on Oil Sands Clay Minerals: A Review. Energy Fuels 2019, 33, 5933–5943. [Google Scholar] [CrossRef]
- Lin, F.; Stoyanov, S.R.; Xu, Y. Recent Advances in Nonaqueous Extraction of Bitumen from Mineable Oil Sands: A Review. Org. Process Res. Dev. 2017, 21, 492–510. [Google Scholar] [CrossRef]
- Tileuberdi, Y.; Ongarbayev, Y.; Imanbayev, Y.; Yermekova, A.; Behrendt, F.; Ismailova, A.; Zhanbekov, K.; Seilkhan, A.; Mansurov, Z. Studying Characteristics of Natural Bitumen of Oil Sand with Comparison to Heavy Crude Oil. ES Mater. Manuf. 2023, 22, 1035. [Google Scholar] [CrossRef]
- Ongarbayev, Y.; Golovko, A.; Krivtsov, E.; Tileuberdi, E.; Imanbayev, Y.; Tuleutayev, B.; Mansurov, Z. Thermocatalytic cracking of Kazakhstan’s natural bitumen. Stud. UBB Chem. 2014, 59, 57–64. [Google Scholar]
- Chrones, J.; Germain, R.R. Bitumen and heavy oil upgrading in Canada. Pet. Sci. Technol. 1988, 7, 783–821. [Google Scholar] [CrossRef]
- Park, Y.C.; Paek, J.Y.; Bae, D.H.; Shun, D. Study of pyrolysis kinetics of Alberta oil sand by thermogravimetric analysis. Korean J. Chem. Eng. 2009, 26, 1608–1612. [Google Scholar] [CrossRef]
- Farnand, J.R.; Meadus, F.W.; Sparks, B.D. Removal of intractable fine solids from bitumen solutions obtained by solvent extraction of oil sands. Fuel Process. Technol. 1985, 10, 131–144. [Google Scholar] [CrossRef]
- Jing, L.; Xin, C.; Santander, C.; Tan, X.; Liu, Q.; Zeng, H. Destabilization of fine solids suspended in oil media through wettability modification and water-assisted agglomeration. Fuel 2019, 254, 115623. [Google Scholar] [CrossRef]
- Saleh, M.; Yani, S.; Nurjannah, N. Hot Water Extraction on Aspal Buton. J. Phys. Conf. Ser. 2022, 2394, 012037. [Google Scholar] [CrossRef]
- Bukharin, N.; El Hassan, M.; Nobes, D.; Omelyanyuk, M. Reducing energy consumption during bitumen separation from oil sand. Energy Rep. 2020, 6, 206–213. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, Q.; Zhao, C.; Zhou, W.; Wang, C. Solvent Extraction for Separation of Indonesian Oil Sands. Int. J. Environ. Res. Public Health 2023, 20, 4527. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, M.; Zhang, Z.; Zhang, D. Pyrolysis of an Indonesian oil sand on a thermogravimetric analyser and a fixed-bed reactor. J. Anal. Appl. Pyrolysis 2016, 117, 191–198. [Google Scholar] [CrossRef]
- Tileuberdi, Y.; Ongarbayev, Y.; Mukatayeva, Z.; Zhanbekov, K.; Mukhambetkaliyev, K.; Akkazin, Y.; Shadin, N.; Imanbayev, Y. Studying Characteristics of Hot Fine-Grained Asphalt Concrete with the Addition of Coked Sands from the Pyrolysis of Oil Sands. Processes 2024, 12, 2540. [Google Scholar] [CrossRef]
- Smith, B. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 2018; p. 288. [Google Scholar] [CrossRef]
- Rudyk, S.; Ongarbayev, Y.; Spirov, P. Feature selection in GC-MS, NMR and MALDI-TOF spectra of tar sand bitumen. Unconv. Resour. 2023, 3, 61–71. [Google Scholar] [CrossRef]
- Daniel, J.; O’Donnell, S.; Sigle, K.; Darrell, B.; Sturm, G.P.; Vogh, J.W. Characterization of high-boiling petroleum distillate fractions by proton and 13C nuclear magnetic resonance spectrometry. Fuel 1980, 59, 166–174. [Google Scholar] [CrossRef]
- Wang, R.; Luo, Y.; Jia, H.; Ferrell, J.R.; Haoxi, B. Development of quantative 13C NMR characterization and simulation of C, H and O content for pyrolysis oils based on 13C NMR analysis. RSC Adv. 2020, 10, 25918–25928. [Google Scholar] [CrossRef]


| № | Oil Sand Samples | Synthetic Oil, wt.% | Mineral Part, wt.% | Separated Gases, wt.% | Water Content, wt.% |
|---|---|---|---|---|---|
| 1 | Beke | 10.2 ± 0.3 | 88.0 ± 0.3 | 1.5 ± 0.1 | 0.3 ± 0.01 |
| 2 | Munaily-Mola | 13.9 ± 0.3 | 84.0 ± 0.3 | 1.7 ± 0.1 | 0.4 ± 0.01 |
| 3 | Dongeleksor | 15.0 ± 0.4 | 83.0 ± 0.4 | 1.6 ± 0.1 | 0.6 ± 0.02 |
| Wavenumber (cm−1) | Band Assignment | Corresponding Functional Group | Interpretation |
|---|---|---|---|
| 2925, 2854 | Valence vibrations of C–H | –CH2, –CH3 | Aliphatic hydrocarbons (paraffins) |
| 1709 | Valence vibrations of C=O | Carbonyl group | Oxygen-containing compounds: ketones, acids, resinous components |
| 1605 | Fluctuations of C=C in the aromatic ring | Aromatic hydrocarbons | Benzene and naphthalene derivatives |
| 1458, 1377 | Deformation vibrations –CH3 and –CH2 | Methyl and methylene groups | Aliphatic chains |
| 965, 909, 743, 722 | Deformation vibrations C–H | Aromatic and cycloalkanes | Indicate the presence of rings, naphthenes, aromatic structures |
| № | Mass Fraction, % | Compound Name | Groups |
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| 1 | 0.04 | 2,4,4-Trimethyl-1-hexene | Alkenes |
| 2 | 0.72 | n-Hexane | Aliphatic alkanes |
| 3 | 0.07 | Cyclopentane, methyl | Aliphatic cycloalkanes |
| 4 | 0.19 | Cyclopentene, methyl | Alkenes |
| 5 | 0.04 | 2(3H)-Furanone, dihydro-5-methyl | Others (furanone, lactone) |
| 6 | 0.10 | Hexane, 3-methyl | Aliphatic alkanes |
| 7 | 0.29 | 1-Heptene | Alkenes |
| 8 | 0.32 | Heptane | Aliphatic alkanes |
| 9 | 0.53 | Cyclopentene, 4,4-dimethyl | Alkenes |
| 10 | 0.30 | Cyclohexane, methyl | Aliphatic cycloalkanes |
| 11 | 0.07 | Cyclopentane, ethyl | Aliphatic cycloalkanes |
| 12 | 0.46 | Cyclohexene, 4-methyl | Alkenes |
| 13 | 0.02 | Cyclobutanone, 2,3,3-trimethyl | Others (ketones) |
| 14 | 0.02 | 1,3,5-Hexatriene, 3-methyl | Alkenes |
| 15 | 0.12 | 2,4-Heptadiene, (E,E) | Alkenes |
| 16 | 0.42 | Heptane, 2-methyl | Aliphatic alkanes |
| 17 | 0.24 | Toluene | Aromatic compounds |
| 18 | 0.08 | Cyclohexane, 1,2-dimethyl | Aliphatic cycloalkanes |
| 19 | 0.17 | 1-Heptene, 2-methyl | Alkenes |
| 20 | 0.59 | 2-Octene | Alkenes |
| 21 | 0.69 | Octane | Aliphatic alkanes |
| 22 | 0.24 | 3-Octene | Alkenes |
| 23 | 0.32 | Cyclopentene, 1,2,3-trimethyl | Alkenes |
| 24 | 0.22 | Cyclohexane, ethenyl | Aliphatic cycloalkanes |
| 25 | 0.10 | Bicyclo[3.1.0]hexane, 1,5-dimethyl | Aliphatic cycloalkanes |
| 26 | 0.04 | Cyclohexanol, 2,6-dimethyl | Others (alcohols) |
| 27 | 0.63 | Cyclohexane, 1,1,3-trimethyl | Aliphatic cycloalkanes |
| 28 | 0.12 | Cyclohexene, 1,6-dimethyl | Alkenes |
| 29 | 0.05 | 1-Octene, 3-methyl | Alkenes |
| 30 | 0.26 | 1-Heptene, 2,6-dimethyl | Alkenes |
| 31 | 0.08 | Nonane, 4-ethyl-5-methyl | Aliphatic alkanes |
| 32 | 0.23 | Cyclopentane, 2-ethylidene-1,1-dimethyl | Aliphatic cycloalkanes |
| 33 | 0.20 | Cyclohexane, 1,2,3-trimethyl | Aliphatic cycloalkanes |
| 34 | 0.33 | p-xylene | Aromatic compounds |
| 35 | 0.05 | Ethylidenecycloheptane | Aliphatic cycloalkanes |
| 36 | 0.15 | 2,4-Dimethyl-1-heptene | Alkenes |
| 37 | 0.13 | Azetidine-2-one, 3-hexyl-3-methyl | Others (azetidine) |
| 38 | 0.04 | Cyclohexane, 1-ethyl-2-methyl | Aliphatic cycloalkanes |
| 39 | 1.07 | 1-Nonene | Alkenes |
| 40 | 0.04 | Cyclohexene, 3,3,5-trimethyl | Alkenes |
| 41 | 1.20 | Nonane | Aliphatic alkanes |
| 42 | 0.10 | 2-Nonene | Alkenes |
| 43 | 0.09 | 1-Octene, 3,7-dimethyl | Alkenes |
| 44 | 0.03 | cis-2-Nonene | Alkenes |
| 45 | 0.07 | 1,3-Hexadiene, 3-ethyl-2-methyl | Alkenes |
| 46 | 0.03 | Cyclohexane, 2-propenyl | Aliphatic cycloalkanes |
| 47 | 0.07 | 4-Nonyne | Alkynes |
| 48 | 0.08 | Cyclohexane, propyl | Aliphatic cycloalkanes |
| 49 | 0.14 | Octane, 2,6-dimethyl | Aliphatic alkanes |
| 50 | 0.05 | 3,6-Nonadien-1-ol | Others (alcohols) |
| δ(1H) ppm | Atom Designation | Functional Group |
|---|---|---|
| 0.5–1.0 | Hγ | CH3 groups of saturated compounds. CH3 groups in γ- and further positions to the aromatic ring |
| 1.0–2.0 | Hβ | CH2 and CH groups of saturated compounds. Hydrogen atoms of β-methyl, β- and further methylene and methine groups at the aromatic ring |
| 2.0–4.0 | Hα | Hydrogen atoms in the α-position to aromatic and carbonyl carbons, heteroatoms |
| 4.5–6.3 | Holef | Hydrogen atoms of olefin groups |
| 6.3–9.0 | Har+ph | Hydrogen atoms of aromatic rings, phenolic hydroxyls |
| δ(13C) ppm | Atom Designation | Functional Group |
|---|---|---|
| 7–17 | Cp,m | Primary carbon atoms at the methylene group |
| 17–25 | Cp,ar | Primary carbon atoms bonded to the CH group or aromatic ring |
| 17–50 | C(sec+quart) | Secondary and quaternary C atoms of saturated compounds |
| 25–65 | Calip | Aliphatic CH groups |
| 25–50 | Cquar | Quaternary carbon atoms of saturated compounds |
| 108–118 | Colef | Olefin fragments |
| 110–135 | Car | Tertiary C atoms of aromatic systems |
| 130–137 | Cm, ar | Methyl-substituted aromatic carbon atoms |
| 137–148 | C(ar+alkyl) | Alkyl- and naphthyl-substituted carbon atoms of aromatic rings |
| 148–170 | Car | Aromatic carbon atoms substituted by a phenol or ether group |
| 170–200 | Ccarb | Carbonyl carbon atoms |
| Type of Atoms | Synthetic Oil Beke | Synthetic Oil Munaily-Mola | Synthetic Oil Dongeleksor |
|---|---|---|---|
| Har | 2.6 | 4.2 | 3.9 |
| Halip | 97.4 | 95.8 | 96.1 |
| Hα | 11.2 | 14.0 | 11.5 |
| Hβ | 62.9 | 63.2 | 58.6 |
| Hγ | 20.6 | 16.7 | 23.3 |
| Holef | 2.7 | 1.9 | 2.7 |
| Cp,m | 14.8 | 13.7 | 12.8 |
| Car | 3.7 | 3.9 | 1.6 |
| C(sec+quart) | 82.2 | 82.3 | 47.7 |
| C(ar+alkyl) | 0 | 0.1 | 37.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yermekova, A.; Tileuberdi, Y.; Seitkan, A.; Gabbassova, A.; Zhatkanbayev, Y.; Nurlybayeva, A.; Totenova, N.; Kotov, S. Advanced Spectroscopic Characterization of Synthetic Oil from Oil Sands via Pyrolysis: An FTIR, GC–MSD, and NMR Study. Molecules 2025, 30, 2927. https://doi.org/10.3390/molecules30142927
Yermekova A, Tileuberdi Y, Seitkan A, Gabbassova A, Zhatkanbayev Y, Nurlybayeva A, Totenova N, Kotov S. Advanced Spectroscopic Characterization of Synthetic Oil from Oil Sands via Pyrolysis: An FTIR, GC–MSD, and NMR Study. Molecules. 2025; 30(14):2927. https://doi.org/10.3390/molecules30142927
Chicago/Turabian StyleYermekova, Ainura, Yerbol Tileuberdi, Ainur Seitkan, Anar Gabbassova, Yerlan Zhatkanbayev, Aisha Nurlybayeva, Nurzada Totenova, and Stanislav Kotov. 2025. "Advanced Spectroscopic Characterization of Synthetic Oil from Oil Sands via Pyrolysis: An FTIR, GC–MSD, and NMR Study" Molecules 30, no. 14: 2927. https://doi.org/10.3390/molecules30142927
APA StyleYermekova, A., Tileuberdi, Y., Seitkan, A., Gabbassova, A., Zhatkanbayev, Y., Nurlybayeva, A., Totenova, N., & Kotov, S. (2025). Advanced Spectroscopic Characterization of Synthetic Oil from Oil Sands via Pyrolysis: An FTIR, GC–MSD, and NMR Study. Molecules, 30(14), 2927. https://doi.org/10.3390/molecules30142927







