Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols
Abstract
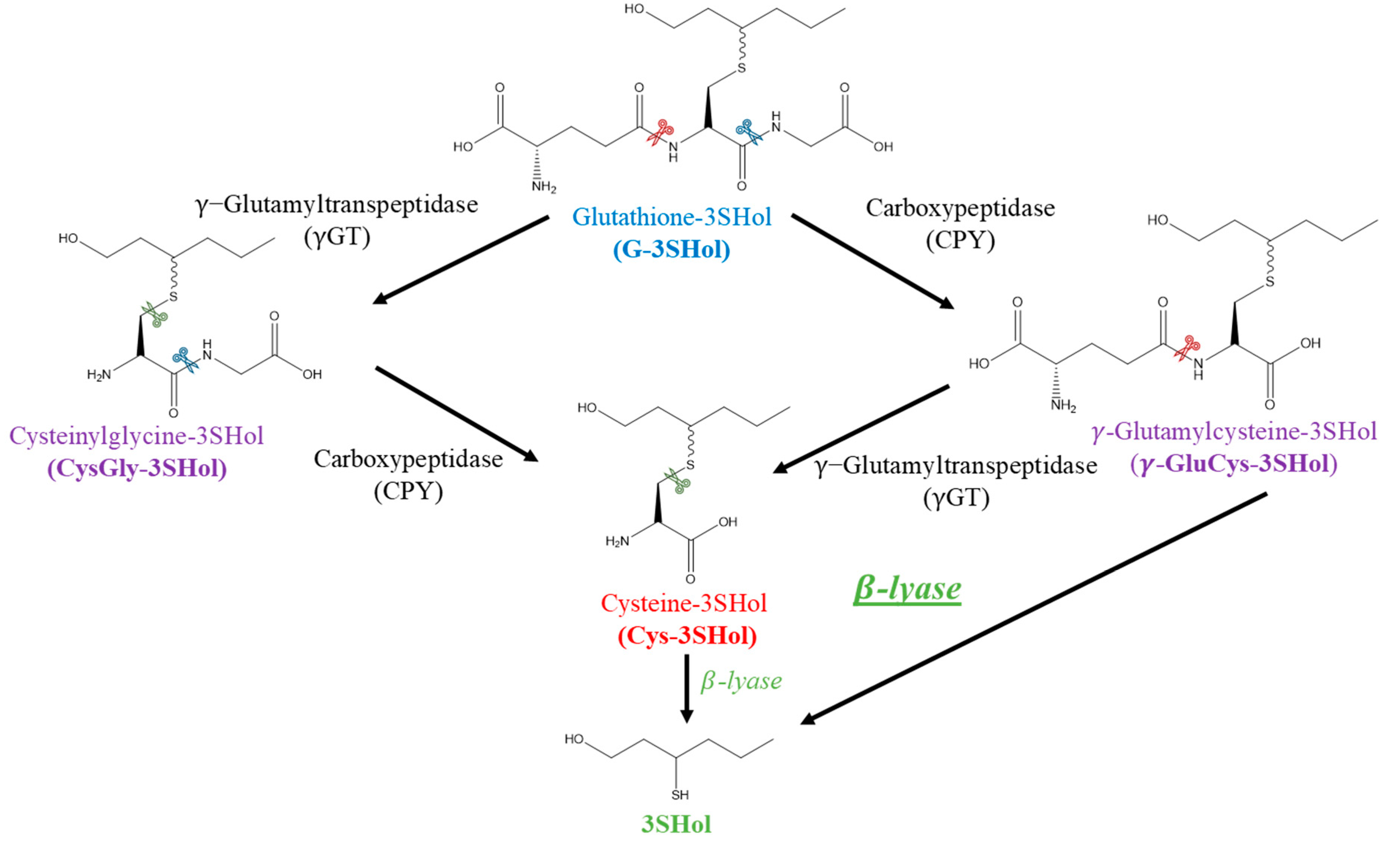
:1. Introduction
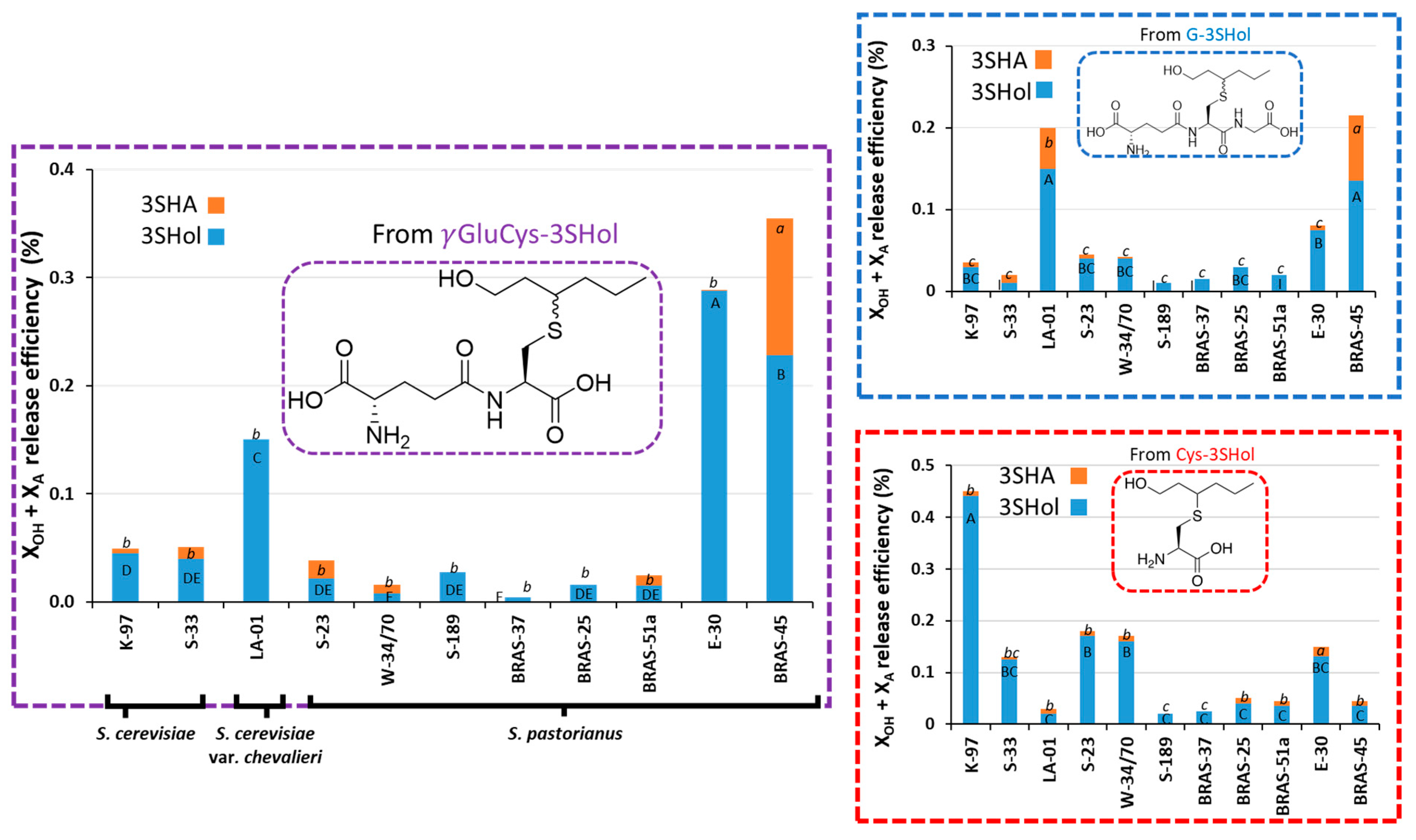
2. Results and Discussion
2.1. Release of Free 3SHol from γ-GluCys-3SHol in Beer Fermentation Trials
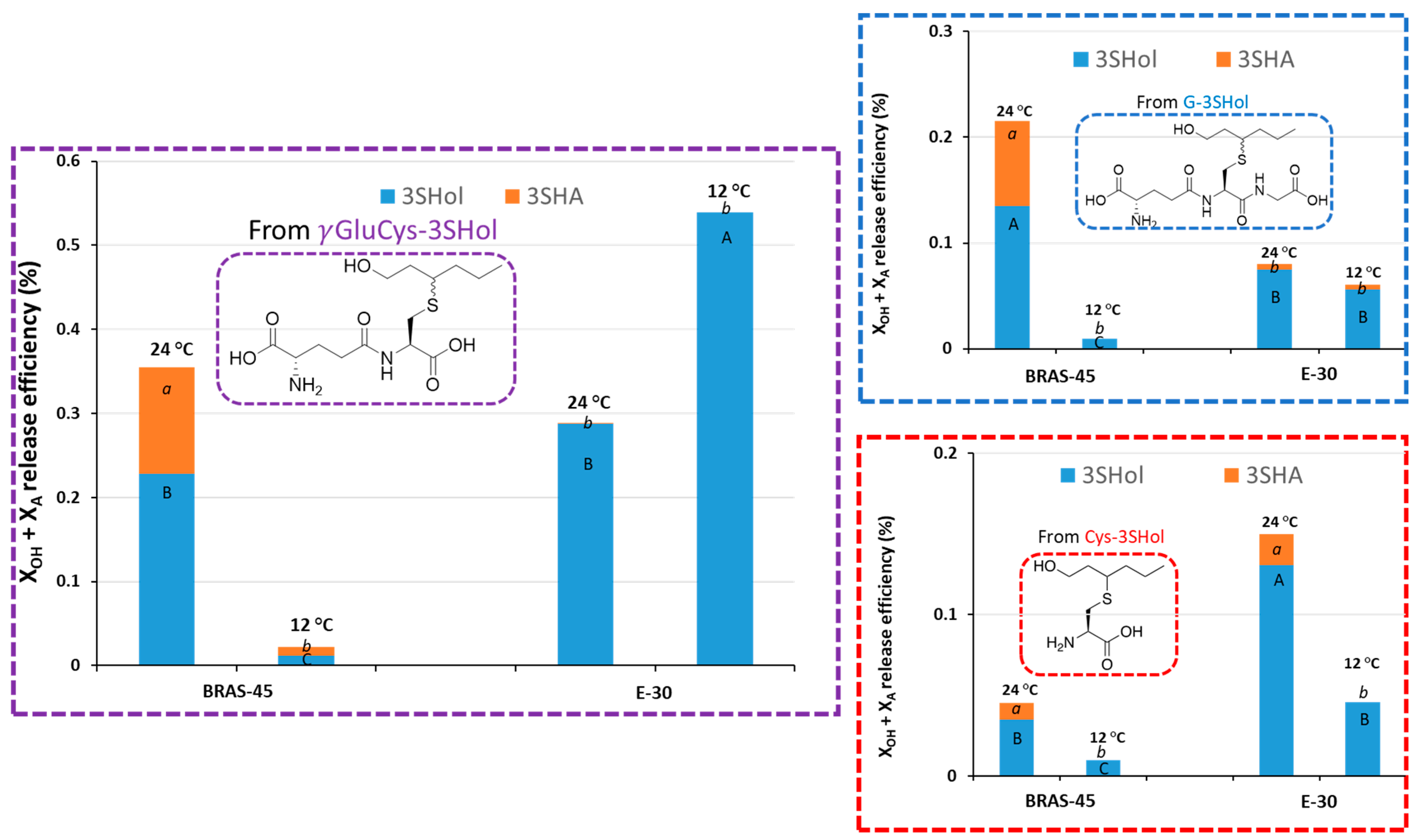
2.2. Impact of Fermentation Temperature
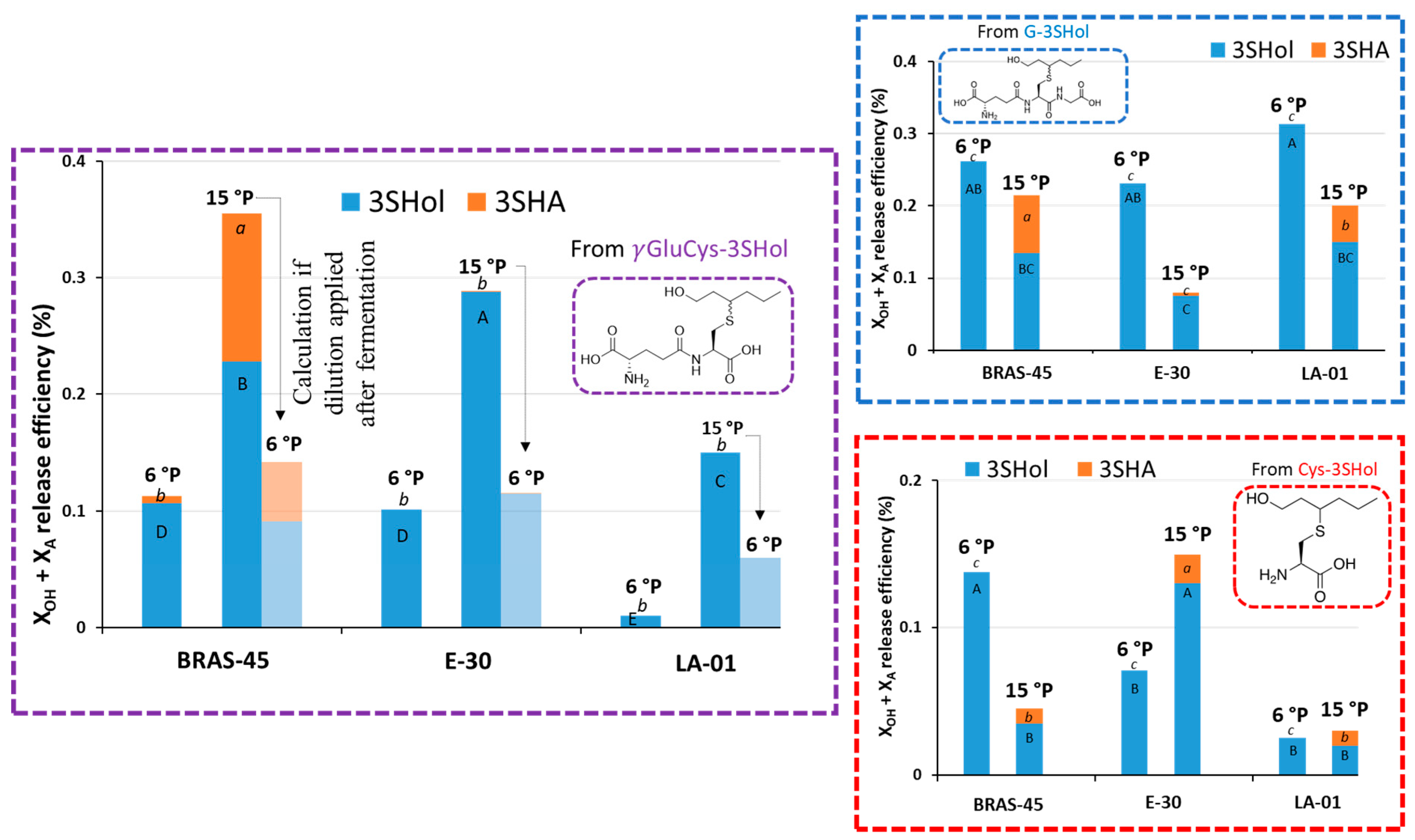
2.3. Impact of Wort Density
3. Materials and Methods
3.1. Chemicals
3.2. Yeasts
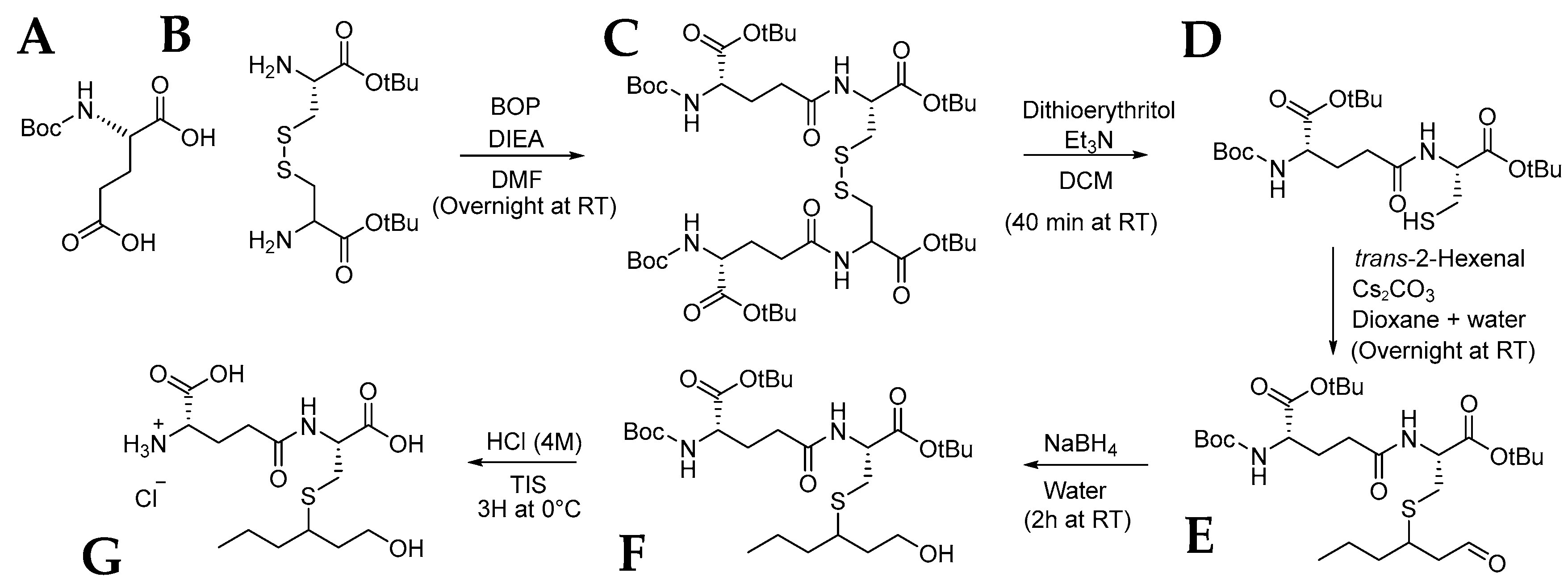
3.3. Synthesis of γ-GluCys-3SHol
3.3.1. Synthesis of N-(N-Boc-l-γ-glutamyl)-l-cystine Di-tert-butyl Ester (C)
3.3.2. Synthesis of N-(N-Boc-l-γ-glutamyl)-l-cysteine tert-Butyl Ester (D)
3.3.3. Synthesis of S-3-(Hexan-1-ol)-N-(N-Boc-l-γ-glutamyl)-l-cysteine tert-Butyl Ester (F)
3.3.4. Synthesis of S-3-(Hexan-1-ol)-N-(-l-γ-glutamyl)-l-cysteine Chloride (G)
3.4. Fermentation of Wort Spiked with γ-GluCys-3SHol
3.5. Extraction of 3SHol from Fermented Spiked Media with Ag Cartridge
3.6. Gas Chromatography–Pulsed Flame Photometric Detection
3.7. Release Efficiency Determination
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Darriet, P.; Tominaga, T.; Lavigne, V.; Boidron, J.N.; Dubourdieu, D. Identification of a Powerful Aromatic Component of Vitis vinifera L. var. Sauvignon Wines: 4-Mercapto-4-methylpentan-2-one. Flavour Fragr. J. 1995, 10, 385–392. [Google Scholar] [CrossRef]
- Guth, H. Quantitation and Sensory Studies of Character Impact Odorants of Different White Wine Varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Picard, M.; Thibon, C.; Redon, P.; Darriet, P.; de Revel, G.; Marchand, S. Involvement of Dimethyl Sulfide and Several Polyfunctional Thiols in the Aromatic Expression of the Aging Bouquet of Red Bordeaux Wines. J. Agric. Food Chem. 2015, 63, 8879–8889. [Google Scholar] [CrossRef]
- Sun, Z.; Hayat, K.; Yu, J.; Karangwa, E.; Duhoranimana, E.; Zhang, X.; Xia, S. Quantification of Free 2-Furfurylthiol in Coffee Brew Using a Prefabricated Coffee Model. Food Anal. Methods 2018, 11, 654–662. [Google Scholar] [CrossRef]
- Vermeulen, C.; Lejeune, I.; Tran, T.T.H.; Collin, S. Occurrence of Polyfunctional Thiols in Fresh Lager Beers. J. Agric. Food Chem. 2006, 54, 5061–5068. [Google Scholar] [CrossRef]
- Kankolongo Cibaka, M.L.; Gros, J.; Nizet, S.; Collin, S. Quantitation of Selected Terpenoids and Mercaptans in the Dual-Purpose Hop Varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace. J. Agric. Food Chem. 2015, 63, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T.; Niclass, Y.; Frérot, E.; Dubourdieu, D. Stereoisomeric Distribution of 3-Mercaptohexan-1-ol and 3-Mercaptohexyl Acetate in Dry and Sweet White Wines Made from Vitis vinifera (Var. Sauvignon Blanc and Semillon). J. Agric. Food Chem. 2006, 54, 7251–7255. [Google Scholar] [CrossRef]
- Carlin, S.; Piergiovanni, M.; Pittari, E.; Lisanti, M.T.; Moio, L.; Piombino, P.; Marangon, M.; Curioni, A.; Rolle, L.; Río Segade, S.; et al. The Contribution of Varietal Thiols in the Diverse Aroma of Italian Monovarietal White Wines. Food Res. Int. 2022, 157, 111404. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Queval, G.; Mhamdi, A.; Chaouch, S.; Foyer, C.H. Glutathione. Arab. Book 2011, 9, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Kankolongo Cibaka, M.L.; Decourrière, L.; Lorenzo-Alonso, C.J.; Bodart, E.; Robiette, R.; Collin, S. 3-Sulfanyl-4-Methylpentan-1-ol in Dry-Hopped Beers: First Evidence of Glutathione S-Conjugates in Hop (Humulus lupulus L.). J. Agric. Food Chem. 2016, 64, 8572–8582. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Deed, R. The Chemical Reaction of Glutathione and trans-2-Hexenal in Grape Juice Media To Form Wine Aroma Precursors: The Impact of pH, Temperature, and Sulfur Dioxide. J. Agric. Food Chem. 2018, 66, 1214–1221. [Google Scholar] [CrossRef]
- Chenot, C.; Collin, S.; Suc, L.; Roland, A. Unusual Profile of Thiol Precursors in Special Malts: First Evidence of Chemical Glutathione-/γ-GluCys- and CysGly-/Cys- Conversions. J. Am. Soc. Brew. Chem. 2024, 82, 15–22. [Google Scholar] [CrossRef]
- Vicente, J.; Kiene, F.; Fracassetti, D.; De Noni, I.; Shemehen, R.; Tarasov, A.; Dobrydnev, A.V.; Marquina, D.; Santos, A.; Rauhut, D.; et al. Precursors Consumption Preferences and Thiol Release Capacity of the Wine Yeasts Saccharomyces cerevisiae, Torulaspora delbrueckii, and Lachancea thermotolerans. Int. J. Food Microbiol. 2024, 425, 110858. [Google Scholar] [CrossRef] [PubMed]
- Chenot, C.; Thibault De Chanvalon, E.; Janssens, P.; Collin, S. Modulation of the Sulfanylalkyl Acetate/Alcohol Ratio and Free Thiol Release from Cysteinylated and/or Glutathionylated Sulfanylalkyl Alcohols in Beer under Different Fermentation Conditions. J. Agric. Food Chem. 2021, 69, 6005–6012. [Google Scholar] [CrossRef] [PubMed]
- Chenot, C.; Donck, W.; Janssens, P.; Collin, S. Malt and Hop as Sources of Thiol S-Conjugates: Thiol-Releasing Property of Lager Yeast during Fermentation. J. Agric. Food Chem. 2022, 70, 3272–3279. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Tran, T.T.H.; Collin, S. Enzymatic Release of Odourant Polyfunctional Thiols from Cysteine Conjugates in Hop: Enzymatic Release of Hop Thiols. J. Inst. Brew. 2013, 119, 221–227. [Google Scholar] [CrossRef]
- Nizet, S.; Peeters, F.; Gros, J.; Collin, S. Odorant Polyfunctional Thiols Issued from Bottle Beer Refermentation. In Flavour Science. Proceedings from XIII Weurman Flavour Research Symposium; Academic Press: Cambridge, MA, USA, 2014; pp. 227–230. [Google Scholar] [CrossRef]
- Santiago, M.; Gardner, R. Yeast Genes Required for Conversion of Grape Precursors to Varietal Thiols in Wine. FEMS Yeast Res. 2015, 15, fov034. [Google Scholar] [CrossRef]
- Lilly, M.; Bauer, F.F.; Lambrechts, M.G.; Swiegers, J.H.; Cozzolino, D.; Pretorius, I.S. The Effect of Increased Yeast Alcohol Acetyltransferase and Esterase Activity on the Flavour Profiles of Wine and Distillates. Yeast 2006, 23, 641–659. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Roland, A.; Rémond, E.; Delpech, S.; Schneider, R.; Cavelier, F. First Identification and Quantification of S-3-(Hexan-1-ol)-γ-Glutamyl-Cysteine in Grape Must as a Potential Thiol Precursor, Using UPLC-MS/MS Analysis and Stable Isotope Dilution Assay. Food Chem. 2017, 237, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Frackenpohl, J.; Arvidsson, P.I.; Schreiber, J.V.; Seebach, D. The Outstanding Biological Stability of β- and γ-Peptides toward Proteolytic Enzymes: An In Vitro Investigation with Fifteen Peptidases. ChemBioChem 2001, 2, 445–455. [Google Scholar] [CrossRef]
- Bonnaffoux, H.; Delpech, S.; Rémond, E.; Schneider, R.; Roland, A.; Cavelier, F. Revisiting the Evaluation Strategy of Varietal Thiol Biogenesis. Food Chem. 2018, 268, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, K.; Thierie, J.; Penninckx, M.J. γ-Glutamyl Transpeptidase in the Yeast Saccharomyces cerevisiae and its Role in the Vacuolar Transport and Metabolism of Glutathione. Biochem. J. 2001, 359, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Cordente, A.G.; Capone, D.L.; Curtin, C.D. Unravelling Glutathione Conjugate Catabolism in Saccharomyces cerevisiae: The Role of Glutathione/Dipeptide Transporters and Vacuolar Function in the Release of Volatile Sulfur Compounds 3-Mercaptohexan-1-ol and 4-Mercapto-4-Methylpentan-2-one. Appl. Microbiol. Biotechnol. 2015, 99, 9709–9722. [Google Scholar] [CrossRef] [PubMed]
- Chenot, C.; Collin, S.; Suc, L.; Roland, A. Evidence of Enzymatic and Chemical Interconversions of Barley Malt 3-Sulfanylhexanol Conjugates during Mashing. J. Agric. Food Chem. 2023, 71, 13107–13113. [Google Scholar] [CrossRef]
- Simon, M.; Christiaens, R.; Janssens, P.; Collin, S. Unexpected Behavior of a Maltose-Negative Saccharomyces cerevisiae Yeast: Higher Release of Polyfunctional Thiols from Glutathionylated Than from Cysteinylated S-Conjugates. Fermentation 2024, 10, 276. [Google Scholar] [CrossRef]
- Piornos, J.A.; Balagiannis, D.P.; Methven, L.; Koussissi, E.; Brouwer, E.; Parker, J.K. Elucidating the Odor-Active Aroma Compounds in Alcohol-Free Beer and Their Contribution to the Worty Flavor. J. Agric. Food Chem. 2020, 68, 10088–10096. [Google Scholar] [CrossRef] [PubMed]
- Piornos, J.A.; Delgado, A.; de La Burgade, R.C.J.; Methven, L.; Balagiannis, D.P.; Koussissi, E.; Brouwer, E.; Parker, J.K. Orthonasal and Retronasal Detection Thresholds of 26 Aroma Compounds in a Model Alcohol-Free Beer: Effect of Threshold Calculation Method. Int. Food Res. J. 2019, 123, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Takazumi, K.; Takoi, K.; Koie, K.; Tuchiya, Y. Quantitation Method for Polyfunctional Thiols in Hops (Humulus lupulus L.) and Beer Using Specific Extraction of Thiols and Gas Chromatography–Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 11598–11604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christiaens, R.; Simon, M.; Robiette, R.; Collin, S. Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols. Molecules 2025, 30, 325. https://doi.org/10.3390/molecules30020325
Christiaens R, Simon M, Robiette R, Collin S. Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols. Molecules. 2025; 30(2):325. https://doi.org/10.3390/molecules30020325
Chicago/Turabian StyleChristiaens, Romain, Margaux Simon, Raphaël Robiette, and Sonia Collin. 2025. "Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols" Molecules 30, no. 2: 325. https://doi.org/10.3390/molecules30020325
APA StyleChristiaens, R., Simon, M., Robiette, R., & Collin, S. (2025). Evidence in Lager Yeasts of β-Lyase Activity Breaking Down γ-GluCys-Conjugates More Efficiently Than Cys-Conjugates to Odorant Beer Polyfunctional Thiols. Molecules, 30(2), 325. https://doi.org/10.3390/molecules30020325





