«Green-Ligand» in Metallodrugs Design—Cu(II) Complex with Phytic Acid: Synthetic Approach, EPR-Spectroscopy, and Antimycobacterial Activity
Abstract
1. Introduction
2. Results and Discussion
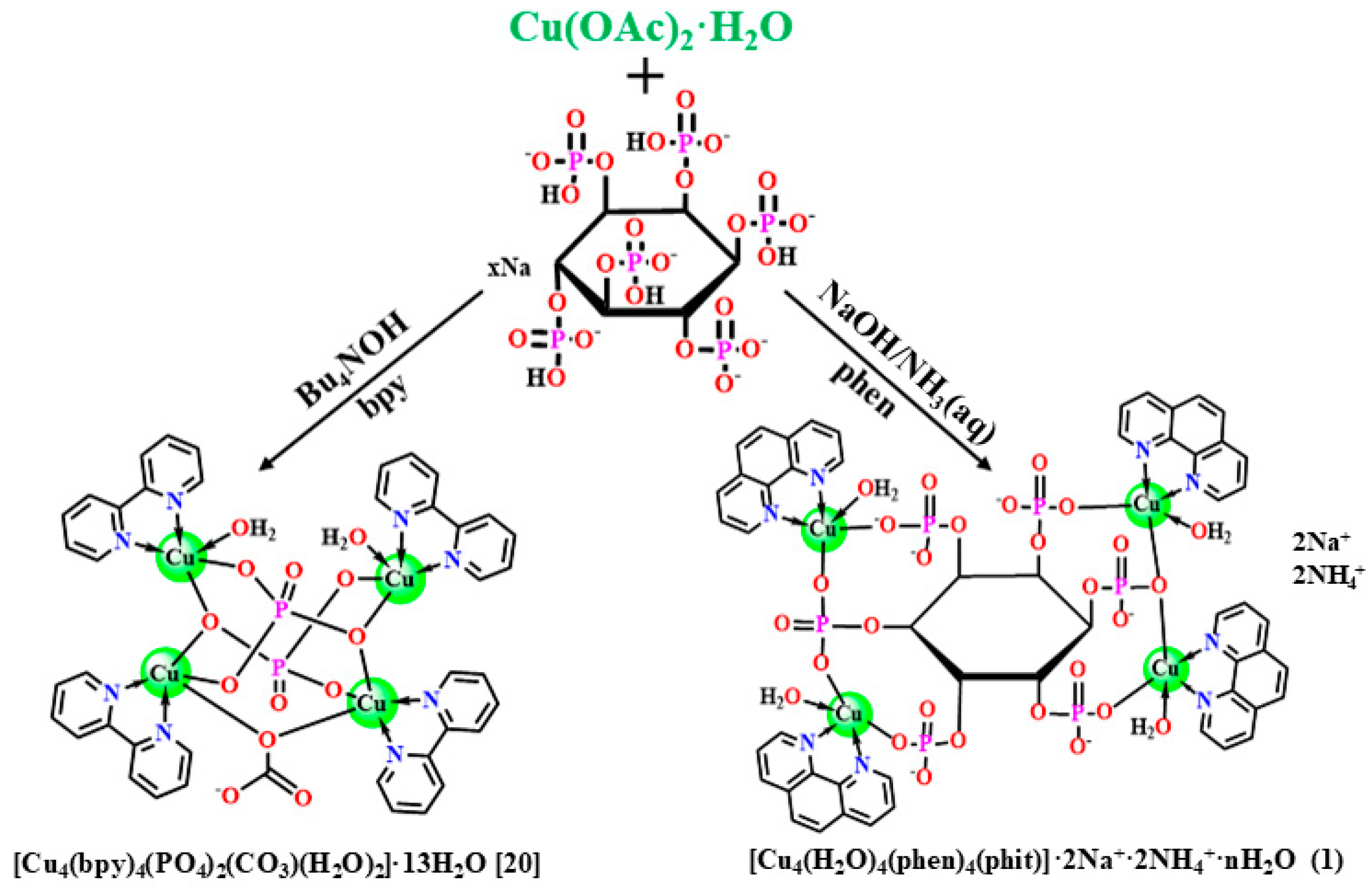
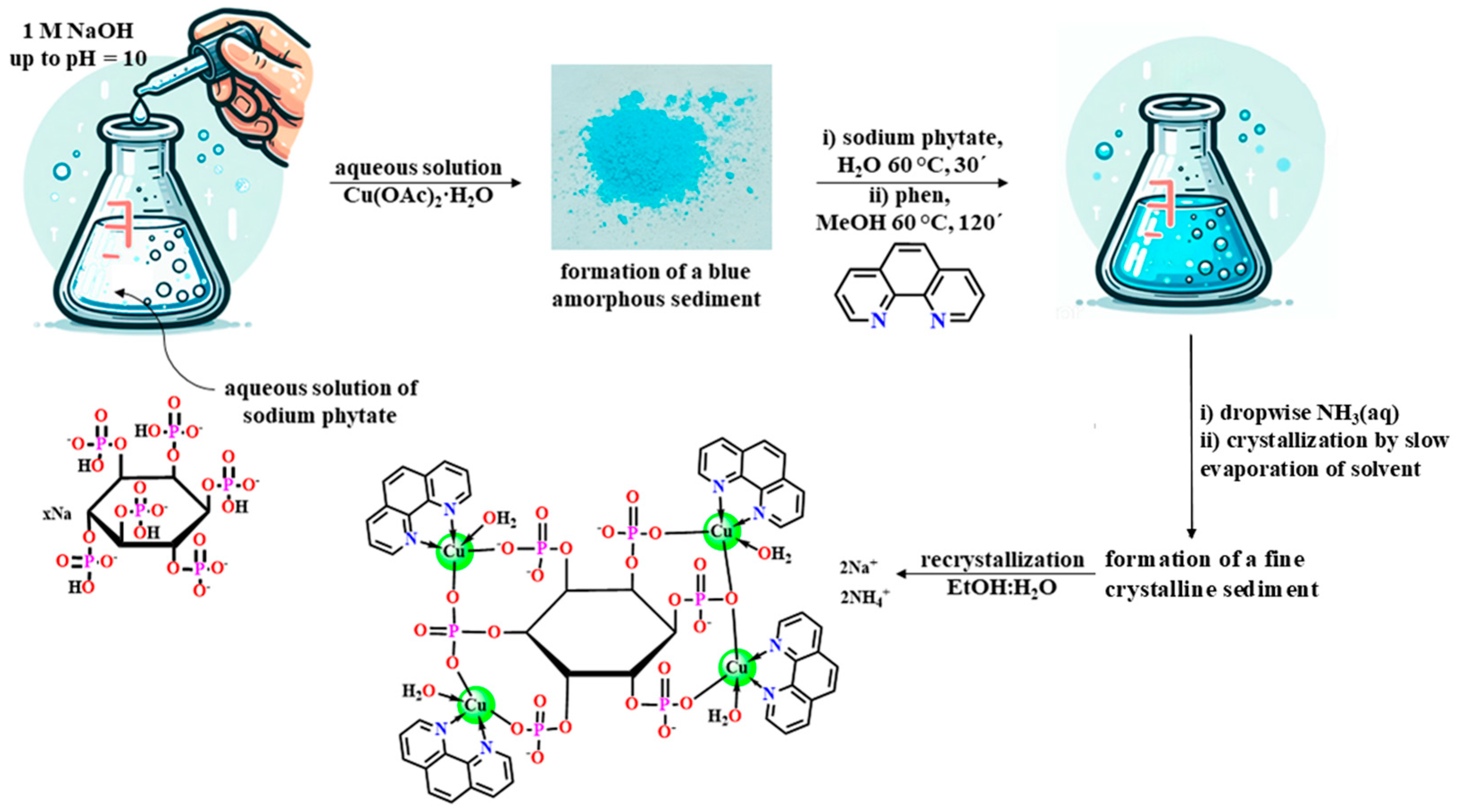
2.1. Synthesis and Characterization
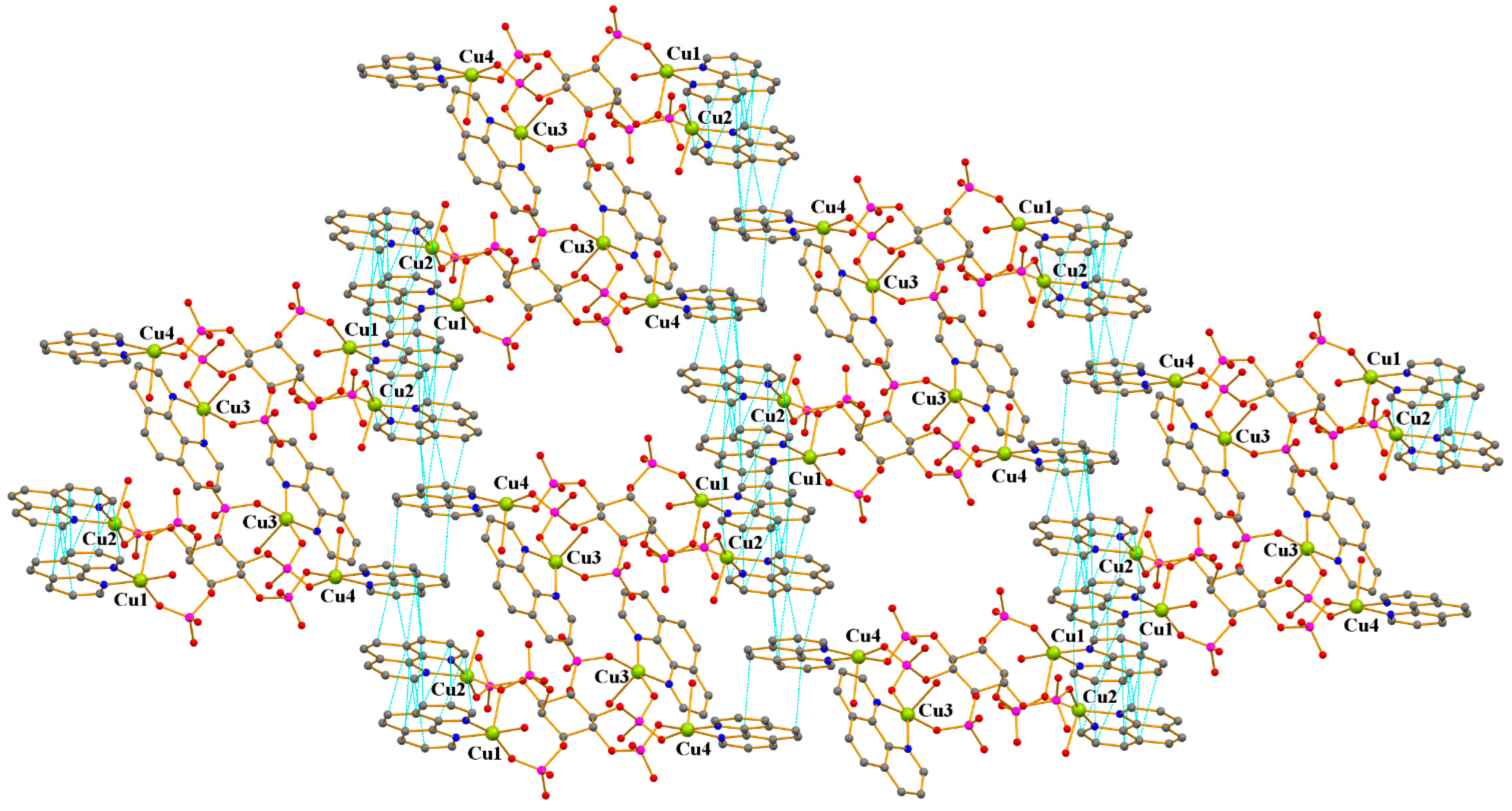
2.2. Crystal Structure of 1
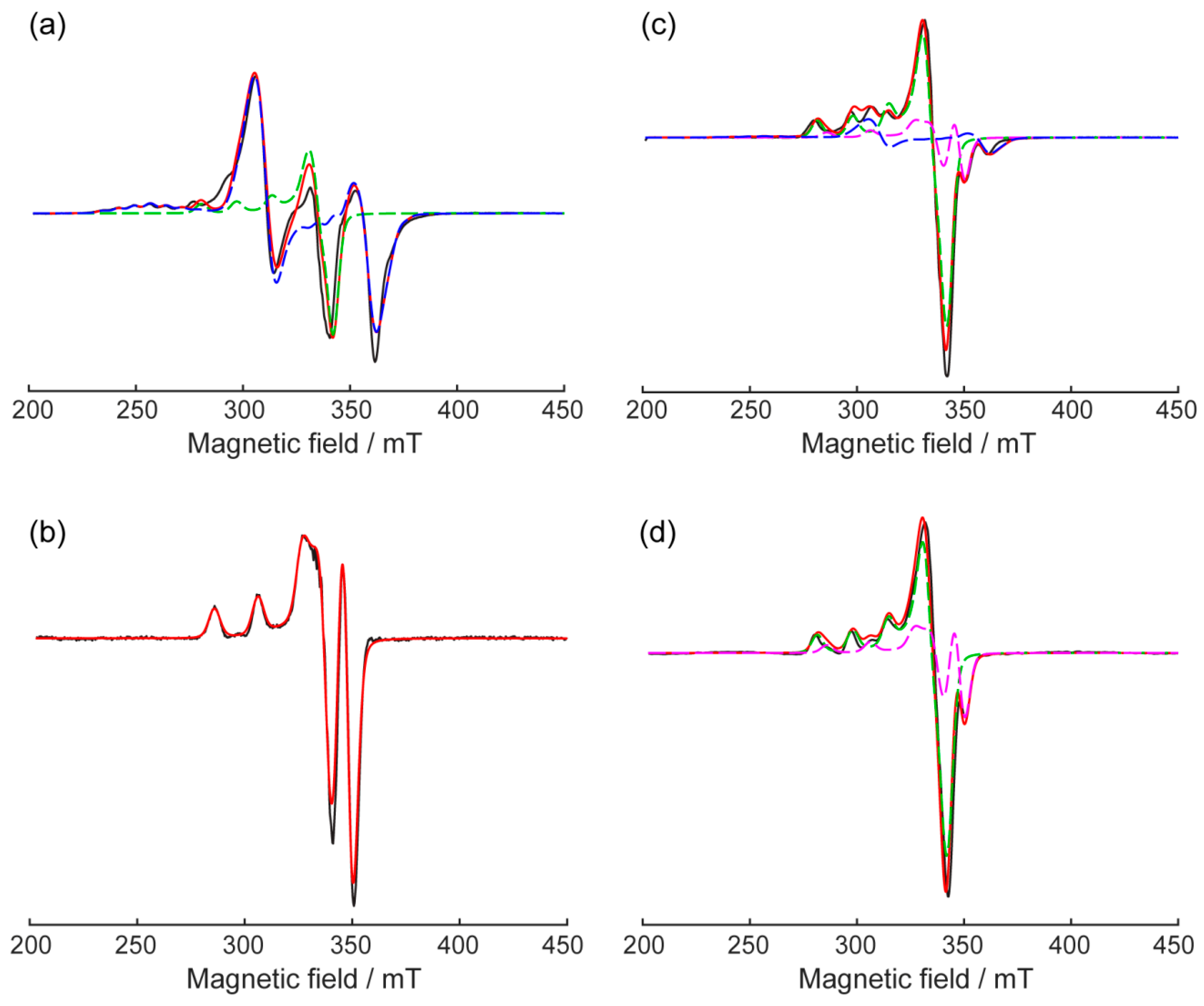
2.3. EPR-Spectroscopy of 1
2.4. Biological Activity of 1
3. Materials and Methods
3.1. General Remarks
3.2. Synthesis of 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ming, J.; Bhatti, M.Z.; Ali, A.; Zhang, Z.; Wang, N.; Mohyuddin, A.; Chen, J.; Zhang, Y.; Rahman, F.U. Vitamin B6 based Pt(II) complexes: Biomolecule derived potential cytotoxic agents for thyroid cancer. Metallomics 2022, 14, mfac053. [Google Scholar] [CrossRef]
- Jeżowska-Bojczuk, M.; Stokowa-Sołtys, K. Peptides having antimicrobial activity and their complexes with transition metal ions. Eur. J. Med. Chem. 2018, 143, 997–1009. [Google Scholar] [CrossRef]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the antioxidant and anti-inflammatory activities of selected plant compounds and their metal ions complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Jiménez, Z.; Espinoza-Guillén, A.; Resendiz-Acevedo, K.; Fuentes-Noriega, I.; Mejía, C.; Ruiz-Azuara, L. The importance of being Casiopeina as polypharmacologycal profile (mixed chelate–copper (II) complexes and their in vitro and in vivo activities). Inorganics 2023, 11, 394. [Google Scholar] [CrossRef]
- Malekshah, R.E.; Shakeri, F.; Khaleghian, A.; Salehi, M. Developing a biopolymeric chitosan supported Schiff-base and Cu(II), Ni(II) and Zn(II) complexes and biological evaluation as pro-drug. Int. J. Biol. Macromol. 2020, 152, 846–861. [Google Scholar] [CrossRef] [PubMed]
- Klarek, M.; Kowalski, K. Chemistry of organometallic nucleic acid components: Personal perspectives and prospects for the future. Dalton Trans. 2024, 53, 18420–18439. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J. A contribution to the chemistry of phytin: I. Composition of barium phytate and phytic acid. II. A study of the properties of phytic acid and its decomposition products. Eighth paper on phytin. J. Biol. Chem. 1914, 17, 171–190. [Google Scholar] [CrossRef]
- Shears, S.B. How versatile are inositol phosphate kinases? Biochem. J. 2004, 377, 265–280. [Google Scholar] [CrossRef]
- Chatree, S.; Thongmaen, N.; Tantivejkul, K.; Sitticharoon, C.; Vucenik, I. Role of inositols and inositol phosphates in energy metabolism. Molecules 2020, 25, 5079. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zhang, Y.; Xu, B.; Ali, A.; Liu, X.; Jia, Q. Inositol hexaphosphate sensitizes hepatocellular carcinoma to oxaliplatin relating inhibition of CCN2-LRP6-β-catenin-ABCG1 signaling pathway. J. Cancer 2021, 12, 6071–6080. [Google Scholar] [CrossRef]
- Sun, T.H.; Heimark, D.B.; Nguygen, T.; Nadler, J.L.; Larner, J. Both myo-inositol to chiro-inositol epimerase activities and chiro-inositol to myo-inositol ratios are decreased in tissues of GK type 2 diabetic rats compared to Wistar controls. Biochem. Biophys. Res. Commun. 2002, 293, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauzá, A.; Calvó, P.; Julià, F.; Dietrich, J.; Gomila, R.M.; Martorell, G.; Sanchis, P. Phytate dephosphorylation products also act as potent inhibitors of calcium oxalate crystallization. Molecules 2022, 27, 5463. [Google Scholar] [CrossRef]
- Saburov, K.A.; Kamilov, K.M. Structure of phytic acid and phytates. Chem. Nat. Compd. 1989, 25, 695–698. [Google Scholar] [CrossRef]
- Barrientos, L.G.; Murthy, P.P.N. Conformational studies of myo-inositol phosphates. Carbohydr. Res. 1996, 296, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Raboy, V. Myo-Inositol-1,2,3,4,5,6-hexakisphosphate. Phytochemistry 2003, 64, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, C.; Giuffre, O.; Milea, D.; Rigano, C.; Sammartano, S. Speciation of phytate ion in aqueous solution. Non covalent interactions with biogenic polyamines. Chem. Speciat. Bioavailab. 2003, 15, 29–36. [Google Scholar] [CrossRef]
- Yu, S.; Cowieson, A.; Gilbert, C.; Plumstead, P.; Dalsgaard, S. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 2012, 90, 1824–1832. [Google Scholar] [CrossRef]
- Nielsen, A.V.F.; Tetens, I.; Meyer, A.S. Potential of phytase-mediated iron release from cereal-based foods: A quantitative view. Nutrients 2013, 5, 3074–3098. [Google Scholar] [CrossRef]
- Massoud, S.S.; Tribolet, R.; Sigel, H. Metal-ion-governed molecular recognition: Extent of intramolecular stack formation in mixed-ligand–copper(II) complexes containing a heteroaromatic N base and an adenosine monophosphate (2′AMP, 3′AMP, or 5′AMP). Eur. J. Biochem. 1990, 187, 387–393. [Google Scholar] [CrossRef]
- Massoud, S.S.; Sigel, H. Ternary Complexes in Solution: Intramolecular Stack Formation in Mixed Ligand Complexes Containing CuII, 2,2′—Bipyridyl, and the 5′-Monophosphate of Inosine (IMP), Guanosine (GMP). CHIMIA 1990, 44, 55. [Google Scholar] [CrossRef]
- Nunes, P.; Correia, I.; Marques, F.; Matos, A.P.; dos Santos, M.M.C.; Azevedo, C.G.; Capelo, J.L.; Santos, H.M.; Gama, S.; Pinheiro, T.; et al. Copper complexes with 1,10-phenanthroline derivatives: Underlying factors affecting their cytotoxicity. Inorg. Chem. 2020, 59, 9116–9134. [Google Scholar] [CrossRef] [PubMed]
- Koshenskova, K.A.; Makarenko, N.V.; Baravikov, D.E.; Dolgushin, F.M.; Bekker, O.B.; Eremenko, I.L.; Lutsenko, I.A. Mixed-ligand complex [Cu4(bpy)4(PO4)2(CO3)(H2O)2]: Synthesis, crystal structure, and biological properties. Russ. J. Coord. Chem. 2023, 49, 792–799. [Google Scholar] [CrossRef]
- Quiñone, D.; Veiga, N.; Torres, J.; Castiglioni, J.; Bazzicalupi, C.; Bianchi, A.; Kremer, C. Synthesis, solid-state characterization and solution studies of new phytate compounds with Cu (II) and 1, 10-phenanthroline: Progress in the structural elucidation of phytate coordinating ability. Dalton Trans. 2016, 45, 12156–12166. [Google Scholar] [CrossRef] [PubMed]
- Veiga, N.; Torres, J.; Bazzicalupi, C.; Bianchi, A.; Kremer, C. The copper(II)–phytate–terpyridine ternary system: The first crystal structures showing the interaction of phytate with bivalent metal and ammonium cations. Chem. Commun. 2014, 50, 14971–14974. [Google Scholar] [CrossRef] [PubMed]
- Quiñone, D.; Veiga, N.; Savastano, M.; Torres, J.; Bianchi, A.; Kremer, C.; Bazzicalupi, C. Supramolecular interaction of inositol phosphates with Cu(II): Comparative study of Ins P6–Ins P3. CrystEngComm 2022, 24, 2126–2137. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Jenkins, R.O.; Goncharov, N.V. Serum albumin in health and disease: From comparative biochemistry to translational medicine. Int. J. Mol. Sci. 2023, 24, 13725. [Google Scholar] [CrossRef]
- Bal, W.; Sokołowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Sendzik, M.; Pushie, M.J.; Stefaniak, E.; Haas, K.L. Structure and affinity of Cu(I) bound to human serum albumin. Inorg. Chem. 2017, 56, 15057–15065. [Google Scholar] [CrossRef] [PubMed]
- Poryvaev, A.S.; Yazikova, A.A.; Polyukhov, D.M.; Chinak, O.A.; Richter, V.A.; Krumkacheva, O.A.; Fedin, M.V. Guest leakage from ZIF-8 particles under drug delivery conditions: Quantitative characterization and guest-induced framework stabilization. J. Phys. Chem. C 2021, 125, 15606–15613. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Chikira, M.; Tomizawa, Y.; Fukita, D.; Sugizaki, T.; Sugawara, N.; Yamazaki, T.; Sasano, A.; Shindo, H.; Palaniandavar, M.; Antholine, W.E. DNA-fiber EPR study of the orientation of Cu(II) complexes of 1,10-Phenanthroline and its derivatives bound to DNA: Mono(phenanthroline)-copper(II) and its ternary complexes with amino acids. J. Inorg. Biochem. 2002, 89, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ramón-García, S.; Ng, C.; Anderson, H.; Chao, J.D.; Zheng, X.; Pfeifer, T.; Av-Gay, Y.; Roberge, M.; Thompson, C.J. Synergistic drug combinations for tuberculosis therapy identified by a novel high-throughput screen. Antimicrob. Agents Chemother. 2011, 55, 3861–3869. [Google Scholar] [CrossRef]
- Bekker, O.B.; Sokolov, D.N.; Luzina, O.A.; Komarova, N.I.; Gatilov, Y.V.; Andreevskaya, S.N.; Smirnova, T.G.; Maslov, D.A.; Chernousova, L.N.; Salakhutdinov, N.F.; et al. Synthesis and activity of (+)-usnic acid and (−)-usnic acid derivatives containing 1,3-thiazole cycle against Mycobacterium tuberculosis. Med. Chem. Res. 2015, 24, 2926–2938. [Google Scholar] [CrossRef]
- Koshenskova, K.A.; Baravikov, D.E.; Kayukova, L.A.; Ergalieva, E.M.; Nelyubina, Y.V.; Nikiforova, M.E.; Dolgushin, F.M.; Fedin, M.V.; Bekker, O.B.; Shender, V.O.; et al. Evaluation of the anionic effect on the formation of biologically active {CuII-phenx; x = 1, 2, 3} fragments-synthetic and structural variations, antimycobacterial and antiblastoma effects. Polyhedron 2024, 251, 116852. [Google Scholar] [CrossRef]
- Uvarova, M.A.; Lutsenko, I.A.; Shmelev, M.A.; Nefedov, S.E.; Bekker, O.B.; Lashkin, A.I.; Shender, V.O.; Eremenko, I.L. Research of the influence of anions in complexes [CuPhen(Hpz)2X2] (X = CF3COO−, Otf−, Cl−) on the structure and bioactivity. New J. Chem. 2024, 48, 717–723. [Google Scholar] [CrossRef]
- Koshenskova, K.A.; Baravikov, D.E.; Nelyubina, Y.V.; Primakov, P.V.; Shender, V.O.; Maljants, I.K.; Bekker, O.B.; Aliev, T.M.; Borodin, E.A.; Kotel’nikov, D.D.; et al. Copper(II) furancarboxylate complexes with 5-nitro-1,10-phenanthroline as promising biological agents. Russ. J. Coord. Chem. 2023, 49, 660–671. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
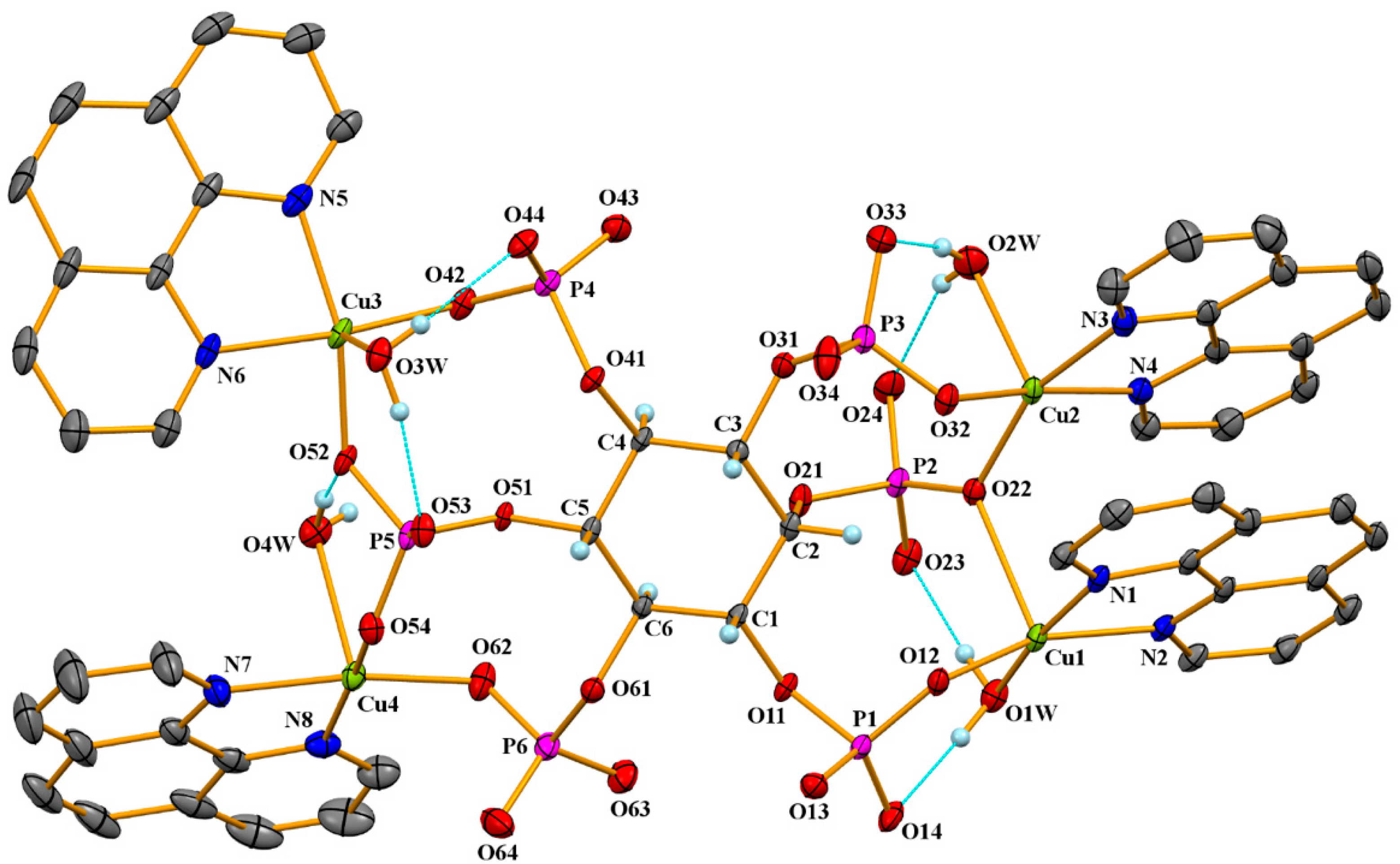
| Cu1 | Cu2 | Cu3 | Cu4 | |
|---|---|---|---|---|
| Cu–N | 2.006(2), 2.012(2) | 2.025(2), 2.033(2) | 2.039(2), 2.021(2) | 2.025(2), 2.030(2) |
| Cu–O(OPO3) | 1.9163(17) | 1.9259(17) | 1.9277(17) | 1.9175(18) |
| Cu–O(O-bridge) Cu–O(OPO-bridge) | 2.3921(17) | 1.9740(17) | 1.9475(17) | 1.9434(18) |
| Cu–O(water) | 1.9697(18) | 2.2681(19) | 2.2704(18) | 2.3460(19) |
| Sample | Fraction | Weight | g-Tensor [gxx, gyy, gzz] | A-Tensor [Axx, Ayy, Azz], MHz | D-Tensor [D, E], MHz |
|---|---|---|---|---|---|
| 1 | Monomer | 30% | [2.064, 2.064, 2.272] | [40, 40, 520] | - |
| Tetramer | 70% | [2.066, 2.076, 2.272] | [80, 150, 470] | [1540, 60] | |
| 1-HSA (1:1) | HSA-Cu(II) | 20% | [2.037, 2.066, 2.190] | [20, 60, 621] | - |
| Tetramer | 20% | [2.066, 2.076, 2.272] | [80, 150, 470] | [1540, 60] | |
| Monomer | 60% | [2.054, 2.078, 2.261] | [40, 40, 525] | - | |
| 1-HSA (1:4) | HSA-Cu(II) | 30% | [2.037, 2.066, 2.190] | [20, 60, 621] | - |
| Monomer | 70% | [2.054, 2.078, 2.261] | [40, 40, 525] | - | |
| HSA-Cu(II) | HSA-Cu(II) | 100% | [2.037, 2.066, 2.190] | [20, 60, 621] | - |
| Compound | MIC, nmol/disk | Zone of Inhibition, mm | Ref. | |
|---|---|---|---|---|
| 24 h | 24 h | 120 h | ||
| 1 | 6 | 6.7 ± 0.3 | 6.6 ± 0.1 * | this work |
| [(Cu4(bpy)4(PO4)2(CO3)(H2O)2]⋅13H2O | 50 | 6.6 ± 0.2 | 6.4 ± 0.2 * | [22] |
| [Cu(2fur)2(phen)] | 5 | 7 ± 0.5 | 7 ± 0.5 * | [34] |
| [Cu(benz)2phen] | 12.5 | 6.5 ± 0.2 | 6.4 ± 0.1 * | [34] |
| [Cu(Hpz)2(OOCCF3)2phen] | 10 | 6.8 ± 0.5 | 6.5 ± 0.1 | [35] |
| [Cu(Hpz)2(Otf)2phen] | 10 | 6.5 ± 0.1 | 6.4 ± 0.1 | [35] |
| [Cu(Hpz)2Clphen]Cl | 8 | 6.6 ± 0.1 | 6.5 ± 0.1 | [35] |
| [Cu2(nfur)4(nphen)2] | 5 | 6.5 ± 0.5 | 6.5 ± 0.2 * | [36] |
| [Cu2(2fur)4(nphen)2]·MeOH | 10 | 7.0 ± 0.5 | 6.4 ± 0.2 * | [36] |
| C6H18O24P6·xNa·yH2O | >500 | 0 | 0 | this work |
| phen | 45 | 7.5 ± 0.5 | 0 | this work |
| Rif | 6 | 7.2 ± 0.3 * | 7.13 ± 0.35 * | this work |
| Parameter | Value |
|---|---|
| Empirical formula | C54H118Cu4N10Na2O60P6 |
| Formula weight | 2353.54 |
| Crystal size (mm) | 0.28 × 0.12 × 0.06 |
| Temperature (K) | 100 |
| Crystal system | triclinic |
| Space group | P-1 |
| a (Å) | 16.8441 (11) |
| b (Å) | 17.8246 (12) |
| c (Å) | 17.9297 (12) |
| α (deg) | 71.620 (2) |
| β (deg) | 72.861 (2) |
| γ (deg) | 64.024 (2) |
| V (Å3) | 4514.5 (5) |
| Z | 2 |
| dcalc (g·cm−3) | 1.731 |
| μ (cm−1) | 11.63 |
| Tmin/Tmax | 0.644/0.933 |
| 2θmax (deg) | 58 |
| no. of unique reflns (Rint) | 23974 (0.0555) |
| no. of obsd reflns (I > 2σ(I)) | 20312 |
| R1 (on F for obsd reflns) | 0.0438 |
| wR2 (on F2 for all reflns) | 0.1220 |
| GOOF | 1.026 |
| diff. peak/hole (e/Å−3) | 1.445/−1.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koshenskova, K.A.; Makarenko, N.V.; Dolgushin, F.M.; Yambulatov, D.S.; Bekker, O.B.; Fedin, M.V.; Dementev, S.A.; Krumkacheva, O.A.; Eremenko, I.L.; Lutsenko, I.A. «Green-Ligand» in Metallodrugs Design—Cu(II) Complex with Phytic Acid: Synthetic Approach, EPR-Spectroscopy, and Antimycobacterial Activity. Molecules 2025, 30, 313. https://doi.org/10.3390/molecules30020313
Koshenskova KA, Makarenko NV, Dolgushin FM, Yambulatov DS, Bekker OB, Fedin MV, Dementev SA, Krumkacheva OA, Eremenko IL, Lutsenko IA. «Green-Ligand» in Metallodrugs Design—Cu(II) Complex with Phytic Acid: Synthetic Approach, EPR-Spectroscopy, and Antimycobacterial Activity. Molecules. 2025; 30(2):313. https://doi.org/10.3390/molecules30020313
Chicago/Turabian StyleKoshenskova, Kseniya A., Natalia V. Makarenko, Fedor M. Dolgushin, Dmitriy S. Yambulatov, Olga B. Bekker, Matvey V. Fedin, Sergei A. Dementev, Olesya A. Krumkacheva, Igor L. Eremenko, and Irina A. Lutsenko. 2025. "«Green-Ligand» in Metallodrugs Design—Cu(II) Complex with Phytic Acid: Synthetic Approach, EPR-Spectroscopy, and Antimycobacterial Activity" Molecules 30, no. 2: 313. https://doi.org/10.3390/molecules30020313
APA StyleKoshenskova, K. A., Makarenko, N. V., Dolgushin, F. M., Yambulatov, D. S., Bekker, O. B., Fedin, M. V., Dementev, S. A., Krumkacheva, O. A., Eremenko, I. L., & Lutsenko, I. A. (2025). «Green-Ligand» in Metallodrugs Design—Cu(II) Complex with Phytic Acid: Synthetic Approach, EPR-Spectroscopy, and Antimycobacterial Activity. Molecules, 30(2), 313. https://doi.org/10.3390/molecules30020313







