Abstract
Gastrodia elata Blume is a well-known medicinal and edible plant in China, celebrated for its extensive history in traditional medicine and functional food applications. Among its key bioactive components, polysaccharides have drawn significant attention from researchers in the fields of health food and medicine due to their potential health benefits. Recent studies have revealed various biological activities associated with G. elata polysaccharides, including antioxidant, anti-tumor, anti-inflammatory, antibacterial, anti-aging, immune regulation, and neuroprotective properties. However, a comprehensive overview of these polysaccharides remains elusive. Specifically, relationship between the structure and activity of G. elata polysaccharides, along with the mechanisms through which various types exert their biological effects, has yet to be fully elucidated. This knowledge gap may impede the further development and utilization of G. elata polysaccharides in medicine, health products, food, and cosmetics. This paper provides a comprehensive overview of recent advancements in extraction, separation, purification, biological activities, and applications of G. elata polysaccharides. Additionally, it delves into structure-activity relationships and pharmacological mechanisms of these polysaccharides, giving support for future research to enhance their application in medicine, food, health products, and cosmetics.
1. Introduction
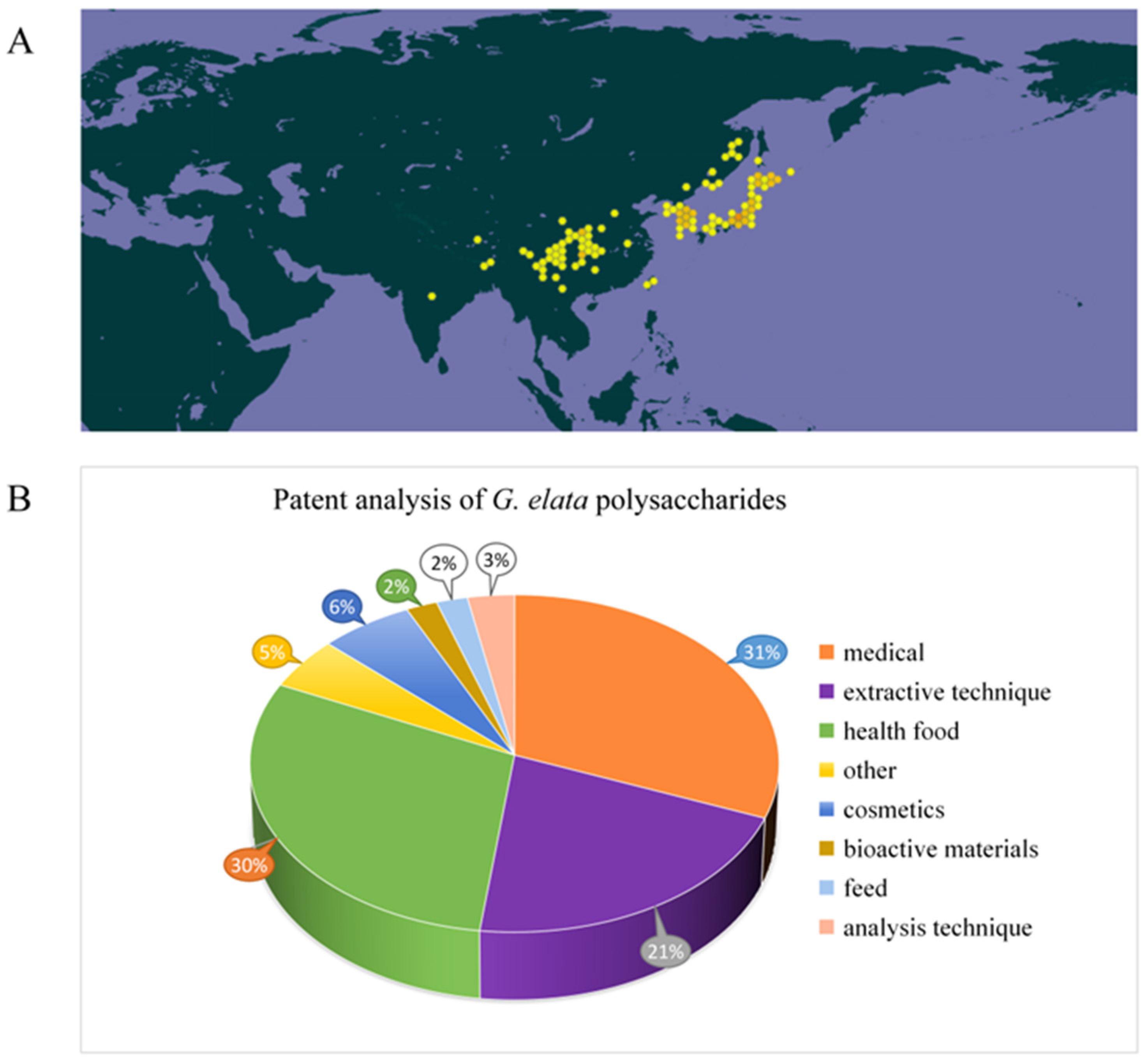
Gastrodiae Rhizoma, derived from the dried tuber of Gastrodia elata Bl., is a traditional Chinese medicine material known for its calming, antispasmodic, and liver-regulating properties [1]. In clinical practice, it is mainly applied for treating diseases like headache, dizziness, memory loss, hypertension, and epilepsy [2,3,4,5,6]. Gastrodiae Rhizoma has a history of over 2000 years of application in China, first documented in the Shennong herbal Scripture [7,8]. Widely distributed in Yunnan, Guizhou, Hubei, Sichuan, Shanxi, and other regions of China (Figure 1A), and traditionally used for both medicine and dietary purposes in these regions for centuries [1,6]. Due to its therapeutic effect and non-toxicity, it is divided in the catalog of homologous drugs and foods, referring from the National Health Commission of the People’s Republic of China and the 2020 edition of the Chinese Pharmacopoeia. According to the characteristics, such as arrow stem color, of G. elata, it can be divided into five types: G. elata f. glauca, G. elata f. elata, G. elata f.alba, G. elata f. elata, and G. elata f. viridis [9]. In these types, G. elata f. glauca and G. elata f. elata are widely used for artificial cultivation. At present, many drugs containing G. elata as the main raw material have been developed. Among them, more than 500 kinds of drugs, such as gastrodin injection, Tianma headache tablets, Tianma capsules, and Tianma tablets, received approval from the State Food and Drug Administration of China for listing [10]. In addition, G. elata is also an important raw material for food and health products. There are about 120 kinds of health foods made from G. elata and its extracts, which have the effects of improving sleep, assisting in lowering blood pressure, and regulating immunity [11]. With people’s pursuit of health, G. elata products are increasingly favored by consumers. Due to its unique growth mode, wild G. elata resources are becoming increasingly scarce. In China, G. elata has emerged as a significant economic crop in Yunnan, Guizhou, Sichuan, and Hubei provinces to fulfill consumer demand [12].
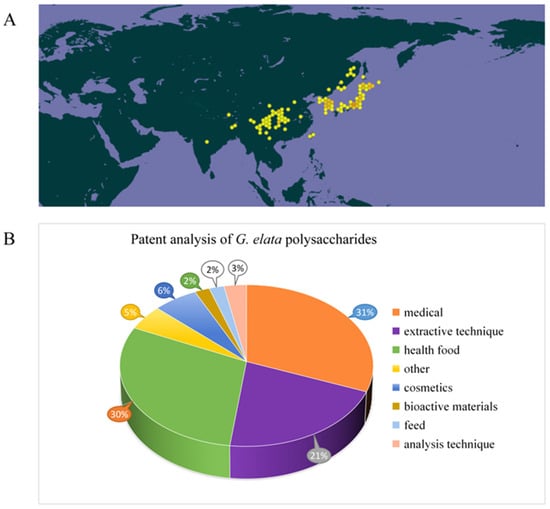
Figure 1.
The distribution of G. elata in the world (https://www.gbif.org/) (accessed on 15 October 2024.) (A) and analysis of related patents on G. elata polysaccharides (B).
G. elata’s biological activity shows close correlation to its phytochemicals. Recent research has noted that chemical compounds isolated and identified from G. elata primarily consist of phenols [13], organic acids, polysaccharides [14], and steroids. These components confer G. elata with various pharmacological properties, including neuroprotection, cardiac protection, vascular regulation, antidepressant effects, anti-cancer activity, sleep enhancement, anticonvulsant properties, anti-inflammatory effects, and analgesic properties [15,16,17,18]. Among them, polysaccharides, as primary active ingredients within G. elata, possess various pharmacological activities, including anti-cancer [19], anti-virus, anti-oxidation, immune regulation, neuroprotection, and cardiovascular system regulation [20,21]. The chemical structure of G. elata polysaccharide is distinguished by a diverse monosaccharide composition. Its diverse physical and chemical properties, along with the associated health benefits, have garnered significant attention from researchers. Currently, more than 50 types of G. elata polysaccharides have been identified, including GaE-B, GaE-R, GEP-3, GEP-4, WGEW, and AGEW [14,22,23,24]. Predominantly, G. elata polysaccharide is classified as a glucan, primarily comprising an α-d-1,4-glycosidic bond as its main chain, potentially supplemented by α-1,3-glycosidic bonds and α-1,4,6-glycosidic bonds. Despite numerous studies focusing on the biological activity and structural characteristics of G. elata polysaccharide, its full potential remains underexplored and warrants further investigation for practical applications [25,26,27]. In addition, studies have found that polysaccharide structure shows close relation to biological activities [28]. However, only a little research has concentrated on the relationship between structural properties and biological activities of G. elata polysaccharides. In-depth research on G. elata polysaccharide is helpful for understanding various biological activities, as well as searching the relation between its function and structure. The patent analysis related to G. elata polysaccharides is shown in Figure 1B, mainly focusing on pharmaceuticals, health products, extraction technology, and other aspects (Figure 1B).
G. elata polysaccharide, as a natural bioactive component, holds significant market potential and development opportunities. However, there is still a lack of systematic and comprehensive summary of these polysaccharides, which may limit the development and utilization of G. elata polysaccharides in medicine, health care products, food, and cosmetics. This review seeks to provide a comprehensive summary of the existing literature on G. elata polysaccharides, covering extracting and purifying techniques, evaluating their respective strengths and limitations, and examining their molecular weight, monosaccharide composition, structural features, structure–activity relationships, and biological activities. Special emphasis is placed on exploring the mechanisms underlying the antitumor, immune regulation, antioxidant, and neuroprotective properties of G. elata polysaccharides; the review delves into potential challenges and future application prospects in G. elata polysaccharide research, offering valuable insights for its development and clinical use (this review collected the literature related to Gastrodia elata polysaccharide in the past 15 years).
2. Extracting and Purifying Techniques of G. elata Polysaccharides
2.1. Extraction and Separation of G. elata Polysaccharides
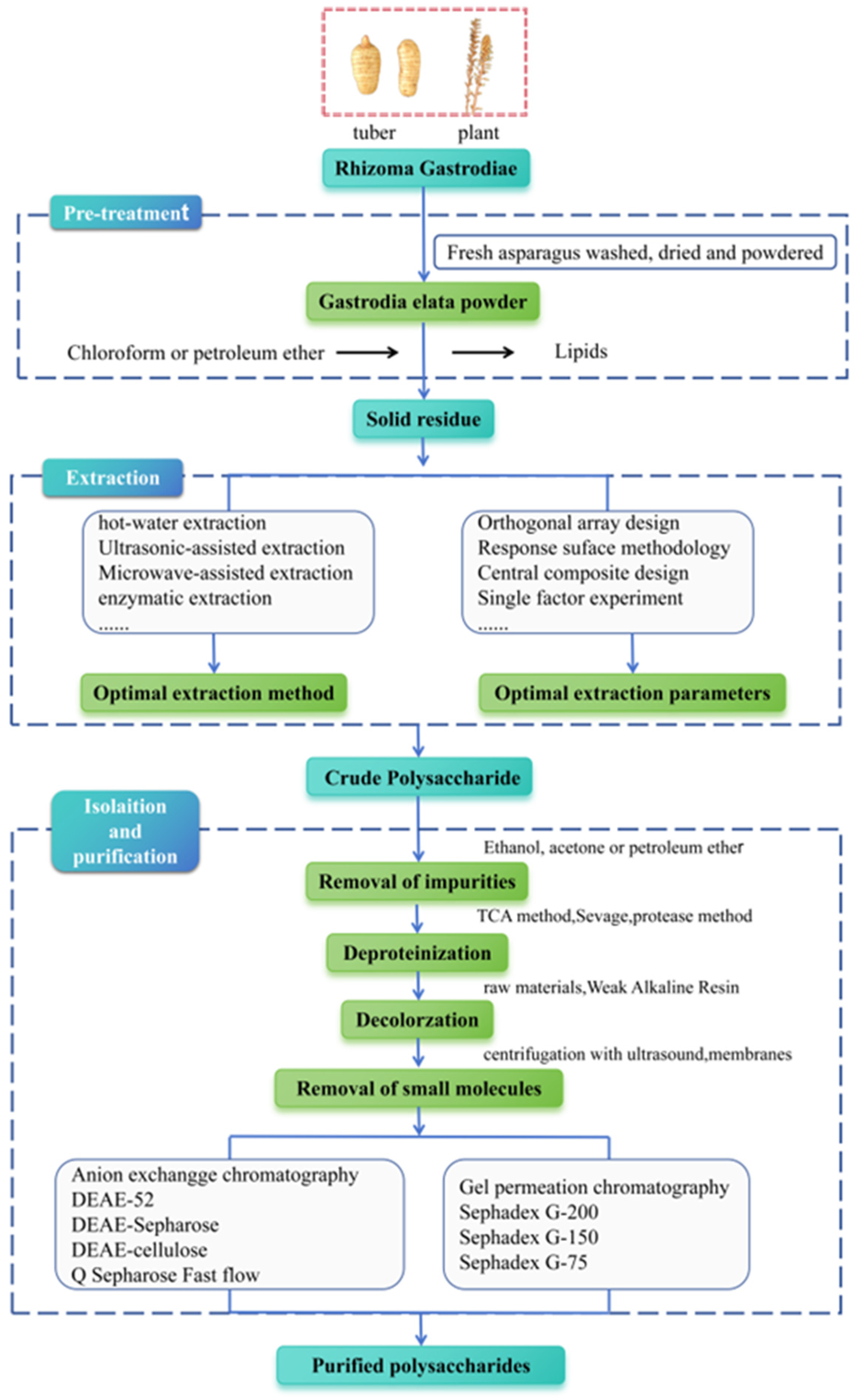
Extracting plant polysaccharides is crucial for subsequent purifying processes, structural analysis, and investigation on pharmacological activities. Therefore, present optimizing extraction techniques for G. elata polysaccharides have become a primary research focus, with methods such as extracting by hot water, ultrasonic, microwave, and enzyme being commonly used [20,29]. The extraction and purification process is shown in Figure 2. Initially, organic solvents like chloroform and petroleum ether are utilized for degreasing to eliminate fat-soluble components, followed by specific extraction methods to extract polysaccharides from G. elata. Among them, the water extraction and alcohol precipitating method is the most extensively applied traditional extraction method for G. elata polysaccharide research [14,24,30,31]. The polarity of polysaccharides is high, and most types of polysaccharides have a large and stable solubility in hot water, while their solubility in organic solvents is small; therefore, hot water is generally applied as an extraction solvent. Moreover, the penetration of hot water into plant tissue is relatively strong, which can improve the extraction efficiency, making it more economical and safer to be used in production. However, this method is cumbersome to operate, and extraction by hot water at high temperature brings polysaccharide degradation, resulting in a relatively low extraction rate.
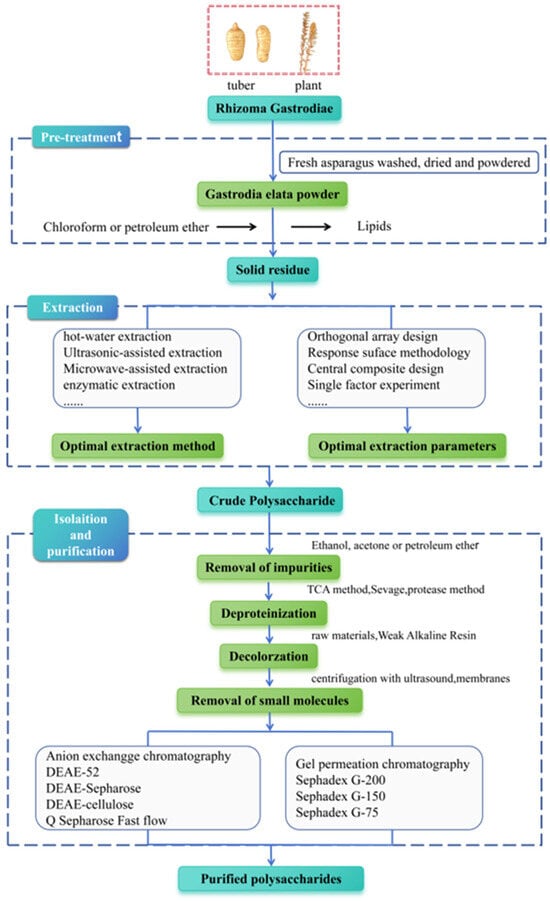
Figure 2.
Schematic representation of extraction, isolation, and purification of G. elata polysaccharides.
Innovative extraction methods, such as enzymatic hydrolysis, offer milder conditions for polysaccharide extraction. Enzymes can effectively break cell walls down, enhance cell permeability, and facilitate the dissolution of intracellular contents. This straightforward process ensures a high extraction rate [32,33]. Moreover, during the extraction of traditional Chinese medicine, impurities such as starch, protein, and pectin can compromise the quality of the extract. Appropriate enzymes (cellulase, pectinase, protease, etc.) can remove these impurities through gentle enzymatic hydrolysis reactions, thus improving the clarity of the extract. Ultrasonic-assisted extraction involves the usage of ultrasonic energy to mechanically destroy the cell wall structure of G. elata, leveraging thermal effects to enhance cell component dissolution. This method provides several advantages over others, including reduced extraction time, greater efficiency of extraction, and optimal preservation of polysaccharide activity, making it a favorable approach for polysaccharide extraction [20,34,35] Microwave-assisted extraction involves the usage of microwave energy to induce friction in plant polar molecules, leading to cell wall rupture and release of contents [36,37]. Wang et al. [38] conducted a study on G. elata using microwave-assisted extraction at 500 W for 120 s, with a material-to-liquid ratio of 1:40, a temperature of 70℃, and a 30-min extraction time, resulting in a 10.40% extraction rate of G. elata polysaccharide. Li et al. [39] observed G. elata polysaccharide’s extraction rate was 6.86% under 500 W, with a solid–liquid ratio of 1:40, and microwave extraction for 120 s at 70 °C. G. elata polysaccharide’s extraction rate obtained by microwave-assisted extraction is low. Notably, a novel methodology utilizing ionic liquid ultrasound-assisted extraction for G. elata polysaccharides has been introduced. Ionic liquids offer several advantages over traditional organic solvents, including low toxicity, volatility, and flammability; effective performance with both non-polar and polar components; recyclability; superior solubility; environmental friendliness; and excellent chemical stability.
2.2. Purification of G. elata Polysaccharides
Separation and purification of polysaccharides are necessary processes to study polysaccharides. The process typically involves separation, purification, and identification of purity. This task is particularly challenging in carbohydrate research due to the presence of impurities, such as pigments, proteins, and oligosaccharides, within crude polysaccharides [40,41]. Because of these impurities, the biological activity of polysaccharides is often affected, which also poses great difficulties for qualitative and quantitative analyses and structural determination of polysaccharides. Moreover, excessive impurities hinder the assessment of the activity–structure relation of polysaccharides. Thus, isolating and purifying crude polysaccharides is essential for obtaining homogeneous samples and accurately determining their structural characteristics. Currently, the main methods used for polysaccharide purification are deproteinization, decolorization, fractionation precipitation, cellulose column chromatography, salting out, quaternary ammonium salt precipitation, ion exchange column chromatography, ultrafiltration, preparative high-performance liquid chromatography, cellulose acetate membrane filtration, gel column chromatography, affinity chromatography, etc. [40].
Following extraction of G. elata polysaccharides, the resultant mixture contains a variety of impurities, including polar impurities such as fatty acids. These weakly polar impurities take advantage of the characteristics of polysaccharides to be insoluble in organic reagents. By using ethanol (≥80%), acetone, or petroleum ether for repeated precipitation and washing, these weakly polar impurities can be eliminated [42]. Furthermore, crude polysaccharides typically contain impurities like proteins, pigments, and small molecules, which can negatively affect both their purity and biological activity. Therefore, it is imperative to extract crude polysaccharides from G. elata for subsequent separation and purification, as outlined in Table 1. Currently, proteins in crude polysaccharide of G. elata can be effectively removed using trichloroacetic acid, Sevag [14,30,43,44,45] (n-butanol: chloroform is 1:5 or 1:4), or protease [46] methods. Among them, the trichloroacetic acid method has a strong effect but can lead to polysaccharide degradation. The Sevag method is milder and better preserves polysaccharide integrity, although its protein removal efficiency is lower, typically requiring multiple repetitions to achieve satisfactory results. The protease method also shows good efficacy, but it may disrupt the peptide chains on glycoprotein and affect the biological activity of polysaccharide. To enhance the purification of crude polysaccharides from G. elata, an effective approach involves combining an improved protease method (papain or trypsin) degradation with the Sevag method for protein removal. This strategy minimizes the need for multiple organic solvent washes, thereby reducing the possibility of polysaccharide loss during gel precipitation. Activated carbon is typically utilized to adsorb pigment substances in crude G. elata; however, it may also inadvertently adsorb some polysaccharides, leading to their loss. The polysaccharides of G. elata may contain phenolic components, which may darken the color. This type of pigment is not captured by activated carbon but can be effectively adsorbed by weak alkaline resin. Small molecules, such as inorganic ions and oligosaccharides, can be eliminated through processes such as dialysis, ultrasonic centrifugation, or ultrafiltration with specialized membranes. Furthermore, homogeneous polysaccharides extracted from G. elata were primarily obtained through gel and cellulose anion exchange column chromatography. Generally, these methods exhibit distinct separation characteristics, with chromatography of ion-exchange being suitable for neutral or acidic polysaccharide separation [47]. Gel filtration chromatography using Sephadex G-200, Sephadex G-100, and Sephadex G-50 is extensively applied as chromatographic media to separate polysaccharides with different molecular weights.

Table 1.
Extraction and purification methods of polysaccharides from G. elata.
3. Structural Characteristics of G. elata Polysaccharide
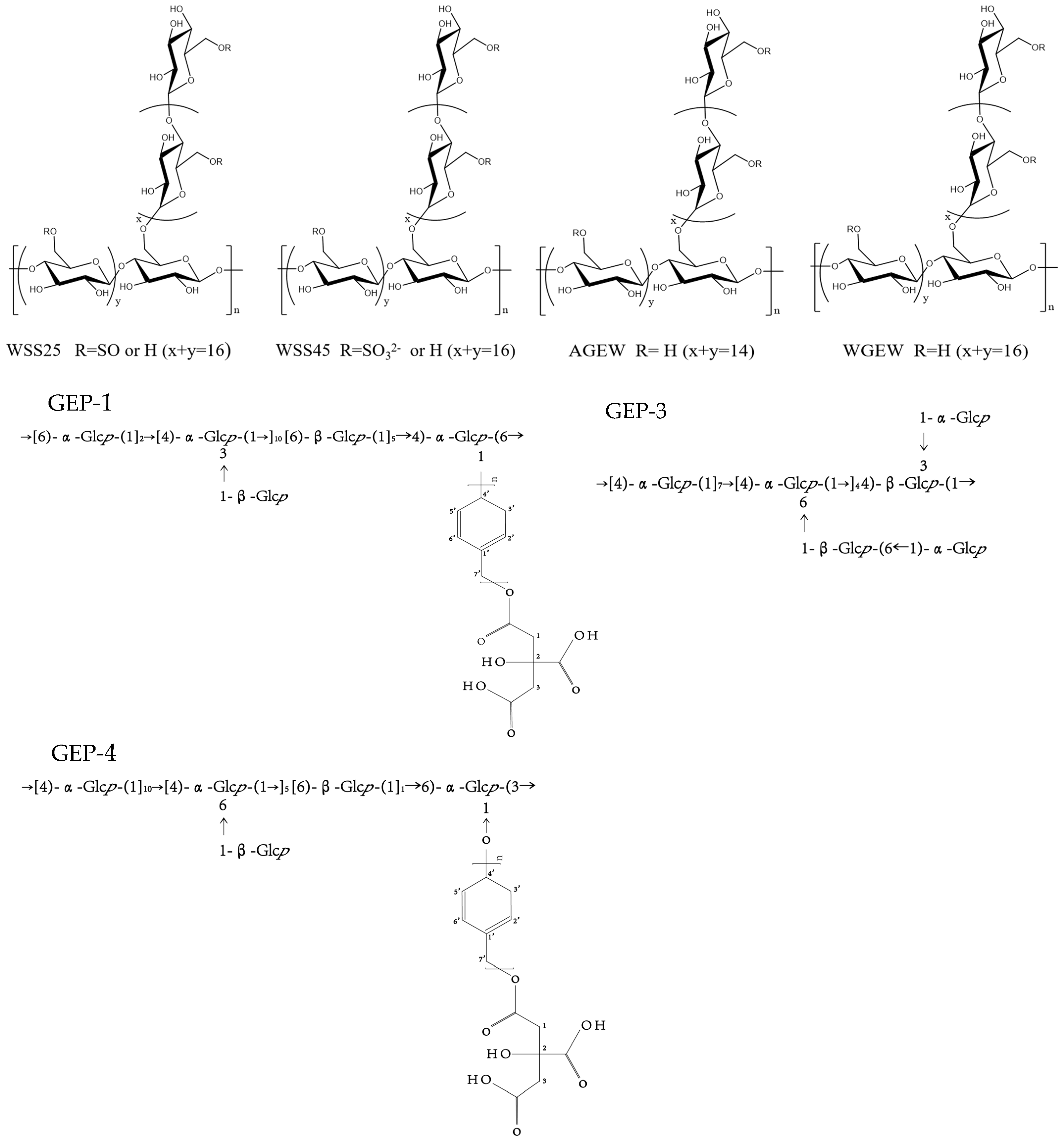
The analysis of the structural characteristics of polysaccharides is crucial; polysaccharide structure diversity directly determines its biological activity [94]. Structural characteristics of polysaccharides were analyzed, including glycosidic bond type, connection mode, high-order structural conformation, relative molecular mass, and monosaccharide composition, which are key factors in determining the structure and function of polysaccharides [95]. The monosaccharide composition of G. elata polysaccharides is diverse, featuring over 50 identified types, including GaE-B, GaE-R, GEP-3, GEP-4, WGEW, and AGEW (Table 2, Figure 3). The polysaccharides derived from G. elata Blume are primarily composed of glucose, with their sugar chains predominantly structured as α-(1,4) pyran-d-glucan [96]. The homogeneous polysaccharide GES extracted from G. elata by Li et al. [49] was found to have a molecular weight of 292.596 kDa and a monosaccharide composition ratio of Glu:Gal:GalA:Arabinose:Fru = 88.21: 4.48: 4.40: 0.87: 0.85 Ning et al. [30] extracted four varieties of GaE polysaccharides: GaE-B (G. elata Bl. f. glauca S. chow polysaccharide), GaE-R (G. elata Bl. f. elata polysaccharide), GaE-G (G. elata Bl. f. viridis Makino polysaccharide), and GaE-Hyb (hybridization of G. elata Bl. f. glauca S. chow and G. elata Bl. f. elata polysaccharides). These polysaccharides share the same monosaccharide composition, primarily consisting of glucose, xylose, and mannose, although the content varies significantly among the different varieties. Huo et al. [14] discovered that GEP-3 is a 1,4-glucan with a molecular weight of 20 kDa. They also identified the homogeneous polysaccharide GEP-4, with a molecular weight of 25 kDa, which features a complex main chain structure. This marks the first time this new polysaccharide has been obtained and characterized. Chen et al. [45] successfully isolated a water-soluble polysaccharide (GEP2-6) of 2.7 × 106 Da from G. elata, achieving a purity of 99.2%. Spectral and chemical analyses indicated that GEP2-6 is a glucan composed of α-(2→6) and α-(1→4) glycosidic bonds. Enzymatic hydrolysis further characterized GEP2-6’s local structure, showing the presence of α-1,6-Glcp, β-1,4,6-Glcp, α-1-Glcp, and α-1,4-Glcp in a molar ratio of 1.32:1.08:0.93:31.27. Zhu et al. [52] separated a homogeneous polysaccharide, PGE, from G. elata by water extraction and Sephadex G-200 separation and purification. Structural analysis revealed that the G. elata polysaccharide was primarily composed of glucose, rhamnose, and mannose in small amounts, with an average relative molecular mass (Mr) of 1.54 × 103 kDa. The main chain is composed of α-(1,4)-d-glucopyranose with 1,3- and 1,4,6-branched glucopyranose. The G. elata polysaccharide PGEB-3H, as obtained by Ming et al. [97], was primarily composed of glucose with a mean molecular weight of 28.8 kDa and a specific optical rotation of +206.3°. It featured a main chain of α-(1,4)-d-glucopyranose and included 1,4- and 1,4,6-connected branches. Chen et al. isolated the G. elata polysaccharide WTMA through processes comprising degreasing, extraction by water, precipitation by alcohol, deproteinization, dialysis, and DEAE column separation. Structural analysis revealed that WTMA consists solely of glucose, with a mean molecular weight of 7.0 × 102 kDa, a specific optical rotation of +382°, and α-(1,4)-glucan as the main chain. Zhu et al. [20] summarized that G. elata polysaccharide mainly consisted of glucose and mostly contained α-1,4-glucan, α-1,4,6-glucan, and α-1,3-glucan. Chen et al. [55] extracted G. elata polysaccharides through water extraction, alcohol precipitation, protein removal, freeze-drying, and additional steps. Structural analysis revealed that the polysaccharide consisted of glucose, with a mean molecular weight of 8.75 × 103 kDa and a polysaccharide content of 91.3%. The G. elata polysaccharide isolated by Liu et al. [98] primarily consisted of glucose, as well as a small amount of mannose, xylose, and arabinose. Qiu et al. [23] isolated two homogeneous α-d-glucans, known as water-extracted polysaccharides WGEW and alkali-extracted polysaccharide AGEW. The average molecular weight of WGEW was 100 kDa, with a specific rotation of +92°. AGEW had an average molecular weight of 280 kDa and a specific rotation of +166.6°. Both WGEW and AGEW were composed of glucose monosaccharides, with the main chain structure as α-d-(1,4)-glucan. They also contained terminal glucose (T-Gle) and glucose linked in 1,4 and 1,4,6 configurations, present in varying molar ratios. Hong et al. [93] utilized a combination of chemical methods, such as sugar composition and methylation analyses, alongside spectroscopic techniques like 13C-NMR and IR, to comprehensively identify the structures of seven homogeneous polysaccharides: GBI-1, GBI-2, GBI-3, GBII, GBIII, GBIV, and GBV. The findings revealed that GBI-1’s main chain comprised α-d-1,4-linked glucose with occasional branches at the 6-position and had a molecular weight of 14600. In contrast, GBI-2 and GBI-3 had molecular weights of 8700 kDa and 7000 kDa, respectively. The authors suggested that, despite the differing molecular weights, GBI-1, GBI-2, and GBI-3 exhibit identical structures and may represent distinct degradation fragments of the same homogeneous polysaccharide. GBII was identified as a glucan comprising 27 glucose units with a molecular weight of 4300, featuring a main chain of α-d-1,4-linked glucoses with branches at the 6-position and some glucuronic acid. GBIII was characterized as a 19,000 molecular weight pectin-type polysaccharide, while GBIV was with a molecular weight of 87,000. Lastly, GBV was identified as a sulfated polysaccharide with a molecular weight of 100,000 and a sulfate content of 25%. The sugars in GBV consisted of galactose and glucose at a molar ratio of 43:57.

Table 2.
Name, molecular weights, monosaccharide composition, and structures of polysaccharides from G. elata.
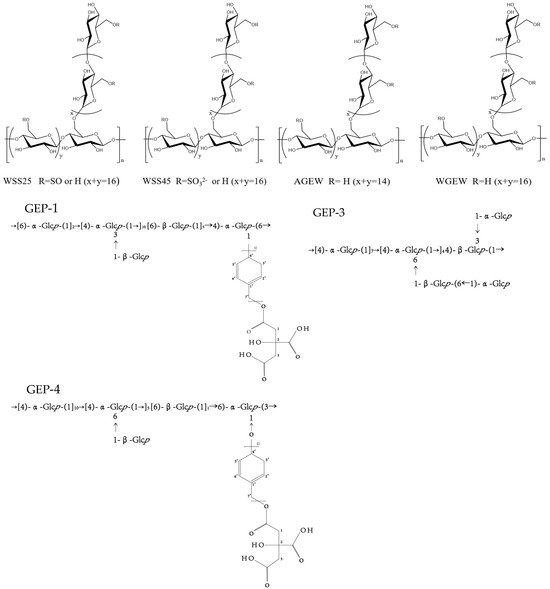
Figure 3.
Polysaccharide structure of WSS25, WSS45, AGEW, WGEW, CEP-4, GEP-3, and CEP-1.
4. Biological Activity of G. elata Polysaccharide
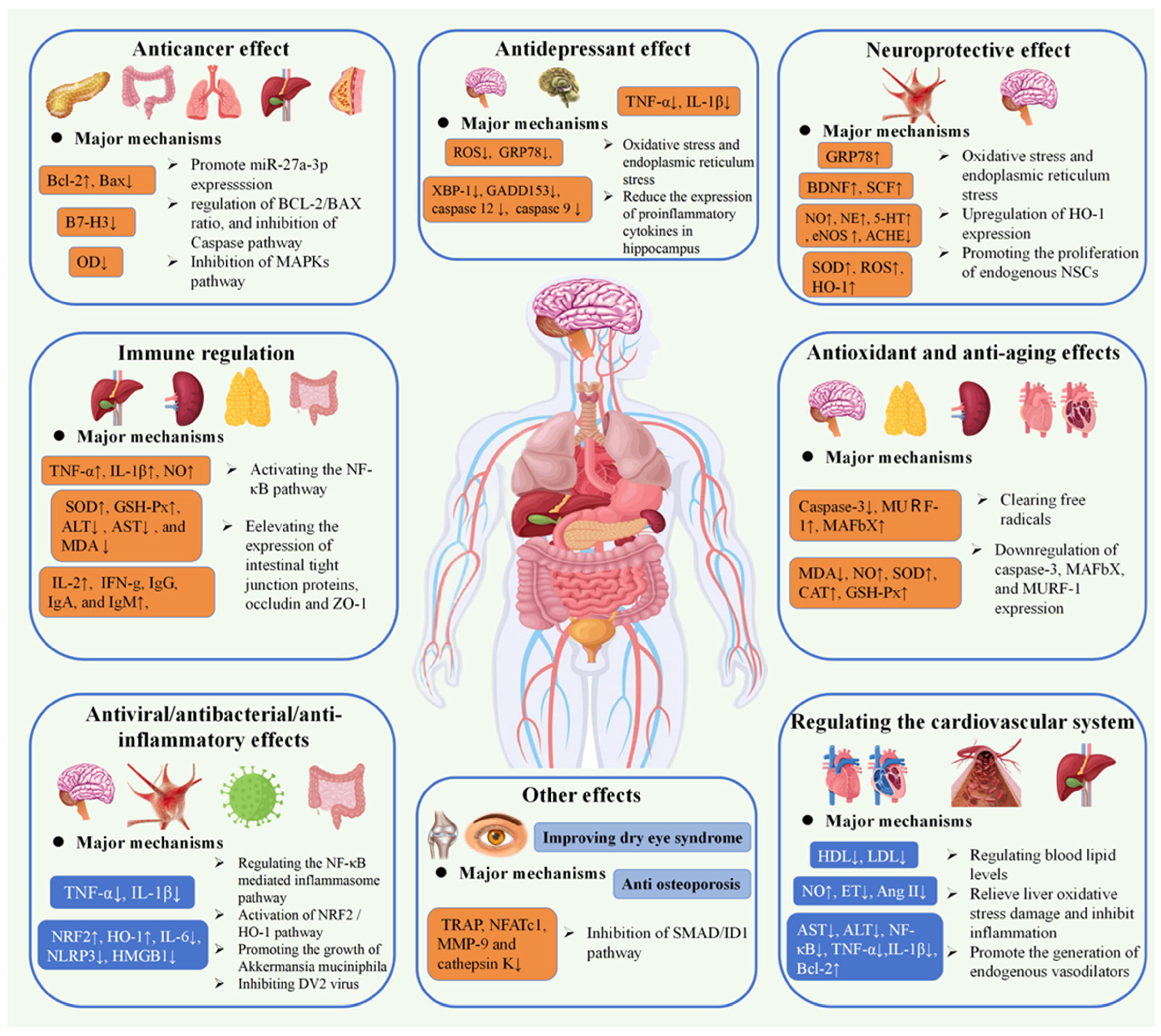
G. elata is a promising edible and medicinal plant with a variety of bioactive components, which has great value for application in the field of medicine and food. G. elata exhibits a variety of biological functions of polysaccharides, comprising anti-oxidation, treatment of cardiovascular system diseases, anti-tumor effects, neuroprotection, immune regulation, anti-inflammatory properties, and so on. Both mushroom polysaccharides and G. elata polysaccharides exhibit immune-enhancing effects. Mushroom polysaccharides are known to stimulate macrophages and enhance cytokine secretion, thereby activating the immune response, while G. elata polysaccharides enhance immune balance by modulating the proportion and function of T cell subsets. The precise regulation of lymphocytes by G. elata polysaccharides is particularly significant in the context of immune disorders [101,102]. Furthermore, both Ganodermalucidum (G.lucidum) and G. elata polysaccharides demonstrate anti-tumor activity. G. lucidum polysaccharides inhibit the signal transduction pathways of tumor cells, whereas G. elata polysaccharides act directly on tumor cells and bolster immune surveillance [103,104]. The potential of G. elata polysaccharides in preventing tumor recurrence and metastasis is noteworthy. Additionally, Lycium barbarum polysaccharides and G. elata polysaccharides exhibit antioxidant properties. Lycium barbarum polysaccharides function by scavenging free radicals to protect cells, while G. elata polysaccharides enhance cellular defense by increasing antioxidant enzyme activity. Particularly noteworthy is the ability of G. elata polysaccharides to provide significant protection to nerve cells against oxidative damage, a crucial aspect in the prevention and treatment of neurodegenerative conditions [105,106]. The similarities and distinctive characteristics in the biological activity of G. elata polysaccharides and other natural polysaccharides provide valuable resources for medicine and health care, contributing to the advancement of human health. Table 3 provides a comprehensive overview of the biological activities associated with polysaccharides from G. elata, and Figure 4 illustrates the pharmacological activities and mechanisms of action associated with G. elata polysaccharides.

Table 3.
Biological activities of G. elata polysaccharides and their underlying mechanisms of actions.
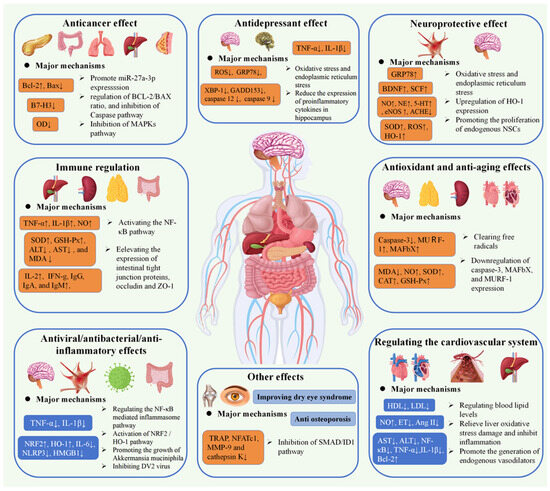
Figure 4.
Schematic representation for the bioactivities of G. elata polysaccharides (↑: It indicates that receptors, hormones, proteins, etc. are up-regulated or increased. ↓: It indicates that receptors, hormones, proteins, etc. are down-regulated or reduction).
4.1. Anti-Cancer Activity
An increasing amount of research indicates that polysaccharides extracted from G. elata exhibit potent anti-cancer properties. Laboratory studies have demonstrated that polysaccharides extracted from G. elata (WTMA) possess the ability to suppress PANC-1 cancer cell proliferation. The products of acid hydrolysis WTMA-AD-O and WTMA-AD-I have significant anti-tumor activity [22]. Specifically, an increase in the molecular size of homogeneous polysaccharides leads to a significant decrease in the growth activity of anti-cancer cells. Furthermore, G. elata polysaccharides (PGEs) have been found to hinder MCF-7 cell growth by inducing late apoptosis and blocking the G2/M phase, with homogeneous polysaccharide PGE-40 exhibiting a notably high suppression rate [107]. PGE-40 has the highest inhibition rate, which may be attributed to its spherical conformation, molar mass distribution, and dense structure. Notably, acetylated and sulfated G. elata polysaccharides (AcGEP and SGEP) have better anti-breast cancer activity [19]. For rat glioma cells (C6), sulfated G. elata polysaccharides (GEPs) could significantly inhibit the growth and migration of glioma cells [108]. This enhancement in activity could potentially be attributed to the structural alterations in GEP that occur during the modification process [90]. In addition, suppressive effects by G. elata polysaccharides on pheochromocytoma were mainly achieved by increasing the ratio of BCL-2/BAX protein and suppressing caspase-related pathways activation [79]. For transplanted tumors, G. elata polysaccharides can induce inflammatory infiltration to impede tumor cell proliferation and enhance the immune response in tumor-bearing mice [109]. G. elata polysaccharide can also suppress the growth and invasion of colon cancer cells under arsenic exposure and promote their apoptosis. The underlying mechanism may be associated with the promotion of miR-27a-3p expression and the inhibition of B7-H3 expression [46].
4.2. Immunomodulatory Activity
Research has shown that G. elata polysaccharides exhibit significant immunomodulatory effects. G. elata polysaccharides (GEP-1) can elevate IL-1β, NO, and TNF-α in macrophages. Experimental evidence supports that its immune regulatory effects are primarily mediated through NF-κB pathway activation [43]. GEP-2, another polysaccharide from G. elata, has been found to decrease TNF-α and IL-1 levels, enhance T and B lymphocytes’ growth, and consequently exhibit immune regulatory properties [115]. Moreover, GEPs have demonstrated the ability to elevate serum levels of IL-2, TNF-α, IFN-γ, IgG, IgA, and IgM, as well as spleen and thymus indices in cyclophosphamide-induced immunosuppressed mice dose-dependently [48,114]. Selenium-enriched G. elata polysaccharides (Se-GEP) have been shown to enhance the body weight and feed intake in immunosuppressed mice, increase spleen and thymus indices, improve phagocytic function, and simultaneously activate Th1 and Th2 lymphocytes, resulting in elevated secretion levels of NO and TNF-α [51]. Compared with the GEP group, high selenium changed the structure of G. elata polysaccharide, thereby enhancing the immunomodulatory activity [51].
4.3. Antioxidant/Anti-Aging Activity
In vitro research demonstrated that polysaccharides extracted from aboveground parts of diverse G. elata varieties possess antioxidant properties. The effectiveness of these polysaccharides in reducing and scavenging DPPH and ABTS free radicals increases with higher concentrations of polysaccharides. Among the above-ground polysaccharides of G. elata f. viridis, the DPPH free radical scavenging rate was found to be the highest at the same concentration of polysaccharide solution [35]. The homogeneous polysaccharides GEP-1 and GEP-2, isolated from G. elata, exhibited a strong DPPH scavenging rate and high inhibition rates for superoxide anion and hydroxyl radical [74]. Zhang et al. [62] and Li et al. [60] utilized the DPPH method and Fenton system to evaluate the scavenging efficiency of DPPH free radicals and hydroxyl radicals in G. elata polysaccharides. The results indicated that G. elata polysaccharides showed significant scavenging effects on both DPPH and hydroxyl radicals, with concentration-dependent scavenging ability. Furthermore, G. elata polysaccharides were found to enhance the learning and memory abilities of d-galactose-induced aging mice and increase SOD activity in the brains of aging mice, elevating GSH-Px activity in blood, reducing MAO activity in brain tissue, and decreasing MDA levels, thereby promoting brain nerve tissue recovery [116]. Selenized G. elata polysaccharides demonstrated higher clearance rates for DPPH and ABTS+ free radicals compared to regular G. elata polysaccharides, with a maximum iron reduction of 0.99% ± 0.24%. [82]. Structural characterization analysis showed that SeGEP had been successfully accomplished, and SeGEP had reduced the particle size, increased the absolute value of Zeta potential, and improved the stability in solution by comparing it with GEP [82].
4.4. Antiviral/Antibacterial/Anti-Inflammatory Activity
4.4.1. Anti-Inflammatory Activity
HO-1 can directly regulate the expression of proinflammatory factors; furthermore, HO-1 can catalyze the decomposition of heme to generate CO, which can inhibit the expression of the classical inflammatory regulator NF-κB, thereby inhibiting the proinflammatory signal. G. elata neutral polysaccharide (NPGE) can downregulate IL-1β, NLRP3, IL-6, HMGB1, and TNF-α and upregulate NRF2 and HO-1 expressions. It also facilitates translocation of NRF2 to the nucleus and suppresses neuroinflammation [118]. Additionally, G. elata polysaccharides (GBP) can mitigate the increase in proinflammatory cytokine levels induced by vincristine (Vin). GBP treatment results in decreased IL-6, IL-8, TNF-α, and IL-1 β mRNA levels within the spinal cord, sciatic nerve, and DRG [99]. Simultaneously, G. elata polysaccharide also alleviates inflammatory injury by regulating the NF-κB-mediated inflammasome pathway and reducing TNF-α and IL-1β, the proinflammatory factors [76].
4.4.2. Antibacterial Activity/Improvement of Intestinal Flora
GEP-3 was 1,4-glucan, GEP-4 comprising a backbone of →[4)-α-Glcp-(1]10→[4)-α-Glcp-(1→]5[6)-β-Glcp-(1]11→6)-α-Glcp-(3→ and two branches of β-Glcp and p-hydroxybenzyl alcohol citrate, with repeating p-hydroxybenzyl alcohol attached to the backbone chain at O-6 position of →4,6)-α-Glcp-(1→ and O-1 position of →3,6)-α-Glcp-(1→. GEP-3 and GEP-4, the homogeneous polysaccharides isolated from G. elata, can notably facilitate Akkermansia muciniphila growth and also promote the myxomycetes in the fecal microbiota of high-fat diets (HFD) [14]. GEP-1 was composed of →[6)-α-Glcp-(1→]2[4)-α-Glcp-(1→]10[6)-β-Glcp-(1]5→4)-α-Glcp-(6→, with three branches of β-Glcp and CA-repeating p-HA attached to the backbone chain at the O-3 position of 1,3,6-linked α-Glcp and the O-1 position of 1,4,6-linked α-Glcp. Bioactivity tests showed that GEP-1 could promote the growth of A. muciniphila and L. paracasei strains [24,44]. Additionally, G. elata polysaccharide (GBP) also restored the imbalance of intestinal microbiota by increasing the levels of Firmicutes, Ligilactobacillus, and Bacteroidetes Alloprevotella, while simultaneously reducing the levels of Proteobacteria-Escherichia coli-Shigella [31].
4.4.3. Antiviral Activity
The structures of two glucans, WGEW and AGEW, isolated from Gastrodia elata Bl. Their structures were deduced as an α-d-(1→4)-glucan. Their sulfate derivatives with distinct degrees of substitution (DS) were prepared. All sulfated derivatives showed strong anti-dengue virus bioactivities. The structure–activity relationships (SAR) between the polysaccharides and their sulfated derivatives were also investigated. Results showed that the higher the DS is, the more potent the impact on the dengue virus infection would be [23,56]. Moreover, WSS45 has been found to effectively inhibit DV2 infection in BHK cells, primarily by disrupting viral adsorption during the early stages of the viral life cycle. WSS45 also enhances the detachment of viruses from cell surfaces in BHK cell lines, effectively inhibiting dengue virus serotype (DV2) by interfering with the interaction between viruses and their target cells [119].
4.5. Neuroprotective Effects
Multiple studies have confirmed the neuroprotective effects of polysaccharides derived from G. elata. For instance, the G. elata polysaccharide GEP can partially reverse the pathological changes induced by corticosterone (Cort) in a depression model using PC12 cells, thereby providing neuronal cell protection. Alongside significant apoptosis in PC12 cells, the expression level of GRP78 also increased, demonstrating a clear dose–response relationship [79,113,130]. Research involving the administration of G. elata polysaccharides (PGB) to rats with focal cerebral ischemia demonstrated that PGB could provide neuroprotective effects by upregulating BDNF and self-consistent field expression [53]. Moreover, G. elata polysaccharides (GRPS) can enhance the memory of young rats with cerebral palsy, with the corresponding mechanism related to increases in contents of NO, NE, and 5-HT in the cerebral cortex and hippocampus, with reduction in ACHE and increase in eNOS expression, thus protecting hippocampal tissue [122]. Using G. elata polysaccharides (GBP) and glutamate (Glu) to co-incubate HT22 hippocampal neurons from mice, researchers have discovered that GBP can reduce Glu-induced damage in HT22 neurons. At the same time, GBP was found to increase the SOD activity and ROS clearance ability of HT22 cells dose-dependently and time-dependently, indicating that the protective effect of GBP on HT22 cells may be linked to its enhanced antioxidant capabilities [123]. The combination of electroacupuncture and G. elata polysaccharides can significantly increase Nestin and BDNF expressions in the CA3 area of ischemic hippocampus from rats with cerebral ischemia, suggesting that it may exert a protective effect on neurons in the ischemic area by promoting endogenous NSCs proliferation [124,125]. After treatment for cerebral palsy, it was found that G. elata polysaccharide could reduce the apoptosis of brain tissue in cerebral palsy rats, playing an effective neuroprotective role [126].
4.6. Treatment of Cardiovascular Diseases
Purified G. elata acidic polysaccharides were mainly composed of xylose, glucose, galacturonic acid, and glucuronic acid. The G. elatas acidic polysaccharide fraction significantly reduced systolic blood pressure in SHR on a high-fat diet. In addition, acidic polysaccharides positively regulated lipid levels in SHR [57]. Meanwhile, G. elata acid polysaccharide also inhibits atherosclerosis by downregulating low-density lipoprotein and total cholesterol levels, thereby regulating the blood lipids of Sprague Dawley rats (SD) [127]. In addition, G. elata polysaccharides inhibited liver cell apoptosis by upregulating anti-apoptotic factor Bcl-2 and inhibiting Bax protein expression. Upregulating the Nrf2/GPx signaling pathway helps alleviate oxidative stress damage, protect, and delay the symptoms of non-alcoholic fatty liver disease (NAFLD) induced by HFD, exhibiting a significant liver protective effect [76]. PGE was composed of glucose, with an average molecular weight of 1.54 × 103 kDa. The structure of PGE was 1→3 and 1→4,6-branched-glucopyranose that had a linear backbone of (1→4)-linked-d-glucopyranose (Glcp). ACE-inhibitory activity results showed that PGE was efficient in inhibiting ACE, and the IC50 value was 0.66 mg/mL [52,128].
4.7. Other Activities
Zhou et al. established a depression model using PC12 cells and observed a protective effect by G. elata polysaccharides (GEP) on cortisol-induced apoptosis in PC12 cells, suggesting their potential as a treatment for depression [113]. Furthermore, G. elata polysaccharides (GEPs) have demonstrated the ability to ameliorate lipopolysaccharide (LPS)-induced depressive behavior in mice, with their potential mechanism associated with reduction in cytokines TNF-α and IL-1β mRNA expression in the hippocampus and improving hippocampal neuronal function [121]. Chen et al. discovered that sulfated polysaccharide (WSS25) suppresses osteoclast formation in RANKL-induced mice by impeding the SMAD/ID1 pathway in the treatment of osteoporosis. This was demonstrated by the establishment of an ovariectomized ICR albino mouse model [112]. A phospholipid complex derived from G. elata polysaccharides significantly enhances tear volume, extends tear film rupture time, and improves the regenerative capacity of corneal epithelial cells, particularly in terms of tear film stability [79].
5. Structure–Activity Relation in G. elata Polysaccharide
In the activity–structure relation of G. elata polysaccharides, the connection between structure and antitumor activity is extensively researched. Chen et al. discovered that the biological activity of three G. elata polysaccharides (WTMA, WTMAE-AD-I, and WTMA-AD-O) is influenced by their molecular size. Specifically, an increase in the molecular size of homogeneous polysaccharides brings a significant drop in the growth activity of anti-cancer cells. Dai et al. [107] observed that G. elata polysaccharides (PGES) can suppress MCF-7 cell growth by facilitating late apoptosis and the blockage in the G2/M phase. Among these, PGE-40 exhibited the highest inhibition rate, likely due to its spherical conformation, molecular weight distribution, and compact structure. However, further investigation is needed to fully understand the relationship between the structural characteristics of PGEs and their antitumor efficacy. Dai et al. [131] found that the inhibitory rate of ultrasound-extracted G. elata polysaccharides (PGE) on MCF-7 cell proliferation was generally higher than that of PGE extracted by hot water. This could be due to the lower molecular weight of the PGE extracted by ultrasound compared to that extracted by hot water. Polysaccharides with appropriate molecular weights tend to exhibit higher anti-tumor activity. The spherical structure of PGE may have a greater impact on inhibiting MCF-7 cell proliferation compared to the loose hyperbranched structure, making it more effective in inhibiting MCF-7 cell growth. Cheng et al. [88] found that high-molecular-weight G. elata polysaccharides were discovered to exhibit a more potent suppressive effect on the in vitro proliferation of tumor cells, specifically on cancer cells HepG2 and Hela.
Apart from that, the relationship between the structure and immunomodulatory activity of G. elata polysaccharides has been extensively researched. GEP-3 was discovered by Huo et al. [14], and GEP-1, discovered by Guan et al. [43], belongs to α-(1→4)-glucan. The key difference lies in the molecular weights, with GEP-3 at 20 kDa and GEP-1 at 76 kDa, indicating a higher degree of polymerization in GEP-1. The molecular weight of polysaccharides is crucial for their activity, and substitution level in branched chains also plays a significant role in immune activity [132]. The substitution level of GEP-1 is less than that of GEP-3, which also indicates that there may be a gap in their immune activity. Chen et al. [56] found that the homogeneous polysaccharide RGP-1b exhibited stronger immune activity compared to RGP-1a, possibly attributed to the varying monosaccharide compositions and structures of the two polysaccharides. Nonetheless, additional investigation is required to fully understand the biological activity and structural characteristics of both RGP-1a and -1b.
Structural modification of G. elata polysaccharides may improve their original activity or generate new activity. For example, Liu et al. [19] found improved anti-breast cancer activity by G. elata polysaccharide following structural modifications, such as sulfation and acetylation, especially for acetylated GEP. This enhancement in activity could potentially be attributed to the structural alterations in GEP that occur during the modification process. Dou [80] sulfated the hydroxyl group of G. elata polysaccharide (SYGEP) and acetylated it (AcYGEP). The findings indicated that the chain conformation of SYGEP was predominantly rigid, irregular, and highly branched, while the chain conformation of AcYGEP was more spherical. The effect of G. elata polysaccharide derivatives on the inhibition of MCF-7 cell proliferation was assessed using the MTT method. The results demonstrated that both modifications enhanced the anti-breast cancer activity of the G. elata polysaccharide, with AcYGEP exhibiting superior activity, possibly due to its specific chain conformation. Ma et al. [51] found that compared with the GEP group, high selenium-modified G. elata polysaccharides (Se-GEP) enhanced the proliferation, phagocytosis, and secretion of NO and interleukin-1β in RAW264.7 cells, thereby enhancing the activity of immune regulation. In addition, Wen et al. [82] found that selenization modification can enhance the antioxidant capacity of G. elata polysaccharides. Zhou et al. [130] found that sulfated G. elata polysaccharide (GEPS) had a certain protective effect on PC12 cell injury induced by corticosterone (CORT), but it was not significantly improved compared to G. elata polysaccharide (GEP), and other biological activities need to be further studied. Qiu et al. [23] identified sulfated derivatives of G. elata polysaccharides with varying degrees of substitution (DS), attributing the substitution position to O-6 based on the (13) C NMR spectrum. These derivatives exhibited significant activity against the dengue virus. Research on the structure–activity relationship revealed that an increased DS is associated with a stronger impact on dengue virus infection.
Our research indicates that the chemical composition and structural characteristics of polysaccharides are critical to their biological activities. Grasping the connection between structure and function is vital for the study of polysaccharides. However, investigations into the structure-function relationship of G. elata polysaccharides remain scarce and lack comprehensive analysis. This limitation largely arises from the intricate chemical composition and structure of polysaccharides, which complicates the assessment of their higher-order structures. To further investigate the connection between the structure and biological activity of G. elata polysaccharides, innovative ideas and methodologies are essential for analyzing their advanced structures in future studies. This approach will not only enhance our understanding of the conformational relationships of these polysaccharides but also offer a more solid theoretical foundation for drug development, disease treatment, and various applications of polysaccharides. Consequently, the exploration of novel techniques for analyzing the higher-order structures of G. elata polysaccharides will be a key priority for future research, paving the way for a deeper understanding of their true conformational relationships.
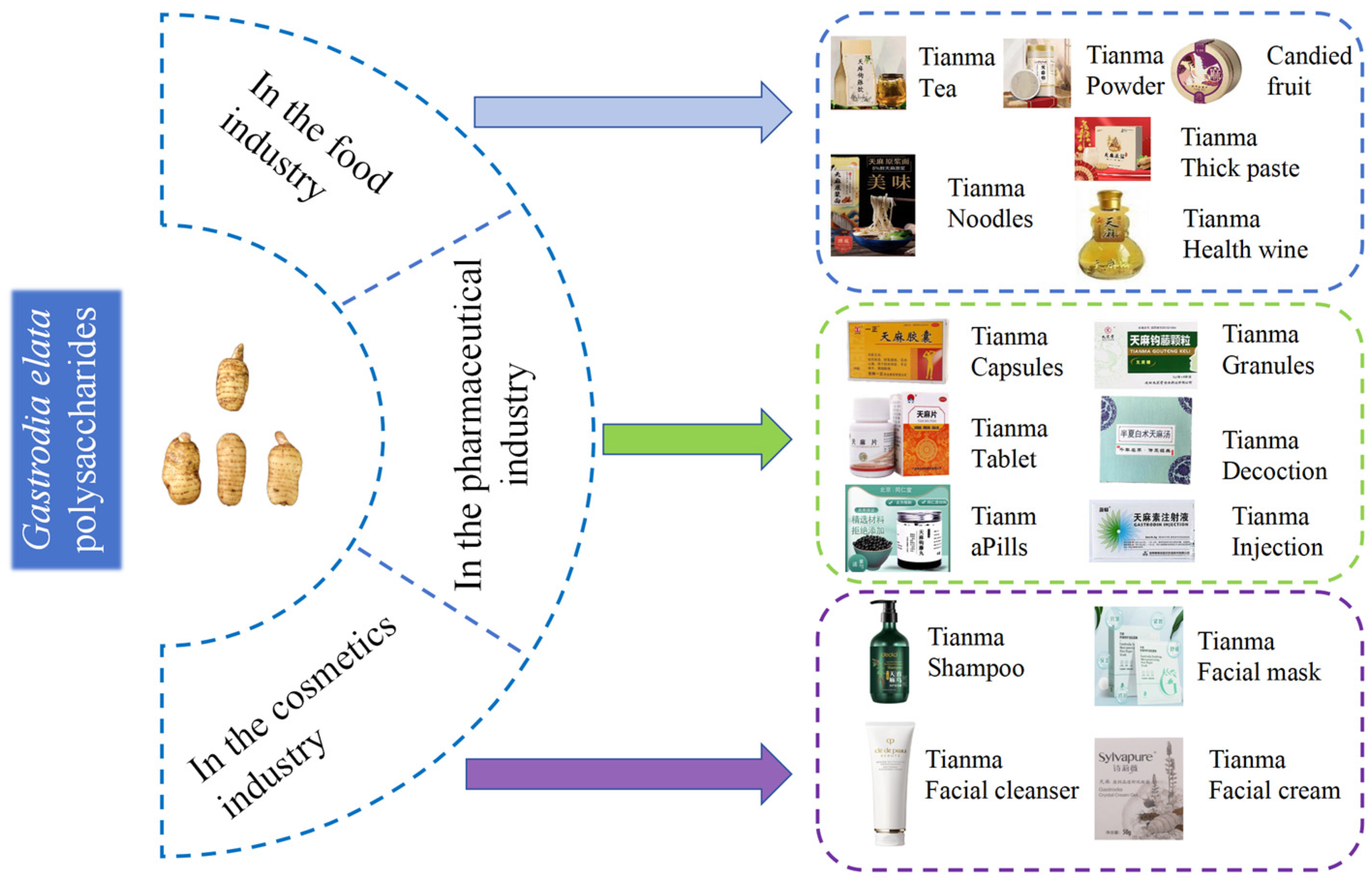
6. Application of G. elata Polysaccharide
G. elata polysaccharides are mainly used in the fields of pharmaceuticals, health foods, and cosmetics, as shown in Figure 5.
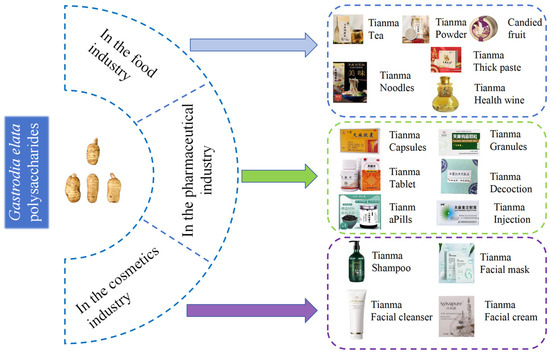
Figure 5.
Practical and potential applications of G. elata polysaccharides.
6.1. In the Food Industry
G. elata is increasingly being used as a health food in folk medicine. Dishes and foods containing G. elata have various health benefits, such as tonifying blood, regulating blood pressure, enhancing immunity, promoting calmness, and improving sleep [11]. Despite its long history of use as a health food in traditional medicine, G. elata is not widely approved for use in the national special food information query platform (http://ypzsx.gsxt.gov.cn/specialfood/#/food) (accessed on 10 october 2024.). Only 120 approved health foods are currently available, with 39 products utilizing G. elata extract as the main ingredient. The remaining products are derived from G. elata [1]. These health foods mainly focus on immune regulation, sleep improvement, anti-aging, blood pressure control, anti-hypoxia, and memory enhancement. They come in various forms, including liquor, hard capsules, tea, tablets, oral liquid, and granules. Hard capsules are the most common form, with 68 different varieties [133,134].
6.2. In the Pharmaceutical Industry
Polysaccharides are the primary active components found in G. elata. An increasing number of medications are incorporating G. elata or its extract as key ingredients. According to the State Food and Drug Administration (https://www.nmpa.gov.cn/) (accessed on 20 September 2024), these medications include G. elata pills, tablets, and capsules. These compound preparations are known for their ability to dispel wind, remove dampness, clear collaterals, relieve pain, and tonify the liver and kidneys. They are commonly used to treat conditions such as memory loss, insomnia, and headaches, and their efficacy is ensured through the establishment of quality standards [8]. For people with low immune function, such as the elderly or long-term patients, G. elata polysaccharide may help to restore and improve immune levels. G. elata polysaccharide can effectively scavenge free radicals in vivo and reduce the damage of oxidative stress to cells. For example, it can delay cell senescence and protect tissues and organs from oxidative damage. G. elata polysaccharide helps to improve insulin resistance and regulate blood glucose levels. For example, in patients with pre-diabetes, it may help prevent the occurrence of diabetes. Combined with hypoglycemic drugs, it may improve the effect of diabetes treatment. G. elata polysaccharide has a protective effect on the nervous system, which may help prevent and treat neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease.Alleviate the symptoms caused by nerve injury, such as improving neurological function after cerebral ischemia–reperfusion injury. It should be noted that although G. elata polysaccharide has a variety of potential applications, it is still in the research and development stage, and its specific application effect and safety need further clinical trials and verification.
6.3. In the Cosmetics Industry
With in-depth research on G. elata polysaccharides, they have significant practical value and promising application prospects in daily cosmetics. Its natural, safe, and functional characteristics provide unique advantages for cosmetics. In recent years, the concept of “plant-based skincare” has gained increasing praise and popularity among consumers. G. elata, a traditional Chinese medicinal herb, extract has been included in the “Catalogue of Cosmetic Raw Materials (2021 Edition)”. Products with mid- to high-end representation, mainly composed of G. elata, are rarely marketed. Gastrodin, polysaccharides, flavonoids, and amino acids in G. elata have the effect of replenishing water and moisturizing, which provides a preliminary material and functional basis for the application of G. elata in cosmetics. Currently, only a few products, such as Shiliwei G. elata cleansing cream, Lifu Queen G. elata herbal moisturizing mask, G. elata polysaccharide moisturizing cream, and G. elata Pleuropterus multiflorus shampoo, are available in the market. Research on G. elata has primarily focused on its medical and edible value, with its potential applications in cosmetics warranting further exploration [135]. The polysaccharide found in G. elata offers distinct advantages as a moisturizing agent, thanks to its abundance of hydrophilic hydroxyl structures that exhibit hygroscopic, moisturizing, high-viscosity, and film-forming properties. It can play a role in nourishing water, moisturizing, improving skin function, and moisturizing the stratum corneum in cosmetic skincare formulations. Simultaneously, it can eliminate free radicals from the skin, promote metabolism, and contribute to anti-wrinkle, whitening, and overall skin function improvement [136].
7. Conclusions and Future Prospects
As a traditional Chinese medicine sourced from food, G. elata is widely utilized in the fields of medicine, health food, and cosmetics. Polysaccharides represent key bioactive constituents in G. elata, exhibiting pharmacological activities such as antioxidant effects, anti-tumor properties, immunomodulation, and memory enhancement. The pharmacological activity of G. elata polysaccharides is closely related to their relative molecular mass, monosaccharide composition, glycosidic linkages, and other structural features. Structurally modified selenated G. elata polysaccharides have been shown to enhance antioxidant capacity. An increase in the molecular size of homogeneous polysaccharides results in a significant decrease in anti-cancer cell growth activity. Among these, α-(1→4)-glucan represents the most important class of G. elata polysaccharides, contributing to the restoration of the human immune system. Furthermore, G. elata polysaccharides are abundant in glucose, xylose, and mannose, which can stimulate the immune system, activate the immunoregulatory network, and enhance immune function.
The extraction, isolation, and purification of G. elatas polysaccharides form the foundation for subsequent research and applications. Currently, the primary methods for extracting G. elatas polysaccharides encompass water extraction, alcohol extraction, ultrasound-assisted extraction, and other traditional techniques. With advancements in modern technology and the ongoing development of polysaccharide research, innovative methods such as microwave-assisted extraction, ionic liquid-assisted extraction, and supercritical fluid extraction are gaining traction for their application in polysaccharide research.
In conclusion, the various beneficial effects of aspalathus polysaccharides have generated significant interest in this area of research. This study systematically summarizes the existing literature on the extraction, purification, structural properties, bioactivity, and conformational relationships of G. elatas polysaccharides, along with an exploration of their potential future applications. Despite the increasing number of studies on G. elatas polysaccharides, investigations are still in their nascent stages, and numerous challenges remain unresolved. Future investigations should prioritize optimizing extraction processes and enhancing purification methods while focusing on the interplay between the structure and function of G. elatas polysaccharides, as well as elucidating their mechanisms of action. Such efforts will further expand their applications in the fields of food, nutraceuticals, and pharmaceuticals. This paper lays a scientific foundation for advancing the application of G. elatas polysaccharides, which are anticipated to possess significant market potential and promising prospects in medicine and functional foods.
Author Contributions
Y.Y. (Yan Yang) and Y.H.: writing—review and editing, writing—original draft, project administration, investigation, formal analysis. Y.Y. (Yongcheng Yang) and R.W.: project administration, investigation, formal analysis. L.W., Y.Q., J.Z. and Y.L.: investigation, formal analysis H.Z. and Z.S.: writing—review and editing, supervision, project administration investigation, formal analysis, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by the Joint Special Fund for Basic Research of Local Undergraduate Universities of Yunnan Province (202101BA070001-207), Doctoral Research Initiation Fund of Dali University (KY2196114240), Research Project of Yunnan Key Laboratory of Gastrodia and Fungi Symbiotic Biology (TMKF2024B08), and Yunnan Engineering Research Center of Medicine and Food Homology, The National Natural Science Foundation of China (No.82360756), Science and technology plan project of Science and Technology Department of Yunnan Province–Basic research plan (202101BA070001-244).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wan, F.; Chen, Z.; Xu, T.; Guan, J.; Cui, X.; Kang, C.; Zhou, T.; Wang, C.; Guo, L.; Yang, Y. Selection and application of aptamers for p-hydroxybenzyl hydrogen sulfite after Gastrodia elata bl. Fumigated with sulfur. Talanta 2024, 269, 125461. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.C.; Wang, J.; Li, M.X.; Liu, Y.; Guo, X.X.; Hao, Q.; An, S.; Xu, T.R.; Yang, Y. Effect of Gastrodia elata on improving sleep in mice and its mechanism. Chin. Tradit. Herb. Drugs 2019, 50, 3140–3146. [Google Scholar]
- Chen, L.; Liu, X.; Wang, H.; Qu, M. Gastrodin attenuates pentylenetetrazole-induced seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses in mice. Neurosci. Bull. 2017, 33, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hao, X.; Yu, L.; Zhang, P.; Cao, W.; Chen, H.; Zhu, D. Gastrodin causes vasodilation by activating k(atp) channels in vascular smooth muscles via pka-dependent signaling pathway. J. Recept. Signal Transduct. Res. 2017, 37, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.F.; Chen, J.B.; Song, W.W.; Zhang, D.F.; Zhang, Y.; Song, X.W.; Dong, X.M.; Su, Y.P.; Lu, W.X.; Li, B. The molecular mechanism of the tianma preparation in the treating migraine by adenosine pathway. J. Apoplexy Nerv. Dis. 2020, 37, 255–260. [Google Scholar]
- Hu, S.; Wang, H.R.; Bai, X.R. Research status and new technology mining of Gastrodia elata analysis and identification methods. Spec. Wild Econ. Anim. Plant Res. 2024, 46, 157–164. [Google Scholar]
- Gong, W.L.; Zhan, Z.L.; Jiang, V.K.; Wang, X.; Chen, K.L.; Huang, B.S.; Liu, Z. Literature researching again on Gastrodia elata. Mod. Chin. Med. 2018, 20, 355–362. [Google Scholar]
- Wang, X.; Zhao, Z.B.; Wang, C.L. Construction and development strategy of “qin medicine” brand in context of great health. Chin. Tradit. Herbal Drugs 2024, 55, 6078–6088. [Google Scholar]
- Yang, S.; Liu, H. Research and development of Gastrodia elata resources and dishes in jinkouhe district. Sci. Technol. Qinghai Agric. 2024, 2, 117–120. [Google Scholar]
- Sun, G.X.; Wang, Z. Overall qualitative and overall quantitative authentic quality control of rhizoma gastrodiae by hplc fingerprints. Cent. South Pharm. 2009, 7, 216–219. [Google Scholar]
- Guo, J.X.; Xie, J.; Jiang, L.S.; Gao, J.H.; Rao, C.L.; Zuo, L.L. Analysis on development status of gastrodiae rhizoma health food. Chin. Tradit. Herbal Drugs 2022, 53, 2247–2254. [Google Scholar]
- Hao, J.L.; Zhao, J.C.; Zhao, M.Y.; Wang, Y.X.; Lu, J.; Shi, X.Y.; Gao, Z.Z.; Xu, Q.Q. Assessment of the cultivation suitability and suitable regions of Gastrodia elata under climate change in china. Forests 2024, 50, 1004–1014. [Google Scholar]
- Wang, Z.W.; Li, Y.; Liu, D.H.; Mu, Y.; Dong, H.J.; Zhou, H.L.; Guo, L.P.; Wang, X. Four new phenolic constituents from the rhizomes of Gastrodia elata Blume. Nat. Prod. Res. 2019, 33, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Lei, M.; Zhou, Y.; Zhong, X.; Liu, Y.; Hou, J.; Long, H.; Zhang, Z.; Tian, M.; Xie, C.; et al. Structural characterization of two novel polysaccharides from Gastrodia elata and their effects on akkermansia muciniphila. Int. J. Biol. Macromol. 2021, 186, 501–509. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcao, A.; Alves, G. Gastrodia elata and epilepsy: Rationale and therapeutic potential. Phytomedicine 2016, 23, 1511–1526. [Google Scholar] [CrossRef]
- Gong, M.Q.; Lai, F.F.; Chen, J.Z.; Li, X.H.; Chen, Y.J.; He, Y. Traditional uses, phytochemistry, pharmacology, applications, and quality control of Gastrodia elata blume: A comprehensive review. J. Ethnopharmacol. 2024, 319, 117128. [Google Scholar] [CrossRef]
- Shao, S.; Xu, C.B.; Chen, C.J.; Shi, G.N.; Guo, Q.L.; Zhou, Y.; Wei, Y.Z.; Wu, L.; Shi, J.G.; Zhang, T.T. Divanillyl sulfone suppresses nlrp3 inflammasome activation via inducing mitophagy to ameliorate chronic neuropathic pain in mice. J. Neuroinflamm. 2021, 18, 142. [Google Scholar] [CrossRef]
- Liu, F.Y.; Wen, J.; Hou, J.; Zhang, S.Q.; Sun, C.B.; Zhou, L.C.; Yin, W.; Pang, W.L.; Wang, C.; Ying, Y.; et al. Gastrodia remodels intestinal microflora to suppress inflammation in mice with early atherosclerosis. Int. Immunopharmacol. 2021, 96, 107758. [Google Scholar] [CrossRef]
- Liu, X.; Dou, Y.; Hao, T.; Wang, M.; Yang, L.; Zheng, H.; Liu, H.; Dou, H. Assessment of the effects of structural modification of Gastrodia elata polysaccharide on anti-breast cancer activity using asymmetrical flow field-flow fractionation. Molecules 2023, 28, 4669. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, C.; Hou, J.; Long, H.; Wang, B.; Guo, D.; Lei, M.; Wu, W. Gastrodia elata blume polysaccharides: A review of their acquisition, analysis, modification, and pharmacological activities. Molecules 2019, 24, 2436. [Google Scholar] [CrossRef]
- Xie, M.; Tao, W.; Wu, F.; Wu, K.; Huang, X.; Ling, G.; Zhao, C.; Lv, Q.; Wang, Q.; Zhou, X.; et al. Anti-hypertensive and cardioprotective activities of traditional chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol. 2021, 185, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, D.; Zhou, L.; Jin, H.; Dong, Q.; Yao, J.; Ding, K. Structure of a polysaccharide from Gastrodia elata bl., and oligosaccharides prepared thereof with anti-pancreatic cancer cell growth activities. Carbohydr. Polym. 2011, 86, 1300–1305. [Google Scholar]
- Qiu, H.; Tang, W.; Tong, X.; Ding, K.; Zuo, J. Structure elucidation and sulfated derivatives preparation of two alpha-d-glucans from Gastrodia elata bl. and their anti-dengue virus bioactivities. Carbohydr. Res. 2007, 342, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Lei, M.; Li, F.; Hou, J.; Zhang, Z.; Long, H.; Zhong, X.; Liu, Y.; Xie, C.; Wu, W. Structural characterization of a polysaccharide from Gastrodia elata and its bioactivity on gut microbiota. Molecules 2021, 26, 4443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.X.; Zhang, Y.; Luo, X.G. Studies on structure characterization of Gastrodia elata bl polysaccharides. Food Res. Dev. 2010, 31, 52–56. [Google Scholar]
- Liu, M.X.; Li, Q.F.; Liu, Q.; Huang, Z.Q.; Qiu, F. Study on extraction technology, structure and free radical scavenging activity of polysaccharides from Gastrodia elata b1. Food Sci. 2009, 30, 29–32. [Google Scholar]
- Zhou, B.H.; Yang, L.; Yuan, Y.; Shen, H.; Feng, Q.; Guo, Z.L.; Liu, G. Isolation and structure identification of a acidic and heteropolysaccharide from Gastrodia elata blume. Chin. J. Hosp. Pharm. 2009, 29, 2002–2006. [Google Scholar]
- Wang, C.; He, Y.; Tang, X.; Li, N. Sulfation, structural analysis, and anticoagulant bioactivity of ginger polysaccharides. J. Food Sci. 2020, 85, 2427–2434. [Google Scholar] [CrossRef]
- Tan, S.; Zhu, R.W.; Zhang, J.; Li, G.F.; Li, L.; Zou, T. Extraction process optimization of polysaccharide in Gastonia elate by enzymatic method. Food. Res. Dev. 2017, 38, 50–53. [Google Scholar]
- Ji, N.; Liu, P.; Zhang, N.; Yang, S.; Zhang, M. Comparison on bioactivities and characteristics of polysaccharides from four varieties of Gastrodia elata blume. Front. Chem. 2022, 10, 956724. [Google Scholar] [CrossRef]
- Xu, D.; Wu, Q.; Liu, W.; Hu, G.; Meng, H.; Wang, J. Therapeutic efficacy and underlying mechanisms of Gastrodia elata polysaccharides on dextran sulfate sodium-induced inflammatory bowel disease in mice: Modulation of the gut microbiota and improvement of metabolic disorders. Int. J. Biol. Macromol. 2023, 248, 125919. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, M.; Luo, A. Extraction, characterization and antioxidant activities of an acidic polysaccharide from Dendrobium devonianum. J. Food Meas. Charact. 2022, 16, 867–879. [Google Scholar] [CrossRef]
- Wang, X.; Luo, S.; Jiang, J.; Ye, J.; Lu, J.; Lu, J.F.; Ke, J.; Ke, Y. Enzymatic extraction of polysaccharides from Gastrodia elata blume. J. Chin. Med. Mater. 2013, 36, 137–140. [Google Scholar]
- Gao, J.; Hu, D.; Shen, Y.; Zheng, Y.; Liang, Y. Optimization of ultrasonic-assisted polysaccharide extraction from hyperici perforati herba using response surface methodology and assessment of its antioxidant activity. Int. J. Biol. Macromol. 2023, 225, 255–265. [Google Scholar] [CrossRef]
- Du, C.; Liu, X.; Algadi, H.; Hou, Y.; Fu, X.; Li, H.; Fan, J.; Singh, M.V.; Li, Y.; Zhang, X.; et al. Polysaccharide extraction optimization, monosaccharide composition, and antioxidant activity analysis of different varieties of Gastrodia elata bl aerial parts. Biomass Convers. Biorefin. 2023, 14, 29353–29365. [Google Scholar] [CrossRef]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohydr. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Ke, L.; Duan, X.; Cui, J.; Song, X.; Ma, W.; Zhang, W.; Liu, Y.; Fan, Y. Research progress on the extraction technology and activity study of epimedium polysaccharides. Carbohydr. Polym. 2023, 306, 120602. [Google Scholar] [CrossRef]
- Wang, R.M.; Zhu, Q.J.; Zhang, C.H.; Yang, X.M.; Zhang, H.M. Effect of different extraction method on antioxidant activity of polysaccharides from Gastrodia elata Blume. Food Sci. Technol. 2015, 40, 208–213. [Google Scholar]
- Li, Z.Y.; Shao, S.M.; Zhang, H.R.; Zhang, J.X.; Zhang, J.X. Study on the Microwave-assisted Extraction Polysaccharide of Gastrodia Elate. J. Shanxi Univ. 2008, 122, 573–576. [Google Scholar]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef]
- Zeng, P.; Li, J.; Chen, Y.; Zhang, L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog. Molec. Biol. Transl. Sci. 2019, 163, 423–444. [Google Scholar]
- Wu, Y.; Li, B.H.; Chen, M.M.; Liu, B.; Jiang, L.L. Research progress on ginger polysaccharides: Extraction, purification and structure-bioactivity relationship. Food Funct. 2023, 14, 10651–10666. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Ling, X.; Xu, J.; Zhu, Y.; Zhang, J.; Liu, X. Structural characterization of polysaccharide derived from Gastrodia elata and its immunostimulatory effect on raw264.7 cells. Molecules 2022, 27, 8059. [Google Scholar] [CrossRef]
- Gan, Q.; Chen, L.; Xian, J.; An, G.; Wei, H.; Ma, Y. Digestive characteristics of gastrodia elata blume polysaccharide and related impacts on human gut microbiota in vitro. J. Ethnopharmacol. 2024, 328, 118064. [Google Scholar] [CrossRef]
- Chen, J.Q.; Miao, W.; Liu, Y.; Zhou, J.; Han, J.; Zhang, L.; Bian, X.Q.; Zhong, T.; Wu, J.L.; Li, N. Structural characterization, molecular dynamic simulation, and conformational visualization of a water-soluble glucan with high molecular weight from Gastrodia elata blume. Int. J. Biol. Macromol. 2024, 263, 130207. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, X. Effect of Gastrodia elata polysaccharide on proliferation, invasion and apoptosis ofcolon cancer cells exposed to arsenic via mir-27α-3p-b7-h3 pathway. Occup. Health 2023, 39, 1596–1603. [Google Scholar]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT Food Sci. Technol. 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Li, N.; Wang, D.; Wen, X.; Chu, R.; Fan, J.; Chen, Y.; Luo, Y. Effects of polysaccharides from Gastrodia elata on the immunomodulatory activity and gut microbiota regulation in cyclophosphamide-treated mice. J. Sci. Food. Agric. 2023, 103, 3390–3401. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.R.; Dou, Y.W.; Ye, H.; Dou, H.Y. Separation and characterization of Gastrodia elata polysaccharides based on asymmetrical flow field-flow fractionation: Steric transition phenomenon. Chin. J. Chromatogr. 2023, 41, 714–721. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, M.; Liu, G.; Li, D.; Zhou, B. Oral absorption mechanism of the polysaccharides from Gastrodia elata blume base on fluorescence labeling. Food Res. Int. 2021, 144, 110342. [Google Scholar] [CrossRef]
- Ma, F.W.; Wen, Q.; Deng, Q.F.; Lu, Y.H.; Zhang, B.Y.; Cheng, Y.Y.; Xu, S. Preparation of high selenized Gastrodia elata blume polysaccharides and its immunomodulatory effects on raw 264.7 macrophages and cyclophosphamide-treated mice. Pak. J. Zool. 2023, 56, 2501–3000. [Google Scholar]
- Zhu, Z.Y.; Chen, C.J.; Sun, H.Q.; Chen, L.J. Structural characterisation and ace-inhibitory activities of polysaccharide from Gastrodia elata blume. Nat. Prod. Res. 2019, 33, 1721–1726. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Wu, F.; Miao, H.C.; Xiong, K.R. Effects of polysaccharide of Gastrodia elata blume and electro-acupuncture on expressions of brain-derived neurotrophic factor and stem cell factor protein in caudate putamen of focal cerebral ischemia rats. Med. Sci. Monit. Basic Res. 2016, 22, 175–180. [Google Scholar] [CrossRef]
- Bao, Q.; Qian, L.; Gong, C.; Shen, X. Immune-enhancing activity of polysaccharides from Gastrodia elata. J. Food Process. Preserv. 2016, 41, 13016. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.P.; Jin, L.X. Preparation, characterization and anti-ageing activity of Gastrodia elata blume polysaccharide. Acta Aliment. 2018, 47, 210–219. [Google Scholar] [CrossRef]
- Chen, J.; Tian, S.; Shu, X.; Du, H.; Li, N.; Wang, J. Extraction, characterization and immunological activity of polysaccharides from rhizoma gastrodiae. Int. J. Mol. Sci. 2016, 17, 1011. [Google Scholar] [CrossRef]
- Lee, O.H.; Kim, K.I.; Han, C.K.; Kim, Y.C.; Hong, H.D. Effects of acidic polysaccharides from gastrodia rhizome on systolic blood pressure and serum lipid concentrations in spontaneously hypertensive rats fed a high-fat diet. Int. J. Mol. Sci. 2012, 13, 698–709. [Google Scholar] [CrossRef]
- Zhuo Ma, N.M.; Liu, T.; Ni, Z. Determination of polysaccharide content in gsatrodiae rhizoma by phenol-sulphoacid method. Guangdong Agric. Sci. 2011, 38, 132–134. [Google Scholar]
- Tian, M.H.; Yang, Z.X.; Tian, Z.J.; Zhang, C.F.; Yu, X.L. Extraction and determination of Gastrodia elata polysaccharides from different regions and grades. Yunnan Chem. Ind. 2016, 43, 73–75. [Google Scholar]
- Li, J.J.; Huang, Y.; Kang, K.J.; Zhang, B.L.; Li, L.L. Study on extraction rate and antioxidant capacity of Zhaotong Gastrodia elata Bl. Glauca polysaccharides with different ph solvents. Acad. Period. Farm Prod. Process. 2024, 5, 18–21. [Google Scholar]
- Zhu, H.Y.; Jiang, R.; He, Z.Y.; Zhao, Y.; Hao, Y.G.; Ying, H.H.; Zhang, L.X. Determination of the contents of gastrodin, 4-hydroxybenzyl alcohol and gastrodia polysaccharides in different processed products of Gastrodia elata Bl. F. glauca S. Chow. J. Chin. Pharm. Sci. 2017, 52, 2062–2065. [Google Scholar]
- Zhang, S.Q.; Liu, L.; He, N.W.; Zhao, Y. Gastrodia elata polysaccharide from shaanxi by ultrasound-assisted hot water extraction: Process optimization and antioxidant activity. Chin. Agric. Sci. Bull. 2021, 37, 131–136. [Google Scholar]
- Cao, X.Y.; Yang, H.T. Study on citrate buffer assisted extraction of polysaccharide from Gastrodia elata and its antioxidant activity. Appl. Chem. Ind. 2016, 45, 1461–1465. [Google Scholar]
- Chen, C.; Li, X.X.; Fu, J.D.; Liu, X.; Zheng, H.X.; Wu, S.Q.; Li, X.D. Research on the antibacterial activity of hanzhong Gastrodia elata blume polysaccharides. Jiangsu Agric. Sci. 2018, 46, 156–159. [Google Scholar]
- Wang, Q.; Li, D.; Pan, Y.; Wu, W. Effect of different extraction methods on the extraction ratio and antioxidant activity of polysaccharides from Gastrodia elata Bl. Food Mach. 2017, 33, 146–150. [Google Scholar]
- Zhou, B.H.; Tan, J.; Zhang, T.; Wu, Y.; Liu, G. Preparation of sulfated polysaccharides from Gastrodia elata blume and its antioxidant activity. Chin. J. Hosp. Pharm. 2017, 37, 1685–1691. [Google Scholar]
- Xu, T.T. Study on the Effect of Sulphur Fumigation on the Structure and Antioxidant Activity of Polysaccharides of Gastrodia elata blume. Master’s Thesis, Kunming University of Science and Technology, Kunming, China, 2023. [Google Scholar]
- Ming, J.; Zeng, K.F.; Wu, S.R.; Fu, A.L.; Zhao, G.H.; Gui, M.Y.; Cheng, Z.D. Effect of soluble polysaccharide pgeb-3-h from Gastrodia elata blume on scopolamine-induced learning and memory disorders in mice. Food Sci. 2010, 31, 246–249. [Google Scholar]
- Ming, J.; Zeng, K.F.; Wu, S.R.; Fu, A.L.; Zhao, G.H.; Gui, M.Y.; Cheng, Z.D. Study on isolation and physicochemical characteristics of water-soluble polysaccharides from Gastrodia elata blume. Food Sci. 2008, 29, 344–347. [Google Scholar]
- Zhu, X.X.; Zhang, Y. Purification of Gastrodia elata Bl. Polysaccharides. Chin. J. Ethnomed. Ethnopharm. 2010, 19, 102–103. [Google Scholar]
- Zhu, X.X.; Zhang, Y. Optiization of extraction param eters for polysaccharides of Gastrodia elata Bl. Lishizhen Med. Mater. Medica Res. 2007, 18, 906–907. [Google Scholar]
- Chen, C.; Li, X.X.; Xu, Y.M.; Lin, B.B.; Zhou, T.H.; Liu, X.; Zheng, H.X.; Wu, S.Q.; Cao, J.Y.; Hu, H.Z. Extraction, purification and antioxidant activity of polysaccharides from Gastrodia elata Bl. Chin. J. Clin. Pharmacol. 2018, 34, 2203–2206. [Google Scholar]
- Zhu, J.P.; Li, F.; Shen, Y.T. Extraction technology of polysaccharides from gastrodia rhizome and its content determination. J. Anhui Agric. Sci. 2012, 40, 9648–9650. [Google Scholar]
- Zheng, J.; Sun, P.P.; Hu, A.J.; Hu, X.H.; Ren, Y.Y.; Yu, X.Y. Extraction of polysaccharide from Gastrodia elata Blume and preparation of its drinks. Food Res. Dev. 2018, 39, 123–129. [Google Scholar]
- Ren, S.L.; Liu, T.S.; Liu, Y.J.; Li, Z.; Nie, L.X. Research of extraction parameters for Gastrodia elata polysaccharides. J. Tradit. Chin. Med. Univ. Hunan 2010, 43, 37–40. [Google Scholar]
- Fan, R.; Ma, G.H.; Yu, S.S. Protective effect of polysaccharide from Gastrodia elata Blume on non-alcoholic fatty liver induced by high fat diet. Sci. Technol. Food Ind. 2022, 43, 381–391. [Google Scholar]
- Zhou, B.H.; Wu, L.N.; Shen, H.; Liu, G.; Jin, L. Protective effects of Gastrodia elata polysaccharides on corticosterone-induced pc12 cell injury. Chin. Pharm. 2012, 15, 595–598. [Google Scholar]
- Zhu, X.X.; Luo, X.G. Optimization of extraction param eters and de-protein process for Gastrodia elata polysaccharides. J. Chin. Med. Mater. 2007, 30, 724–726. [Google Scholar]
- Gao, Q.Q. Study of Ophthalmic Gel with Phospholipid Complex of Gastrodia elata Polysaccharide. Master’s Thesis, Tianjin Journal of Tra-ditional Chinese Medicine, Tianjin, China, 2023. [Google Scholar]
- Wei, D.Y. Study on Structural Characterization and Anti-Breast Cancer Activity of Gastrodia elata Polysaccharide and Its Derivatives. Master’s Thesis, Hebei University, Baoding, China, 2022. [Google Scholar]
- Liu, Y.T.; Liang, H.; Cai, Y.P.; Lin, Y.; Zhan, S.H. Prmary research on isolation purification and character of polysaccharide of Gastrodia elata Bl. Acta Laser Biol. Sin. 2007, 16, 495–500. [Google Scholar]
- Hua, W.Q.; Lu, Y.H.; Yang, F. Preparation, structure characterization and antioxidant activity evaluation of selenized Gastrodia elata polysaccharides. Sci. Technol. Food Ind. 2024, 45, 18–30. [Google Scholar]
- Ma, Y.J.; Yu, L.L.; Liu, R.X.; Chen, T.Z. Optimization of ultrasonic extraction process of Gastrodia elata using response surface methodology. Mod. Chin. Med. 2018, 20, 599–603. [Google Scholar]
- Li, C.; Wang, J.R.; Ji, X.H.; Lu, X.L. Isolation of Gastrodia elata Bl. Polysaccharides and a nalysis of its composition of monosaccharide. Chin. Agric. Sci. Bull. 2008, 24, 89–92. [Google Scholar]
- Zhu, P.F.; Chen, T.; Gu, W.; Zhao, R.H. Determination of gastrodia polysaccharide content in Gastrodia elata produced in zhaotong, yunnan province. Chin. J. Spectrosc. Lab. 2013, 30, 2960–2964. [Google Scholar]
- Chen, C.J. Structural Characterization and Ace Inhibiory Activity of Polysaccharide from Gastrodia elata Blum. Master’s Thesis, Tianjin University of Science and Technology, Tianjin, China, 2018. [Google Scholar]
- Zhang, M.J. Extraction, Purification and Activity of Polysaccharides from Gastrodia elata Blume. Master’s Thesis, Northwest A&F University, Xianyang, China, 2007. [Google Scholar]
- Pan, C.S. Purification Strcture Characterization and Antitumor Activity Studyof Polysaccharides from Gastrodiae elata. Master’s Thesis, Shandong University of Traditional Chinese Medicine, Jinan, China, 2019. [Google Scholar]
- Sun, Y.N. Study on Separation and Purification and Antioxidant Activity of Polysaccharides from Gastrodia elata. Master’s Thesis, Southwest University, Chongqing, China, 2007. [Google Scholar]
- Zhang, G.C. Separation and Purification of Gastrodia elata Blume Polysaccharide and Its Antioxidant Activity. Master’s Thesis, Northwest A&F University, Xianyang, China, 2013. [Google Scholar]
- Hang, J.Y. Extraction Technology and Application of Gastrodin and Gastrodia elata Polysaccharide. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2022. [Google Scholar]
- Liu, Y.T. Research on the Accumulation Law of Effective Components and Structure of Polysaccharides of Gastrodia elata Blume. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2007. [Google Scholar]
- Hong, Q.M. Study on the Structure and Their Biological Activities of Polysaccharides of Gastrodia elata Bl. Master’s Thesis, Shanghai University of Traditional Chinese Medicine, Shanghai, China, 2009. [Google Scholar]
- Ai, X.; Yu, P.; Li, X.; Lai, X.; Yang, M.; Liu, F.; Luan, F.; Meng, X. Polysaccharides from Spirulina platensis: Extraction methods, structural features and bioactivities diversity. Int. J. Biol. Macromol. 2023, 231, 123211. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Liu, G.; Li, Y.; Liang, L.; Liu, X.; Xu, X.; Wen, C. Advance in morchella sp. Polysaccharides: Isolation, structural characterization and structure-activity relationship: A review. Int. J. Biol. Macromol. 2023, 247, 125819. [Google Scholar] [CrossRef]
- Zhan, H.D.; Zhou, H.Y.; Sui, Y.P.; Du, X.L.; Wang, W.H.; Dai, L.; Sui, F.; Huo, H.R.; Jiang, T.L. The rhizome of Gastrodia elata blume—An ethnopharmacological review. J. Ethnopharmacol. 2016, 189, 361–385. [Google Scholar] [CrossRef]
- Ming, J.; Liu, J.; Wu, S.; Guo, X.; Chen, Z.; Zhao, G. Structural characterization and hypolipidemic activity of a polysaccharide pgeb-3h from the fruiting bodies of Gastrodia elata blume. Procedia Eng. 2012, 37, 169–173. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, G. The chemical composition, pharmacological effects, clinical applications and market analysis of Gastrodia elata. Pharm. Chem. J. 2017, 51, 211–215. [Google Scholar] [CrossRef]
- Xie, H.; Chen, Y.; Wu, W.; Feng, X.; Du, K. Gastrodia elata blume polysaccharides attenuate vincristine-evoked neuropathic pain through the inhibition of neuroinflammation. Mediat. Inflamm. 2021, 2021, 9965081. [Google Scholar] [CrossRef]
- Gan, Q.X.; Peng, M.Y.; Wei, H.B.; Chen, L.L.; Chen, X.Y.; Li, Z.H.; An, G.Q.; Ma, Y.T. Gastrodia elata polysaccharide alleviates parkinson’s disease via inhibiting apoptotic and inflammatory signaling pathways and modulating the gut microbiota. Food Funct. 2024, 15, 2920–2938. [Google Scholar] [CrossRef]
- Maity, P.; Sen, I.K.; Chakraborty, I.; Mondal, S.; Bar, H.; Bhanja, S.K.; Mandal, S.; Maity, G.N. Biologically active polysaccharide from Edible mushrooms: A review. Int. J. Biol. Macromol. 2021, 172, 408–417. [Google Scholar] [CrossRef]
- Govindan, S.; Shanmugam, J.; Rajendran, G.; Ramani, P.; Unni, D.; Venkatachalam, B.; Janardhanan, A.; Aswini, K.; Rajendran, R.L.; Gangadaran, P.; et al. Antidiabetic activity of polysaccharide from Hypsizygus ulmarius in streptozotocin-nicotinamide induced diabetic rats. Bioact. Carbohydr. Diet. Fibre. 2023, 29, 100350. [Google Scholar] [CrossRef]
- Lu, J.; He, R.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (lingzhi), a review. Int. J. Biol. Macromol. 2020, 150, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Unlu, A.; Nayir, E.; Kirca, O.; Ozdogan, M. Ganoderma lucidum (Reishi mushroom) and cancer. J. Buon 2016, 21, 792–798. [Google Scholar] [PubMed]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Thakur, K.; Zhang, J.G.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Sun, Z.; Zheng, X.; Zhang, H.; Xu, H.; Wang, L. Dietary lycium barbarum polysaccharide modulates growth performance, antioxidant capacity, and lipid metabolism in common carp (Cyprinus carpio) fed with high-fat diet. Antioxidants 2024, 13, 540. [Google Scholar] [CrossRef]
- Dai, S.; Zhang, W.; Dou, Y.; Liu, H.; Chen, X.; Shi, J.; Dou, H. Towards a better understanding of the relationships between the structure and antitumor activity of Gastrodia elata polysaccharides by asymmetrical flow field-flow fractionation. Food Res. Int. 2021, 149, 110673. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Tan, J.; Guo, X.X.; Liu, G.; Zhou, B.H. Studies on anti-c6 glioma cells activity of sulfated polysaccharides from Gastrodia elata Blume. Chin. Pharm. 2020, 23, 227–231. [Google Scholar]
- Zhao, W.C.; Guan, Z.F.; Jiang, P. Inhibitory effects of Gastrodia elata polysaccharides on transplanted tumours in mice. J. Minzu Univ. China. 2008, 17, 77–80. [Google Scholar]
- Wang, Q.; Zhang, Y.; Li, J.; Ren, T.L. The inhibition effect of polysaccharides from Gastrodia elata Bl. On tumor growth though immune system. Cell. Mol. Immunol. 2014, 30, 566–568. [Google Scholar]
- Liu, X.F.; Guo, X.N.; Zhan, J.P.; Xie, Z.L.; Wang, J.M.; Zhang, Y.T.; Chen, Y.L.; Li, X.B. The effects of polysaccharide from Gastrodia elata Bl. On cell cycle and caspase proteins activity in h22 tumor bearing mice. Chin. J. Gerontol. 2015, 35, 5681–5682. [Google Scholar]
- Chen, C.; Qin, Y.; Fang, J.P.; Ni, X.Y.; Yao, J.; Wang, H.Y.; Ding, K. Wss25, a sulfated polysaccharide, inhibits rankl-induced mouse osteoclast formation by blocking smad/id1 signaling. Acta Pharmacol. Sin. 2015, 36, 1053–1064. [Google Scholar] [CrossRef]
- Zhou, B.; Tan, J.; Zhang, C.; Wu, Y. Neuroprotective effect of polysaccharides from Gastrodia elata blume against corticosterone-induced apoptosis in pc12 cells via inhibition of the endoplasmic reticulum stress-mediated pathway. Mol. Med. Rep. 2018, 17, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zhan, J.P.; Zhang, Y.T.; Xie, Z.L.; Zhu, Q.Y.; Chen, Y.L.; Cheng, K.; Wang, J.M.; Li, R.Q. Effect of polysaccharide from Gastrodia elata Bl. On humoral immune function in immunosuppressed mice induced by cyclophosphamide. Chin. J. Gerontol. 2016, 36, 1027–1028. [Google Scholar]
- Hu, D.K.; Shen, Y.S. Protection of polysaccharide gep-2 from Gastrodia elata on the immunity mediated liver injury induced by bcg+lps in mice. Cell. Mol. Immunol. 2007, 23, 912–914. [Google Scholar]
- Xie, X.Y.; Chao, Y.M.; Zhang, Z.; Du, Y. Effects of polysaccharides from Gastrodia elata bl. On anti-aging of ageing mice. Pharm. J. Chin. People’s Lib. Army. 2010, 26, 206–209. [Google Scholar]
- Zhang, Y.; Ye, P.; Zhu, H.; Gu, L.; Li, Y.; Feng, S. Experimental study of the effects of Gastrodia elata polysaccharide on delay in skeletal muscle aging. J. Zunyi Med. Univ. 2019, 42, 135–140. [Google Scholar]
- Zhang, Y.; Ye, P.; Zhu, H.; Gu, L.; Li, Y.; Feng, S.; Zeng, Z.; Chen, Q.; Zhou, B.; Xiong, X. Neutral polysaccharide from Gastrodia elata alleviates cerebral ischemia-reperfusion injury by inhibiting ferroptosis-mediated neuroinflammation via the nrf2/ho-1 signaling pathway. CNS Neurosci. Ther. 2024, 30, e14456. [Google Scholar] [CrossRef]
- Tong, X.K.; Qiu, H.; Zhang, X.; Shi, L.P.; Wang, G.F.; Ji, F.H.; Ding, H.Y.; Tang, W.; Ding, K.; Zuo, J.P. Wss45, a sulfated alpha-d-glucan, strongly interferes with dengue 2 virus infection in vitro. Acta Pharmacol. Sin. 2010, 31, 585–592. [Google Scholar] [CrossRef]
- Lin, B.; Zhao, G.H. Effect of polysaccharide pgeb-3-h from Gastrodia elata Blume on short chain fatty acids in the intestinal tract of rats. Food Sci. 2011, 32, 284–287. [Google Scholar]
- Liu, M.M.; Zhang, Y.; Zhang, Y.Y.; Wang, G.H.; Zhou, B.H. Effects and potential mechanisms of Gastrodia elata polysaccharides in lps-induced depression model mice. Chin. Pharm. 2021, 24, 2018–2023. [Google Scholar]
- Shi, H.; He, Q.; Lou, Y.J.; Shao, S.J. Effect of gastrodiae rhizoma polysaccharide on brain neutral transmitter in immature rats with cerebral palsy. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 140–145. [Google Scholar]
- Xie, R.Z.; Yuan, X. Protective effect of gastrodia polysaccharides against ht22 cell damage induced by glutamate acid. Pharm. J. Chin. People’s Lib. Army. 2016, 32, 120–123. [Google Scholar]
- Huang, R.; Zhao, J.; Wu, F.; Miao, H.C.; Ding, J.; Xiong, R. Effect of electroacupuncture plus polysaccharide of Gastrodia elata Blume on expression of nestin and brain derived neurotrophic factor in the basolateral amygdala of focal cerebral ischemia rats. Acupunct. Res. 2016, 41, 230–234. [Google Scholar]
- Liao, H.C.; Wu, F.; Ding, J.; Xiong, K.R. Effeets of electroacupuneture intervention combined with polysaccharide of Gastrodia elata Blume on expression of nestin and cytokines of neural stem cells in the dentate gyrus of cerebral ischemia rats. Chin. J. Histochem. Cytochem. 2014, 23, 35–39. [Google Scholar]
- Wang, X.L.; Liu, P.D.; Zhang, L.; Xie, Y.Y.; Yang, C.; Yu, Q. Effect of gastrodiae rhizoma polysaccharide on neuronal apoptosis gene expression in brain tissue of cerebral palsy newborn rats. Chin. J. Clin. Pharmacol. 2019, 35, 2062–2064. [Google Scholar]
- Kim, K.J.; Lee, O.H.; Han, C.K.; Kim, Y.C.; Hong, H.D. Acidic polysaccharide extracts from gastrodia rhizomes suppress the atherosclerosis risk index through inhibition of the serum cholesterol composition in sprague dawley rats fed a high-fat diet. Int. J. Mol. Sci. 2012, 13, 1620–1631. [Google Scholar] [CrossRef]
- Miu, H.C.; Shen, Y.S. Antihypertensive effect of polysaccharides substracted from Gastrodia elata blume. Chin. J. Hypertens. 2006, 14, 531–534. [Google Scholar]
- Yu, L.; Shen, Y.S.; Miao, H.C. Study on the anti-vertigo function of polysaccharides of Gastrodia elata and polysaccharides of armillaria mellea. China J. Tradit. Chin. Med. Pharm. 2006, 13, 29–36. [Google Scholar]
- Zhou, B.H.; Tan, J.; Zeng, M.L.; Wu, Y. Protective effect of sulfated Gastrodia elata polysaccharides on the apoptosis of pc12 cells induced by corticosterone. Chin. Pharm. 2017, 20, 1005–1009. [Google Scholar]
- Dai, S.S. Extraction, Study on Extraction, Characterization and Anti-Human Breast Cancer mcf-7 Cells of Gastrodia elata Polysaccharide. Master’s Thesis, Hebei University, Baoding, China, 2020. [Google Scholar]
- Brade, H.; Brade, L.; Rietschel, E.T. Structure-activity relationships of bacterial lipopolysaccharides (endotoxins). Current and future aspects. Zentralblatt Bakteriol. Mikrobiol. Hyg. A 1988, 268, 151–179. [Google Scholar]
- Deng, Y.Y.; Wu, W.D.; Chen, N.N.; Chen, B.; Yuan, P.L. Study on formula application of gastrodia rhizoma health food. Beverage Ind. 2023, 26, 72–78. [Google Scholar]
- Duan, H.; Zhou, Y.X.; Zhou, S.Q.; Yan, W.J. Application of Gastrodia elata in functional food in china. Sci. Technol. Food Ind. 2023, 44, 403–412. [Google Scholar]
- Hu, F.; Chen, H.; Huang, B.L.; Fang, S.L.; Liu, Y.F.; Shen, K.Z. Application potential of Gastrodia elata in cosmetics. Acad. Period. Farm Prod. Process. 2023, 96–100. [Google Scholar] [CrossRef]
- Shi, S.J.; Chen, D.L. Extraction of polysaccharides from Gastrodia elata stem and preparation of moisturizer. Yunnan Chem. Ind. 2018, 45, 102–104. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).