Abstract
Natural deep eutectic solvents (NADESs) are emerging green solvents widely applied to improve the extraction of essential oil (EO) through plant tissue pretreatment. Various NADESs, formulated from polyalcohols, sugars, and organic acids, were employed as pretreatment solvents prior to microwave-assisted hydrodistillation (MAHD) to facilitate plant cell wall breakdown and improve the efficiency of EO extraction. The findings revealed that the most effective pretreatment conditions for enhancing EO extraction involved using a NADES composed of choline chloride and glycerol (in a 1:2 molar ratio), applied to fennel seed powder at a solid-to-NADES ratio of 1:6 g/mL. Optimal performance was achieved with 20% water content in the NADES, microwave irradiation at 400 W for 6 min, followed by 96 min of MAHD. Under these conditions, the NADESs-based MAHD achieved the highest EO yield, increasing it from 1.33% with water-based MAHD to 2.70%. Fennel EO demonstrated the strongest antimicrobial activity against S. pyogenes and C. albicans., while the EO obtained from NADES-MAHD using Ch:Gly (1:2) showed the highest antioxidant activity, with 72.41% inhibition. Finally, GC-MS phytochemical analysis of the extracted EOs revealed anethole as the major compound. Notably, the application of NADES, particularly Ch:Gly (1:2), enhanced the relative content of monoterpene hydrocarbons. These findings highlight the superior effectiveness of deep eutectic solvents during the pretreatment stage in enhancing EO production.
1. Introduction
Fennel (Foeniculum vulgare) is a flowering plant originating from the Mediterranean but has become naturalized in many parts of the world [1], as shown in Figure 1. It is widely recognized for its broad range of applications in numerous fields, and traditional medicine frequently prescribes it for kidney stones, vomiting, diarrhea and neurological disorders. In addition to its antiulcer and antiseptic properties, this plant is a good carminative and antispasmodic [2,3,4]. Because fennel seeds contain a number of nutrients, including vitamins and minerals, they are frequently utilized in cooking recipes. It is also used in the culinary, cosmetics, fragrance, and pharmaceutical industries, mostly in beverages and confections [5,6]. In addition, this plant offers added value through the production of essential oils (EOs), which are aromatic compounds extracted and separated from the aqueous phase after distillation.

Figure 1.
Morphological characteristics of fennel (Foeniculum vulgare) seeds.
EOs are concentrated natural oils extracted rich in aromatic compounds, commonly used in aromatherapy for their therapeutic benefits. EOs from Foeniculum vulgare exhibit hepatoprotective and antidiabetic effects in vivo models. Owing to their high trans-anethole content, these oils demonstrate potent antioxidant activity. Additionally, fennel EOs show antimicrobial effects against Gram-positive and Gram-negative bacteria, as well as fungi, highlighting their potential application in food preservation [7]. Given the diverse benefits of EOs, advancing efficient and sustainable extraction methods has become increasingly important.
EOs are commonly extracted using traditional methods such as hydrodistillation and steam distillation. However, these methods often require long extraction times and yield relatively small amounts of oil, which can lead to the loss of valuable components through hydrolysis or thermal degradation, especially in the case of unsaturated compounds. To address these issues, MAHD has emerged as a green, eco-friendly, and efficient alternative that combines microwave heating with traditional hydrodistillation principles. The development and adoption of MAHD EO extraction is highly desirable. Due to its advantages including shorter extraction time, faster heating, improved product quality, energy efficiency, and no direct contact between the plant and heat source, MAHD is widely used for extracting volatile compounds from various plants [8].
Additionally, pretreatment is essential for overcoming the natural resistance of plant cell walls and enhancing the release of intracellular bioactive compounds. Recently, deep eutectic solvents (DESs) have emerged as a novel class of green solvents offering a promising alternative for such applications. They are typically made by mixing hydrogen bond donors (HBD) and hydrogen bonds acceptors (HBA) in various molar ratios; this results in a combination that has a lower melting point because of the hydrogen bonding. Low toxicity, affordability, biodegradability, broad liquid range, low vapor pressure, non-flammability, chemical and thermal stability, ease of synthesis with excellent purity, water compatibility, and strong solubilizing power are only a few benefits of DESs. DESs made from solely natural substances are termed natural deep eutectic solvents (NADESs) [9]. NADESs have emerged as versatile green solvents, enabling the efficient extraction of diverse bioactive compounds from plants through a variety of advanced extraction techniques. Antioxidant compounds from Thai pigmented rice bran [10], Anthocyanins from chokeberry fruit [11] and from black raspberry [12], hydrophilic and lipophilic compounds from brown seaweeds [13], and coumarins from Angelicae pubescentis [14] were obtained by ultrasonically assisted extraction (UAE) with the assistance of NADES. Curcuminoids were reported to be extracted from turmeric by heating turmeric powder in the presence of NADES [15]. NADES were demonstrated as promising green solvents that can efficiently extract triterpene saponins from Aralia elata roots, in some cases outperforming conventional alcohol-based methods [16]. Microwave-assisted hydrodistillation (MAHD) is an advanced technique that overcomes limitations of traditional methods by enabling faster and more efficient extraction of essential oils from diverse plant materials. MAHD utilizes microwave radiation to heat the water within plant cells, leading to evaporation and expansion that generate high internal pressure. This pressure stretches and may rupture the oil gland cell walls, thereby facilitating the release of essential oils or bioactive compounds. The process benefits from a synergistic effect of heat and mass transfer, which work together to enhance extraction efficiency. Compared to conventional methods, MAHD is a green and innovative extraction technique that offers rapid extraction, higher essential oil quality and yield, energy efficiency, cost-effectiveness, and minimal solvent usage, thereby reducing both cost and environmental impact [17]. However, the technique also has limitations, particularly the risk of thermal degradation of thermo-sensitive compounds under excessive microwave power or prolonged heating that influence yield and product quality [18]. NADESs have been mostly used with microwave-assisted extraction method [19]. Rapid heating by microwave together with the advantages of NADES make this combination attractive. The microwave hydrodistillation based on deep eutectic solvent has been applied in the extraction of EOs from Amomum Species [20], Liquidambar formosana leaves and fruits [21], and turmeric [22]. In addition, we previously reported the use of DES-based microwave-assisted hydrodistillation (DES-MHD) and ultrasonic-assisted DES pretreatment followed by microwave-assisted hydrodistillation (U-DES-MHD) of clove [23].
Despite the recognized potential of EO extraction from F. vulgare, studies focusing on green pretreatment techniques to enhance EO yield are still limited to only a few reports. To address this research gap, the objective of this study is to evaluate a novel green extraction method, employing NADES-based pretreatment with microwave-assisted hydrodistillation (MAHD) for the efficient recovery of EOs from F. vulgare seeds. The investigation will focus on varying parameters in the pretreatment stage. Those parameters include the type of NADES, solid-to-NADES ratio, water content, microwave power, and pretreatment duration. Furthermore, the extracted EOs were evaluated for their antimicrobial and antioxidant activities, along with an analysis of their phytochemical compositions.
2. Results
2.1. Effects of NADES Components
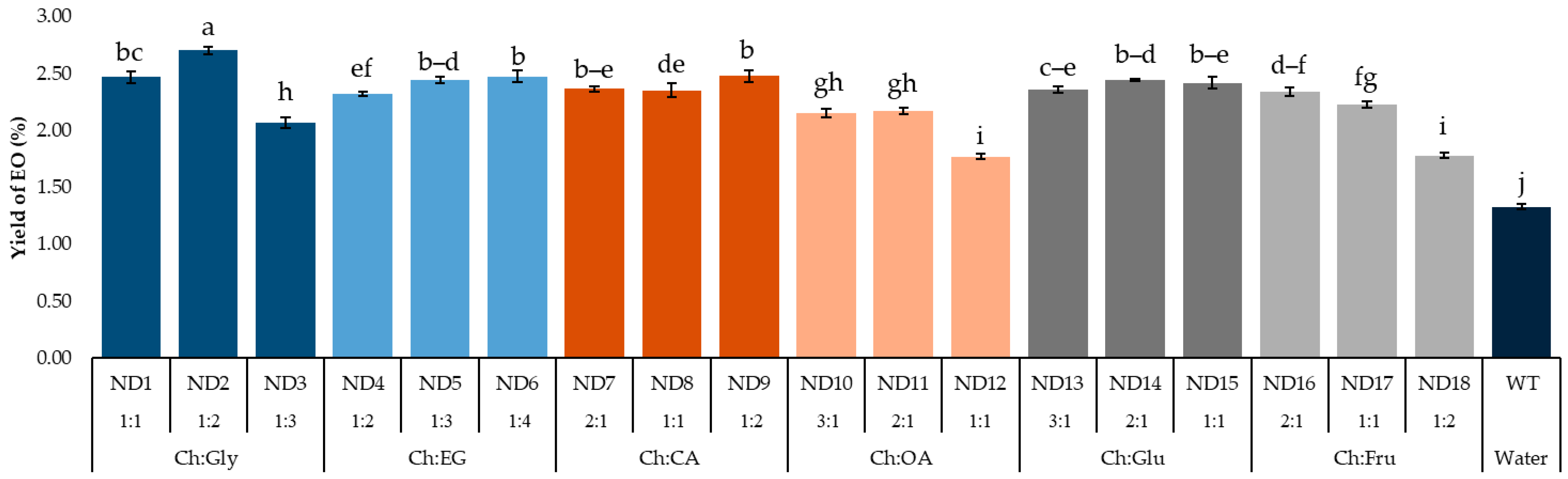
Efficiency of NADES-MAHD using different types and molar ratios of NADESs represented as EO extraction yields shown in Figure 2. When microwave irradiation is used in an extraction, the dipole moment of the medium directly affects the extraction efficiency. This is because polar molecules can absorb microwave irradiation, which results in fast heating and breaking of cell structure. This phenomenon results in releasing compounds from the cell [24,25]. Furthermore, variations in the chemical structures of DESs affect EO yields due to their differing polarities and cellulose-dissolving capabilities [26].
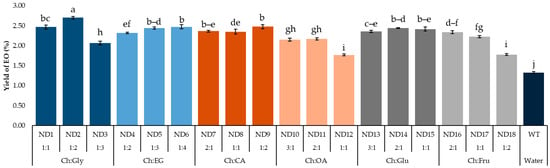
Figure 2.
EO yield from F. vulgare with different NADESs. Data are presented as mean ± standard deviation (SD) from n = 3 independent experiments. Abbreviations are as follows—Ch: Choline chloride; Gly: Glycerol; EG: Ethylene glycol; CA: Citric acid; OA: Oxalic acid; Glu: Glucose; Fru: Fructose; EOs: Essential oils. Different lowercase letters indicate significant differences among groups based on Tukey’s pairwise comparison test (p < 0.05); bars sharing the same letter are not significantly different, while bars with different letters are significantly different in the measured response.
EO yields were in the range of 1.33–2.70%. The highest yield was obtained using Ch:Gly at a molar ratio of 1:2 (ND2) as NADES. Therefore, it was selected for further investigation. It is worth mentioning that, although not giving the highest extraction yield, NADES composed of Ch:EG, Ch:CA and Ch:Glu provided remarkable high yield at 2.32–2.48% for all ratios in this study.
Due to the similar dipole moments of glycerol and ethylene glycol, NADESs based on Ch:Gly and Ch:EG showed comparable extraction yields. For carboxylic acids, an overall yield of EOs from Ch:CA was found to be higher than that of Ch:OA. This effect can be attributed to the intrinsic properties of citric acid, which exhibits a relatively high dipole moment and an abundance of hydroxyl and carboxyl groups, thereby promoting extensive hydrogen-bonding interactions that enhance the structural stability of the system [27].
Glucose was reported slightly higher dipole moment than fructose. This is due to the different ring size and arrangement of hydroxyl groups. As a result, when used as NADESs in NADES-MAHD, Ch:Glu represented a higher average yield of EOs than that from Ch:Fru. The polarity of DESs varies depending on the hydrogen bond donor (HBD) component used. Organic acid-based DESs are generally the most polar, while sugar- and polyalcohol-based DESs exhibit lower polarity, similar to that of methanol. Additionally, the polarity of a NADES can be fine-tuned by adjusting the molar ratio of its components. Since solvent polarity strongly influences the extraction efficiency of EO compounds, matching the polarity of the NADES with the oil composition typically leads to higher yields [28].
All types of NADES-based MAHD resulted in higher EO yields (1.77–2.70%.) compared to water-based MAHD without pretreatment (1.33%), indicating that the use of NADESs as a pretreatment for turmeric had a significant impact on enhancing EO yield. These interactions increase cell wall permeability, thereby facilitating EO extraction. As NADES are composed of natural plant-compatible components, they represent a promising green solvent for improving EO isolation [29].
Although Ch:Gly at a 1:2 molar ratio (ND2) provided the highest yield (2.70%), increasing the ratio to 1:3 (ND3) led to a significant decline (2.07%). Similar trends were observed for Ch:OA (ND12), with a yield of 1.77%, and Ch:Fru (ND18), with a yield of 1.78%. This reduction is likely due to the decreased amount of choline chloride, which lowered the proportion of hydrogen bond acceptors in the NADES system. Since hydrogen bonding is crucial for solubilizing bioactive compounds, especially those with polar functional groups like hydroxyls, this imbalance may impair extraction efficiency. These findings highlight the importance of maintaining an optimal donor-to-acceptor ratio in NADES formulations [30].
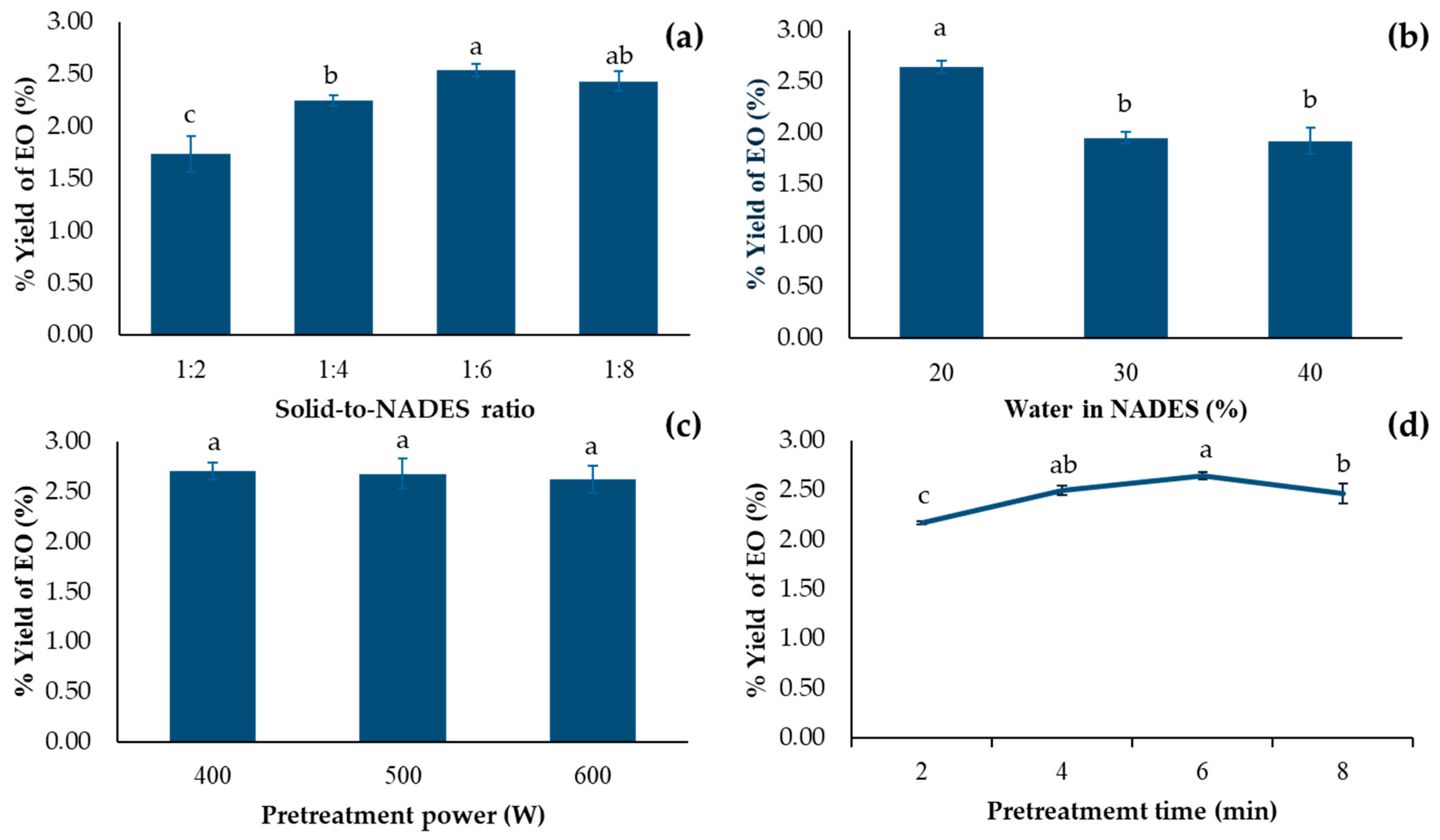
2.2. Effects of Solid-to-NADES Ratio
Solid-to-NADES (S/N) ratio is one of the most important parameters in the pretreatment step of EO extraction, as shown in Figure 3a. With the small amount of NADES, i.e., high S/N ratio (1:2 and 1:4), low EO yield were obtained. The reason behind this was the saturation of EO in solvent, which led to an incomplete extraction. In addition, too little amount of solvent could lead to a complete evaporation, which resulted in burning of the seeds [31]. The higher yield was obtained when increasing quantity of NADES-lowering S/N ratio. This is probably because of a larger concentration gradient of EO between matrix and the solvent [32]. However, too low S/N ratio (1:8) resulted in dropping of extracted yield. This is because, with a low S/N ratio, longer time is required due to high dilution [31]. With pretreatment in this condition, it was possible that the pretreatment step was not completed. Therefore, the optimal S/N ratio for NADES-MAHD of EO from fennel seed was 1:6 g/mL.
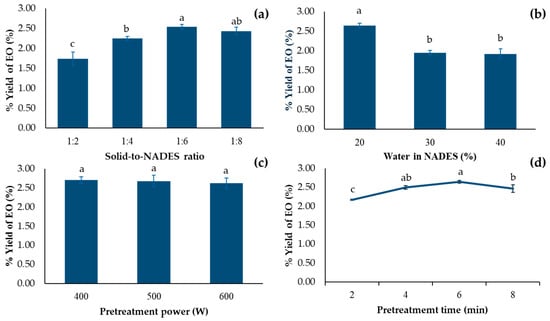
Figure 3.
EO yield from F. vulgare with different (a) amount NADESs (b) water content in NADES (c) pretreatment power and (d) pretreatment time. Abbreviations are as follows—NADES: Natural deep eutectic solvent; EOs: Essential oils. Data are presented as mean ± standard deviation (SD) from n = 3 independent experiments. Different lowercase letters indicate significant differences among groups based on Tukey’s pairwise comparison test (p < 0.05); bars sharing the same letter are not significantly different, while bars with different letters are significantly different in the measured response.
2.3. Effect of Water Content in NADES
Water plays a major role in NADES properties. High viscosity is one of the distinct characteristics of NADES due to the extensive hydrogen bonding. Adding water to NADES decreased this viscosity, making solvent molecules easier to contact fennel seeds [33]. However, excessive water results in decreasing interaction between NADES and targeted molecules [24].
A water content of 20–30% in NADES is generally recommended, as it can enhance the extraction efficiency of both polar and non-polar compounds [30]. However, the optimal water level should be tailored to suit each specific application. With 3 different levels of water in NADES (20%, 30% and 40%), the 20% formulation provided the highest extraction yield (2.65%), as shown in Figure 3b.
In addition to decreasing viscosity, water in NADES also affected the hydrogen-bond network of NADES, a phenomenon that disrupts the plant cell wall or improves the solubility of EOs. With too high water content, NADES loses this network, hence the decreased extracted yield [34,35]. Different water content also alternated NADES polarity. Higher water content made NADES more polar, which was undesired for the extraction of non-polar EOs.
Recent studies have shown that water content influences not only the viscosity of lactic acid-based NADES but also several physicochemical and antimicrobial properties. Dilution with water weakens hydrogen bonding, decreases density and refractive index, increases pH, and significantly reduces viscosity and surface tension. Moreover, higher water content increases water activity and leads to a reduction in antimicrobial activity. These findings highlight the importance of controlling water content when designing NADES for green extraction and antimicrobial applications [36].
2.4. Effect of Pretreatment Power
Microwave power was suspected to improve extraction efficiency by inducing molecular vibrations that disrupt plant cell walls during the pretreatment stage, thereby facilitating dissolution and allowing EOs to diffuse out [26]. Previous study found the obvious different extracted yield when using different microwave power in MAHD step [37]. However, as shown in Figure 3c, no significant difference in EO yield was observed when the microwave power was increased from 400 to 600 W, with yields ranging from 2.65% to 2.71%. Therefore, 400 W was the optimum microwave power for this step.
2.5. Effect of Pretreatment Time
The extraction procedure consisted of two steps: NADES pretreatment and MAHD. Besides NADES compositions, parameters including pretreatment duration were investigated. Pretreatment time directly affected the yield of EO. As shown in Figure 3d, increasing the pretreatment time from 2 to 6 min resulted in an increase in EO recovery yield from 2.17% to 2.65%. Prolonging the pretreatment to 8 min resulted in a reduced recovery yield of 2.47%, which may be attributed to the thermal degradation of the EOs [38]. Considering power consumption and the lack of significant difference between pretreatment durations of 4 min and 6 min, the 4 min pretreatment was identified as the most suitable option.
2.6. Antimicrobial Activities
All fennel EOs were examined for their antimicrobial activity against four Gram-positive and four Gram-negative bacteria, as outlined in Table 1. In comparison to the antibiotic tetracycline, which served as positive control, all EOs exhibited significantly lower antimicrobial activities. Notably, every EOs extracted using MAHD, including the sample that did not undergo NADES pretreatment, demonstrated the strongest antimicrobial activity against S. pyogenes. Specifically, the EOs extracted with Ch:Gly (1:2) and Ch:CA (1:2) were particularly effective, showing the most potent antibacterial effects. Additionally, all EOs were active against L. monocytogenes and Salmonella Typhi. However, none of the EOs demonstrated the ability to inhibit P. aeruginosa. These findings indicate that NADESs did not alter the antimicrobial activity of the EOs. Previous studies have described fennel oil as being antimicrobial against S. aureus and E. coli [39,40]. Although Naaz et al. reported that fennel oil inhibited P. aeruginosa, this activity was not observed in the current study [40]. Minimum inhibitory concentration (MIC) of EOs ranged from 1.56 to 25 mg/mL (Table 2). EO extracted with Ch:CA (1:2) pretreatment seems to be the most active against bacteria. All EOs were found to be most active against Str. Pyogenes and least active against P. aeruginosa. These results correspond to an agar disk diffusion experiment.

Table 1.
Agar disk diffusion of EOs extracted by NADES-MAHD and without NADES.

Table 2.
Minimum inhibitory concentration (MIC) of EOs extracted by NADES-MAHD and without NADES.
The antifungal activities of fennel EO extract are detailed in Table 1. For each type of NADES, only the EO with the highest extraction yield was used to evaluate antifungal activity. The most effective extract against C. albicans was obtained from MAHD without NADES pretreatment. Although NADES pretreatment resulted in slightly lower antifungal inhibition compared to MAHD alone, it still offered advantages in extraction efficiency. Notably, EOs obtained using NADESs maintained antifungal activity that exceeded that of the positive control, nystatin. Among the NADES extracts, Ch:Fru (2:1) exhibited the highest inhibition activity at 11.40 ± 0.27 mm, followed closely by Ch:CA (1:2) at 11.35 ± 0.25 mm, and Ch:Gly (1:2) at 10.68 ± 0.15 mm. These findings align with previous research [41], which also demonstrated the antifungal properties of fennel oil against C. albicans. EOs are recognized for their effective antifungal activity against C. albicans and show great potential for future medical applications due to their low toxicity and multifunctional properties [42].
In addition to their antimicrobial properties, it is important to consider the cytotoxic potential of fennel essential oil. Sharopov et al. (2017) reported that the essential oil of fennel seeds exhibits low cytotoxicity against various cancer cell lines, including HeLa (human cervical cancer), Caco-2 (human colorectal adenocarcinoma), MCF-7 (human breast adenocarcinoma), CCRF-CEM (human T lymphoblast leukemia), and CEM/ADR5000 (adriamycin-resistant leukemia), compared to the chemotherapy drug doxorubicin [43].
2.7. Antioxidant Activity
Antioxidant capacity of EOs is shown in Table 3. It appears that, besides elevating extraction yield, NADES also affects the activity of the resulting EOs. While most of the EOs exhibited inhibition in the same range as that from MAHD without NADES, the EO obtained from NADES-MAHD using Ch:Gly (1:2) as NADES showed a higher activity, with 72.41% inhibition and an IC50 value of 8.03 ± 0.16 mg/mL. In contrast, when NADES was Ch:CA (1:2), the resulting EO had less activity, showing only 60.81% inhibition. Effects of NADES on EOs’ antioxidant capacity were not surprising as we previously found that NADES affected DPPH radical scavenging activity of EO from clove [23]. Nevertheless, the fennel seed EO exhibited lower antioxidant activity compared with the positive control, BHT, which showed an IC50 of 8.05 ± 0.95 µg/mL. However, according to [44], fennel EOs exhibited notable antioxidant activity, with IC50 values ranging from 11.83 to 36.90 mg/mL in the DPPH assay. The radical scavenging capacity of the EOs may be attributed to certain compounds that are capable of donating a hydrogen atom to the DPPH• free radical, thereby converting it to the reduced form, DPPH-H [44].

Table 3.
Antioxidant activity of EOs extracted by NADES-MAHD and without NADES.
Nevertheless, previous studies have suggested that compounds such as fenchone and anethole contribute to the phenolic content and antioxidant activity of fennel, while monoterpene hydrocarbons (e.g., those present in thyme and oregano) play a significant role in inhibiting oxidation–peroxidation [45]. Beyond the DPPH assay used in the present work, other evaluations have also demonstrated the antioxidant potential of fennel seed EO, including its inhibitory efficacy against lipid peroxidation and remarkable Fe-binding capacity, indicative of metal-chelating activity. Collectively, these findings reinforce the role of fennel EO as both a free radical scavenger and an oxidation protector through multiple mechanisms [40].
2.8. Volatile Phytochemical Compositions of Fennel Seed EOs
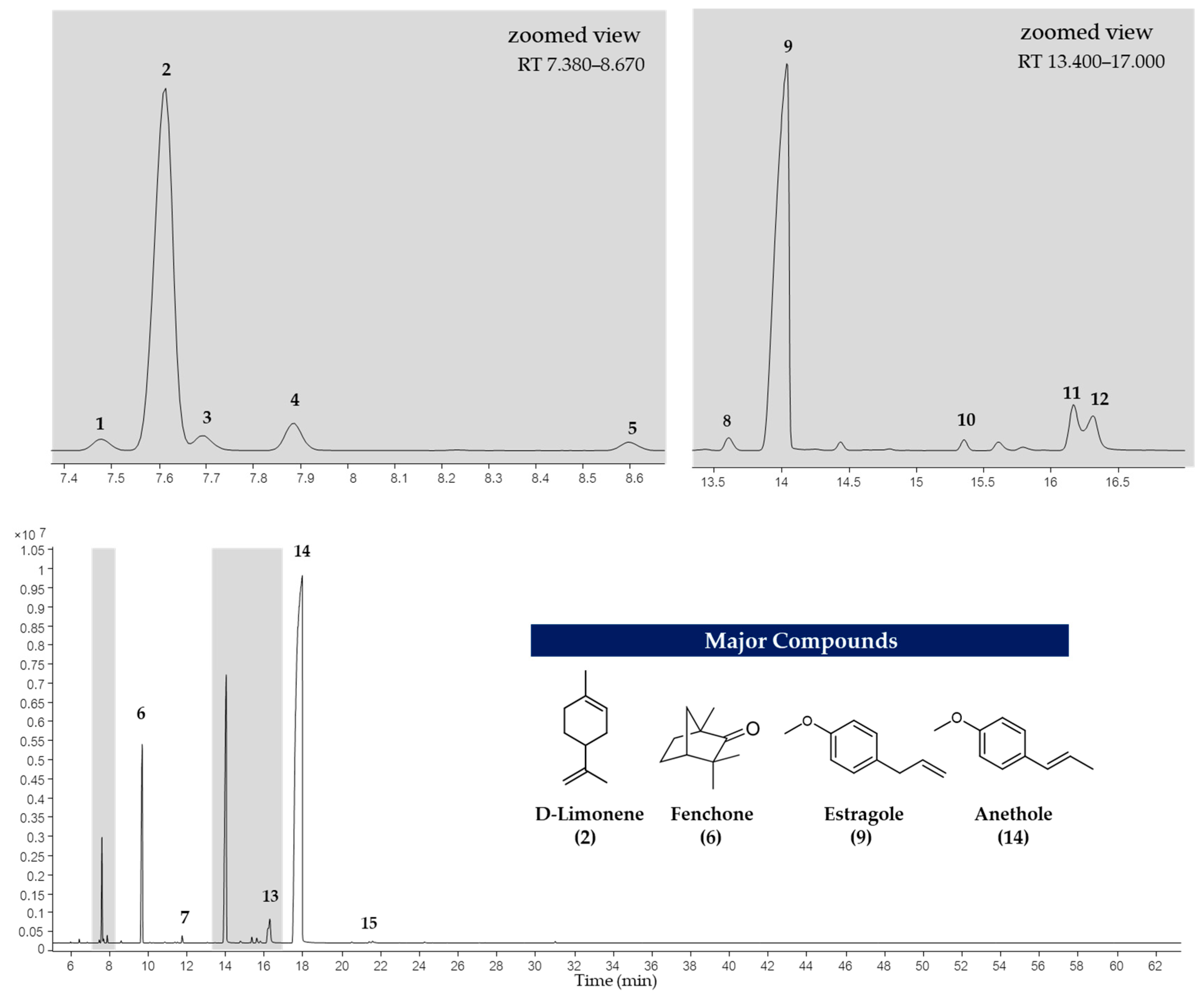
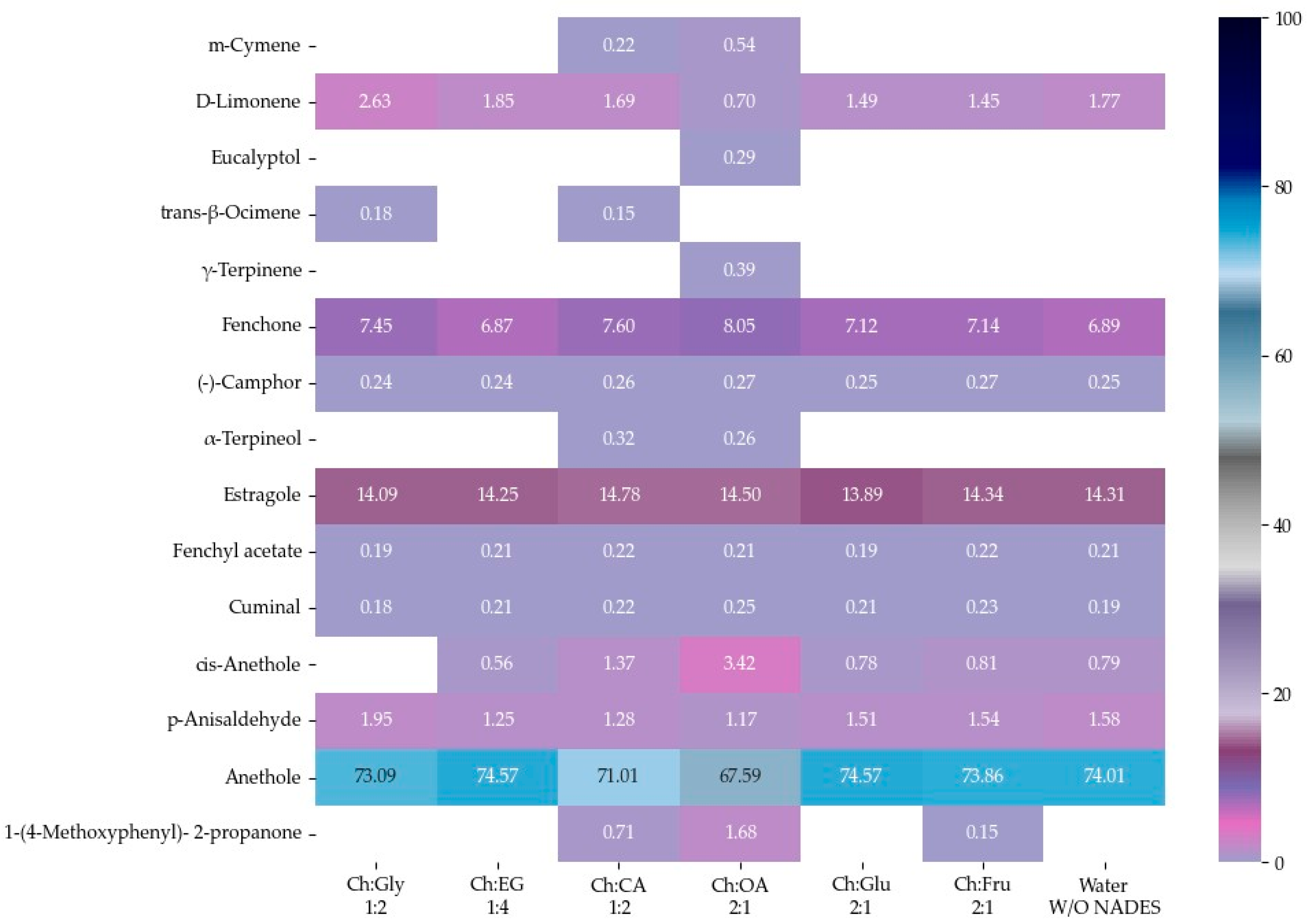
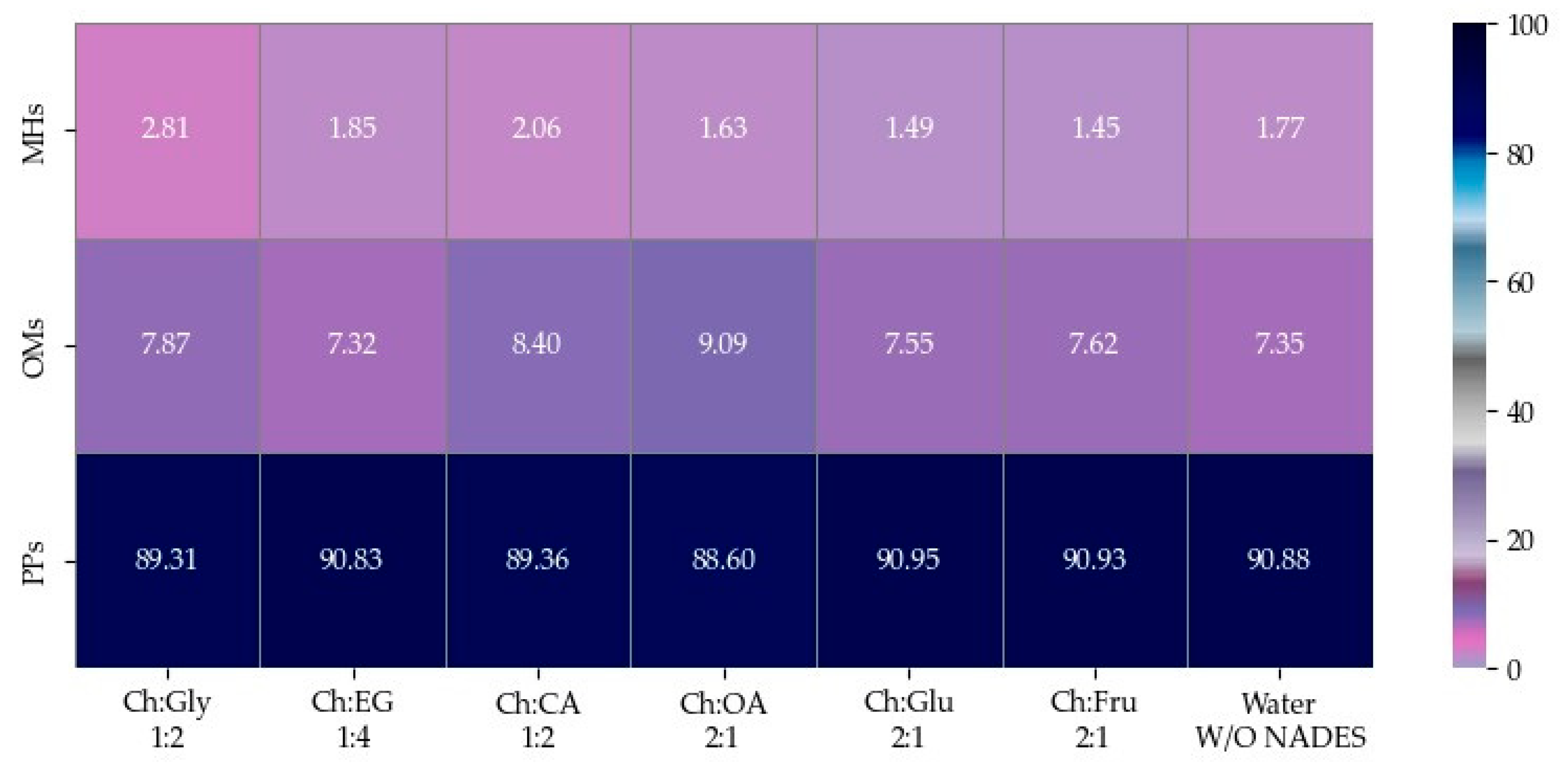
The EOs of F. vulgare extracted using each type of NADES, as well as those extracted with water (without NADES), were analyzed for their chemical constituents using GC-MS. The total ion chromatogram is shown in Figure 4, and the results are summarized in Figure 5 and Figure 6. Based on the data (Table 4), a total of 15 volatile compounds were identified and quantified across the three groups, including 4 monoterpene hydrocarbons (MHs), 5 oxygenated monoterpenes (OMs), and 6 phenylpropanoids (PPs), as shown in Figure 6. Of all EOs, the major compounds were phenylpropanoids (PPs), specifically anethole and estragole. It is worth noting that all EOs consisted of anethole at the minimum level of 71.01% except that obtained by using Ch:OA as NADES, in which anethole was only 67.59%.
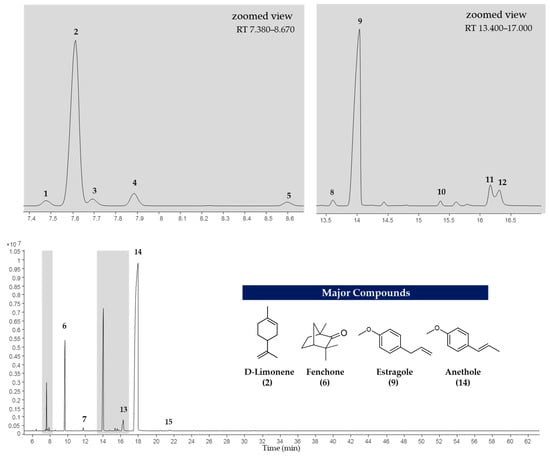
Figure 4.
Total ion chromatogram of EO extracted using NADES-MAHD.
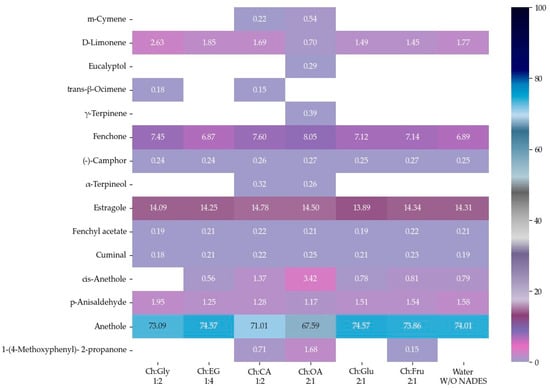
Figure 5.
Heat map of relative area percentage of volatile phytochemical compositions in EOs.
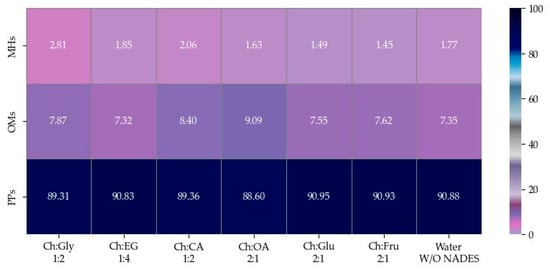
Figure 6.
Heat map of relative area percentages of phytochemical classes in EOs.

Table 4.
Assignment compounds identified in EOs of F. vulgare using GC-MS.
In addition, oxygen atoms in fenchone probably made the difference by interacting with NADES, making the compound more accessible to solvents. EO obtained by Ch:CA (1:2) and Ch:OA (2:1) also demonstrated other interesting constituents besides a higher number of compounds compared to other EOs.
The 4 major compounds (anethole, estragole, fenchone and D-limonene) extracted by MAHD, without any NADES, were found identical to those reported by Diao et al. [46], in which fennel seeds were extracted by conventional hydrodistillation (HD). Diao et al. had found that D-limonene was slightly higher than fenchone (5.45% to 6.24%) while this study found fenchone (6.87% to 8.05%) [46] much higher than D-limonene (0.70–2.63%). It is proposed that two reasons behind this phenomenon are that fenchone possesses a higher dipole moment than D-limonene and therefore interacts more effectively with microwaves.
Considering, α-terpineol was a compound found only when carboxylic acids were used as HBDs, Ch:OA (2:1). trans-β-Ocimene was found in EOs extracted using Ch:Gly 1:2 and ChCA (1:2). 1-(4-Methoxyphenyl)-2-propanone was observed in EOs obtained from systems using carboxylic acids as HBDs as well as Ch:Fru (2:1). The EO extracted using Ch:Gly (1:2) contained the highest levels of monoterpenes, particularly D-limonene and trans-β-ocimene, highlighting its remarkable efficiency in capturing key aromatic compounds. Consistent with [20], the application of DES led to an increase in the relative content of hydrocarbons in most EOs, indicating that the enhanced EO yield resulted primarily from the release of more intracellular bioactive compounds rather than from oxidation of EO components.
Multiple studies have demonstrated the antimicrobial effects and underlying mechanisms of D-limonene against a range of bacterial and fungal species, while β-ocimene, a key component in EOs, has been associated with anticonvulsant, antifungal, and antitumor activities, along with pest-resistant properties [47]. These observations indicate that suitable NADES can enhance the extraction of target compounds. Their superior performance may result from stronger hydrogen bonding or specific interactions with analytes, improving solubility and diffusion. Thus, tailoring NADES compositions to match compound structures can boost yield and selectivity, promoting greener extraction technologies [34].
3. Discussion
In recent years, growing environmental awareness has led companies to adjust their ethical standards and production practices. The drive toward global sustainability and the concept of a “green industry” calls for practical solutions that reduce environmental impact while preserving product quality [48]. This study successfully developed an innovative and eco-friendly extraction strategy using NADES pretreatment to significantly enhance the efficiency of MAHD for isolating essential oils from Foeniculum vulgare. These green NADESs facilitate cell wall disruption and enhance the release of volatile compounds, resulting in a substantially higher yield. NADES are natural solvents composed of primary or secondary metabolites mainly derived from living organisms, especially plants, such as sugars, amino acids, organic acids, fatty acids, or chlorine derivatives. Due to their natural origin, these solvents exhibit low toxicity, high biodegradability, and stability when mixed. Such properties confirm the suitability of NADES as environmentally friendly solvents and highlight their potential as a promising and sustainable alternative to conventional extraction methods for the valorization of plant materials [49]. One of the most remarkable features of NADES is their high tunability, which allows their properties to be tailored by selecting appropriate components and molar ratios. This tunability affects both physical properties (e.g., viscosity, pH) and chemical characteristics (e.g., polarity), enabling their customization for specific applications [34]. In hydrophilic DES systems, the choice of HBD plays a key role in determining viscosity. Systems containing phenol, glycols, or ethylene glycol generally exhibit reduced viscosity, whereas those composed of choline chloride with urea, polycarboxylic acids, or sugars tend to have medium to high viscosity [50]. For DESs containing 30% water at a 1:1 HBA:HBD ratio, the viscosity follows the order: sugar-based DES > glycerol-based DES > organic acid-based DES > ethylene glycol-based DES > amide-based DES. Correspondingly, the pH values vary, with organic acid-based DESs showing the lowest pH (<1), followed by sugar-based (3.39–4.15), alcohol-based (4.91–5.37), and amide-based DESs (7.08–7.35). Such tunability not only governs their solvent characteristics but also enables interactions with specific compounds, including essential oil constituents, through non-covalent forces such as hydrogen bonding and electrostatic (both repulsive and attractive) interactions [10].
The higher extraction yield observed in NADES-based MAHD compared to water-based MAHD can be attributed to the superior penetration capacity of NADES constituents. These components absorb microwave radiation more effectively than water and convert it into thermal energy, which facilitates the dissolution of cell wall components and the extraction of soluble substances from the plant material. This enhanced performance is largely due to the unique role of hydrogen bond donors (HBD) under microwave irradiation, which promotes compound release through efficient microwave absorption, disruption of the cell wall, and cellulose dissolution [51,52].
The integration of NADES with microwave-assisted hydrodistillation (NADES-MAHD) not only enhances essential oil yield but also significantly improves energy efficiency compared to conventional hydrodistillation (HD). By reducing extraction time and lowering electricity consumption, NADES-MAHD minimizes the overall energy demand of the process. For instance, during the extraction of essential oils from Litsea cubeba fruits, conventional HD required 0.76 kWh, while MAHD and NADES-MAHD consumed only 0.40 and 0.39 kWh, respectively. This substantial reduction highlights the benefit of combining green solvents with microwave technology, as it translates into lower operational costs, reduced carbon emissions, and improved sustainability. Thus, NADES-MAHD offers a promising pathway for scalable essential oil production with minimized environmental impact [24].
Interest in essential oils has steadily increased in recent years due to their high economic value and expanding market demand. These aromatic compounds are widely applied in the food, cosmetics, and pharmaceutical industries. Despite their potential, scaling up essential oil extraction using microwave-assisted hydrodistillation (MAHD) remains challenging, primarily because of the limited penetration of microwaves into plant materials [53]. Nevertheless, the study by Lamberti et al. demonstrated the feasibility of scaling MAHD from laboratory to pilot scale. Using the ETHOS XL system, they obtained yields comparable to those achieved at the laboratory scale while processing more than six times the biomass within the same time frame. This significantly enhanced productivity, with yields from pellets increasing nearly fourfold and those from dry cones almost doubling, underscoring the strong potential of MAHD for industrial application, particularly given the industry’s preference for dried and pelletized hops [54].
Beyond extraction efficiency, an important consideration for the industrial application of NADES is their toxicological safety, particularly the potential risk of co-extracting toxic trace elements from plant matrices. Although NADES are composed of generally recognized as safe (GRAS) components, their strong solubilizing power may lead to the unintended extraction of metals or other impurities. This concern has been recently addressed in a study on Glycyrrhiza glabra roots, where acid-based NADES were shown to co-extract glycyrrhizic acid along with trace elements. Interestingly, the recovery of all trace elements (except Li) was relatively low (<6%), and statistical analysis indicated that the hydrogen bond donor type was the decisive factor influencing element extraction. More importantly, comprehensive health risk assessments, including the metal pollution index, hazard quotient, hazard index, and chronic daily intake, confirmed that all tested NADES extracts were nontoxic and posed no health risk for either ingestion or topical application [55].
4. Materials and Methods
4.1. Materials and Chemicals
Choline chloride (98%) was purchased from Loba Chemie Pvt Ltd., Mumbai, India. Oxalic acid dihydrate (≥99.5%) was purchased from Qrec (Asia), Rawang, Malaysia. Glycerol (≥99.5%), ethylene glycol (≥99.9%), citric acid 1-hydrate (99.5–100.5%), D-glucose-hydrate (97.5–102%) and D-fructose (98.0–102.0%) were purchased from Elago Enterprises Pty Ltd., Cherrybrook, Australia. 2-Diphenyl-1-picrylhydrazyl (DPPH) and alkane standard solution were bought from Sigma Aldrich Chemical Co. (St Louis, MO, USA). The compound 2,6-di-tert-butyl-4-methylphenol (BHT) was provided from Acros, Dreieich, Germany. Yeast malt broth and mueller hinton broth were from HiMedia, Kennett Square, PA, USA, while agar powder was from Krungthepchemical, Bangkok, Thailand. The material was authenticated by the Faculty of Science and Technology, Pibulsongkram Rajabhat University (PSRU), Phitsanulok, Thailand. Foeniculum vulgare Mill. (Fennel) was preserved with a specimen number (PSRU1233). Fennel seeds were oven-dried at 40 °C until a constant weight was achieved. Dried seeds were grounded and filtered through a 250-µm sieve.
4.2. Preparation of NADESs
The selection of hydrogen bond donors (HBDs) was guided by previous studies demonstrating their effectiveness in forming NADES with choline chloride for essential oil extraction. Ethylene glycol, glycerol, oxalic acid, citric acid, glucose, and fructose were selected as hydrogen bond donors (HBD) along with choline chloride (HBA) as NADESs because previous research utilized ethylene glycol, glycerol, oxalic acid, and fructose along with choline chloride as a DES-based microwave-assisted hydrodistillation method (DES-MAHD) to extract clove essential oils [23], while other studies employed oxalic acid, citric acid, and glucose along with choline chloride as a DES-based MAHD to extract essential oils from fruits of Litsea cubeba (Lour.) [24].
Both glucose and fructose were selected as HBDs along with choline chloride (HBA) due to previous findings in the literature. Yu et al. (2017) employed glucose, fructose, ethylene glycol, glycerol, and urea as HBDs with choline chloride to extract essential oils from pepper fruits using the DES-based MAHD method and observed that fructose as HBD gave the highest essential oil yield [56]. In contrast, Xu et al. (2021) used glucose, fructose, and oxalic acid as HBDs with choline chloride to extract turmeric oils by the DES-based MAHD method, finding that glucose provided a higher yield than fructose [22]. Based on these studies, choline chloride (Ch) was selected as the sole HBA in our work, while the HBDs were varied as glycerol (Gly), ethylene glycol (EG), citric acid (CA), oxalic acid (OA), glucose (Glu), and fructose (Fru) to investigate their effects on essential oil extraction. The HBA and HBD were mixed at varying molar ratios, as summarized in Table 5. Each mixture was heated with magnetic agitation until clear liquid was formed.

Table 5.
List of NADESs for pretreatment process.
4.3. NADES-MAHD for EO Extraction
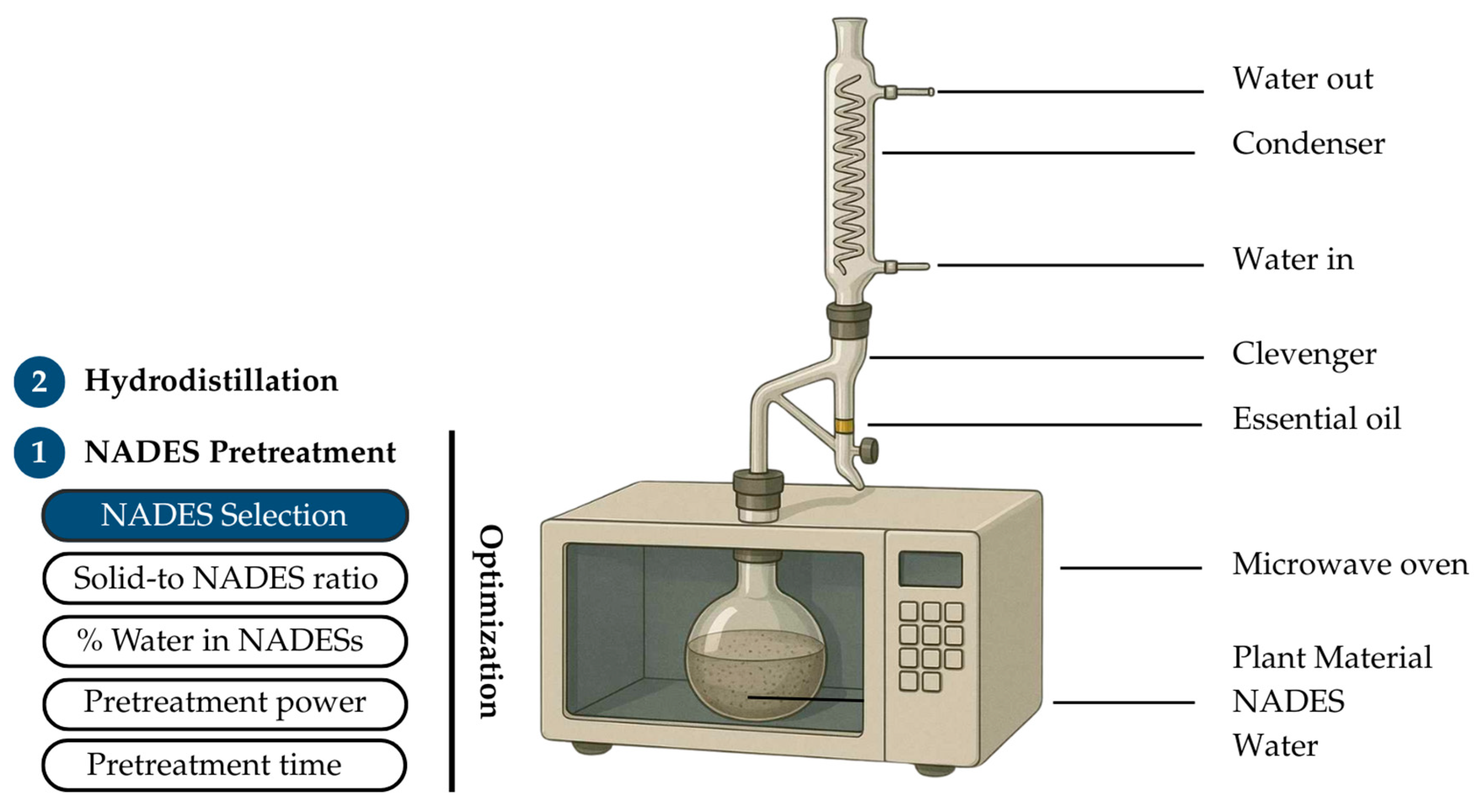
Microwave-assisted natural deep eutectic solvent pretreatment coupled with hydrodistillation (NADES-MAHD) was selected as the extraction method. The schematic diagram of the extraction setup is shown in Figure 7. The apparatus consisted of a microwave oven (TOSHIBA ER-SGS34(S)TH 34, Toshiba Thailand Co., Ltd., Bangkok, Thailand), connected to a Clevenger-type apparatus for the collection of EOs. This configuration allowed for efficient energy transfer during pretreatment and facilitated the separation and condensation of volatile compounds during the hydrodistillation step. The process consisted of 2 main steps: pretreatment and hydrodistillation. In step 1, the pretreatment stage using NADESs facilitated the release of EO components from the fennel seed matrix, enabling step 2, hydrodistillation, to effectively separate the EOs from the extraction medium. During the pretreatment step, key parameters, including the type of NADES, solid-to-NADES ratio, water content in the NADES, pretreatment time, and microwave power, were systematically optimized. NADESs with 20% water content was mixed with grounded fennel seed at the solid-to-NADES ratio of 1:6 (g/mL). The mixture was heated with microwave irradiation at 400 W for 4 min. During hydrodistillation, 270 mL of deionized water was added to the mixture, followed by microwave heating at 400 W for 96 min, based on the optimized MAHD conditions previously reported by our group [37]. After hydrodistillation, the fennel EO was separated from aqueous phase and was weighed. The yield of EOs in each extraction condition was calculated according to Equation (1)
where WE is the weight of fennel EO, and WF is the weight of fennel seed powder.
Yield of EO(%) = WE/WF × 100%
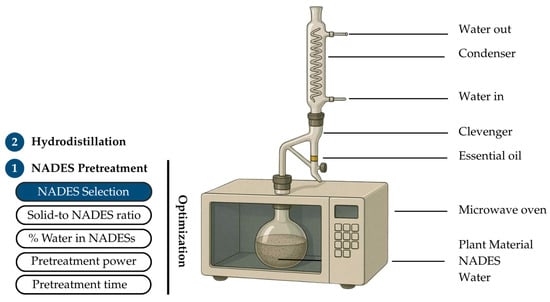
Figure 7.
Overview of the NADES-MAHD extraction process, highlighting 2 major steps and key pretreatment optimization parameters.
4.4. Antimicrobial Assay
The antimicrobial activities of fennel EO extract were evaluated using the agar disk-diffusion method. A suspension containing a final inoculation size of 0.5 McFarland was prepared for four Gram-positive bacteria (Staphylococcus aureus DMST 8840, Streptococcus pyogenes DMST 30563, Bacillus cereus DMST 5040, and Listeria monocytogenes DMST 17303) and four Gram-negative bacteria (Escherichia coli DMST 4212, Salmonella Typhi DMST 5784, Pseudomonas aeruginosa DMST 4739, and Enterobacter aerogenes DMST 8841). These suspensions were spread-plated on Mueller Hinton Agar (MHA) (HiMedia, Thane, India). For each test bacterium, a 6.0 mm diameter filter paper disk was placed on the agar surface, and 10 µL of EO was added. Additionally, tetracycline (Oxoid, Ogdensburg, NY, USA) at a concentration of 30 µg was used as a positive control. The agar plates were incubated at 37 °C for 24 h, and the sizes of the inhibition zones were measured. All experiments were conducted in triplicate.
A suspension of Candida albicans was prepared to reach a final inoculation size of 0.5 McFarland and was then spread-plated on Potato Dextrose Agar (PDA) (HiMedia, Thane, India). For each EO tested, a 6.0 mm diameter filter paper disk was placed on the surface of the agar, and 15 µL of the EO was applied to the disk. The agar plates were incubated at 25 °C for 48 h, and the size of the inhibition zone was measured afterward. Nystatin (Alfa Aesar, Lancashire, UK) at a concentration of 50 µg served as the positive control. All experiments were performed in triplicate.
Minimum inhibitory concentrations (MIC) were determined using broth dilution method in 96-well plates. A series of two-fold dilutions of each EO, ranging from 25 to 0.01 mg/mL, was prepared in MHB with the volume of 50 µL. The solution was then added 50 µL of bacteria culture containing approximately 1.5 × 106 CFU/mL. The plates were incubated at 37 °C for 24 h. The MICs were determined as the lowest concentration of EO inhibiting visible growth of each organism. Tetracycline was used as a positive control, and the experiment was carried out in triplicate.
4.5. DPPH Radical Scavenging Assay
A 50 μL aliquot of EO stock solution (diluted 5-fold) was mixed with 150 μL of DPPH solution (0.417 mM). Methanol was used as the blank. After incubation for 30 min at room temperature in the dark, the absorbance was measured at 517 nm using a microplate reader (Metertech, Taipei, Taiwan). The percentage of DPPH radical inhibition was calculated using Equation (2), as follows:
where Acontrol was the absorbance of the control and Asample was the absorbance of the EO solution.
% DPPH radical scavenging = (Acontrol − Asample)/Acontrol × 100
4.6. GC–MS-Based Analysis of Fennel Seed Phytochemicals
EO samples were diluted with dichloromethane, and anhydrous sodium sulfate was added to eliminate moisture from the solution. The phytochemical compositions in the EOs were identified and quantified by gas chromatography–mass spectrometry (GC–MS) using an Agilent 6890N GC coupled with a 5973 MSD (Agilent Technologies, Santa Clara, CA, USA), equipped with an HP-5MS capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. Sample injection was performed in split mode with a split ratio of 20:1, using an injection volume of 1 μL and an injector temperature of 230 °C. The column oven temperature was initially set at 60 °C and then ramped to 250 °C at a rate of 3 °C/min. Mass spectrometric detection was conducted using electron ionization (EI) at 70 eV, with a scan range of m/z. The ion source and quadrupole temperatures were maintained at 230 °C and 150 °C, respectively.
Compound identification in fennel seed EO was carried out by matching the acquired mass spectra with those available in the NIST Mass Spectral Search Program database. Additionally, confirmation was supported by comparing the Kovats retention indices (RIs), calculated based on the retention times of the target compounds relative to a homologous series of n-alkanes (C9–C26), with reference values reported in the literature and included in the NIST database. A compound was considered positively identified when both the mass spectral data and RI showed strong agreement. Quantification was achieved by applying the peak area normalization method.
4.7. Statistica Analysis
All experimental data are expressed as mean ± standard deviation. Data analysis was performed using the trial version of Minitab 18 software, including analysis of variance (ANOVA) and Tukey’s post hoc test to determine statistically significant differences among means (p < 0.05).
5. Conclusions
This study successfully developed a novel green extraction strategy combining NADES pretreatment with MAHD to isolate essential oils from Foeniculum vulgare. By employing NADES of natural origin, characterized by low toxicity and high biodegradability, the method enhances extraction efficiency while reinforcing its role as a sustainable alternative to conventional techniques. The highest EO yield was achieved using Ch:Gly (1:2 molar ratio) under optimal conditions, including a solid-to-NADES ratio of 1:6 g/mL, 20% water content, microwave power of 400 W, and 6 min of microwave duration, resulting in a significantly higher EO yield compared to conventional water-based MAHD. NADESs are recognized as effective pretreatment solvents owing to their remarkable ability to disrupt cellulose, hemicellulose, and lignin structures. The EO exhibited antimicrobial activity against S. pyogenes and C. albicans, as well as DPPH radical scavenging activity.
Although the overall EO composition remained consistent, NADES pretreatment led to a higher content of monoterpene hydrocarbons, with D-limonene and trans-β-ocimene uniquely detected in the extract obtained using Ch:Gly (1:2). The increased hydrocarbon content and EO yield observed with this NADES formulation could be attributed to its cellulose-dissolving capability, which facilitated greater release of intracellular compounds. The developed method offers an environmentally friendly strategy for producing fennel EO with a targeted composition. NADESs, as tailor-made solvents, possess excellent physicochemical properties that can be fine-tuned to selectively enhance the extraction of specific compounds, allowing precise control over EO profiles. In addition, NADES extracts are non-toxic and are not expected to pose any health risks from either topical application or ingestion, further supporting their potential as safe and sustainable solvents for greener and more efficient pretreatment processes, particularly in the food industry.
Despite the promising results of NADES-assisted MAHD for essential oil extraction, comprehensive optimization of process parameters is still required to fully enhance extraction efficiency and reproducibility. In addition, further studies are needed to assess the stability of essential oil components in NADES and to evaluate the feasibility of solvent reuse. Addressing these challenges will be crucial for meeting the stringent quality standards of the pharmaceutical, cosmetic, and food industries, and for supporting the industrial-scale adoption of NADES-MAHD as a sustainable extraction technology.
Author Contributions
Conceptualization, P.S.; methodology, S.N., N.W. and J.M.; software, P.S.; investigation, S.P. and S.L.; Resources, K.T.; data curation, A.C.; writing—original draft preparation, S.P.; writing—review and editing, P.S.; visualization, S.P.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Science, Research and Innovation Fund (NSRF) and King Mongkut’s University of Technology North Bangkok under Contract No. KMUTNB-FF-65-32.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Additional support for this study was provided by the Faculty of Science, Energy and Environment, King Mongkut’s University of Technology North Bangkok.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NADES | Natural deep eutectic solvent |
| MAHD | Microwave-assisted hydrodistillation |
| HD | Hydrodistillation |
| EOs | Essential oils |
| S/N | Solid-to-NADES |
| Ch | Choline chloride |
| Gly | Glycerol |
| EG | Ethylene glycol |
| CA | Citric acid |
| OA | Oxalic acid |
| Glu | Glucose |
| Fru | Fructose |
| MHs | Monoterpene hydrocarbons |
| OMs | Oxygenated monoterpenes |
| PPs | Phenylpropanoids |
References
- Malo, C.; Gil, L.; Cano, R.; González, N.; Luño, V. Fennel (Foeniculum vulgare) provides antioxidant protection for boar semen cryopreservation. Andrologia 2012, 44, 710–715. [Google Scholar] [CrossRef]
- Salama, Z.A.; El Baz, F.K.; Gaafar, A.A.; Zaki, M.F. Antioxidant activities of phenolics, flavonoids and vitamin C in two cultivars of fennel (Foeniculum vulgare Mill.) in responses to organic and bio-organic fertilizers. J. Saudi Soc. Agric. Sci. 2015, 14, 91–99. [Google Scholar] [CrossRef]
- Ahmad, B.S.; Talou, T.; Saad, Z.; Hijazi, A.; Cerny, M.; Kanaan, H.; Merah, O. Fennel oil and by-products seed characterization and their potential applications. Ind. Crops Prod. 2018, 111, 92–98. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Jagan Mohan Rao, L.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Emami, S.A. Ethnobotany, phytochemistry and traditional uses of Curcuma spp. and pharmacological profile of two important species (C. longa and C. zedoaria): A review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Meklati, B.Y.; Chemat, F. A new process for extraction of essential oil from Citrus peels: Microwave hydrodiffusion and gravity. J. Food Eng. 2009, 90, 409–413. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Valková, V.; Ďúranová, H.; Vuković, N.; Vukić, M.; Kačániová, M. Biological activity of essential oil from Foeniculum vulgare. Acta Hortic. Regiotect. 2021, 24, 148–152. [Google Scholar] [CrossRef]
- Boudraa, H.; Kadri, N.; Mouni, L.; Madani, K. Microwave-assisted hydrodistillation of essential oil from fennel seeds: Optimization using Plackett–Burman design and response surface methodology. J. Appl. Res. Med. Aromat. Plants 2021, 23, 100307. [Google Scholar] [CrossRef]
- Troter, D.Z.; Todorović, Z.B.; Đokić-Stojanović, D.R.; Stamenković, O.S.; Veljković, V.B. Application of ionic liquids and deep eutectic solvents in biodiesel production: A review. Renew. Sustain. Energy Rev. 2016, 61, 473–500. [Google Scholar] [CrossRef]
- Ratanasongtham, P.; Bunmusik, W.; Luangkamin, S.; Mahatheeranont, S.; Suttiarporn, P. Optimizing green approach to enhanced antioxidants from Thai pigmented rice bran using deep eutectic solvent-based ultrasonic-assisted extraction. Heliyon 2024, 10, e23525. [Google Scholar] [CrossRef]
- Gerçek, Y.C.; Kutlu, N.; Çelik, S.; Bayram, S.; Kırkıncı, S.; Bayram, N.E. Optimized ultrasonic-NaDES extraction of anthocyanins, polyphenolics, and organic acids from chokeberry fruit with blueness and antimicrobial evaluation. Microchem. J. 2025, 210, 113061. [Google Scholar] [CrossRef]
- Krgović, N.; Jovanović, M.S.; Nedeljković, S.K.; Šavikin, K.; Lješković, N.J.; Ilić, M.; Živković, J.Č.; Menković, N. Natural deep eutectic solvents extraction of anthocyanins–effective method for valorisation of black raspberry (Rubus occidentalis L.) pomace. Ind. Crops Prod. 2025, 223, 120237. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of natural deep eutectic solvents for extraction of hydrophilic and lipophilic compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, N.; Li, Q. Ultrasonic assisted extraction of coumarins from Angelicae Pubescentis Radix by betaine-based natural deep eutectic solvents. Arab. J. Chem. 2024, 17, 105542. [Google Scholar] [CrossRef]
- Jovanović, J.; Jović, M.; Trifković, J.; Smiljanić, K.; Gašić, U.; Krstić Ristivojević, M.; Ristivojević, P. Green extraction of bioactives from Curcuma longa using natural deep eutectic solvents: Unlocking antioxidative, antimicrobial, antidiabetic, and skin depigmentation potentials. Plants 2025, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Petrochenko, A.A.; Orlova, A.; Frolova, N.; Serebryakov, E.B.; Soboleva, A.; Flisyuk, E.V.; Frolov, A.; Shikov, A.N. Natural deep eutectic solvents for the extraction of triterpene saponins from Aralia elata var. mandshurica (Rupr. & Maxim.) J. Wen. Molecules 2023, 28, 3614. [Google Scholar]
- Nitthiyah, J.; Nour, A.H.; Kantasamy, R.; Akindoyo, J.O. Microwave assisted hydrodistillation—An overview of mechanism and heating properties. Aust. J. Basic Appl. Sci. 2017, 11, 22–29. [Google Scholar]
- Crescente, G.; Cascone, G.; Sorrentino, A.; Volpe, M.G.; Boscaino, F.; Moccia, S. Influence of extraction techniques on chemical composition, antioxidant and antifungal activities of Mentha spicata L. essential oil: A comparative study of microwave-assisted hydrodistillation and steam distillation. Food Biosci. 2025, 69, 106939. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel extraction of polyphenols from sour cherry pomace using natural deep eutectic solvents—Ultrafast microwave-assisted NADES preparation and extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Yu, G.W.; Cheng, Q.; Nie, J.; Wang, X.J.; Wang, P.; Li, Z.G.; Lee, M.R. Microwave hydrodistillation based on deep eutectic solvent for extraction and analysis of essential oil from three Amomum species using gas chromatography—Mass spectrometry. Chromatographia 2018, 81, 657–667. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.; Yang, Y.; Ping, Y.; Tian, H.; Gu, H.; Yang, L. Natural acidic deep eutectic solvent-mediated microwave-assisted simultaneous hydrodistillation, hydrolysis, and extraction for obtaining essential oils, gallic acid and ellagic acid from Liquidambar formosana leaves and fruits. Sustain. Chem. Pharm. 2025, 43, 101870. [Google Scholar] [CrossRef]
- Xu, F.X.; Zhang, J.Y.; Jin, J.; Li, Z.G.; She, Y.B.; Lee, M.R. Microwave-assisted natural deep eutectic solvents pretreatment followed by hydrodistillation coupled with GC-MS for analysis of essential oil from turmeric (Curcuma longa L.). J. Oleo Sci. 2021, 70, 1481–1494. [Google Scholar] [CrossRef] [PubMed]
- Suttiarporn, P.; Taithaisong, T.; Namkhot, S.; Luangkamin, S. Enhanced eugenol composition in clove essential oil by deep eutectic solvent-based ultrasonic extraction and microwave-assisted hydrodistillation. Molecules 2025, 30, 504. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Li, Z.; Jiang, L.; Cao, X.; Gao, W.; Chen, F. Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation of essential oil from Litsea cubeba (Lour.) Pers. fruits and its chemical composition and biological activity. J. Chromatogr. A 2021, 1646, 462089. [Google Scholar] [CrossRef] [PubMed]
- Muley, P.D.; Mobley, J.K.; Tong, X.; Novak, B.; Stevens, J.; Moldovan, D.; Shi, J.; Boldor, D. Rapid microwave-assisted biomass delignification and lignin depolymerization in deep eutectic solvents. Energy Convers. Manag. 2019, 196, 1080–1088. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, F.; Pang, M.; Jin, X.; Lv, H.; Li, Z.; Lee, M. Microwave-assisted hydrodistillation extraction based on microwave-assisted preparation of deep eutectic solvents coupled with GC-MS for analysis of essential oils from clove buds. Sustain. Chem. Pharm. 2022, 27, 100695. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Coscarella, M.; Nardi, M.; Alipieva, K.; Bonacci, S.; Popova, M.; Procopio, A.; Scarpelli, R.; Simeonov, S. Alternative assisted extraction methods of phenolic compounds using NaDESs. Antioxidants 2023, 13, 62. [Google Scholar] [CrossRef]
- Stanojević, L.P.; Todorović, Z.B.; Stanojević, K.S.; Stanojević, J.S.; Troter, D.Z.; Nikolić, L.B.; Đorđević, B. The influence of natural deep eutectic solvent glyceline on the yield, chemical composition and antioxidative activity of essential oil from rosemary (Rosmarinus officinalis L.) leaves. J. Essent. Oil Res. 2021, 33, 247–255. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, I.R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Jeyaratnam, N.; Nour, A.H.; Akindoyo, J.O. Comparative study between hydrodistillation and microwave-assisted hydrodistillation for extraction of Cinnamomum cassia oil. ARPN J. Eng. Appl. Sci. 2016, 11, 2647–2652. [Google Scholar]
- Alanon, M.E.; Ivanovic, M.; Pimentel-Mora, S.; Borras-Linares, I.; Arraez-Roman, D.; Segura-Carretero, A. A novel sustainable approach for the extraction of value-added compounds from Hibiscus sabdariffa L. calyces by natural deep eutectic solvents. Food Res. Int. 2020, 137, 109716. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Acosta-Vega, L.; Cifuentes, A.; Ibáñez, E.; Galeano Garcia, P. Exploring natural deep eutectic solvents (NADES) for enhanced essential oil extraction: Current insights and applications. Molecules 2025, 30, 284. [Google Scholar] [CrossRef] [PubMed]
- Kivela, H.; Salomaki, M.; Vainikka, P.; Makila, E.; Poletti, F.; Ruggeri, S.; Terzi, F.; Lukkari, J. Effect of water on a hydrophobic deep eutectic solvent. J. Phys. Chem. B 2022, 126, 513–527. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikova, V.A.; Flisyuk, E.V.; Vishnyakov, E.V.; Makarevich, E.V.; Shikov, A.N. Physicochemical and antimicrobial properties of lactic acid-based natural deep eutectic solvents as a function of water content. Appl. Sci. 2024, 14, 10409. [Google Scholar] [CrossRef]
- Noyraksa, S.; Wichianwat, K.; Punpuk, S.; Aiemyeesun, S.; Maitip, J.; Suttiarporn, P. Optimization of microwave-assisted hydrodistillation of essential oils from fennel seeds. Mater. Today Proc. 2023, 77, 1079–1085. [Google Scholar] [CrossRef]
- Yu, F.; Wan, N.; Zheng, Q.; Li, Y.; Yang, M.; Wu, Z. Effects of ultrasound and microwave pretreatments on hydrodistillation extraction of essential oils from Kumquat peel. Food Sci. Nutr. 2021, 9, 2372–2380. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Giedrys-Kalemba, S.; Mizielinska, M.; Bartkowiak, A.J.H.P. Antibacterial activity of rosemary, caraway and fennel essential oils. Herba Pol. 2015, 61, 31–39. [Google Scholar] [CrossRef]
- Naaz, S.; Ahmad, N.; Qureshi, M.I.; Hashmi, N.; Akhtar, M.S.; Khan, M.M.A. Antimicrobial and antioxidant activities of fennel oil. Bioinformation 2022, 18, 795. [Google Scholar] [CrossRef]
- Bassyouni, R.H.; Wali, I.E.; Kamel, Z.; Kassim, M.F. Fennel oil: A promising antifungal agent against biofilm forming fluconazole resistant Candida albicans causing vulvovaginal candidiasis. J. Herb. Med. 2019, 15, 100227. [Google Scholar] [CrossRef]
- Hou, G.W.; Huang, T. Essential oils as promising treatments for treating Candida albicans infections: Research progress, mechanisms, and clinical applications. Front. Pharmacol. 2024, 15, 1400105. [Google Scholar] [CrossRef] [PubMed]
- Sharopov, F.; Valiev, A.; Satyal, P.; Gulmurodov, I.; Yusufi, S.; Setzer, W.N.; Wink, M. Cytotoxicity of the essential oil of Foeniculum vulgare from Tajikistan. Foods 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Sabzi Nojadeh, M.; Pouresmaeil, M.; Younessi-Hamzekhanlu, M.; Venditti, A. Phytochemical profile of fennel essential oils and possible applications for natural antioxidant and controlling Convolvulus arvensis L. Nat. Prod. Res. 2021, 35, 4164–4168. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Lin, H.; Li, Z.; Sun, Y.; Zhang, Y.; Wang, S.; Zhang, Q.; Tang, J. D-Limonene: Promising and sustainable natural bioactive compound. Appl. Sci. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.-S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Affat, S. A review of deep eutectic solvents (DESs), preparation, classification, physicochemical properties, advantages and disadvantages. Univ. Thi-Qar J. Sci. 2024, 11, 167–175. [Google Scholar] [CrossRef]
- Vo, T.P.; Pham, T.V.; Tran, T.N.H.; Vo, L.T.V.; Vu, T.T.; Pham, N.D.; Nguyen, D.Q. Ultrasonic-assisted and microwave-assisted extraction of phenolics and terpenoids from Abelmoschus sagittifolius (Kurz) Merr roots using natural deep eutectic solvents. ACS Omega 2023, 8, 29704–29716. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.S.; Moreira, L.S.; Silva, A.M.; Silva, R.J.; dos Santos, M.P.; da Silva, E.G.P.; Grassi, M.T.; Gonzalez, M.H.; Amaral, C.D.B. Natural deep eutectic solvent-based microwave-assisted extraction in the medicinal herb sample preparation and elemental determination by ICP OES. J. Food Compos. Anal. 2022, 109, 104510. [Google Scholar] [CrossRef]
- Masum, Z.; Mahfud, M.; Altway, A. Parameter for scale-up of extraction of Cymbopogon nardus dry leaf using microwave-assisted hydro-distillation. J. Appl. Eng. Sci. 2019, 17, 126–133. [Google Scholar]
- Lamberti, L.; Grillo, G.; Gallina, L.; Carnaroglio, D.; Chemat, F.; Cravotto, G. Microwave-assisted hydrodistillation of hop (Humulus lupulus L.) terpenes: A pilot-scale study. Foods 2021, 10, 2726. [Google Scholar] [CrossRef]
- Shikov, A.N.; Shikova, V.A.; Whaley, A.O.; Burakova, M.A.; Flisyuk, E.V.; Whaley, A.K.; Terninko, I.I.; Generalova, Y.E.; Gravel, I.V.; Pozharitskaya, O.N. The ability of acid-based natural deep eutectic solvents to co-extract elements from the roots of Glycyrrhiza glabra L. and associated health risks. Molecules 2022, 27, 7690. [Google Scholar] [CrossRef]
- Yu, G.W.; Cheng, Q.; Nie, J.; Wang, P.; Wang, X.J.; Li, Z.G.; Lee, M.R. DES-based microwave hydrodistillation coupled with GC-MS for analysis of essential oil from black pepper (Piper nigrum) and white pepper. Anal. Methods 2017, 9, 6777–6784. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).