Enhanced Antibacterial Activity of Hydrophobic Modified Lysozyme Against Gram-Negative Bacteria Without Accumulated Resistance
Abstract
1. Introduction
2. Results and Discussion
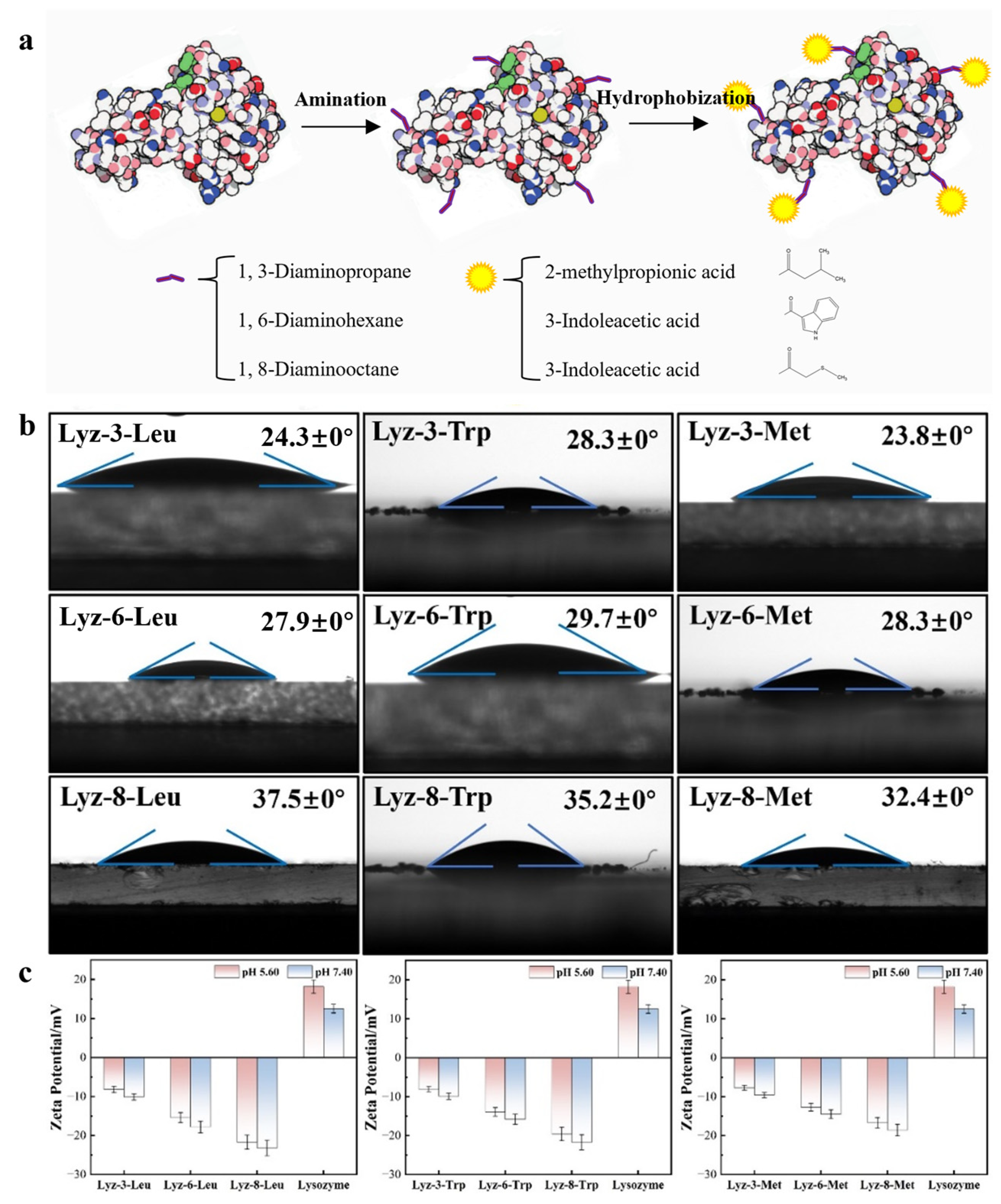
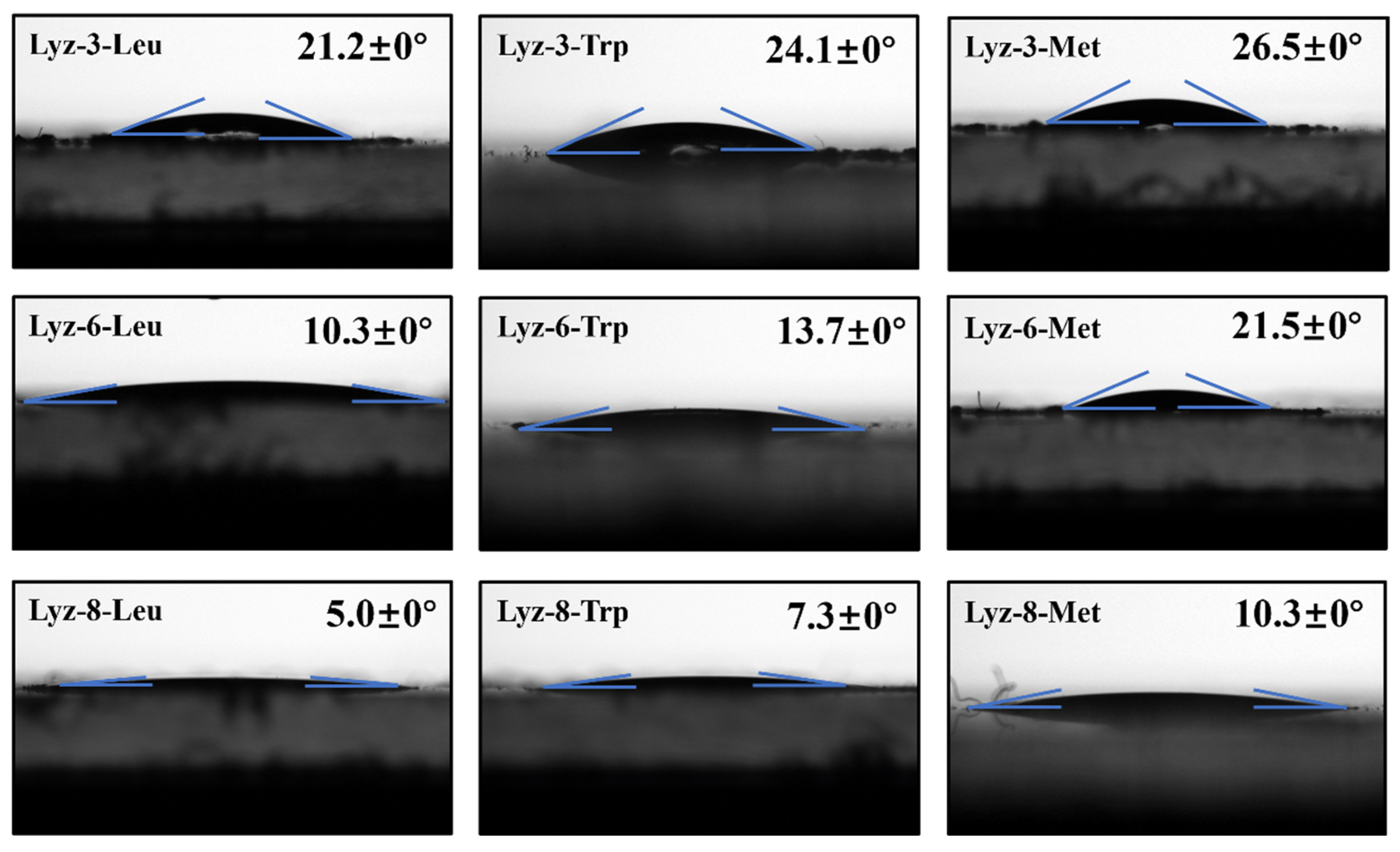
2.1. Construction and Characterization of HML
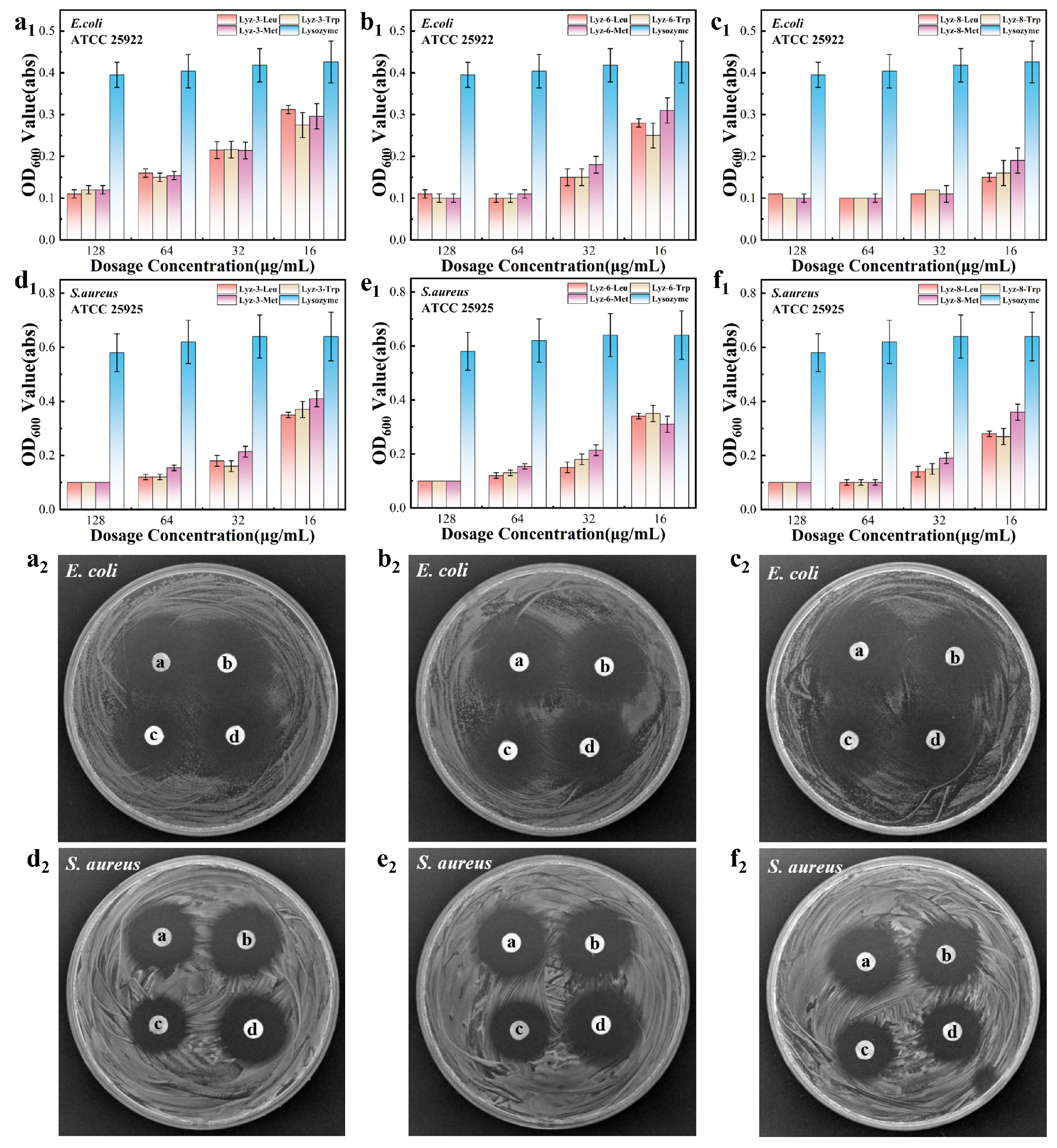
2.2. Antibacterial Ability of HML Against Gram-Negative Bacteria
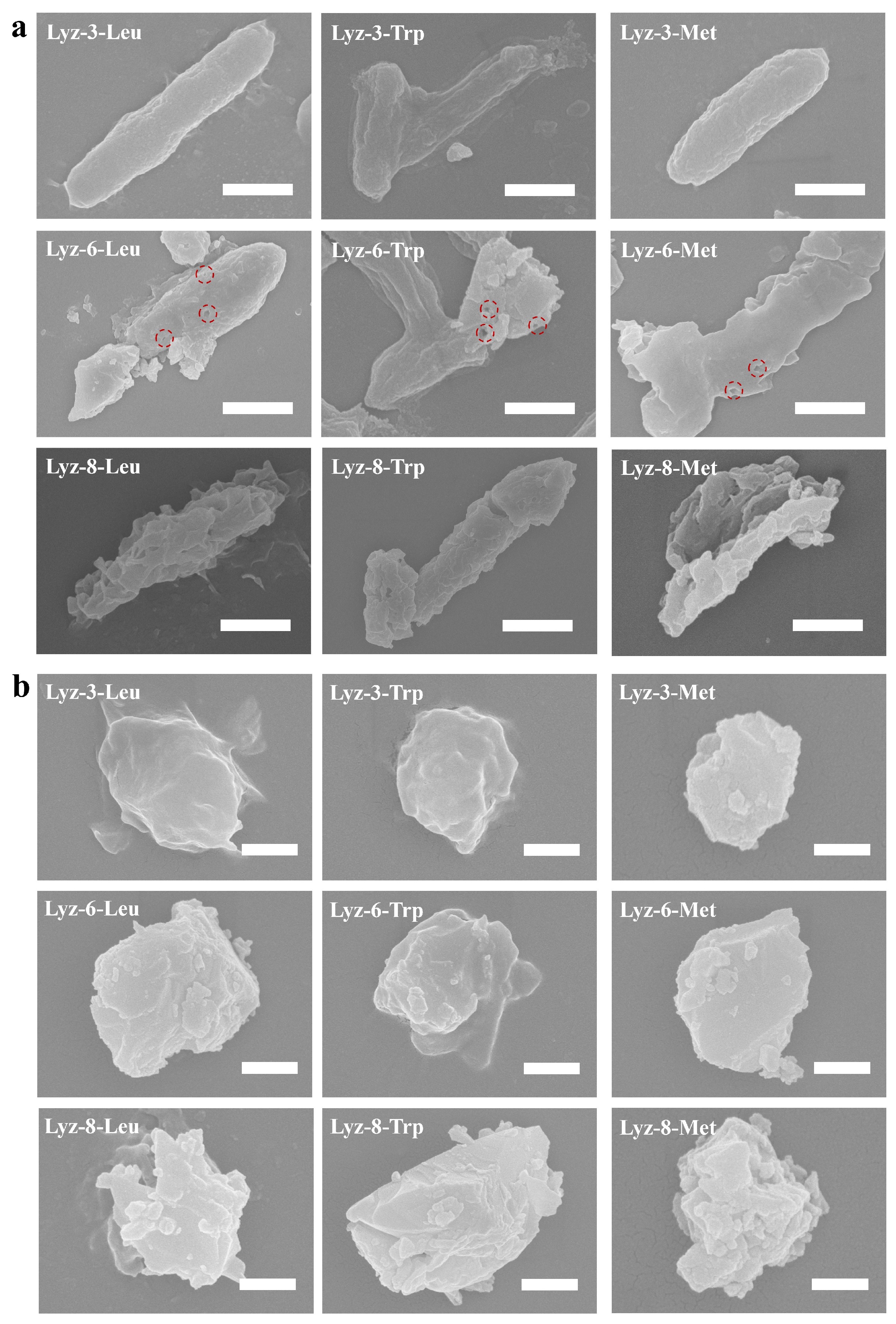
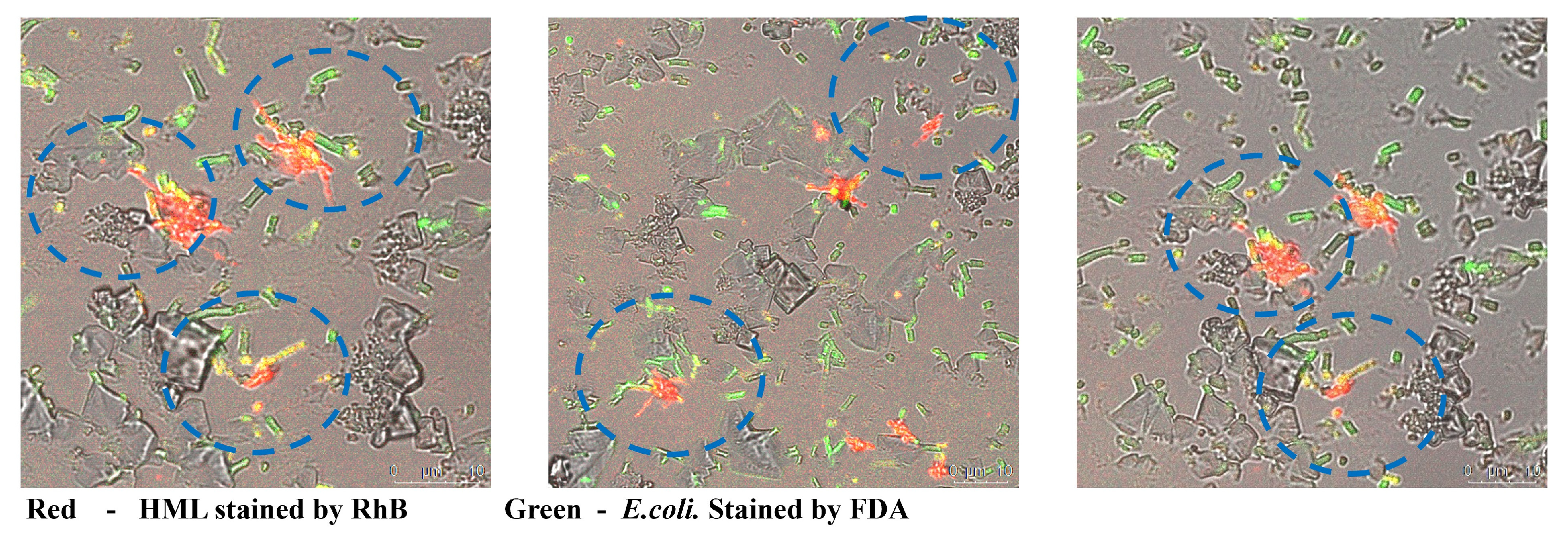
2.3. Mechanism of HML Antibacterial Ability
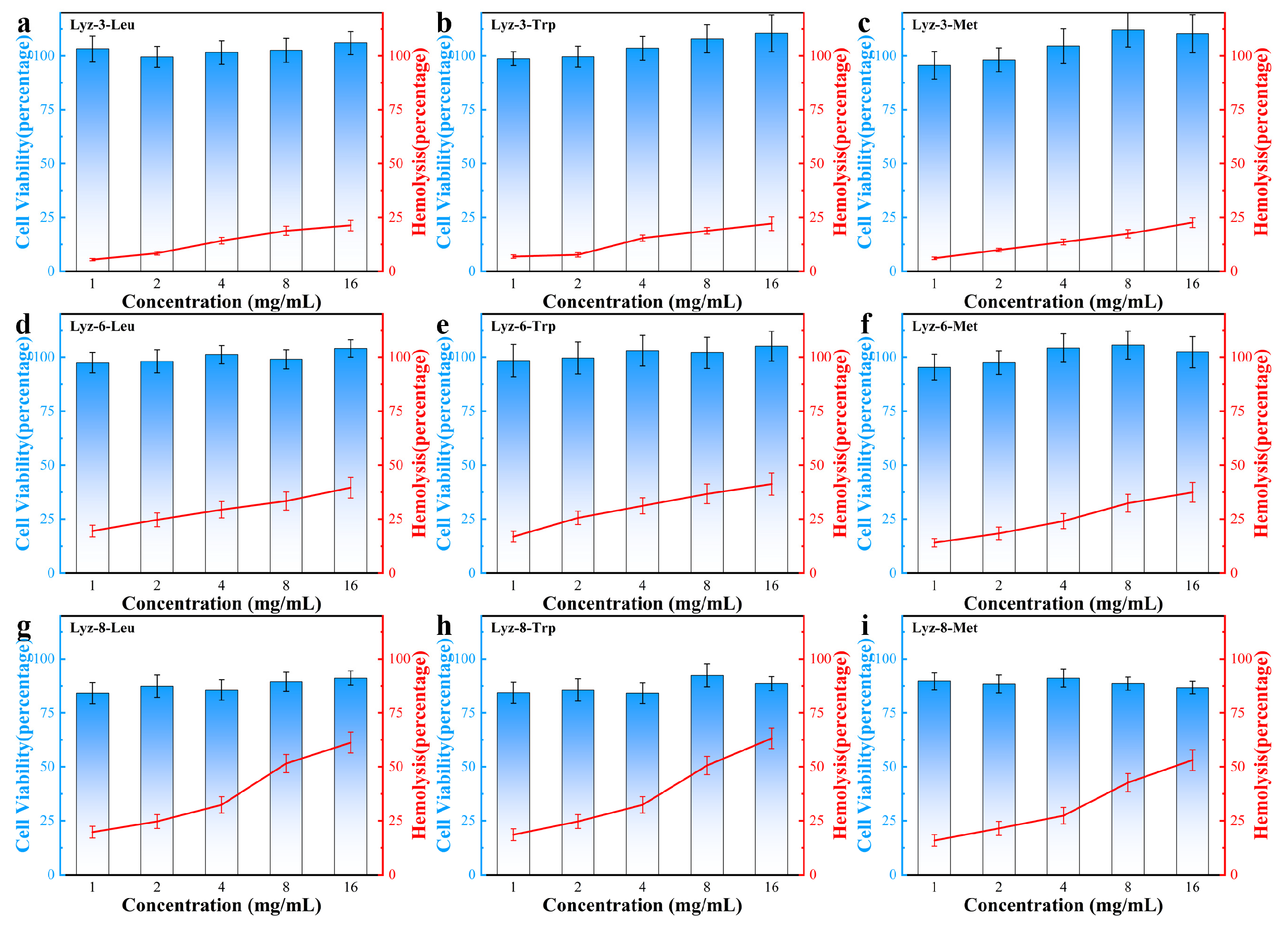
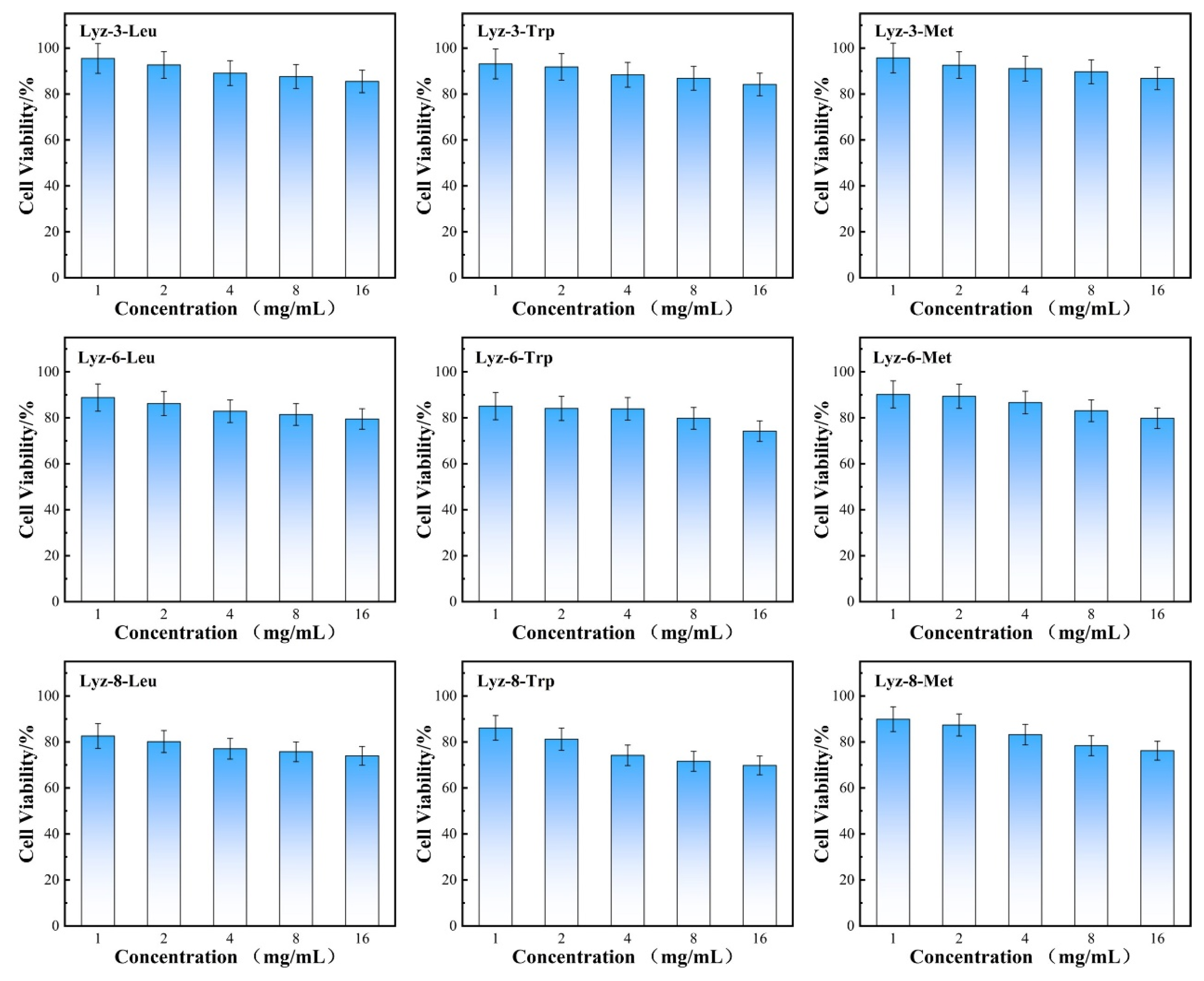
2.4. Therapeutic Selectivity and Hemolysis Activity of HML Bactericide Against Pathogenic Bacteria
2.5. Bacterial Resistance Development Against Antibiotics Versus HML
3. Materials and Methods
3.1. Material
3.2. Amination Process of Lysozyme
3.3. Conjugation Process of HML
3.4. Function Group Assay of HML
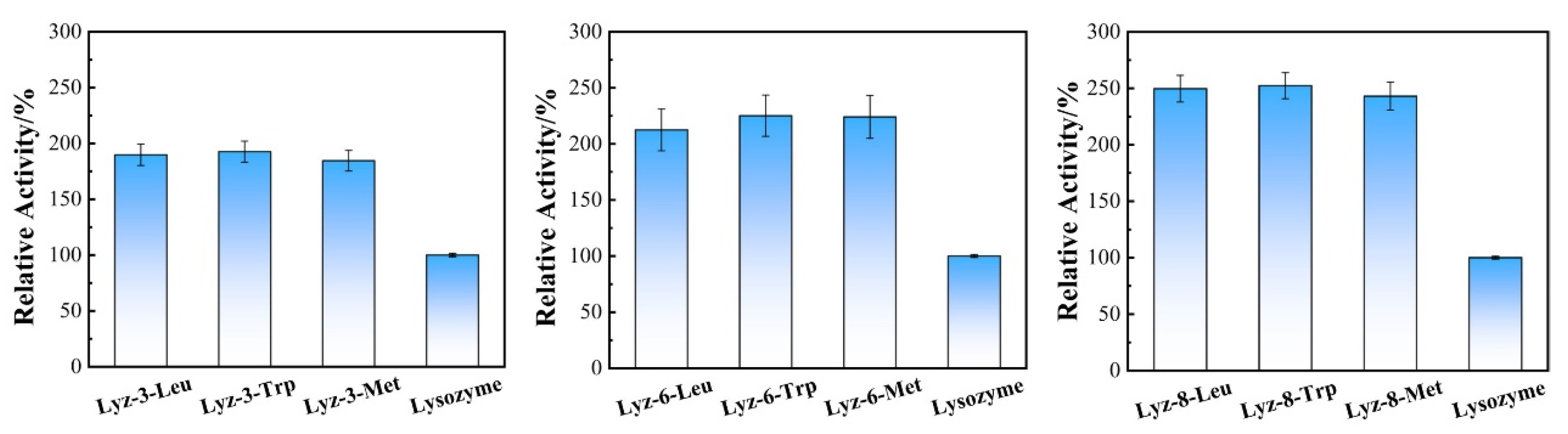
3.5. Enzyme Activity Assay of HML
- I—Enzyme activity;
- —The D-value of absorbance at 450 nm per minute;
- Ew—The mass (mg or g) or volume (mL) of the tested enzyme solution contained in 0.5 mL tested solution;
- 0.001—An activity unit is defined as a decrease of 0.001 in absorbance at 450 nm per minute.
3.6. Antibacterial Assay of HML
3.7. Scanning Electron Microscopy (SEM)
3.8. Determination of Viability of NIH-3T3 Stem Cell Co-Cultured with HML
3.9. Determination of Hemolysis of HML
3.10. Determination of Bacterial Resistance Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, T.; Wang, Y.; Xie, J.; Qu, X.; Liu, C. Lysozyme Amyloid Fibril-Integrated PEG Injectable Hydrogel Adhesive with Improved Antiswelling and Antibacterial Capabilities. Biomacromolecules 2022, 23, 1376–1391. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Kummer, N.; Ren, Q.; Campioni, S.; Nystrom, G. Assembly of Cellulose Nanocrystal-Lysozyme Composite Films with Varied Lysozyme Morphology. Biomacromolecules 2020, 21, 5139–5147. [Google Scholar] [CrossRef]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Jia, X.Y.; Liu, W.Y.; Huang, G.Q.; Xiao, J.X. Antibacterial activity of lysozyme after association with carboxymethyl konjac glucomannan. Food Chem. 2024, 449, 139229. [Google Scholar] [CrossRef]
- Jiang, Z.; Fu, L.; Wei, C.; Fu, Q.; Pan, S. Antibacterial micro/nanomotors: Advancing biofilm research to support medical applications. J. Nanobiotechnol. 2023, 21, 388. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; Zhu, M.; Wang, L.; Liu, X.; Huang, X. Enhanced antibacterial activity of surface re-engineered lysozyme against Gram-negative bacteria without accumulated resistance. Biomater. Sci. 2022, 10, 4474–4478. [Google Scholar] [CrossRef]
- Liang, J.; Li, W.; Chen, J.; Huang, X.; Liu, Y.; Zhang, X.; Shu, W.; Lei, B.; Zhang, H. Carbon dots as an electron extractant for enhanced photocatalytic antibacterial activity of covalent organic frameworks. J. Mater. Chem. A 2022, 10, 23384–23394. [Google Scholar] [CrossRef]
- Nardulli, P.; Hall, G.G.; Quarta, A.; Fruscio, G.; Laforgia, M.; Garrisi, V.M.; Ruggiero, R.; Scacco, S.; De Vito, D. Antibiotic Abuse and Antimicrobial Resistance in Hospital Environment: A Retrospective Observational Comparative Study. Medicina 2022, 58, 1257. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.M.; Goswami, J.; Davis, V.A. Comparison of Attachment and Antibacterial Activity of Covalent and Noncovalent Lysozyme-Functionalized Single-Walled Carbon Nanotubes. ACS Omega 2020, 5, 2254–2259. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.; Mostovaya, O.; Ovchinnikov, D.; Shiabiev, I.; Pysin, D.; Akhmedov, A.; Mukhametzyanov, T.; Lyubina, A.; Voloshina, A.; Stoikov, I. Combined antimicrobial agents based on self-assembled PAMAM-calix-dendrimers/lysozyme nanoparticles: Design, antibacterial properties and cytotoxicity. J. Mol. Liq. 2023, 389, 122838. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, S.; Cong, Z.; Zhang, H.; Lu, Z.; Shao, N.; Bhatti, S.A.; Zhou, C.; Cheng, J.; Gellman, S.H.; et al. Secondary Amine Pendant beta-Peptide Polymers Displaying Potent Antibacterial Activity and Promising Therapeutic Potential in Treating MRSA-Induced Wound Infections and Keratitis. J. Am. Chem. Soc. 2022, 144, 1690–1699. [Google Scholar] [CrossRef]
- Sahu, I.; Verma, J.; Bera, A.K.; Pande, S.; Bhavsar, A.; Pati, F.; Chakraborty, P. Synergistic Coassembly of Folic Acid-Based Supramolecular Polymer with a Covalent Polymer Toward Fabricating Functional Antibacterial Biomaterials. ACS Appl. Mater. Interfaces 2024, 16, 34141–34155. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, A.; Dang, F.; Ferrell, A.; Barton, H.A.; Joy, A. Peptidomimetic Polyurethanes Inhibit Bacterial Biofilm Formation and Disrupt Surface Established Biofilms. J. Am. Chem. Soc. 2021, 143, 9440–9449. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, S.; Liang, Y.; Qin, C.; Wang, F.; Wang, H.; Chang, C.; Inoue, A. Flexible free-standing antibacterial nanoporous Ag ribbon. J. Colloid Interface Sci. 2023, 645, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Wang, T.-Y. Study on Antibacterial Activity and Structure of Chemically Modified Lysozyme. Molecules 2022, 28, 95. [Google Scholar] [CrossRef]
- Wei, Z.; Wu, S.; Xia, J.; Shao, P.; Sun, P.; Xiang, N. Enhanced Antibacterial Activity of Hen Egg-White Lysozyme against Staphylococcus aureus and Escherichia coli due to Protein Fibrillation. Biomacromolecules 2021, 22, 890–897. [Google Scholar] [CrossRef]
- Worthington, R.J.; Melander, C. Combination approaches to combat multidrug-resistant bacteria. Trends Biotechnol. 2013, 31, 177–184. [Google Scholar] [CrossRef]
- Wu, Y.; Garren, M.R.; Estes Bright, L.M.; Maffe, P.; Brooks, M.; Brisbois, E.J.; Handa, H. Enhanced antibacterial efficacy against antibiotic-resistant bacteria via nitric oxide-releasing ampicillin polymer substrates. J. Colloid. Interface Sci. 2024, 653 Pt B, 1763–1774. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Liu, J.; Huang, Y.; Peng, Y.; Xu, Y.; Ren, L. Photo-crosslinked GelMA/collagen membrane loaded with lysozyme as an antibacterial corneal implant. Int. J. Biol. Macromol. 2021, 191, 1006–1016. [Google Scholar] [CrossRef]
- Yang, Y.; Qian, Y.; Zhang, M.; Hao, S.; Wang, H.; Fan, Y.; Liu, R.; Xu, D.; Wang, F. Host defense peptide-mimicking β-peptide polymer displaying strong antibacterial activity against cariogenic Streptococcus mutans. J. Mater. Sci. Technol. 2023, 133, 77–88. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, J.; Liu, C.; Li, L.; Xu, C.; Li, Y.; Li, Y.; Tian, H. Antibacterial activity of guanidinium-based ionic covalent organic framework anchoring Ag nanoparticles. J. Hazard. Mater. 2022, 435, 128965. [Google Scholar] [CrossRef]
- Zhang, T.; Nickerson, R.; Zhang, W.; Peng, X.; Shang, Y.; Zhou, Y.; Luo, Q.; Wen, G.; Cheng, Z. The impacts of animal agriculture on One Health-Bacterial zoonosis, antimicrobial resistance, and beyond. One Health 2024, 18, 100748. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Pei, Z.; Wang, Z.-H.; Wang, C.-E.; Liang, F.-F.; Wei, L.-Q. ZIF-8 modified by isocyanate as a photocatalytic antibacterial agent. Rare Met. 2024, 43, 2708–2718. [Google Scholar] [CrossRef]


| Sample | Conc.A340=0.40 | Conc.A340=0.40 | Conc.A340=0.40 | Amountamino (CGly/Cx) | AmountHydrophobic |
|---|---|---|---|---|---|
| Glycine | 0.610 | 0.835 | 1.06 | - | - |
| Lysozyme | 0.0313 | 0.0523 | 0.0733 | 16.64 | - |
| Lyz-3-Leu | 0.0162 | 0.0292 | 0.0422 | 30.45 | 13.81 |
| Lyz-3-Trp | 0.0160 | 0.0263 | 0.0366 | 32.94 | 16.30 |
| Lyz-3-Met | 0.0210 | 0.0319 | 0.0428 | 26.66 | 9.96 |
| Lyz-6-Leu | 0.0178 | 0.0288 | 0.0398 | 29.96 | 13.32 |
| Lyz-6-Trp | 0.0143 | 0.0244 | 0.0345 | 35.87 | 19.23 |
| Lyz-6-Met | 0.0210 | 0.0263 | 0.0366 | 29.92 | 13.28 |
| Lyz-8-Leu | 0.0184 | 0.0293 | 0.0401 | 30.20 | 13.56 |
| Lyz-8-Trp | 0.0184 | 0.0292 | 0.0401 | 29.39 | 12.75 |
| Lyz-8-Met | 0.0237 | 0.0354 | 0.0471 | 23.94 | 7.30 |
| Sample | MICE.coli (μg/mL) | MBCE.coli (μg/mL) | MICS. aureus (μg/mL) | MBCS. aureus (μg/mL) |
|---|---|---|---|---|
| Lyz-3-Leu | 128 | 128 | 128 | 256 |
| Lyz-3-Trp | 128 | 128 | 128 | 256 |
| Lyz-3-Met | 128 | 256 | 128 | 256 |
| Lyz-6-Leu | 64 | 128 | 128 | 128 |
| Lyz-6-Trp | 64 | 128 | 128 | 128 |
| Lyz-6-Met | 128 | 128 | 128 | 256 |
| Lyz-8-Leu | 32 | 64 | 64 | 64 |
| Lyz-8-Trp | 32 | 64 | 64 | 64 |
| Lyz-8-Met | 64 | 64 | 64 | 64 |
| Lyz-3-Gua | 256 | 512 | 256 | 512 |
| Lyz-6-Gua | 128 | 256 | 128 | 256 |
| Lyz-8-Gua | 128 | 128 | 128 | 256 |
| Sample | TIE. coli | TiS. aureus | Sample | TIE. coli | TiS. aureus | Sample | TIE. coli | TiS. aureus |
|---|---|---|---|---|---|---|---|---|
| Lyz-3-Leu | >500 | >500 | Lyz-6-Leu | >500 | >500 | Lyz-8-Leu | 250 | 250 |
| Lyz-3-Trp | >500 | >500 | Lyz-6-Trp | >500 | >500 | Lyz-8-Trp | 500 | 500 |
| Lyz-3-Met | >500 | >500 | Lyz-6-Met | >500 | >500 | Lyz-8-Met | >500 | >500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Lin, S.; Zhu, M.; Liu, X.; Huang, X. Enhanced Antibacterial Activity of Hydrophobic Modified Lysozyme Against Gram-Negative Bacteria Without Accumulated Resistance. Molecules 2025, 30, 232. https://doi.org/10.3390/molecules30020232
Li Z, Lin S, Zhu M, Liu X, Huang X. Enhanced Antibacterial Activity of Hydrophobic Modified Lysozyme Against Gram-Negative Bacteria Without Accumulated Resistance. Molecules. 2025; 30(2):232. https://doi.org/10.3390/molecules30020232
Chicago/Turabian StyleLi, Zhenhui, Song Lin, Mei Zhu, Xiaoman Liu, and Xin Huang. 2025. "Enhanced Antibacterial Activity of Hydrophobic Modified Lysozyme Against Gram-Negative Bacteria Without Accumulated Resistance" Molecules 30, no. 2: 232. https://doi.org/10.3390/molecules30020232
APA StyleLi, Z., Lin, S., Zhu, M., Liu, X., & Huang, X. (2025). Enhanced Antibacterial Activity of Hydrophobic Modified Lysozyme Against Gram-Negative Bacteria Without Accumulated Resistance. Molecules, 30(2), 232. https://doi.org/10.3390/molecules30020232






