Ex Vivo Biosafety and Efficacy Assessment of Advanced Chlorin e6 Nanoemulsions as a Drug Delivery System for Photodynamic Antitumoral Application
Abstract
:1. Introduction
2. Results
2.1. Physical–Chemical Analysis: Particle Size, PdI, and Zeta Potential
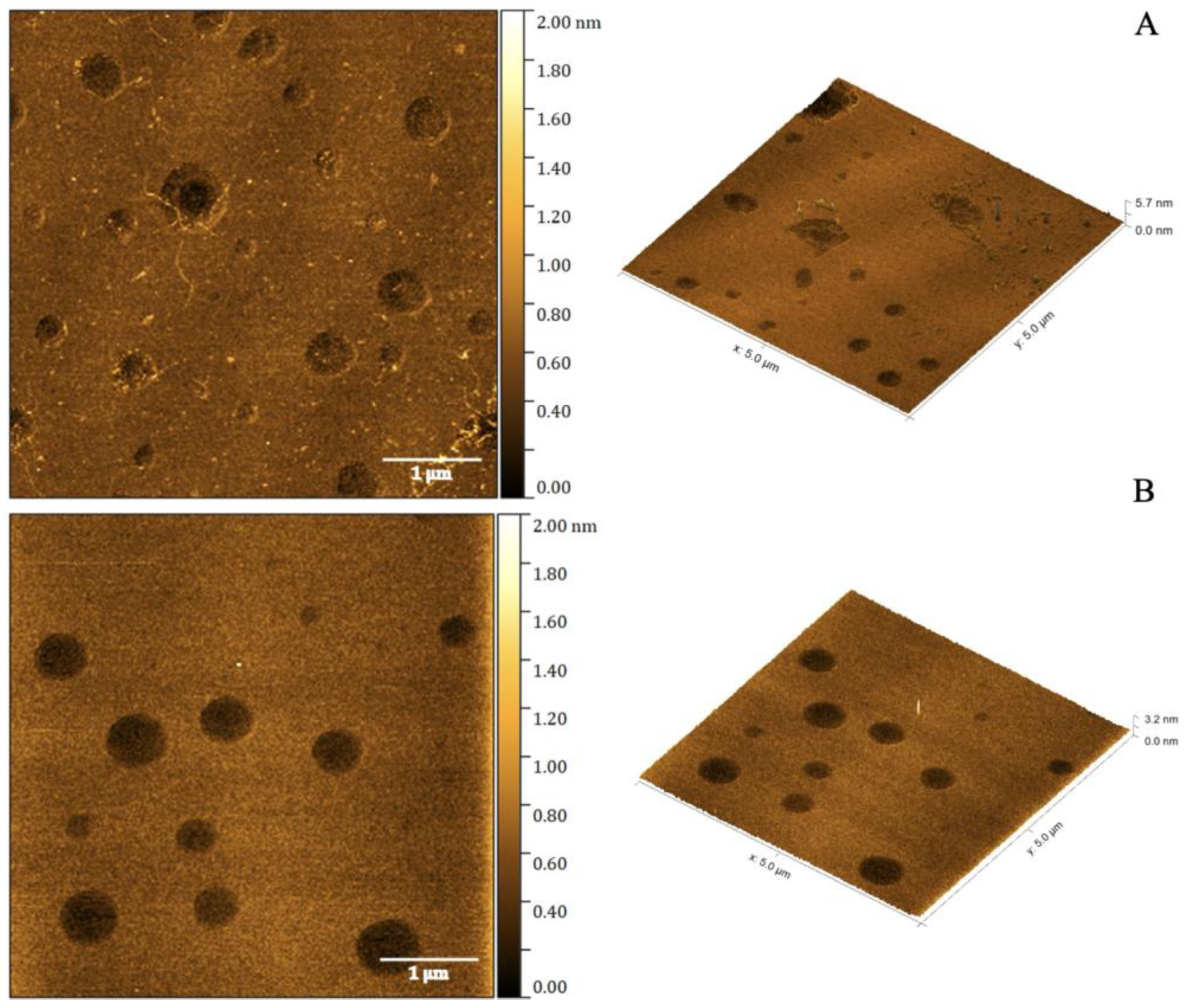
2.2. Atomic Force Microscopy
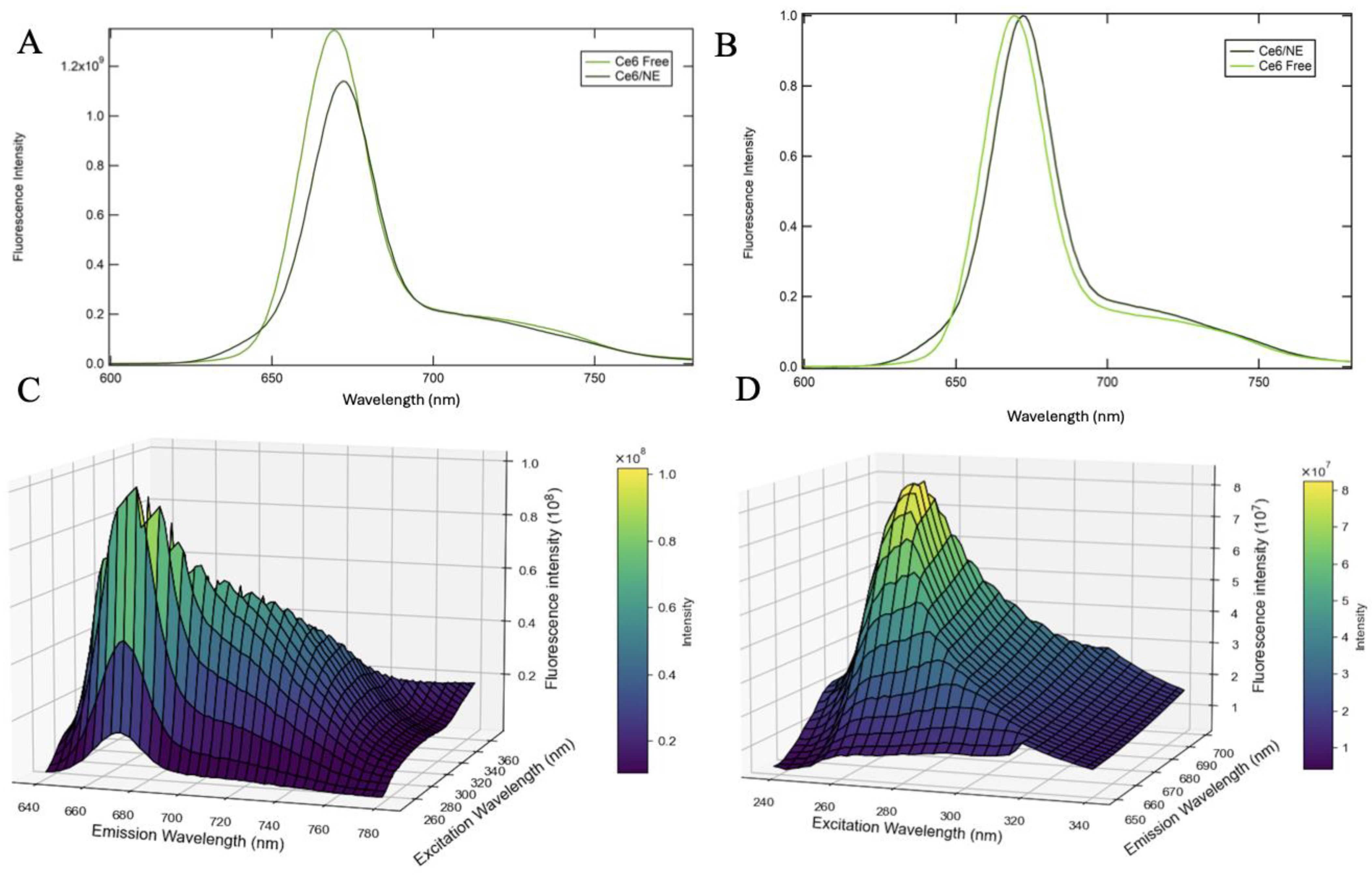
2.3. Fluorescence Characterization of Chlorin e6 Nanoemulsions
2.3.1. Two-Dimensional and Three-Dimensional Fluorescence Spectroscopy
2.3.2. Fluorescence Quantum Yield
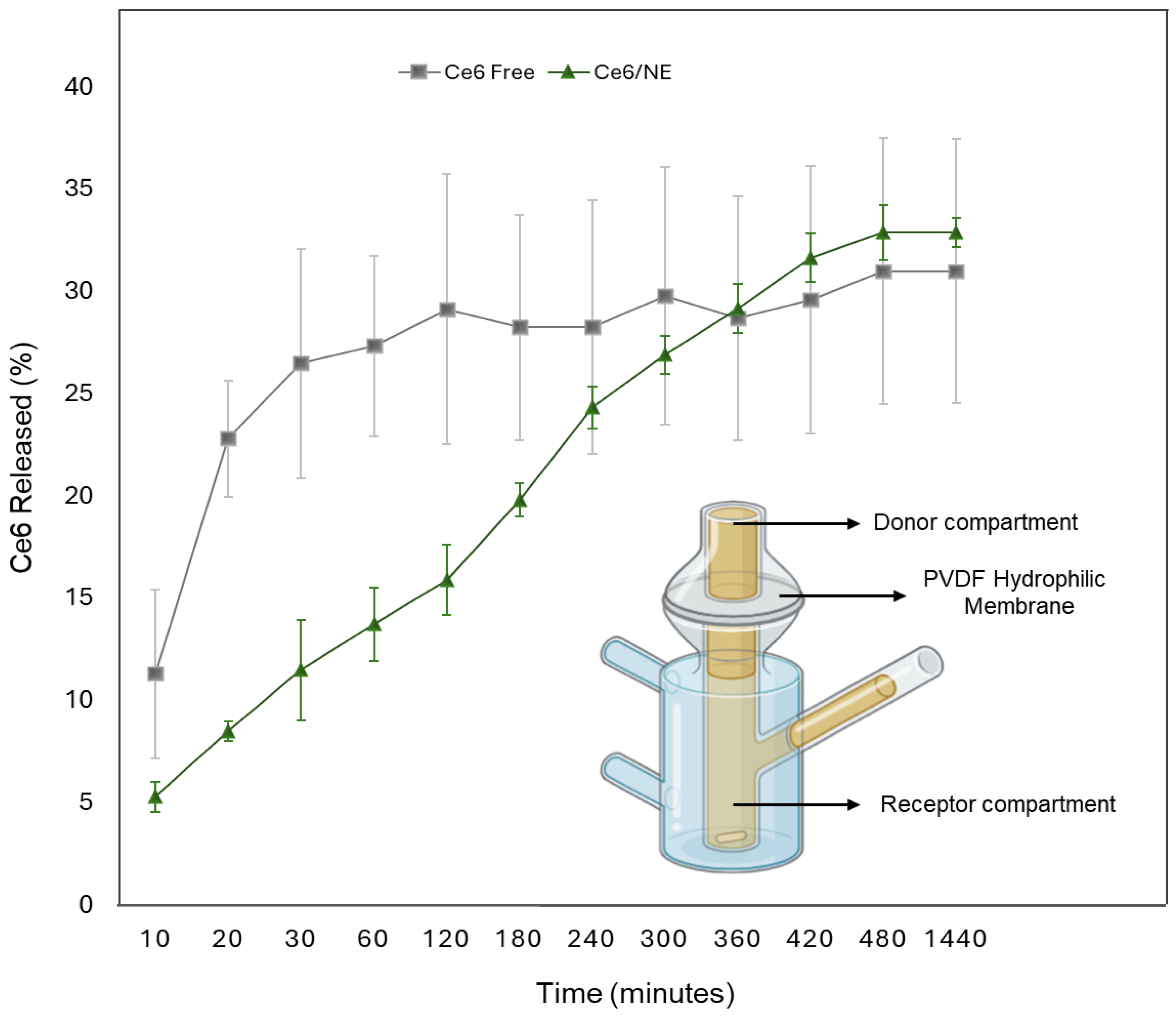
2.4. In Vitro Release Study
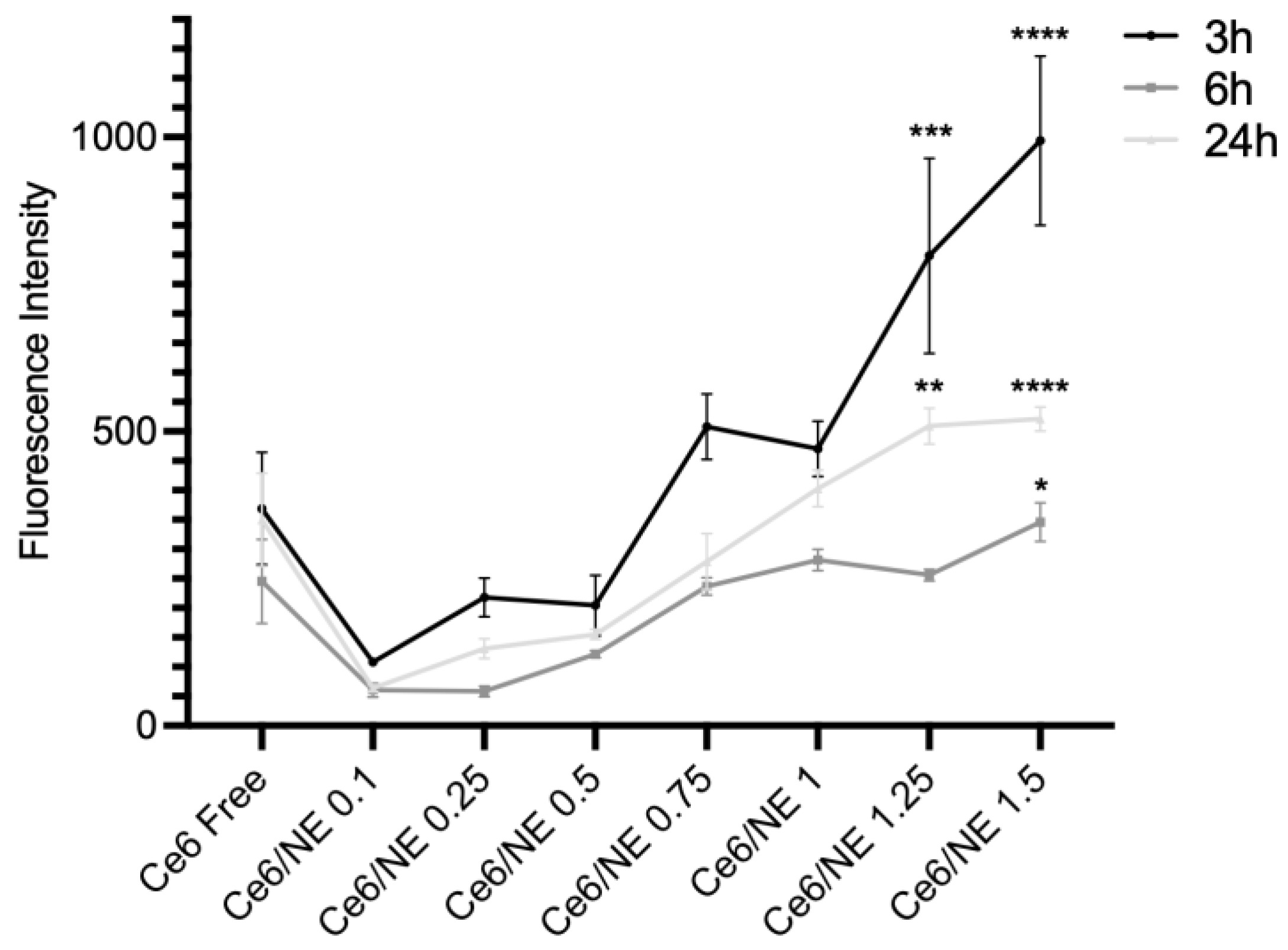
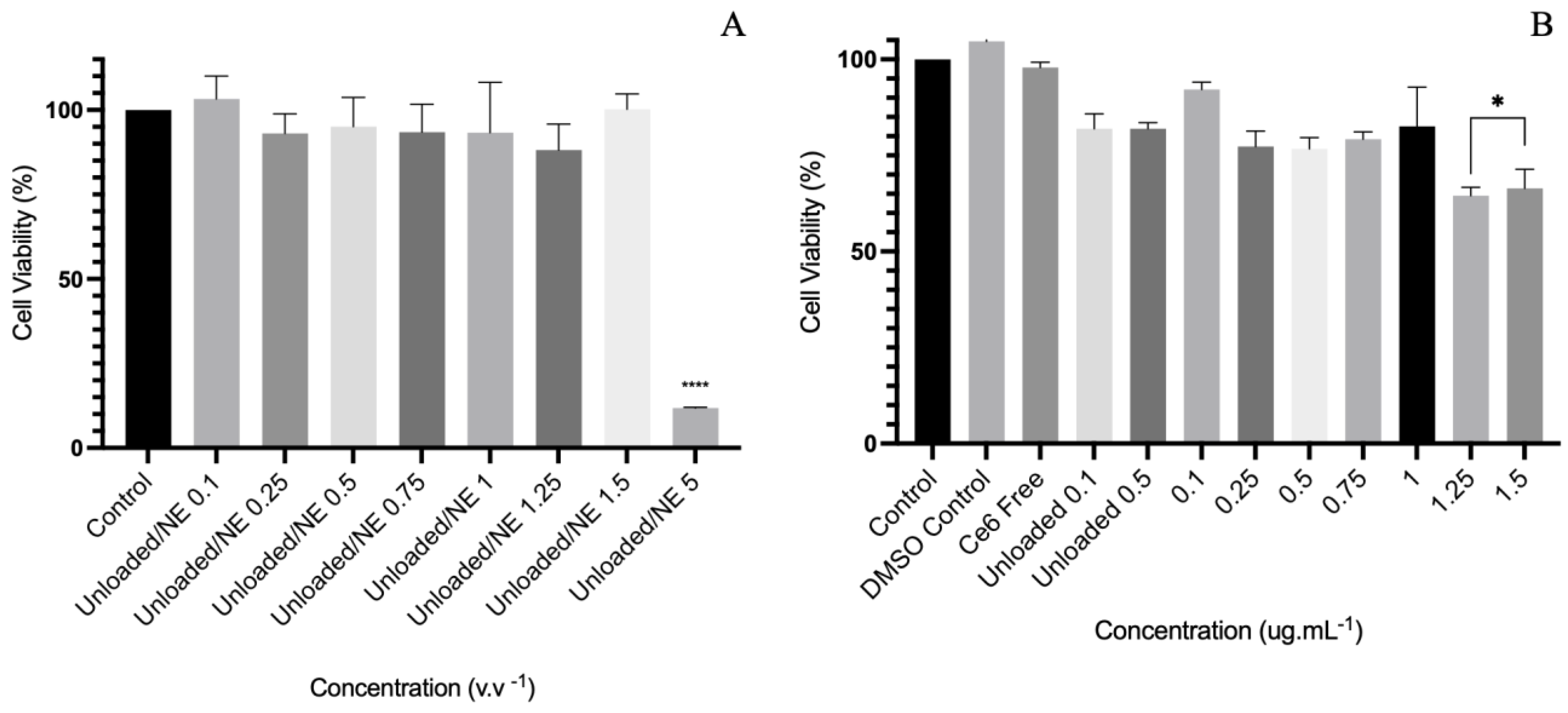
2.5. Cellular Uptake and Cytotoxicity Assay
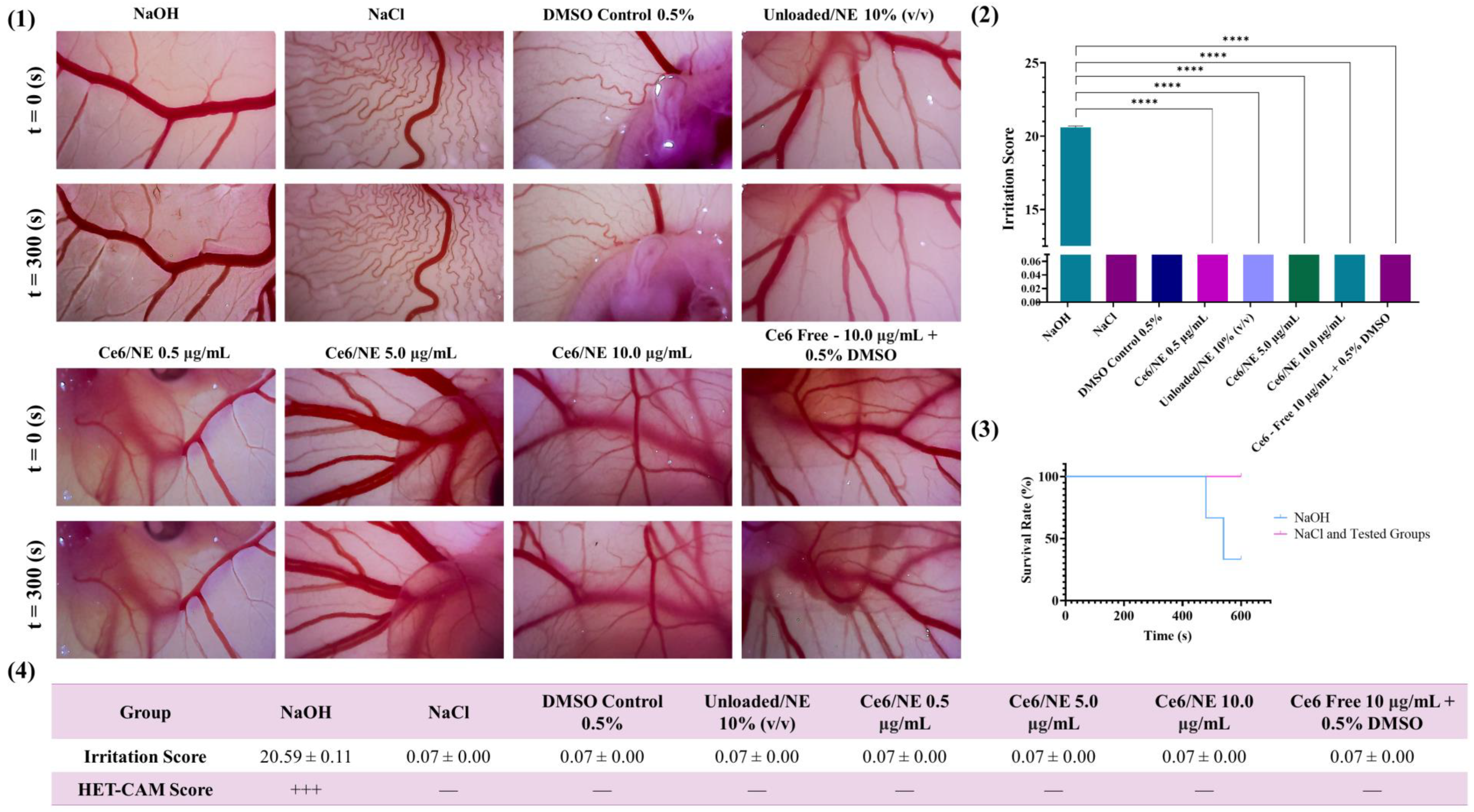
2.6. Ex Vivo Evaluation of the Biocompatibility and Antiangiogenic Activity of Ce6/NE Using the Hen’s Egg Chorioallantoic Membrane Assay (HET-CAM)
2.6.1. Biocompatibility Assay
2.6.2. Antitumoral Efficacy: Antiangiogenic Activity
3. Discussion
4. Materials and Methods
4.1. Development of Ce6 Nanoemulsions Using the Low-Energy Method
4.2. Characterization of Particle Size, Polydispersity Index (PdI), and Zeta Potential
4.3. Atomic Force Microscopy
4.4. Fluorescence Characterization
4.4.1. Two-Dimensional and Three-Dimensional Fluorescence Spectroscopy UV-Vis
4.4.2. Fluorescence Quantum Yield
4.5. In Vitro Drug Release Analysis
4.5.1. Solubility Analysis
4.5.2. In Vitro Release
4.6. Cell Culture
4.7. Cellular Uptake
4.8. Metabolic Activity Assay—Resazurin Based
4.9. Ex Vivo Evaluation of the Biocompatibility and Antiangiogenic Activity of Ce6/NE Using the Hen’s Egg Chorioallantoic Membrane Assay (HET-CAM)
4.9.1. Biocompatibility
4.9.2. Antiangiogenic Assessment
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin e6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhang, H.; Xu, D.; Yan, G.; Wu, Y.; Zhang, G.; Zeng, Q.; Wang, X. A chlorin e6 derivative-mediated photodynamic therapy inhibits cutaneous squamous cell carcinoma cell proliferation via Akt/mTOR signaling pathway. Photodiagnosis Photodyn. Ther. 2023, 42, 103332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Peng, H.; Shi, W.; Gan, L.; Zhong, L.; He, J.; Xie, L.; Wu, P.; Zhao, Y.; Deng, Z.; et al. Application of photodynamic therapy in cancer: Challenges and advancements. Biocell 2021, 45, 489–500. [Google Scholar] [CrossRef]
- Kim, G.L.; Park, B.; Jang, E.H.; Gu, J.; Seo, S.R.; Cheung, H.; Lee, H.J.; Lee, S.; Kim, J.-H. Dual Effect of Chemo-PDT with Tumor Targeting Nanoparticles Containing iRGD Peptide. Pharmaceutics 2023, 15, 614. [Google Scholar] [CrossRef]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, Q.; Zhao, Q.; Wang, F.; Wang, H.; Zhi, J.; Shan, C. Photosensitizer Functionalized Nanodiamonds for Raman Imaging and Photodynamic Therapy of Cancer Cells. Langmuir 2021, 37, 4308–4315. [Google Scholar] [CrossRef]
- Mata, A.I.; Pereira, N.A.; Cardoso, A.L.; Nascimento, B.F.; Pineiro, M.; Schaberle, F.A.; Gomes-Da-Silva, L.C.; Brito, R.M.; e Melo, T.M.P. Novel Foscan®-derived ring-fused chlorins for photodynamic therapy of cancer. Bioorg. Med. Chem. 2023, 93, 117443. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Bagaria, A.; Poddar, N.K. Photodynamic therapy—A novel and promising treatment for cancer. Mater Today Proc. 2021, 43, 2861–2863. [Google Scholar] [CrossRef]
- Torkhovskaya, T.I.; Kostryukova, L.V.; Tereshkina, Y.A.; Tikhonova, E.G.; Morozevich, G.E.; Plutinskaya, A.D.; Lupatov, A.Y.; Pankratov, A.A. Chlorin e6 embedded in phospholipid nanoparticles equipped with specific peptides: Interaction with tumor cells with different aminopeptidase N expression. Biomed. Pharmacother. 2021, 134, 111154. [Google Scholar] [CrossRef]
- Preeti, R.; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K.; Chaudhary, R.P.; Sharma, A.; Yadav, J.P.; Pathak, P.; Grishina, M.; Pathak, K.; Kumar, P. Unraveling the multifaceted role of nanoemulsions as drug delivery system for the management of cancer. J. Drug Deliv. Sci. Technol. 2024, 100, 106056. [Google Scholar] [CrossRef]
- Park, C.; Yoo, J.; Lee, D.; Jang, S.; Kwon, S.; Koo, H. Chlorin e6-Loaded PEG-PCL Nanoemulsion for Photodynamic Therapy and In Vivo Drug Delivery. Int. J. Mol. Sci. 2019, 20, 3958. [Google Scholar] [CrossRef]
- Aloss, K.; Hamar, P. Augmentation of the EPR effect by mild hyperthermia to improve nanoparticle delivery to the tumor. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 1879, 189109. [Google Scholar] [CrossRef]
- Varenne, F.; Rustique, E.; Botton, J.; Coty, J.-B.; Lanusse, G.; Lahcen, M.A.; Rio, L.; Zandanel, C.; Lemarchand, C.; Germain, M.; et al. Towards quality assessed characterization of nanomaterial: Transfer of validated protocols for size measurement by dynamic light scattering and evaluation of zeta potential by electrophoretic light scattering. Int. J. Pharm. 2017, 528, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Birla, D.; Khandale, N.; Bashir, B.; Alam, S.; Vishwas, S.; Gupta, G.; Dureja, H.; Kumbhar, P.S.; Disouza, J.; Patravale, V.; et al. Application of quality by design in optimization of nanoformulations: Principle, perspectives and practices. Drug Deliv. Transl. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Paul, M.; Padaga, S.G.; Dubey, S.K.; Biswas, S.; Singhvi, G. Dual-Drug-Loaded Topical Delivery of Photodynamically Active Lipid-Based Formulation for Combination Therapy of Cutaneous Melanoma. Mol. Pharm. 2023, 20, 3653–3671. [Google Scholar] [CrossRef]
- Ho, T.M.; Abik, F.; Mikkonen, K.S. An overview of nanoemulsion characterization via atomic force microscopy. Crit. Rev. Food Sci. Nutr. 2022, 62, 4908–4928. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B 2023, 248, 112796. [Google Scholar] [CrossRef] [PubMed]
- Logue, B.A.; Manandhar, E. Percent residual accuracy for quantifying goodness-of-fit of linear calibration curves. Talanta 2018, 189, 527–533. [Google Scholar] [CrossRef]
- Pan, Z.; Li, R.H.; Cui, Y.Y.; Wu, X.J.; Zhang, Y.Y.; Wang, Y.T. A simple and quick method to detect adulterated sesame oil using 3D fluorescence spectra. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 245, 118948. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Isakau, H.A.; Parkhats, M.V.; Knyukshto, V.N.; Dzhagarov, B.M.; Petrov, E.P.; Petrov, T. Toward understanding the high PDT efficacy of chlorin e6–polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer–polymer interaction in vitro. J. Photochem. Photobiol. B 2008, 92, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020, 577, 119009. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Ritger, L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Kujur, P.K.; Singh, R.P. Angiogenesis Assays. Methods Mol. Biol. 2016, 1379, 107–115. [Google Scholar] [CrossRef]
- Cimpean, A.M.; Ribatti, D.; Raica, M. The chick embryo chorioallantoic membrane as a model to study tumor metastasis. Angiogenesis 2008, 11, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane in the Study of Angiogenesis and Metastasis; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Amantino, C.F.; Amaral, S.R.D.; Aires-Fernandes, M.; Oliani, S.M.; Tedesco, A.C.; Primo, F.L. Development of 3D skin equivalents for application in photodynamic biostimulation therapy assays using curcumin nanocapsules. Heliyon 2024, 10, e32808. [Google Scholar] [CrossRef] [PubMed]
- Mudalige, T.; Qu, H.; Van Haute, D.; Ansar, S.M.; Paredes, A.; Ingle, T. Characterization of Nanomaterials. In Nanomaterials for Food Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 313–353. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Z.; Xu, H.; Zhang, C.; Sun, M.; Wang, C.; Zhang, S. Investigation on the precipitate morphology and fraction characterization by atomic force microscopy. Ultramicroscopy 2023, 253, 113796. [Google Scholar] [CrossRef]
- de Paula, C.S.; Tedesco, A.C.; Primo, F.L.; Vilela, J.M.C.; Andrade, M.S.; Mosqueira, V.C.F. Chloroaluminium phthalocyanine polymeric nanoparticles as photosensitisers: Photophysical and physicochemical characterisation, release and phototoxicity in vitro. Eur. J. Pharm. Sci. 2013, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Heng, P.W.S.; Chan, L.W. Optimization in Solvent Selection for Chlorin e6 in Photodynamic Therapy. J. Fluoresc. 2013, 23, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Trivedi, P. In vitro and in vivo characterization of pharmaceutical topical nanocarriers containing anticancer drugs for skin cancer treatment. In Lipid Nanocarriers for Drug Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–627. [Google Scholar] [CrossRef]
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; de Holanda e Silva, K.G.; Mansur, C.R.E. Formulation characterization and in vitro drug release of hydrogel-thickened nanoemulsions for topical delivery of 8-methoxypsoralen. Mater. Sci. Eng. C 2018, 92, 245–253. [Google Scholar] [CrossRef]
- US Food and Drug Administration. In Vitro Release Test Studies for Topical Drug Products Submitted in ANDAs; US Food and Drug Administration: Silver Spring, MD, USA, 2022. [Google Scholar]
- Demisli, S.; Mitsou, E.; Pletsa, V.; Xenakis, A.; Papadimitriou, V. Development and Study of Nanoemulsions and Nanoemulsion-Based Hydrogels for the Encapsulation of Lipophilic Compounds. Nanomaterials 2020, 10, 2464. [Google Scholar] [CrossRef]
- Latif, M.S.; Nawaz, A.; Asmari, M.; Uddin, J.; Ullah, H.; Ahmad, S. Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels 2022, 9, 3. [Google Scholar] [CrossRef]
- Nagaraju, P.G.; Sindhu, P.; Dubey, T.; Chinnathambi, S.; G, P.P.C.; Rao, P.J. Influence of sodium caseinate, maltodextrin, pectin and their Maillard conjugate on the stability, in vitro release, anti-oxidant property and cell viability of eugenol-olive oil nanoemulsions. Int. J. Biol. Macromol. 2021, 183, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Alhamdany, A.T.; Saeed, A.M.H.; Alaayedi, M. Nanoemulsion and Solid Nanoemulsion for Improving Oral Delivery of a Breast Cancer Drug: Formulation, Evaluation, and a Comparison Study. Saudi Pharm. J. 2021, 29, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Mojzisova, H.; Bonneau, S.; Vever-Bizet, C.; Brault, D. Cellular uptake and subcellular distribution of chlorin e6 as functions of pH and interactions with membranes and lipoproteins. Biochim. Biophys. Acta (BBA) Biomembr. 2007, 1768, 2748–2756. [Google Scholar] [CrossRef] [PubMed]
- Huerta-García, E.; Márquez-Ramírez, S.G.; Ramos-Godinez, M.d.P.; López-Saavedra, A.; Herrera, L.A.; Parra, A.; Alfaro-Moreno, E.; Gómez, E.O.; López-Marure, R. Internalization of titanium dioxide nanoparticles by glial cells is given at short times and is mainly mediated by actin reorganization-dependent endocytosis. Neurotoxicology 2015, 51, 27–37. [Google Scholar] [CrossRef]
- Szurko, A.; Rams-Baron, M.; Montforts, F.-P.; Bauer, D.; Kozub, P.; Gubernator, J.; Altmann, S.; Stanek, A.; Sieroń, A.; Ratuszna, A. Photodynamic performance of amphiphilic chlorin e6 derivatives with appropriate properties: A comparison between different-type liposomes as delivery systems. Photodiagnosis Photodyn. Ther. 2020, 30, 101799. [Google Scholar] [CrossRef]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- De Jong, W.H.; Carraway, J.W.; Geertsma, R.E. In vivo and in vitro testing for the biological safety evaluation of biomaterials and medical devices. In Biocompatibility and Performance of Medical Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 123–166. [Google Scholar] [CrossRef]
- Magar, T.B.T.; Mallik, S.K.; Gurung, P.; Lim, J.; Kim, Y.-T.; Shrestha, R.; Kim, Y.-W. Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. Int. J. Mol. Sci. 2023, 24, 13468. [Google Scholar] [CrossRef]
- Chapman, K.L.; Holzgrefe, H.; Black, L.E.; Brown, M.; Chellman, G.; Copeman, C.; Couch, J.; Creton, S.; Gehen, S.; Hoberman, A.; et al. Pharmaceutical toxicology: Designing studies to reduce animal use, while maximizing human translation. Regul. Toxicol. Pharmacol. 2013, 66, 88–103. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-based 3D cell culture models in cancer research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Ayoub, A.M.; Atya, M.S.; Abdelsalam, A.M.; Schulze, J.; Amin, M.U.; Engelhardt, K.; Wojcik, M.; Librizzi, D.; Yousefi, B.H.; Nasrullah, U.; et al. Photoactive Parietin-loaded nanocarriers as an efficient therapeutic platform against triple-negative breast cancer. Int. J. Pharm. 2023, 643, 123217. [Google Scholar] [CrossRef] [PubMed]
- Dario, M.F.; Oliveira, C.A.; Cordeiro, L.R.; Rosado, C.; Mariz, I.d.F.A.; Maçôas, E.; Santos, M.S.C.; da Piedade, M.E.M.; Baby, A.R.; Velasco, M.V.R. Stability and safety of quercetin-loaded cationic nanoemulsion: In vitro and in vivo assessments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 591–599. [Google Scholar] [CrossRef]
- Migotto, A.; Carvalho, V.F.M.; Salata, G.C.; da Silva, F.W.M.; Yan, C.Y.I.; Ishida, K.; Costa-Lotufo, L.V.; Steiner, A.A.; Lopes, L.B. Multifunctional nanoemulsions for intraductal delivery as a new platform for local treatment of breast cancer. Drug Deliv. 2018, 25, 654–667. [Google Scholar] [CrossRef]
- Tirpakova, Z.; Demcisakova, Z.; Luptakova, L.; Hurnikova, J.; Coma, M.; Urban, L.; Gal, P.; Medvecky, L.; Petrovova, E. Novel approach for biomaterial assessment: Utilizing the Ex Ovo quail cam assay for biocompatibility pre-screening. Vet. Res. Commun. 2025, 49, 24. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Bhatta, A.; Marimuthu, N.; Dihingia, A.; Manna, P.; Biswal, H.T.; Das, M.; Krishnamoorthy, G. Chlorin e6 decorated doxorubicin encapsulated chitosan nanoparticles for photo-controlled cancer drug delivery. Int. J. Biol. Macromol. 2019, 136, 951–961. [Google Scholar] [CrossRef]
- Krivosheeva, O.P.; Doctor, M.A.; Larkina, E.A.; Vedenkin, A.S.; Nikolskaya, T.A. Effect of substituents in chlorin e6 derivatives on the loading efficiency of the photosensitizer into the liposome membrane and their biological activity. Photodiagnosis Photodyn Ther. 2023, 42, 103328. [Google Scholar] [CrossRef] [PubMed]
- Salim, N.; García-Celma, M.J.; Escribano, E.; Nolla, J.; Llinàs, M.; Basri, M.; Solans, C.; Esquena, J.; Tadros, T.F. Formation of Nanoemulsion Containing Ibuprofen by PIC Method for Topical Delivery. Mater. Today Proc. 2018, 5, S172–S179. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guidance for Industry Q2B Validation of Analytical Procedures: Methodology. 1996. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2b-validation-analytical-procedures-methodology (accessed on 9 January 2025).
- Vieira-da-Silva, B.; Castanho, M.A.R.B. Resazurin Reduction-Based Assays Revisited: Guidelines for Accurate Reporting of Relative Differences on Metabolic Status. Molecules 2023, 28, 2283. [Google Scholar] [CrossRef]
- Steiling, W.; Bracher, M.; Courtellemont, P.; de Silva, O. The HET–CAM, a Useful In Vitro Assay for Assessing the Eye Irritation Properties of Cosmetic Formulations and Ingredients. Toxicol. Vitr. 1999, 13, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Zacharakis, N.; Tone, P.; Flordellis, C.S.; Maragoudakis, M.E.; Tsopanoglou, N.E. Methylene blue inhibits angiogenesis in chick chorioallontic membrane through a nitric oxide-independent mechanism. J. Cell Mol. Med. 2006, 10, 493–498. [Google Scholar] [CrossRef]

| Samples | Particle Size Average (nm) | ||
|---|---|---|---|
| Day 1 | Day 180 | Average | |
| Ce6/NE | 67.55 ± 14.81 | 72.36 ± 5.94 | 62.07 ± 10.46 |
| unloaded/NE | 67.41 ± 15.69 | 62.12 ± 16.73 | 59.74 ± 10.77 |
| PdI | |||
| Day 1 | Day 180 | Average | |
| Ce6/NE | 0.182 ± 0.01 | 0.124 ± 0.08 | 0.178 ± 0.04 |
| unloaded/NE | 0.145 ± 0.06 | 0.180 ± 0.01 | 0.185 ± 0.03 |
| Zeta Potential | |||
| Day 1 | Day 180 | Average | |
| Ce6/NE | −29.58 ± 2.05 | −31.350 ± 4.08 | −31.75 ± 3.41 |
| unloaded/NE | −28.867 ± 1.18 | −26.250 ± 6.29 | −30.362 ± 4.52 |
| Ce6 PBS 7.4 | Ce6 DMSO | Ce6/NE | |
|---|---|---|---|
| λ Soret max. (nm) | 403 | 407 | 406 |
| λ Excitation (nm) | 407 | 407 | 407 |
| λ Emission max. (nm) | 662.4 | 669.0 | 668.6 |
| Φf | 0.18 * | 0.36 | 0.16 |
| Global Adjustment | Inflexion Point Adjustment | |||
|---|---|---|---|---|
| Mathematical Models | Ce6/NE | Free Ce6 | Ce6/NE | Free Ce6 |
| Weibull | R2 = 0.9834 | R2 = 0.9786 | R2 = 0.9818 | R2 = 0.9898 |
| k = 4.6475 | k = 4.3040 | k = 46.6752 | k = 3.9929 | |
| b = 1.9344 | b = 0.2180 | b = 0.4796 | b = 0.5353 | |
| Korsmeyer–Peppas | R2 = 0.8792 | R2 = 0.6141 | R2 = 0.9819 | R2 = 0.6130 |
| k = 0.6132 | k = 2.2074 | k = 0.3082 | k = 1.9373 | |
| n = 0.3008 | n = 0.1047 | n = 0.4341 | n = 0.1357 | |
| Baker and Lonsdale | R2 = 0.6226 | R2 = −4.5063 | R2 = 0.9706 | R2 = −1.8142 |
| k = 5.3685 × 10−7 | k = 6.5515 × 10−7 | k = 7.9361 × 10−7 | k = 1.3635 × 10−6 | |
| Higuchi | R2 = 0.6108 | R2 = −4.5950 | R2 = 0.9698 | R2 = −1.8491 |
| k = 0.1773 | k = 0.1952 | k = 0.2166 | k = 0.2828 | |
| First Order | R2 = −1.0314 | R2 = −13.5953 | R2 = 0.5453 | R2 = −6.1378 |
| k = 5.5912 × 10−5 | k = 5.7098 × 10−5 | k = 0.0001 | k = 0.0002 | |
| Hixon and Crowell | R2 = −1.0546 | R2 = −13.6907 | R2 = 0.5414 | R2 = −6.1701 |
| k = 1.8326 × 10−5 | k = 1.8690 × 10−5 | k = 4.4599 × 10−5 | k = 5.3941 × 10−5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
do Amaral, S.R.; Aires-Fernandes, M.; Haddad, F.F.; Gini, A.L.R.; Scarim, C.B.; Primo, F.L. Ex Vivo Biosafety and Efficacy Assessment of Advanced Chlorin e6 Nanoemulsions as a Drug Delivery System for Photodynamic Antitumoral Application. Molecules 2025, 30, 544. https://doi.org/10.3390/molecules30030544
do Amaral SR, Aires-Fernandes M, Haddad FF, Gini ALR, Scarim CB, Primo FL. Ex Vivo Biosafety and Efficacy Assessment of Advanced Chlorin e6 Nanoemulsions as a Drug Delivery System for Photodynamic Antitumoral Application. Molecules. 2025; 30(3):544. https://doi.org/10.3390/molecules30030544
Chicago/Turabian Styledo Amaral, Stéphanie Rochetti, Mariza Aires-Fernandes, Felipe Falcão Haddad, Ana Luísa Rodriguez Gini, Cauê Benito Scarim, and Fernando Lucas Primo. 2025. "Ex Vivo Biosafety and Efficacy Assessment of Advanced Chlorin e6 Nanoemulsions as a Drug Delivery System for Photodynamic Antitumoral Application" Molecules 30, no. 3: 544. https://doi.org/10.3390/molecules30030544
APA Styledo Amaral, S. R., Aires-Fernandes, M., Haddad, F. F., Gini, A. L. R., Scarim, C. B., & Primo, F. L. (2025). Ex Vivo Biosafety and Efficacy Assessment of Advanced Chlorin e6 Nanoemulsions as a Drug Delivery System for Photodynamic Antitumoral Application. Molecules, 30(3), 544. https://doi.org/10.3390/molecules30030544








