Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides
Abstract
1. Introduction
2. Results
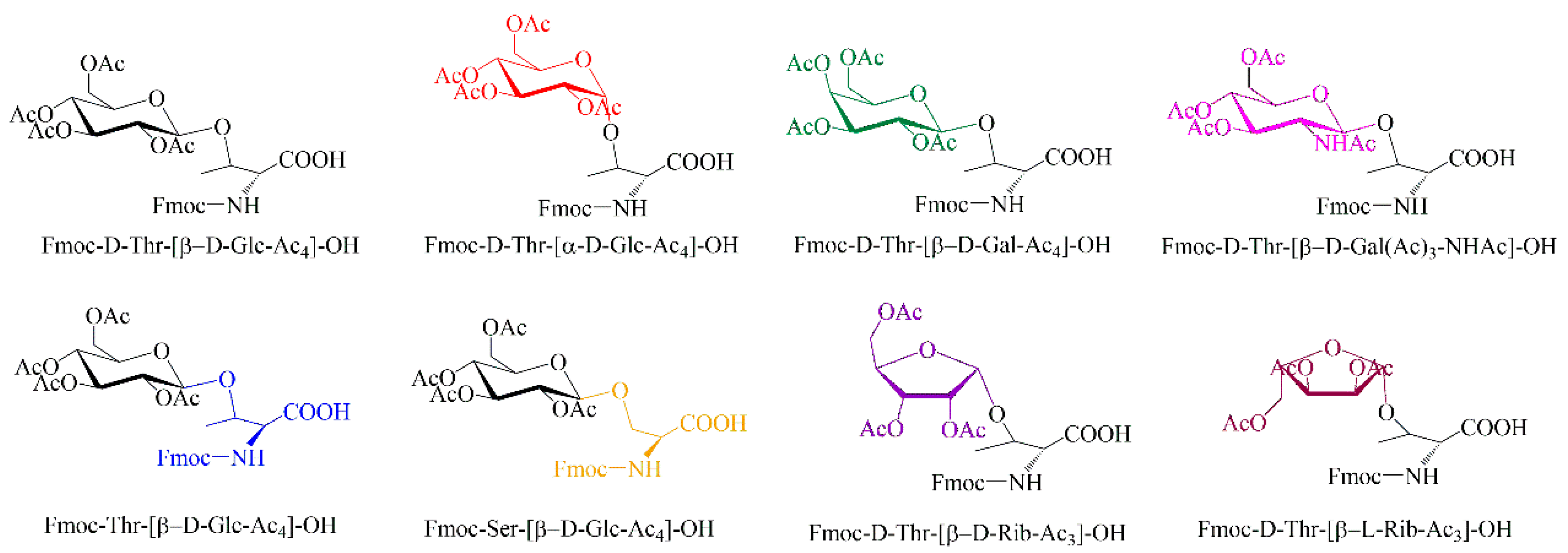
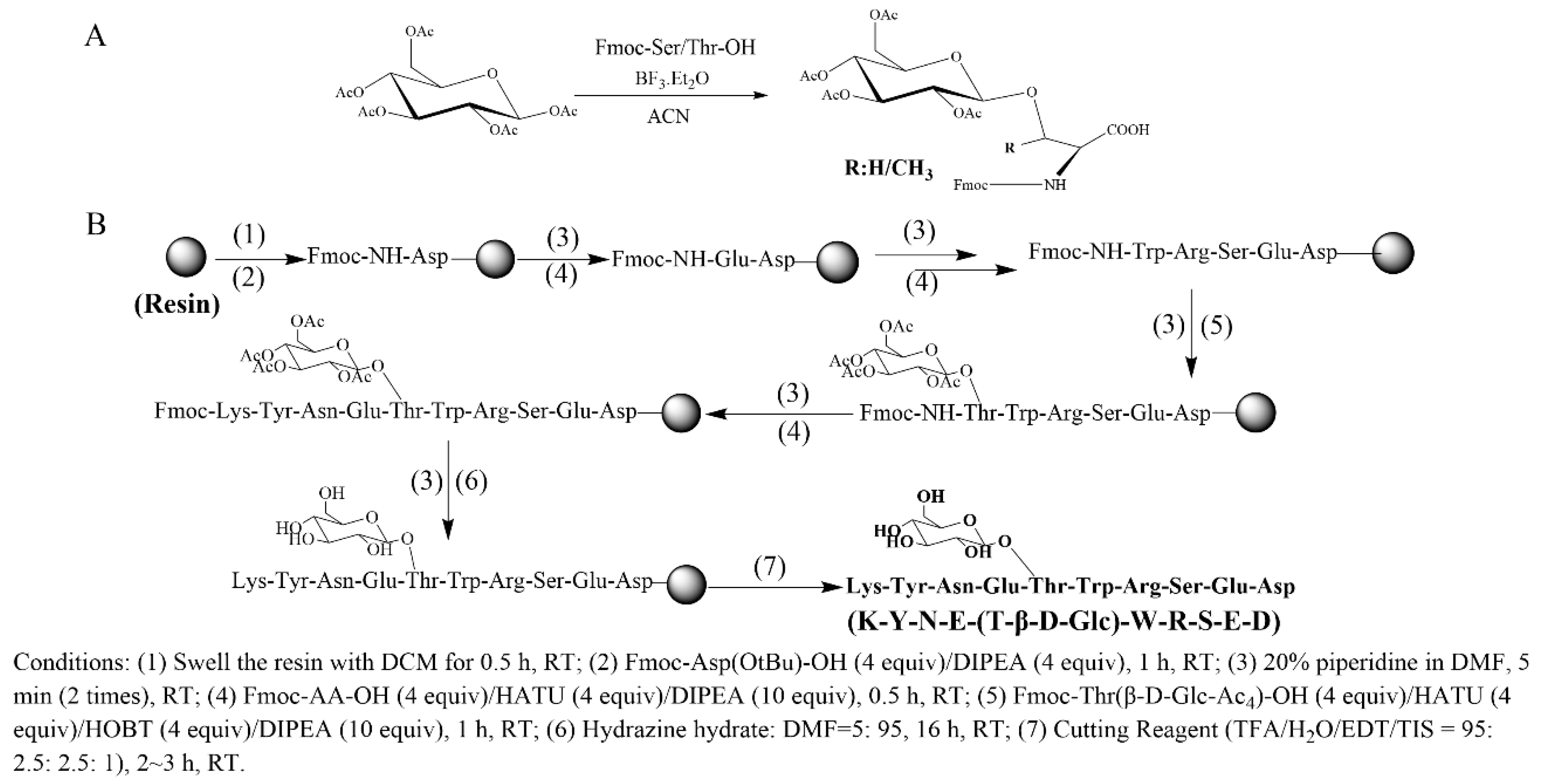
2.1. Design and Synthesis of Glycosylated Peptides
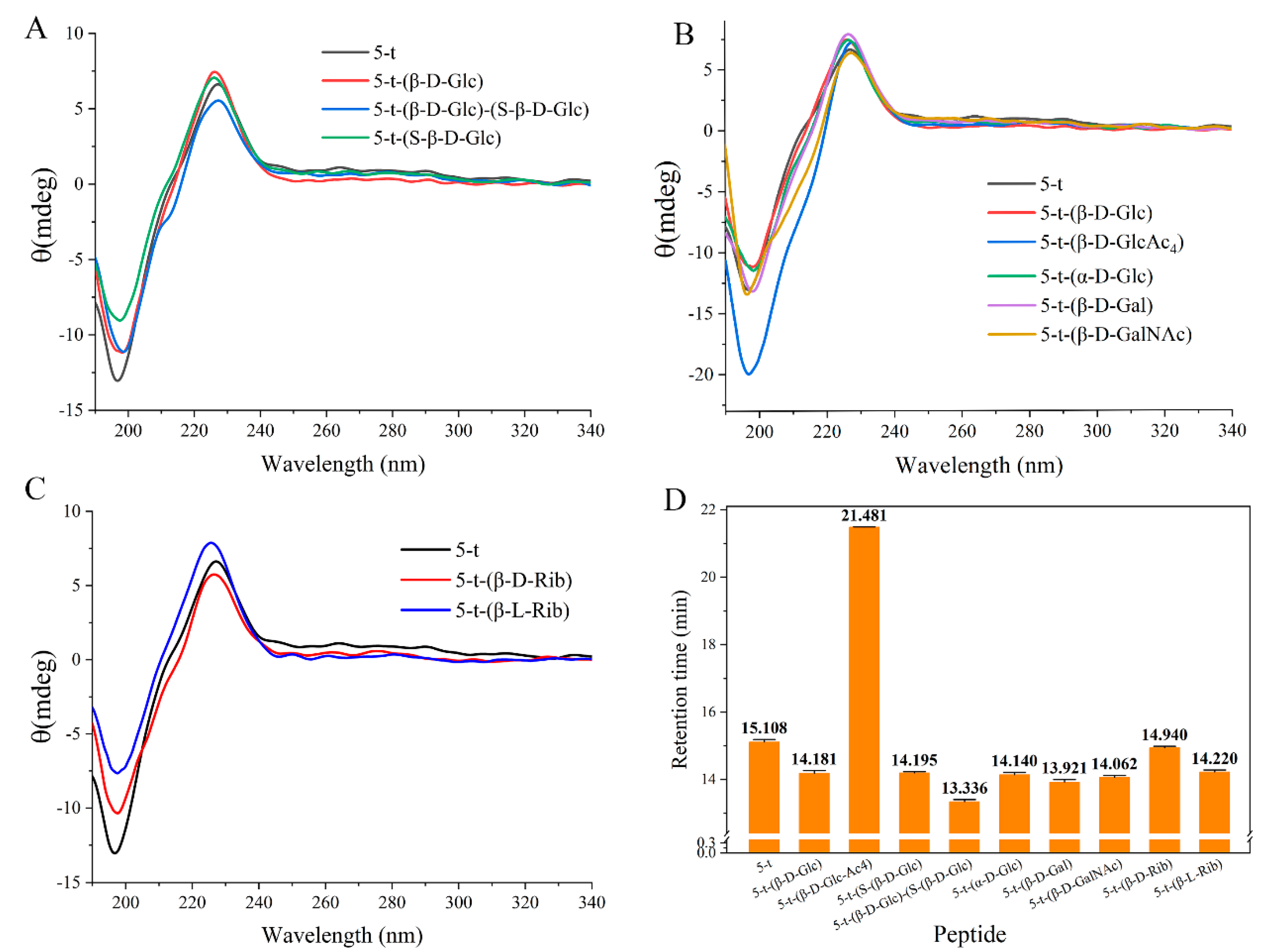
2.2. Effect of Monosaccharides on Peptide Structure
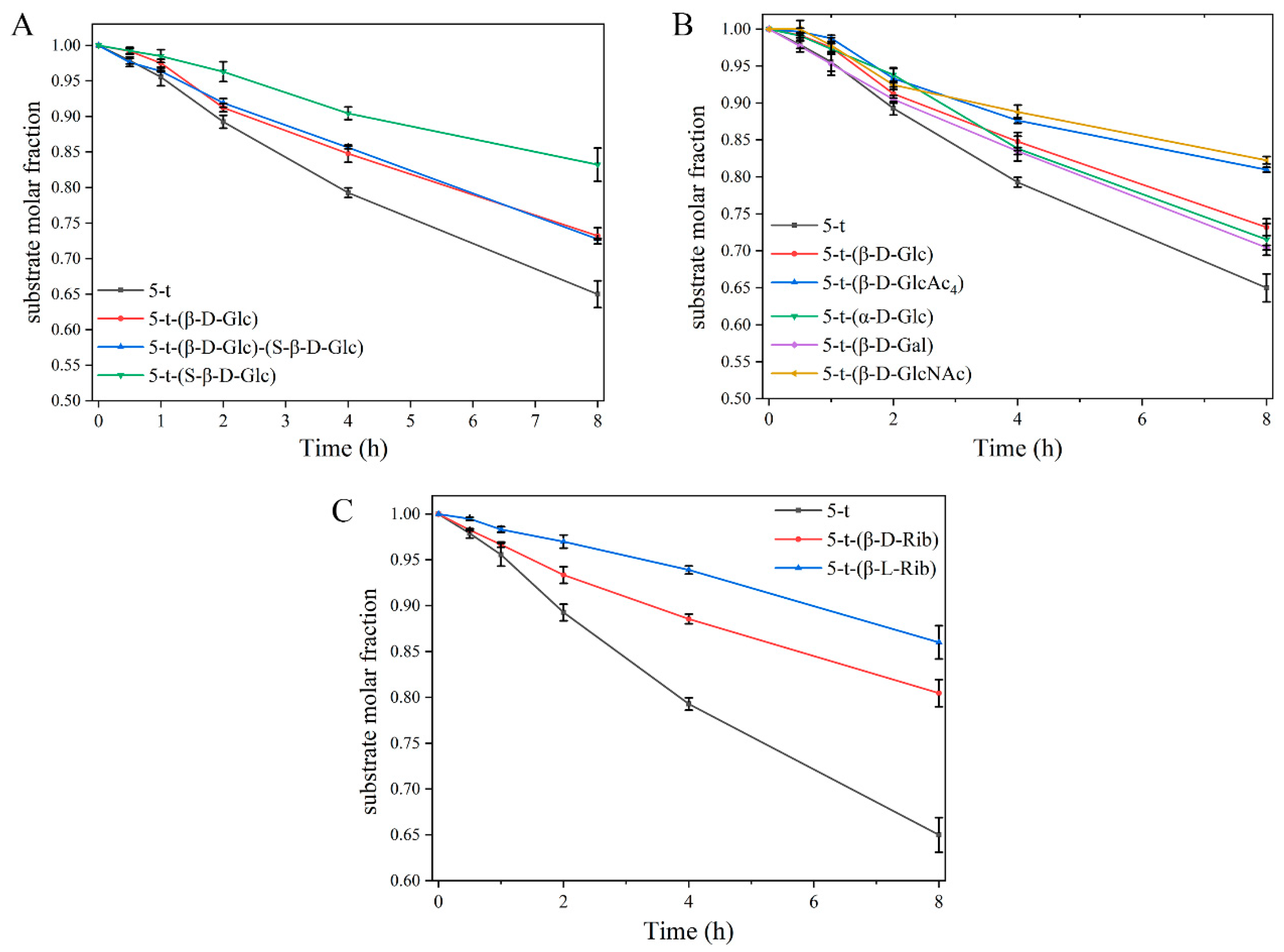
2.3. Effect of Glycosylation on Enzymolysis Kinetics of Peptides
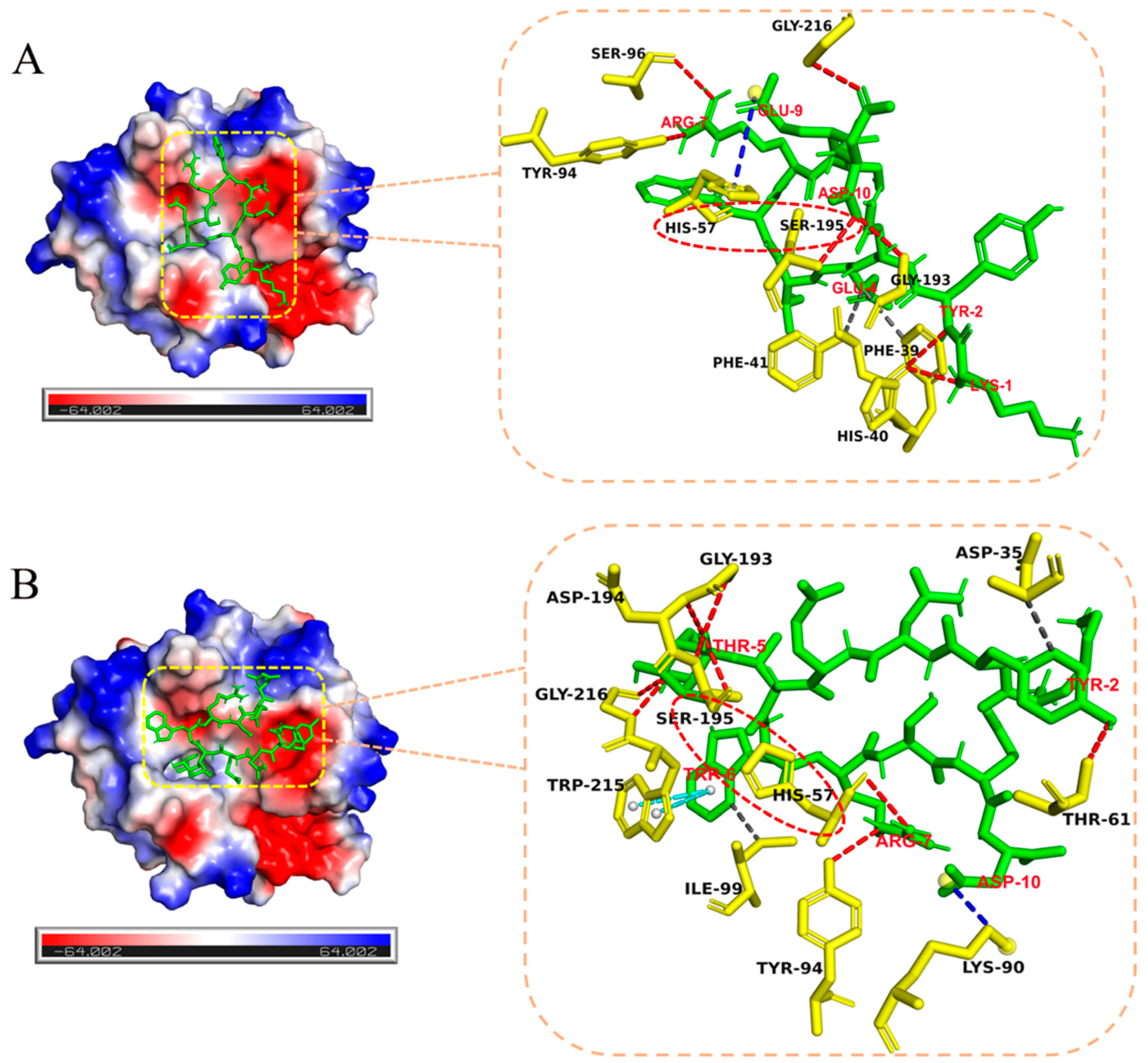
2.4. Effect of Glycosylation on Enzymatic Cleavage Site of 5-t
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Synthesis of Glycosylated Amino Acids
4.3. Peptide Synthesis
4.4. Purification of Peptides by Reversed-Phase HPLC
4.5. High-Resolution Mass Spectra
4.6. Circular Dichroism
4.7. Protease Resistance
4.8. HPLC Analysis of the Enzymatic Digestion Results
4.9. Kinetic Analysis
4.10. Docking Calculations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, T.Y.; Suh, J. Target-selective peptide-cleaving catalysts as a new paradigm in drug design. Chem. Soc. Rev. 2009, 38, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Prakash, J.; Kodanko, J.J. Metal-based methods for protein inactivation. Curr. Opin. Chem. Biol. 2013, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A.; Sasaki, D.; Shiohara, A.; Iwatsubo, T.; Tomita, T.; Sohma, Y.; Kanai, M. Attenuation of the Aggregation and Neurotoxicity of Amyloid-β Peptides by Catalytic Photooxygenation. Angew. Chem. Int. Ed. 2014, 53, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Arnau, J.; Lauritzen, C.; Petersen, G.E.; Pedersen, J. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 2006, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Seki, Y.; Tanabe, K.; Sasaki, D.; Sohma, Y.; Oisaki, K.; Kanai, M. Serine-selective aerobic cleavage of peptides and a protein using a water-soluble copper-organoradical conjugate. Angew. Chem. Int. Ed. 2014, 53, 6501–6505. [Google Scholar] [CrossRef]
- Yan, L.; Ke, Y.; Kan, Y.; Lin, D.; Yang, J.; He, Y.; Wu, L. New insight into enzymatic hydrolysis of peptides with site-specific amino acid d-isomerization. Bioorganic Chem. 2020, 105, 104389. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, M.; Qiu, S.; Wang, J.; Peng, J.; Zhao, P.; Zhu, R.; Wang, H.; Li, Y.; Wang, K. Antimicrobial activity and stability of the D-amino acid substituted derivatives of antimicrobial peptide polybia-MPI. AMB Express 2016, 6, 122. [Google Scholar] [CrossRef]
- Tochio, N.; Murata, T.; Utsunomiya-Tate, N. Effect of site-specific amino acid D-isomerization on beta-sheet transition and fibril formation profiles of Tau microtubule-binding repeat peptides. Biochem. Biophys. Res. Commun. 2019, 508, 184–190. [Google Scholar] [CrossRef]
- Lin, C.H.; Yang, H.T.; Lane, H.Y. D-glutamate, D-serine, and D-alanine differ in their roles in cognitive decline in patients with Alzheimer’s disease or mild cognitive impairment. Pharmacol. Biochem. Behav. 2019, 185, 172760. [Google Scholar] [CrossRef]
- Fujii, N.; Takata, T.; Fujii, N.; Aki, K.; Sakaue, H. D-Amino acids in protein: The mirror of life as a molecular index of aging. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2018, 1866, 840–847. [Google Scholar] [CrossRef]
- Kita, Y.; Nishii, Y.; Higuchi, T.; Mashima, K. Zinc-catalyzed amide cleavage and esterification of β-hydroxyethylamides. Angew. Chem. Int. Ed. 2012, 51, 5723–5726. [Google Scholar] [CrossRef] [PubMed]
- Ariani, H.H.; Polkowska-Nowakowska, A.; Bal, W. Effect of D-amino acid substitutions on Ni(II)-assisted peptide bond hydrolysis. Inorg. Chem. 2013, 52, 2422–2431. [Google Scholar] [CrossRef] [PubMed]
- Hie, L.; Fine Nathel, N.F.; Shah, T.K.; Baker, E.L.; Hong, X.; Yang, Y.F.; Liu, P.; Houk, K.N.; Garg, N.K. Conversion of amides to esters by the nickel-catalysed activation of amide C-N bonds. Nature 2015, 524, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Kassai, M.; Ravi, R.G.; Shealy, S.J.; Grant, K.B. Unprecedented Acceleration of Zirconium(IV)-Assisted Peptide Hydrolysis at Neutral pH. Inorg. Chem. 2004, 43, 6130–6132. [Google Scholar] [CrossRef]
- Zhang, T.; Sharma, G.; Paul, T.J.; Hoffmann, Z.; Prabhakar, R. Effects of Ligand Environment in Zr(IV) Assisted Peptide Hydrolysis. J. Chem. Inf. Model. 2017, 57, 1079–1088. [Google Scholar] [CrossRef]
- Belczyk-Ciesielska, A.; Zawisza, I.A.; Mital, M.; Bonna, A.; Bal, W. Sequence-specific Cu(II)-dependent peptide bond hydrolysis: Similarities and differences with the Ni(II)-dependent reaction. Inorg. Chem. 2014, 53, 4639–4646. [Google Scholar] [CrossRef]
- Ni, J.; Sohma, Y.; Kanai, M. Scandium(iii) triflate-promoted serine/threonine-selective peptide bond cleavage. Chem. Commun. 2017, 53, 3311–3314. [Google Scholar] [CrossRef]
- Kita, Y.; Nishii, Y.; Onoue, A.; Mashima, K. Combined Catalytic System of Scandium Triflate and Boronic Ester for Amide Bond Cleavage. Adv. Synth. Catal. 2013, 355, 3391–3395. [Google Scholar] [CrossRef]
- Degani, Y.; Patchornik, A. Cyanylation of sulfhydryl groups by 2-nitro-5-thiocyanobenzoic acid. High-yield modification and cleavage of peptides at cysteine residues. Biochemistry 1974, 13, 1–11. [Google Scholar] [CrossRef]
- Tanabe, K.; Taniguchi, A.; Matsumoto, T.; Oisaki, K.; Sohma, Y.; Kanai, M. Asparagine-selective cleavage of peptide bonds through hypervalent iodine-mediated Hofmann rearrangement in neutral aqueous solution. Chem. Sci. 2014, 5, 2747. [Google Scholar] [CrossRef]
- Elashal, H.E.; Raj, M. Site-selective chemical cleavage of peptide bonds. Chem. Commun. 2016, 52, 6304–6307. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Metzka, L. Enhancement of Cyanogen Bromide Cleavage Yields for Methionyl-Serine and Methionyl-Threonine Peptide Bonds. Anal. Biochem. 1999, 266, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Gayle, C.J.C.; Hamley, I.W. Self-assembly of bioactive peptides, peptide conjugates, and peptide mimetic materials. Org. Biomol. Chem. 2017, 15, 5867–5876. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, S.; Zhang, D.; Zhang, W.; Zhang, H.; Zou, J.; Mao, Z.; Yuan, Y.; Gao, C.; Liu, R. Impact of Antifouling PEG Layer on the Performance of Functional Peptides in Regulating Cell Behaviors. J. Am. Chem. Soc. 2019, 141, 16772–16780. [Google Scholar] [CrossRef]
- Nielsen, J.E.; König, N.; Yang, S.; Skoda, M.W.A.; Maestro, A.; Dong, H.; Cárdenas, M.; Lund, R. Lipid membrane interactions of self-assembling antimicrobial nanofibers: Effect of PEGylation. RSC Adv. 2020, 10, 35329–35340. [Google Scholar] [CrossRef]
- Shin, E.; Lim, C.; Kang, U.J.; Kim, M.; Park, J.; Kim, D.; Choi, W.; Hong, J.; Baig, C.; Lee, D.W.; et al. Mussel-Inspired Copolyether Loop with Superior Antifouling Behavior. Macromolecules 2020, 53, 3551–3562. [Google Scholar] [CrossRef]
- Eldeeb, M.A.; Fahlman, R.P.; Ragheb, M.A.; Esmaili, M. Does N-Terminal Protein Acetylation Lead to Protein Degradation? BioEssays 2019, 41, 1800167. [Google Scholar] [CrossRef]
- Bell, R.; Thrush, R.J.; Castellana-Cruz, M.; Oeller, M.; Staats, R.; Nene, A.; Flagmeier, P.; Xu, C.K.; Satapathy, S.; Galvagnion, C.; et al. N-Terminal Acetylation of α-Synuclein Slows down Its Aggregation Process and Alters the Morphology of the Resulting Aggregates. Biochemistry 2022, 61, 1743–1756. [Google Scholar] [CrossRef]
- Guan, X.; Chaffey, P.K.; Zeng, C.; Greene, E.R.; Chen, L.; Drake, M.R.; Chen, C.; Groobman, A.; Resch, M.G.; Himmel, M.E.; et al. Molecular-scale features that govern the effects of O-glycosylation on a carbohydrate-binding module. Chem. Sci. 2015, 6, 7185–7189. [Google Scholar] [CrossRef]
- Dwivedi, R.; Aggarwal, P.; Bhavesh, N.S.; Kaur, K.J. Design of therapeutically improved analogue of the antimicrobial peptide, indolicidin, using a glycosylation strategy. Amino Acids 2019, 51, 1443–1460. [Google Scholar] [CrossRef]
- Yan, L.; Ke, Y.; Wang, Y.; Yang, J.; He, Y.; Wu, L. Effect of Mini-PEGs Modification on the Enzymatic Digestion of D-Amino Acid-Containing Peptides under the Action of PROK. Chem. Eur. J. 2023, 29, e202203524. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wu, M.; Chang, Q.; Hu, H.; Zhao, X. Improving Selectivity, Proteolytic Stability, and Antitumor Activity of Hymenochirin-1B: A Novel Glycosylated Staple Strategy. ACS Chem. Biol. 2019, 14, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Muthana, S.M.; Farnsworth, D.; Ludek, O.; Adams, K.; Barchi, J.J., Jr.; Gildersleeve, J.C. Enhanced Epimerization of Glycosylated Amino Acids During Solid-Phase Peptide Synthesis. J. Am. Chem. Soc. 2012, 134, 6316–6325. [Google Scholar] [CrossRef]
- Polt, R.; Dhanasekaran, M.; Keyari, C.M. Glycosylated neuropeptides: A new vista for neuropsychopharmacology? Med. Res. Rev. 2005, 25, 557–585. [Google Scholar] [CrossRef]
- Varamini, P.; Mansfeld, F.M.; Blanchfield, J.T.; Wyse, B.D.; Smith, M.T.; Toth, I. Synthesis and biological evaluation of an orally active glycosylated endomorphin-1. J. Med. Chem. 2012, 55, 5859–5867. [Google Scholar] [CrossRef]
- Mehta, S.; Meldal, M.; Duus, J.; Bock, K. Evaluation of the effect of glycosylation on the enzymic hydrolysis of peptides. J. Chem. Soc. Perkin Trans. 1999, 1, 1445–1451. [Google Scholar] [CrossRef]
- He, C.; Wu, S.; Liu, D.; Chi, C.; Zhang, W.; Ma, M.; Lai, L.; Dong, S. Glycopeptide Self-Assembly Modulated by Glycan Stereochemistry through Glycan-Aromatic Interactions. J. Am. Chem. Soc. 2020, 142, 17015–17023. [Google Scholar] [CrossRef]
- Wu, M.H.; Ai, S.; Chen, Q.; Chen, X.Y.; Li, H.J.; Li, Y.L.; Zhao, X. Effects of Glycosylation and d-Amino Acid Substitution on the Antitumor and Antibacterial Activities of Bee Venom Peptide HYL. Bioconjugate Chem. 2020, 31, 2293–2302. [Google Scholar] [CrossRef]
- Jin, Y.; Penning, T.M. Aldo-Keto Reductases and Bioactivation/Detoxication. Annu. Rev. Pharmacool. Toxicol. 2007, 47, 263–292. [Google Scholar] [CrossRef]
- Hansen, L.H.; Madsen, T.D.; Goth, C.K.; Clausen, H.; Chen, Y.; Dzhoyashvili, N.; Iyer, S.R.; Sangaralingham, S.J.; Burnett, J.C., Jr.; Rehfeld, J.F.; et al. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J. Biol. Chem. 2019, 294, 12567–12578. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, H.; Blow, D.M. Structure of α-chymotrypsin refined at 1.68 Å resolution. J. Mol. Biol. 1985, 184, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dauter, M.; Dauter, Z. What can be done with a good crystal and an accurate beamline? Acta Crystallogr. Sect. D 2006, 62, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef]

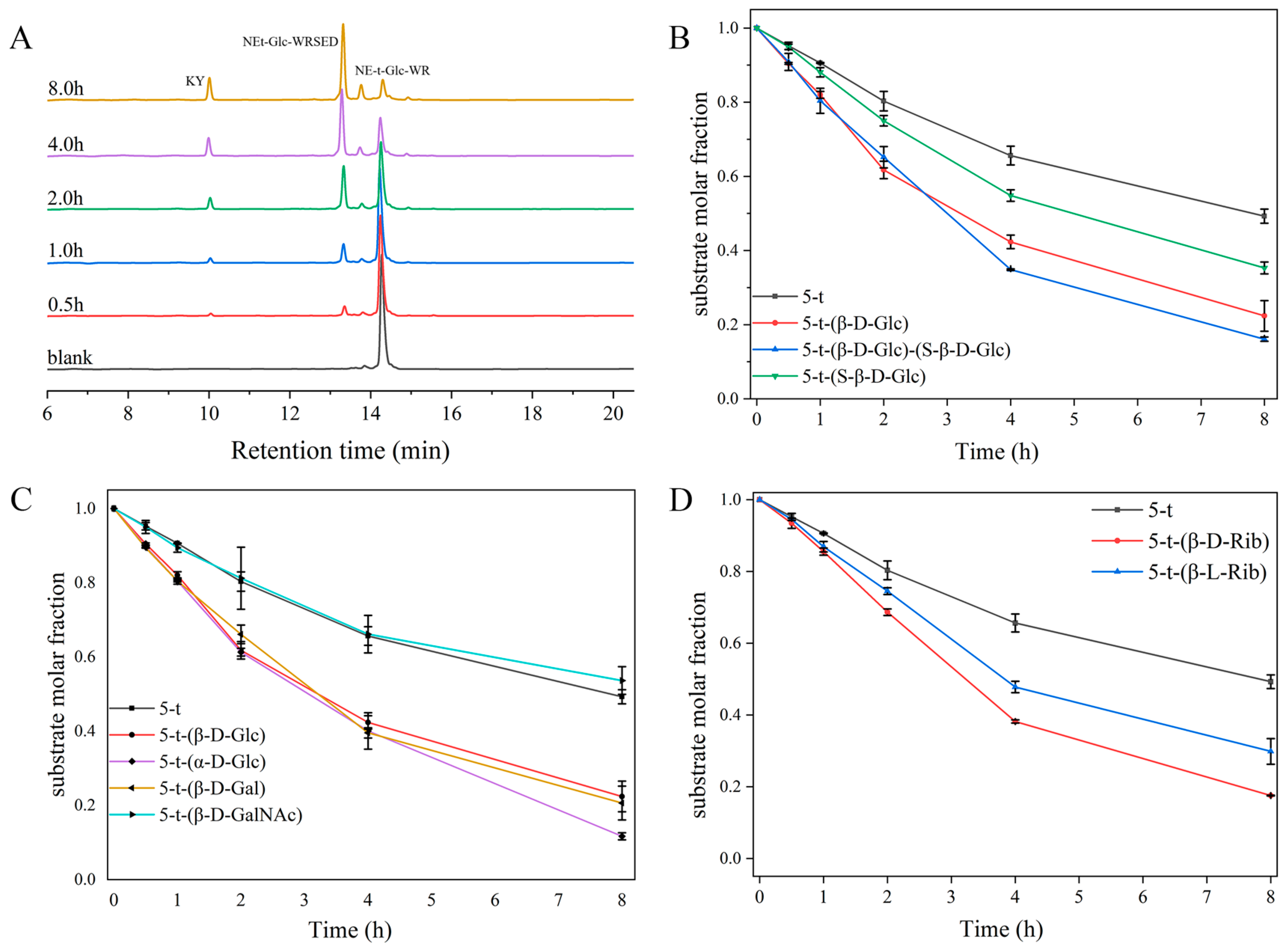

| Peptides | Sequence |
|---|---|
| all-L | KYNETWRSED |
| all-L-(T-β-D-Glc) | KYNE(T-β-D-Glc)WRSED |
| all-L-(T-β-D-Glc)-(S-β-D-Glc) | KYNE(T-β-D-Glc)WR(S-β-D-Glc)ED |
| 5-t | KYNEtWRSED |
| 5-t-(β-D-Glc) | KYNE(t-β-D-Glc)WRSED |
| 5-t-(β-D-Glc)-(S-β-D-Glc) | KYNE(t-β-D-Glc)WR(S-β-D-Glc)ED |
| 5-t-(S-β-D-Glc) | KYNEtWR(S-β-D-Glc)ED |
| 5-t-(β-D-GlcAc4) | KYNE(t-β-D-Glc-Ac4)WRSED |
| 5-t-(α-D-Glc) | KYNE(t-α-D-Glc)WRSED |
| 5-t-(β-D-Gal) | KYNE(t-β-D-Gal)WRSED |
| 5-t-(β-D-GalNAc) | KTNE(t-β-D-Gal-NAc)WRSED |
| 5-t-(β-D-Rib) | KYNE(t-β-D-Rib)WRSED |
| 5-t-(β-L-Rib) | KYNE(t-β-L-Rib)WRSED |
| Peptides | Enzymatic Fragments | Cleavage Sites |
|---|---|---|
| 5-t | KY, NE-t-WR, NE-t-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-D-Glc) | KY, NE-(t-β-D-Glc)-WR, NE-(t-β-D-Glc)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-D-Glc)-(S-β-D-Glc) | KY, NE-(t-β-D-Glc)-WR, NE-(t-β-D-Glc)-WR-(S-β-D-Glc)-ED, (S-β-D-Glc)-ED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(S-β-D-Glc) | KY, KYNE-t-WR, NE-t-WR, NE-t-WR-(S-β-D-Glc)-ED, (S-β-D-Glc)-ED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(α-D-Glc) | KY, NE-(t-α-D-Glc)-WR, NE-(t-α-D-Glc)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-D-Gal) | KY, NE-(t-β-D-Gal)-WR, NE-(t-β-D-Gal)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-D-GalNAc) | KY, NE-(t-β-D-GalNAc)-WR, NE-(t-β-D-Gal-NAc)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-D-Rib) | KY, KYNE-(t-β-D-Rib)-WR, NE-(t-β-D-Rib)-WR, NE-(t-β-D-Rib)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
| 5-t-(β-L-Rib) | KY, KYNE(t-β-L-Rib)-WR, NE-(t-β-L-Rib)-WR, NE-(t-β-L-Rib)-WRSED, SED | Tyr2-Asn3, Arg7-Ser8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Jin, Z.; Yu, T.; Guo, C.; He, Y.; Kan, Y.; Yan, L.; Wu, L. Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides. Molecules 2025, 30, 441. https://doi.org/10.3390/molecules30030441
Cui S, Jin Z, Yu T, Guo C, He Y, Kan Y, Yan L, Wu L. Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides. Molecules. 2025; 30(3):441. https://doi.org/10.3390/molecules30030441
Chicago/Turabian StyleCui, Shuaishuai, Zhaoyang Jin, Tonglin Yu, Cunxin Guo, Yujian He, Yuhe Kan, Liang Yan, and Li Wu. 2025. "Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides" Molecules 30, no. 3: 441. https://doi.org/10.3390/molecules30030441
APA StyleCui, S., Jin, Z., Yu, T., Guo, C., He, Y., Kan, Y., Yan, L., & Wu, L. (2025). Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides. Molecules, 30(3), 441. https://doi.org/10.3390/molecules30030441






