A Photocontrolled Molecular Rotor Based on Azobenzene-Strapped Mixed (Phthalocyaninato)(Porphyrinato) Rare Earth Triple-Decker
Abstract
:1. Introduction
2. Results
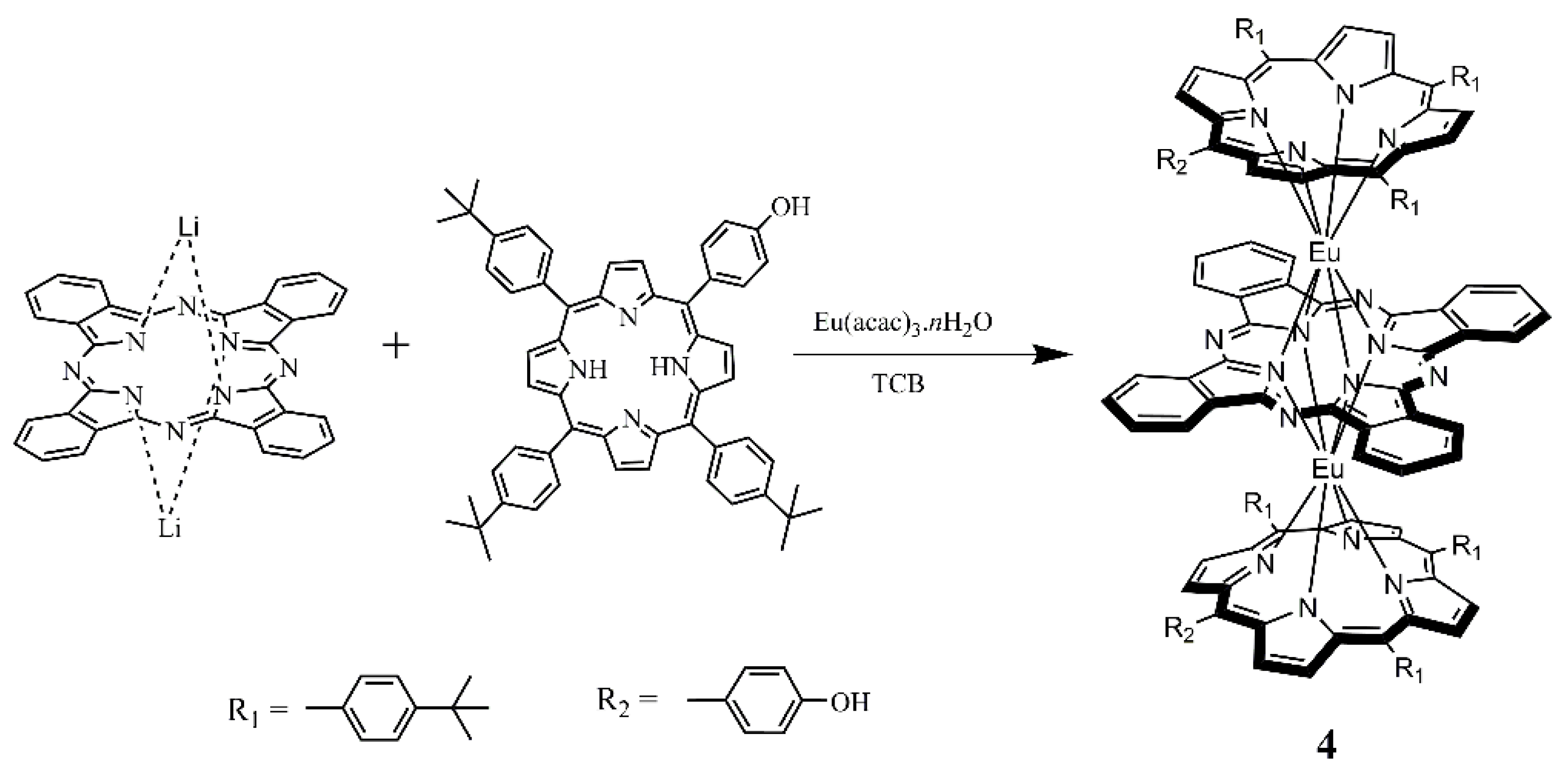
2.1. Molecular Design and Synthesis
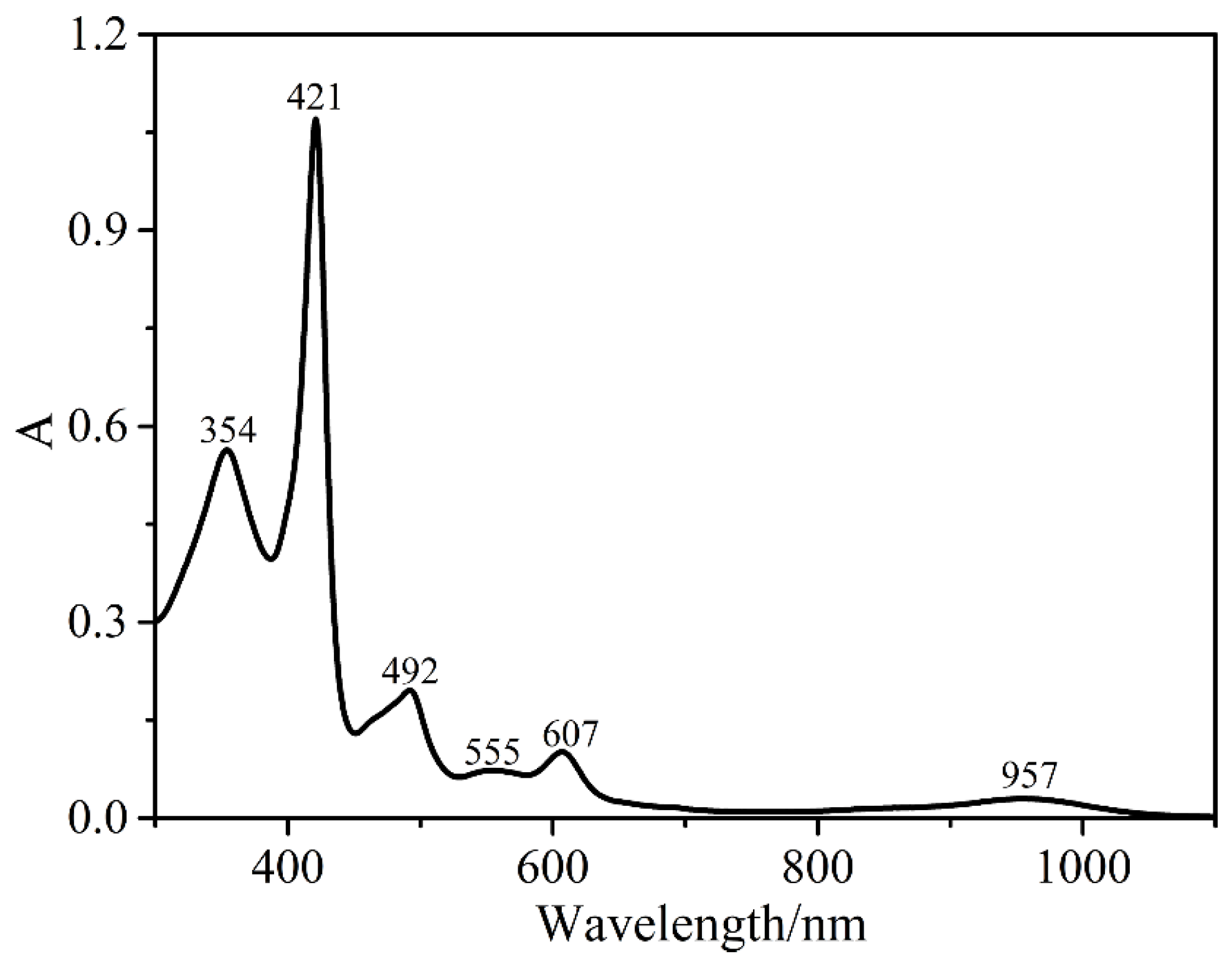
2.2. Electronic Absorption Spectroscopy
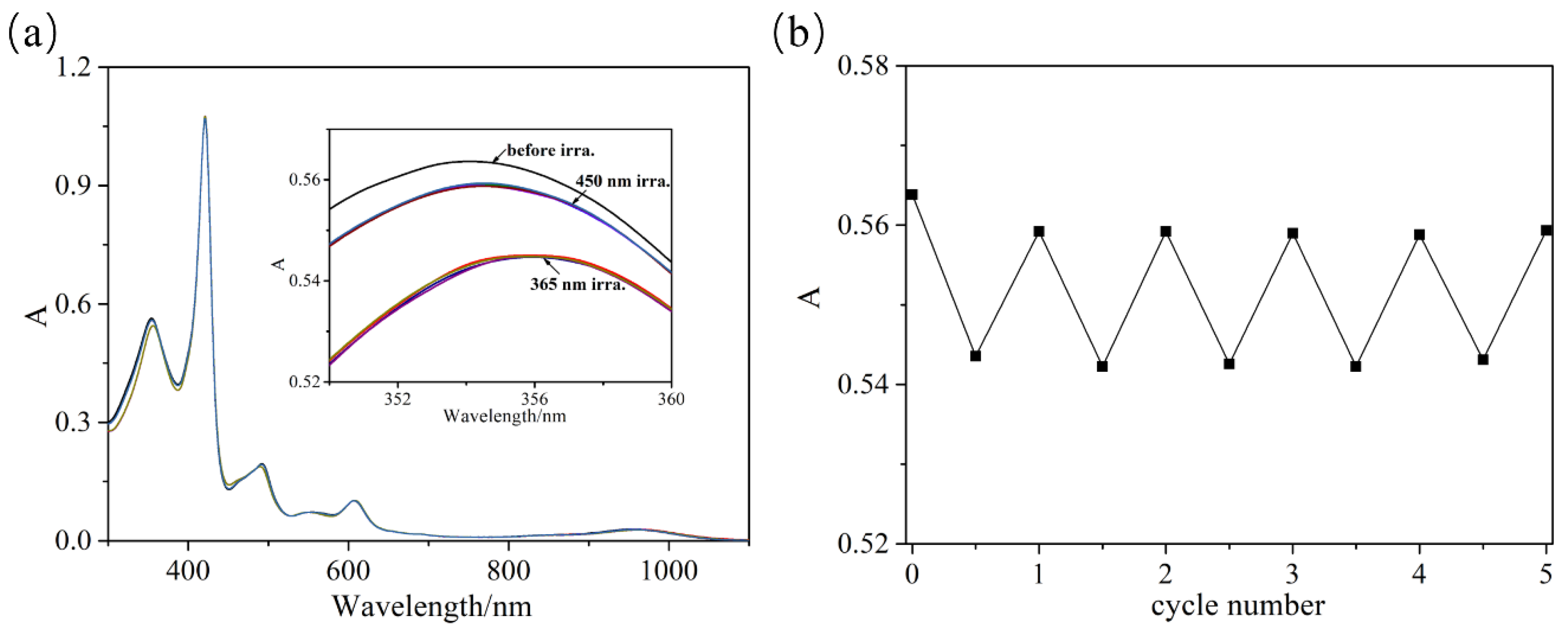
2.3. The Cis–Trans Reversible Isomerization
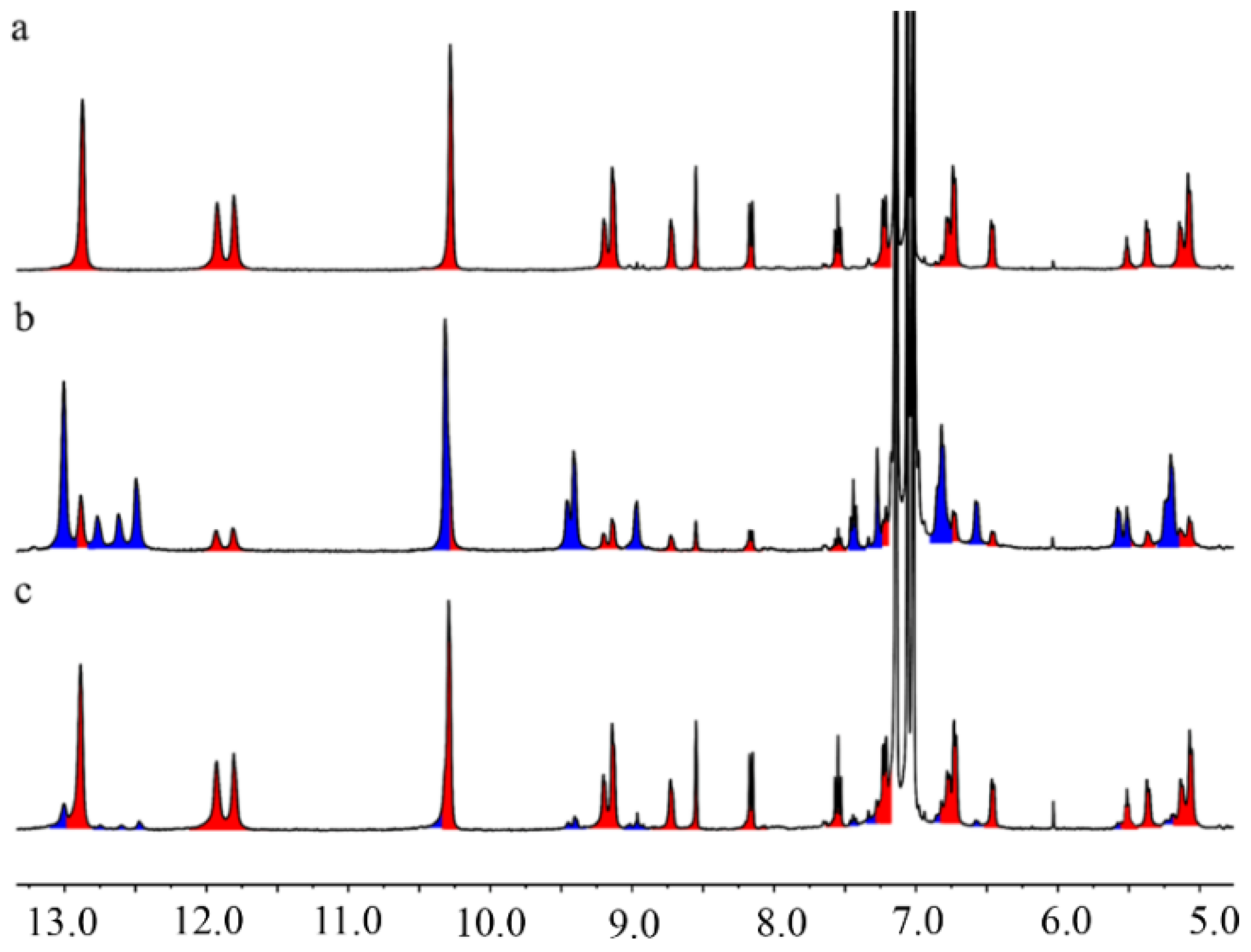
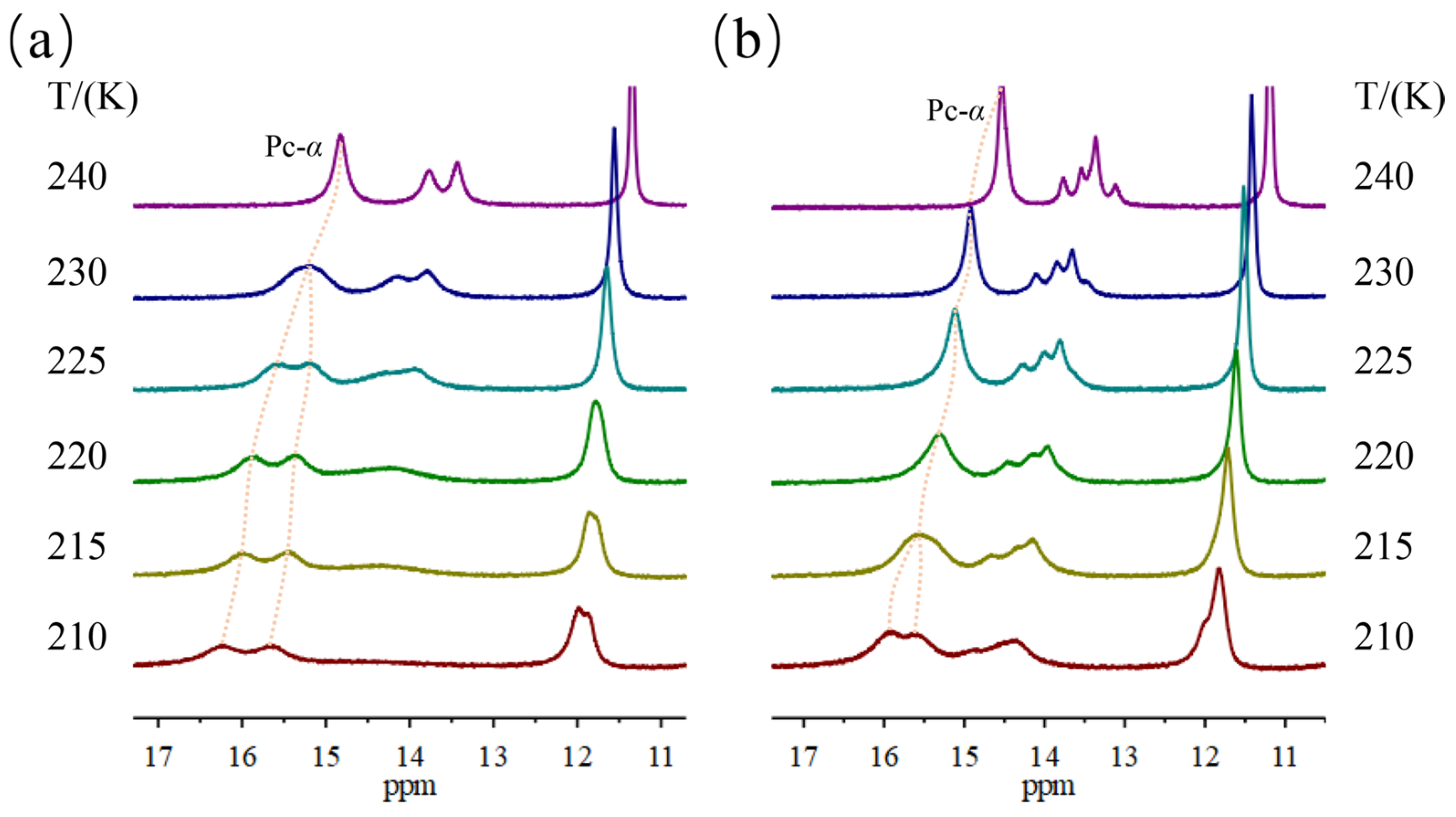
2.4. Investigating the Rotational Dynamics of Photo-Responsive Rotor Through Variable Temperature 1H NMR Spectroscopy
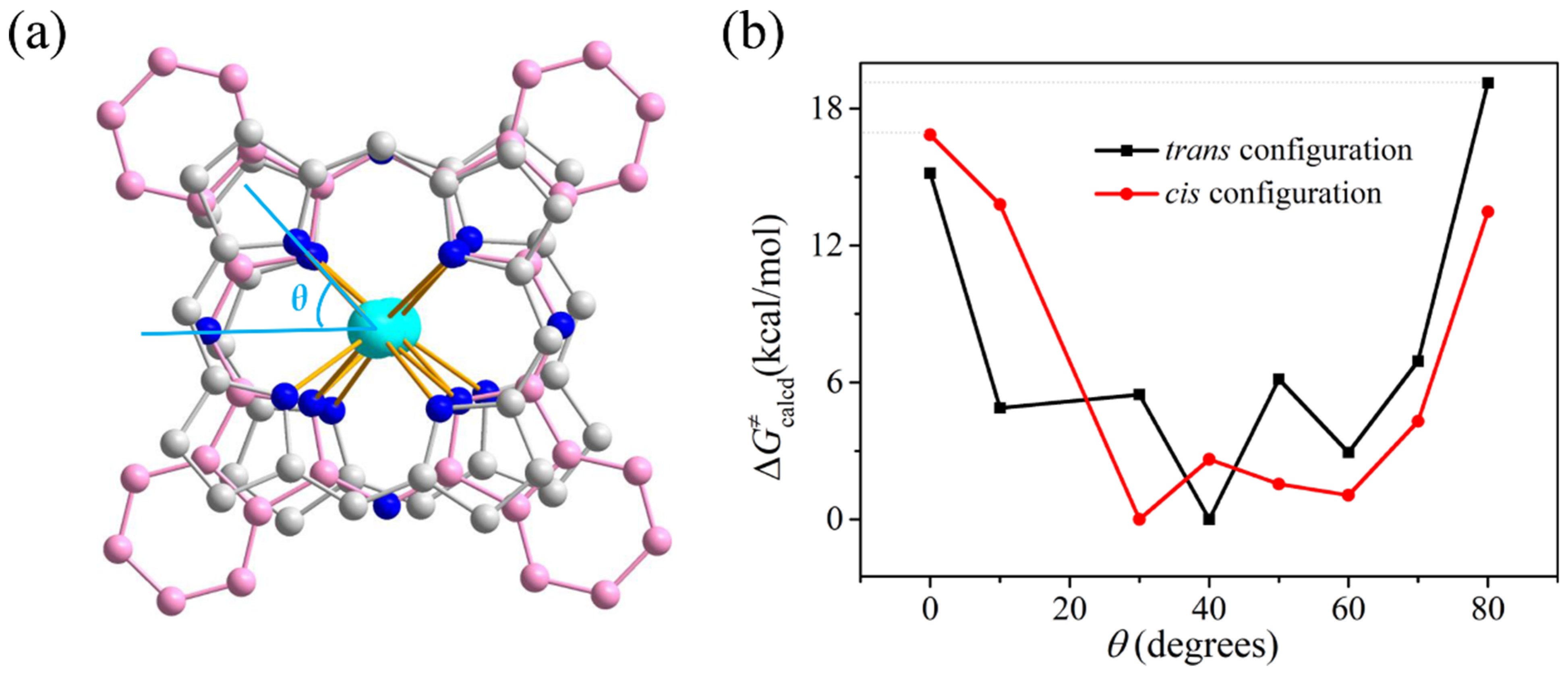
2.5. Density Function Theory Calculations
3. Experimental Section
3.1. Chemicals and Instruments
3.2. Synthesis and Characterization
3.2.1. Synthesis of 4,4’-Dihydroxylazobenzene (2)
3.2.2. Synthesis of 4,4’-Di(3-bromopropoxy) Azobenzene (3)
3.2.3. Synthesis of (TTBPP)Eu(Pc)Eu(TTBPP) (4)
3.2.4. Synthesis of Azo-(TTBPP)Eu(Pc)Eu(TTBPP) (Azo-1)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goodsell, D.S. The Machinery of Life, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef]
- Dattler, D.; Fuks, G.; Heiser, J.; Moulin, E.; Perrot, A.; Yao, X.Y.; Giuseppone, N. Design of Collective Motions from Synthetic Molecular Switches, Rotors, and Motors. Chem. Rev. 2020, 120, 310–433. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Nie, T.; Bao, C.; Wang, C.; Xu, T.; Lin, Q.; Qu, D.-H.; Gong, X.; Yang, Y.; et al. An Artificial Molecular Shuttle Operates in Lipid Bilayers for Ion Transport. J. Am. Chem. Soc. 2018, 140, 17992–17998. [Google Scholar] [CrossRef]
- Krause, S.; Feringa, B.L. Towards artificial molecular factories from framework-embedded molecular machines. Nat. Rev. Chem. 2020, 4, 550–562. [Google Scholar] [CrossRef]
- Goswami, A.; Saha, S.; Elramadi, E.; Ghosh, A.; Schmittel, M. Off-Equilibrium Speed Control of a Multistage Molecular Rotor: 2-Fold Chemical Fueling by Acid or Silver(I). J. Am. Chem. Soc. 2021, 143, 14926–14935. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, G.; Li, Q.; Xiang, J.; Jiang, H.; Wang, Y. A multistage rotational speed changing molecular rotor regulated by pH and metal cations. Nat. Commun. 2018, 9, 23641. [Google Scholar] [CrossRef]
- Civita, D.; Kolmer, M.; Simpson, G.J.; Li, A.P.; Hecht, S.; Grill, L. Control of long-distance motion of single molecules on a surface. Science 2020, 370, 957–960. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Chen, J.W.; Luan, Y.F.; Vainikka, P.A.; Thallmair, S.; Marrink, S.J.; Feringa, B.L.; Rijn, P.V. Unidirectional rotating molecular motors dynamically interact with adsorbed proteins to direct the fate of mesenchymal stem cells. Sci. Adv. 2020, 6, 2756. [Google Scholar] [CrossRef]
- Dong, J.Q.; Wee, V.; Peh, S.B.; Zhao, D. Molecular-Rotor-Driven Advanced Porous Materials. Angew. Chem. Int. Ed. 2021, 60, 16279–16292. [Google Scholar] [CrossRef]
- Bracco, S.; Miyano, T.; Negroni, M.; Bassanetti, I.; Marchio, L.; Sozzani, P.; Tohnai, N.; Comotti, A. CO2 regulates molecular rotor dynamics in porous materials. Chem. Commun. 2017, 53, 7776–7779. [Google Scholar] [CrossRef]
- Schmitt, S.; Renzer, G.; Benrath, J.; Best, A.; Jiang, S.; Landfester, K.; Butt, H.J.; Simonutti, R.; Crespy, D.; Koynov, K. Monitoring the Formation of Polymer Nanoparticles with Fluorescent Molecular Rotors. Macromolecules 2022, 55, 7284–7293. [Google Scholar] [CrossRef]
- Ellis, E.; Moorthy, S.; Chio, W.I.K.; Lee, T.C. Artificial molecular and nanostructures for advanced nanomachinery. Chem. Commun. 2018, 54, 4075–4090. [Google Scholar] [CrossRef]
- Kaleta, J.; Kaletová, E.; Císařová, I.; Teat, S.J.; Michl, J. Synthesis of Triptycene-Based Molecular Rotors for Langmuir Blodgett Monolayers. J. Org. Chem. 2015, 80, 10134–10150. [Google Scholar] [CrossRef]
- Páez-Pérez, M.; López-Duarte, I.; Vyšniauskas, A.; Brooks, N.J.; Kuimova, M.K. Imaging non-classical mechanical responses of lipid membranes using molecular rotors. Chem. Sci. 2021, 12, 2604–2613. [Google Scholar] [CrossRef]
- Ma, L.J.; Geng, Y.J.; Zhang, G.Y.; Hu, Z.W.; James, T.D.; Wang, X.F.; Wang, Z. Near-Infrared Bodipy-Based Molecular Rotors for β-Amyloid Imaging In Vivo. Adv. Healthc. Mater. 2023, 12, 2300733. [Google Scholar] [CrossRef]
- Fares, M.; Li, Y.; Liu, Y.; Miao, K.; Gao, Z.; Zhai, Y.; Zhang, X. A Molecular Rotor-Based Halo-Tag Ligand Enables a Fluorogenic Proteome Stress Sensor to Detect Protein Misfolding in Mildly Stressed Proteome. Bioconjug. Chem. 2018, 29, 215–224. [Google Scholar] [CrossRef]
- Chen, W.; Han, J.; She, J.; Wang, F.; Zhu, L.; Yu, R.-Q.; Jiang, J.H. Simultaneous imaging of lysosomal and mitochondrial viscosity during mitophagy using molecular rotors with dual-color emission. Chem. Commun. 2020, 56, 7797–7800. [Google Scholar] [CrossRef]
- Prando, G.; Perego, J.; Negroni, M.; Riccò, M.; Bracco, S.; Comotti, A.; Sozzani, P.; Carretta, P. Molecular Rotors in a Metal-Organic Framework: Muons on a Hyper-Fast Carousel. Nano Lett. 2020, 20, 7613–7618. [Google Scholar] [CrossRef]
- Perego, J.; Bezuidenhout, C.X.; Bracco, S.; Piva, S.; Prando, G.; Aloisi, C.; Carretta, P.; Kaleta, J.; Le, T.P.; Sozzani, P.; et al. Benchmark Dynamics of Dipolar Molecular Rotors in Fluorinated Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2023, 62, e202215893. [Google Scholar] [CrossRef]
- Li, J.; Wan, Y.; Jiang, G.; Ozaki, Y.; Pi, F. Hygrosensitive Dynamic Covalent Organic Framework Films: Harnessing Molecular Rotors for Moisture-Driven Actuation in Wearable Health Monitoring. Adv. Funct. Mater. 2024, 34, 2408778. [Google Scholar] [CrossRef]
- Tsurunaga, M.; Inagaki, Y.; Momma, H.; Kwon, E.; Yamaguchi, K.; Yoza, K.; Setaka, W. Dielectric Relaxation of Powdered Molecular Gyrotops Having a Thiophene Dioxide-diyl as a Dipolar Rotor. Org. Lett. 2018, 20, 6934–6937. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Inagaki, Y.; Yamaguchi, K.; Setaka, W. Structure and Dynamics of Crystalline Molecular Gyrotops with a Difluorophenylene Rotor. J. Org. Chem. 2021, 86, 2423–2430. [Google Scholar] [CrossRef]
- Yu, C.; Ma, L.; He, J.; Xiang, J.; Deng, X.; Wang, Y.; Chen, X.; Jiang, H. Flexible, Linear Chains Act as Baffles to Inhibit the Intramolecular Rotation of Molecular Turnstiles. J. Am. Chem. Soc. 2016, 138, 15849–15852. [Google Scholar] [CrossRef]
- Dong, J.Q.; Li, X.; Zhang, K.; Yuan, Y.D.; Wang, Y.X.; Zhai, L.Z.; Liu, G.L.; Yuan, D.Q.; Jiang, J.W.; Zhao, D. Confinement of Aggregation-Induced Emission Molecular Rotors in Ultrathin Two-Dimensional Porous Organic Nanosheets for Enhanced Molecular Recognition. J. Am. Chem. Soc. 2018, 140, 4035–4046. [Google Scholar] [CrossRef]
- Dong, J.Q.; Zhang, K.; Li, X.; Qian, Y.H.; Zhu, H.; Yuan, D.Q.; Xu, Q.H.; Jiang, J.W.; Zhao, D. Ultrathin two-dimensional porous organic nanosheets with molecular rotors for chemical sensing. Nat. Commun. 2017, 8, 1142. [Google Scholar] [CrossRef]
- Paez-Perez, M.; Kuimova, M.K. Molecular Rotors: Fluorescent Sensors for Microviscosity and Conformation of Biomolecules. Angew. Chem. Int. Ed. 2023, 63, e202311233. [Google Scholar] [CrossRef]
- Polita, A.; Toliautas, S.; Žvirblis, R.; Vyšniauskas, A. The effect of solvent polarity and macromolecular crowding on the viscosity sensitivity of a molecular rotor BODIPY-C10. Phys. Chem. Chem. Phys. 2020, 22, 8296–8303. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Y. Illuminating Protein Phase Separation: Reviewing Aggregation-Induced Emission, Fluorescent Molecular Rotor and Solvatochromic Fluorophore Based Probes. Chem. Eur. J. 2021, 27, 14564–14576. [Google Scholar] [CrossRef]
- Wu, Y.L.; Frasconi, M.; Liu, W.G.; Young, R.M.; Goddard, W.A.; Wasielewski, M.R.; Stoddart, J.F. Electrochemical Switching of a Fluorescent Molecular Rotor Embedded within a Bistable Rotaxane. J. Am. Chem. Soc. 2020, 142, 11835–11846. [Google Scholar] [CrossRef]
- Inukai, M.; Tamura, M.; Horike, S.; Higuchi, M.; Kitagawa, S.; Nakamura, K. Storage of CO into Porous Coordination Polymer Controlled by Molecular Rotor Dynamics. Angew. Chem. Int. Ed. 2018, 57, 8687–8690. [Google Scholar] [CrossRef]
- Feng, L.; Xie, Y.; Au-Yeung, S.K.; Hailu, H.B.; Liu, Z.; Chen, Q.; Zhang, J.; Pang, Q.; Yao, X.; Yang, M.; et al. A fluorescent molecular rotor probe for tracking plasma membranes and exosomes in living cells. Chem. Commun. 2020, 56, 8480–8483. [Google Scholar] [CrossRef] [PubMed]
- Fam, K.T.; Saladin, L.; Klymchenko, A.S.; Collot, M. Confronting molecular rotors and self-quenched dimers as fluorogenic BODIPY systems to probe biotin receptors in cancer cells. Chem. Commun. 2021, 57, 4807–4810. [Google Scholar] [CrossRef] [PubMed]
- Zsila, F. Comment on “Emissive H-Aggregates of an Ultrafast Molecular Rotor: A Promising Platform for Sensing Heparin”. ACS Appl. Mater. Inter. 2021, 13, 50585–50588. [Google Scholar] [CrossRef] [PubMed]
- Szymański, W.; Beierle, J.M.; Kistemaker, H.A.V.; Velema, W.A.; Feringa, B.L. Reversible Photocontrol of Biological Systems by the Incorporation of Molecular Photoswitches. Chem. Rev. 2013, 113, 6114–6178. [Google Scholar] [CrossRef]
- Tylkowski, B.; Trojanowska, A.; Marturano, V.; Nowak, M.; Marciniak, L.; Giamberini, M.; Ambrogi, V.; Cerruti, P. Power of light-Functional complexes based on azobenzene molecules. Coord. Chem. Rev. 2017, 351, 205–217. [Google Scholar] [CrossRef]
- Adam, A.; Haberhauer, G. Switching Process Consisting of Three Isomeric States of an Azobenzene Unit. J. Am. Chem. Soc. 2017, 139, 9708–9713. [Google Scholar] [CrossRef]
- Islam Sk, A.; Kundu, K.; Kundu, P.K. Azobenzene Isomerization-Induced Photomodulation of Electronic Properties of N-Heterocyclic Carbenes. Chem. Eur. J. 2020, 26, 4214–4219. [Google Scholar] [CrossRef]
- Kawamoto, M.; Shiga, N.; Takaishi, K.; Yamashita, T. Non-destructive erasable molecular switches and memory using light-driven twisting motions. Chem. Commun. 2010, 46, 8344. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Friss, T.R.; Enriquez, M.M.; Isley, W.; Incarvito, C.; Frank, H.A.; Gascon, J.; Burdette, S.C. Proof for the Concerted Inversion Mechanism in the trans→cis Isomerization of Azobenzene Using Hydrogen Bonding to Induce Isomer Locking. J. Org. Chem. 2010, 75, 4817–4827. [Google Scholar] [CrossRef]
- Tkachenko, I.M.; Kurioz, Y.I.; Kravchuk, R.M.; Kobzar, Y.L.; Litoshenko, D.V.; Glushchenko, A.V.; Shevchenko, V.V.; Nazarenko, V.G. Photoinduced Birefringence and Liquid Crystal Orientation on Polymers with Different Azobenzene Content in the Main Chain. ACS Appl. Mater. Inter. 2024, 16, 52945–52957. [Google Scholar] [CrossRef]
- Zheng, M.; Yuan, J. Polymeric nanostructures based on azobenzene and their biomedical applications: Synthesis, self-assembly and stimuli-responsiveness. Org. Biomol. Chem. 2022, 20, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.B.; Zhang, S.; Qi, J.; Liang, X.J.; Yoon, J. Advances in Application of Azobenzene as a Trigger in Biomedicine: Molecular Design and Spontaneous Assembly. Adv. Mater. 2021, 33, 2007290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, H.; Du, Q.; Zhang, G.; Zhu, S.; Wu, Z.; Luo, X. Photoliquefiable Azobenzene Surfactants toward Solar Thermal Fuels that Upgrade Photon Energy Storage via Molecular Design. Small 2022, 19, 2206623. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, W.; Zhang, Y.X.; Jiang, J.Z. Quintuple-Decker Heteroleptic Phthalocyanine Heterometallic Samarium-Cadmium Complexes. Synthesis, Crystal Structure, Electrochemical Behavior, and Spectroscopic Investigation. Inorg. Chem. 2020, 59, 17591–17599. [Google Scholar] [CrossRef]
- Liu, W.B.; Pan, H.H.; Wang, Z.Q.; Wang, K.; Qi, D.D.; Jiang, J.Z. Sandwich rare earth complexes simultaneously involving aromatic phthalocyanine and antiaromatic hemiporphyrazine ligands showing a predominantly aromatic nature. Chem. Commun. 2017, 53, 3765–3768. [Google Scholar] [CrossRef]
- Martynov, A.G.; Horii, Y.; Katoh, K.; Bian, Y.Z.; Jiang, J.Z.; Yamashita, M.; Gorbunova, Y.G. Rare-earth based tetrapyrrolic sandwiches: Chemistry, materials and applications. Chem. Soc. Rev. 2022, 51, 9262–9339. [Google Scholar] [CrossRef]
- Tashiro, K.; Konishi, K.; Aida, T. Enantiomeric Resolution of Chiral Metallobis(porphyrin)s: Studies on Rotatability of Electronically Coupled Porphyrin Ligands. Angew. Chem. Int. Ed. 1997, 36, 856–858. [Google Scholar] [CrossRef]
- Tashiro, K.; Fujiwara, T.; Konishi, K.; Aida, T. Rotational oscillation of two interlocked porphyrins in cerium bis(5,15-diarylporphyrinate) double-deckers. Chem. Commun. 1998, 10, 1121–1122. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, Y.; Ou, Z.; Jiang, J. Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific: Singapore, 2011; Volume 14, pp. 249–460. [Google Scholar]
- Sun, X.; Li, R.; Wang, D.; Dou, J.; Zhu, P.; Lu, F.; Ma, C.; Choi, C.F.; Cheng, D.Y.Y.; Ng, D.K.P.; et al. Synthesis and Characterization of Mixed Phthalocyaninato and meso-Tetrakis (4-chlorophenyl) porphyrinato Triple-Decker Complexes-Revealing the Origin of Their Electronic Absorptions. Eur. J. Inorg. Chem. 2004, 19, 3806–3813. [Google Scholar] [CrossRef]
- Das, G.; Prakasam, T.; Addicoat, M.A.; Sharma, S.K.; Rayaux, F.; Mathew, R.; Baias, M.; Jagannathan, R.; Olson, M.A.; Trabolsi, A. Azobenzene-Equipped Covalent Organic Framework: Light-Operated Reservoir. J. Am. Chem. Soc. 2019, 141, 19078–19087. [Google Scholar] [CrossRef]
- Birin, K.P.; Gorbunova, Y.G.; Tsivadze, A.Y. NMR investigation of intramolecular dynamics of heteroleptic triple-decker (porphyrinato)(phthalocyaninato) lanthanides. Dalton Trans. 2011, 40, 11474–11479. [Google Scholar] [CrossRef] [PubMed]
- Huggins, M.T.; Kesharwani, T.; Buttrick, J.; Nicholson, C. Variable Temperature NMR Experiment Studying Restricted Bond Rotation. J. Chem. Educ. 2020, 97, 1425–1429. [Google Scholar] [CrossRef]
- Lu, W.X.; Li, L.K.; Yang, H.F.; Zhao, L.Y.; Qi, D.D.; Bian, Y.Z.; Jiang, J.Z. Intramolecular chirality induction and intermolecular chirality modulation in BINOL bridged bisporphyrin hosts. Dyes Pigments 2017, 137, 608–614. [Google Scholar] [CrossRef]
- Cao, W.; Wang, H.; Wang, X.; Lee, H.K.; Ng, D.K.P.; Jiang, J. Constructing Sandwich-Type Rare Earth Double-Decker Complexes with N-Confused Porphyrinato and Phthalocyaninato Ligands. Inorg. Chem. 2012, 51, 9265–9272. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 mode. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Chen, X.; Chen, T.T.; Li, W.L.; Lu, J.B.; Zhao, L.J.; Jian, T.; Hu, H.S.; Wang, L.S.; Li, J. Lanthanides with Unusually Low Oxidation States in the PrB3– and PrB4– Boride Clusters. Inorg. Chem. 2019, 58, 411–418. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Hay, P.J. Modern Theoretical Chemistry; Schaefer, H.F., III, Ed.; Plenum: New York, NY, USA, 1977; Volume 3, pp. 1–28. [Google Scholar]
- Peng, C.; Ayala, P.Y.; Schlegel, H.B.; Frisch, M.J. Using redundant internal coordinates to optimize equilibrium geometries and transition states. J. Comput. Chem. 1996, 17, 49. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, W.; Mu, T.; Zhang, Y.; Chen, B.; Guo, H.; Zhao, L.; Wang, P.; Bian, Y. A Photocontrolled Molecular Rotor Based on Azobenzene-Strapped Mixed (Phthalocyaninato)(Porphyrinato) Rare Earth Triple-Decker. Molecules 2025, 30, 326. https://doi.org/10.3390/molecules30020326
Lu W, Mu T, Zhang Y, Chen B, Guo H, Zhao L, Wang P, Bian Y. A Photocontrolled Molecular Rotor Based on Azobenzene-Strapped Mixed (Phthalocyaninato)(Porphyrinato) Rare Earth Triple-Decker. Molecules. 2025; 30(2):326. https://doi.org/10.3390/molecules30020326
Chicago/Turabian StyleLu, Wenxin, Tiantian Mu, Yuehong Zhang, Bo Chen, Huantao Guo, Luyang Zhao, Peng Wang, and Yongzhong Bian. 2025. "A Photocontrolled Molecular Rotor Based on Azobenzene-Strapped Mixed (Phthalocyaninato)(Porphyrinato) Rare Earth Triple-Decker" Molecules 30, no. 2: 326. https://doi.org/10.3390/molecules30020326
APA StyleLu, W., Mu, T., Zhang, Y., Chen, B., Guo, H., Zhao, L., Wang, P., & Bian, Y. (2025). A Photocontrolled Molecular Rotor Based on Azobenzene-Strapped Mixed (Phthalocyaninato)(Porphyrinato) Rare Earth Triple-Decker. Molecules, 30(2), 326. https://doi.org/10.3390/molecules30020326






