Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF)
Abstract
:1. Introduction
- Evaluate TXRF’s effectiveness, focusing on sensitivity, precision, detection, and quantitation limits for various elements in mussel tissues.
- Characterize the elemental profiles of mussels from three different aquaculture farms regarding nutrients and contaminants and analyze the correlation between them.
2. Results and Discussion
2.1. TXRF Method Validation
2.2. Elemental Composition of Mussels
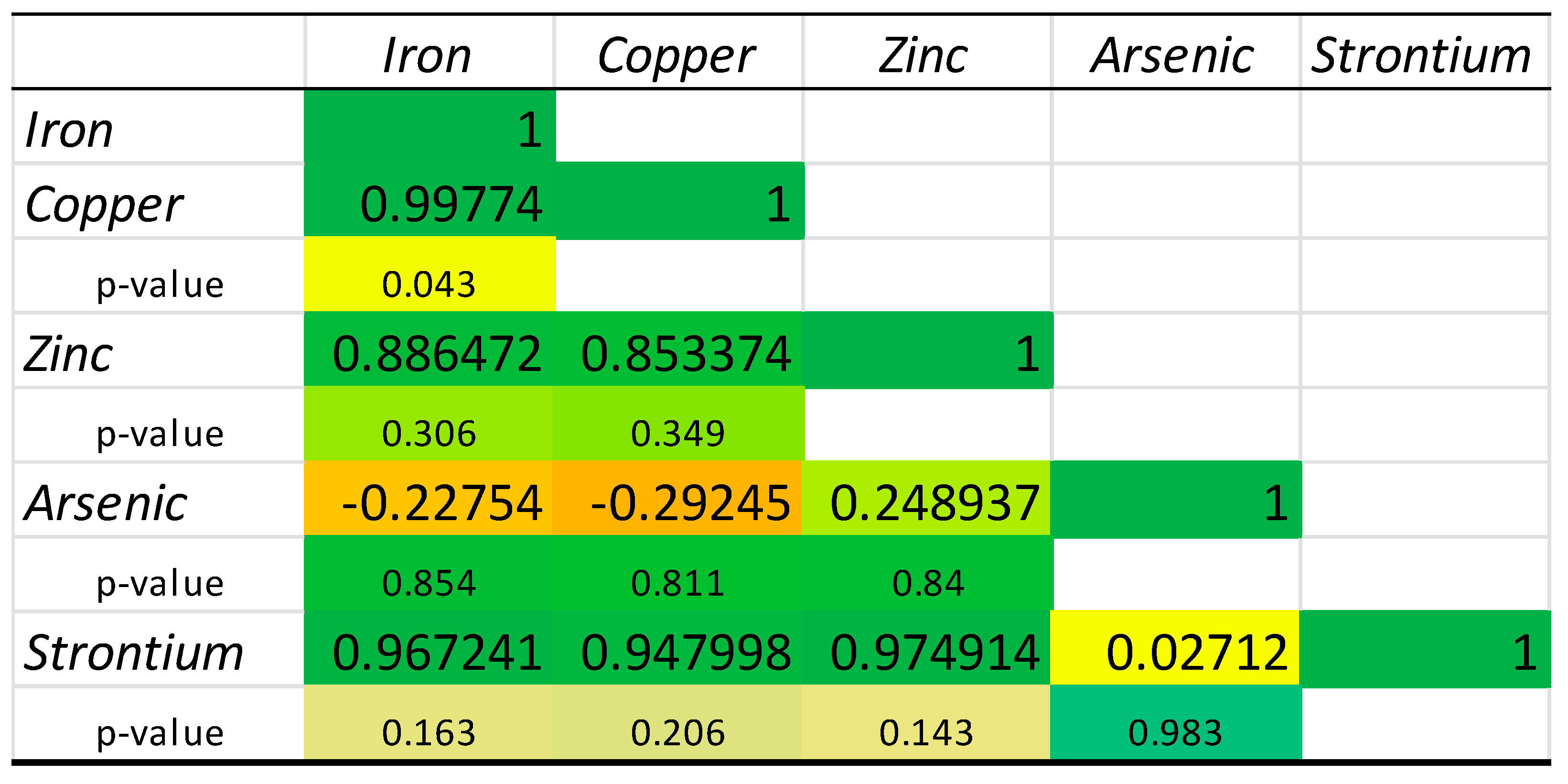
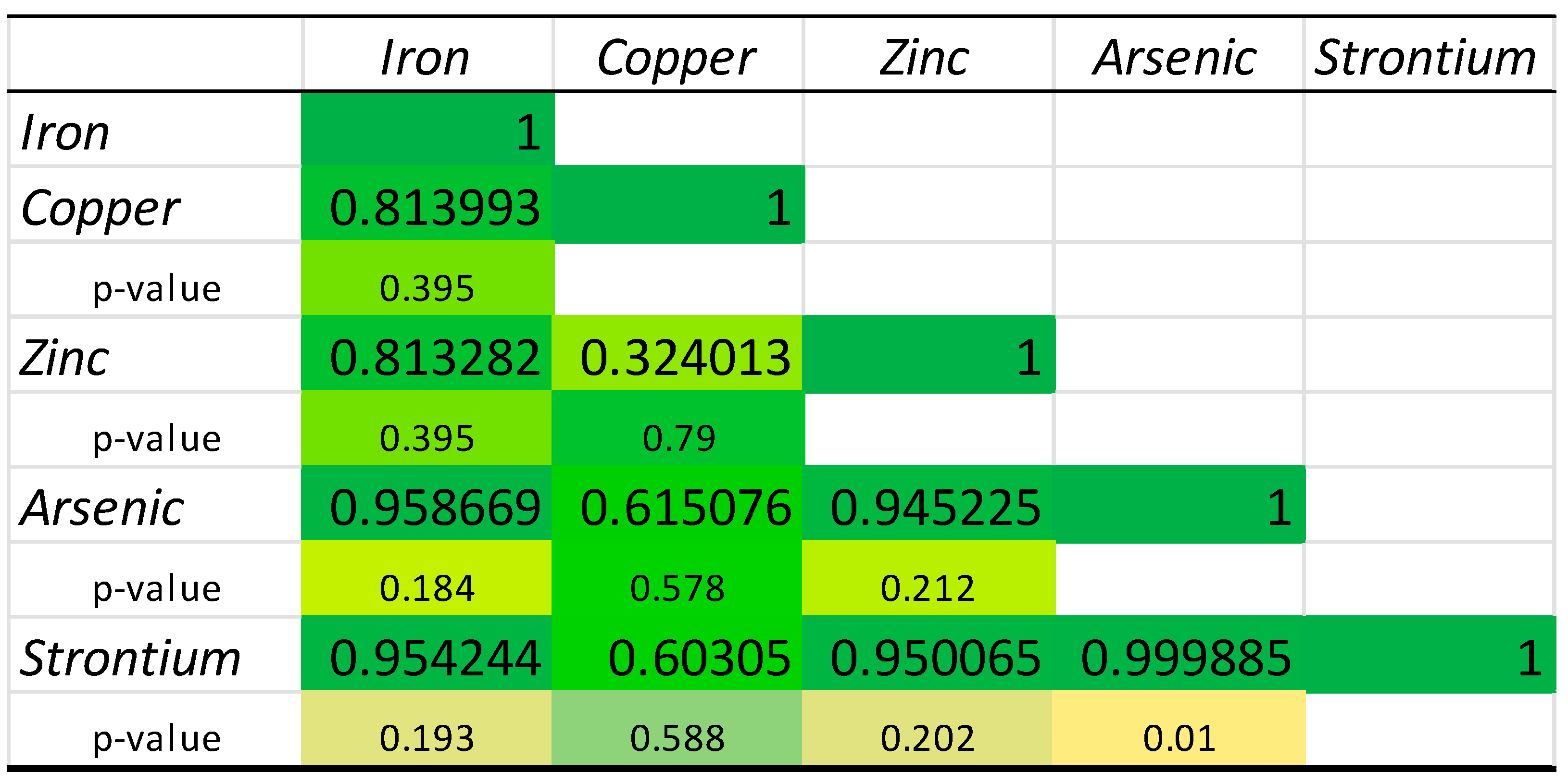
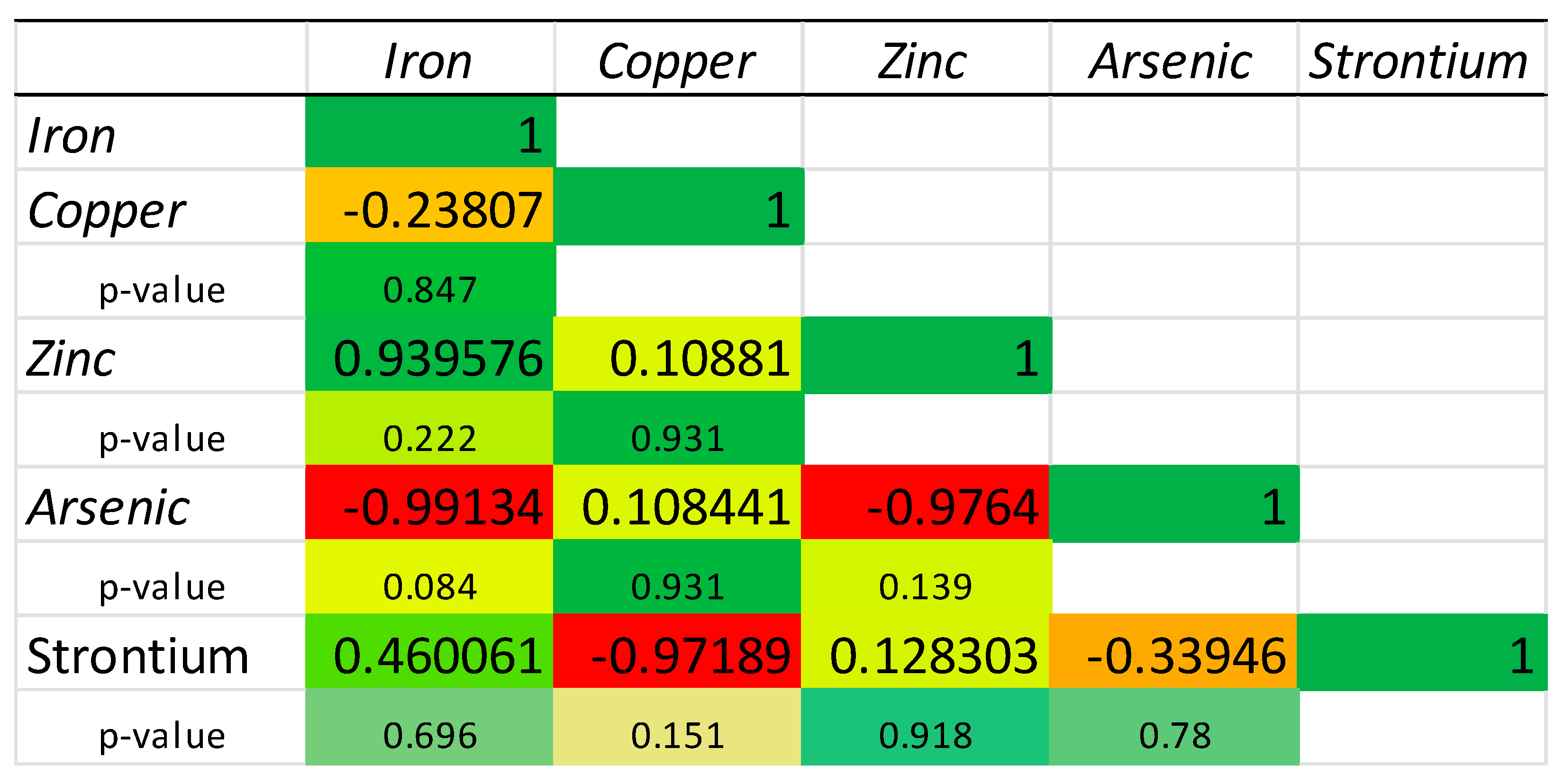
2.3. Chemical Elements’ Correlation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Validation of TXRF Method
3.3. Mussel Tissue Initial Preparation
3.4. Total Reflection X-Ray Spectrometry Analysis
3.4.1. Mussel Sample Preparation
3.4.2. Instrumentation
3.4.3. Calculation of Elements Concentration
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Consumption—European Commission. Available online: https://oceans-and-fisheries.ec.europa.eu/facts-and-figures/facts-and-figures-common-fisheries-policy/consumption_en (accessed on 25 January 2024).
- The European Market for Mussels|GLOBEFISH|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/in-action/globefish/news-events/news/news-detail/The-European-market-for-mussels/en (accessed on 25 January 2024).
- Dalipi, R.; Borgese, L.; Marguí, E.; Sangiorgi, E.; Depero, L.E. Total Reflection X-Ray Fluorescence Technique for Multi-Elemental. Analysis of Food. Spectrosc. Eur. 2017, 29, 1. [Google Scholar]
- Simionov, I.-A.; Călmuc, M.; Iticescu, C.; Călmuc, V.; Georgescu, P.-L.; Faggio, C.; Petrea, Ş.-M. Human Health Risk Assessment of Potentially Toxic Elements and Microplastics Accumulation in Products from the Danube River Basin Fish Market. Environ. Toxicol. Pharmacol. 2023, 104, 104307. [Google Scholar] [CrossRef] [PubMed]
- Simionov, I.-A.; Cristea, V.; Petrea, S.-M.; Sirbu, E.B. Evaluation of heavy metals concentration dynamics in fish from the black sea coastal area: An overview. Environ. Eng. Manag. J. 2019, 18, 1097–1110. [Google Scholar] [CrossRef]
- Caruso, J.A.; Montes-Bayon, M. Elemental Speciation Studies—New Directions for Trace Metal Analysis. Ecotoxicol. Environ. Saf. 2003, 56, 148–163. [Google Scholar] [CrossRef]
- Pröfrock, D.; Prange, A. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS) for Quantitative Analysis in Environmental and Life Sciences: A Review of Challenges, Solutions, and Trends. Appl. Spectrosc. 2012, 66, 843–868. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Marguí, E.; Queralt, I.; de Almeida, E. X-Ray Fluorescence Spectrometry for Environmental Analysis: Basic Principles, Instrumentation, Applications and Recent Trends. Chemosphere 2022, 303, 135006. [Google Scholar] [CrossRef]
- Fernández-Ruiz, R. TXRF Spectrometry in the Bioanalytical Sciences: A Brief Review. X-Ray Spectrom. 2022, 51, 279–293. [Google Scholar] [CrossRef]
- Borgese, L.; Bilo, F.; Dalipi, R.; Bontempi, E.; Depero, L.E. Total Reflection X-Ray Fluorescence as a Tool for Food Screening. Spectrochim. Acta Part B At. Spectrosc. 2015, 113, 1–15. [Google Scholar] [CrossRef]
- Schmeling, M. X-Ray Fluorescence and Emission|Total Reflection X-Ray Fluorescence. In Encyclopedia of Analytical Science, 3rd. ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Oxford, UK, 2019; pp. 449–458. ISBN 978-0-08-101984-9. [Google Scholar]
- Bohuss, I.; Varga, A.; Barkács, K.; Oertel, N.; Záray, G. Microanalytical determination of metals in selected organs of zebra mussels by total reflection X-Ray fluorescence spectrometry. J. Trace Microprobe Tech. 2001, 19, 177–182. [Google Scholar] [CrossRef]
- Brauer, H.; Wagner, A.; Boman, J.; Binh, D.V. Use of Total-Reflection X-Ray Fluorescence in Search of a Biomonitor for Environmental Pollution in Vietnam. At. Spectrosc. 2001, 56, 2147–2155. [Google Scholar] [CrossRef]
- Marguí, E.; De Fátima Marques, A.; De Lurdes Prisal, M.; Hidalgo, M.; Queralt, I.; Carvalho, M.L. Total Reflection X-Ray Spectrometry (TXRF) for Trace Elements Assessment in Edible Clams. Appl. Spectrosc. 2014, 68, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Boman, J. Biomonitoring of Trace Elements in Vietnamese Freshwater Mussels. Spectrochim. Acta Part B At. Spectrosc. 2004, 59, 1125–1132. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Escriche, I.; Serra, J.A. Comparison of Physico-Chemical Parameters and Composition of Mussels (Mytilus galloprovincialis Lmk.) from Different Spanish Origins. Food Chem. 2009, 112, 295–302. [Google Scholar] [CrossRef]
- Yildirim, Ş.; Erdem, Ö.A.; Sabanci, F.; Gökvardar, A.; Serdar, S.; Ertan, A. Effects of Different Culture Methods on Growth, Meat Yield, and Nutritional Composition of Mediterranean Mussels (Mytilus galloprovincialis), South Side of Marmara Sea, Türkiye. Eur. Zool. J. 2024, 91, 869–889. [Google Scholar] [CrossRef]
- Beliaeff, B.; O’Connor, T.P.; Claisse, D. Comparison of Chemical Concentrations in Mussels and Oysters from the United States and France. Environ. Monit. Assess 1998, 49, 87–95. [Google Scholar] [CrossRef]
- Lyubas, A.A.; Kuznetsova, I.A.; Bovykina, G.V.; Eliseeva, T.A.; Gofarov, M.Y.; Khrebtova, I.S.; Kondakov, A.V.; Malkov, A.V.; Mavromatis, V.; Shevchenko, A.R.; et al. Trace Element Patterns in Shells of Mussels (Bivalvia) Allow to Distinguish between Fresh- and Brackish-Water Coastal Environments of the Subarctic and Boreal Zone. Water 2023, 15, 3625. [Google Scholar] [CrossRef]
- Özden, Ö.; Erkan, N.; Doğruyol, H.; Ortaboy, S.; Tunçelli, İ.C. Examination of Toxic Element Levels in Seafood from Aquaculture and Associated Risks for European Consumers. Aquaculture 2024, 585, 740688. [Google Scholar] [CrossRef]
- Rao, T.N.; Stauffer, M.T. Validation of Analytical Methods. In Calibration and Validation of Analytical Methods—A Sampling of Current Approaches; IntechOpen: London, UK, 2018; ISBN 978-1-78923-085-7. [Google Scholar]
- Shukla, S.S.; Pandey, R.K.; Gidwani, B.; Kalyani, G. General Principles of Validation. In Pharmaceutical Calibration, Validation and Qualification: A Comprehensive Approach; Shukla, S.S., Pandey, R.K., Gidwani, B., Kalyani, G., Eds.; Springer Nature: Singapore, 2023; pp. 111–119. ISBN 978-981-19900-2-1. [Google Scholar]
- Badran, M.; Morsy, R.; Elnimr, T.; Badran, M.; Soliman, H. Assessment of Wet Acid Digestion Methods for ICP-MS Determination of Trace Elements in Biological Samples by Using Multivariate Statistical Analysis. J. Elem. 2017, 23, 179–189. [Google Scholar] [CrossRef]
- Somer, G.; Ünlü, A.N. The Effect of Acid Digestion on the Recoveries of Trace Elements: Recommended Policies for the Elimination of Losses. Turk. J. Chem. 2006, 30, 10. [Google Scholar]
- Nurhaini, F.F.; Lestiani, D.D.; Syahfitri, W.Y.N.; Kusmartini, I.; Sari, D.K.; Kurniawati, S.; Santoso, M. Determination of Nutrient and Toxic Elements in Food Reference Materials by Suspension Preparation and TXRF Analysis. IFRJ 2023, 30, 463–471. [Google Scholar] [CrossRef]
- Ishak, I.; Rosli, F.D.; Mohamed, J.; Mohd Ismail, M.F. Comparison of Digestion Methods for the Determination of Trace Elements and Heavy Metals in Human Hair and Nails. Malays. J. Med. Sci. 2015, 22, 11–20. [Google Scholar] [PubMed]
- Beltrán, B.G.; Martínez-Serrano, I.; Ramos-Sanchez, V.; Chávez-Flores, D.; Nevárez-Rodríguez, M.C.; Suárez-Domínguez, E.A. Development and Validation of a New Method for Determination of Pb and Cr in Marine Organisms by Total Reflection X-Ray Fluorescence (TXRF) Spectroscopy. J. Anal. Methods Chem. 2019, 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. AOAC Guidelines for Single Laboratory. Validation of Chemical Methods for Dietary Supplements and Botanicals, Appendix F: Guidelines for Standard Method Performance Requirements. Available online: https://www.aoac.org/wp-content/uploads/2019/08/app_f.pdf (accessed on 4 October 2024).
- Lossow, K.; Schlörmann, W.; Tuchtenhagen, M.; Schwarz, M.; Schwerdtle, T.; Kipp, A.P. Measurement of Trace Elements in Murine Liver Tissue Samples: Comparison between ICP-MS/MS and TXRF. J. Trace Elem. Med. Biol. 2023, 78, 127167. [Google Scholar] [CrossRef]
- Varga, I. Iodine Determination in Dietary Supplement Products by TXRF and ICP-AES Spectrometry. Microchem. J. 2007, 85, 127–131. [Google Scholar] [CrossRef]
- Muehlbauer, F.; Fraser, D.; Brenner, M.; Van Nieuwenhove, K.; Buck, B.H.; Strand, O.; Mazurié, J.; Thorarinsdottir, G.; Dolmer, P.; O’Beirn, F.; et al. Bivalve Aquaculture Transfers in Atlantic Europe. Part A: Transfer Activities and Legal Framework. Ocean Coast. Manag. 2014, 89, 127–138. [Google Scholar] [CrossRef]
- Selected Papers on Mollusc Culture. Available online: https://www.fao.org/4/ab737e/ab737e04.htm (accessed on 4 October 2024).
- della Malva, A.; Santillo, A.; Francavilla, M.; Caroprese, M.; Marino, R.; Sevi, A.; Albenzio, M. Mussel Culture Farming Systems in the Northern Gargano Coast (Adriatic Sea): Changes in the Nutritional Profile of the Mytilus Galloprovincialis. Foods 2024, 13, 2205. [Google Scholar] [CrossRef]
- Tamburini, E.; Turolla, E.; Fano, E.A.; Castaldelli, G. Sustainability of Mussel (Mytilus galloprovincialis) Farming in the Po River Delta, Northern Italy, Based on a Life Cycle Assessment Approach. Sustainability 2020, 12, 3814. [Google Scholar] [CrossRef]
- Costas-Rodríguez, M.; Lavilla, I.; Bendicho, C. Classification of Cultivated Mussels from Galicia (Northwest Spain) with European Protected Designation of Origin Using Trace Element Fingerprint and Chemometric Analysis. Anal. Chim. Acta 2010, 664, 121–128. [Google Scholar] [CrossRef]
- FAO. Mytilus Galloprovincialis. Available online: https://www.fao.org/fishery/docs/DOCUMENT/aquaculture/CulturedSpecies/file/en/en_mediterraneanmussel.htm (accessed on 4 October 2024).
- An Overview of Mussel Culture in Chile—Responsible Seafood Advocate. Available online: https://www.globalseafood.org/advocate/overview-mussel-culture-chile/ (accessed on 7 October 2024).
- Karayücel, S.; Karayücel, I.; Erdem, M.; Saygun, S.; Uyan, O. Growth And Production In Long-Line Cultivated Mediterranean Mussel (Mytilus Galloprovincialis) In Sinop, Black Sea. Isr. J. Aquac. Bamidgeh. 2003, 55, 169–178. [Google Scholar] [CrossRef]
- Bloecher, N.; Floerl, O. Efficacy Testing of Novel Antifouling Coatings for Pen Nets in Aquaculture: How Good Are Alternatives to Traditional Copper Coatings? Aquaculture 2020, 519, 734936. [Google Scholar] [CrossRef]
- Hobbs, W.O.; McCall, M.; Lanksbury, J.; Seiders, K.; Sandvik, P.; Jones, M.; Chuhran, H.; Momohara, D.; Norton, D. A Baseline of Copper Associated with Antifouling Paint in Marinas within a Large Fjord Estuary. Mar. Pollut. Bull. 2022, 178, 113547. [Google Scholar] [CrossRef] [PubMed]
- Sievers, M.; Dempster, T.; Keough, M.J.; Fitridge, I. Methods to Prevent and Treat Biofouling in Shellfish Aquaculture. Aquaculture 2019, 505, 263–270. [Google Scholar] [CrossRef]
- Birch, G.F.; Apostolatos, C. Use of Sedimentary Metals to Predict Metal Concentrations in Black Mussel (Mytilus Galloprovincialis) Tissue and Risk to Human Health (Sydney Estuary, Australia). Environ. Sci. Pollut. Res. 2013, 20, 5481–5491. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, S.; Jovic, M.; Stankovic, A.R.; Katsikas, L. Heavy Metals in Seafood Mussels. Risks for Human Health. In Environmental Chemistry for a Sustainable World; Lichtfouse, E., Schwarzbauer, J., Robert, D., Eds.; Environmental Chemistry for a Sustainable World; Springer: Dordrecht, The Netherlands, 2012; pp. 311–373. ISBN 978-94-007-2441-9. [Google Scholar]
- Stankovic, S.; Jovic, M. Health Risks of Heavy Metals in the Mediterranean Mussels as Seafood. Environ. Chem. Lett. 2012, 10, 119–130. [Google Scholar] [CrossRef]
- Young, T.; Alfaro, A.C.; Villas-Bôas, S.G. Metabolic Profiling of Mussel Larvae: Effect of Handling and Culture Conditions. Aquacult. Int. 2016, 24, 843–856. [Google Scholar] [CrossRef]
- Jansen, H.M.; Strand, Ø.; van Broekhoven, W.; Strohmeier, T.; Verdegem, M.C.; Smaal, A.C. Feedbacks from Filter Feeders: Review on the Role of Mussels in Cycling and Storage of Nutrients in Oligo- Meso- and Eutrophic Cultivation Areas. In Goods and Services of Marine Bivalves; Smaal, A.C., Ferreira, J.G., Grant, J., Petersen, J.K., Strand, Ø., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 143–177. ISBN 978-3-319-96776-9. [Google Scholar]
- Chavan, S.D.; Desai, D.M. Analytical Method Validation: A Brief Review. World J. Adv. Res. Rev. 2022, 16, 389–402. [Google Scholar] [CrossRef]
- Shrivastava, A. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Huber, L. Validation and Qualification in Analytical Laboratories, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-19122-0. [Google Scholar]
- Manjunatha; Bennal, A.S.; Badiger, N.M.; Stosnach, H.; Kariduraganavar, M.Y. Effect of Sample Carriers on Analytical Parameters in Total Reflection X-Ray Fluorescence—A Tool for Multi-Trace Elemental Analysis. Spectrosc. Lett. 2024, 57, 45–58. [Google Scholar] [CrossRef]
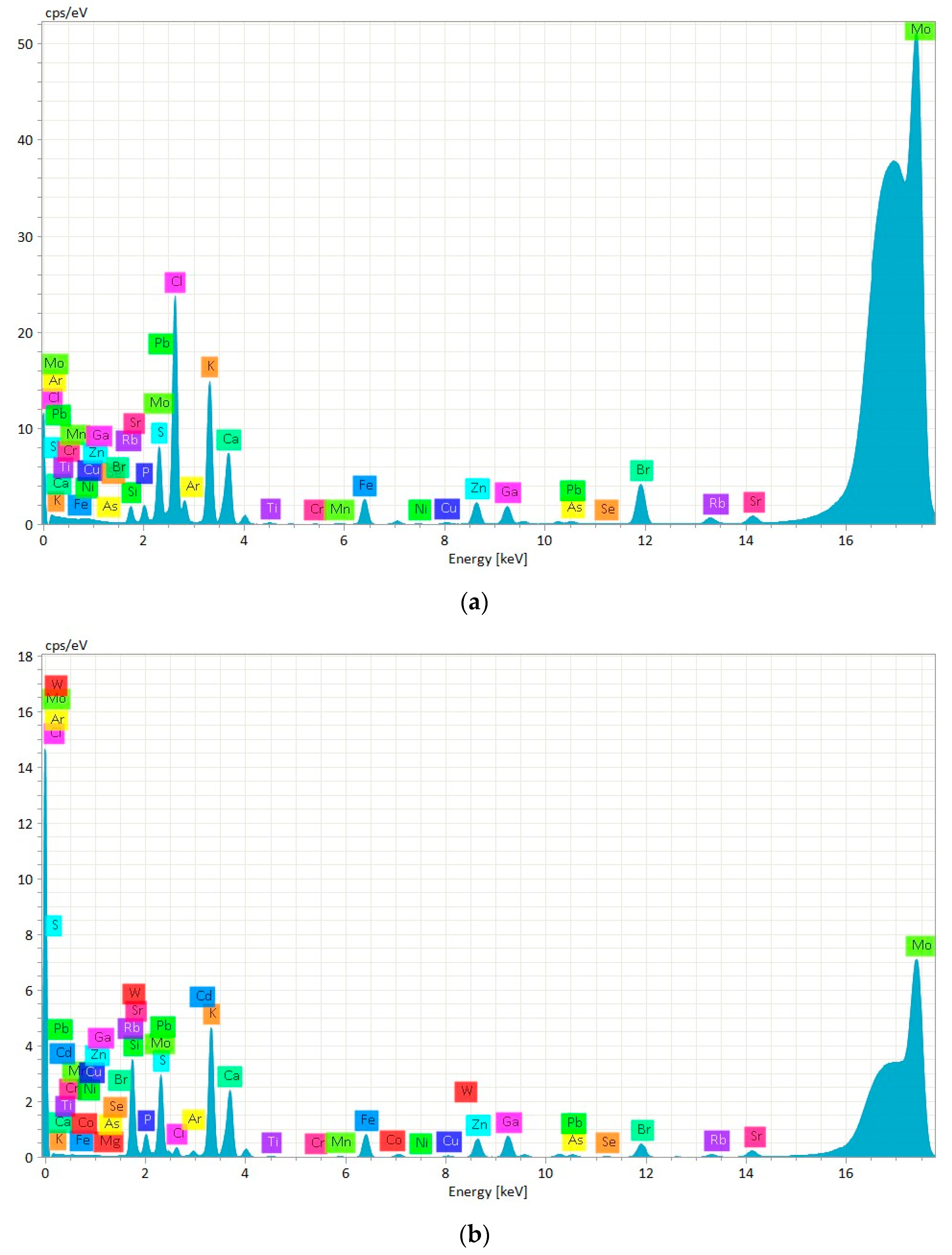
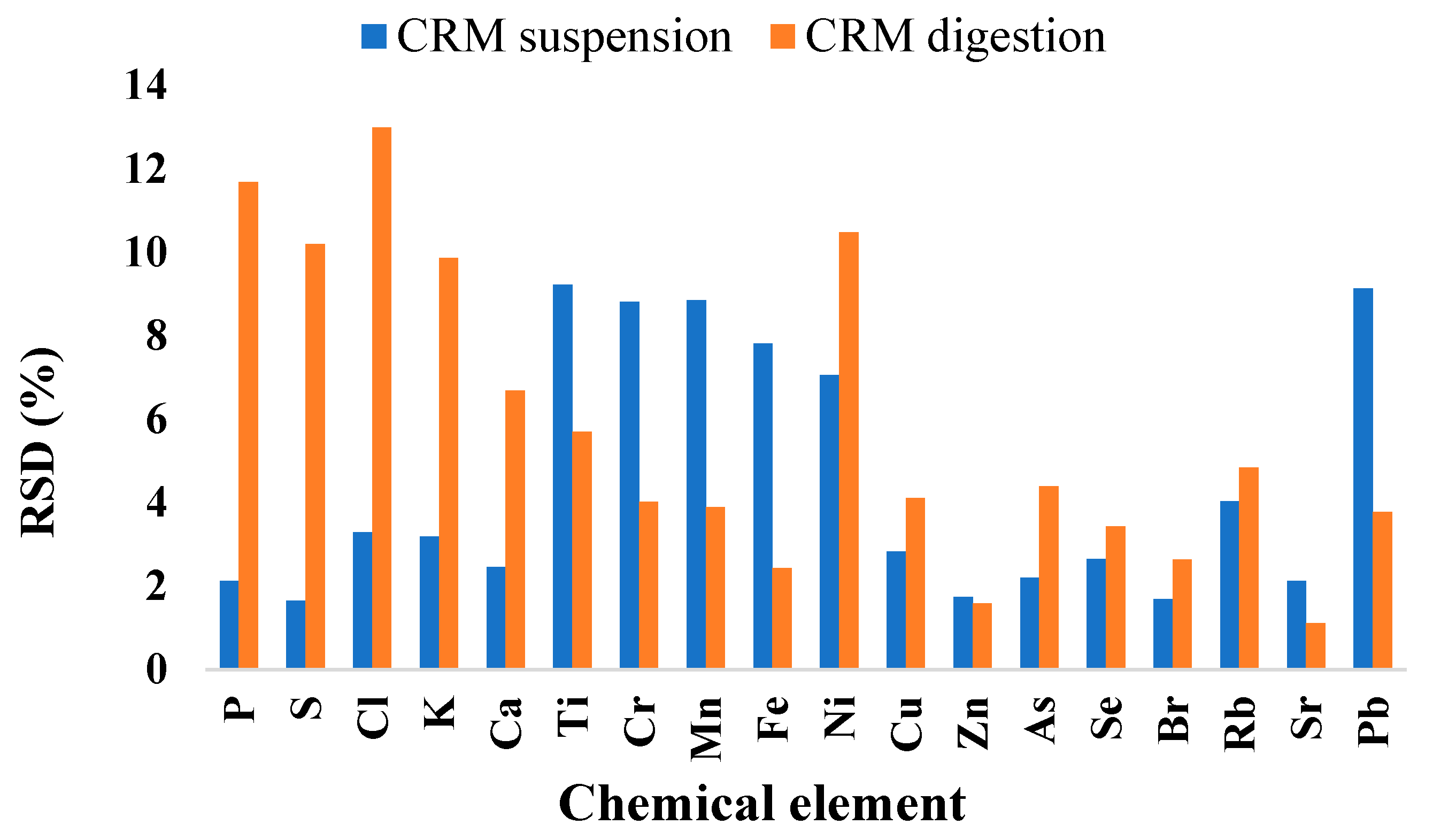
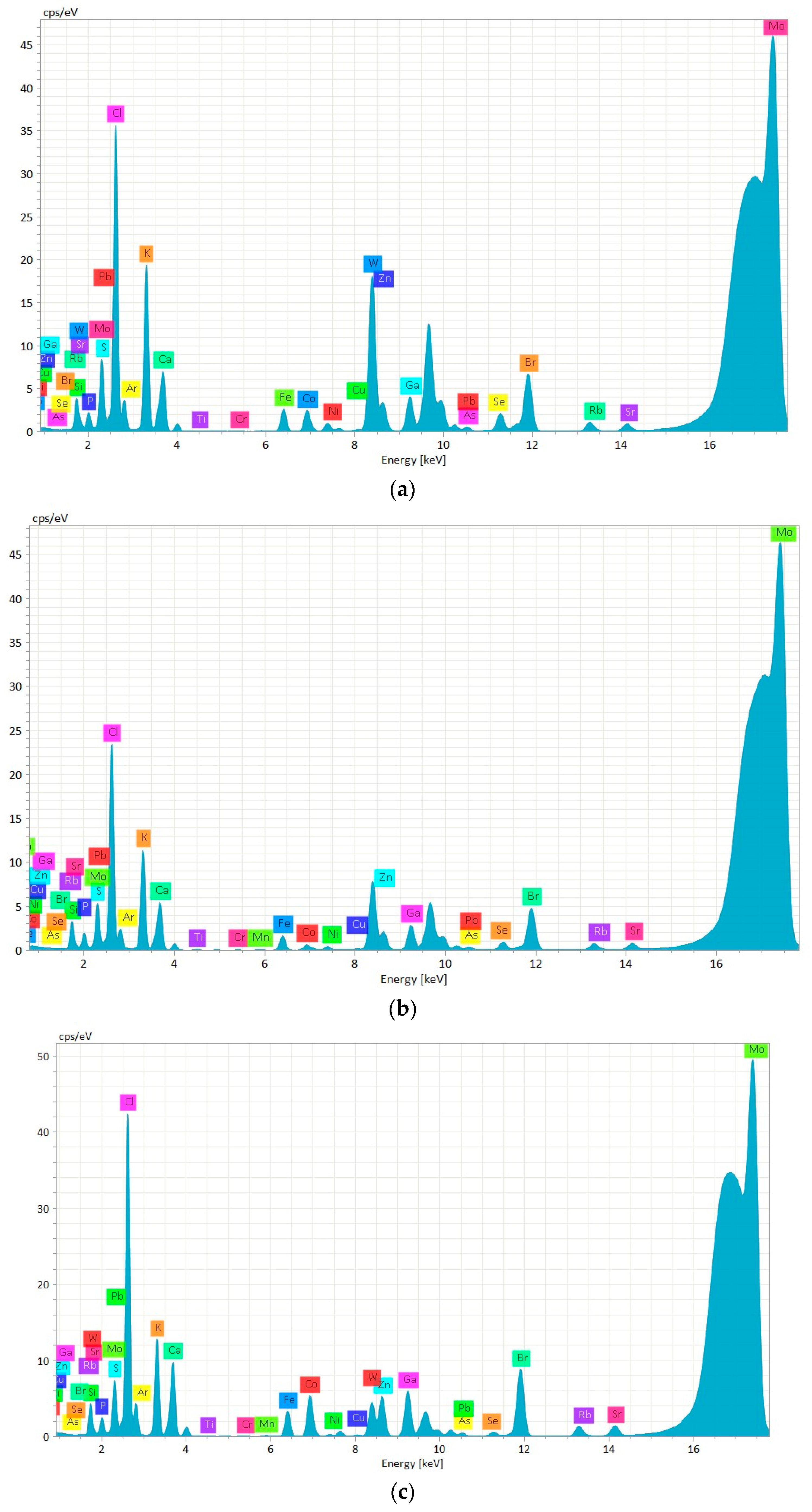
| Chemical Element | Certified Value | CRM Suspension | CRM Digested | ||||
|---|---|---|---|---|---|---|---|
| Concentration | LLD | LOQ | Concentration | LLD | LOQ | ||
| P (g/kg) | - | 1.30 ± 0.03 a | 0.003 | 0.01 | 1.16 ± 0.14 a | 0.003 | 0.01 |
| S (g/k) | - | 3.69 ± 0.06 a | 0.002 | 0.01 | 3.26 ± 0.33 b | 0.002 | 0.01 |
| Cl (g/kg) | 22.8 | 8.80 ± 0.29 a | 0.002 | 0.01 | 0.30 ± 0.04 b | 0.001 | 0.004 |
| K (g/kg) | 5.37 | 3.43 ± 0.11 a | 0.001 | 0.003 | 3.04 ± 0.30 b | 0.001 | 0.002 |
| Ca (g/kg) | 1.83 | 1.31 ± 0.03 a | 0.001 | 0.002 | 1.19 ± 0.08 b | 0.001 | 0.002 |
| Ti (mg/g) | - | 14.30 ± 1.32 a | 0.25 | 0.83 | 7.63 ± 0.43 b | 0.21 | 0.71 |
| Cr (mg/kg) | 0.73 ± 0.22 | 1.31 ± 0.12 a | 0.15 | 0.48 | 0.96 ± 0.04 b | 0.13 | 0.42 |
| Mn (mg/kg) | 4.88 ± 0.24 | 3.16 ± 0.28 a | 0.11 | 0.38 | 3.06 ± 0.12 a | 0.10 | 0.33 |
| Fe (mg/kg) | 161 ± 8 | 143.63 ± 11.22 a | 0.10 | 0.32 | 131.27 ± 3.19 b | 0.08 | 0.28 |
| Ni (mg/kg) | 0.69 ± 0.15 | 0.90 ± 0.06 b | 0.07 | 0.23 | 1.08 ± 0.11 a | 0.06 | 0.19 |
| Cu (mg/kg) | 5.98 ± 0.27 | 6.04 ± 0.17 a | 0.06 | 0.20 | 5.08 ± 0.21 b | 0.05 | 0.17 |
| Zn (mg/kg) | 71 ± 4 | 67.38 ± 1.17 a | 0.06 | 0.19 | 60.62 ± 0.96 b | 0.05 | 0.15 |
| As (mg/kg) | 6.7 ± 0.4 | 6.35 ± 0.14 a | 0.05 | 0.15 | 5.20 ± 0.23 b | 0.04 | 0.12 |
| Se (mg/kg) | 1.62 ± 0.12 | 1.01 ± 0.03 a | 0.05 | 0.15 | 1.04 ± 0.04 a | 0.04 | 0.12 |
| Br (mg/kg) | - | 95.06 ± 1.60 a | 0.04 | 0.14 | 25.70 ± 0.68 b | 0.03 | 0.11 |
| Rb (mg/kg) | 2.46 ± 0.16 | 1.80 ± 0.07 a | 0.05 | 0.17 | 1.59 ± 0.08 b | 0.04 | 0.13 |
| Sr (mg/kg) | 19 ± 1.2 | 16.08 ± 0.34 a | 0.06 | 0.19 | 13.79 ± 0.15 b | 0.03 | 0.10 |
| Pb (mg/kg) | 2.18 ± 0.18 | 0.88 ± 0.08 b | 0.05 | 0.16 | 1.60 ± 0.06 a | 0.04 | 0.13 |
| Cd (mg/kg) | 0.33 ± 0.02 | ND | - | - | ND | - | - |
| Hg (mg/kg) | 0.07 ± 0.00 | ND | - | - | ND | - | - |
| Co (mg/kg) | 0.21 | ND | - | - | ND | - | - |
| Mg (g/kg) | 1.51 | ND | - | - | ND | - | - |
| Na (g/kg) | 13.9 | ND | - | - | ND | - | - |
| Chemical Element | Recovery Rates (R%) | |
|---|---|---|
| CRM Suspension | CRM Digested | |
| Cl (g/kg) | 38.60 ± 1.27 a | 1.32 ± 0.17 b |
| K (g/kg) | 63.96 ± 2.04 a | 56.52 ± 5.58 b |
| Ca (g/kg) | 71.64 ± 1.76 a | 65.04 ± 4.35 b |
| Cr (mg/kg) | 179.59 ± 15.82 a | 131.68 ± 5.29 b |
| Mn (mg/kg) | 64.70 ± 5.73 a | 62.75 ± 2.44 a |
| Fe (mg/kg) | 89.21 ± 6.97 a | 81.53 ± 1.98 b |
| Ni (mg/kg) | 130.07 ± 9.18 b | 152.06 ± 6.98 a |
| Cu (mg/kg) | 100.95 ± 2.85 a | 84.96 ± 3.49 b |
| Zn (mg/kg) | 94.91 ± 1.65 a | 85.38 ± 1.36 b |
| As (mg/kg) | 94.83 ± 2.09 a | 77.61 ± 3.41 b |
| Se (mg/kg) | 62.32 ± 1.65 a | 64.14 ± 2.20 a |
| Rb (mg/kg) | 73.24 ± 2.95 a | 64.63 ± 3.13 b |
| Sr (mg/kg) | 84.61 ± 1.79 a | 72.60 ± 0.81 b |
| Pb (mg/kg) | 40.52 ± 3.70 b | 73.22 ± 2.77 a |
| Sample | Fe (g/kg DW) | Cu (mg/kg DW) | Zn (g/kg DW) | As (mg/kg DW) | Sr (mg/kg DW) |
|---|---|---|---|---|---|
| M.G.I.1 | 1.00 ± 0.07 c | 19.52 ± 0.30 c | 0.59 ± 0.03 e | 69.26 ± 0.81 b | 120.00 ± 2.13 e |
| M.G.I.2 | 0.71 ± 0.07 d | 7.84 ± 0.14 d | 0.99 ± 0.00 c | 66.80 ± 3.97 b | 106.33 ± 0.46 f |
| M.G.I.3 | 1.45 ± 0.06 b | 19.93 ± 0.25 c | 2.31 ± 0.02 a | 87.42 ± 1.12 a | 238.63 ± 4.12 a |
| M.C.C.1 | 0.46 ± 0.02 e | 21.13 ± 0.58 c | 0.47 ± 0.02 ef | 41.64 ± 0.16 c | 79.08 ± 2.34 h |
| M.C.C.2 | 0.46 ± 0.01 e | 22.34 ± 0.99 c | 0.41 ± 0.02 f | 28.46 ± 0.53 e | 75.06 ± 2.32 h |
| M.C.C.3 | 0.79 ± 0.04 d | 34.03 ± 0.49 a | 0.55 ± 0.00 ef | 32.60 ± 0.23 de | 91.39 ± 2.16 g |
| M.G.S.1 | 1.03 ± 0.03 c | 28.81 ± 2.11 b | 0.77 ± 0.03 d | 46.52 ± 2.18 c | 141.34 ± 8.38 d |
| M.G.S.2 | 1.59 ± 0.06 b | 27.72 ± 0.52 b | 0.91 ± 0.02 cd | 37.56 ± 2.15 cd | 173.88 ± 5.04 b |
| M.G.S.3 | 1.99 ± 0.08 a | 28.64 ± 1.68 b | 1.28 ± 0.09 b | 27.42 ± 1.62 e | 153.59 ± 1.20 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazăr, N.-N.; Simionov, I.-A.; Călmuc, M.; Călmuc, V.-A.; Iticescu, C.; Georgescu, P.-L.; Timofti, M.; Drăgan, S. Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF). Molecules 2025, 30, 283. https://doi.org/10.3390/molecules30020283
Lazăr N-N, Simionov I-A, Călmuc M, Călmuc V-A, Iticescu C, Georgescu P-L, Timofti M, Drăgan S. Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF). Molecules. 2025; 30(2):283. https://doi.org/10.3390/molecules30020283
Chicago/Turabian StyleLazăr, Nina-Nicoleta, Ira-Adeline Simionov, Mădălina Călmuc, Valentina-Andreea Călmuc, Cătălina Iticescu, Puiu-Lucian Georgescu, Mihaela Timofti, and Silvia Drăgan. 2025. "Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF)" Molecules 30, no. 2: 283. https://doi.org/10.3390/molecules30020283
APA StyleLazăr, N.-N., Simionov, I.-A., Călmuc, M., Călmuc, V.-A., Iticescu, C., Georgescu, P.-L., Timofti, M., & Drăgan, S. (2025). Cross-Regional Elemental Comparison of Mussels Using Total Reflection X-Ray Fluorescence (TXRF). Molecules, 30(2), 283. https://doi.org/10.3390/molecules30020283








