MXene-Reinforced Composite Cryogel Scaffold for Neural Tissue Repair
Abstract
:1. Introduction
2. Results and Discussion
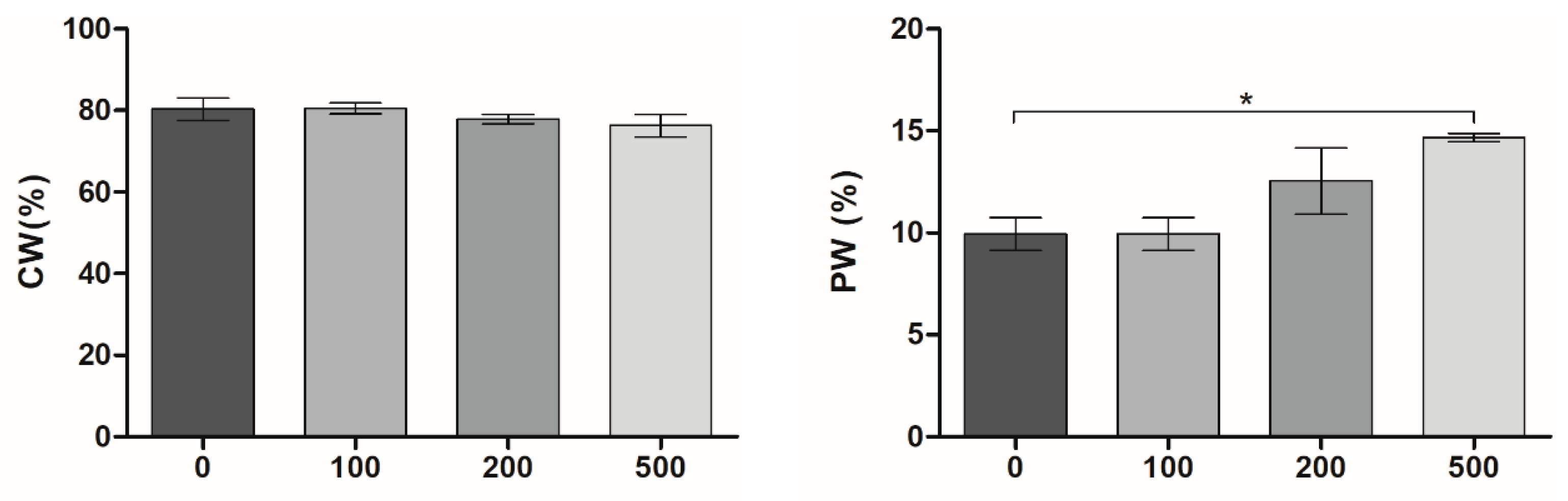
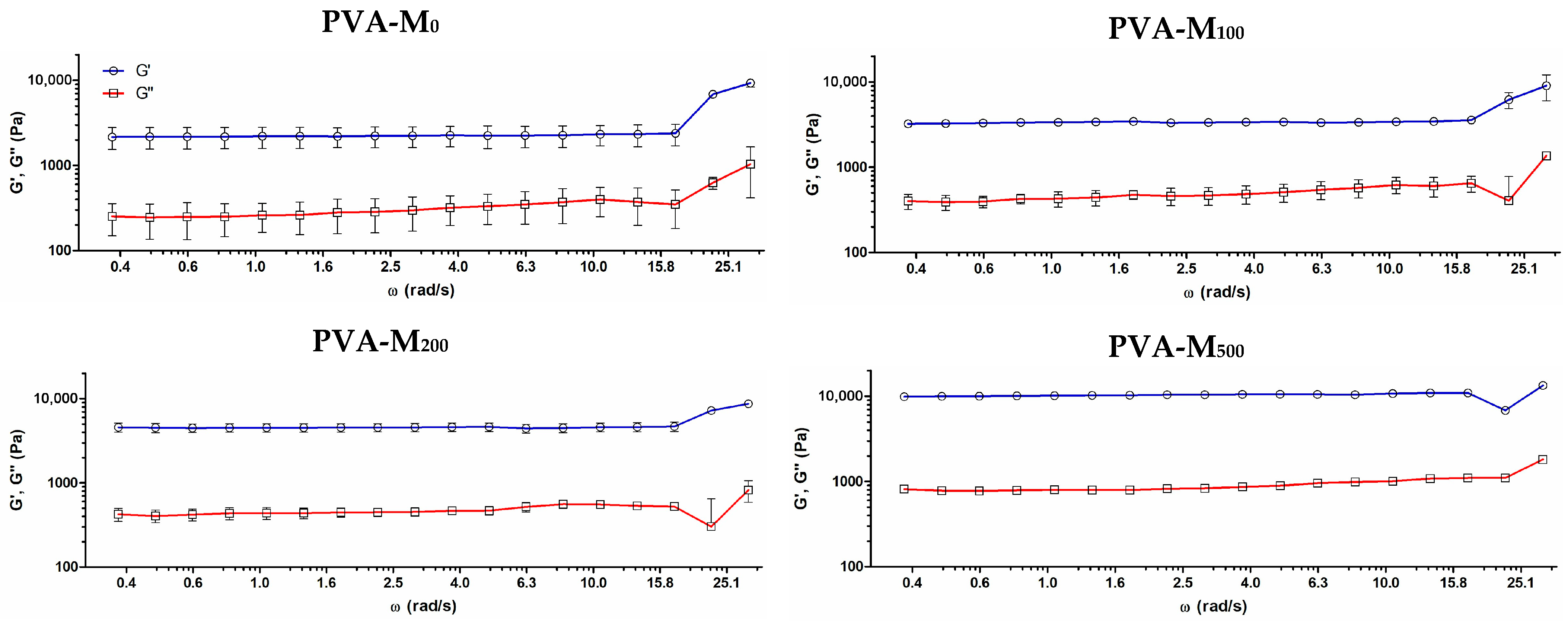
2.1. Preparation and Characterization of MXene/PVA Cryogels
2.2. Cell Behavior
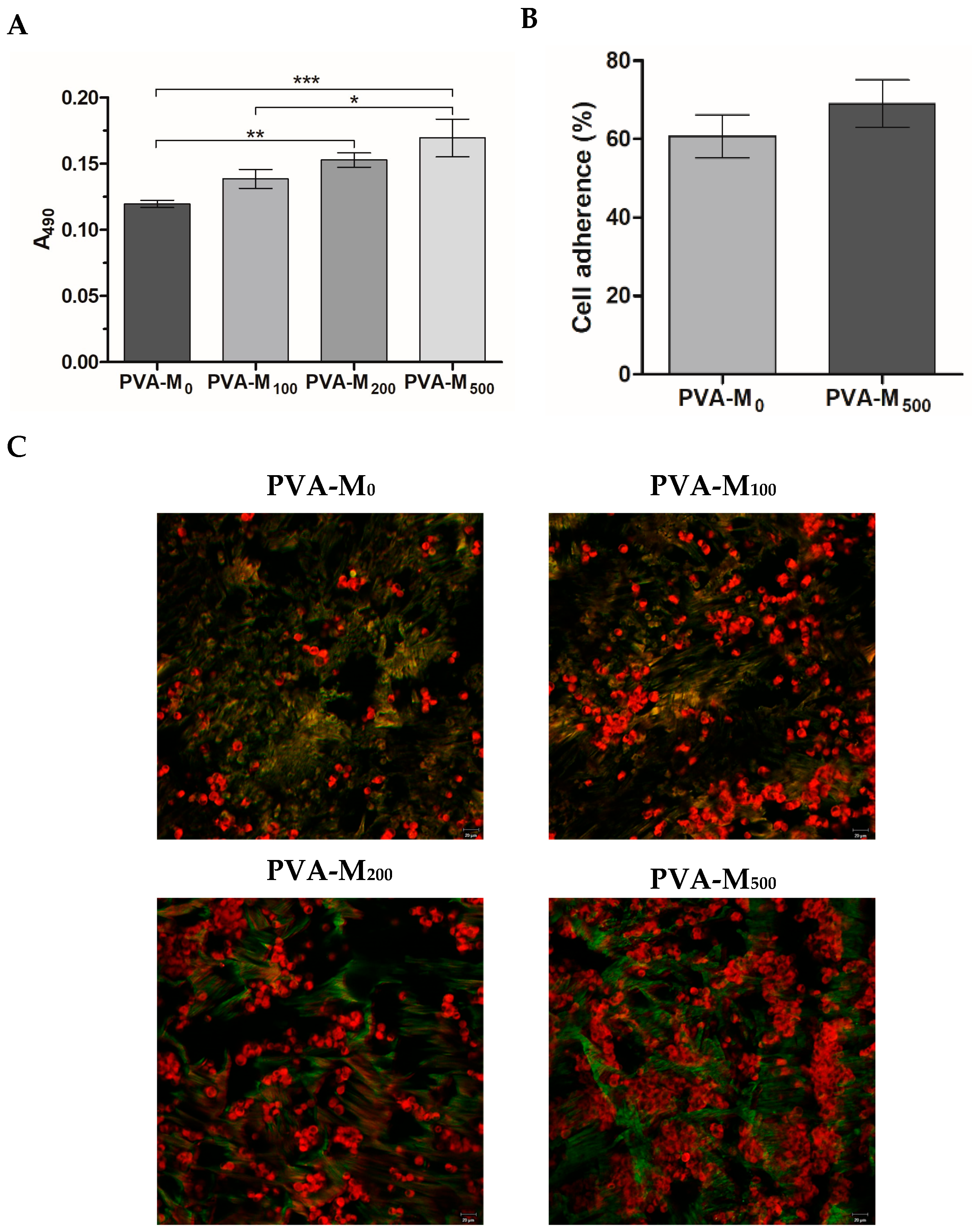
2.2.1. Cell Proliferation
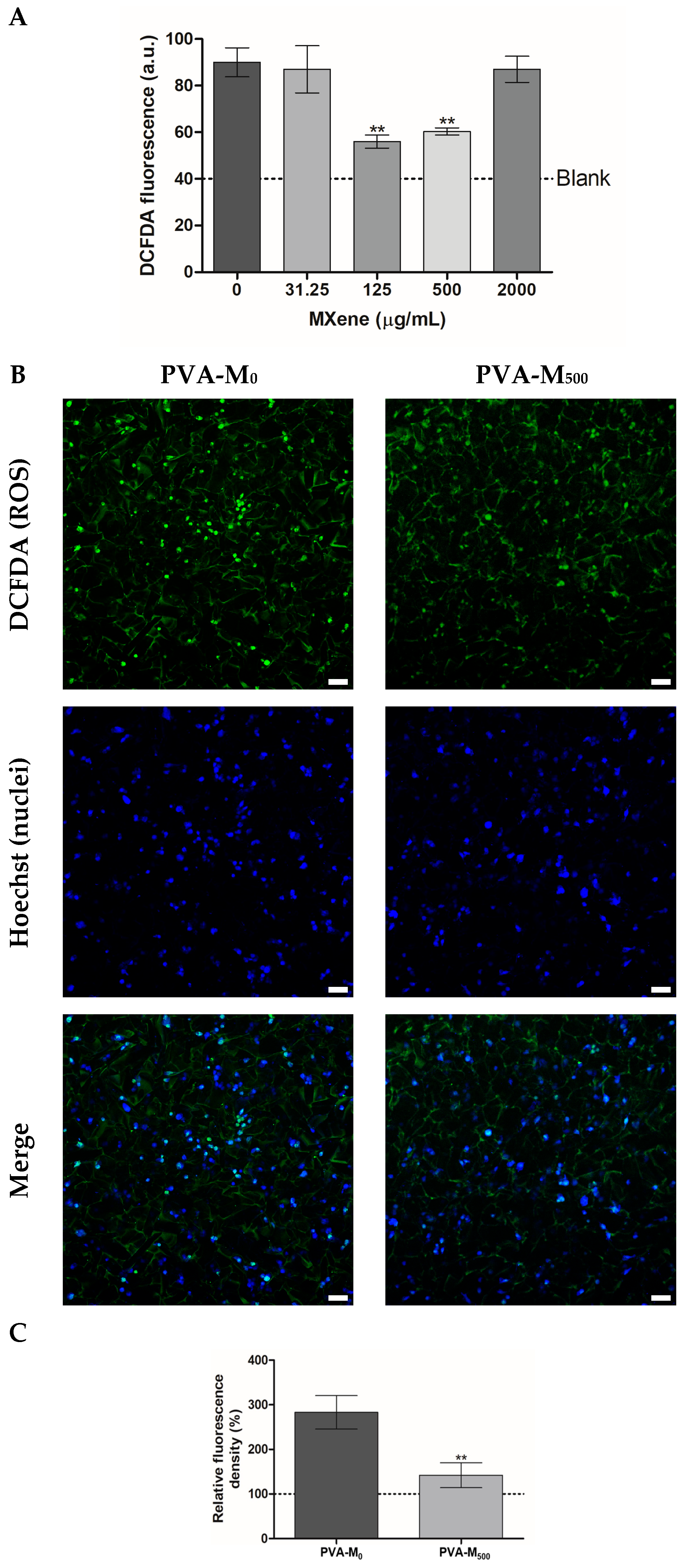
2.2.2. ROS-Modulating Effects of MXene-Containing Cryogels
2.2.3. Neurogenic Differentiation
3. Materials and Methods
3.1. Reagents
3.2. Ti3AlC2 MAX Synthesis
3.3. Ti3C2Tx MXene Synthesis
3.4. Preparation of MXene-Containing PVA Cryogels
3.5. Scanning Electron Microscopy
3.6. Swelling and Viscoelastic Properties of the Cryogels
3.7. Cell Culture and Viability
3.8. Cell Seeding and Detection in the Cryogels
3.8.1. Cell Proliferation Analysis
3.8.2. Cell Visualization
3.9. Detection of Intracellular ROS
3.10. Immunocytochemistry
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sjeklocha, L.; Gatz, J.D. Traumatic Injuries to the Spinal Cord and Peripheral Nervous System. Emerg. Med. Clin. N. Am. 2021, 39, 1–28. [Google Scholar] [CrossRef]
- Wang, B.; Lu, C.-F.; Liu, Z.-Y.; Han, S.; Wei, P.; Zhang, D.-Y.; Kou, Y.-H.; Jiang, B.-G. Chitin scaffold co.bined with autologous small nerve repairs sciatic nerve defects. Neural Regen. Res. 2022, 17, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, T.; Borger, A.; Radtke, C. Reconstruction of Critical Nerve Defects Using Allogenic Nerve Tissue: A Review of Current Approaches. Int. J. Mol. Sci. 2021, 22, 3515. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.-P.; Lin, Z.; Wang, F.; Jin, X.-H.; Fang, J.-Q.; Xu, B.; Liu, S.-H.; Zhang, F.; Wu, Z.; Zhou, Z.-F.; et al. Ti3C2Tx MXene-Coated Electrospun PCL Conduits for Enhancing Neurite Regeneration and Angiogenesis. Front. Bioeng. Biotechnol. 2022, 10, 850650. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Huang, Y.-Y. Biomaterials and Strategies for Nerve Regeneration. Artif. Organs 2006, 30, 514–522. [Google Scholar] [CrossRef]
- Yergeshov, A.; Zoughaib, M.; Dayob, K.; Kamalov, M.; Luong, D.; Zakirova, A.; Mullin, R.; Salakhieva, D.; Abdullin, T.I. Newly Designed PCL-Wrapped Cryogel-Based Conduit Activated with IKVAV Peptide Derivative for Peripheral Nerve Repair. Pharmaceutics 2024, 16, 1569. [Google Scholar] [CrossRef]
- Kaplan, B.; Levenberg, S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. Int. J. Mol. Sci. 2022, 23, 1244. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhang, Z.; Su, Y.; Wang, Z.; Dong, M.; Chen, M. An injectable high-conductive bimaterial scaffold for neural stimulation. Colloids Surf. B Biointerfaces 2020, 195, 111210. [Google Scholar] [CrossRef]
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 1801983. [Google Scholar] [CrossRef]
- Zhang, Z.; Rouabhia, M.; Wang, Z.; Roberge, C.; Shi, G.; Roche, P.; Li, J.; Dao, L.H. Electrically Conductive Biodegradable Polymer Composite for Nerve Regeneration: Electricity-Stimulated Neurite Outgrowth and Axon Regeneration. Artif. Organs 2007, 31, 13–22. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Zangene, E.; Manouchehri, S.; Amirabad, L.M.; Baheiraei, N.; Hadjighasem, M.R.; Farokhi, M.; Ganjali, M.R.; Walker, B.W.; Saeb, M.R.; et al. Conductive biomaterials as nerve conduits: Recent advances and future challenges. Appl. Mater. Today 2020, 20, 100784. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Byers, J.C.; O’Neil, K.D.; Semenikhin, O.A. Directed differentiation of embryonic P19 cells and neural stem cells into neural lineage on conducting PEDOT–PEG and ITO glass substrates. Arch. Biochem. Biophys. 2012, 528, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Qiao, F.; Hou, W.; Yang, L.; Lv, Y. Graphene-based conductive fibrous scaffold boosts sciatic nerve regeneration and functional recovery upon electrical stimulation. Appl. Mater. Today 2020, 21, 100870. [Google Scholar] [CrossRef]
- Liu, X.; Kim, J.C.; Miller, A.L.; Waletzki, B.E.; Lu, L. Electrically conductive nanocomposite hydrogels embedded with functionalized carbon nanotubes for spinal cord injury. New J. Chem. 2018, 42, 17671–17681. [Google Scholar] [CrossRef]
- Yadav, S.; Singh Raman, A.P.; Meena, H.; Goswami, A.G.; Bhawna; Kumar, V.; Jain, P.; Kumar, G.; Sagar, M.; Rana, D.K.; et al. An Update on Graphene Oxide: Applications and Toxicity. ACS Omega 2022, 7, 35387–35445. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Malaiya, A.; Kesharwani, P.; Soni, D.; Jain, A. Biomedical applications and toxicities of carbon nanotubes. Drug Chem. Toxicol. 2022, 45, 435–450. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. MXenes and MXene-based materials for tissue engineering and regenerative medicine: Recent advances. Mater. Adv. 2021, 2, 2906–2917. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. TrAC Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Seidi, F.; Arabi Shamsabadi, A.; Dadashi Firouzjaei, M.; Elliott, M.; Saeb, M.R.; Huang, Y.; Li, C.; Xiao, H.; Anasori, B. MXenes Antibacterial Properties and Applications: A Review and Perspective. Small 2023, 19, 2206716. [Google Scholar] [CrossRef]
- Unal, M.A.; Bayrakdar, F.; Fusco, L.; Besbinar, O.; Shuck, C.E.; Yalcin, S.; Erken, M.T.; Ozkul, A.; Gurcan, C.; Panatli, O.; et al. 2D MXenes with antiviral and immunomodulatory properties: A pilot study against SARS-CoV-2. Nano Today 2021, 38, 101136. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Ahmad, T.; Manzoor, M.H.; Laiba; Verpoort, F. MXenes as theranostics: Diagnosis and therapy including in vitro and in vivo applications. Appl. Mater. Today 2023, 35, 102002. [Google Scholar] [CrossRef]
- Noriega, N.; Shekhirev, M.; Shuck, C.E.; Salvage, J.; VahidMohammadi, A.; Dymond, M.K.; Lacey, J.; Sandeman, S.; Gogotsi, Y.; Patel, B.A. Pristine Ti3C2Tx MXene Enables Flexible and Transparent Electrochemical Sensors. ACS Appl. Mater. Interfaces 2024, 16, 6569–6578. [Google Scholar] [CrossRef] [PubMed]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically conductive 3D printed Ti3C2Tx MXene-PEG composite constructs for cardiac tissue engineering. Acta Biomater. 2022, 139, 179–189. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, S.; Feng, Z.; Fu, Y.; Mo, A. Recent advances and trends in the applications of MXene nanomaterials for tissue engineering and regeneration. J. Biomed. Mater. Res. Part A 2022, 110, 1840–1859. [Google Scholar] [CrossRef]
- Guo, R.; Xiao, M.; Zhao, W.; Zhou, S.; Hu, Y.; Liao, M.; Wang, S.; Yang, X.; Chai, R.; Tang, M. 2D Ti3C2TxMXene couples electrical stimulation to promote proliferation and neural differentiation of neural stem cells. Acta Biomater. 2022, 139, 105–117. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Wei, H.; Cao, W.; Qi, Y.; Zhou, S.; Zhang, P.; Li, H.; Li, G.-L.; Chai, R. Two-dimensional Ti3C2Tx MXene promotes electrophysiological maturation of neural circuits. J. Nanobiotechnol. 2022, 20, 398. [Google Scholar] [CrossRef]
- Wu, W.; Ge, H.; Zhang, L.; Lei, X.; Yang, Y.; Fu, Y.; Feng, H. Evaluating the Cytotoxicity of Ti3C2 MXene to Neural Stem Cells. Chem. Res. Toxicol. 2020, 33, 2953–2962. [Google Scholar] [CrossRef]
- Wei, H.; Gu, Y.; Li, A.; Song, P.; Liu, D.; Sun, F.; Ma, X.; Qian, X. Conductive 3D Ti3C2Tx MXene-Matrigel hydrogels promote proliferation and neuronal differentiation of neural stem cells. Colloids Surf. B Biointerfaces 2024, 233, 113652. [Google Scholar] [CrossRef]
- Liao, M.; Hu, Y.; Zhang, Y.; Wang, K.; Fang, Q.; Qi, Y.; Shen, Y.; Cheng, H.; Fu, X.; Tang, M.; et al. 3D Ti3C2Tx MXene–Matrigel with Electroacoustic Stimulation to Promote the Growth of Spiral Ganglion Neurons. ACS Nano 2022, 16, 16744–16756. [Google Scholar] [CrossRef]
- Yu, Q.; Jin, S.; Wang, S.; Xiao, H.; Zhao, Y. Injectable, adhesive, self-healing and conductive hydrogels based on MXene nanosheets for spinal cord injury repair. Chem. Eng. J. 2023, 452, 139252. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, H.; Hu, Y.; Huang, Z.; Wang, Y.; Xia, Y.; Chen, X.; Guo, J.; Cheng, H.; Xia, L.; et al. GelMA-MXene hydrogel nerve conduits with microgrooves for spinal cord injury repair. J. Nanobiotechnol. 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Ioniță, D.-A.; Sîrmon, A.-M.; Neacșu, I.A.; Ficai, A. Chapter 3—Natural, synthetic, and hybrid and composite biomaterials for neural tissue engineering. In Biomaterials for Neural Tissue Engineering; Gunduz, O., Ustundag, C.B., Sengor, M., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 21–58. [Google Scholar]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Mathis, T.S.; Maleski, K.; Goad, A.; Sarycheva, A.; Anayee, M.; Foucher, A.C.; Hantanasirisakul, K.; Shuck, C.E.; Stach, E.A.; Gogotsi, Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. [Google Scholar] [CrossRef]
- Downes, M.; Shuck, C.E.; McBride, B.; Busa, J.; Gogotsi, Y. Comprehensive synthesis of Ti3C2Tx from MAX phase to MXene. Nat. Protoc. 2024, 19, 1807–1834. [Google Scholar] [CrossRef]
- Shekhirev, M.; Shuck, C.E.; Sarycheva, A.; Gogotsi, Y. Characterization of MXenes at every step, from their precursors to single flakes and assembled films. Prog. Mater. Sci. 2021, 120, 100757. [Google Scholar] [CrossRef]
- Zheng, Y.; Gun’ko, V.M.; Howell, C.A.; Sandeman, S.R.; Phillips, G.J.; Kozynchenko, O.P.; Tennison, S.R.; Ivanov, A.E.; Mikhalovsky, S.V. Composites with Macroporous Poly(vinyl alcohol) Cryogels with Attached Activated Carbon Microparticles with Controlled Accessibility of a Surface. ACS Appl. Mater. Interfaces 2012, 4, 5936–5944. [Google Scholar] [CrossRef]
- Luong, T.D.; Zoughaib, M.; Garifullin, R.; Kuznetsova, S.; Guler, M.O.; Abdullin, T.I. In Situ functionalization of Poly(hydroxyethyl methacrylate) Cryogels with Oligopeptides via β-Cyclodextrin–Adamantane Complexation for Studying Cell-Instructive Peptide Environment. ACS Appl. Bio Mater. 2020, 3, 1116–1128. [Google Scholar] [CrossRef]
- Zoughaib, M.; Dayob, K.; Avdokushina, S.; Kamalov, M.I.; Salakhieva, D.V.; Savina, I.N.; Lavrov, I.A.; Abdullin, T.I. Oligo (Poly (Ethylene Glycol) Fumarate)-Based Multicomponent Cryogels for Neural Tissue Replacement. Gels 2023, 9, 105. [Google Scholar] [CrossRef]
- Li, W.; Huang, A.; Zhong, Y.; Huang, L.; Yang, J.; Zhou, C.; Zhou, L.; Zhang, Y.; Fu, G. Laminin-modified gellan gum hydrogels loaded with the nerve growth factor to enhance the proliferation and differentiation of neuronal stem cells. RSC Adv. 2020, 10, 17114–17122. [Google Scholar] [CrossRef]
- dos Santos, J.F.; Couceiro, R.; Concheiro, A.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C. Poly(hydroxyethyl methacrylate-co-methacrylated-beta-cyclodextrin) hydrogels: Synthesis, cytocompatibility, mechanical properties and drug loading/release properties. Acta Biomater. 2008, 4, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Kroeker, S.G.; Morley, P.L.; Jones, C.F.; Bilston, L.E.; Cripton, P.A. The development of an improved physical surrogate model of the human spinal cord—Tension and transverse compression. J. Biomech. 2009, 42, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Eigel, D.; Werner, C.; Newland, B. Cryogel biomaterials for neuroscience applications. Neurochem. Int. 2021, 147, 105012. [Google Scholar] [CrossRef]
- Shahriari, D.; Koffler, J.Y.; Tuszynski, M.H.; Campana, W.M.; Sakamoto, J.S. Hierarchically Ordered Porous and High-Volume Polycaprolactone Microchannel Scaffolds Enhanced Axon Growth in Transected Spinal Cords. Tissue Eng. Part A 2017, 23, 415–425. [Google Scholar] [CrossRef]
- Kubinová, Š.; Horák, D.; Hejčl, A.; Plichta, Z.; Kotek, J.; Proks, V.; Forostyak, S.; Syková, E. SIKVAV-modified highly superporous PHEMA scaffolds with oriented pores for spinal cord injury repair. J. Tissue Eng. Regen. Med. 2015, 9, 1298–1309. [Google Scholar] [CrossRef]
- Martín-López, E.; Nieto-Díaz, M.; Nieto-Sampedro, M. Differential Adhesiveness and Neurite-promoting Activity for Neural Cells of Chitosan, Gelatin, and Poly-l-Lysine Films. J. Biomater. Appl. 2012, 26, 791–809. [Google Scholar] [CrossRef]
- Dayob, K.; Zengin, A.; Garifullin, R.; Guler, M.O.; Abdullin, T.I.; Yergeshov, A.; Salakhieva, D.V.; Cong, H.H.; Zoughaib, M. Metal-Chelating Self-Assembling Peptide Nanofiber Scaffolds for Modulation of Neuronal Cell Behavior. Micromachines 2023, 14, 883. [Google Scholar] [CrossRef]
- Wu, J.; Yu, Y.; Su, G. Safety Assessment of 2D MXenes: In Vitro and In Vivo. Nanomaterials 2022, 12, 828. [Google Scholar] [CrossRef]
- Cui, D.; Kong, N.; Ding, L.; Guo, Y.; Yang, W.; Yan, F. Ultrathin 2D Titanium Carbide MXene (Ti3C2T) Nanoflakes Activate WNT/HIF-1α-Mediated Metabolism Reprogramming for Periodontal Regeneration. Adv. Healthc. Mater. 2021, 10, 2101215. [Google Scholar] [CrossRef]
- Song, R.; Xie, H.; Liu, G. Advances of MXene-based hydrogels for chronic wound healing. Chin. Chem. Lett. 2024, 110442. [Google Scholar] [CrossRef]
- Jurga, M.; Dainiak, M.B.; Sarnowska, A.; Jablonska, A.; Tripathi, A.; Plieva, F.M.; Savina, I.N.; Strojek, L.; Jungvid, H.; Kumar, A. The performance of laminin-containing cryogel scaffolds in neural tissue regeneration. Biomaterials 2011, 32, 3423–3434. [Google Scholar] [CrossRef] [PubMed]
- Yergeshov, A.A.; Zoughaib, M.; Ishkaeva, R.A.; Savina, I.N.; Abdullin, T.I. Regenerative Activities of ROS-Modulating Trace Metals in Subcutaneously Implanted Biodegradable Cryogel. Gels 2022, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, V.; Kamalov, M.; Abdullin, T.I.; Salakhieva, D.; Chasov, V.; Rogov, A.; Zoughaib, M. Evaluation of GHK peptide-heparin interactions in multifunctional liposomal covering. J. Liposome Res. 2024, 34, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, T.; Zhu, Y.; Qin, H.; Qian, J.; Wang, Q.; Zhang, P.; Liu, P.; Xiong, A.; Li, N.; et al. Enhanced osteogenic and ROS-scavenging MXene nanosheets incorporated gelatin-based nanocomposite hydrogels for critical-sized calvarial defect repair. Int. J. Biol. Macromol. 2024, 269, 131914. [Google Scholar] [CrossRef]
- Wei, C.; Tang, P.; Tang, Y.; Liu, L.; Lu, X.; Yang, K.; Wang, Q.; Feng, W.; Shubhra, Q.T.H.; Wang, Z.; et al. Sponge-Like Macroporous Hydrogel with Antibacterial and ROS Scavenging Capabilities for Diabetic Wound Regeneration. Adv. Healthc. Mater. 2022, 11, 2200717. [Google Scholar] [CrossRef]
- Fan, L.; Lin, X.; Hong, L.; Li, L.; Lin, R.; Ren, T.; Tian, J.; Chen, M. Simultaneous antioxidant and neuroprotective effects of two-dimensional (2D) MXene-loaded isoquercetin for ischemic stroke treatment. J. Mater. Chem. B 2024, 12, 2795–2806. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano 2022, 16, 7486–7502. [Google Scholar] [CrossRef]
- Li, T.; Ma, J.; Wang, W.; Lei, B. Bioactive MXene Promoting Angiogenesis and Skeletal Muscle Regeneration through Regulating M2 Polarization and Oxidation Stress. Adv. Healthc. Mater. 2023, 12, 2201862. [Google Scholar] [CrossRef]
- Kang, Y.; Park, H.; Shim, S.; Karima, G.; Lee, S.; Yang, K.; Kim, H.D. MXene Nanoparticles: Orchestrating Spherioidogenesis for Targeted Osteogenic and Neurogenic Differentiation. Adv. NanoBiomed Res. 2025, 2400100. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Hu, Y.; Tian, L.; Cheng, H.; Wang, Y.; Gao, X.; Cui, Q.; Zheng, S.; Feng, P.; et al. Decellularized umbilical cord wrapped with conductive hydrogel for peripheral nerve regeneration. Aggregate 2024, e674. [Google Scholar] [CrossRef]
- Wu, P.; Chen, P.; Xu, C.; Mu, C.; Zou, X.; Yang, K.; Xu, Y.; Li, X.; Li, X.; Liu, Z.; et al. Biodegradable conductive hydrogels generating magnetic-field-driven wireless electrical stimulation enhance the spinal cord injury repair. Nano Energy 2024, 130, 110123. [Google Scholar] [CrossRef]
- Kim, H.-S.; Baby, T.; Lee, J.-H.; Shin, U.S.; Kim, H.-W. Biomaterials-enabled electrical stimulation for tissue healing and regeneration. Med-X 2024, 2, 7. [Google Scholar] [CrossRef]
- Qi, F.; Liao, R.; Wu, P.; Li, H.; Zan, J.; Peng, S.; Shuai, C. An electrical microenvironment constructed based on electromagnetic induction stimulates neural differentiation. Mater. Chem. Front. 2023, 7, 1671–1683. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zoughaib, M.; Avdokushina, S.; Savina, I.N. MXene-Reinforced Composite Cryogel Scaffold for Neural Tissue Repair. Molecules 2025, 30, 479. https://doi.org/10.3390/molecules30030479
Zoughaib M, Avdokushina S, Savina IN. MXene-Reinforced Composite Cryogel Scaffold for Neural Tissue Repair. Molecules. 2025; 30(3):479. https://doi.org/10.3390/molecules30030479
Chicago/Turabian StyleZoughaib, Mohamed, Svetlana Avdokushina, and Irina N. Savina. 2025. "MXene-Reinforced Composite Cryogel Scaffold for Neural Tissue Repair" Molecules 30, no. 3: 479. https://doi.org/10.3390/molecules30030479
APA StyleZoughaib, M., Avdokushina, S., & Savina, I. N. (2025). MXene-Reinforced Composite Cryogel Scaffold for Neural Tissue Repair. Molecules, 30(3), 479. https://doi.org/10.3390/molecules30030479









