Discovery of Quinazolone Pyridiniums as Potential Broad-Spectrum Antibacterial Agents
Abstract
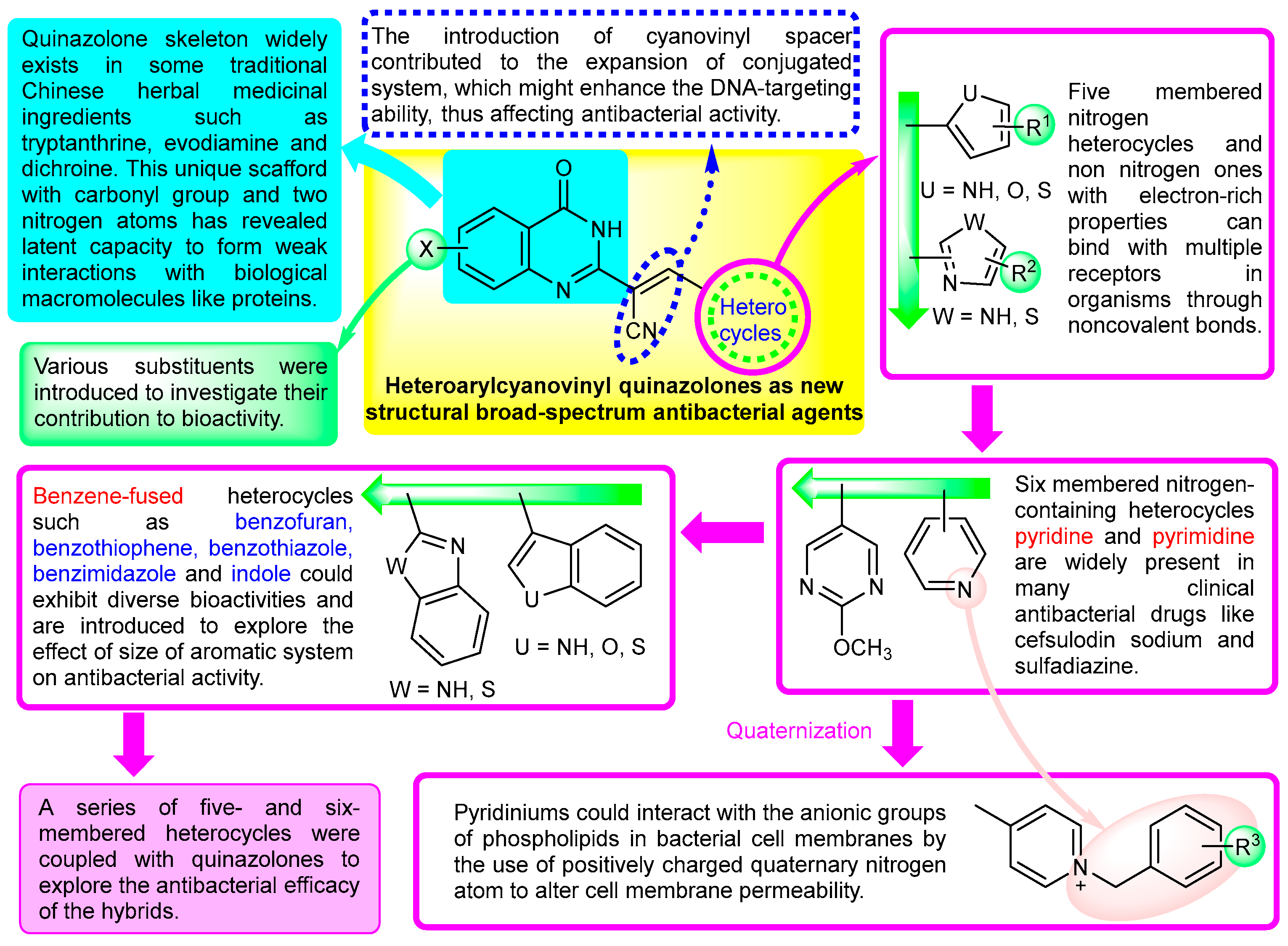
:1. Introduction
2. Results and Discussion
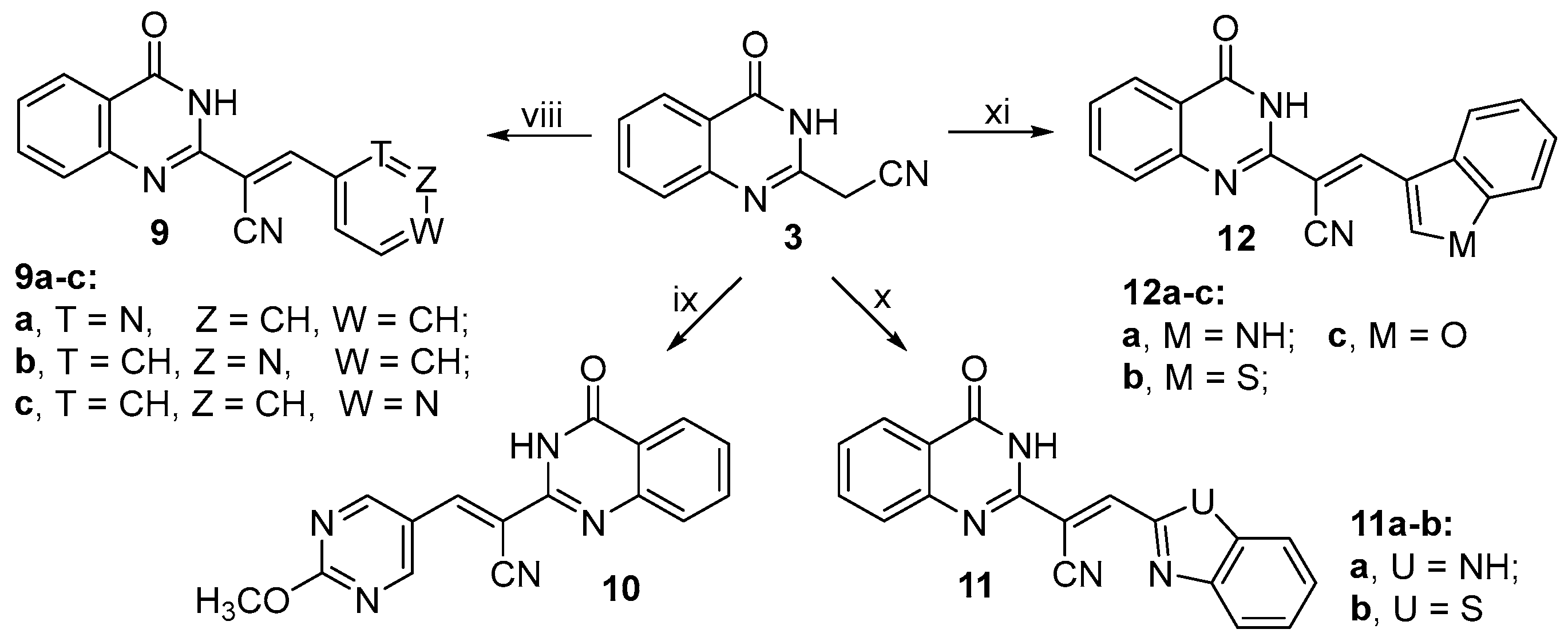
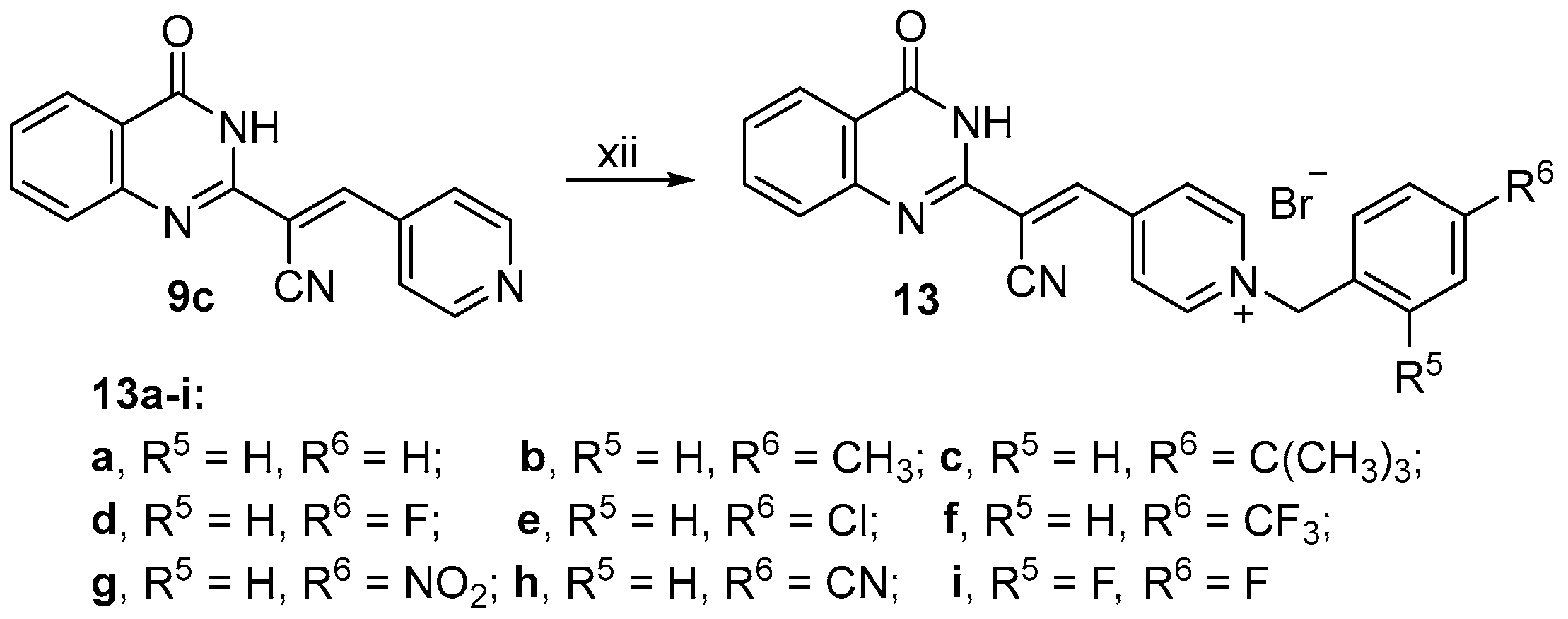
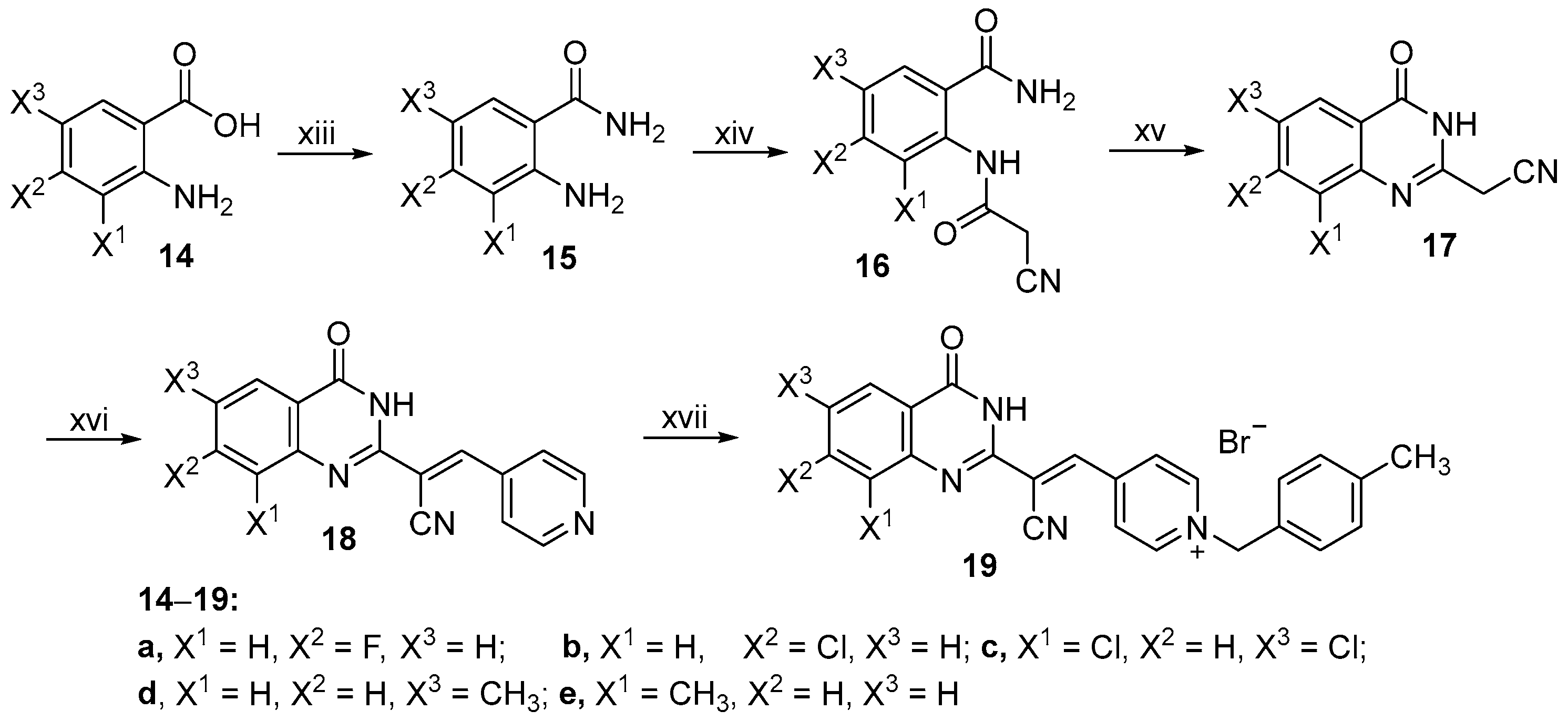
2.1. Chemistry
2.2. Antibacterial Activity
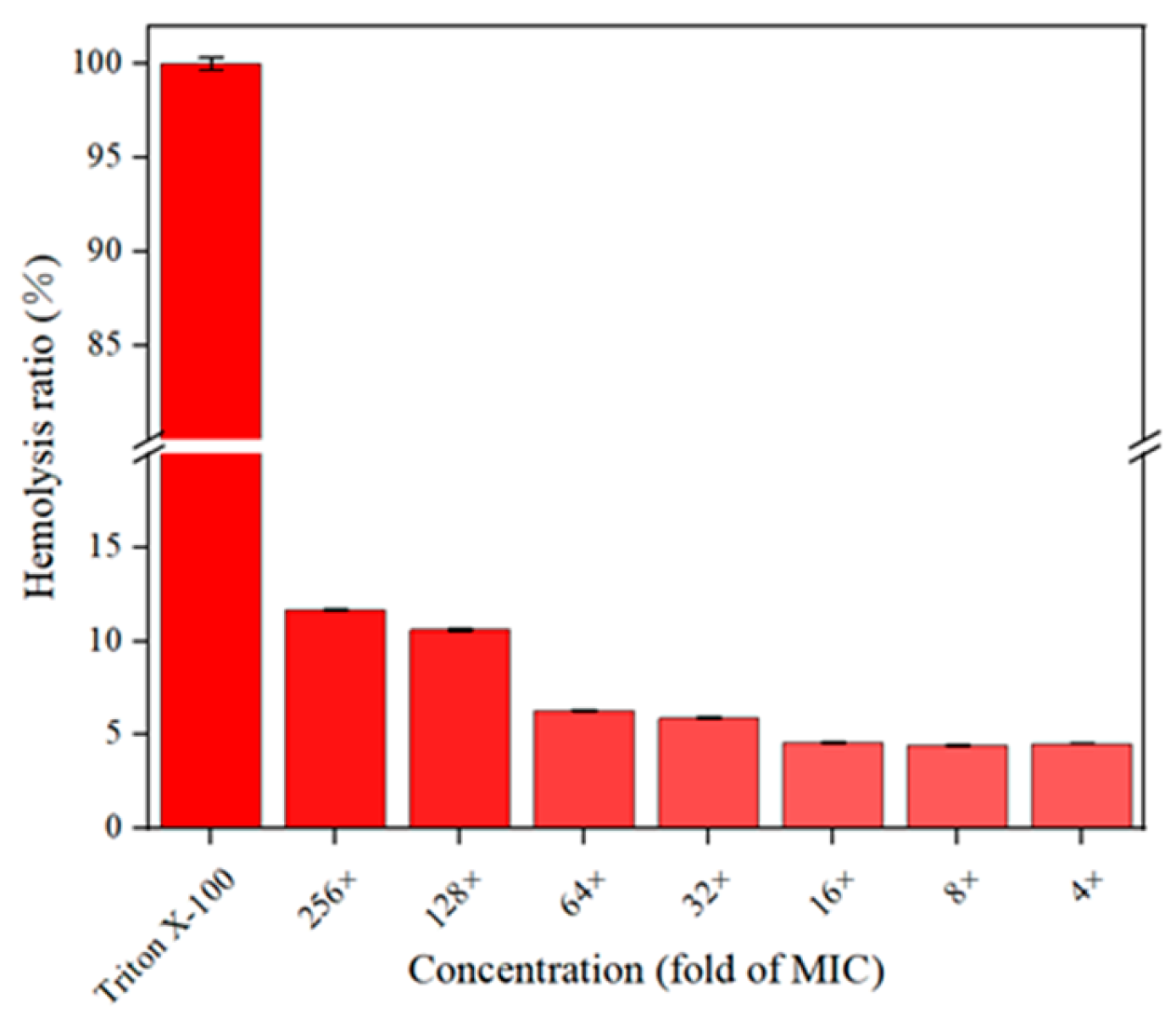
2.3. Hemolytic Assay
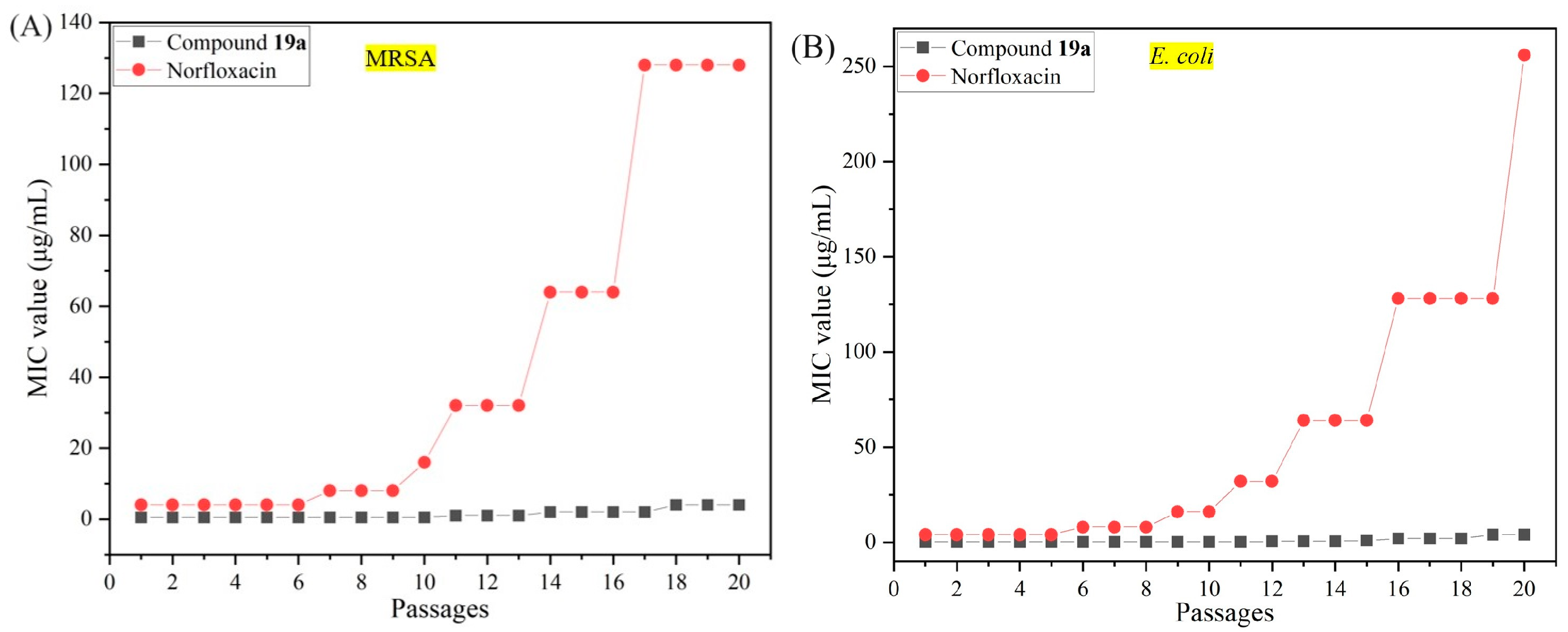
2.4. Resistance Study
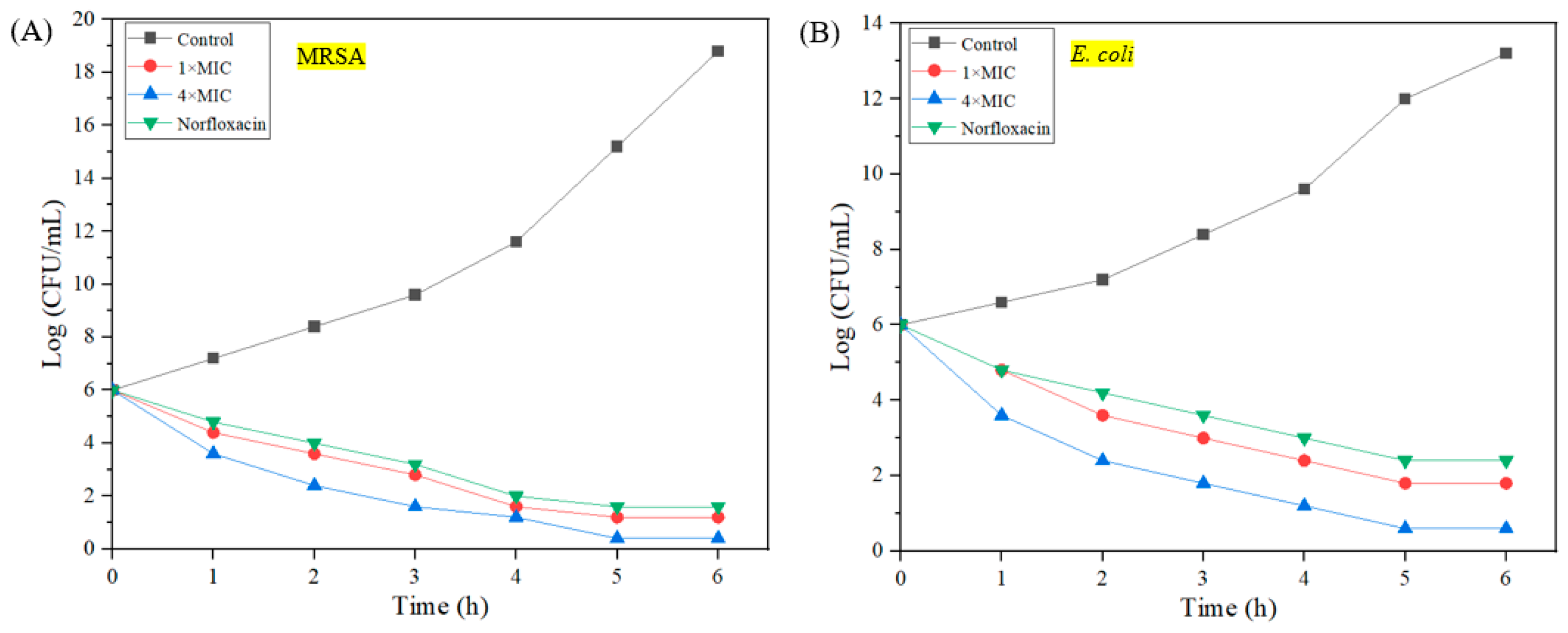
2.5. Bactericidal Kinetics
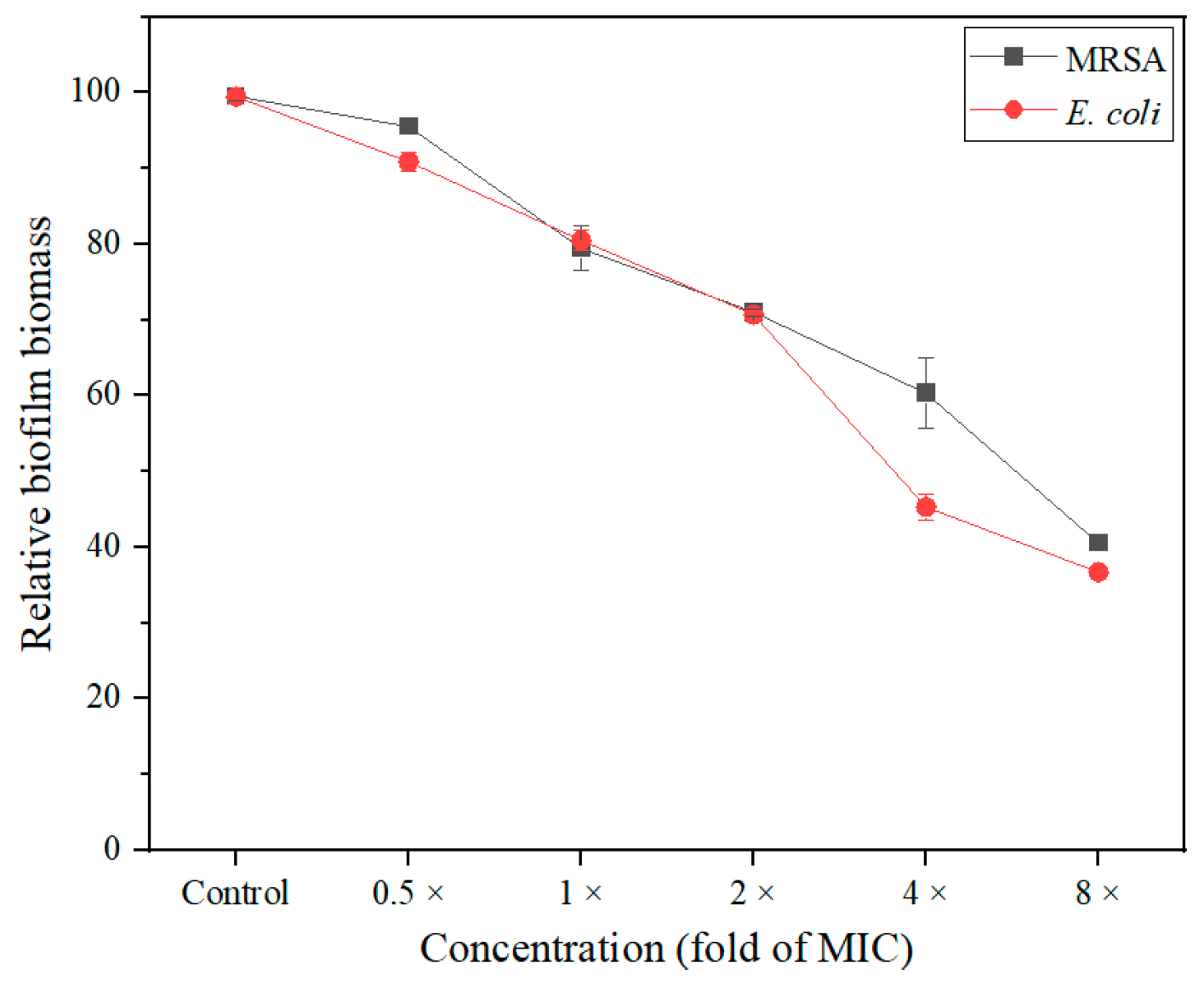
2.6. Antibiofilm Activity
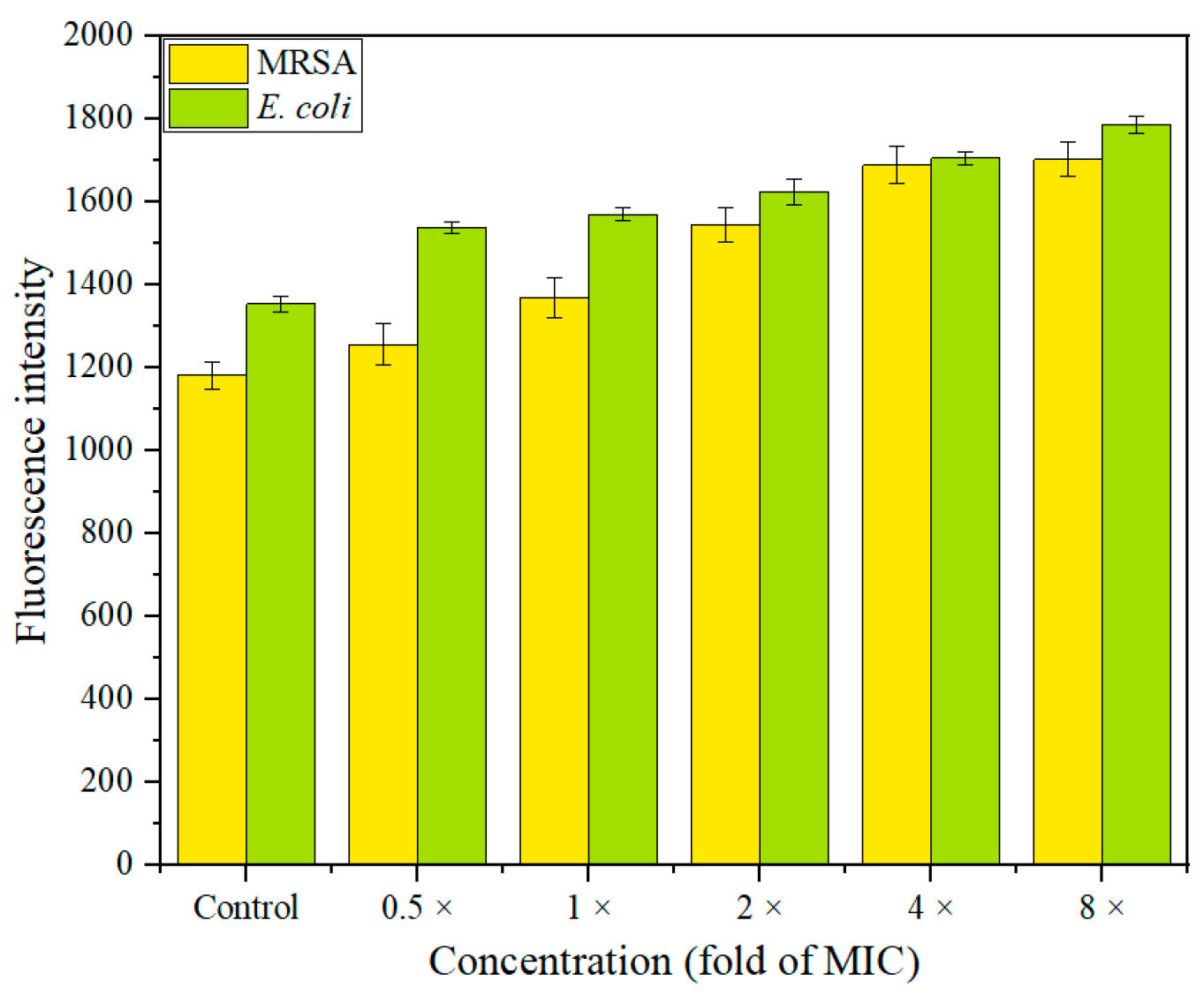
2.7. Membrane Damage Assay
2.7.1. Membrane Depolarization Assay
2.7.2. Assay of Outer Membrane Damage
2.7.3. Study of Inner Membrane Permeabilization
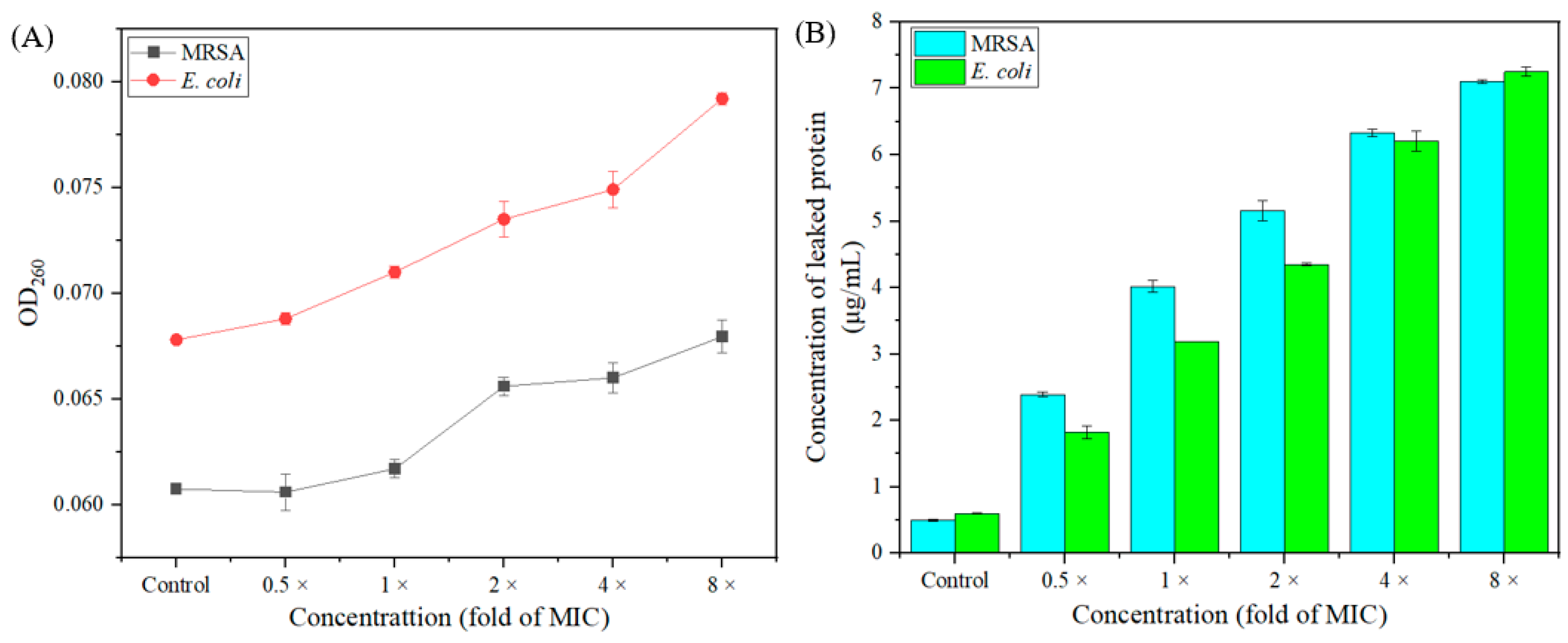
2.8. The Leakage of Intracellular Protein and Nucleic Acid
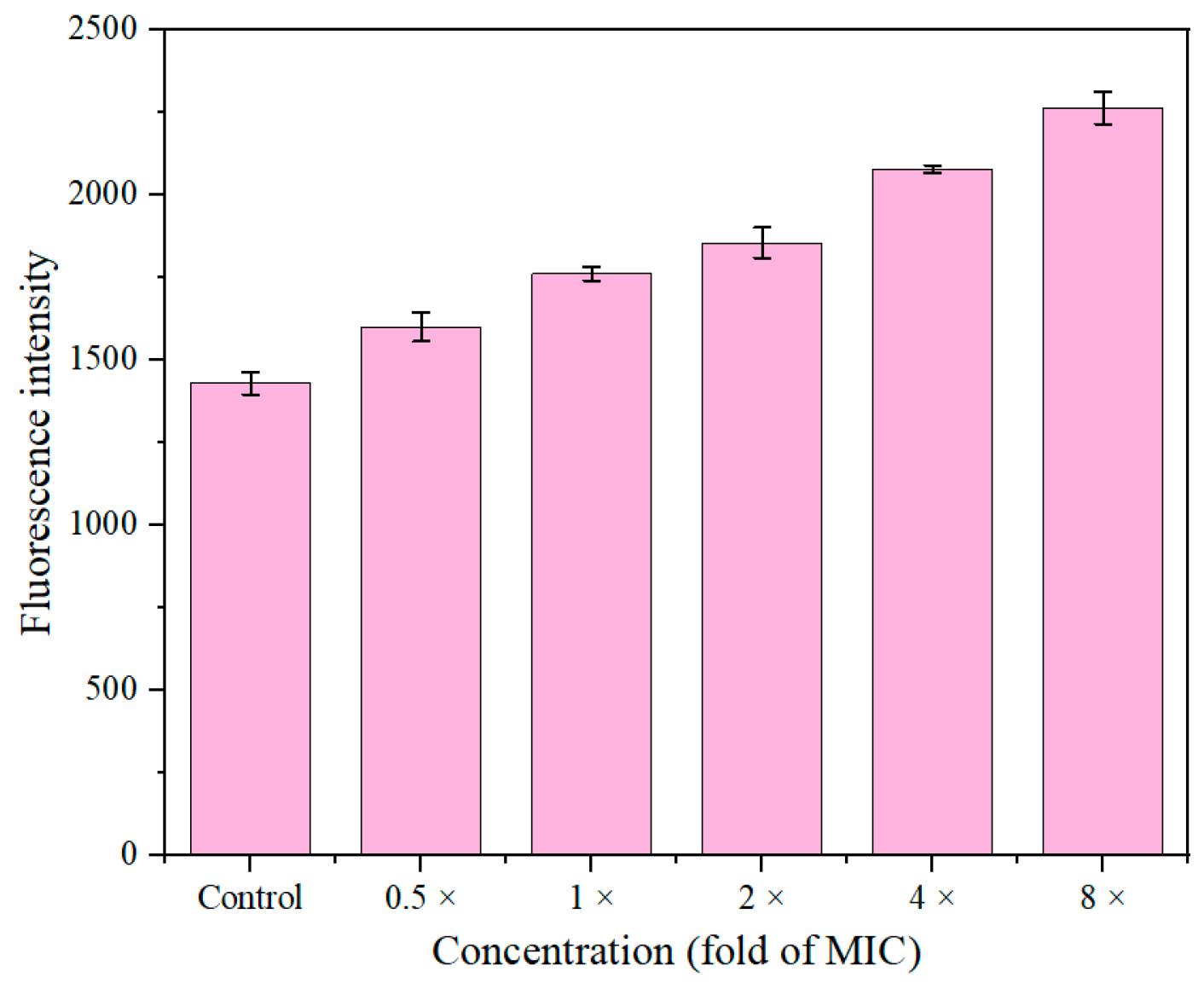
2.9. Intracellular ROS Accumulation
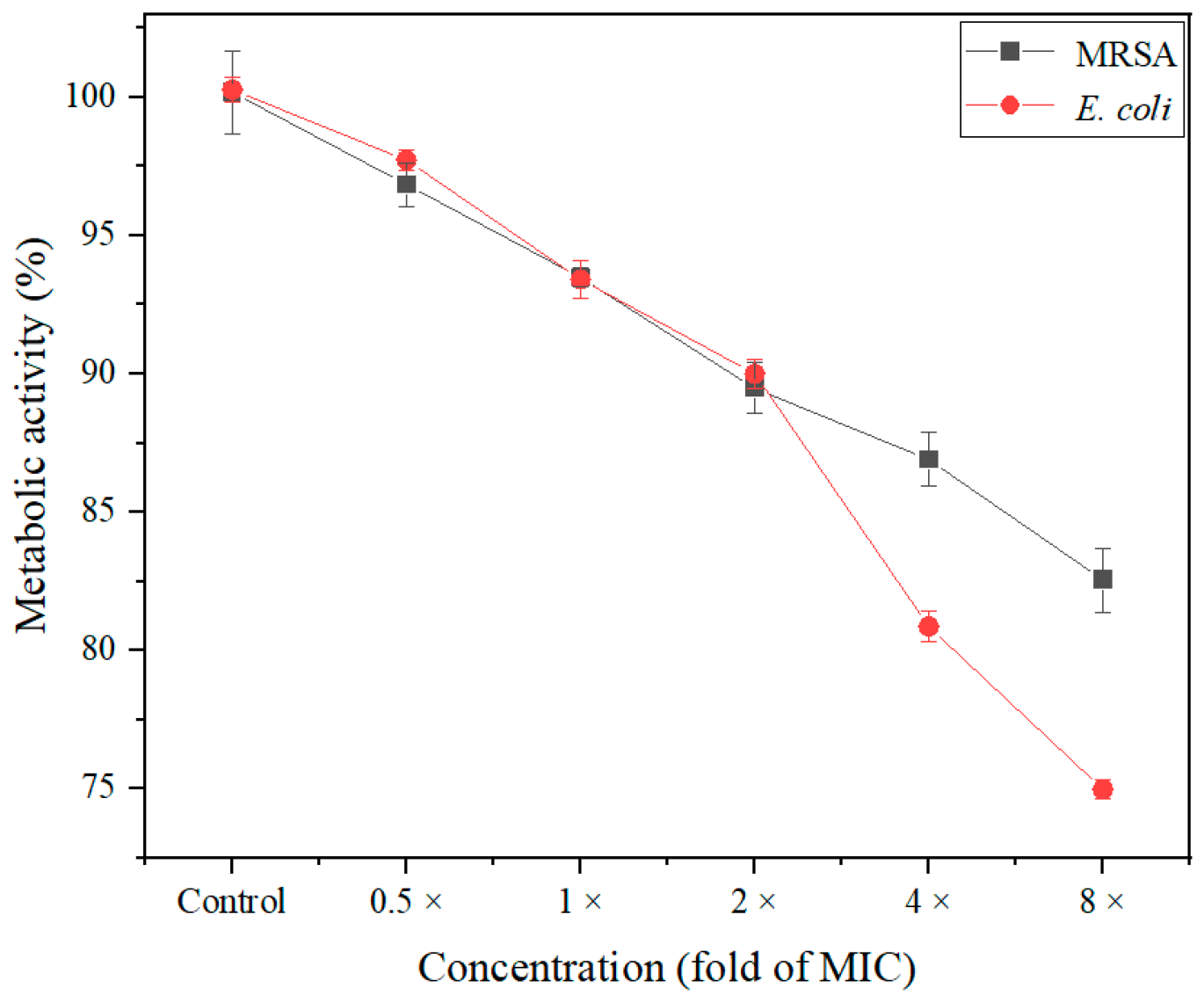
2.10. Detection of Metabolic Activity
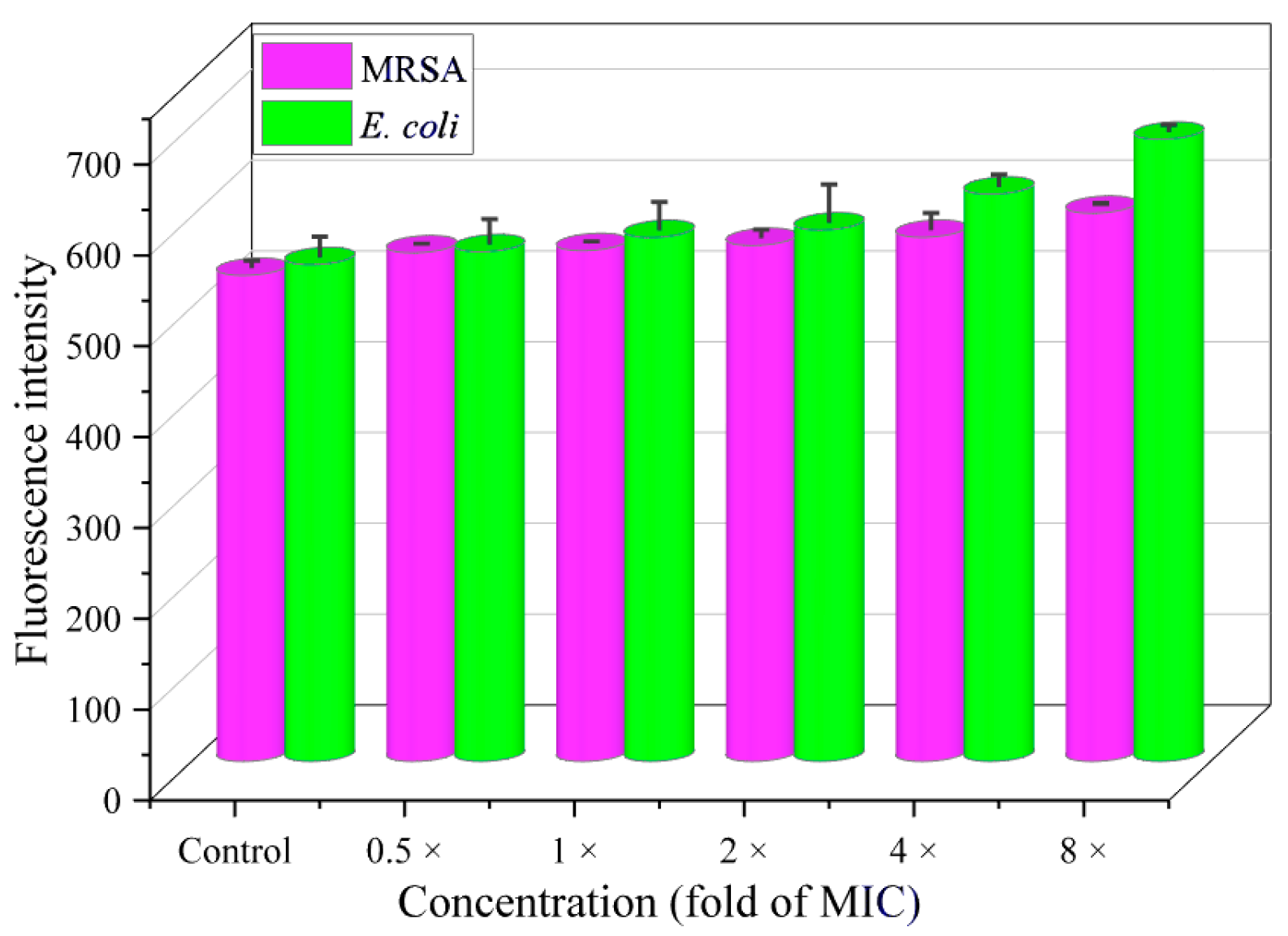
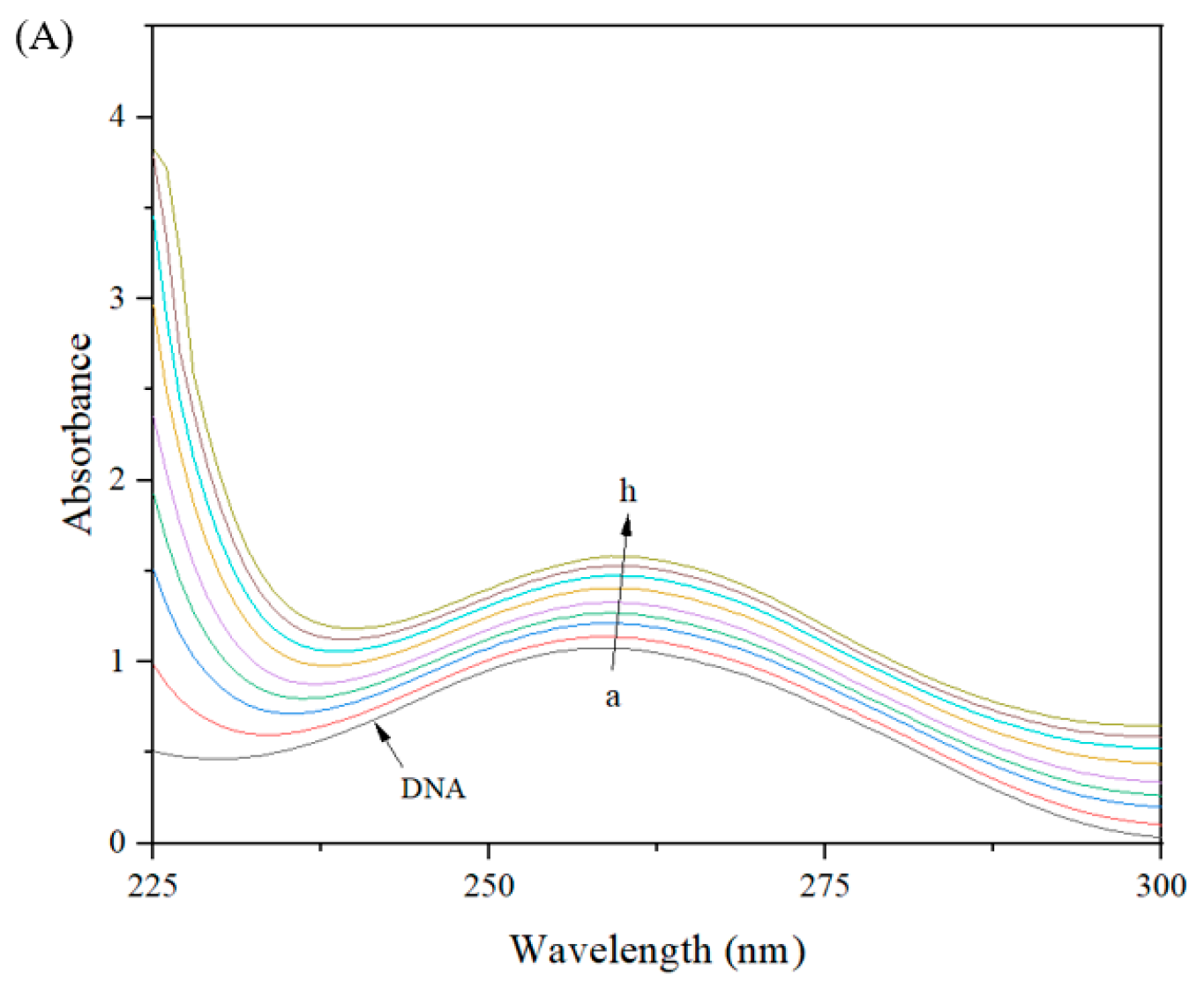
2.11. Interaction Between Compound 19a and DNA
2.11.1. DNA Binding Study
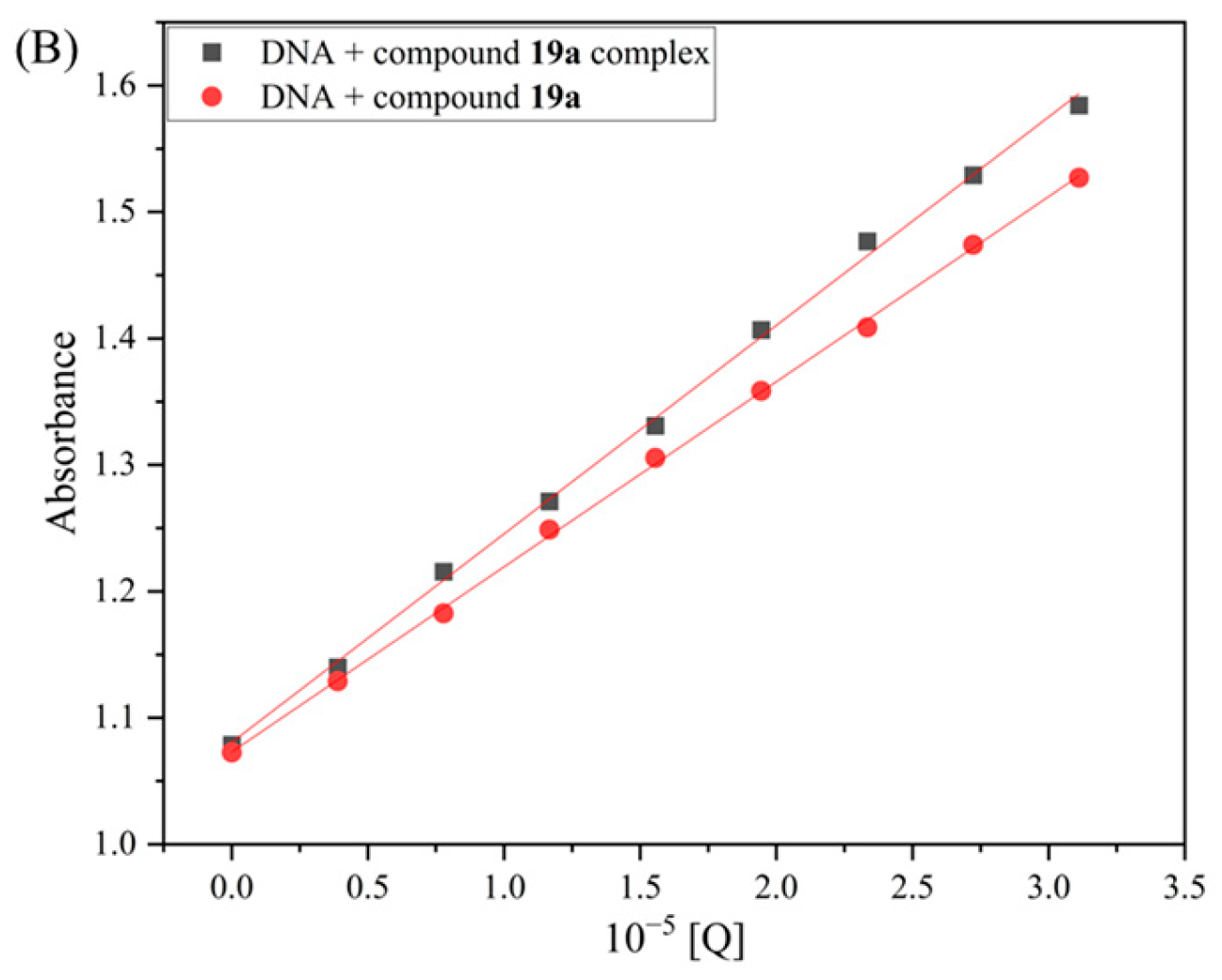
2.11.2. Competitive Binding Study
2.12. Allosteric Modulation of Compound 19a with PBP2a
2.12.1. Contents of PBP2a
2.12.2. Allosteric Site Binding Affinity of PBP2a
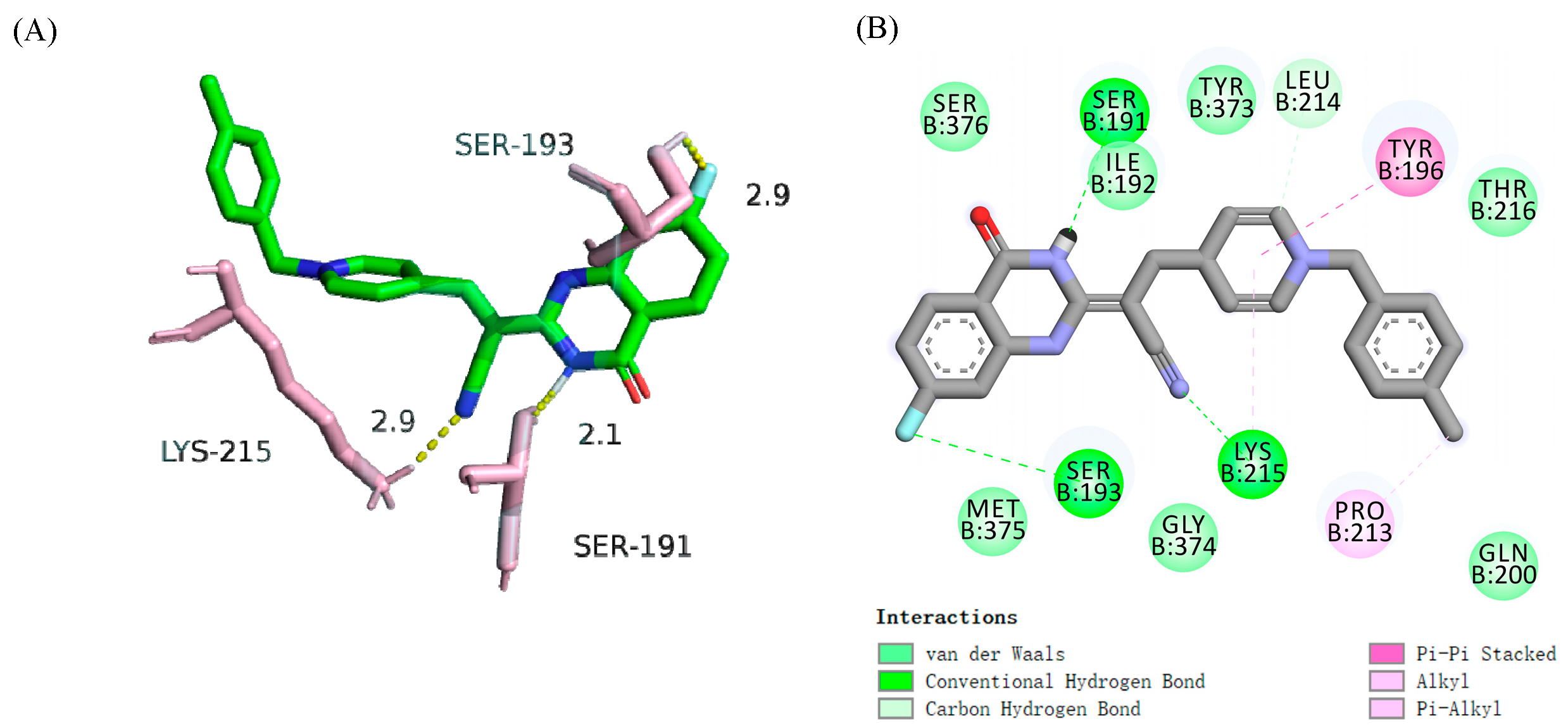
2.12.3. Molecular Docking
3. Materials and Methods
3.1. Instruments and Chemicals
3.2. Synthesis of Intermediates and Target Molecules
3.2.1. Synthesis of Intermediate 2
3.2.2. Synthesis of Intermediate 3
3.2.3. Synthesis of (E)-3-(Furan-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (4a)
3.2.4. Synthesis of (E)-3-(5-Methylfuran-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (4b)
3.2.5. Synthesis of (E)-3-(5-(Hydroxymethyl)furan-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (4c)
3.2.6. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(thiophen-2-yl)acrylonitrile (5a)
3.2.7. Synthesis of (E)-3-(3-Methylthiophen-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (5b)
3.2.8. Synthesis of (E)-3-(1H-Imidazol-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (6a)
3.2.9. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(thiazol-2-yl)acrylonitrile (6b)
3.2.10. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(1H-pyrrol-2-yl)acrylonitrile (6c)
3.2.11. Synthesis of (E)-3-(2-Methylthiazol-5-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (7)
3.2.12. Synthesis of (E)-3-(1H-Imidazol-4-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (8a)
3.2.13. Synthesis of (E)-3-(2-Butyl-5-chloro-1H-imidazol-4-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (8b)
3.2.14. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(pyridin-2-yl)acrylonitrile (9a)
3.2.15. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(pyridin-3-yl)acrylonitrile (9b)
3.2.16. Synthesis of (E)-2-(4-Oxo-3,4-dihydroquinazolin-2-yl)-3-(pyridin-4-yl)acrylonitrile (9c)
3.2.17. Synthesis of (E)-3-(2-Methoxypyrimidin-5-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (10)
3.2.18. Synthesis of (E)-3-(1H-Benzo[d]imidazol-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (11a)
3.2.19. Synthesis of (E)-3-(Benzo[d]thiazol-2-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (11b)
3.2.20. Synthesis of (E)-3-(1H-Indol-3-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (12a)
3.2.21. Synthesis of (E)-3-(Benzo[b]thiophen-3-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (12b)
3.2.22. Synthesis of (E)-3-(Benzofuran-3-yl)-2-(4-oxo-3,4-dihydroquinazolin-2-yl)acrylonitrile (12c)
3.2.23. Synthesis of (E)-1-Benzyl-4-(2-cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)pyridin-1-ium (13a)
3.2.24. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-methylbenzyl)pyridin-1-ium (13b)
3.2.25. Synthesis of (E)-1-(4-(tert-Butyl)benzyl)-4-(2-cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)pyridine -1-ium (13c)
3.2.26. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-fluorobenzyl)pyridin-1-ium (13d)
3.2.27. Synthesis of (E)-1-(4-Chlorobenzyl)-4-(2-cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)pyridin-1-ium (13e)
3.2.28. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-(trifluoromethyl)benzyl) pyridin-1-ium (13f)
3.2.29. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-nitrobenzyl)pyridin-1-ium (13g)
3.2.30. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-cyanobenzyl)pyridin-1-ium (13h)
3.2.31. Synthesis of (E)-4-(2-Cyano-2-(4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(2,4- difluorobenzyl)pyridin-1-ium (13i)
3.2.32. Synthesis of Intermediates 15a–e
3.2.33. Synthesis of (E)-4-(2-cyano-2-(7-fluoro-4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4-methylbenzyl) pyridin-1-ium (19a)
3.2.34. Synthesis of (E)-4-(2-(7-Chloro-4-oxo-3,4-dihydroquinazolin-2-yl)-2-cyanovinyl)-1-(4- methylbenzyl)pyridin-1-ium (19b)
3.2.35. Synthesis of (E)-4-(2-Cyano-2-(6,8-dichloro-4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4- methylbenzyl)pyridin-1-ium (19c)
3.2.36. Synthesis of (E)-4-(2-Cyano-2-(6-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4- methylbenzyl)pyridin-1-ium (19d)
3.2.37. Synthesis of (E)-4-(2-Cyano-2-(8-methyl-4-oxo-3,4-dihydroquinazolin-2-yl)vinyl)-1-(4- methylbenzyl)pyridin-1-ium (19e)
3.3. Biological Assay
3.3.1. Antibacterial Evaluation
3.3.2. Hemolytic Toxicity
3.3.3. Drug-Resistance Assay
3.3.4. Inhibition of Bacterial Growth
3.3.5. Inhibition of Biofilm
3.3.6. Membrane Depolarization
3.3.7. Assay for Outer Membrane Permeability
3.3.8. Assay for Inner Membrane Damage
3.3.9. Leakage of Cellular Protein
3.3.10. Assessment of Metabolic Inactivation
3.3.11. Reactive Oxygen Species (ROS) Production
3.3.12. Absorption Spectra of DNA
3.3.13. AO Binding Assay
3.3.14. Measurement of PBP2a Contents
3.3.15. Interaction with PBP2a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, H.; Jakob, R.P.; Marzinek, J.K.; Robert, G.; Imai, Y.; Bolla, J.R.; Agustoni, E.; Robinson, C.V.; Bond, P.J.; Lewis, K.; et al. The antibiotic darobactin mimics a β-strand to inhibit outer membrane insertase. Nature 2021, 593, 125–129. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Koirala, B.; Hernandez, Y.; Zimmerman, M.; Brady, S.F. Bioinformatic prospecting and synthesis of a bifunctional lipopeptide antibiotic that evades resistance. Science 2022, 376, 991–996. [Google Scholar]
- Gerlach, D.; Guo, Y.L.; Castro, C.D.; Kim, S.H.; Schlatterer, K.; Xu, F.F.; Pereira, C.; Seeberger, P.H.; Ali, S.; Codée, J.; et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 2018, 563, 705–709. [Google Scholar] [CrossRef]
- Lewis, K. The science of antibiotic discovery. Cell 2020, 181, 29–45. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, M.X.; Yao, X.; Li, Y.; Tan, J.; Wang, L.Z.; Qiao, W.T.; Geng, Y.Q.; Liu, Y.X.; Wang, Q.M. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar] [CrossRef]
- Plescia, F.; Maggio, B.; Daidone, G.; Raffa, D. 4-(3H)-quinazolinones N-3 substituted with a five membered heterocycle: A promising scaffold towards bioactive molecules. Eur. J. Med. Chem. 2021, 213, 113070. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Fu, G.H.; Zhong, W.H. Natural quinazolinones: From a treasure house to promising anticancer leads. Eur. J. Med. Chem. 2023, 245, 114915. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhao, L.; Bian, Y.Q.; Li, Y.; Qu, J.; Song, F. The antibacterial activity of quinazoline and quinazolinone hybrids. Curr. Top. Med. Chem. 2022, 22, 1035–1044. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Identification of unique quinazolinone thiazoles as novel structural scaffolds for potential gram-negative bacterial conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Dai, J.; Battini, N.; Zang, Z.L.; Luo, Y.; Zhou, C.H. Discovery of alkaloid quinazolone-derived imidazolenones with novel structural scaffolds of multitargeting antibacterial potential. Chin. J. Chem. 2023, 41, 3645–3661. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, Y.; Chen, X.H.; Li, C.H. Antibacterial activity evaluation of pleuromutilin derivatives with 4 (3H)-quinazolinone scaffold against methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2022, 246, 114960. [Google Scholar] [CrossRef]
- Wang, J.; Battini, N.; Ansari, M.F.; Zhou, C.H. Synthesis and biological evaluation of quinazolinonethiazoles as new potential conquerors towards Pseudomonas aeruginosa. Chin. J. Chem. 2021, 39, 1093–1103. [Google Scholar] [CrossRef]
- Ambade, S.S.; Gupta, V.K.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. A review on five and six-membered heterocyclic compounds targeting the penicillin-binding protein 2 (PBP2A) of methicillin-resistant Staphylococcus aureus (MRSA). Molecules 2023, 28, 7008. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Battini, N.; Zang, Z.L.; Luo, Y.; Zhou, C.H. Novel thiazolylketenyl quinazolinones as potential anti-MRSA agents and allosteric modulator for PBP2a. Molecules 2023, 28, 4240. [Google Scholar] [CrossRef]
- Janardhanan, J.; Bouley, R.; Martinez-Caballero, S.; Peng, Z.H.; Batuecas-Mordillo, M.; Meisel, J.E.; Ding, D.R.; Schroeder, V.A.; Wolter, W.R.; Mahasenan, K.V.; et al. The quinazolinone allosteric inhibitor of PBP 2a synergizes with piperacillin and tazobactam against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2019, 63, e02637-18. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Xue, Z.H.; Huang, M.J.; Wang, T.T.; Zhang, M.M.; Yang, R.G.; Guo, Y. Current development of thiazole-containing compounds as potential antibacterials against methicillin-resistant Staphylococcus aureus. ACS Infect. Dis. 2024, 10, 350–370. [Google Scholar] [CrossRef]
- Zhou, X.M.; Hu, Y.Y.; Fang, B.; Zhou, C.H. Benzenesulfonyl thiazoloimines as unique multitargeting antibacterial agents towards Enterococcus faecalis. Eur. J. Med. Chem. 2023, 248, 115088. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.V.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Li, S.R.; Tan, Y.M.; Zhang, L.; Zhou, C.H. Comprehensive insights into medicinal research on imidazole-based supramolecular complexes. Pharmaceutics 2023, 15, 1348. [Google Scholar] [CrossRef]
- Ahmed, K.; Choudhary, M.I.; Saleem, R.S.Z. Heterocyclic pyrimidine derivatives as promising antibacterial agents. Eur. J. Med. Chem. 2023, 259, 115701. [Google Scholar] [CrossRef]
- Tan, Y.M.; Wang, Y.; Li, S.; Zhang, S.L.; Zhou, C.H. Azolylpyrimidinediols as novel structural scaffolds of DNA-groove binders against intractable Acinetobacter baumannii. J. Med. Chem. 2023, 66, 4910–4931. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.B.; Islam, M.I.; Nath, N.; Emran, T.B.; Rahman, M.R.; Sharma, R.; Matin, M.M. Recent advances in pyridine scaffold: Focus on chemistry, synthesis, and antibacterial activities. BioMed Res. Int. 2023, 2023, 9967591. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Zang, Z.L.; Wang, Y.X.; Battini, N.; Gao, W.W.; Zhou, C.H. Synthesis and antibacterial medicinal evaluation of carbothioamido hydrazonyl thiazolylquinolone with multitargeting antimicrobial potential to combat increasingly global resistance. Eur. J. Med. Chem. 2024, 275, 116626. [Google Scholar] [CrossRef]
- Wang, Y.X.; Deng, Z.; Bibi, A.; Fang, B.; Zhou, C.H. Unique azolyl acylhydrazonyl hybridization of aloe emodins to access potential antibacterial agents. Chin. J. Chem. 2024, 42, 1741–1758. [Google Scholar] [CrossRef]
- Wang, Y.X.; Du, Y.F.; Huang, H. A survey of the role of nitrile groups in protein–ligand interactions. Future Med. Chem. 2018, 10, 2713–2727. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Y.; Zhang, Y.Q.; Chen, Y.; Zhao, Y.F.; Shao, W.Y.; Ma, Z.H. Characterization of the fludioxonil and phenamacril dual resistant mutants of Fusarium graminearum. Pestic. Biochem. Physiol. 2024, 200, 105815. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary ammonium salts and their antimicrobial potential: Targets or nonspecific interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Saczewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomasic, T.; Skok, Z.; Savijoki, K.; Morawska, M.; et al. Synthesis and biological evaluation of hybrid quinolone-based quaternary ammonium antibacterial agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Sowmiah, S.; Esperanca, J.M.S.S.; Rebelo, L.P.N.; Afonso, C.A.M. Pyridinium salts: From synthesis to reactivity and applications. Org. Chem. Front. 2018, 5, 453–493. [Google Scholar] [CrossRef]
- Fromm-Dornieden, C.; Rembe, J.; Schafer, N.; Bohm, J.; Stuermer, E.K. Cetylpyridinium chloride and miramistin as antiseptic substances in chronic wound management-prospects and limitations. J. Med. Microbiol. 2015, 64, 407–414. [Google Scholar] [CrossRef]
- Wang, N.; Yu, H.; Zhu, Z.H.; Wang, H.; Wei, Y.F.; Wang, Q.; Zhou, Y.L.; Fang, T.Q.; Zhang, Y.; Cui, M.H.; et al. Novel methyldithiocarbazate derivatives as NDM-1 inhibitors to combat multidrug-resistant bacterial infection with β-lactams. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.H.; Wen, T.Y.; Yan, X.T.; Han, M.Y.; Bai, L.P.; Fu, X.J.; Liu, J.F.; Qin, S.S. Development of membrane-active honokiol/magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, Y.M.; Li, S.R.; Battini, N.; Zhang, S.L.; Lin, J.M.; Zhou, C.H. Discovery of benzopyridone cyanoacetates as new type of potential broad-spectrum antibacterial candidates. Eur. J. Med. Chem. 2024, 265, 116107. [Google Scholar] [CrossRef]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef]
- García-Castro, M.; Sarabia, F.; Díaz-Morilla, A.; López-Romero, J.M. Approved antibacterial drugs in the last 10 years: From the bench to the clinic. Explor. Drug Sci. 2023, 1, 180–209. [Google Scholar] [CrossRef]
- Yang, X.C.; Zeng, C.M.; Avula, S.R.; Peng, X.M.; Geng, R.X.; Zhou, C.H. Novel coumarin aminophosphonates as potential multitargeting antibacterial agents against Staphylococcus aureus. Eur. J. Med. Chem. 2023, 245, 114891. [Google Scholar] [CrossRef]
- Li, F.F.; Zhao, W.H.; Tangadanch, V.K.R.; Meng, J.P.; Zhou, C.H. Discovery of novel phenylhydrazone-based oxindole-thiolazoles as potent antibacterial agents toward Pseudomonas aeruginosa. Eur. J. Med. Chem. 2022, 239, 114521. [Google Scholar] [CrossRef]
- Cai, Q.N.; Yu, Q.; Liang, W.X.; Li, H.Z.; Liu, J.Y.; Li, H.X.; Chen, Y.Z.; Fang, S.F.; Zhong, R.C.; Liu, S.P.; et al. Membrane-active nonivamide derivatives as effective broad-spectrum antimicrobials: Rational design, synthesis, and biological evaluation. J. Med. Chem. 2022, 65, 16754–16773. [Google Scholar] [CrossRef]
- Zhou, X.M.; Li, Q.Y.; Lu, X.; Bheemanaboina, R.R.Y.; Fang, B.; Cai, G.X.; Zhou, C.H. Identification of unique indolylcyanoethylenyl sulfonylanilines as novel structural scaffolds of potential antibacterial agents. Eur. J. Med. Chem. 2023, 260, 115773. [Google Scholar] [CrossRef]
- Lin, S.M.; Koh, J.J.; Aung, T.T.; Sin, W.L.; Lim, F.H.; Wang, L.; Lakshminarayanan, R.; Zhou, L.; Tan, D.T.H.; Cao, D.R.; et al. Semisynthetic flavone-derived antimicrobials with therapeutic potential against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2017, 60, 6152–6165. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, X.; Ahmad, N.; Zhang, S.L.; Zhou, C.H. Novel aminothiazoximone-corbelled ethoxycarbonylpyrimidones with antibiofilm activity to conquer gram-negative bacteria through potential multitargeting effects. Eur. J. Med. Chem. 2024, 268, 116219. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, X.Y.; Zhong, X.L.; Huang, Y.J.; Lin, J.; Chen, W.M. Design and synthesis of 3-hydroxy-pyridin-4(1H)-ones–ciprofloxacin conjugates as dual antibacterial and antibiofilm agents against Pseudomonas aeruginosa. J. Med. Chem. 2023, 66, 2169–2193. [Google Scholar] [CrossRef] [PubMed]
- Li, S.R.; Zeng, C.M.; Peng, X.M.; Chen, J.P.; Li, S.; Zhou, C.H. Benzopyrone-mediated quinolones as potential multitargeting antibacterial agents. Eur. J. Med. Chem. 2023, 262, 115878. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, P.; Mudgil, P. The Cell wall, cell membrane and virulence factors of Staphylococcus aureus and their role in antibiotic resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Deng, Z.; Bheemanaboina, R.R.Y.; Luo, Y.; Zhou, C.H. Aloe emodin-conjugated sulfonyl hydrazones as novel type of antibacterial modulators against S. aureus 25923 through multifaceted synergistic effects. Bioorg. Chem. 2022, 127, 106035. [Google Scholar] [CrossRef]
- Liang, W.X.; Yu, Q.; Zheng, Z.X.; Liu, J.Y.; Cai, Q.M.; Liu, S.P.; Lin, S.M. Design and synthesis of phenyl sulfide-based cationic amphiphiles as membrane-targeting antimicrobial agents against Gram-positive pathogens. J. Med. Chem. 2022, 65, 14221–14236. [Google Scholar] [CrossRef]
- Sun, J.W.; Rutherford, S.T.; Silhavy, T.J.; Huang, K.C. Physical properties of the bacterial outer membrane. Nat. Rev. Microbiol. 2022, 20, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Genet, S.; Costalat, R.; Burger, J. The influence of plasma membrane electrostatic properties on the stability of cell ionic composition. Biophys. J. 2001, 81, 2442–2457. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, H.G.; Li, H.Z.; Fang, S.F.; Shi, J.G.; Chen, Y.Z.; Zhong, R.C.; Liu, S.P.; Lin, S.M. Rational design of dipicolylamine-containing carbazole amphiphiles combined with Zn2+ as potent broad-spectrum antibacterial agents with a membrane-disruptive mechanism. J. Med. Chem. 2021, 64, 10429–10444. [Google Scholar] [CrossRef]
- Yang, X.; Sun, H.; Maddili, S.K.; Li, S.; Yang, R.G.; Zhou, C.H. Dihydropyrimidinone imidazoles as unique structural antibacterial agents for drug-resistant Gram-negative pathogens. Eur. J. Med. Chem. 2022, 232, 114188. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Kasson, P.M. Antibiotic uptake across Gram-negative outer membranes: Better predictions towards better antibiotics. ACS Infect. Dis. 2019, 5, 2096–2104. [Google Scholar] [CrossRef]
- Rojas, E.R.; Billings, G.; Odermatt, P.D.; Auer, G.K.; Zhu, L.; Miguel, A.; Chang, F.; Weibel, D.B.; Theriot, J.A.; Huang, K.C. The outer membrane is an essential load-bearing element in Gram-negative bacteria. Nature 2018, 559, 617–621. [Google Scholar] [CrossRef]
- Sun, H.; Li, Z.Z.; Jeyakkumar, P.; Zang, Z.L.; Fang, B.; Zhou, C.H. A new discovery of unique 13-(benzimidazolylmethyl)berberines as promising broad-spectrum antibacterial agents. J. Agric. Food Chem. 2022, 70, 12320–12329. [Google Scholar] [CrossRef]
- Zhao, J.S.; Ahmad, N.; Li, S.; Zhou, C.H. Hydrazyl hydroxycoumarins as new potential conquerors towards Pseudomonas aeruginosa. Bioorg. Med. Chem. Lett. 2024, 103, 129709. [Google Scholar] [CrossRef]
- Shi, Y.G.; Zhang, R.R.; Zhu, C.M.; Xu, M.F.; Gu, Q.; Ettelaie, R.; Lin, S.; Wang, Y.F.; Leng, X.Y. Antimicrobial mechanism of alkyl gallates against Escherichia coli and Staphylococcus aureus and its combined effect with electrospun nanofibers on Chinese Taihu icefish preservation. Food Chem. 2021, 346, 128949. [Google Scholar] [CrossRef]
- Zang, Z.L.; Gao, W.W.; Zhou, C.H. Unique aminothiazolyl coumarins as potential DNA and membrane disruptors towards Enterococcus faecalis. Bioorg. Chem. 2024, 148, 107451. [Google Scholar] [CrossRef]
- Yao, C.Y.; Li, X.D.; Bi, W.W.; Jiang, C. Relationship between membrane damage, leakage of intracellular compounds, and inactivation of Escherichia coli treated by pressurized CO2. J. Basic Microbiol. 2014, 54, 858–865. [Google Scholar] [CrossRef]
- Song, J.F.; Wang, M.M.; Tao, H.Y.; Yang, A.M.; Zhu, Z.H.; Bai, S.L.; Luo, M.M.; Xu, J.P.; Liu, X.K.; Sun, Y.C.; et al. Manipulation of bacterial ROS production leads to self-escalating DNA damage and resistance-resistant lethality for intracellular mycobacteria. Microbiology 2023, 7, 548098. [Google Scholar]
- Zou, L.L.; Wang, J.; Gao, Y.; Ren, X.Y.; Rottenberg, M.E.; Lu, J.; Holmgren, A. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Sci. Rep. 2018, 8, 11131. [Google Scholar] [CrossRef]
- Tan, Y.M.; Zhang, J.; Wei, Y.J.; Hu, Y.G.; Li, S.R.; Zhang, S.L.; Zhou, C.H. Cyanomethylquinolones as a new class of potential multitargeting broad-spectrum antibacterial agents. J. Med. Chem. 2024, 67, 9028–9053. [Google Scholar] [CrossRef]
- Zhang, P.L.; Laiche, M.H.; Li, Y.L.; Gao, W.W.; Lin, J.M.; Zhou, C.H. An unanticipated discovery of novel naphthalimidopropanediols as potential broad-spectrum antibacterial members. Eur. J. Med. Chem. 2022, 241, 114657. [Google Scholar] [CrossRef]
- Zhao, W.H.; Xu, J.H.; Tangadanchu, V.K.R.; Zhou, C.H. Thiazolyl hydrazineylidenyl indolones as unique potential multitargeting broad-spectrum antimicrobial agents. Eur. J. Med. Chem. 2023, 256, 115452. [Google Scholar] [CrossRef]
- Yahia, E.; Mohammad, H.; Abdelghany, T.M.; Fayed, E.; Seleem, M.N.; Mayhoub, A.S. Phenylthiazole antibiotics: A metabolism-guided approach to overcome short duration of action. Eur. J. Med. Chem. 2017, 126, 604–613. [Google Scholar] [CrossRef]
- Yang, X.C.; Zhang, P.L.; Kumar, K.V.; Li, S.; Geng, R.X.; Zhou, C.H. Discovery of unique thiazolidinone-conjugated coumarins as novel broad spectrum antibacterial agents. Eur. J. Med. Chem. 2022, 232, 114192. [Google Scholar] [CrossRef]
- Farag, N.A.H.; El-Tayeb, W. Design, synthesis and docking studies of new furobenzopyranones and pyranobenzopyranones as photoreagent towards DNA and as antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 317–325. [Google Scholar] [CrossRef]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New efforts toward aminothiazolylquinolones with multitargeting antibacterial potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef]
- Chen, J.P.; Battini, N.; Ansari, M.F.; Zhou, C.H. Membrane active 7-thiazoxime quinolones as novel DNA binding agents to decrease the genes expression and exert potent anti-methicillin-resistant Staphylococcus aureus activity. Eur. J. Med. Chem. 2021, 217, 113340. [Google Scholar] [CrossRef]
- Cui, S.F.; Addla, D.; Zhou, C.H. Novel 3-aminothiazolquinolones: Design, synthesis, bioactive evaluation, SARs, and preliminary antibacterial mechanism. J. Med. Chem. 2016, 59, 4488–4510. [Google Scholar] [CrossRef]
- Bouley, R.; Kumarasiri, M.; Peng, Z.; Otero, L.H.; Song, W.; Suckow, M.A.; Schroeder, V.A.; Wolter, W.R.; Lastochkin, E.; Antunes, N.T.; et al. Discovery of antibiotic (E)-3-(3-carboxyphenyl)-2-(4-cyanostyryl)quinazolin-4(3H)-one. J. Am. Chem. Soc. 2015, 137, 1738–1741. [Google Scholar] [CrossRef]
- Zhang, P.L.; Lavanya, G.; Zhang, S.L.; Cai, G.X.; Zhou, C.H. An unanticipated discovery towards novel naphthalimide corbelled aminothiazoximes as potential anti-MRSA agents and allosteric modulators for PBP2a. Eur. J. Med. Chem. 2022, 229, 114050. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhang, P.L.; Gopala, L.; Lv, J.S.; Lin, J.M.; Zhou, C.H. A unique hybridization route to access hydrazylnaphthalimidols as novel structural scaffolds of multitargeting broad-spectrum antifungal candidates. J. Med. Chem. 2024, 67, 8932–8961. [Google Scholar] [CrossRef]
- Nathubhai, A.; Wood, P.J.; Lloyd, M.D.; Thompson, A.S.; Threadgill, M.D. Design and discovery of 2-arylquinazolin-4-ones as potent and selective inhibitors of Tankyrases. ACS Med. Chem. Lett. 2013, 4, 1173–1177. [Google Scholar] [CrossRef]




| Compds. | Gram-Positive Bacteria a | Gram-Negative Bacteria b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSA | S. a. | S. a. 25923 | S. a. 29213 | E. f. | K. p. | E. c. | E. c. 25922 | P. a. | P. a. 27853 | A. b. | |
| 4a | 4 | 16 | 8 | 32 | 64 | 64 | 2 | 16 | 32 | 16 | 64 |
| 4b | 8 | 16 | 16 | 16 | 4 | 128 | 2 | 2 | 4 | 128 | 64 |
| 4c | 8 | 32 | 32 | 32 | 16 | 128 | 4 | 4 | 8 | 32 | 128 |
| 5a | 16 | 4 | 1 | 32 | 64 | 8 | 8 | 2 | 1 | 64 | 16 |
| 5b | 16 | 32 | 4 | 16 | 8 | 32 | 32 | 4 | 8 | 8 | 8 |
| 6a | 8 | 64 | 32 | 16 | 32 | 32 | 4 | 16 | 8 | 16 | 16 |
| 6b | 16 | 64 | 8 | 16 | 128 | 128 | 1 | 16 | 1 | 32 | 16 |
| 6c | 32 | 8 | 1 | 16 | 128 | 64 | 4 | 16 | 4 | 32 | 8 |
| 7 | 64 | 64 | 32 | 16 | 128 | 128 | 128 | 8 | 16 | 128 | 32 |
| 8a | 16 | 32 | 32 | 8 | 64 | 32 | 2 | 4 | 8 | 128 | 32 |
| 8b | 8 | 8 | 4 | 8 | 2 | 8 | 4 | 4 | 2 | 4 | 128 |
| 9a | 8 | 4 | 32 | 8 | 4 | 128 | 16 | 8 | 16 | 256 | 64 |
| 9b | 2 | 16 | 32 | 16 | 2 | 256 | 8 | 16 | 16 | 256 | 64 |
| 9c | 4 | 4 | 32 | 1 | 4 | 256 | 1 | 4 | 1 | 64 | 64 |
| 10 | 256 | 128 | 2 | 16 | 16 | 8 | 8 | 16 | 2 | 16 | 16 |
| 11a | 32 | 16 | 4 | 2 | 64 | 32 | 16 | 32 | 1 | 128 | 32 |
| 11b | 8 | 64 | 1 | 32 | 64 | 16 | 32 | 16 | 32 | 16 | 32 |
| 12a | 8 | 32 | 16 | 128 | 2 | 32 | 32 | 32 | 4 | 16 | 16 |
| 12b | 16 | 16 | 64 | 16 | 128 | 8 | 64 | 4 | 64 | 32 | 16 |
| 12c | 4 | 32 | 32 | 32 | 64 | 16 | 128 | 16 | 16 | 64 | 32 |
| 13a | 2 | 8 | 64 | 64 | 128 | 32 | 64 | 32 | 16 | 64 | 128 |
| 13b | 1 | 4 | 32 | 16 | 8 | 8 | 0.5 | 32 | 2 | 16 | 8 |
| 13c | 8 | 8 | 16 | 16 | 8 | 4 | 16 | 1 | 16 | 16 | 64 |
| 13d | 64 | 8 | 64 | 2 | 4 | 16 | 32 | 128 | 32 | 8 | 128 |
| 13e | 32 | 16 | 64 | 32 | 64 | 64 | 8 | 8 | 64 | 32 | 128 |
| 13f | 64 | 4 | 128 | 64 | 32 | 32 | 32 | 32 | 4 | 32 | 64 |
| 13g | 32 | 8 | 8 | 16 | 8 | 32 | 4 | 32 | 2 | 16 | 8 |
| 13h | 64 | 128 | 64 | 16 | 4 | 32 | 4 | 16 | 8 | 64 | 32 |
| 13i | 128 | 16 | 64 | 128 | 8 | 64 | 8 | 128 | 16 | 32 | 64 |
| 19a | 0.5 | 1 | 1 | 2 | 2 | 2 | 0.5 | 8 | 2 | 4 | 2 |
| 19b | 4 | 4 | 8 | 16 | 16 | 4 | 16 | 16 | 4 | 8 | 4 |
| 19c | 8 | 8 | 4 | 16 | 4 | 16 | 8 | 16 | 4 | 16 | 2 |
| 19d | 8 | 4 | 4 | 8 | 4 | 2 | 4 | 16 | 0.5 | 4 | 4 |
| 19e | 16 | 8 | 8 | 4 | 8 | 8 | 8 | 8 | 4 | 16 | 8 |
| Norfloxacin | 4 | 8 | 4 | 2 | 2 | 4 | 4 | 2 | 4 | 8 | 2 |
| Compounds | PBP2a Contents in Treated MRSA (ng/mL) |
|---|---|
| Control | 17.19 ± 0.76 |
| Compound 19a | 8.46 ± 0.18 |
| Cefdinir | 10.48 ± 0.12 |
| Compound 19a + Cefdinir | 3.79 ± 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Li, Q.; Li, Z.; Zang, Z.; Luo, Y.; Zhou, C. Discovery of Quinazolone Pyridiniums as Potential Broad-Spectrum Antibacterial Agents. Molecules 2025, 30, 243. https://doi.org/10.3390/molecules30020243
Dai J, Li Q, Li Z, Zang Z, Luo Y, Zhou C. Discovery of Quinazolone Pyridiniums as Potential Broad-Spectrum Antibacterial Agents. Molecules. 2025; 30(2):243. https://doi.org/10.3390/molecules30020243
Chicago/Turabian StyleDai, Jie, Qianyue Li, Ziyi Li, Zhonglin Zang, Yan Luo, and Chenghe Zhou. 2025. "Discovery of Quinazolone Pyridiniums as Potential Broad-Spectrum Antibacterial Agents" Molecules 30, no. 2: 243. https://doi.org/10.3390/molecules30020243
APA StyleDai, J., Li, Q., Li, Z., Zang, Z., Luo, Y., & Zhou, C. (2025). Discovery of Quinazolone Pyridiniums as Potential Broad-Spectrum Antibacterial Agents. Molecules, 30(2), 243. https://doi.org/10.3390/molecules30020243





