Metal-Free Synthesis of α-H Chlorine Alkylaromatic Hydrocarbons Driven by Visible Light
Abstract
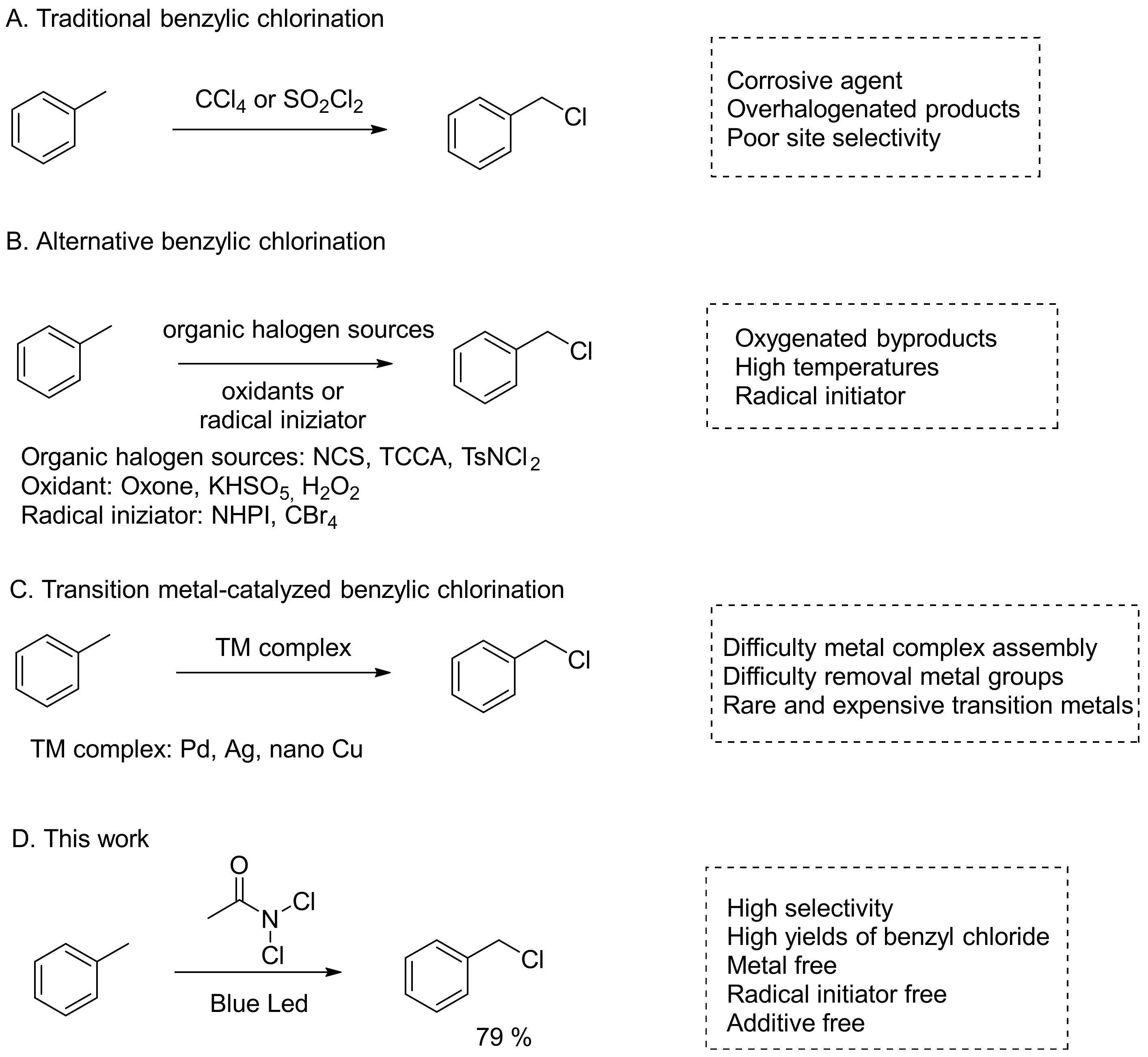
1. Introduction
2. Results
2.1. Optimization of Reaction Conditions
2.2. Scale-Up of Our Methodology
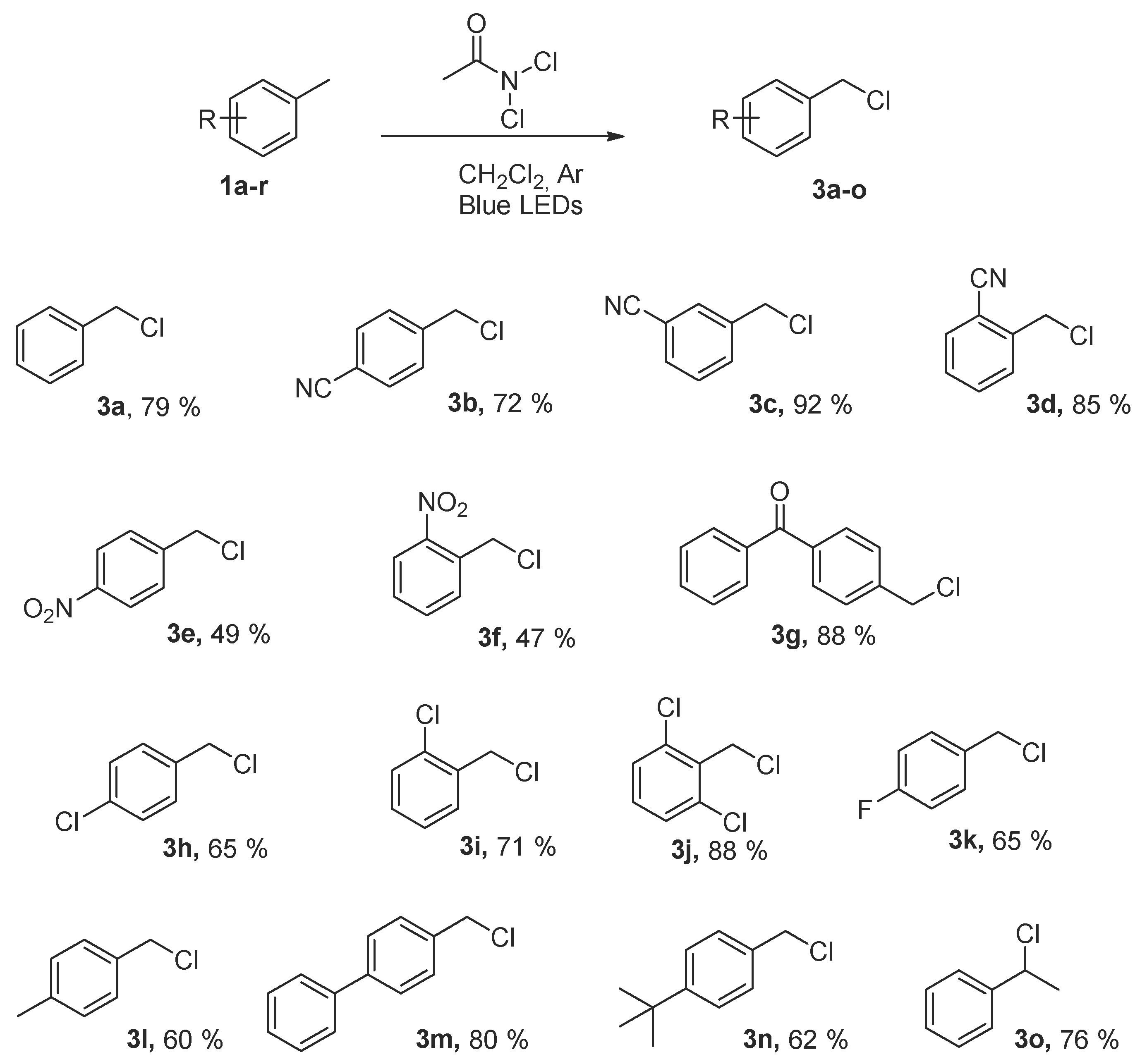
2.3. Scope of the Reaction
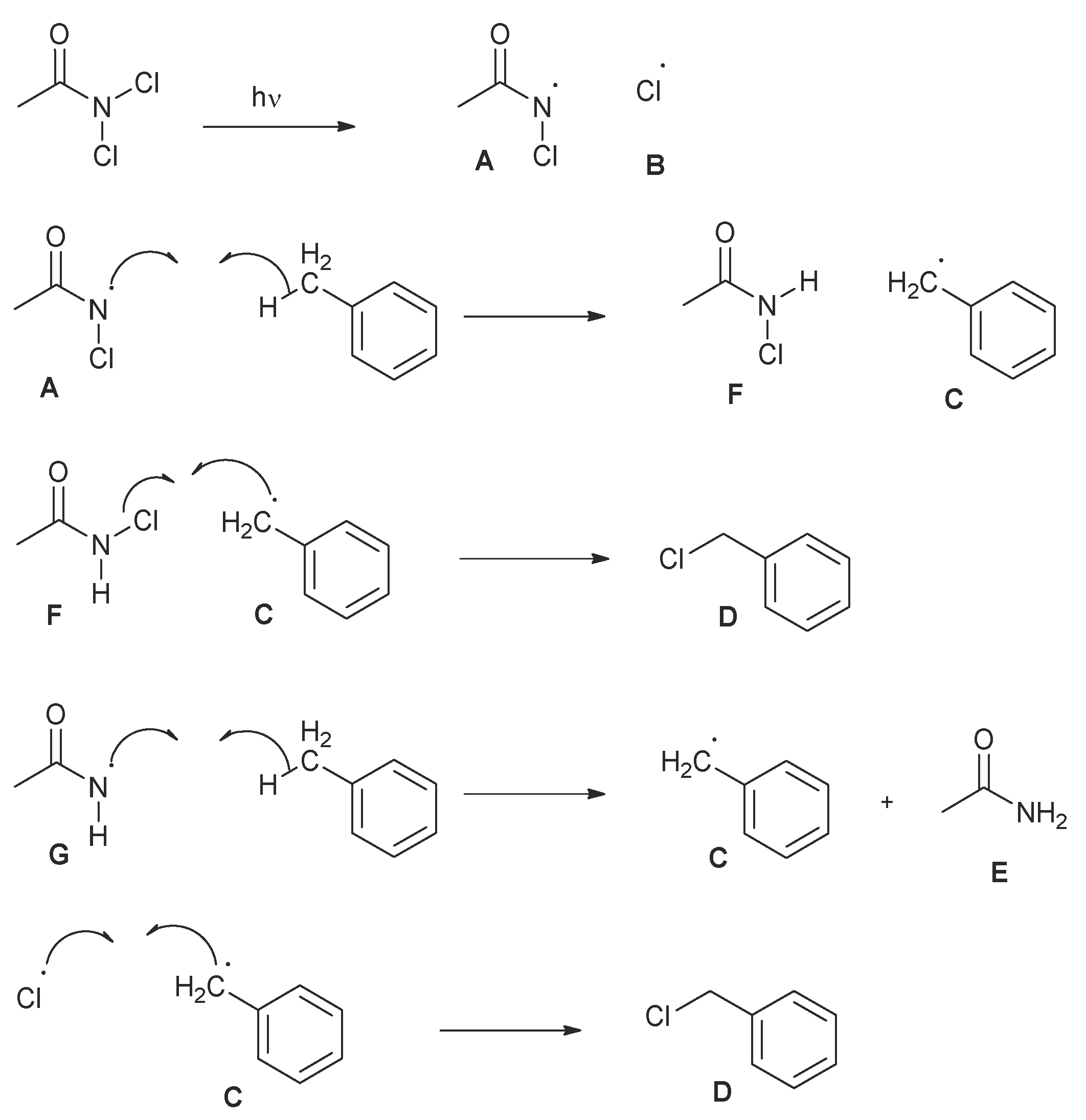
2.4. Proposed Mechanism
3. Materials and Methods
3.1. General Information
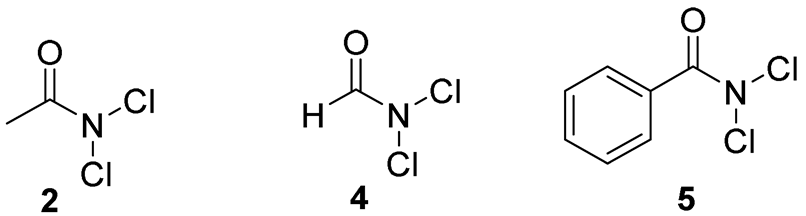
3.2. General Procedure for N,N-Dichloroamides 2, 4, 5
3.3. General Procedure for Compounds 3a–3o
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gribble, G.W. Natural Organohalogens: A New Frontier for Medicinal Agents? J. Chem. Educ. 2004, 81, 1441. [Google Scholar] [CrossRef]
- Lin, R.; Amrute, A.P.; Pérez-Ramírez, J. Halogen-Mediated Conversion of Hydrocarbons to Commodities. Chem. Rev. 2017, 117, 4182–4247. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Kambe, N. Cross-Coupling Reaction of Alkyl Halides with Grignard Reagents Catalyzed by Ni, Pd, or Cu Complexes with π-Carbon Ligand(s). Acc. Chem. Res. 2008, 41, 1545–1554. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern Transition-Metal-Catalyzed Carbon–Halogen Bond Formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- Podgoršek, A.; Zupan, M.; Iskra, J. Oxidative Halogenation with “Green” Oxidants: Oxygen and Hydrogen Peroxide. Angew. Chem. Int. Ed. 2009, 48, 8424–8450. [Google Scholar] [CrossRef]
- Rossberg, M.; Lendle, W.; Pfleiderer, G.; Tögel, A.; Dreher, E.-L.; Langer, E.; Rassaerts, H.; Kleinschmidt, P.; Strack, H.; Cook, R.; et al. Chlorinated Hydrocarbons. In Ullmann’s Encyclopedia of Industrial Chemistry; United States Environmental Protection Agency: Washington, DC, USA, 2006. [Google Scholar]
- Zhang, L.; Hu, X. Room temperature C(sp2)–H oxidative chlorination via photoredox catalysis. Chem. Sci. 2017, 8, 7009–7013. [Google Scholar] [CrossRef]
- Ozawa, J.; Kanai, M. Silver-Catalyzed C(sp3)–H Chlorination. Org. Lett. 2017, 19, 1430–1433. [Google Scholar] [CrossRef]
- Furniss, B.S.H.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textbook of Practical Organic Chemistry, 5th ed.; Longman: Harlow, UK, 1989; p. 864. [Google Scholar]
- Whitmore, F.C.; Ginsburg, A.; Rueggeberg, W.; Tharp, I.; Nottorf, H.; Cannon, M.; Carnahan, F.; Cryder, D.; FLeming, G.; Goldberg, G.; et al. Production of Benzyl Chloride by Chloromethylation of Benzene. Laboratory and Pilot Plant Studies. Ind. Eng. Chem. 1946, 38, 478–485. [Google Scholar] [CrossRef]
- Lei, A.S.W.; Liu, C.; Liu, W.; Zhang, H.; He, C. Oxidative Cross-Coupling Reactions; Wiley-VCH: Hoboken, NJ, USA, 2016. [Google Scholar]
- Delaude, L.; Laszlo, P. Versatility of zeolites as catalysts for ring or side-chain aromatic chlorinations by sulfuryl chloride. J. Org. Chem. 1990, 55, 5260–5269. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Horaguchi, T. Chlorination of aliphatic hydrocarbons, aromatic compounds, and olefins in subcritical carbon tetrachloride. Tetrahedron Lett. 2008, 49, 6419–6422. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Brown, H.C. Chlorinations with Sulfuryl Chloride. I. The Peroxide-Catalyzed Chlorination of Hydrocarbons. J. Am. Chem. Soc. 1939, 61, 2142–2150. [Google Scholar] [CrossRef]
- Xiang, M.; Zhou, C.; Yang, X.-L.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Visible Light-Catalyzed Benzylic C–H Bond Chlorination by a Combination of Organic Dye (Acr+-Mes) and N-Chlorosuccinimide. J. Org. Chem. 2020, 85, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Combe, S.H.; Hosseini, A.; Parra, A.; Schreiner, P.R. Mild Aliphatic and Benzylic Hydrocarbon C–H Bond Chlorination Using Trichloroisocyanuric Acid. J. Org. Chem. 2017, 82, 2407–2413. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhao, Y.; Kyne, S.H.; Farshadfar, K.; Ariafard, A.; Chan, P.W.H. Copper(I)-catalysed site-selective C(sp3)–H bond chlorination of ketones, (E)-enones and alkylbenzenes by dichloramine-T. Nat. Commun. 2021, 12, 4065. [Google Scholar] [CrossRef] [PubMed]
- Thirumamagal, B.T.S.; Narayanasamy, S.; Venkatesan, R. Regiospecific Chlorination of Xylenes Using K-10 Montmorrillonite Clay. Synth. Commun. 2008, 38, 2820–2825. [Google Scholar] [CrossRef]
- Bucos, M.; Villalonga-Barber, C.; Micha-Screttas, M.; Steele, B.R.; Screttas, C.G.; Heropoulos, G.A. Microwave assisted solid additive effects in simple dry chlorination reactions with n-chlorosuccinimide. Tetrahedron 2010, 66, 2061–2065. [Google Scholar] [CrossRef]
- Gaspa, S.; Valentoni, A.; Mulas, G.; Porcheddu, A.; De Luca, L. Metal-Free Preparation of α-H-Chlorinated Alkylaromatic Hydrocarbons by Sunlight. ChemistrySelect 2018, 3, 7991–7995. [Google Scholar] [CrossRef]
- Li, Z.-H.; Fiser, B.; Jiang, B.-L.; Li, J.-W.; Xu, B.-H.; Zhang, S.-J. N-Hydroxyphthalimide/benzoquinone-catalyzed chlorination of hydrocarbon C–H bond usingN-chlorosuccinimide. Org. Biomol. Chem. 2019, 17, 3403–3408. [Google Scholar] [CrossRef]
- Nikitas, N.F.; Apostolopoulou, M.K.; Skolia, E.; Tsoukaki, A.; Kokotos, C.G. Photochemical Activation of Aromatic Aldehydes: Synthesis of Amides, Hydroxamic Acids and Esters. Chem.–A Eur. J. 2021, 27, 7915–7922. [Google Scholar] [CrossRef]
- Çimen, Y.; Akyüz, S.; Türk, H. Facile, efficient, and environmentally friendly α- and aromatic regioselective chlorination of toluene using KHSO5 and KCl under catalyst-free conditions. N. J. Chem. 2015, 39, 3894–3899. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Tian, X.; Fan, S.; Huang, J.; Whiting, A. Highly selective halogenation of unactivated C(sp3)–H with NaX under co-catalysis of visible light and Ag@AgX. Green Chem. 2018, 20, 4729–4737. [Google Scholar] [CrossRef]
- Lu, W.; Zhao, M.; Li, M. Visible-Light-Driven Oxidative Mono- and Dibromination of Benzylic sp3 C–H Bonds with Potassium Bromide/Oxone at Room Temperature. Synthesis 2018, 50, 4933–4939. [Google Scholar] [CrossRef]
- Zhao, M.; Lu, W. Visible Light-Induced Oxidative Chlorination of Alkyl sp3 C–H Bonds with NaCl/Oxone at Room Temperature. Org. Lett. 2017, 19, 4560–4563. [Google Scholar] [CrossRef]
- Stephenson, C.; Yoon, T.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH Verlag: Weinheim, Germany, 2017. [Google Scholar]
- Das, R.; Kapur, M. Transition-Metal-Catalyzed Site-Selective C−H Halogenation Reactions. Asian J. Org. Chem. 2018, 7, 1524–1541. [Google Scholar] [CrossRef]
- Abderrazak, Y.; Bhattacharyya, A.; Reiser, O. Visible-Light-Induced Homolysis of Earth-Abundant Metal-Substrate Complexes: A Complementary Activation Strategy in Photoredox Catalysis. Angew. Chem. Int. Ed. 2021, 60, 21100–21115. [Google Scholar] [CrossRef]
- Paik, A.; Paul, S.; Bhowmik, S.; Das, R.; Naveen, T.; Rana, S. Recent Advances in First-Row Transition-Metal-Mediated C−H Halogenation of (Hetero)arenes and Alkanes. Asian J. Org. Chem. 2022, 11, e202200060. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Racowski, J.M.; Ball, N.D.; Sanford, M.S. C–H Bond Activation at Palladium(IV) Centers. J. Am. Chem. Soc. 2011, 133, 18022–18025. [Google Scholar] [CrossRef]
- Chen, C.; Tong, X. Synthesis of organic halides via palladium(0) catalysis. Org. Chem. Front. 2014, 1, 439–446. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Tian, X.; Ni, S.-F.; Li, S.; Zhang, Z.-H.; Li, D.; Liu, S. Visible light-induced FeCl3-catalyzed chlorination of C–H bonds with MgCl2. Green Chem. 2024, 26, 6559–6569. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, H.; Yang, Y.; Tian, X.; Whiting, A. Corrigendum: A Visible-Light-Induced α-H Chlorination of Alkylarenes with Inorganic Chloride under NanoAg@AgCl. Chem.—A Eur. J. 2015, 21, 10932. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.A.; Buss, J.A.; Stahl, S.S. Cu-Catalyzed Site-Selective Benzylic Chlorination Enabling Net C–H Coupling with Oxidatively Sensitive Nucleophiles. Org. Lett. 2021, 24, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, S.; Tian, X.; Liu, Y.; Fan, S.; Huang, B.; Whiting, A. Cu@CuCl-visible light co-catalysed chlorination of C(sp3)–H bonds with MCln solution and photocatalytic serial reactor-based synthesis of benzyl chloride. Green Chem. 2022, 24, 384–393. [Google Scholar] [CrossRef]
- Zimmer, H.; Audrieth, L.F. Tertiary Butyl Hypochlorite as N-Chlorinating Agent. J. Am. Chem. Soc. 1954, 76, 3856–3857. [Google Scholar] [CrossRef]
- Gómez, J.E.; Guo, W.; Gaspa, S.; Kleij, A.W. Copper-Catalyzed Synthesis of γ-Amino Acids Featuring Quaternary Stereocenters. Angew. Chem. Int. Ed. 2017, 56, 15035–15038. [Google Scholar] [CrossRef]
- Dellisanti, A.; Chessa, E.; Porcheddu, A.; Carraro, M.; Pisano, L.; De Luca, L.; Gaspa, S. Visible Light-Promoted Oxidative Cross-Coupling of Alcohols to Esters. Molecules 2024, 29, 570. [Google Scholar] [CrossRef]
- Gaspa, S.; Raposo, I.; Pereira, L.; Mulas, G.; Ricci, P.C.; Porcheddu, A.; De Luca, L. Visible light-induced transformation of aldehydes to esters, carboxylic anhydrides and amides. N. J. Chem. 2019, 43, 10711–10715. [Google Scholar] [CrossRef]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Visible light induced ‘on water’ benzylic bromination with N-bromosuccinimide. Tetrahedron Lett. 2006, 47, 1097–1099. [Google Scholar] [CrossRef]
- Venkatachalapathy, C.; Pitchumani, K. Selectivity in bromination of alkylbenzenes in the presence of montmorillonite clay. Tetrahedron 1997, 53, 2581–2584. [Google Scholar] [CrossRef]
- Zhang, H.; Muñiz, K. Selective Piperidine Synthesis Exploiting Iodine-Catalyzed Csp3–H Amination under Visible Light. ACS Catal. 2017, 7, 4122–4125. [Google Scholar] [CrossRef]
- Saikia, I.; Borah, A.J.; Phukan, P. Use of Bromine and Bromo-Organic Compounds in Organic Synthesis. Chem. Rev. 2016, 116, 6837–7042. [Google Scholar] [CrossRef] [PubMed]
- Vaillard, S.E.; Schulte, B.; Studer, A. Radical-Based Arylation Methods; Wiley: Hoboken, NJ, USA, 2009; pp. 475–511. ISBN 9783527627325. [Google Scholar]
- Guru, M.M.; Shima, T.; Hou, Z. Conversion of Dinitrogen to Nitriles at a Multinuclear Titanium Framework. Angew. Chem. Int. Ed. 2016, 55, 12316–12320. [Google Scholar] [CrossRef] [PubMed]
- Huy, P.H.; Motsch, S.; Kappler, S.M. Formamides as Lewis Base Catalysts in SN Reactions—Efficient Transformation of Alcohols into Chlorides, Amines, and Ethers. Angew. Chem. Int. Ed. 2016, 55, 10145–10149. [Google Scholar] [CrossRef]
- Ding, R.; He, Y.; Wang, X.; Xu, J.; Chen, Y.; Feng, M.; Qi, C. Treatment of Alcohols with Tosyl Chloride Does Not always Lead to the Formation of Tosylates. Molecules 2011, 16, 5665–5673. [Google Scholar] [CrossRef]
- Ogawa, D.; Hyodo, K.; Suetsugu, M.; Li, J.; Inoue, Y.; Fujisawa, M.; Iwasaki, M.; Takagi, K.; Nishihara, Y. Palladium-catalyzed and copper-mediated cross-coupling reaction of aryl- or alkenylboronic acids with acid chlorides under neutral conditions: Efficient synthetic methods for diaryl ketones and chalcones at room temperature. Tetrahedron 2013, 69, 2565–2571. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Yin, F.; Su, Z.; Li, Z.; Li, C. Silver-Catalyzed Decarboxylative Chlorination of Aliphatic Carboxylic Acids. J. Am. Chem. Soc. 2012, 134, 4258–4263. [Google Scholar] [CrossRef]
- Heijnen, D.; Tosi, F.; Vila, C.; Stuart, M.C.A.; Elsinga, P.H.; Szymanski, W.; Feringa, B.L. Oxygen Activated, Palladium Nanoparticle Catalyzed, Ultrafast Cross-Coupling of Organolithium Reagents. Angew. Chem. Int. Ed. 2017, 56, 3354–3359. [Google Scholar] [CrossRef]

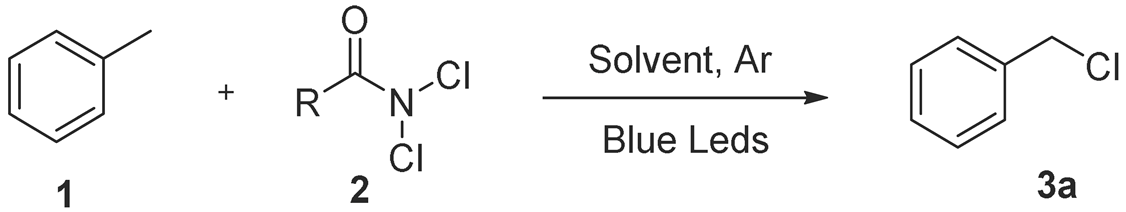 | |||||
|---|---|---|---|---|---|
| Entry 1 | Toluene (mmol) | Cl Source (mmol) | Solvent | Time (h) | Yield 2,5 (%) |
| 1 | 2 | (2) 1 | CH2Cl2 | 8 | 64% |
| 2 | 2 | (2) 1.2 | CH2Cl2 | 8 | 70% |
| 3 | 2 | (2) 1.3 | CH2Cl2 | 8 | 79% |
| 4 | 2 | (2) 2 | CH2Cl2 | 8 | 70% |
| 5 | 2 | (2) 1.3 | CH2Cl2 | 0.5 | 36% |
| 6 | 2 | (2) 1.3 | CH2Cl2 | 1 | 44% |
| 7 | 2 | (2) 1.3 | CH2Cl2 | 2 | 47% |
| 8 | 2 | (2) 1.3 | CH2Cl2 | 3 | 57% |
| 9 | 2 | (2) 1.3 | CH2Cl2 | 5 | 65% |
| 10 | 2 | (2) 1.3 | CH2Cl2 | 12 | 66% |
| 11 | 2 | (2) 1.3 | CPME | 8 | - |
| 12 | 2 | (2) 1.3 | CH3CN | 8 | - |
| 13 | 2 | (2) 1.3 | AcOEt | 8 | trace |
| 14 | 2 | (2) 1.3 | 2-MeTHF | 8 | - |
| 15 | 2 | (2) 1.3 | THF | 8 | - |
| 16 | 2 | (2) 1.3 | DCE | 8 | - |
| 17 3 | 2 | (2) 1.3 | CH2Cl2 | 8 | - |
| 18 4 | 2 | (2) 1.3 | CH2Cl2 | 8 | - |
| 19 | 2 | (4) 1.3 | CH2Cl2 | 8 | 47% |
| 20 | 2 | (5) 1.3 | CH2Cl2 | 8 | 60% |
 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, L.; Ledda, L.; Porcheddu, A.; Carraro, M.; Pisano, L.; Gaspa, S. Metal-Free Synthesis of α-H Chlorine Alkylaromatic Hydrocarbons Driven by Visible Light. Molecules 2025, 30, 312. https://doi.org/10.3390/molecules30020312
De Luca L, Ledda L, Porcheddu A, Carraro M, Pisano L, Gaspa S. Metal-Free Synthesis of α-H Chlorine Alkylaromatic Hydrocarbons Driven by Visible Light. Molecules. 2025; 30(2):312. https://doi.org/10.3390/molecules30020312
Chicago/Turabian StyleDe Luca, Lidia, Luca Ledda, Andrea Porcheddu, Massimo Carraro, Luisa Pisano, and Silvia Gaspa. 2025. "Metal-Free Synthesis of α-H Chlorine Alkylaromatic Hydrocarbons Driven by Visible Light" Molecules 30, no. 2: 312. https://doi.org/10.3390/molecules30020312
APA StyleDe Luca, L., Ledda, L., Porcheddu, A., Carraro, M., Pisano, L., & Gaspa, S. (2025). Metal-Free Synthesis of α-H Chlorine Alkylaromatic Hydrocarbons Driven by Visible Light. Molecules, 30(2), 312. https://doi.org/10.3390/molecules30020312








