Biomass-Based Hydrogen Extraction and Accompanying Hazards—Review
Abstract
1. Introduction
2. Methods
3. Various Hazards Related to Hydrogen, Biohydrogen, and Biomass
- Wood and its processing waste—firewood, wood pellets, and chips; lumber and furniture mill sawdust and waste; and black liquor from pulp and paper mills.
- Farming crops and waste materials—corn, soybeans, sugar cane, switchgrass, woody plants, algae, crop, and food processing residues, applicable to biofuels’ production.
- Biogenic substances in municipal solid waste-paper products; cotton and wool products; and food, yard, and wood waste.
- Animal manure and human sewage for BG production.
- Direct combustion (burning) to produce heat.
- TC transformation for producing solid, gaseous, and liquid fuels.
- Chemical transformation for producing liquid fuels.
- Biological transformation for producing liquid and gaseous fuels.
3.1. Various Hazards Related to Hydrogen and Biohydrogen
- ▪
- During accidental outdoor operation, H2 disperses faster than the other fuels.
- ▪
- H2 has the greatest flammability range in the air (4–77% by volume) with no safety issues.
- ▪
- The H2 flame speed (346 cm/s) exceeds that of CH4 8-fold (43.25 cm/s).
- ▪
- H2 burns with low docility, so its fast consumption is accompanied by little damage to the adjacent elements.
- ▪
- The H2 explosion range exceeded those of the other two fuels but H2 deflagrate at higher volume concentrations.
- ▪
- The H2 energy per unit mass (LHV = 120 MJ/kg) thrice exceeds that from gasoline combustion (40 MJ/kg).
- ▪
- Ability to ignite easily.
- ▪
- Dangers posed by high-pressure and low-temperature storage conditions.
- ▪
- Potential to penetrate small gaps or porous materials due to its small molecular size [37].
- When handling CH4, all risks of ignition and explosion must be removed from the surrounding area, and smoking is prohibited nearby.
- When CH4 might exist in an enclosed area, its levels ought to be regulated and assessed alongside O2 levels.
- CH4 contained in a cylinder needs to be safeguarded against harm and maintained in an upright stance. When stored in facilities, CH4 must be placed in a cool, well-aired area away from direct sunlight and other potential ignition sources.
- If there is a suspicion of a CH4 leak, it is essential to clear the area right away.
- If a person is exposed to CH4, wearable units can notify them to seek fresh air to avoid methane gas poisoning.
- Attempting to heat frozen tissues and obtaining medical assistance.
- Staying clear of heat, hot surfaces, sparks, open flames, and other sources of ignition. No use of tobacco products.
- Do not attempt to extinguish a fire caused by leaking gas unless the leak can be safely closed. Remove all ignition sources if it is safe to do so.
- Shielding tanks containing C2H6 from sunlight and keeping them in a properly ventilated area.
3.2. Various Hazards Related to Biomass Storage
4. Methods for Renewable-H2 Production from Biomass
4.1. Biological Methods Used for Hydrogen Production from Biomass and Hazards Related to It
4.1.1. Bio-Photolysis
- Keeping solid S in a ventilated space, away from substances that may react with it.
- Implementing suitable engineering measures or respiratory safeguards.
- It is advised to wear safety goggles when exposed to high levels of dust.
- Wearing a face shield for safety from molten S.
- Steering clear of continuous or extended skin contact.
- To safeguard against molten S, it is advised to use gloves and skin protection made from leather or heat-resistant materials.
- Avoiding open flames, sparks, and smoking.
- Storing in a closed system, dusting explosion-proof electrical equipment and lighting, and preventing the deposition of dust.
- Preventing the buildup of electrostatic charges (e.g., by grounding).
- Wearing safety goggles.
| Microorganism/Substrate/Operating Conditions | Information of Hazards | Refs. |
|---|---|---|
| Chlorella sp.; 30 mM glucose; T: 25–42 °C; LI: 120 μmol/m2/s; IT: 70 h; pH: 8.6; Medium: MA; Reactor type: serum bottle reactor. | MA-Medium for fresh-H2O, terrestrial, hot-spring, and salt-H2O algae. Some types of algae are harmful when they grow too quickly or make toxins. Toxins from algal cells or those released into H2O can make people and animals ill when they encounter these toxins via food or H2O. At times, algal blooms can grow so thick that they block sunlight, preventing other aquatic plants and animals from obtaining the amount needed for their survival. Thick blooms can similarly obstruct the gills of fish, shellfish, and other creatures, hindering their ability to breathe. When a bloom fades away, the decomposition process can consume all the O2 in the H2O, leading to the suffocation of other aquatic life. When a bloom deteriorates, it might emit gases like CH4 and H2S that can pose dangers to humans. The mitigation of hazards comprises the following measures:
| [115,116,117] |
| Chlorella sp.—The cells of Chlorella sp. do not create any harmful substances and are regarded as safe for people to eat (when used as food supplements based on such microalgae). There are serious worries about the quality of products made from such microalgae because they may be contaminated with toxic metals, inorganic arsenic, or cyanotoxins. The mitigation of hazards comprises the following measures: Use only premium Chlorella products and make sure that the food is well formulated to prevent any possible negative impacts on fish health and productivity. | [118] | |
| Chlorella sp. has no negative impact on fish health, growth, or immune system function, but more research is necessary to understand its long-term effects on fish. | [119] | |
| Glucose is a safe substance or mixture under Regulation (EC) No 1272/2008 [120]. | [121] | |
| Serum bottles are commonly made of transparent polyester (PET) material (inedible). This substance is harmless, has no flavor, and is highly transparent. | [122] | |
| Serum bottles made from Wheaton 400 borosilicate molded glass can also meet USP Type I requirements. | [123] | |
| Cyanobacteria, also known as blue-green algae, induce most toxic algal blooms in fresh H2O. Diatoms or dinoflagellates (red tides) are responsible for inducing the most harmful algal blooms in salt H2O. Prevention: Similar to the case of MA. | [116] | |
| Excessive algal growth deprives or restricts other types of marine life and obstructs the sunlight needed for their rapid proliferation. Issues with taste and smell in drinking H2O and fish deaths are linked to high levels of planktonic algae blooms. Prevention: Similar to the case of MA | [124] | |
| C. vulgaris; Crude glycerol; Temp.: 30 °C; LI: 48 μmol/m2/s, IT: 72 h; pH: 6.8; Medium: Modified TAP; Reactor type: 1 L bioreactor | Administering Chlorella vulgaris orally to mice in both acute and multiple doses did not result in any toxicity or adverse reactions. | [125] |
| No physical or behavioral changes were observed with different doses of C. vulgaris, and there were no signs of pain or distress, suggesting that C. vulgaris is not toxic. A study based on OECD Guideline 420 found no acute liver damage in female SD rats when given Chlorella vulgaris at 2000 mg kg−1 BW. Therefore, C. vulgaris falls into the unclassified category within the classification of GHS. | [126] | |
| Crude C3H8O3/Glycerin can induce skin irritation (H315) and organ damage (H370) and is dangerous if ingested (H302). Excessive mist buildup can cause irritation of the respiratory tract. It can cause temporary eye discomfort (burning, stinging, and tearing). Possible health impacts relate to the following: Eyes: Contact can lead to slight eye discomfort. Skin: Exposure might lead to skin irritation. Ingestion: It is of minimal toxicity. It could be dangerous if swallowed. It can result in nausea, headaches, and diarrhea. Inhalation: Due to its low vapor pressure, it is improbable that vapor would be inhaled at room temperature. Breathing in mist can lead to irritation of the respiratory system. The mitigation of hazards comprises the following measures:
| [127,128] | |
| C. reinhardtii; -; T: 25 °C; LI: 200 μmol/m2/s; IT: 140 h; pH: 7.2; Medium: TAP; Reactor type: 250 mL Erlenmeyer flasks | Dried biomass powder of C. reinhardtii (THN 6) did not show mutagenic properties in the bacterial reverse mutation test at the highest recommended concentration for soluble non-cytotoxic substances and did not exhibit clastogenic effects in the chromosomal aberrations test at the maximum cytotoxic concentration. The micronucleus test showed that THN 6 dried biomass powder did not exhibit genotoxic effects in vivo when tested at the maximum dose. In the end, the administration of 6 dried biomass powders via gavage to male and female HSD showed no specific organ effects or toxicity. Han Wistar rats were fed 4000 mg/kg bw/day doses. | [129] |
| Erlenmeyer flasks made of PTFE: According to CLP regulation (EC) No. 1272/2008 [120], this product is not classified as an unsafe substance/unsafe mixture. Products can induce burns in hot conditions. Heating PTFE above 400 °C can induce unsafe vapors. The latter can induce irritation in the eyes, nose, throat, and lungs. The mitigation of hazards comprises the following measures:
| [130] | |
| TAP-TAP (Tris-Acetate Phosphate) Medium induces skin irritation (H315). It also induces serious eye irritation (H319). In a blend including, i.a., CuSO4.5H2O and ZnSO4·7H2O, it might be harmful to the user or the environment or may be presumed to be so. The mitigation of hazards comprises the following measures:
| [131] | |
| C. vulgaris; Corn stalk; T: 30 °C; LI: 108 μmol/m2/s; IT: 144 h; pH: 7.0; Medium: Modified BG-11; Reactor type: 500 mL bioreactor | C. vulgaris—as mentioned. | |
| Corn stalk-The cornstalk plant is moderately toxic to pets. In dogs, it induces signs of gastrointestinal upset with vomiting and diarrhea. Mitigation of hazards: Avoid contacting pets with the corn stalk. If necessary, contact a veterinarian. | [132] | |
| Corn plant sap is toxic, especially to inquisitive kids and pets. Consuming the corn plant may result in nausea, vomiting, and diarrhea. The intensity of these symptoms frequently depends on the quantity ingested. Continuous contact with the toxins in the corn plant can lead to long-term health problems. The mitigation of hazards comprises the following measures:
| [133] | |
| Cornstalk plants are toxic to dogs, cats, and horses. It can cause vomiting (seldom with blood), depression, anorexia, hypersalivation, and dilated pupils in cats. | [134] | |
| Modified BG-11 (Blue-Green) Medium can harm fertility or the developing baby (H360). It causes irritation of the skin (H315) and eyes (severely) (H319). It can cause irritation of the respiratory system (H335). The mitigation of hazards comprises the following measures:
| [135] | |
| C. sorokiniana; Acetate; T: 30 °C; LI: 120 μmol/m2/s; IT: 222 h; pH: 7.2; Medium: BG-11; Reactor type: 500 mL Erlenmeyer flasks | Chlorella sorokiniana can contain heavy metals (Hg, Pb, Cd) if its culture medium is not strictly controlled, such as culture in a non-glass tube. The composition of samples of material containing Chlorella sorokiniana should be systematically monitored. | [136] |
| Some people may experience gastrointestinal signs such as nausea, diarrhea, gas, or abdominal pain. The mitigation of hazards comprises the following measures: People who are allergic to I2 or have thyroid problems and troubles need to be extremely careful or avoid chlorella containing naturally present I2. Chlorella should also be avoided by people with autoimmune diseases and by pregnant and breastfeeding women. | [137] | |
| Ethyl acetate is an extremely flammable liquid and vapor (H225). It causes severe irritation of the eye (H319). It can cause drowsiness or dizziness (H336). Repeated exposure can cause skin dryness or cracking (EUH066). The mitigation of hazards comprises the following measures:
| [138] | |
| BG11 Broth-An oxidizer able to amplify fire (H272). It causes severe irritation of the eye (H319). The mitigation of hazards comprises the following measures:
| [139] | |
| Erlenmeyer flasks-as mentioned. | ||
| C. reinhardtii; Starch; T: 28 °C; LI: 50 μmol/m2/s; IT: 144 h; pH: 7.5; Medium: TAP-C; Reactor type: 500 mL Erlenmeyer flasks | C. reinhardtii—as mentioned. | |
| Starch is not an unsafe substance or mixture. It may form an explosible dust–air mixture if dispersed. The mitigation of hazards comprises the following measures:
| [140] | |
| Starch has the potential to create flammable dust levels in the air (while being processed). The mitigation of hazards comprises the following measures:
| [141] | |
| TAP—as mentioned. However, TAP-C—no information. | ||
| Erlenmeyer flasks—as mentioned. | ||
| P. boryanum; DCMU; T: 22 °C; LI: 50 μmol/m2/s; IT: 188 h; pH: 7.5; Medium: 0.5 mM N; Reactor type: Roux bottle | Administration of lyophilized microalgal biomass suspension of P. boryanum at doses of 300 and 2000 mg.kg−1 showed no toxicity signs, indicating its safety according to its OECD classification as “Minimal Toxicity or Secure”. | [142] |
| DCMU is toxic when ingested (H302). It is believed to cause cancer (H351). Prolonged or repeated inhalation of the substance may induce harm to organs (blood) (H373). It has high toxicity to aquatic organisms with long-lasting effects (H410). The mitigation of hazards comprises the following measures:
| [143] | |
| Roux bottle—Roux laboratory bottles made of Borosil are chemically resistant and stable. | [144] | |
| C. reinhardtii; -; T: 24 °C; LI: 60 μmol/m2/s; IT: 204 h; pH: 7.2, Medium: TAP-S; Reactor type: 500 mL Duran glass bottles. | C. reinhardtii—as mentioned. | |
| TAP—as mentioned. However, TAP-S—no information. | ||
| Duran glass bottles are made from 3.3 borosilicate glass, exhibiting exceptional thermal shock resistance and chemical compatibility, ensuring safe handling of diverse substances. | [145] | |
| Anabaena sp.; Glucose; T: 24 °C; LI: 4400 lux; IT: 156 h; pH: 9.2; Medium: BG-11, Reactor type: 500 mL Duran glass bottles. | Anabaena sp. is non-toxic and not pathogenic (hazard class: 1). | [146] |
| Anabaena spp. can generate anatoxin-a, anatoxin-a(s), saxitoxin, and microcystins. Anatoxin-a affects nerve synapses. It works as a postsynaptic cholinergic nicotinic agonist, inducing a depolarizing neuromuscular blockade. | [147] | |
| Glucose—as mentioned. | ||
| BG-11—as mentioned. | ||
| Duran glass bottles—as mentioned. | ||
| Arthrospira sp.; 0.10% glucose; T: 30 °C; LI: 40 μmol/m2/s; IT: 156 h; pH: 9.0; Medium: ZnO. | Arthrospira sp. is safe to ingest by animals and humans. | [148] |
| ZN0—a complex medium, no information about hazards. | [148] | |
| Glucose—as mentioned. | ||
| Chlamydomonas sp.; -; T: 24 °C; LI: 60 μmol/m2/s; IT: 372 h; pH: 7.2; Medium: TAP-S; Reactor type: 500 mL Duran glass bottles. | Chlamydomonas sp. is non-toxic. | [149] |
| TAP—as mentioned. However, TAP-S—no information. | ||
| Duran glass bottles—as mentioned. |
| Parameters | Effects on H2 Yield in Bio-Photolysis | Refs. |
|---|---|---|
| Temperature | The ideal temperature for H2 production significantly differs between species. | [39] |
| The peak H2 yields for cyanobacteria are found at temperatures between 30 and 40 °C; however, certain strains show maximum production within a cooler temperature range of 20–25 °C. | [150,151] | |
| pH | Cyanobacteria demonstrated the greatest H2 production at a pH of 8.0, while an acidic pH of 4.5 led to an 83% decrease in H2 output. The ideal pH range for achieving peak H2 production in most species lies between 6.0 and 8.0. H2 production significantly declined at a starting acidic pH (5.0) and gradually increased under alkaline conditions, attaining peak yield at pH 9.0. | [150] |
| O2 content | O2 in the milieu hinders renewable-H2 production by the enzyme hydrogenase, and production stops entirely when O2 levels completely deactivate the catalytic activity. Producing O2 and H2 simultaneously necessitates the extraction of O2 from the milieu to maintain process efficacy. | [89] |
| Light intensity | H2 production by C. reinhardtii demonstrated a gradual increase in yield from 60 to 200 µE m−2 s−1, ultimately achieving peak production. Raising the light intensity to 300 µE m−2 s−1 showed light saturation accompanied by a reduction in H2 production. | [95] |
| N2 and S limitation | It was reported that ongoing H2 production from cyanobacteria achieves the greatest yield when deprived of N2 and S. | [103] |
| The S absence creates an anaerobic setting, boosting the capacity of microalgae to generate H2. | [152] | |
| Organic carbon | Organic carbon sources enhance the growth of mixotrophic cyanobacteria and aid in H2 production by fostering an anaerobic milieu suitable for achieving greater process efficacy. | [101] |
| Cell density and culture age | Elevated cell density within the photobioreactor limits light access for each cell, diminishing photosynthesis and elevating respiration. Even younger cultures with lower biomass demonstrate greater H2 production because the exponentially growing cells are more metabolically active than the older cells. | [101] |
4.1.2. Photo-Fermentation
- Minimal efficiency in converting solar energy (3–10%).
- The requirement for extensive anaerobic photo-bioreactors.
- The reliance on nitrogenase enzymes, which require significant energy for activation.
4.1.3. Dark Fermentation
| Fermentation Type | Inoculum/Substrate/ Operating Conditions | Information of Hazards | Refs. |
|---|---|---|---|
| PF | C. butyricum and R. palustris; Rice straw; T: 30 °C; pH 7.0; LI: 6000 lux | C. butyricum—The presence of Clostridium bacteria in food products threatens human health and life. There are many poisonings and deaths due to the ingestion of Clostridium spp. toxins. The mitigation of hazards comprises the following measures: Regularly clean areas that may be contaminated with C. difficile. Wear protective clothing and gloves when it is essential to have direct skin contact with infected materials or animals. Eye protection should be worn when there is a recognized or possible risk of splash exposure. Any procedures that could generate aerosols or that entail high concentrations or substantial volumes must be performed within a biological safety cabinet (BSC). The use of needles, syringes, and other sharp instruments must be highly restricted. Extra measures should be considered when dealing with animals or large-scale operations. | [212,213] |
| Some rare strains of Clostridium butyricum comprise the gene encoding the botulinal type E neurotoxin and promote hazards in certain types of food. The control of toxigenic C. butyricum in the food industry needs to allow for the great pH tolerance of this species. Clostridium butyricum—German TRBA Risk group: 2. (Agents that are associated with human disease that is rarely serious and for which preventive or therapeutic interventions are often available. They pose a medium risk to a person but a minimal risk to the community.) | [214] | ||
| R. palustris’s metabolic effectiveness and growth rate are truly low. Very few genetic manipulation tools are achievable for R. palustris to raise its performance. | [215] | ||
| Rice straw—Besides inducing air pollution, burning paddy straw leads to the loss of soil organic matter and essential nutrients, lowers microbial activities, and makes the land more vulnerable to soil erosion. Mitigation of hazards: Avoid burning paddy straw. | [216] | ||
| Post-harvest straw is often burned, releasing several pollutants into the environment. CO2 dominates at 70%, accompanied by CH4 at 0.66%, CO at 7%, and N2O at 2.09%. Mitigation of hazards: Avoid burning post-harvest straw. | [217] | ||
| Rhodopseudomonas palustris—German TRBA Risk group: 1. (Agents that are not associated with disease in healthy adult humans. This group has a record of animal viral etiologic agents in common use. They represent no or little risk to an individual and no or little risk to the community). | [218] | ||
| R. pseudopalustris DSM 123; Tequila vinasses; T: 30 °C; pH 7.0; LI: LED lamp (13.5 W/m2); Inoculum: 3.3 g/L cell suspension | Rhodopseudomonas pseudopalustris (DSM 123)—German TRBA Risk group: 1. | [215] | |
| Tequila vinasses pose a significant threat to surface aquatic ecosystems when discharged without proper treatment or with insufficient treatment. The disposal of highly concentrated Tequila vinasses poses an ecological risk. Mitigation of hazards: Utilizing AD methods to decrease organic material while generating BG. | [219,220,221] | ||
| Improperly disposing of untreated Tequila vinasses (TVs) can result in significant environmental harm to soil and H2O sources and the production of elevated levels of GHG emissions. Using TV for field fertilization may not always be effective since not all the nutrients (N, P, K) on TV are readily available for crops. Mitigation of hazards: TV adjustment to crop parameters. | [222] | ||
| HAU-M1 (R. sphaeroides (9%), R. palustris (28%), R. rubrum (27%), R. capsulata (25%) and R. capsulatus (11%); Corn stover; The 1st study: pH 7.0; LI: 3000 lux; Inoculum: 30% 150 mg/g TS; TiO2/AC fiber addition of 100 mg/L. The 2nd study: T: 30 °C; pH 7.0; LI: 3000 lux; Substrate concentration: 25 gDM/L. | Rhodobacter sphaeroides, Rhodopseudomonas palustris, Rhodospirillum rubrum, and Rhodopseudomonas capsulata—German TRBA Risk group: 1. | [215] | |
| Corn straw roots can easily absorb Cu and Zn in the soil, which can be harmful to human health, especially to children. The levels of heavy metals in soil and flue gas from burning corn straw have reached a very high ecological risk. The mitigation of hazards comprises the following measures:
| [223] | ||
| When crop straw is added to PAH-contaminated agricultural soils, particularly corn straw, the accumulation of polycyclic aromatic hydrocarbons (PAHs) in winter wheat, as well as the ecological and human health risks, seems to decrease due to increased PAH dissipation in the rhizosphere soil. | [224] | ||
| R. sphaeroides NCIMB8253; Combination of palm oil (25%, v/v), pulp and paper (75%, v/v) mill effluents; T: 30 °C; LI: 7000 lux; Combined substrate (25 vol.% POME and 75 vol.% PPME). | Rhodobacter sphaeroides—German TRBA Risk group: 1. | [215] | |
| Inhaling palm oil could be dangerous. It can cause irritation of the respiratory tract and skin. Absorbing it via the skin could be dangerous. The mitigation of hazards comprises the following measures:
| [225] | ||
| Palm oil contains high levels of saturated fat, which can increase LDL cholesterol levels and increase the likelihood of developing heart disease. The oil may be associated with inflammation, specific cancer risks, and type 2 diabetes. It is damaging to the environment. The sector has a record of unsustainable farming methods, is known for unjust labor practices, and has led to extensive deforestation. | [226] | ||
| Palm oil, often grown on plantations after rainforests are flattened and burned, is environmentally destructive. Following some initial advances, the reemergence of deforestation linked to palm oil production in Indonesia is evident once more. | [227] | ||
| The pulp and paper industry uses a significant quantity of energy and releases pollutants and GHGs into the atmosphere. The waste produced by the pulp and paper industry causes significant damage to aquatic life, disrupts the food chain, and leads to various health issues. This garbage creates significant issues for both H2O- and land-dwelling creatures. Health risks associated with waste H2O range from skin irritation to genetic abnormalities. Toxic substances in waste H2O demonstrate mutagenic and genotoxic effects. Mitigation of hazards: The waste from the pulp and paper industry must be directed (under strict control) to appropriate treatment plants. | [228] | ||
| DF | Sludge from an anaerobic digester of a waste-H2O treatment plant; Food waste; T: 35 °C; pH 5.3; HRT: 36 h | Toxic substances in the AD system, whether from influent waste streams or bacterial metabolism, can hinder the digestion process. Mitigation of hazards: Systematic control of sources of consumed substances. | [229] |
| Wasted food releases damaging gases like CO2, H2S, CH4, N2O, and PM2.5, which are harmful to human health. Mitigating hazards: Provide necessary ventilation for the zone with people present. | [230] | ||
| E. Coli XL1-Blue/Enterobacter cloacae DSM 16657; Beverage waste H2O; T: 37 °C; pH 6.5 | E. coli XL-1-Blue—no known hazards. | ||
| Enterobacter cloacae—There is a risk to immunocompromised patients when in direct or indirect contact with contaminated persons/objects. Pathogens can be transmitted via contaminated infusion solutions or blood products. Mitigation of hazards: Avoid contact with contaminated people. Use proper protective clothing, gloves, masks, and glasses. | [231] | ||
| Enterob. cl.—a biosafety level 1 organism in the USA/level 2 in Canada. | [232] | ||
| Enterobacter infections are serious ones with a high mortality rate, even with appropriate treatment. Enterobacter species induce many nosocomial infections and, less frequently, community-acquired infections, including urinary tract infections (UTIs), respiratory infections, soft tissue infections, osteomyelitis, and endocarditis, among many others. | [233] | ||
| Waste H2O in the beverage industry contains raw materials used in beverage fabrication, such as diverse sugars, ethanol, fruit concentrates, malts, hops, syrups, acids, and mineral salts. Due to the raw materials used in beverages, the concentration of organic matter is high in the waste H2O. Mitigation of hazards: Waste H2O from the beverage industry must be directed (under strict control) to appropriate treatment plants. | [234] | ||
| Fermentative consortium MC 1 (Firmicutes and Bacteroidota phyla); Food waste + Fe-modified biochar; T: 55 °C; pH 7.0; Inoculation ratio: 10 vol.% | Firmicutes—A higher ratio of Firmicutes is tied to Type 1 and Type 2 diabetes, heart disease, certain cancers, Alzheimer’s, and obesity. | [235] | |
| Firmicutes and Bacteroidetes can influence diseases related to obesity, which are also risk factors for breast cancer. It may be necessary to adopt an appropriate diet and change your lifestyle habits toward being more active. | [236] | ||
| Unsafe waste can contaminate the soil, H2O, and air, disrupting ecosystems and harming wildlife. The mitigation of hazards comprises the following measures:
| [237] | ||
| Human exposure to unsafe waste can lead to acute or chronic health issues, ranging from respiratory problems to cancer. Mitigation of hazards: Avoid contact with unsafe waste. | [238] | ||
| Food waste—1/3 of all human-caused GHG emissions and 8% of GHG annually, a significant waste of fresh H2O and ground H2O resources. Emissions from food waste, like H2S, CH4, and volatile organic carbons, can affect human endocrine, respiratory, nervous, and olfactory systems. The mitigation of hazards comprises the following measures:
| [230] | ||
| Fe-modified biochar feedstock and temperature mainly affect biochar (BC) contamination and toxicity. PAHs, heavy metals, pH, and EC affect BC toxicity. Mitigation of hazards: Systematic control of biochar parameters. | [239] | ||
| Biochar is a flammable material (H228). After inhalation (H333) or swallowing (H305), it may be harmful. It induces eye irritation (H320). If heated, it may induce a fire (H242). Excessive C dust from handling biochar may produce allergenic responses in a few sensitive individuals. Overexposure to biochar dust may cause skin/eye and upper respiratory tract irritation, allergenic responses, and asthma. The mitigation of hazards comprises the following measures:
| [240] | ||
| The production of Pinus patula raw biochar—the source of energy utilized during this process—accompanied by the generation of gases and polycyclic aromatic hydrocarbons. For Fe-modified biochar, the potential environmental effects differed only in the stage of biomass modification with the metal. They depend on the extraction of Fe and the generation of waste H2O. | [241] | ||
| Sludge from waste-H2O treatment facility; Corn stover+ therm.-modified maifanite; T: 35 °C | Corn stover—as mentioned | ||
| Thermally modified maifanite—no hazards found. | |||
| The MicroStart waste-H2O bacterial inoculum is harmful if swallowed. It can induce harm if it contacts the skin or is breathed in. It may induce eye irritation. The mitigation of hazards comprises the following measures:
| [242] | ||
| Anaerobic granules from an anaerobic digester of a waste-H2O treatment plant; Cassava pulp and processing waste H2O; T: 35 °C; pH 6.0; HRT: 132 h | Organoarsenic feed additives (roxarsone) induce organoarsenicals in livestock waste H2O and anaerobic waste-H2O treatment systems. | [243] | |
| AD—as mentioned. | |||
| Cassava production worsens soil fertility via crop removal of nutrients, a more serious and long-term effect on environmental erosion. Mitigation of hazards: Limit areas for cassava production. | [244] | ||
| Cassava leaves contain toxins from cyanogenic glucosides (which may cause cyanide poisoning, resulting in signs like headaches, nausea, dizziness, diarrhea, vomiting, and potentially fatal outcomes) as well as antinutritional components (such as high fiber, tannins, polyphenols, and phytic acid) that lower the absorption and digestion of nutrients. Mitigation of hazards: Avoid eating improperly prepared Cassava leaves. | [245] | ||
| Improperly cooked Cassava may have substances that change into cyanide in the body. This could induce cyanide poisoning and result in specific paralysis conditions. Some individuals may experience an allergic response to cassava. Mitigation of hazards: Follow good Cassava cooking practices. | [245] | ||
| Sludge from primary anaerobic digester; Pruning wastes + food-rich MSW; T: 37 °C; pH 5.0 and 7.0; HRT: 72 h | AD—as mentioned. | ||
| Burning waste from tree pruning outdoors decreases air quality and adds to the greenhouse effect. Mitigating hazards: Avoid burning waste from tree pruning. | [246] | ||
| Food waste—as mentioned | |||
| Sludge from upflow anaerobic sludge blanket treating papermaking waste H2O; Corn straw + excess sludge; T: 35 °C; pH 7.0; HRT: 17 days | Toxic pollutants in papermaking waste H2O have carcinogenic, genotoxic, and mutagenic effects. Gaseous compounds are toxic to human health and the environment. The mitigation of hazards comprises the following measures: Waste H2O from the paper industry must be directed (under strict control) to appropriate treatment plants. Provide enough ventilation for zones with people present. | [247] | |
| Corn straw—as mentioned. | |||
| Anaerobic sludge—A problem with AD occurs since the feedstock may contain heavy metals or persistent organic pollutants (POPs). Heavy metals cannot be destroyed by digestion. The mitigation of hazards comprises the following measures: Systematic control of AD parameters. Limit its contact with people. Proper personal protective gear should be used. | [248] | ||
| Anaerobic sludge from an anaerobic digester; Swine manure + food waste; T: 35 °C; pH: 5.5–6; HRT: 4 days | AD—as mentioned. | ||
| The concentration of Cu in swine manure exceeded the Cu limit; likewise, the concentration of Zn in swine manure exceeded the Zn limit. All livestock manure showed elevated levels of Zn, Cu, and Cr, suggesting possible ecological hazards. Swine manure exhibits the maximum potential ecological risk for agronomic application. Swine manure poses a non-carcinogenic risk to kids and an unacceptable carcinogenic risk to kids. The mitigation of hazards comprises the following measures:
| [249] | ||
| Food waste—as mentioned. |
| Parameters | Effect on H2 Yield in PF and DF | Refs. |
|---|---|---|
| Inoculum | In PF, incorporating purple non-S bacteria such as Rhodobacter sp. and increased light intensity can boost H2 production, while using mixed strains can further enhance the yield. In DF, H2 production relies on the use of strict (e.g., Clostridium sp.) or facultative anaerobic bacteria and can be improved through methods like immobilization and the addition of metal ions or oxide nanoparticles (NPs). | [155] |
| Temperature | The fermentative bacterial community generates H2 across a broad temperature spectrum, with mesophilic (35–40 °C) and thermophilic (50–60 °C) conditions frequently utilized due to their effects on pH and VFA generation. | [251] |
| pH | In PF, an acidic pH promotes H2 production, while in DF, a nearly neutral pH is more effective for H2 generation. Raising the pH might improve the capacity of H2-generating bacteria; nonetheless, elevated pH levels could lower H2 production. | [252,253] |
| Type of substrate | The selection of the substrate is based on factors like expense, accessibility, carbohydrate levels, and ease of fermentation. Although glucose is widely used, solid waste and industrial waste H2O present attractive options for economic and sustainability factors, with little pretreatment, affecting the best substrate choice for H2 generation. | [254] |
| Substrate concentration | Optimal H2 production is positively associated with the substrate concentration, ensuring adequate nutrition for photosynthetic bacteria to sustain H2 production. | [175] |
| In PF, a high substrate concentration can greatly elevate butyric acid levels, which reduces the pH and halts H2 production. | [255] |
4.1.4. Integrated Systems (ISs)
4.1.5. Projects Focused on Biological Technologies
4.2. Thermochemical Methods for H2 Extraction from Biomass and Hazards Related to It
4.2.1. Biomass Pyrolysis
| Methods | Information of Hazards | Refs. | |
|---|---|---|---|
| PY | Efficient separation and purification of end products are necessary because of high temperatures and long residence times, leading to high energy consumption. | [308] | |
| [309] | ||
| Certain biomass samples are not environmentally friendly during PY. | [310] | ||
| Biomass dominated by lignin produces products dominated by char, significantly impacting two environmental categories. Cellulose-rich biomass has an impact on six other categories by generating oil-rich products. Biomass rich in hemicellulose produces gases with high levels and minimal environmental impacts. | [311] | ||
| HT processes | HTC | Process water (PW) with a short retention time contains toxic phenols, furfurals, and their derivatives, which enable AD to produce biogas (BG). | [312] |
| The PW is contaminated by both organic and inorganic sources and requires treatment. The issues concerning the management of stable and toxic organic substances such as phenols, phenolic compounds, furfural, and 5-HMF remain unresolved. These compounds can sometimes be hard to break down through biological processes (having high COD-BOD5 ratios), which could create challenges in treating PW. Mitigation of hazards: Post-PW should be directed to an appropriate sewage treatment plant. | [89] | ||
| HTL |
| [313] | |
| HTG | High levels of energy consumption and technological requirements for the process are costly. | [314] | |
| GA | The safety of startup processes is influenced by the temperature used for heating. The possibility of fire and explosion, along with the release of environmental pollutants through various routes, is a concern due to the presence of a flammable gas mixture with a high amount of H2 gas produced under high-temperature and high-pressure conditions. | [315,316] | |
| The specific dangers include the possible release of harmful gases such as CO, SOx, NOx, and particles. | [317] | ||
| CO can enter the bloodstream and bind with hemoglobin to inhibit O2 absorption and circulation. Prolonged exposure to CO can lead to asthma, inflammation of the lungs, schizophrenia, and heart defects. Harmful gases such as SOx, NOx, and volatile organics can harm human respiratory, digestive, and skin systems. | [318] | ||
| CO is an extremely poisonous gas. It is referred to as a toxic (blood) asphyxiant, indicating that it diminishes the blood’s ability to transport O2. Low-level doses of CO can lead to headaches and dizziness; however, if the person is taken to fresh air, no lasting harm will occur. Elevated levels, however, can saturate an individual’s blood within minutes and rapidly result in respiratory failure or demise. The existing allowable exposure limit for CO is determined by a Time-Weighted Average (TWA) of 30 ppm. Even though extremely high levels of CO can be acutely harmful, potentially causing immediate respiratory failure or death, it is the long-term health impacts from chronic exposure at lower concentrations that have the most significant effect on affected workers. Exposure levels are insufficient to cause immediate symptoms; however, frequent small doses gradually diminish the blood’s ability to carry O2 to dangerously low levels. The mitigation of hazards comprises the following measures:
| [319,320] | ||
| In elevated amounts, gaseous SOx can negatively impact trees and plants by harming leaves and inhibiting growth. SO2 and various sulfur oxides may lead to acid rain that can damage delicate ecosystems. SO2 may lead to respiratory issues like bronchitis and can irritate your nose, throat, and lungs. It can lead to coughing, wheezing, mucus production, and asthma episodes. The impacts are more severe during physical activity. SO2 has been associated with heart disease. The mitigation of hazards comprises the following measures:
| [321,322,323] | ||
| Low concentrations of NOx in the atmosphere can irritate your eyes, nose, throat, and lungs, potentially leading to coughing and symptoms such as shortness of breath, fatigue, and nausea. Being exposed to low levels can also lead to fluid accumulation in the lungs one or two days following the exposure. Inhaling elevated amounts of nitrogen oxides may lead to quick combustion, spasms, and inflammation of the throat and upper respiratory area, decreased O2 supply to body tissues, fluid accumulation in the lungs, and death. Skin or eye contact with high levels of nitrogen oxide gases or liquid nitrogen dioxide would probably result in severe burns. Possible reduction in hazards: Reducing NOx and N2O emissions is a significant issue since these compounds can inflict considerable harm to the atmosphere, soil, H2O, and human health. Currently, selective catalytic reduction is the most efficient and common technology for eliminating NOx from flue gases. Additional approaches are utilized selectively, particularly those below:
| [324,325] | ||
| Particle emission (PM2.5) induces cancer. Their ability to soak up various soluble organic compounds such as alkanes, carboxylic acid, and aromatic compounds can harm the lungs and livers of humans. Short-term exposure to PM2.5 (lasting up to 24 h) is linked to early death, heart or lung issues, both acute and chronic bronchitis, asthma episodes, respiratory problems, and limited activity. PM2.5 particles in the air can penetrate deeply into the respiratory system, reaching the lungs and leading to immediate health issues such as irritation in the eyes, nose, throat, and lungs, along with symptoms like coughing, sneezing, a runny nose, and difficulty breathing. The mitigation of hazards comprises the following measures:
| [326,327,328,329] | ||
| Environmental issues can arise when ashes and condensates produced from biomass gasification are not appropriately disposed of. Dealing with a toxic condensate that has a high tar content is challenging and presents greater hazards. | [330] | ||
- Potential dangers of fire and explosion caused by the existence of extremely flammable gases (H2 and CO).
- Dangerous release of toxic gas, with potentially severe outcomes, caused by the abundant presence of CO [309].
- Risks to human health may be linked to long-term exposure to bio-oils.
- Severe harmful impacts on humans and eco-toxic impacts on H2O environmental ecosystems might also occur in the event of a loss of containment.
- A slight carcinogenic risk might arise from the existence of cancer-causing substances such as catechol and PAHs. Therefore, evaluating and managing the risks associated with bio-oils is a crucial factor to consider for ensuring the safe and sustainable utilization of products derived from biomass PY.
- Remove all ignition sources near the spilled material.
- Steer clear of heat, sparks, flames, and oxidizers.
- Steer clear of exposure to mineral acid/alkali.
- Halt the origin of the release if you can accomplish it safely. Perform release containment to avoid additional pollution of soil, surface H2O, or ground H2O.
- Use protective gear to avoid eye contact and skin exposure.
- Typically, no respiratory protection is needed. Nevertheless, utilize a positive-pressure air-supplying respirator in situations where air-purifying respirators are insufficient.
4.2.2. Hydrothermal Processes of Biomass
- A major challenge is the existence of heteroatoms, like sulfur and nitrogen, in HTL biocrudes. Effectively controlling these heteroatoms via desulfurization and denitrogenation processes is needed. Specifically, it is essential to create strong and economical techniques to lower the sulfur and nitrogen levels in biocrudes.
- Despite numerous HTL studies being performed at laboratory and pilot levels, moving to full-scale industrial operations is complicated. Difficulties involve creating sizable reactors, guaranteeing a steady supply of feedstock, and tackling financial factors. Research needs to concentrate on expanding HTL and hydrotreatment techniques for commercial use.
- While often challenging, securing a steady supply of sustainable biomass feedstocks is crucial. The sustained availability and continuity of feedstocks are essential for commercial viability and process consistency.
- Although different catalysts have demonstrated potential in enhancing biocrude yield and quality, obstacles persist in creating highly efficient, reusable, and feedstock-flexible catalysts. Catalyst innovation represents a crucial area of research focus.
- Enhancing the hydrotreatment process is essential for attaining optimal biocrude upgrading efficiency. Additional studies are necessary to enhance selectivity, yield, and energy usage in the hydrotreatment process.
4.2.3. Biomass Gasification
| Parameters | Effect on H2 Yield in Biomass Steam GA | Refs. |
|---|---|---|
| Temperature | Increasing the temperature encourages endothermic processes like hydrocarbon reforming (Formula (17)), methane reforming (Formula (21)), and carbon gasification (Formula (23)), leading to enhanced gas production and a higher volumetric fraction of H2 in syngas. | [391], |
| Raising the temperature further reduces the tar content in syngas. | [392] | |
| Temperatures exceeding 950 °C can inhibit the WGS reaction (Formula (23)), an exothermic process, resulting in a reduction in the H2 content in syngas. | [393,394] | |
| Steam to biomass ratio (S/B) | The S/B ratio plays a crucial role in determining the syngas composition and energy input in biomass gasification. A low S/B ratio results in the creation of solid C and CH4. Augmenting steam availability enhances the reforming processes of C and CH4 into CO and H2. An S/B ratio exceeding 1.3 leads to excess steam, which decreases solid C and CH4 while increasing H2 and CO2 production. Steam aids in lowering CO through the WGS (Formula (23)) and hydrocarbon reforming reactions (Formulas (17), (21), and (22)). Ideal S/B ratios of up to 1.3 promote H2 production, whereas ratios above 1.3 lower temperature and elevate tar creation. | [282,314,395] |
| Biomass characteristics | Biomass type: A composition high in cellulose and lignin increases the yield of gaseous products and the H2 percentage in syngas. | [314,396] |
| Particle size: Reduced particle sizes enhance the surface area, facilitating better heat transfer, increasing gasification rates, and elevating H2 content while minimizing tar production. Particles less than 1 mm may raise energy usage. | [283], | |
| Moisture content: The ideal range is 10–15% by weight; levels over 40% result in lower temperatures, increased energy consumption, and decreased GA efficacy and H2 levels. | [395,397] | |
| Ash content: Elevated ash levels boost coke and particulate generation in syngas, requiring efficient gas-cleaning methods. | [396,397] | |
| Catalysts | Boost H2 and CO yields by optimizing heat and mass transfer efficiency during the GA process (Formulas (23)–(27)). | [306] |
| Catalysts additionally assist in tar elimination, enhancing H2 yield and overall GA efficacy, thereby augmenting renewable-H2 generation. | [282] | |
| Typical catalysts consist of Ni-based compounds, alkali metals (such as K2CO3, Na2CO3), alumina, aluminosilicate, ZnCl2, and dolomite. | [283] |
- I.
- A process called aqueous-phase reforming (APR) involves converting biomass-derived compounds (C6H12O6, C6H14O6, C3H8O3, CH3OH, and C2H6O2) into mainly H2 and CO2 at temperatures between 215 and 265 °C with the help of a catalyst (initially Pt, later also Ni, Ru, Rh, Pd, and Ir).
- II.
- The catalyzed near-critical GA of biomass or organic compounds at approximately 350 °C in the liquid phase or 400 °C in the supercritical state using a heterogenous catalyst converts them mainly to CH4 and CO2 for combustion.
- III.
- Supercritical H2O gasification (SCWG) involves the gasification of biomass or organic compounds to produce mainly H2 as a burnable gas and CO2, without requiring the use of a solid catalyst or the presence of C or other solid catalysts.
4.2.4. Initiatives Focused on TC Methods
4.3. The Hydrogen Extraction via Glycerol Steam Reforming and Hazards Related to It
4.3.1. Catalysts
4.3.2. Promotors
| Material Category | Substance | Information on Hazards | Refs. |
|---|---|---|---|
| Substrate | C3H8O3 | Coming into contact with it can result in skin and eye irritation. Inhaling Glycerol-alpha-Monochlorohydrin may lead to irritation in the nose and throat, resulting in coughing and wheezing. Exposure to Glycerol-alpha-Monochlorohydrin may lead to nausea, vomiting, dizziness, lack of coordination, and potentially coma. The mitigation of hazards comprises the following measures:
| [445] |
| It is flammable (H228) and emits irritating or toxic fumes (or gases) when exposed to fire. The mitigation of hazards comprises the following measures:
| [446] | ||
| When taken by mouth, headaches, dizziness, bloating, nausea, and diarrhea might occur. When applied to the skin, it might cause redness, itching, and burning. | [447] | ||
| Catalyst | Pt | Highly fragmented Pt powder can ignite easily, explode, and emit toxic fumes when burned. Coming into contact with it may cause irritation to the skin and eyes. Inhaling it may cause irritation to the nose and throat. It could lead to a skin rash. The mitigation of hazards comprises the following measures:
| [454,455] |
| Pt powder is a flammable solid (H228). Pt powder may react violently or explosively upon contact with H2O. It may be ignited by friction, heat, sparks, or flames. Its dust or fumes may form explosive mixtures in the air. The mitigation of hazards comprises the following measures:
| [456] | ||
| Pt coordination complexes are carcinogenic and genotoxic in mammalian and bacterial cells. | [457] | ||
| Rh | It is flammable when in dust or powder form, and toxic gases are generated during a fire. Breathing in Rh powder can have an impact on humans. Exposure to Rh powder may cause irritation to the skin and eyes. It has the potential to lead to a skin allergic reaction. If an allergy develops, even minimal future exposure can lead to itching and a skin rash. The mitigation of hazards comprises the following measures:
| [458] | |
| All Rh compounds are highly toxic and carcinogenic. They stain the skin very strongly. They can be flammable. A dust explosion can occur if the Rh powder or granular form is mixed with air. Mitigation of hazards: In addition to those previously mentioned, do not allow the material to be released into the environment. | [459] | ||
| Rh, when not managed effectively, can have negative effects on both human health and the environment. Rh solutions, which include Rh2(SO4)3, can be harmful, causing skin and eye irritation and respiratory issues if breathed in. | [460] | ||
| Rh is a catalyst and can result in a combustible and explosive danger when exposed to various organic and inorganic substances. Mitigation of hazards: In addition to those previously mentioned, use local exhaust or breathing protection. Remove inappropriate substances from places with Rh present. | [461] | ||
| Re | Re can lead to skin irritation. Its liquid state has the potential to inflict burns on the skin and eyes. Following consumption, it can lead to irritation in the digestive system. Inhaling it could result in irritation of the respiratory tract. | [462] | |
| If exposed to alcohol or similar organic substances, it could spontaneously combust. The Re dust can result in minor to moderate eye and skin irritation. Breathing in fine Re dust or fumes can cause irritation in the nose and lungs. The mitigation of hazards comprises the following measures:
| [463] | ||
| Re is a flammable solid. It is a physical irritant to the gastrointestinal tract. The mitigation of hazards comprises the following measures:
| [464] | ||
| Re2O7 is an oxidizer, and its contact with combustible material may cause fire. Its inhalation may lead to irritation of the respiratory tract. It may lead to irritation of the nose, throat, and lungs and potential chemical burns to mucous membranes. If swallowed, it may lead to chemical burns in the mouth, throat, and digestive system. When in contact with skin, it is a strong irritant and corrosive. It may result in intense irritation and chemical burns. In contact with the eye, it is a corrosive substance and a powerful irritant. It may result in chemical burns to eye tissue and visual disturbances, including blindness. The mitigation of hazards comprises the following measures:
| [465] | ||
| Pd | When consumed, the body does not absorb Pd well. It can lead to irritation of the skin, eyes, or respiratory tract and can also cause skin sensitization. Liquid has the potential to inflict burns on the skin and eyes. All compounds containing Pd are extremely harmful and can cause cancer. PdCl2 is poisonous and can cause harm if ingested, breathed in, or taken in through the skin. It results in harm to the bone marrow, liver, and kidneys of lab animals. It is irritating. Low concentrations of Pd salts can eliminate the H2O hyacinth, while most plants can withstand levels under 3 ppm. | [466] | |
| Powdered Pd is a solid that can catch fire easily (H228). It results in skin irritation (H315) and severe eye irritation (H319), possibly leading to respiratory irritation (H335). The mitigation of hazards comprises the following measures:
| [467] | ||
| Solid-state Pd alloys are typically considered non-hazardous. Nonetheless, if the procedure includes grinding, melting, cutting, or any other method that results in the emission of dust or fumes, dangerous quantities of airborne particulates may be produced. Contact dermatitis can be caused by Pd alloy. In case of dust or fume generation, the following measures should be taken:
| [468] | ||
| Pd powder can catch fire when coming into contact with air, especially if there is adsorbed H2 present. It easily ignites flammable solvents when they are exposed to air. Pd can catch fire easily in the form of fine powder or dust. Many of Pd’s compounds act as oxidizing agents, and others can react explosively with organic materials. Pd exhibits high toxicity over an extended period and at the cellular level in the liver and kidney. Elevated levels of Pd can be toxic and potentially cancer-causing for mammals. Long-term exposure to Pd particles found in dust can cause harmful impacts on the blood and respiratory systems. With Pd/C catalysts, the finely divided carbon can cause irritation to mucous membranes and the upper respiratory tract. Pd/C catalysts with absorbed H2 can ignite easily, especially when devoid of moisture and exposed to high temperatures. Catalysts prepared by reducing CH2O are not as pyrophoric as those reduced with H2. C in a fine powder form has the potential to cause a dust explosion. Catalysts made on supports with a large surface area are very effective and easily ignite mixtures of H2/air and solvent/air. CH3OH is highly volatile, which makes it prone to easily catch fire. The introduction of a catalyst into a H4B solution could lead to the combustion of the released H2. | [469] | ||
| Ru | All Ru substances are extremely poisonous and can cause cancer. They leave a very intense stain on the skin. Ru that is consumed is firmly held in bones. RuO4 is extremely dangerous and easily evaporates, so it should be stayed away from. Mitigation of hazards: Avoid the ingestion of Ru and its contact with skin and eyes. | [470] | |
| Ruthenium is a solid substance that can easily catch fire (H228). The mitigation of hazards comprises the following measures:
| [471] | ||
| Ir | Ir is very combustible. It might lead to irritation in the eyes. When consumed, it can lead to irritation of the gastrointestinal system. The mitigation of hazards comprises the following measures:
| [472,510] | |
| Extracting and processing Ir has negative impacts on the environment. Ir extraction and processing have a negative impact on ecosystems and add to carbon emissions. The mitigation of hazards comprises the following measures:
| [473] | ||
| Co | Coming into contact with Co dust can cause irritation to the skin, eyes, nose, and throat. Co may trigger an asthma-like allergic reaction, leading to asthma attacks characterized by difficulty breathing, wheezing, coughing, and chest tightness upon subsequent exposure. Co may impact the heart, thyroid, liver, and kidneys. The mitigation of hazards comprises the following measures: Limit or avoid contact with Co in all forms. In case of Co dust generation, take the following measures:
| [474] | |
| Excessive amounts of inorganic Co can lead to considerable toxicity. Infrequent occurrence of acute toxicity can be attributed to the excessive intake of nutrients. Mitigation of hazards: Avoid excessive intake of nutrients with Co. | [475] | ||
| Soluble Co salts have a negative impact on cell division, permanently attach to nucleic acids in the nucleus, cause chromosome abnormalities in plants, and show mild mutagenic effects in certain in vitro tests with cultured animal cells, bacteria, and yeast. | [476] | ||
| Cu | Consuming high levels of Cu can result in serious health issues, including kidney and liver damage. Inhaling Cu dust, sprays, or crystals can result in nasal and throat irritation, as well as lead to dizziness and headaches. The mitigation of hazards comprises the following measures:
| [477] | |
| Highly fragmented Cu can ignite or burst in the presence of O2. Toxic fumes are generated during a fire. Exposure to Cu powder may cause skin and eye irritation and burns. Breathing Cu can lead to irritation in the nose and throat, possibly resulting in a sore or perforation in the septum of the inner nose. Cu can lead to headaches, nausea, vomiting, diarrhea, and abdominal pain. Exposure to Cu can result in a flu-like sickness known as “metal fume fever”. Cu has the potential to trigger a skin allergy and impact the functioning of the liver and kidneys. The mitigation of hazards comprises the following measures:
| [478] | ||
| Being exposed to increased levels of Cu can be damaging. Prolonged exposure to Cu dust can lead to irritation of the nose, mouth, and eyes, as well as headaches, dizziness, nausea, and diarrhea. It is necessary to avoid prolonged exposure to Cu dust. | [479] | ||
| Excessive consumption of Cu regularly can lead to liver damage, abdominal pain, cramps, nausea, diarrhea, and vomiting. Cu toxicity may develop in individuals with Wilson’s disease, an uncommon genetic condition. The level of Cu in diet should be controlled and Cu overdosing is prohibited. | [480] | ||
| Cu does not degrade in the environment, leading to its accumulation in plants and animals from the soil. Only a few plants can thrive on Cu-rich soil. Hence, the presence of Cu-disposing factories restricts plant diversity in the surrounding area. It is necessary to systematically control the Cu level in the soil. | [481] | ||
| Ni | The activated catalyst Raney Nickel in a 50% slurry of H2O can spontaneously combust, leading to a potential fire risk (H251). It can result in a skin allergy (H317). There are suspicions that it may lead to cancer (H351). It can also harm organs with long-term or repeated contact (H372). Harming aquatic organisms with lasting consequences (H412) is detrimental. The mitigation of hazards comprises the following measures:
| [482] | |
| Promotor | K | Coming into contact with solid K can result in serious burns. Inhaling K fumes can cause irritation to the nose, throat, and lungs, resulting in sneezing and coughing. Extended exposure to K fumes can lead to ulcers in the inner nasal passages. K is a chemical that is both flammable and reactive, posing risks of fire and explosions. The mitigation of hazards comprises the following measures:
| [484] |
| In some cases, too much K may cause muscle weakness, confusion, irregular heartbeat, or difficulty breathing. The K level in the diet should be controlled. | [485] | ||
| Excessive consumption of K led to issues with neuromuscular functioning such as weakness, paralysis, nausea, vomiting, and diarrhea. These symptoms do not always appear before life-threatening heart rhythm disturbances. K overdosing is prohibited. | [486] | ||
| Ca | Ca can have an explosive reaction with H2O, steam, moisture, and potent acids like hydrochloric, sulfuric, and nitric, resulting in the production of flammable H2 gas. Finely powdered Ca can catch fire when exposed to air or when coming into contact with halogens such as Cl and F. The mitigation of hazards comprises the following measures:
| [487] | |
| High levels of Ca in the bloodstream and urine can lead to weak muscle tone, impaired kidney function, decreased phosphate levels, constipation, nausea, weight loss, severe fatigue, frequent urination, irregular heartbeats, and a significant risk of heart disease-related death. | [488] | ||
| The human body requires an abundance of Ca in CaCO3, rather than in its pure metallic state. Ca reacts vigorously with H2O and can be harmful to the tongue and esophagus due to its corrosive nature. It is necessary to avoid direct Ca contact with the tongue and esophagus. | [489] | ||
| Elevated levels of Ca can result in severe disruptions in heart rhythm, as well as the formation of kidney stones and the impairment of kidney function. Repeatedly using something for a long time can have worse consequences than taking too much of it at once. It is necessary to avoid the overconsumption of Ca and CaCO3. | [490] | ||
| Sr | Inhaling Sr can have an impact on humans. It has the potential to irritate the skin and eyes. Extensive exposure to strontium could impact the heart. High levels of Sr exposure can lead to accumulation in the bones and potentially impact their functionality. The mitigation of hazards comprises the following measures:
| [491] | |
| Exposure to significant levels of radioactive Sr may cause cancer. Leukemia has occurred in humans exposed to substantial amounts of radioactive Sr. Leukemia and cancers of the bone, nose, lung, and skin also occurred in laboratory animals. It is necessary to avoid elevated levels of radioactive Sr. | [492] | ||
| Sr does not burn but produces flammable gas when exposed to H2O or moist air. It is necessary to avoid Sr contact with H2O or moist air. | [493] | ||
| Ce | Ce is a solid that can easily catch fire (H228). It ignites via friction and combusts in fire situations. It could potentially release H2 gas when exposed to H2O during a fire situation. Exposure to acids can produce H2 gas. The mitigation of hazards comprises the following measures:
| [494] | |
| Contact with CeO2 can lead to irritation of the eyes and abraded skin. It can also lead to irritation of the lungs. Compounds of Ce2(CO3)3 display varying degrees of toxicity, ranging from mild to moderate depending on the specific compound. In an animal study, Ce2(CO3)3, CeF3, and CeO2 did not show acute toxicity, exhibited no dermal irritation, and caused minimal eye irritation. CeCl3 showed higher acute toxicity and caused intense irritation to the skin. The mitigation of hazards comprises the following measures:
| [495] | ||
| Ce is particularly hazardous in the workplace because of the presence of vapors and gases that can be breathed in along with air. This could lead to pulmonary embolisms, particularly with prolonged exposure. Accumulation in the human body can pose a threat to the liver. It is necessary to provide enough ventilation and avoid prolonged exposure to Ce and contact Ce with H2O. | [496] | ||
| Ce NPs showed high toxicity in all toxicity tests performed (more than 80% inhibition at low concentrations in the bioluminescence test and an LC50 of 0.012 mg/mL in Daphnia magna assays). It is necessary to avoid the intake of CE NPs. | [497] | ||
| La | La can become inflammable when exposed to heat, sparks, or flames. It reacts vigorously with acids and may react with H2O under fire conditions, in each case releasing flammable H2 gas. In regular use and handling, solid forms of this substance pose minimal health risks. Additional actions like grinding, melting, or welding can create dangerous dust or fumes that may be breathed in or contact the skin or eyes. The mitigation of hazards comprises the following measures:
| [498] | |
| La cannot pass through the unbroken blood–brain barrier. The most frequent negative reactions include slight to moderate nausea, diarrhea, and flatulence. The La amount in the human body should be controlled, and La overdosing and excessive exposure to La are prohibited. | [499] | ||
| The use of Fosrenol (La2(CO3)3) medicine may cause serious stomach or bowel problems, including blockage or perforation (tear or hole) or severe constipation. The use of Fosrenol should be controlled, and its overdosing is prohibited. | [500] | ||
| La compound (Fosrenol) can cause blockage of the stomach, intestines, or rectum, which can be very dangerous. The risk is higher in people with a history of changes to the digestive tract’s anatomy or constipation problems or who are taking medications that can also cause blockage. The use of Fosrenol should be controlled and carefully selected for each individual. | [501] | ||
| Cr | Exposure to Cr(VI) can lead to occupational asthma, eye irritation and damage, perforated eardrums, respiratory irritation, kidney damage, liver damage, pulmonary congestion and edema, upper abdominal pain, nose irritation and damage, respiratory cancer, skin irritation, and erosion and discoloration of the teeth. Some employees may experience an allergic skin reaction known as allergic contact dermatitis. This is evident when dealing with liquids or solids that contain Cr(VI), such as Portland cement. This dermatitis persists for a long time and becomes more intense with frequent contact with the skin. Skin ulcers (chrome ulcers) can be caused by contact with damaged skin. Ulcers on the skin caused by chromium are characterized by crusty, painless sores with a depressed center filled with fluid. The mitigation of hazards comprises the following measures:
| [495] | |
| Long-term exposure to Cr(VI) compounds increases the likelihood of developing cancer in the lungs, nasal passages, and sinus cavities. Contact with Cr(VI) compounds can lead to severe dermatitis and typically painless skin ulcers. Cr compounds can act as both sensitizers and irritants. It is necessary to avoid long-term exposure to Cr(VI) compounds. | [503] | ||
| Exposure to different oxidation states of Cr results in varying levels of health hazards, with the metal form being less toxic and the hexavalent form being highly toxic. | [504] | ||
| The process of Cr plating involves certain risks. Cr contains carcinogenic Cr(VI). The plating process involves Pb, which has the potential to be soaked up through the skin and leads to harm to the liver, organs, and brain. Cyanide is extremely poisonous. It is utilized in the process of Cr plating and has the potential to be lethal to humans. Additionally, Cd utilized during the procedure has the potential to induce cancer as well as issues with kidney and lung functionality. The mitigation of hazards comprises the following measures:
| [505] | ||
| Cr is a significant pollutant found in numerous toxic waste locations globally, such as the Superfund sites in America. | [506] | ||
| Employees in sectors that utilize Cr are more likely to experience the negative health impacts of the element. It is necessary to avoid excessive exposure to Cr(VI) compounds | [507] | ||
| Fe | Fe powder or dust is a solid that can catch fire easily. It can lead to irritation of the eyes and skin by mechanical means. It can result in abnormalities in the blood. It can also lead to harm in the lungs. Breathing in Fe fumes can lead to metal fume fever. It can lead to heart issues and harm the liver. The ingestion of Fe powder can result in gastrointestinal irritation accompanied by symptoms like nausea, vomiting, and diarrhea. Continuous exposure can lead to damage to the pancreas, diabetes, and abnormal heart function. The mitigation of hazards comprises the following measures:
| [508] | |
| Excessive intake of Fe by humans can lead to serious symptoms, liver damage, and possibly death. Signs progress via different stages, starting with vomiting, diarrhea, and abdominal pain. Liver failure may manifest itself several days after the initial onset. It is necessary to control Fe levels in the diet, and Fe overdosing is prohibited. | [509] |
4.4. Electrochemical Methods
4.4.1. Water Electrolysis
- Minimize contact with pure O2.
- Stay away from gas that is expanding quickly. Proper protective gear and attire are essential in the event of exposure.
- Attempt to heat the frozen areas and obtain medical assistance.
- Avoid contact with garments and other flammable substances. Ensure that reduction valves, valves, and fittings are kept clean and free of oil and grease.
- If it is safe, stop the leak in the event of a fire.
- Shield storage devices from direct sunlight. Keep in a location with good air circulation.
4.4.2. Microbial Electrolysis Cells
- Avoiding areas with increased Cl concentration.
- Controlling the Cl concentration in the air.
- Ensuring the adequate ventilation of rooms.
- Wearing appropriate protective clothing, gloves, respiratory masks, and goggles, especially in areas with increased Cl concentration.
4.4.3. Proton Exchange Membrane Electrolysis Cells
4.4.4. Projects on Electrochemical Techniques
5. Hazards Related to Separation and Purification of Hydrogen
6. Techno-Economic and Environmental Aspects of Renewable-H2 Production Technologies
| Process | Feedstock | Capital Costs (M-EUR) | H2 Cost (EUR/kg) | Efficiency (%) | TRL | Ref. |
|---|---|---|---|---|---|---|
| Biomass PY | Biomass + Heat + Steam | 53.4–3.1 | 1.3–2.2 | 17–33 | 4–5 | [538] |
| Biomass GA | Biomass + H2O | 149.3–6.4 | 1.8–2.1 | 35–50 | 7–8 | [539] |
| HT GA | Biomass + Heat + Steam | - | 1.5–3.2 | 70 | 2–3 | [540] |
| DbP | Sun + H2O + Algae | 50 USD/m2 | 2.13 | 12.2 | 2–3 | [541] |
| i-DbP | H2O + Algae | 135 USD/m2 | 1.42 | 4.1 | 2–3 | |
| DF | Biomass + Anaerobic bacteria | - | 2.57 | 12 | 4–5 | |
| PF | Sunlight + Biomass | - | 2.83 | 8.5 | 4–5 | |
| Electrolysis | H2O + Electricity | - | 10.3 | 60–80 | 9 | [542] |
| MEC | Waste H2O + Electricity | - | - | 67–90 | <5 | [542] |
| PEMEC | Waste H2O + Electricity | - | - | 70–80 | 7–8 (9) | [542] |
6.1. Techno-Economical Aspects of Renewable-H2 Production Technologies
6.1.1. Biological Technologies
6.1.2. Thermochemical Technologies
6.1.3. Electrochemical Technologies
6.2. Barrier to Commercialization of Renewable-H2 Production Technologies
6.2.1. Technical Challenges
| Thermochemical Conversion | Technical Obstacles | Financial Obstacles | Potential Strategies for Overcoming These Barriers | Ref. |
|---|---|---|---|---|
| GA | Energy usage | Expense of CO2 | [571] | |
| SR | - | - | The improved efficiency and longer life of a precious metal catalyst balance the higher cost per catalyst unit experienced in the catalytic process. | [572] |
| GA | Problems like corrosion, fouling, and catalyst deactivation, along with the absence of broad industrial acceptance and standardization of the product, can impede the effectiveness of catalyst applications. | The requirement for high temperatures leads to substantial capital and operational costs when executing certain procedures. | Membrane reactors can improve the efficiency of thermochemical processes by using different H2-production methods. | [573] |
| SCWG | - | The viability of a project depends on the financial factors related to obtaining algal biomass and the yield produced. | Optimization plays a vital role in research to improve fuel production. When a payment is made from a carbon dioxide emitter to an algal conversion plant, the expense of H2 reduces. | [413] |
6.2.2. Financial Hurdles
| Biochemical Process | Technical Obstacles | Financial Obstacles | Potential Strategies for Overcoming Such Barriers | Ref. |
|---|---|---|---|---|
| DF | Establishing, constructing, operating, and managing an appropriate bioreactor. | The primary factor impacting the expense of bio-H2 is the cost of substrates. | Feedback inhibition decreases when dark fermentation and photo-fermentation are integrated. | [566] |
| DF | Because pretreatment methods differ based on the feedstock, conducting pretreatment before fermentation poses a major challenge. | Expensive process. | Extensive, high-level research to overcome financial and technological challenges. | [576] |
| AD | Variations in H2 yield result from differences in biomass, process inhibition, bacteria that utilize H2, high levels of heavy metal ions, optimization challenges, and H2 storage issues. | Cost of H2 storing as a liquid. | The effectiveness of H2 production can be enhanced by incorporating chemical additives. | [577] |
| DF | Thermodynamic limitations exist on the quantity of H2 generated through microbial fermentation, in addition to the design and operation of active bioreactors. The primary technological obstacle to the application of DF in practice is its restricted H2 production of 4 mol H2/mol of glucose. | Elevated expenses associated with the raw materials. | The extraction of energy from the substrate is enhanced when DF is combined with other energy-producing systems. | [269] |
| The process of integrated DF and PF techniques | A key challenge in the pretreatment process is the presence of inhibitory chemicals. The substrate restricts one or both of the processes. | Due to the harmful nature of waste-H2O treatment effluents, the costs of processing increase. The expenses of the procedure are raised in a sequential reactor due to the reactor’s operation and upkeep. The treatment of DF waste H2O leads to a rise in operating expenses. | Selecting appropriate H2 producers enhances the efficiency of H2 production through genetic or metabolic engineering in the integrated DF and PF process. | [578] |
| PF | - | Increased production at a higher energy expense. | The notable progress in the bio-H2 process can be counterbalanced by metabolic engineering. By investigating the effects of nutrient restriction and substrate use, scientists discovered the chromosomal genes in microalgae that play a role in boosting H2 production. Advancements in photobioreactor design should be performed with maximum efficacy. | [579] |
6.3. Environmental Impacts of Renewable-H2 Production Technologies
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanty, M. Green Growth as a Pathway to Sustainable Development. 2015. Available online: https://www.studocu.com/row/document/the-university-of-the-south-pacific-solomon-islands/geography/mohanty-manoranjan-2015-green-growth-as-a-pathway-to-sustainable-development/37836548 (accessed on 9 September 2024).
- Song, Y.; Ji, Q.; Du, Y.-J.; Geng, J.-B. The Dynamic Dependence of Fossil Energy, Investor Sentiment and Renewable Energy Stock Markets. Energy Econ. 2019, 84, 104564. [Google Scholar] [CrossRef]
- Gladchenko, M.A.; Gaydamaka, S.N.; Kornilov, V.I.; Chernov, V.V.; Kornilova, A.A. Anaerobic Conversion of Waste of Alcohol Production with Animal and Poultry Waste into Methane as a Substrate for Hydrogen Production. Int. J. Hydrogen Energy 2024, 51, 37–48. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Lopez-Sanchez, J.A. Review of Hydrogen Production by Catalytic Aqueous-Phase Reforming. ChemistrySelect 2017, 2, 6563–6576. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A Review on Biomass Based Hydrogen Production Technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Developments and Issues in Renewable Ecofuels and Feedstocks. Energies 2024, 17, 3560. [Google Scholar] [CrossRef]
- Jarosz, Z.; Kapłan, M.; Klimek, K.; Anders, D.; Dybek, B.; Herkowiak, M.; Hołaj-Krzak, J.T.; Syrotyuk, S.; Korobka, S.; Syrotyuk, H.; et al. Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies 2024, 17, 2524. [Google Scholar] [CrossRef]
- Fasolini, A.; Cucciniello, R.; Paone, E.; Mauriello, F.; Tabanelli, T. A Short Overview on the Hydrogen Production Via Aqueous Phase Reforming (APR) of Cellulose, C6-C5 Sugars and Polyols. Catalysts 2019, 9, 917. [Google Scholar] [CrossRef]
- Del Mundo, D.M.N.; Sutheerawattananonda, M. Influence of Fat and Oil Type on the Yield, Physico-Chemical Properties, and Microstructure of Fat, Oil, and Grease (FOG) Deposits. Water Res. 2017, 124, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P. Effect of Biomedical Waste Co-Feeding in the Steam Gasification of Indian Palm Kernel Shell in Fluidized Bed Gasifier. Environ. Sci. Pollut. Res. 2022, 29, 36788–36800. [Google Scholar] [CrossRef] [PubMed]
- Halba, A.; Arora, P. Pine Needle Gasification–Based Electricity Production: Understanding the Effect of Supply Chain. Environ. Sci. Pollut. Res. 2024, 1–19. [Google Scholar] [CrossRef]
- Karthikeyan, P.K.; Bandulasena, H.C.H.; Radu, T. A Comparative Analysis of Pre-Treatment Technologies for Enhanced Biogas Production from Anaerobic Digestion of Lignocellulosic Waste. Ind. Crops Prod. 2024, 215, 118591. [Google Scholar] [CrossRef]
- Viswanathan, K.; Wang, S. Experimental Investigation on the Application of Preheated Fish Oil Ethyl Ester as a Fuel in Diesel Engine. Fuel 2021, 285, 119244. [Google Scholar] [CrossRef]
- Viswanathan, K.; Ikhsan Taipabu, M.; Wu, W. Novel Petit Grain Bitter Orange Waste Peel Oil Biofuel Investigation in Diesel Engine with Modified Fuel Injection Pressure and Bowl Geometry. Fuel 2022, 319, 123660. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Illathukandy, B.; Wu, W.; Chang, J.-S. Plasma Gasification Performances of Various Raw and Torrefied Biomass Materials Using Different Gasifying Agents. Bioresour. Technol. 2020, 314, 123740. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-M.; Sun, Y.-L.; Lin, C.-H.; Lin, C.-H.; Wu, H.-T.; Lin, C.-S. Cultivation and Biorefinery of Microalgae (Chlorella sp.) for Producing Biofuels and Other Byproducts: A Review. Sustainability 2021, 13, 13480. [Google Scholar] [CrossRef]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Abedi, H. Biodiesel Production Integrated with Glycerol Steam Reforming Process, Solid Oxide Fuel Cell (SOFC) Power Plant. Energy Convers. Manag. 2020, 206, 112467. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Varuvel, E.; Karthickeyan, V.; Sonthalia, A.; Kumar, G.; Saravanan, C.G.; Dhinesh, B.; Pugazhendhi, A. Effect of Hydrogen on Compression-Ignition (CI) Engine Fueled with Vegetable Oil/Biodiesel from Various Feedstocks: A Review. Int. J. Hydrogen Energy 2022, 47, 37648–37667. [Google Scholar] [CrossRef]
- Alizadeh, S.M.; Khalili, Y.; Ahmadi, M. Comprehensive Review of Carbon Capture and Storage Integration in Hydrogen Production: Opportunities, Challenges, and Future Perspectives. Energies 2024, 17, 5330. [Google Scholar] [CrossRef]
- Health and Safety Authority Hazard and Risk. 2023. Available online: https://www.hsa.ie/eng/topics/hazards/ (accessed on 9 September 2024).
- Lu, Y.; Guo, L.; Ji, C.; Zhang, X.; Hao, X.; Yan, Q. Hydrogen Production by Biomass Gasification in Supercritical Water: A Parametric Study. Int. J. Hydrogen Energy 2006, 31, 822–831. [Google Scholar] [CrossRef]
- Ozbas, E.E.; Aksu, D.; Ongen, A.; Aydin, M.A.; Ozcan, H.K. Hydrogen Production via Biomass Gasification, and Modeling by Supervised Machine Learning Algorithms. Int. J. Hydrogen Energy 2019, 44, 17260–17268. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, Y.; Fan, H.; Ji, J. Hydrogen Production from Biomass Pyrolysis in Molten Alkali. AASRI Procedia 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.I.; Puello, P.; Cabarcas, A. Process Analysis of Hydrogen Production via Biomass Gasification under Computer-Aided Safety and Environmental Assessments. ACS Omega 2020, 5, 19667–19681. [Google Scholar] [CrossRef] [PubMed]
- PMA Stainless Steel. Safety Data Sheet. 2024. Available online: https://s29571.pcdn.co/wp-content/uploads/2021/01/SDS-Prime-Stainless-Steel-2020.pdf (accessed on 9 October 2024).
- HSE Network Managing Health and Safety Risks in Engineering. HSE Network, HSE News, 29 November 2023. Available online: https://www.hse-network.com/managing-health-and-safety-risks-in-engineering/ (accessed on 9 September 2024).
- Chen, C.; Reniers, G. Risk Assessment of Processes and Products in Industrial Biotechnology. In Sustainability and Life Cycle Assessment in Industrial Biotechnology; Fröhling, M., Hiete, M., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2018; Volume 173, pp. 255–279. ISBN 978-3-030-47065-4. [Google Scholar]
- Eckebrecht, T. Occupational Standards for the Protection of Employees in Biotechnology. Int. Arch. Occup. Environ. Health 2000, 73, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Ghittori, S.; Ferrari, M.; Negri, S.; Serranti, P.; Sacco, P.; Biffi, R.; Imbriani, M. Recenti strategie per la prevenzione e l’analisi dei rischi in ambito occupazionale: Control banding e Sobane [Recent prevention strategies and occupational risk analysis: Control Banding and So-bane]. G. Ital. Med. Lav. Ergon. 2006, 28, 30–43. [Google Scholar] [PubMed]
- Ferrari, M.; Colombi, A.; Imbriani, M. Rischio professionale e prevenzione nell’industria bio-tecnologica: Revisione della letteratura [Occupational risk and prevention in the biotech-nology industry: A review]. Med. Lav. 2006, 97, 651–675. [Google Scholar] [PubMed]
- Ghasemi, A.; Nikafshan Rad, H.; Akrami, M. Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods. Hydrogen 2024, 5, 474–493. [Google Scholar] [CrossRef]
- National Grid Energy Explained. What Is Hydrogen? 2023. Available online: https://www.nationalgrid.com/stories/energy-explained/what-is-hydrogen (accessed on 9 September 2024).
- Hassan, Q.; Hafedh, S.A.; Mohammed, H.B.; Abdulrahman, I.S.; Salman, H.M.; Jaszczur, M. A Review of Hydrogen Production from Bio-Energy, Technologies and Assessments. Energy Harvest. Syst. 2024, 11, 20220117. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Gnansounou, E. Agroresidue-Based Biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–258. ISBN 978-0-12-818996-2. [Google Scholar] [CrossRef]
- Sivaranjani, R.; Veerathai, S.; Jeoly Jenifer, K.; Sowmiya, K.; Rupesh, K.J.; Sudalai, S.; Arumugam, A. A Comprehensive Review on Biohydrogen Production Pilot Scale Reactor Technologies: Sustainable Development and Future Prospects. Int. J. Hydrogen Energy 2023, 48, 23785–23820. [Google Scholar] [CrossRef]
- Morya, R.; Raj, T.; Lee, Y.; Kumar Pandey, A.; Kumar, D.; Rani Singhania, R.; Singh, S.; Prakash Verma, J.; Kim, S.-H. Recent Updates in Biohydrogen Production Strategies and Life–Cycle Assessment for Sustainable Future. Bioresour. Technol. 2022, 366, 128159. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Ganeshan, P.; Gohil, N.; Kumar, V.; Singh, V.; Rajendran, K.; Harirchi, S.; Solanki, M.K.; Sindhu, R.; Binod, P.; et al. Advanced Approaches for Resource Recovery from Wastewater and Activated Sludge: A Review. Bioresour. Technol. 2023, 384, 129250. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.C.; Mateos-Pedrero, C.; Longo, A.; Rijo, B.; Brito, P.; Ferreira, P.; Nobre, C. Renewable Hydrogen from Biomass: Technological Pathways and Economic Perspectives. Energies 2024, 17, 3530. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration Biomass Explained. 2024. Available online: https://www.eia.gov/energyexplained/biomass/ (accessed on 9 September 2024).
- International Energy Agency (IEA). The Future of Hydrogen; International Energy Agency: Paris, France, 2019. [Google Scholar]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A Technical and Environmental Comparison between Hydrogen and Some Fossil Fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Ruiz Pérez, S.; Bohacikova, V. Hydrogen Storage Safety in Urban Settings: Analysing Soci-Etal Risk Using RiskCurves; Gexcon: Bergen, Norway, 2024; Available online: https://www.gexcon.com/blog/hydrogen-storage-safety-in-urban-settings-analysing-societal-risk-using-riskcurves/ (accessed on 9 October 2024).
- Neville, A. Power, 1 May 2009. Available online: https://www.powermag.com/lessons-learned-from-a-hydrogen-explosion/ (accessed on 9 October 2024).
- Crowl, D.A.; Jo, Y.-D. The Hazards and Risks of Hydrogen. J. Loss Prev. Process Ind. 2007, 20, 158–164. [Google Scholar] [CrossRef]
- Barbir, F. Safety Issues of Hydrogen in Vehicles. 2012. Available online: https://api.semanticscholar.org/CorpusID:235432870 (accessed on 9 October 2024).
- MineARC System Risks and Safety Hazards of Methane. 2024. Available online: https://minearc.com (accessed on 9 October 2024).
- NevadaNano. The Dangers of Methane Gas Poisoning and Exposure. 2024. Available online: https://nevadanano.com (accessed on 9 October 2024).
- Airgas USA, LLC. Ethane CAS Number 74-84-0. 2020. Available online: https://www.airgas.com/msds/001183.pdf (accessed on 9 October 2024).
- EERE. Safe Use of Hydrogen; Energy Efficiency & Renewable Energy (EERE), Hydrogen and Fuel Cell Technologies Office: Washington, DC, USA, 2024. Available online: https://www.energy.gov/eere/fuelcells/safe-use-hydrogen (accessed on 9 October 2024).
- Lenntech Hydrogen—H. Chemical Properties of Hydrogen—Health Effects of Hydrogen—Environmental Effects of Hydrogen. 2024. Available online: https://www.lenntech.com/periodic/elements/h.htm (accessed on 9 October 2024).
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Qi, C.; Huang, J.; Wang, B.; Deng, S.; Wang, Y.; Yu, G. Contaminants of Emerging Concern in Landfill Leachate in China: A Review. Emerg. Contam. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Zhang, X.; Ali, N.; Yuan, J.; Wang, K. Pyrolysis Coupled Anaerobic Digestion Process for Food Waste and Recalcitrant Residues: Fundamentals, Challenges, and Considerations. Energy Sci. Eng. 2019, 7, 2250–2264. [Google Scholar] [CrossRef]
- Gao, A.; Tian, Z.; Wang, Z.; Wennersten, R.; Sun, Q. Comparison between the Technologies for Food Waste Treatment. Energy Procedia 2017, 105, 3915–3921. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah, X.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of Heavy Metals from Soil and Aquatic Environment: An Overview of Principles and Criteria of Fundamental Processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy Metal Contamination: An Alarming Threat to Environment and Human Health. In Environmental Biotechnology: For Sustainable Future; Sobti, R.C., Arora, N.K., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 103–125. ISBN 978-981-10-7283-3. [Google Scholar]
- Khan, A.; Khan, S.; Khan, M.A.; Qamar, Z.; Waqas, M. The Uptake and Bioaccumulation of Heavy Metals by Food Plants, Their Effects on Plants Nutrients, and Associated Health Risk: A Review. Environ. Sci Pollut Res 2015, 22, 13772–13799. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.M.; Kumar, S.G.; Jyothsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead Induced Changes in Antioxidant Metabolism of Horsegram (Macrotyloma uniflorum (Lam.) Verdc.) and Bengalgram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Chia, S.R.; Chia, W.Y.; Cheah, W.Y.; Munawaroh, H.S.H.; Ong, W.-J. Abatement of Hazardous Materials and Biomass Waste via Pyrolysis and Co-Pyrolysis for Environmental Sustainability and Circular Economy. Environ. Pollut. 2021, 278, 116836. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Zhang, J. The Role of Biochar in Organic Waste Composting and Soil Improvement: A Review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Meng, H.; Zhao, L.; Shen, Y.; Hou, Y.; Cheng, H.; Song, L. Effect of Biochar and Humic Acid on the Copper, Lead, and Cadmium Passivation During Composting. Bioresour. Technol. 2018, 258, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in Heavy Metal Mobility and Availability from Contaminated Wetland Soil Remediated with Combined Biochar-Compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Thi, T.X.; Nguyen, P.Q.P.; Tran, V.D.; Ağbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Pham, N.D.K. Recent Advances in Hydrogen Production from Biomass Waste with a Focus on Pyrolysis and Gasification. Int. J. Hydrogen Energy 2024, 54, 127–160. [Google Scholar] [CrossRef]
- Dolle, C.; Neha, N.; Coutanceau, C. Electrochemical Hydrogen Production from Biomass. Curr. Opin. Electrochem. 2022, 31, 100841. [Google Scholar] [CrossRef]
- Chung, Y.T.; Rohani, R.; Mohamad, I.N.; Mastar@Masdar, M.S.; Takriff, M.S. Biohydrogen Purification from Palm Oil Mill Effluent Fermentation for Fuel Cell Application. Malays. J. Anal. Sci. 2019, 23, 80–89. [Google Scholar]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Mohsen, A.; Elwakeel, K.Z. Biomass-to-Sustainable Biohydrogen: Insights into the Production Routes, and Technical Challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Vernick, D. What Is Green Hydrogen, and How Can It Help Tackle the Climate Crisis? 2024. Available online: https://www.worldwildlife.org/stories/what-is-green-hydrogen-and-how-can-it-help-tackle-the-climate-crisis (accessed on 9 October 2024).
- Pirelli. Cars. Hydrogen as a Fuel: The Pros and Cons. 2024. Available online: https://www.pirelli.com/global/en-ww/road/cars/hydrogen-as-a-fuel-the-pros-and-cons-53908/ (accessed on 9 October 2024).
- Gupta, U. Critical Challenges in Biohydrogen Production Processes from Organic Feedstocks. 2024. Available online: https://www.linkedin.com/pulse/critical-challenges-biohydrogen-production-processes-utkarsh-zdimc (accessed on 9 October 2024).
- Hurwitz, Z.; Bujak, N.; Tapia, M.; Daza, E.; Gischler, C. Key Aspects for Managing the En-Vironmental and Social Risks of Green Hydrogen. Hablemeos De Sostenibilidad Y Cambio Climático 2023. Available online: https://blogs.iadb.org/sostenibilidad/en/key-aspects-for-managing-the-environmental-and-social-risks-of-green-hydrogen/ (accessed on 9 October 2024).
- Mullerova, J. Health and Safety Hazards of Biomass Storage. 20 June 2014. Available online: https://www.researchgate.net/profile/Jana-Muellerova-5/publication/273061578_Health_and_Safety_hazards_of_biomass_storage/links/54f60e0f0cf27d8ed71d4cf2/Health-and-Safety-hazards-of-biomass-storage (accessed on 9 October 2024).
- Ennis, T. Fire and Explosion Hazards in the Biomass Industries. IChemE Symp. Ser. 2016, 161, 64. Available online: https://www.icheme.org/media/11801/hazards-26-paper-64-fire-and-explosion-hazards-in-the-biomass-industries.pdf (accessed on 9 October 2024).
- Márquez-Reyes, L.A.; Sánchez-Saavedra, M.D.P.; Valdez-Vazquez, I. Improvement of Hydrogen Production by Reduction of the Photosynthetic Oxygen in Microalgae Cultures of Chlamydomonas Gloeopara and Scenedesmus Obliquus. Int. J. Hydrogen Energy 2015, 40, 7291–7300. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Ena, A.; Johanningmeier, U. Increased Hydrogen Photoproduction by Means of a Sulfur-Deprived Chlamydomonas Reinhardtii D1 Protein Mutant. Int. J. Hydrogen Energy 2009, 34, 4529–4536. [Google Scholar] [CrossRef]
- Al- Janabi, S.K.; Barron, A.R.; Shabbani, H.J.K.; Othman, M.R.; Kim, J. Advances in Hydrogen Production from Sustainable Resources through Biological and Thermochemical Pathways: Review and Bibliometric Analysis. Int. J. Hydrogen Energy 2024, 60, 28–45. [Google Scholar] [CrossRef]
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-Hydrogen Production by Chlorella Vulgaris under Diverse Photoperiods. Bioresour. Technol. 2011, 102, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Mona, S.; Kaushik, A.; Kaushik, C.P. Hydrogen Production and Metal-Dye Bioremoval by a Nostoc Linckia Strain Isolated from Textile Mill Oxidation Pond. Bioresour. Technol. 2011, 102, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Hitam, C.N.C.; Jalil, A.A. A Review on Biohydrogen Production through Photo-Fermentation of Lignocellulosic Biomass. Biomass Conv. Bioref. 2023, 13, 8465–8483. [Google Scholar] [CrossRef]
- Zhang, X.; Sherman, D.M.; Sherman, L.A. The Uptake Hydrogenase in the Unicellular Diazotrophic Cyanobacterium Cyanothece Sp. Strain PCC 7822 Protects Nitrogenase from Oxygen Toxicity. J. Bacteriol. 2014, 196, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Melis, A. Photobiological Hydrogen Production: Recent Advances and State of the Art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Zafar, A.M.; Aly Hassan, A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The Role of Oxygen Regulation and Algal Growth Parameters in Hydrogen Production via Biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Rahman, S.N.A.; Masdar, M.S.; Rosli, M.I.; Majlan, E.H.; Husaini, T.; Kamarudin, S.K.; Daud, W.R.W. Overview Biohydrogen Technologies and Application in Fuel Cell Technology. Renew. Sustain. Energy Rev. 2016, 66, 137–162. [Google Scholar] [CrossRef]
- Mona, S.; Kumar, S.S.; Kumar, V.; Parveen, K.; Saini, N.; Deepak, B.; Pugazhendhi, A. Green Technology for Sustainable Biohydrogen Production (Waste to Energy): A Review. Sci. Total Environ. 2020, 728, 138481. [Google Scholar] [CrossRef]
- New Jersey Department of Health and Senior Services Sulfur CAS Number 7704-34-9. 2011. Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/1757.pdf (accessed on 9 October 2024).
- Boone, C.; Bond, C.; Cross, A.; Jenkins, J. Sulfur. 2017. Available online: https://npic.orst.edu/factsheets/sulfurgen.html (accessed on 9 October 2024).
- Hovensa LLC. Sulfur CAS No. 7704-34-9. 2006. Available online: http://westliberty.edu/health-and-safety/files/2012/08/Sulfur.pdf (accessed on 9 October 2024).
- International Chemical Safety Cards (ICSCs) Carbon CAS No.: 7440-44-0 2017. Available online: https://chemicalsafety.ilo.org/dyn/icsc/showcard.display?p_card_id=0702 (accessed on 9 October 2024).
- Kossalbayev, B.D.; Tomo, T.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Alwasel, S.; Allakhverdiev, S.I. Determination of the Potential of Cyanobacterial Strains for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 2627–2639. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Sun, J.; Zhong, J.-J. Biofuel Production by in Vitro Synthetic Enzymatic Pathway Biotransformation. Curr. Opin. Biotechnol. 2010, 21, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.P.; Evans, B.R.; Mielenz, J.R.; Hopkins, R.C.; Adams, M.W.W. High-Yield Hydrogen Production from Starch and Water by a Synthetic Enzymatic Pathway. PLoS ONE 2007, 2, e456. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.A.; Tam, T.K.; Zhang, Y.-H.P. New Biotechnology Paradigm: Cell-Free Biosystems for Biomanufacturing. Green Chem. 2013, 15, 1708. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen Production by Immobilized Chlorella Sp. Using Cycles of Oxygenic Photosynthesis and Anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef]
- Sengmee, D.; Cheirsilp, B.; Suksaroge, T.T.; Prasertsan, P. Biophotolysis-Based Hydrogen and Lipid Production by Oleaginous Microalgae Using Crude Glycerol as Exogenous Carbon Source. Int. J. Hydrogen Energy 2017, 42, 1970–1976. [Google Scholar] [CrossRef]
- Pyokim, J.; Dukkang, C.; Hyunpark, T.; Sunkim, M.; Junsim, S. Enhanced Hydrogen Production by Controlling Light Intensity in Sulfur-Deprived Chlamydomonas Reinhardtii Culture. Int. J. Hydrogen Energy 2006, 31, 1585–1590. [Google Scholar] [CrossRef]
- Bala Amutha, K.; Murugesan, A.G. Biological Hydrogen Production by the Algal Biomass Chlorella Vulgaris MSU 01 Strain Isolated from Pond Sediment. Bioresour. Technol. 2011, 102, 194–199. [Google Scholar] [CrossRef]
- Chader, S.; Hacene, H.; Agathos, S.N. Study of Hydrogen Production by Three Strains of Chlorella Isolated from the Soil in the Algerian Sahara. Int. J. Hydrogen Energy 2009, 34, 4941–4946. [Google Scholar] [CrossRef]
- Hong, M.E.; Shin, Y.S.; Kim, B.W.; Sim, S.J. Autotrophic Hydrogen Photoproduction by Operation of Carbon-Concentrating Mechanism in Chlamydomonas Reinhardtii under Sulfur Deprivation Condition. J. Biotechnol. 2016, 221, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.H.; Hausmann, T.S.; Carter, B.M.; Gerschler, J.J.; Benemann, J.R. Hydrogen Generation Through Indirect Biophotolysis in Batch Cultures of the Nonheterocystous Nitrogen-Fixing Cyanobacterium Plectonema Boryanum. Appl. Biochem Biotechnol 2010, 162, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.R.; Santos, P.V.D.; Giraldi, L.A.; Zaiat, M.; Calijuri, M.D.C. Anaerobic Phototrophic Processes of Hydrogen Production by Different Strains of Microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365, fny073. [Google Scholar] [CrossRef]
- Vargas, S.R.; Zaiat, M.; Calijuri, M.D.C. Influence of Culture Age, Ammonium and Organic Carbon in Hydrogen Production and Nutrient Removal by Anabaena Sp. in Nitrogen-Limited Cultures. Int. J. Hydrogen Energy 2020, 45, 30222–30231. [Google Scholar] [CrossRef]
- Vargas, S.R.; Santos, P.V.D.; Zaiat, M.; Calijuri, M.D.C. Optimization of Biomass and Hydrogen Production by Anabaena Sp. (UTEX 1448) in Nitrogen-Deprived Cultures. Biomass Bioenergy 2018, 111, 70–76. [Google Scholar] [CrossRef]
- Raksajit, W.; Satchasataporn, K.; Lehto, K.; Mäenpää, P.; Incharoensakdi, A. Enhancement of Hydrogen Production by the Filamentous Non-Heterocystous Cyanobacterium Arthrospira Sp. PCC 8005. Int. J. Hydrogen Energy 2012, 37, 18791–18797. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Ajeesh, C.P.M.; Sundaramoorthy, B.; Shalini, M.; Siva, R.; Das, R.; Fulzele, D.; Thiagarajan, K. Effect of Modified Zarrouk’s Medium on Growth of Different Spirulina Strains. Walailak J. Sci. Technol. 2016, 13, 67–75. [Google Scholar]
- Vargas, S.R.; Zaiat, M.; Calijuri, M.D.C. Chlamydomonas Strains Respond Differently to Photoproduction of Hydrogen and By-Products and Nutrient Uptake in Sulfur-Deprived Cultures. J. Environ. Chem. Eng. 2021, 9, 105930. [Google Scholar] [CrossRef]
- Poudyal, R.S.; Tiwari, I.; Koirala, A.R.; Masukawa, H.; Inoue, K.; Tomo, T.; Najafpour, M.M.; Allakhverdiev, S.I.; Veziroğlu, T.N. Hydrogen Production Using Photobiological Methods. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2015; pp. 289–317. ISBN 978-1-78242-361-4. [Google Scholar] [CrossRef]
- Cohen, I.; Knopf, J.A.; Irihimovitch, V.; Shapira, M. A Proposed Mechanism for the Inhibitory Effects of Oxidative Stress on Rubisco Assembly and Its Subunit Expression. Plant Physiol. 2005, 137, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Photosynthetic H2 Metabolism in Chlamydomonas Reinhardtii (Unicellular Green Algae). Planta 2007, 226, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Paramesh, K.; Chandrasekhar, T. Improvement of Photobiological Hydrogen Production in Chlorococcum Minutum Using Various Oxygen Scavengers. Int. J. Hydrogen Energy 2020, 45, 7641–7646. [Google Scholar] [CrossRef]
- Wei, L.; Yi, J.; Wang, L.; Huang, T.; Gao, F.; Wang, Q.; Ma, W. Light Intensity Is Important for Hydrogen Production in NaHSO3 -Treated Chlamydomonas reinhardtii. Plant Cell Physiol 2017, 58, 451–457. [Google Scholar] [CrossRef]
- Surzycki, R.; Cournac, L.; Peltier, G.; Rochaix, J.-D. Potential for Hydrogen Production with Inducible Chloroplast Gene Expression in Chlamydomonas. Proc. Natl. Acad. Sci. USA 2007, 104, 17548–17553. [Google Scholar] [CrossRef] [PubMed]
- Bothe, H.; Schmitz, O.; Yates, M.G.; Newton, W.E. Nitrogen Fixation and Hydrogen Metabolism in Cyanobacteria. Microbiol. Mol. Biol. Rev. 2010, 74, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Toepel, J.; Burgess, S.; Uhmeyer, A.; Blifernez, O.; Doebbe, A.; Hankamer, B.; Nixon, P.; Wobbe, L.; Kruse, O. Time-Course Global Expression Profiles of Chlamydomonas Reinhardtii during Photo-Biological H2 Production. PLoS ONE 2011, 6, e29364. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, C.N.; Jose Gilbert, J.; Lindblad, P.; Heidorn, T.; Borgvang, S.A.; Skjanes, K.; Das, D. Recent Trends on the Development of Photobiological Processes and Photobioreactors for the Improvement of Hydrogen Production. Int. J. Hydrogen Energy 2010, 35, 10218–10238. [Google Scholar] [CrossRef]
- MCC MA—Media for Freshwater, Terrestrial, Hot Spring and Salt Water Algae. 2024. Available online: https://mcc.nies.go.jp/02medium-e.html#ma (accessed on 9 October 2024).
- US Centers for Disease Control and Prevention. Types of Harmful Algal Blooms. Harmful Algal Bloom (HAB)-Associated Illness. 2024. Available online: https://www.cdc.gov/harmful-algal-blooms/about/types-of-harmful-algal-blooms.html (accessed on 9 October 2024).
- US Centres of Disease Control and Prevention (CDC). Harmful Algal Blooms: Contributing Factors and Impacts. Harmful Algal Bloom (HAB)-Associated Illness. 2024. Available online: https://www.cdc.gov/harmful-algal-blooms/about/harmful-algal-blooms-contributing-factors-and-impacts.html (accessed on 9 October 2024).
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The Multidisciplinary Approach to Safety and Toxicity Assessment of Microalgae-Based Food Supplements Following Clinical Cases of Poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Aly, S.M.; ElBanna, N.I.; Fathi, M. Chlorella in Aquaculture: Challenges, Opportunities, and Disease Prevention for Sustainable Development. Aquacult. Int. 2024, 32, 1559–1586. [Google Scholar] [CrossRef]
- European Parliament and of the Council Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EC, and Amending Regulation (EC) No 1907/2006 (Text with EEA Relevance). 2008. Available online: https://eur-lex.europa.eu/eli/reg/2008/1272/oj/eng (accessed on 9 October 2024).
- Sigma-Aldrich D-(+)-Glucose. CAS No. 50-99-7. Safety Data Sheet. 2024. Available online: https://www.sigmaaldrich.com/US/en/sds/sigma/g8270 (accessed on 9 October 2024).
- Li, L. Features of Serum Bottles. 2023. Available online: https://www.linkedin.com/pulse/features-serum-bottles-jason-wong/ (accessed on 9 October 2024).
- Sigma-Aldrich WHEATON Type I Crimp Top Serum Bottle. 2024. Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/dwk223738 (accessed on 9 October 2024).
- LGSonic Algae Problems. Algae Problems. 2024. Available online: https://www.lgsonic.com/algae-problems/ (accessed on 9 October 2024).
- Yadav, M.; Sharma, P.; Kushwah, H.; Sandal, N.; Chauhan, M.K. Assessment of the Toxicological Profile of Chlorella (C. Vulgaris) Powder by Performing Acute and Sub-Acute Oral Toxicity Studies in Mice. J. Appl. Phycol. 2022, 34, 363–373. [Google Scholar] [CrossRef]
- Zainul Azlan, N.; Abd Ghafar, N.; Mohd Yusof, Y.A.; Makpol, S. Toxicity Study of Chlorella Vulgaris Water Extract on Female Sprague Dawley Rats by Using the Organization for Economic Cooperation and Development (OECD) Guideline 420. J. Appl. Phycol. 2020, 32, 3063–3075. [Google Scholar] [CrossRef]
- Cargill Crude Glycerin. Material Safety Data Sheet. 2007. Available online: https://www.cargill.com/doc/1432075994011/glycerin-crude-msds.pdf (accessed on 9 October 2024).
- RBFuels Crude Glycerol/Glycerin. CAS No. 56-81-5. Safety Data Sheet. 2015. Available online: http://www.rbfuels.com/wp-content/uploads/2016/09/Crude-Glycerin_RBF-002_Final.pdf (accessed on 9 October 2024).
- Murbach, T.S.; Glávits, R.; Endres, J.R.; Hirka, G.; Vértesi, A.; Béres, E.; Szakonyiné, I.P. A Toxicological Evaluation of Chlamydomonas Reinhardtii, a Green Algae. Int. J. Toxicol. 2018, 37, 53–62. [Google Scholar] [CrossRef]
- Bohlender Polytetrafluorethylene (PTFE), Sintered. Safety Data Sheet. 2016. Available online: https://www.bola.de/msdb/Englisch/MSDS_PTFE_e.pdf (accessed on 9 October 2024).
- Smart Science TAP (Tris-Acetate-Phosphate) Medium. Safety Data Sheet. 2015. Available online: https://phytotechlab.com/mwdownloads/download/link/id/2275 (accessed on 9 October 2024).
- Wag! Cornstalk Plant Poisoning in Dogs. 2017. Available online: https://wagwalking.com/condition/cornstalk-plant-poisoning (accessed on 9 October 2024).
- Rankel, K. Dracaena Fragrans “Massangeana” (Corn Plant) Is Toxic To Humans. 2024. Available online: https://greg.app/dracaena-elegance-toxic-to-humans/ (accessed on 9 October 2024).
- ASPCA Corn Plant. 2024. Available online: https://www.aspca.org/pet-care/animal-poison-control/toxic-and-non-toxic-plants/corn-plant (accessed on 9 October 2024).
- 1st BASE Modified BG-11 (Blue-Green) Medium, Premix Powder Biotechnology Grade. Material Safety Data Sheet. 2022. Available online: https://base-asia.com/downloads/products/msds/SDS-7001-10L-Modified-BG-11-Blue-green-Medium-Premix-Powder.pdf (accessed on 9 October 2024).
- eChlorial. The 7 Lies about Chlorella: Dangers, Organic, Properties…. eChlorial. 2024. Available online: https://www.echlorial.com/blog/lies-dangers-chlorella/ (accessed on 9 October 2024).
- Jelonek, L. Chlorella Side Effects, Benefits, and Contraindications Natu.Care. Natu.Care. 2024. Available online: https://natu.care/uk/plants/chlorella (accessed on 9 October 2024).
- Sigma-Aldrich Ethyl Acetate. CAS No. 141-78-6. Safety Data Sheet. 2024. Available online: https://www.sigmaaldrich.com/PL/en/sds/sial/270989 (accessed on 9 October 2024).
- Sigma-Aldrich BG11 Broth. 2024. Available online: https://www.sigmaaldrich.com/US/en/product/sial/73816 (accessed on 9 October 2024).
- Sigma-Aldrich Starch, from Corn. CAS No. 9005-25-8. Safety Data Sheet. 2024. Available online: https://www.sigmaaldrich.com/DE/de/sds/SIAL/S4180%3Fsrsltid%3DAfmBOoqu8bSMlsamm9ixtAAyt0UazjJTdwiHBMiEvQIUpjbFu7cFo2Is (accessed on 9 October 2024).
- Fisher Science Education Starch, Solube, Lab Grade. Safety Data Sheet. 2015. Available online: https://www.fishersci.com/store/msds?partNumber=S25582A&productDescription=STARCH+SOLUBLE+500G&vendorId=VN00115888&countryCode=US&language=en (accessed on 9 October 2024).
- Fonseca, A.; Almeida, K.; Tavella, R.; Da Silva Júnior, F.; Dora, C.; Giroldo, D.; Maidana, M.; Muccillo-Baisch, A.L. Evaluation of Acute Toxicity of the Microalgae Pediastrum Boryanum. VITTALLE—Rev. Ciências Saúde. 2016, 8, 90–102. [Google Scholar]
- Sigma-Aldrich Diuron (DCMU). CAS No. 330-54-1. Safety Data Sheet. 2024. Available online: https://www.sigmaaldrich.com/PL/en/sds/aldrich/s279013 (accessed on 9 October 2024).
- Sigma-Aldrich Borosil Reagent Bottle. 2024. Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/671/804/foxx-life-sciences-1500017-1.pdf (accessed on 9 October 2024).
- Sigma-Aldrich Duran Laboratory Bottles, with Caps. 2024. Available online: https://www.sigmaaldrich.com/PL/en/product/aldrich/z305227?srsltid=AfmBOorv0ucXzMnHaDtcOOT8WoCktY71P_0gDlfi4tBthSGjucV3fq_w (accessed on 9 October 2024).
- Culture Collection of Algae and Protozoa Anabaena sp. 2024. Available online: https://www.ccap.ac.uk/catalogue/strain-1453-30 (accessed on 9 October 2024).
- Washington State Department of Health Cyanobacteria Technical Information. Cyanobacterial Toxins and Symptoms. Anabaena spp. 2024. Available online: https://doh.wa.gov/community-and-environment/contaminants/blue-green-algae/technical-information (accessed on 9 October 2024).
- Fleurence, J.; Levine, I.A. Antiallergic and Allergic Properties. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 307–315. ISBN 978-0-12-811405-6. [Google Scholar] [CrossRef]
- Culture Collection of Algae and Protozoa Chlamydomonas sp. 2024. Available online: https://www.ccap.ac.uk/catalogue/strain-11-154 (accessed on 9 October 2024).
- Kaushik, A.; Anjana, K. Biohydrogen Production by Lyngbya Perelegans: Influence of Physico-Chemical Environment. Biomass Bioenergy 2011, 35, 1041–1045. [Google Scholar] [CrossRef]
- Tiwari, A.; Pandey, A. Cyanobacterial Hydrogen Production—A Step towards Clean Environment. Int. J. Hydrogen Energy 2012, 37, 139–150. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. Parameters Affecting the Growth and Hydrogen Production of the Green Alga Chlamydomonas Reinhardtii. Int. J. Hydrogen Energy 2011, 36, 7872–7876. [Google Scholar] [CrossRef]
- Loyte, A.; Suryawanshi, J.; Bellala, S.S.K.; Marode, R.V.; Devarajan, Y. Current Status and Obstacles in the Sustainable Synthesis of Biohydrogen from Microalgal Species. Results Eng. 2024, 24, 103455. [Google Scholar] [CrossRef]
- Saifuddin, N.; Priatharsini, P. Developments in Bio-Hydrogen Production from Algae: A Review. Res. J. Appl. Sci. Eng. 2016, 12, 968–982. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strategy Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Das, S.R.; Basak, N. Molecular Biohydrogen Production by Dark and Photo Fermentation from Wastes Containing Starch: Recent Advancement and Future Perspective. Bioprocess Biosyst. Eng. 2021, 44, 1–25. [Google Scholar] [CrossRef]
- Koku, H. Aspects of the Metabolism of Hydrogen Production by Rhodobacter Sphaeroides. Int. J. Hydrogen Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from Food Processing Wastes via Photofermentation Using Purple Non-Sulfur Bacteria (PNSB)—A Review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Sakurai, H.; Masukawa, H.; Kitashima, M.; Inoue, K. Photobiological Hydrogen Production: Bioenergetics and Challenges for Its Practical Application. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 1–25. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Coupling Dark Fermentation with Biochemical or Bioelectrochemical Systems for Enhanced Bio-Energy Production: A Review. Int. J. Hydrogen Energy 2017, 42, 26667–26686. [Google Scholar] [CrossRef]
- Basak, N.; Das, D. The Prospect of Purple Non-Sulfur (PNS) Photosynthetic Bacteria for Hydrogen Production: The Present State of the Art. World J. Microbiol. Biotechnol. 2007, 23, 31–42. [Google Scholar] [CrossRef]
- Tao, Y. Characteristics of a New Photosynthetic Bacterial Strain for Hydrogen Production and Its Application in Wastewater Treatment. Int. J. Hydrogen Energy 2008, 33, 963–973. [Google Scholar] [CrossRef]
- Zhu, H.; Suzuki, T.; Tsygankov, A.A.; Asada, Y.; Miyake, J. Hydrogen Production from Tofu Wastewater by Rhodobacter Sphaeroides Immobilized in Agar Gels. Int. J. Hydrogen Energy 1999, 24, 305–310. [Google Scholar] [CrossRef]
- Yetis, M. Photoproduction of Hydrogen from Sugar Refinery Wastewater by Rhodobacter Sphaeroides O.U. 001. Int. J. Hydrogen Energy 2000, 25, 1035–1041. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, Y.; Wu, Y.; He, Y.; Zhou, Z. High Hydrogen Yield from a Two-Step Process of Dark- and Photo-Fermentation of Sucrose. Int. J. Hydrogen Energy 2007, 32, 200–206. [Google Scholar] [CrossRef]
- Hakobyan, L.; Gabrielyan, L.; Trchounian, A. Biohydrogen by Rhodobacter Sphaeroides during Photo-Fermentation: Mixed vs. Sole Carbon Sources Enhance Bacterial Growth and H2 Production. Int. J. Hydrogen Energy 2019, 44, 674–679. [Google Scholar] [CrossRef]
- Fedorov, A.S.; Tsygankov, A.A.; Rao, K.K.; Hall, D.O. Hydrogen Photoproduction by Rhodobacter Sphaeroides Immobilised on Polyurethane Foam. Biotechnol. Lett. 1998, 20, 1007–1009. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Role of Chemicals Addition in Affecting Biohydrogen Production through Photofermentation. Energy Convers. Manag. 2018, 165, 509–527. [Google Scholar] [CrossRef]
- Cheng, J.; Su, H.; Zhou, J.; Song, W.; Cen, K. Microwave-Assisted Alkali Pretreatment of Rice Straw to Promote Enzymatic Hydrolysis and Hydrogen Production in Dark- and Photo-Fermentation. Int. J. Hydrogen Energy 2011, 36, 2093–2101. [Google Scholar] [CrossRef]
- García-Sánchez, R.; Ramos-Ibarra, R.; Guatemala-Morales, G.; Arriola-Guevara, E.; Toriz-González, G.; Corona-González, R.I. Photofermentation of Tequila Vinasses by Rhodopseudomonas Pseudopalustris to Produce Hydrogen. Int. J. Hydrogen Energy 2018, 43, 15857–15869. [Google Scholar] [CrossRef]
- Yue, T.; Sun, Y.; Zhang, Q.; Jiang, D.; Zhang, Z.; Zhang, H.; Li, Y.; Zhang, Y.; Zhang, T. Enhancement of Biohydrogen Production by Photo-Fermentation of Corn Stover via Visible Light Catalyzed Titanium Dioxide/Activated Carbon Fiber. Bioresour. Technol. 2024, 399, 130459. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wang, G.; Zhang, Q.; Yang, X.; Yu, J.; Liu, T.; Petracchini, F.; Zhang, Z.; Sun, Y.; Jiang, D.; et al. Comparison of Biorefinery Characteristics: Photo-Fermentation Biohydrogen, Dark Fermentation Biohydrogen, Biomethane, and Bioethanol Production. Appl. Energy 2023, 347, 121463. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J.M. Improving Photofermentative Biohydrogen Production by Using Intermittent Ultrasonication and Combined Industrial Effluents from Palm Oil, Pulp and Paper Mills. Energy Convers. Manag. 2017, 132, 110–118. [Google Scholar] [CrossRef]
- Rezania, S.; Din, M.F.M.; Taib, S.M.; Sohaili, J.; Chelliapan, S.; Kamyab, H.; Saha, B.B. Review on Fermentative Biohydrogen Production from Water Hyacinth, Wheat Straw and Rice Straw with Focus on Recent Perspectives. Int. J. Hydrogen Energy 2017, 42, 20955–20969. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, D.; Zhang, H.; Jing, Y.; Tahir, N.; Zhang, Y.; Zhang, Q. Comparative Study on Bio-Hydrogen Production from Corn Stover: Photo-Fermentation, Dark-Fermentation and Dark-Photo Co-Fermentation. Int. J. Hydrogen Energy 2020, 45, 3807–3814. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.-J.; Lee, D.-W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, P.C.; Abo-Hashesh, M.; Ghosh, D. Strategies for Improving Biological Hydrogen Production. Bioresour. Technol. 2012, 110, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Wu, J. Enhancement of Methane Production in Anaerobic Digestion Process: A Review. Appl. Energy 2019, 240, 120–137. [Google Scholar] [CrossRef]
- Ángel Siles López, J.; Li, Q.; Thompson, I.P. Biorefinery of Waste Orange Peel. Crit. Rev. Biotechnol. 2010, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Lepp, P.W.; Brinig, M.M.; Ouverney, C.C.; Palm, K.; Armitage, G.C.; Relman, D.A. Methanogenic Archaea and Human Periodontal Disease. Proc. Natl. Acad. Sci. USA 2004, 101, 6176–6181. [Google Scholar] [CrossRef] [PubMed]
- Sogodogo, E.; Drancourt, M.; Grine, G. Methanogens as Emerging Pathogens in Anaerobic Abscesses. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, M.; Gunsalus, R.P.; Rao, S.S.; Zhang, H. Methanogens in Human Health and Disease. Am. J. Gastroenterol. Suppl. 2012, 1, 28–33. [Google Scholar] [CrossRef]
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen Sulfide Induces Direct Radical-Associated DNA Damage. Mol. Cancer Res. 2007, 5, 455–459. [Google Scholar] [CrossRef]
- Modern Water How Sulfate-Reducing Bacteria Impact Corrosion. 2023. Available online: https://www.modernwater.com/2023/01/25/how-sulfate-reducing-bacteria-impact-corrosion/ (accessed on 9 October 2024).
- Druzhinin, V.G.; Matskova, L.V.; Fucic, A. Induction and Modulation of Genotoxicity by the Bacteriome in Mammals. Mutat. Res./Rev. Mutat. Res. 2018, 776, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, J.; Sawmiller, D.; Shytle, R.D. Propionate and Alzheimer’s Disease. Front. Aging Neurosci. 2021, 12, 580001. [Google Scholar] [CrossRef]
- Sweeney, C. Could a Popular Food Ingredient Raise the Risk for Diabetes and Obesity? The Harvard Gazette, Health, The Dietary Factor, 24 April 2019. Available online: https://news.harvard.edu/gazette/story/2019/04/could-a-popular-food-ingredient-raise-the-risk-for-diabetes-and-obesity/ (accessed on 9 October 2024).
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.; Clark, J.H. (Eds.) Handbook of Biofuels Production: Processes and Technologies; Woodhead Publishing series in energy 2044-9364; Woodhead Pub: Oxford Philadelphia, PA, USA, 2011; Volume 15, ISBN 978-0-85709-049-2. Available online: https://www.shakes.cz/ebook/978-0-85709-049-2 (accessed on 9 October 2024).
- Demirel, B.; Yenigün, O. Two-phase Anaerobic Digestion Processes: A Review. J Chem. Tech. Amp. Biotech. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A Review of Anaerobic Digestion Systems for Biodegradable Waste: Configurations, Operating Parameters, and Current Trends. Environ. Eng. Res. 2019, 25, 1–17. [Google Scholar] [CrossRef]
- Kumar, J.C.R.; Majid, M.A. Renewable Energy for Sustainable Development in India: Current Status, Future Prospects, Challenges, Employment, and Investment Opportunities. Energy Sustain. Soc. 2020, 10, 2. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Rajendran, K.; Taherzadeh, M.J. A Comparative Study between Single- and Two-Stage Anaerobic Digestion Processes: Effects of Organic Loading Rate and Hydraulic Retention Time. Int. Biodeterior. Biodegrad. 2014, 95, 181–188. [Google Scholar] [CrossRef]
- Pinkard, B.R.; Gorman, D.J.; Tiwari, K.; Rasmussen, E.G.; Kramlich, J.C.; Reinhall, P.G.; Novosselov, I.V. Supercritical Water Gasification: Practical Design Strategies and Operational Challenges for Lab-Scale, Continuous Flow Reactors. Heliyon 2019, 5, e01269. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Martínez-Merino, V.; Gil, M.J.; Cornejo, A. Biomass Sources for Hydrogen Production. In Renewable Hydrogen Technologies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–110. ISBN 978-0-444-56352-1. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Kim, K.-Y.; Shin, H.-S. Experience of a Pilot-Scale Hydrogen-Producing Anaerobic Sequencing Batch Reactor (ASBR) Treating Food Waste. Int. J. Hydrogen Energy 2010, 35, 1590–1594. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Sivagurunathan, P.; Kim, S.-H.; Nemestóthy, N.; Bélafi-Bakó, K.; Lin, C.-Y. Enhanced Biohydrogen Production from Beverage Industrial Wastewater Using External Nitrogen Sources and Bioaugmentation with Facultative Anaerobic Strains. J. Biosci. Bioeng. 2015, 120, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bu, J.; Tiong, Y.W.; Xu, S.; Zhang, J.; He, Y.; Zhu, M.; Tong, Y.W. Enhanced Thermophilic Dark Fermentation of Hydrogen Production from Food Waste by Fe-Modified Biochar. Environ. Res. 2024, 244, 117946. [Google Scholar] [CrossRef]
- Zhao, B.; Yuan, A.; Cao, S.; Dong, Z.; Sha, H.; Song, Z. Advancing Two-Stage Hydrogen Production from Corn Stover via Dark Fermentation: Contributions of Thermally Modified Maifanite to Microbial Proliferation and pH Self-Regulation. Bioresour. Technol. 2024, 403, 130853. [Google Scholar] [CrossRef] [PubMed]
- Chantawan, N.; Moungprayoon, A.; Lunprom, S.; Reungsang, A.; Salakkam, A. High-Solid Dark Fermentation of Cassava Pulp and Cassava Processing Wastewater for Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 40672–40682. [Google Scholar] [CrossRef]
- Kazemi, R.; Mirmohamadsadeghi, S.; Amiri, H. Efficient Bio-Hydrogen Production by Dark Co-Fermentation of Food-Rich Municipal Solid Waste and Urban Pruning Wastes of Pine, Cypress, and Berry. Process Saf. Environ. Prot. 2024, 187, 398–407. [Google Scholar] [CrossRef]
- Ban, Q.; Wang, J.; Guo, P.; Yue, J.; Zhang, L.; Li, J. Improved Biohydrogen Production by Co-Fermentation of Corn Straw and Excess Sludge: Insights into Biochemical Process, Microbial Community and Metabolic Genes. Environ. Res. 2024, 256, 119171. [Google Scholar] [CrossRef] [PubMed]
- Hussien, M.; Jadhav, D.A.; Le, T.T.Q.; Jang, J.H.; Jang, J.K.; Chae, K.J. Tuning Dark Fermentation Operational Conditions for Improved Biohydrogen Yield during Co-Digestion of Swine Manure and Food Waste. Process Saf. Environ. Prot. 2024, 187, 1496–1507. [Google Scholar] [CrossRef]
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Bilska, A.; Wochna, K.; Habiera, M.; Serwańska-Leja, K. Health Hazard Associated with the Presence of Clostridium Bacteria in Food Products. Foods 2024, 13, 2578. [Google Scholar] [CrossRef]
- Government of Canada. Public Health Agency of Canada Pathogen Safety Data Sheets: Infectious Substances—Clostridium spp. 2014. Available online: https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/clostridium.html (accessed on 9 October 2024).
- Ghoddusi, H.B.; Sherburn, R.E.; Aboaba, O.O. Growth Limiting pH, Water Activity, and Temperature for Neurotoxigenic Strains of Clostridium butyricum. ISRN Microbiol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed]
- TRBA. TRBA 466 Classification of Prokaryotes (Bacteria and Archaea) into Risk Groups. 2010. Available online: https://www.certifico.com/sicurezza-lavoro/documenti-sicurezza/64-documenti-enti/17642-technical-rules-for-biological-agents-trba?tmpl=component (accessed on 9 October 2024).
- Li, M.; Ning, P.; Sun, Y.; Luo, J.; Yang, J. Characteristics and Application of Rhodopseudomonas Palustris as a Microbial Cell Factory. Front. Bioeng. Biotechnol. 2022, 10, 897003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kushwaha, K.K.; Singh, S.; Shivay, Y.S.; Meena, M.C.; Nain, L. Effect of Paddy Straw Burning on Soil Microbial Dynamics in Sandy Loam Soil of Indo-Gangetic Plains. Environ. Technol. Innov. 2019, 16, 100469. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, S.; Kumar, U.; Sihag, P.; Balyan, P.; Singh, K.P.; Dhankher, O.P. Strategies for Economic Utilization of Rice Straw Residues into Value-Added by-Products and Prevention of Environmental Pollution. Sci. Total Environ. 2024, 906, 167714. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, A.; Montoya, A.; Sulbarán-Rangel, B.; Zurita, F. Possible Pollution of Surface Water Bodies with Tequila Vinasses. Water 2023, 15, 3773. [Google Scholar] [CrossRef]
- Zurita, F.; Tejeda, A.; Montoya, A.; Carrillo, I.; Sulbarán-Rangel, B.; Carreón-Álvarez, A. Generation of Tequila Vinasses, Characterization, Current Disposal Practices and Study Cases of Disposal Methods. Water 2022, 14, 1395. [Google Scholar] [CrossRef]
- Mendiola-Rodriguez, T.A.; Ricardez-Sandoval, L.A. Robust Control for Anaerobic Digestion Systems of Tequila Vinasses under Uncertainty: A Deep Deterministic Policy Gradient Algorithm. Digit. Chem. Eng. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Orozco-Nunnelly, D.A.; Yebra-Montes, C.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. Using Yeast Cultures to Valorize Tequila Vinasse Waste: An Example of a Circular Bioeconomy Approach in the Agro-Industrial Sector. Biomass Bioenergy 2022, 161, 106471. [Google Scholar] [CrossRef]
- Cui, Y.; Bai, L.; Li, C.; Du, R. Source and Migration Patterns of Heavy Metals and Human Health Risk Assessment of Heavy Metals in Soil-Corn Straw-Flue Gas System. Environ. Geochem. Health 2023, 45, 8043–8061. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhang, H.; Wu, X.; Liu, Y.; Wu, Y.; Wang, J.; Zhang, C.; Sun, B.; Wu, F. Straw Return Decreases Polycyclic Aromatic Hydrocarbon (PAH) Accumulation in Winter Wheat and Human Health Risk by Enhancing PAH Dissipation in Rhizosphere Soil. Pedosphere 2024, 34, 699–708. [Google Scholar] [CrossRef]
- Ventura Foods Palm Oil. Finished Product Safety Data Sheet (SDS). 2020. Available online: https://www.venturafoods.com/wp-content/uploads/2022/01/VF-SDS-Palm-Oil-1.pdf (accessed on 9 October 2024).
- Lake Champlain Chocolates Why Is Palm Oil Bad? Lake Champlain Chocolates. 2024. Available online: https://www.lakechamplainchocolates.com/palm-oil/ (accessed on 9 October 2024).
- Andreoni, M. Why Palm Oil Is Still a Big Problem? New York Times, Climate, 26 March 2024. Available online: https://www.nytimes.com/2024/03/26/climate/why-palm-oil-is-still-a-big-problem.html (accessed on 9 October 2024).
- Mandeep; Gupta, G.K.; Liu, H.; Shukla, P. Pulp and Paper Industry–Based Pollutants, Their Health Hazards and Environmental Risks. Curr. Opin. Environ. Sci. Health 2019, 12, 48–56. [Google Scholar] [CrossRef]
- Stronach, S.M.; Rudd, T.; Lester, J.N. Toxic Substances in Anaerobic Digestion. In Anaerobic Digestion Processes in Industrial Wastewater Treatment; Biotechnology Monographs; Springer: Berlin/Heidelberg, Germany, 1986; Volume 2, pp. 71–92. ISBN 978-3-642-71217-3. [Google Scholar] [CrossRef]
- Rudziak, P.; Batung, E.; Luginaah, I. The Effects of Gases from Food Waste on Human Health: A Systematic Review. PLoS ONE 2024, 19, e0300801. [Google Scholar] [CrossRef] [PubMed]
- University College Cork Risk Assessment for Genetically Modified Micro-Organisms [GMMs] Class 1 & Class 2. 2001. Available online: https://www.ucc.ie/en/media/research/biosafety/GMM_C1.pdf (accessed on 9 October 2024).
- Prevent & Protect Enterobacter Cloacae. 2024. Available online: https://prevent-and-protect.com/pathogen/enterobacter-cloacae-en/ (accessed on 9 October 2024).
- Ramirez, D.; Giron, M. Enterobacter Infections. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rinne, C. Factors Affecting Wastewater Characteristics in Beverage Industry: Reduction of Environmental Load and Wastewater Treatment. Master’s Thesis, Lahti University of Technology, Lahti, Finland, 2024. [Google Scholar]
- Cornell, T. Supercharge Your Gut Health. 2024. Available online: https://www.sutterhealth.org/health/how-gut-health-affects-weight (accessed on 9 October 2024).
- An, J.; Kwon, H.; Kim, Y.J. The Firmicutes/Bacteroidetes Ratio as a Risk Factor of Breast Cancer. JCM 2023, 12, 2216. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. How Does Food Waste Affect the Environment? Earth, 17 October 2022. Available online: https://earth.org/how-does-food-waste-affect-the-environment/ (accessed on 9 October 2024).
- Move for Hunger The Environmental Impact of Food Waste. Move for Hunger. 2024. Available online: https://www.send2press.com/wire/profile/move-for-hunger/ (accessed on 9 October 2024).
- Godlewska, P.; Ok, Y.S.; Oleszczuk, P. The Dark Side of Black Gold: Ecotoxicological Aspects of Biochar and Biochar-Amended Soils. J. Hazard. Mater. 2021, 403, 123833. [Google Scholar] [CrossRef] [PubMed]
- Newman, M. Biochar. Safety Data Sheet. 2019. Available online: https://ariescleantech.com/wp-content/uploads/2020/11/Aries-GREENE284A2-Biochar-Safety-Data-Sheet.pdf (accessed on 9 October 2024).
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Life Cycle Assessment of Raw and Fe-Modified Biochars: Contributing to Circular Economy. Materials 2023, 16, 6059. [Google Scholar] [CrossRef]
- Environmental Business Specialists MicroStart. Safety Data Sheet. 2021. Available online: https://www.ebsbiowizard.com/wp-content/uploads/2021/11/EBS-SDS-MicroStart%E2%84%A2.pdf (accessed on 9 October 2024).
- Tang, R.; Luo, H.; Prommer, H.; Yue, Z.; Wang, W.; Su, K.; Hu, Z.-H. Response of Anaerobic Granular Sludge to Long-Term Loading of Roxarsone: From Macro- to Micro-Scale Perspective. Water Res. 2021, 204, 117599. [Google Scholar] [CrossRef] [PubMed]
- FAO and IFAD Strategic Environmental Assessment. An Assessment of the Impact of Cassava Production and Processing on the Environment and Biodiversity. In Proceedings of the Validation Forum on the Global Cassava Development Strategy; Food and Agriculture Organization of the United Nations International Fund for Agricultural Development, Rome, Italy, 26 April 2000; Volume 5. [Google Scholar]
- Pereira, I.G.; Vagula, J.M.; Marchi, D.F.; Barão, C.E.; Almeida, G.R.S.; Visentainer, J.V.; Maruyama, S.A.; Santos Júnior, O.O. Easy Method for Removal of Cyanogens from Cassava Leaves with Retention of Vitamins and Omega-3 Fatty Acids. J. Braz. Chem. Soc. 2016, 27, 1290–1296. [Google Scholar] [CrossRef]
- Alves, C.A.; Vicente, E.D.; Evtyugina, M.; Vicente, A.; Pio, C.; Amado, M.F.; Mahía, P.L. Gaseous and Speciated Particulate Emissions from the Open Burning of Wastes from Tree Pruning. Atmos. Res. 2019, 226, 110–121. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Chandra, R. Environmental Pollutants of Paper Industry Wastewater and Their Toxic Effects on Human Health and Ecosystem. Bioresour. Technol. Rep. 2022, 20, 101250. [Google Scholar] [CrossRef]
- Stuart, P. The Advantages and Disadvantages of Anaerobic Digestion as a Renewable Energy Source. 2022. Available online: https://www.maths.tcd.ie/~jlennon/miniprojects/biomass.doc (accessed on 9 October 2024).
- Duan, B.; Feng, Q. Comparison of the Potential Ecological and Human Health Risks of Heavy Metals from Sewage Sludge and Livestock Manure for Agricultural Use. Toxics 2021, 9, 145. [Google Scholar] [CrossRef]
- Lubentsov, V.; Ozhogova, E.; Lubentsova, E.; Shakhray, E. Selection and Justification of Priority Tasks of Biogas Plant Management Taking into Account Technological Risks. E3S Web Conf. 2021, 285, 04006. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, R.; Lal, B.; Haque, S. Evaluation of Bioprocess Parameters for Pilot Scale Fermentative Biohydrogen Production Using Organic Waste: Environmental Remediation, Techno-Economic Challenges & Future Solutions. Int. J. Hydrogen Energy 2024, S0360319924018986. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Srikanth, S. Biohydrogen Production from Industrial Effluents. In Biofuels: Alternative Feedstocks and Conversion Processes; Pandey, A., Ed.; Academic Press: Oxford, UK; Burlington, MA, USA, 2011; Chapter 22; pp. 499–524. ISBN 978-0-12-385099-7. Available online: https://shop.elsevier.com/books/biofuels/unknown/978-0-12-385099-7 (accessed on 9 October 2024).
- Wang, J.; Yin, Y. Influencing Factors for Biohydrogen Production. In Biohydrogen Production from Organic Wastes; Green Energy and Technology; Springer: Singapore, 2017; pp. 197–268. ISBN 978-981-10-4675-9. [Google Scholar] [CrossRef]
- Ananthi, V.; Bora, A.; Ramesh, U.; Yuvakkumar, R.; Raja, K.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on the Technologies for Sustainable Biohydrogen Production. Process Saf. Environ. Prot. 2024, 186, 944–956. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Zhang, Q.; Zhu, S.; Yang, S.; Zhang, Z. Effect of Substrate Concentration on Photo-Fermentation Bio-Hydrogen Production Process from Starch-Rich Agricultural Leftovers under Oscillation. Sustainability 2020, 12, 2700. [Google Scholar] [CrossRef]
- Aathika, A.R.S.; Kubendran, D.; Yuvarani, M.; Thiruselvi, D.; Amudha, T.; Karthik, P.; Sivanesan, S. Enhanced Biohydrogen Production from Leather Fleshing Waste Co-Digested with Tannery Treatment Plant Sludge Using Anaerobic Hydrogenic Batch Reactor. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 586–593. [Google Scholar] [CrossRef]
- Sharma, R.K.; Nazari, M.A.; Haydary, J.; Singh, T.P.; Mandal, S. A Review on Advanced Processes of Biohydrogen Generation from Lignocellulosic Biomass with Special Emphasis on Thermochemical Conversion. Energies 2023, 16, 6349. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Bioenergy from Microorganisms: An Overview. In Microbial Bioenergy: Hydrogen Production; Zannoni, D., De Philippis, R., Eds.; Advances in photosynthesis and respiration; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2014; Volume 38, pp. 3–21. ISBN 978-94-017-8554-9. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Barupal, D.K.; Fan, X.; Gustafson, R.; Guo, R.; Fiehn, O. Metabolomics of Photobiological Hydrogen Production Induced by CCCP in Chlamydomonas Reinhardtii. Int. J. Hydrogen Energy 2014, 39, 150–158. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Ren, Y.; Wang, J.; Chen, H.; Wang, Q. Biological Hydrogen with Industrial Potential: Improvement and Prospection in Biohydrogen Production. J. Clean. Prod. 2023, 387, 135777. [Google Scholar] [CrossRef]
- McNeely, K.; Xu, Y.; Bennette, N.; Bryant, D.A.; Dismukes, G.C. Redirecting Reductant Flux into Hydrogen Production via Metabolic Engineering of Fermentative Carbon Metabolism in a Cyanobacterium. Appl. Environ. Microbiol. 2010, 76, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of Biohydrogen Production by Photobiological, Fermentation and Electrochemical Processes: A Review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Singh, A.; Sevda, S.; Abu Reesh, I.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen Production from Lignocellulosic Biomass: Technology and Sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Moon, C.; Jang, S.; Yun, Y.-M.; Lee, M.-K.; Kim, D.-H.; Kang, W.-S.; Kwak, S.-S.; Kim, M.-S. Effect of the Accuracy of pH Control on Hydrogen Fermentation. Bioresour. Technol. 2015, 179, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Obiora, N.K.; Ujah, C.O.; Asadu, C.O.; Kolawole, F.O.; Ekwueme, B.N. Production of Hydrogen Energy from Biomass: Prospects and Challenges. Green Technol. Sustain. 2024, 2, 100100. [Google Scholar] [CrossRef]
- Willey, J.M.; Sherwood, L.; Woolverton, C.J.; Prescott, L.M.; Willey, J.M. Prescott’s Microbiology, 8th ed.; McGraw-Hill: New York, NY, USA, 2011; ISBN 978-0-07-337526-7. [Google Scholar] [CrossRef]
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Enhancing Hydrogen Production from Chlorella Sp. Biomass by Pre-Hydrolysis with Simultaneous Saccharification and Fermentation (PSSF). Energies 2019, 12, 908. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative Hydrogen Production Using Various Biomass-Based Materials as Feedstock. Renew. Sustain. Energy Rev. 2018, 92, 284–306. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.-J.; Lee, K.-S.; Lin, C.-Y.; Chen, C.-Y.; Chang, J.-S. Biomass Based Hydrogen Production by Dark Fermentation—Recent Trends and Opportunities for Greener Processes. Curr. Opin. Biotechnol. 2018, 50, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, L. Stability and Activity of a Co-Precipitated Mg Promoted Ni/Al2O3 Catalyst for Supercritical Water Gasification of Biomass. Int. J. Hydrogen Energy 2019, 44, 15842–15852. [Google Scholar] [CrossRef]
- Asadu, C.O.; Aneke, N.G.; Egbuna, S.O.; Agulanna, A.C. Comparative Studies on the Impact of Bio-Fertilizer Produced from Agro-Wastes Using Thermo-Tolerant Actinomycetes on the Growth Performance of Maize (Zea-Mays) and Okro (Abelmoschus Esculentus). Environ. Technol. Innov. 2018, 12, 55–71. [Google Scholar] [CrossRef]
- Asadu, C.O.; Ike, I.S.; Onu, C.E.; Egbuna, S.O.; Onoh, M.; Mbah, G.O.; Eze, C.N. Investigation of the Influence of Biofertilizer Synthesized Using Microbial Inoculums on the Growth Performance of Two Agricultural Crops. Biotechnol. Rep. 2020, 27, e00493. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Han, F.; Chen, Y.; Yang, H.; Chen, H. Greenhouse Gas Emissions of a Biomass-Based Pyrolysis Plant in China. Renew. Sustain. Energy Rev. 2016, 53, 1580–1590. [Google Scholar] [CrossRef]
- Forest Research Carbon Emissions of Different Fuels. 2024. Available online: https://www.forestresearch.gov.uk/tools-and-resources/fthr/biomass-energy-resources/reference-biomass/facts-figures/carbon-emissions-of-different-fuels/ (accessed on 9 October 2024).
- El-Shafie, M.; Kambara, S.; Hayakawa, Y. Hydrogen Production Technologies Overview. J. Power Energy Eng. 2019, 07, 107–154. [Google Scholar] [CrossRef]
- Ramdon Safety Protocols in CO2 Handling: Ensuring Workplace Safety and Compliance. 2024. Available online: https://ramdon.com/safety-protocols-in-co2-handling/ (accessed on 9 October 2024).
- Zhao, X.-Y.; Ren, J.; Cao, J.-P.; Wei, F.; Zhu, C.; Fan, X.; Zhao, Y.-P.; Wei, X.-Y. Catalytic Reforming of Volatiles from Biomass Pyrolysis for Hydrogen-Rich Gas Production over Limonite Ore. Energy Fuels 2017, 31, 4054–4060. [Google Scholar] [CrossRef]
- Dong, L.; Wu, C.; Ling, H.; Shi, J.; Williams, P.T.; Huang, J. Promoting Hydrogen Production and Minimizing Catalyst Deactivation from the Pyrolysis-Catalytic Steam Reforming of Biomass on Nanosized NiZnAlOx Catalysts. Fuel 2017, 188, 610–620. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhu, B.; Wang, C.; Jia, X.; Guan, G.; Zeng, X.; Hu, E.; Han, Z.; Xu, G. Pyrolysis of Biomass to Produce H-Rich Gas Facilitated by Simultaneously Occurring Magnesite Decomposition. Carbon Resour. Convers. 2024, 100265. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Chen, W.-H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T.; Saidina Amin, N.A. Current Status of Biohydrogen Production from Lignocellulosic Biomass, Technical Challenges and Commercial Potential through Pyrolysis Process. Energy 2021, 226, 120433. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. [Google Scholar] [CrossRef]
- Airgas USA, LLC. Nitrogen CAS Number 7727-37-9. 2021. Available online: https://www.airgas.com/msds/001040.pdf (accessed on 9 October 2024).
- Naqvi, S.R.; Prabhakara, H.M.; Bramer, E.A.; Dierkes, W.; Akkerman, R.; Brem, G. A Critical Review on Recycling of End-of-Life Carbon Fibre/Glass Fibre Reinforced Composites Waste Using Pyrolysis towards a Circular Economy. Resour. Conserv. Recycl. 2018, 136, 118–129. [Google Scholar] [CrossRef]
- Arregi, A.; Lopez, G.; Amutio, M.; Barbarias, I.; Bilbao, J.; Olazar, M. Hydrogen Production from Biomass by Continuous Fast Pyrolysis and In-Line Steam Reforming. RSC Adv. 2016, 6, 25975–25985. [Google Scholar] [CrossRef]
- Byrd, A.J.; Kumar, S.; Kong, L.; Ramsurn, H.; Gupta, R.B. Hydrogen Production from Catalytic Gasification of Switchgrass Biocrude in Supercritical Water. Int. J. Hydrogen Energy 2011, 36, 3426–3433. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, B.-G.; Kim, B.-K.; Han, J.-I.; Yang, J.-W. Continuous Microalgae Recovery Using Electrolysis with Polarity Exchange. Bioresour. Technol. 2012, 111, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Zhou, Z.; Yuan, H.; Zheng, T.; Chen, Y. Effect of Carbon Structure on Hydrogen Release Derived from Different Biomass Pyrolysis. Fuel 2020, 271, 117638. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Adam, J.; Blazso, M.; Meszaros, E.; Stocker, M.; Nilsen, M.; Bouzga, A.; Hustad, J.; Gronli, M.; Oye, G. Pyrolysis of Biomass in the Presence of Al-MCM-41 Type Catalysts. Fuel 2005, S0016236105000578. [Google Scholar] [CrossRef]
- Prasertcharoensuk, P.; Bull, S.J.; Phan, A.N. Gasification of Waste Biomass for Hydrogen Production: Effects of Pyrolysis Parameters. Renew. Energy 2019, 143, 112–120. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Yue, Y.; Lin, Q.; Irfan, M.; Chen, Q.; Zhao, X. Characteristics and Potential Values of Bio-Oil, Syngas and Biochar Derived from Salsola Collina Pall. in a Fixed Bed Slow Pyrolysis System. Bioresour. Technol. 2016, 220, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Shu, J.; Xia, H.; Wang, S.; Zhang, L.; Peng, J.; Li, C.; Jiang, X.; Zhang, Q. Pyrolysis of Crofton Weed for the Production of Aldehyde Rich Bio-Oil and Combustible Matter Rich Bio-Gas. Appl. Therm. Eng. 2019, 148, 1164–1170. [Google Scholar] [CrossRef]
- Rusin, A.; Stolecka-Antczak, K. Assessment of Syngas Storage Tank Hazards Taking Account of the Domino Effect. Energies 2024, 17, 1857. [Google Scholar] [CrossRef]
- Work Safe BC Reducing Risk of Syngas Fires Explosions When Drying Wood Fibre. 2024. Available online: https://www.worksafebc.com/resources/health-safety/hazard-alerts/reducing-risk-of-syngas-fires-explosions-when-drying-wood-fibre%3Flang%3Den%26direct (accessed on 9 October 2024).
- Stolecka, K.; Rusin, A. Analysis of Hazards Related to Syngas Production and Transport. Renew. Energy 2020, 146, 2535–2555. [Google Scholar] [CrossRef]
- Nandan, A.; Siddiqui, N.A.; Mondal, P.; Chaudhary, K.; Pandey, R. Hazards Associated to Synthesis Gas and Its Mitigation Measures. Res. J. Eng. Tech. 2014, 5, 144–146. [Google Scholar]
- Safdari, M.-S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating Rate and Temperature Effects on Pyrolysis Products from Live Wildland Fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Bakhtyari, A.; Makarem, M.A.; Rahimpour, M.R. Hydrogen Production Through Pyrolysis. In Fuel Cells and Hydrogen Production; Lipman, T.E., Weber, A.Z., Eds.; Springer: New York, NY, USA, 2019; pp. 947–973. ISBN 978-1-4939-7788-8. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Chen, M.; Xin, W.; Yang, Z.; Kong, L. Hydrogen Production via Catalytic Pyrolysis of Biomass in a Two-Stage Fixed Bed Reactor System. Int. J. Hydrogen Energy 2014, 39, 13128–13135. [Google Scholar] [CrossRef]
- Navarro, R.M.; Sanchez-Sanchez, M.C.; Alvarez-Galvan, M.C.; Fierro, J.L.G.; Al-Zaharani, S.M. Hydrogen Production from Renewables. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-1-119-95143-8. [Google Scholar] [CrossRef]
- Czernik, S.; Evans, R.; French, R. Hydrogen from Biomass-Production by Steam Reforming of Biomass Pyrolysis Oil. Catal. Today 2007, 129, 265–268. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of Thermochemical Routes for Hydrogen Production from Biomass: A Review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Lan, P.; Xu, Q.; Zhou, M.; Lan, L.; Zhang, S.; Yan, Y. Catalytic Steam Reforming of Fast Pyrolysis Bio-Oil in Fixed Bed and Fluidized Bed Reactors. Chem. Eng. Amp. Technol. 2010, 33, 2021–2028. [Google Scholar] [CrossRef]
- Kintek Solution Ltd. The Advantages and Disadvantages of Different Biomass Feedstocks for Pyrolysis. 2024. Available online: https://kindle-tech.com/articles/the-advantages-and-disadvantages-of-different-biomass-feedstocks-for-pyrolysis (accessed on 9 October 2024).
- Elsdon, R.; Pal, D. Waste-to-Energy Plant Process Safety Challenges. IChemE Symp. Ser. 2011, 156, 356–360. [Google Scholar]
- Ndirangu, S.M.; Liu, Y.; Xu, K.; Song, S. Risk Evaluation of Pyrolyzed Biochar from Multiple Wastes. J. Chem. 2019, 2019, 1–28. [Google Scholar] [CrossRef]
- Pang, Y.X.; Foo, D.C.Y.; Yan, Y.; Sharmin, N.; Lester, E.; Wu, T.; Pang, C.H. Analysis of Environmental Impacts and Energy Derivation Potential of Biomass Pyrolysis via Piper Diagram. J. Anal. Appl. Pyrolysis 2021, 154, 104995. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Zanon Costa, C.; Falabella Sousa-Aguiar, E.; Peixoto Gimenes Couto, M.A.; Souza De Carvalho Filho, J.F. Hydrothermal Treatment of Vegetable Oils and Fats Aiming at Yielding Hydrocarbons: A Review. Catalysts 2020, 10, 843. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable Hydrogen Production through Biomass Gasification: A Review and Future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Henriksen, U.B.; Ciolkosz, D. Startup Process, Safety and Risk Assessment of Biomass Gasification for off-Grid Rural Electrification. Sci. Rep. 2023, 13, 21395. [Google Scholar] [CrossRef]
- Herdin, G.; Robitschko, R.; Klausner, J. Gaseous Emissions Experience of GE Jenbacher with Wood Gas. In Proceedings of the International Workshop of Health, Safety and Environment of Biomass Gasification, Innsbruck, Austria, 28 September 2005; pp. 30–47. [Google Scholar]
- San Miguel, G.; Domínguez, M.P.; Hernández, M.; Sanz-Pérez, F. Characterization and Potential Applications of Solid Particles Produced at a Biomass Gasification Plant. Biomass Bioenergy 2012, 47, 134–144. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human Health Effects of Air Pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- CAC. GAS & Instrumentation Carbon Monoxide (CO)—Gas Hazards & Workplace Safety. 2025. Available online: https://cacgas.com.au/blog/carbon-monoxide-co-toxic-gas-workplace-safety/ (accessed on 9 October 2024).
- CDC National Institute for Occupational Safety and Health (NIOSH). Workplace Carbon Monoxide Hazards. 2024. Available online: https://www.cdc.gov/niosh/carbon-monoxide/about/index.html (accessed on 9 October 2024).
- EPA Sulfur Dioxide Basics. 2024. Available online: https://www.epa.gov/so2-pollution/sulfur-dioxide-basics (accessed on 9 October 2024).
- NZ Ministry for the Environment Sulphur Dioxide. 2021. Available online: https://environment.govt.nz/facts-and-science/air/air-pollutants/sulphur-dioxide-and-effects-on-health/ (accessed on 9 October 2024).
- Mitra, D.; Basak, R.; Nilanjan, S.; Deepak, P. A Note on Prevention and Control of Sulfur Oxide Polution. Int. J. Eng. Sci. Technol. 2010, 2, 3926–3928. [Google Scholar]
- Agency for the Registry of Toxic Substances and Diseases ToxFAQs for Nitrogen Oxides. 2024. Available online: https://www.atsdr.cdc.gov/index.html (accessed on 9 October 2024).
- Alves, L.; Holz, L.I.V.; Fernandes, C.; Ribeirinha, P.; Mendes, D.; Fagg, D.P.; Mendes, A. A Comprehensive Review of NOx and N2O Mitigation from Industrial Streams. Renew. Sustain. Energy Rev. 2022, 155, 111916. [Google Scholar] [CrossRef]
- Lewtas, J. Air Pollution Combustion Emissions: Characterization of Causative Agents and Mechanisms Associated with Cancer, Reproductive, and Cardiovascular Effects. Mutat. Res./Rev. Mutat. Res. 2007, 636, 95–133. [Google Scholar] [CrossRef] [PubMed]
- CARB. Inhalable Particulate Matter and Health (PM2.5 and PM10). 2025. Available online: https://ww2.arb.ca.gov/resources/inhalable-particulate-matter-and-health (accessed on 9 October 2024).
- N.Y. Department of Health. Particle Pollution and Health. 2024. Available online: https://www.health.ny.gov/environmental/indoors/air/pmq_a.htm (accessed on 9 October 2024).
- Consensus Task Force, E.; Shi, X.; Duan, G. China CDC Key Laboratory of Environment and Population Health, National Institute of Environmental Health, Chinese Center for Disease Control and Prevention, Beijing, China; School of Public Health, Zhengzhou University, Zhengzhou, Henan, China Recommendations of Controlling and Preventing Acute Health Risks of Fine Particulate Matter Pollution—China, 2021. China CDC Wkly. 2022, 4, 329–341. [Google Scholar] [CrossRef]
- Luo, X.; Wu, T.; Shi, K.; Song, M.; Rao, Y. Biomass Gasification: An Overview of Technological Barriers and Socio-Environmental Impact. In Gasification for Low-Grade Feedstock; Yun, Y., Ed.; InTech: London, UK, 2018; ISBN 978-1-78923-288-2. [Google Scholar]
- Cordella, M.; Torri, C.; Adamiano, A.; Fabbri, D.; Barontini, F.; Cozzani, V. Bio-Oils from Biomass Slow Pyrolysis: A Chemical and Toxicological Screening. J. Hazard. Mater. 2012, 231–232, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chewron Renevable Energy Group REG Bio-Residual Oil®. 2023. Available online: https://www.regi.com/products/other-products/bio-residual-oil (accessed on 9 October 2024).
- Shiva Kumar, S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Teoh, R.H.; Mahajan, A.S.; Moharir, S.R.; Abdul Manaf, N.; Shi, S.; Thangalazhy-Gopakumar, S. A Review on Hydrothermal Treatments for Solid, Liquid and Gaseous Fuel Production from Biomass. Energy Nexus 2024, 14, 100301. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and Characterization of Activated Carbon from Barley Straw by Physical Activation with Carbon Dioxide and Steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- El Bast, M.; Allam, N.; Abou Msallem, Y.; Awad, S.; Loubar, K. A Review on Continuous Biomass Hydrothermal Liquefaction Systems: Process Design and Operating Parameters Effects on Biocrude. J. Energy Inst. 2023, 108, 101260. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Nanda, S.; Castello, D.; Dalai, A.K.; Kozinski, J.A. Modeling and Process Optimization of Hydrothermal Gasification for Hydrogen Production: A Comprehensive Review. J. Supercrit. Fluids 2021, 173, 105199. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Hunter, H.N.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrothermal Catalytic Processing of Waste Cooking Oil for Hydrogen-Rich Syngas Production. Chem. Eng. Sci. 2019, 195, 935–945. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M. Chemistry and Materials Options of Sustainable Carbon Materials Made by Hydrothermal Carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S. Nanostructured Carbohydrate—Derived Carbonaceous Materials. Tanso 2013, 2013, 232–233. [Google Scholar] [CrossRef]
- Pandey, B.; Prajapati, Y.K.; Sheth, P.N. Recent Progress in Thermochemical Techniques to Produce Hydrogen Gas from Biomass: A State of the Art Review. Int. J. Hydrogen Energy 2019, 44, 25384–25415. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Manandhar, A.; Shah, A. Hydrothermal Carbonization: Upgrading Waste Biomass to Char. 2021. Available online: https://ohioline.osu.edu/factsheet/fabe-6622 (accessed on 9 October 2024).
- Jaruwat, D.; Udomsap, P.; Chollacoop, N.; Fuji, M.; Eiad-ua, A. Effects of Hydrothermal Temperature and Time of Hydrochar from Cattail Leaves. AIP Conf. Proc. 2018, 2010, 020016. [Google Scholar] [CrossRef]
- Surup, G.R.; Leahy, J.J.; Timko, M.T.; Trubetskaya, A. Hydrothermal Carbonization of Olive Wastes to Produce Renewable, Binder-Free Pellets for Use as Metallurgical Reducing Agents. Renew. Energy 2020, 155, 347–357. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Z.; Shen, B.; Liu, L. Insights into Biochar and Hydrochar Production and Applications: A Review. Energy 2019, 171, 581–598. [Google Scholar] [CrossRef]
- Ma, J.; Chen, M.; Yang, T.; Liu, Z.; Jiao, W.; Li, D.; Gai, C. Gasification Performance of the Hydrochar Derived from Co-Hydrothermal Carbonization of Sewage Sludge and Sawdust. Energy 2019, 173, 732–739. [Google Scholar] [CrossRef]
- Khoshbouy, R.; Takahashi, F.; Yoshikawa, K. Preparation of High Surface Area Sludge-Based Activated Hydrochar via Hydrothermal Carbonization and Application in the Removal of Basic Dye. Environ. Res. 2019, 175, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Shyam, S.; Reddy, B.R.; Govindaraju, K.; Vinu, R. Microwave-Assisted Pyrolysis and Analytical Fast Pyrolysis of Macroalgae: Product Analysis and Effect of Heating Mechanism. Sustain. Energy Fuels 2019, 3, 3009–3020. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A Review of the Hydrothermal Carbonization of Biomass Waste for Hydrochar Formation: Process Conditions, Fundamentals, and Physicochemical Properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Heidari, M.; Dutta, A.; Acharya, B.; Mahmud, S. A Review of the Current Knowledge and Challenges of Hydrothermal Carbonization for Biomass Conversion. J. Energy Inst. 2019, 92, 1779–1799. [Google Scholar] [CrossRef]
- Yang, L.; Lu, C.; Gao, Y.; Lin, Y.; Xu, J.; Xu, H.; Zhang, X.; Wang, M.; Zhao, Y.; Yu, C.; et al. Hydrogen-Rich Gas Production from the Gasification of Biomass and Hydrothermal Carbonization (HTC) Aqueous Phase. Biomass Convers. Bioref. 2023, 13, 1529–1538. [Google Scholar] [CrossRef]
- Ender, T.; Ekanthalu, V.S.; Jalalipour, H.; Sprafke, J.; Nelles, M. Process Waters from Hydrothermal Carbonization of Waste Biomasses like Sewage Sludge: Challenges, Legal Aspects, and Opportunities in EU and Germany. Water 2024, 16, 1003. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. From Biomass to Biocrude: Innovations in Hydrothermal Liquefaction and Upgrading. Energy Convers. Manag. 2024, 302, 118093. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Grigoras, I.F.; Hoffmann, J.; Toor, S.S.; Daraban, I.M.; Jensen, C.U.; Iversen, S.B.; Madsen, R.B.; Glasius, M.; Arturi, K.R.; et al. Continuous Hydrothermal Co-Liquefaction of Aspen Wood and Glycerol with Water Phase Recirculation. Appl. Energy 2016, 162, 1034–1041. [Google Scholar] [CrossRef]
- Masoumi, S.; Dalai, A.K. Techno-Economic and Life Cycle Analysis of Biofuel Production via Hydrothermal Liquefaction of Microalgae in a Methanol-Water System and Catalytic Hydrotreatment Using Hydrochar as a Catalyst Support. Biomass Bioenergy 2021, 151, 106168. [Google Scholar] [CrossRef]
- Alherbawi, M.; Parthasarathy, P.; Al-Ansari, T.; Mackey, H.R.; McKay, G. Potential of Drop-in Biofuel Production from Camel Manure by Hydrothermal Liquefaction and Biocrude Upgrading: A Qatar Case Study. Energy 2021, 232, 121027. [Google Scholar] [CrossRef]
- DeRose, K.; DeMill, C.; Davis, R.W.; Quinn, J.C. Integrated Techno Economic and Life Cycle Assessment of the Conversion of High Productivity, Low Lipid Algae to Renewable Fuels. Algal Res. 2019, 38, 101412. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumar, V.; Kumar, P.; Mohanty, K. Hydrothermal Liquefaction of Biomass for Bio-Crude Production: A Review on Feedstocks, Chemical Compositions, Operating Parameters, Reaction Kinetics, Techno-Economic Study, and Life Cycle Assessment. Fuel 2022, 316, 123377. [Google Scholar] [CrossRef]
- Geng, J.; Sun, H. Optimization and Analysis of a Hydrogen Liquefaction Process: Energy, Exergy, Economic, and Uncertainty Quantification Analysis. Energy 2023, 262, 125410. [Google Scholar] [CrossRef]
- Gholami, T.; Pirsaheb, M. Review on Effective Parameters in Electrochemical Hydrogen Storage. Int. J. Hydrogen Energy 2021, 46, 783–795. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsumura, Y. Gasification of Cellulose, Xylan, and Lignin Mixtures in Supercritical Water. Ind. Eng. Chem. Res. 2001, 40, 5469–5474. [Google Scholar] [CrossRef]
- Bircan, S.Y.; Matsumoto, K.; Kitagaw, K. Environmental Impacts of Hydrogen Production by Hydrothermal Gasification of a Real Biowaste. In Gasification for Practical Applications; Yun, Y., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0818-4. [Google Scholar] [CrossRef]
- Antal, M.J.; Allen, S.G.; Schulman, D.; Xu, X.; Divilio, R.J. Biomass Gasification in Supercritical Water. Ind. Eng. Chem. Res. 2000, 39, 4040–4053. [Google Scholar] [CrossRef]
- Deniz, I.; Vardar-Sukan, F.; Yüksel, M.; Saglam, M.; Ballice, L.; Yesil-Celiktas, O. Hydrogen Production from Marine Biomass by Hydrothermal Gasification. Energy Convers. Manag. 2015, 96, 124–130. [Google Scholar] [CrossRef]
- Aziz, M. Integrated Supercritical Water Gasification and a Combined Cycle for Microalgal Utilization. Energy Convers. Manag. 2015, 91, 140–148. [Google Scholar] [CrossRef]
- Kruse, A. Supercritical Water Gasification. Biofuels Bioprod. Biorefin. 2008, 2, 415–437. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical Biofuel Production in Hydrothermal Media: A Review of Sub- and Supercritical Water Technologies. Energy Environ. Sci. 2008, 1, 32. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, L.; Zhang, X.; Yan, Q. Thermodynamic Modeling and Analysis of Biomass Gasification for Hydrogen Production in Supercritical Water. Chem. Eng. J. 2007, 131, 233–244. [Google Scholar] [CrossRef]
- Matsumura, Y. Evaluation of Supercritical Water Gasification and Biomethanation for Wet Biomass Utilization in Japan. Energy Convers. Manag. 2002, 43, 1301–1310. [Google Scholar] [CrossRef]
- Ro, K.S.; Cantrell, K.; Elliott, D.; Hunt, P.G. Catalytic Wet Gasification of Municipal and Animal Wastes. Ind. Eng. Chem. Res. 2007, 46, 8839–8845. [Google Scholar] [CrossRef]
- Calzavara, Y.; Joussot-Dubien, C.; Boissonnet, G.; Sarrade, S. Evaluation of Biomass Gasification in Supercritical Water Process for Hydrogen Production. Energy Convers. Manag. 2005, 46, 615–631. [Google Scholar] [CrossRef]
- Crocker, M. (Ed.) Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; The Royal Society of Chemistry: London, UK, 2010; ISBN 978-1-84973-035-8. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I. Pyrolysis, Combustion and Gasification Characteristics of Miscanthus and Sewage Sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Cengel, Y.A.; Boles, M.A.; Kanoglu, M. Thermodynamics: An Engineering Approach, 9th ed.; McGraw-Hill: New York, NY, USA, 2019; ISBN 978-981-315-787-3. [Google Scholar]
- Elliott, D.C. Catalytic Hydrothermal Gasification of Biomass. Biofuels Bioprod. Bioref. 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.; Prins, W.; Vanswaaij, W.; Vandebeld, B.; Elliott, D.; Neuenschwander, G.; Kruse, A. Biomass Gasification in Near- and Super-Critical Water: Status and Prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Wei, L.; Xu, S.; Zhang, L.; Liu, C.; Zhu, H.; Liu, S. Steam Gasification of Biomass for Hydrogen-Rich Gas in a Free-Fall Reactor. Int. J. Hydrogen Energy 2007, 32, 24–31. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Influence of Operating Conditions on the Steam Gasification of Biomass in a Conical Spouted Bed Reactor. Chem. Eng. J. 2014, 237, 259–267. [Google Scholar] [CrossRef]
- Koppatz, S.; Pfeifer, C.; Hofbauer, H. Comparison of the Performance Behaviour of Silica Sand and Olivine in a Dual Fluidised Bed Reactor System for Steam Gasification of Biomass at Pilot Plant Scale. Chem. Eng. J. 2011, 175, 468–483. [Google Scholar] [CrossRef]
- Herguido, J.; Corella, J.; Gonzalez-Saiz, J. Steam Gasification of Lignocellulosic Residues in a Fluidized Bed at a Small Pilot Scale. Effect of the Type of Feedstock. Ind. Eng. Chem. Res. 1992, 31, 1274–1282. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical Water Gasification of Biomass for Hydrogen Production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Khlifi, S.; Pozzobon, V.; Lajili, M. A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion. Energies 2024, 17, 3646. [Google Scholar] [CrossRef]
- Copa Rey, J.R.; Tamayo Pacheco, J.J.; António Da Cruz Tarelho, L.; Silva, V.; Cardoso, J.S.; Silveira, J.L.; Tuna, C.E. Evaluation of Cogeneration Alternative Systems Integrating Biomass Gasification Applied to a Brazilian Sugar Industry. Renew. Energy 2021, 178, 318–333. [Google Scholar] [CrossRef]
- Briones-Hidrovo, A.; Copa, J.; Tarelho, L.A.C.; Gonçalves, C.; Pacheco Da Costa, T.; Dias, A.C. Environmental and Energy Performance of Residual Forest Biomass for Electricity Generation: Gasification vs. Combustion. J. Clean. Prod. 2021, 289, 125680. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J. Hydrogen Production from Biomass Steam Gasification: Experiment and Simulation. Chem. Eng. Sci. 2024, 292, 120011. [Google Scholar] [CrossRef]
- Shen, Y. Biomass Pretreatment for Steam Gasification toward H2-Rich Syngas Production—An Overview. Int. J. Hydrogen Energy 2024, 66, 90–102. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Huo, C.; Hu, M.; Guo, D.; Xiao, B. Effect of Bioleaching on Hydrogen-Rich Gas Production by Steam Gasification of Sewage Sludge. Energy Convers. Manag. 2015, 106, 1212–1218. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Kutty, P.; Pachapur, P.; Brar, S.K.; Le Bihan, Y.; Galvez-Cloutier, R.; Buelna, G. Seed Pretreatment for Increased Hydrogen Production Using Mixed-Culture Systems with Advantages over Pure-Culture Systems. Energies 2019, 12, 530. [Google Scholar] [CrossRef]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-Rich Gas Production by Steam Gasification of Hydrochar Derived from Sewage Sludge. Int. J. Hydrogen Energy 2016, 41, 3363–3372. [Google Scholar] [CrossRef]
- Niu, Y.; Han, F.; Chen, Y.; Lyu, Y.; Wang, L. Experimental Study on Steam Gasification of Pine Particles for Hydrogen-Rich Gas. J. Energy Inst. 2017, 90, 715–724. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Yang, Z.; Yan, Y.; Pu, G.; Guo, M. Hydrogen-Rich Gas Production from Wet Biomass Steam Gasification with CaO/MgO. Int. J. Hydrogen Energy 2015, 40, 8816–8823. [Google Scholar] [CrossRef]
- Chan, Y.H.; Cheah, K.W.; How, B.S.; Loy, A.C.M.; Shahbaz, M.; Singh, H.K.G.; Yusuf, N.R.; Shuhaili, A.F.A.; Yusup, S.; Ghani, W.A.W.A.K.; et al. An Overview of Biomass Thermochemical Conversion Technologies in Malaysia. Sci. Total Environ. 2019, 680, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Barbuzza, E.; Buceti, G.; Pozio, A.; Santarelli, M.; Tosti, S. Gasification of Wood Biomass with Renewable Hydrogen for the Production of Synthetic Natural Gas. Fuel 2019, 242, 520–531. [Google Scholar] [CrossRef]
- Kruse, A. Hydrothermal Biomass Gasification. J. Supercrit. Fluids 2009, 47, 391–399. [Google Scholar] [CrossRef]
- Huber, G.W.; Dumesic, J.A. An Overview of Aqueous-Phase Catalytic Processes for Production of Hydrogen and Alkanes in a Biorefinery. Catal. Today 2006, 111, 119–132. [Google Scholar] [CrossRef]
- Cortright, R.D.; Davda, R.R.; Dumesic, J.A. Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature 2002, 418, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Tanksale, A.; Beltramini, J.N.; Lu, G.M. A Review of Catalytic Hydrogen Production Processes from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 166–182. [Google Scholar] [CrossRef]
- Davda, R.R.; Shabaker, J.W.; Huber, G.W.; Cortright, R.D.; Dumesic, J.A. A Review of Catalytic Issues and Process Conditions for Renewable Hydrogen and Alkanes by Aqueous-Phase Reforming of Oxygenated Hydrocarbons over Supported Metal Catalysts. Appl. Catal. B Environ. 2005, 56, 171–186. [Google Scholar] [CrossRef]
- He, L.; Yang, J.; Chen, D. Hydrogen from Biomass. In Renewable Hydrogen Technologies; Elsevier: Amsterdam, The Netherlands, 2013; pp. 111–133. ISBN 978-0-444-56352-1. [Google Scholar] [CrossRef]
- Harrison, D.P. Sorption-Enhanced Hydrogen Production: A Review. Ind. Eng. Chem. Res. 2008, 47, 6486–6501. [Google Scholar] [CrossRef]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Steam Gasification of Biomass in a Conical Spouted Bed Reactor with Olivine and γ-Alumina as Primary Catalysts. Fuel Process. Technol. 2013, 116, 292–299. [Google Scholar] [CrossRef]
- Balat, H.; Kırtay, E. Hydrogen from Biomass—Present Scenario and Future Prospects. Int. J. Hydrogen Energy 2010, 35, 7416–7426. [Google Scholar] [CrossRef]
- Rennard, D.; French, R.; Czernik, S.; Josephson, T.; Schmidt, L. Production of Synthesis Gas by Partial Oxidation and Steam Reforming of Biomass Pyrolysis Oils. Int. J. Hydrogen Energy 2010, 35, 4048–4059. [Google Scholar] [CrossRef]
- Pirez, C.; Fang, W.; Capron, M.; Paul, S.; Jobic, H.; Dumeignil, F.; Jalowiecki-Duhamel, L. Steam Reforming, Partial Oxidation and Oxidative Steam Reforming for Hydrogen Production from Ethanol over Cerium Nickel Based Oxyhydride Catalyst. Appl. Catal. A Gen. 2016, 518, 78–86. [Google Scholar] [CrossRef]
- Tóth, M.; Varga, E.; Oszkó, A.; Baán, K.; Kiss, J.; Erdőhelyi, A. Partial Oxidation of Ethanol on Supported Rh Catalysts: Effect of the Oxide Support. J. Mol. Catal. A Chem. 2016, 411, 377–387. [Google Scholar] [CrossRef]
- Salge, J.; Deluga, G.; Schmidt, L. Catalytic Partial Oxidation of Ethanol over Noble Metal Catalysts. J. Catal. 2005, 235, 69–78. [Google Scholar] [CrossRef]
- Gandia, L.M.; Arzamedi, G.; Dieguez, P.M. Hydrogen from Biomass: Advances in Ther-Mochemical Processes; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A Comparative Analysis of Hydrogen Production from the Thermochemical Conversion of Algal Biomass. Int. J. Hydrogen Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Osada, M.; Sato, T.; Watanabe, M.; Shirai, M.; Arai, K. Catalytic Gasification of Wood Biomass in Subcritical and Supercritical Water. Combust. Sci. Technol. 2006, 178, 537–552. [Google Scholar] [CrossRef]
- Resende, F.L.P.; Savage, P.E. Effect of Metals on Supercritical Water Gasification of Cellulose and Lignin. Ind. Eng. Chem. Res. 2010, 49, 2694–2700. [Google Scholar] [CrossRef]
- Razuan, R.; Chen, Q.; Zhang, X.; Sharifi, V.; Swithenbank, J. Pyrolysis and Combustion of Oil Palm Stone and Palm Kernel Cake in Fixed-Bed Reactors. Bioresour. Technol. 2010, 101, 4622–4629. [Google Scholar] [CrossRef] [PubMed]
- Razuan, R.; Finney, K.N.; Chen, Q.; Sharifi, V.N.; Swithenbank, J. Pelletised Fuel Production from Palm Kernel Cake. Fuel Process. Technol. 2011, 92, 609–615. [Google Scholar] [CrossRef]
- Razuan, R.; Chen, Q.; Finney, K.N.; Russell, N.V.; Sharifi, V.N.; Swithenbank, J. Combustion of Oil Palm Stone in a Pilot-Scale Fluidised Bed Reactor. Fuel Process. Technol. 2011, 92, 2219–2225. [Google Scholar] [CrossRef]
- Güngören Madenoğlu, T.; Boukis, N.; Sağlam, M.; Yüksel, M. Supercritical Water Gasification of Real Biomass Feedstocks in Continuous Flow System. Int. J. Hydrogen Energy 2011, 36, 14408–14415. [Google Scholar] [CrossRef]
- Azadi, P.; Farnood, R. Review of Heterogeneous Catalysts for Sub- and Supercritical Water Gasification of Biomass and Wastes. Int. J. Hydrogen Energy 2011, 36, 9529–9541. [Google Scholar] [CrossRef]
- Watanabe, M.; Inomata, H.; Arai, K. Catalytic Hydrogen Generation from Biomass (Glucose and Cellulose) with ZrO2 in Supercritical Water. Biomass Bioenergy 2002, 22, 405–410. [Google Scholar] [CrossRef]
- Park, K.C.; Tomiyasu, H. Gasification Reaction of Organic Compounds Catalyzed by RuO2 in Supercritical Water. Chem. Commun. 2003, 6, 694–695. [Google Scholar] [CrossRef] [PubMed]
- Sınaǧ, A.; Kruse, A.; Schwarzkopf, V. Key Compounds of the Hydropyrolysis of Glucose in Supercritical Water in the Presence of K2 CO3. Ind. Eng. Chem. Res. 2003, 42, 3516–3521. [Google Scholar] [CrossRef]
- Sutton, D.; Kelleher, B.; Ross, J.R.H. Review of Literature on Catalysts for Biomass Gasification. Fuel Process. Technol. 2001, 73, 155–173. [Google Scholar] [CrossRef]
- Paida, V.R.; Kersten, S.R.A.; Van Der Ham, A.G.J.; Brilman, D.W.F. A Two-Step Approach to the Hydrothermal Gasification of Carbohydrate-Rich Wastes: Process Design and Economic Evaluation. Int. J. Hydrogen Energy 2019, 44, 25524–25541. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Xu, P.; Liu, B.; Shuai, Y.; Li, B. Hydrogen Production through Biomass Gasification in Supercritical Water: A Review from Exergy Aspect. Int. J. Hydrogen Energy 2019, 44, 15727–15736. [Google Scholar] [CrossRef]
- Lethner, F.; Timmerer, H. Overview on Risk Assessment of Biomass Gasification Plants. In Proceedings of the International Workshop Health, Safety and Environment of Biomass Gasification, Innsbruck, Austria, 18 September 2005; Furnsinn, S., Buhler, R., Hofbauer, H., Eds.; Graz University of Technology: Graz, Austria, 2005; Volume 3, pp. 106–115. [Google Scholar]
- Timmerer, H.; Lethner, F. Explosion Parameters and Explosion Protection in Biomass Gasification Plants. Risk Assessment and Permission Procedure. In Proceedings of the International Workshop Health, Safety and Environment of Biomass Gasification, Innsbruck, Austria, 18 September 2005; Furnsinn, S., Buhler, R., Hofbauer, H., Eds.; Graz University of Technology: Graz, Austria, 2005; Volume 3, pp. 114–127. [Google Scholar]
- Yan, F.; Xu, K.; Li, D.; Zhang, X. Hazard Assessment for Biomass Gasification Station Using General Set Pair Analysis. BioResources 2016, 11, 8307–8324. [Google Scholar] [CrossRef]
- Chen, G.; Li, W.; Chen, H.; Yan, B. Progress in the Aqueous-Phase Reforming of Different Biomass-Derived Alcohols for Hydrogen Production. J. Zhejiang Univ. Sci. A 2015, 16, 491–506. [Google Scholar] [CrossRef]
- Glushkov, D.; Nyashina, G.; Shvets, A.; Pereira, A.; Ramanathan, A. Current Status of the Pyrolysis and Gasification Mechanism of Biomass. Energies 2021, 14, 7541. [Google Scholar] [CrossRef]
- Salam, M.A.; Ahmed, K.; Akter, N.; Hossain, T.; Abdullah, B. A Review of Hydrogen Production via Biomass Gasification and Its Prospect in Bangladesh. Int. J. Hydrogen Energy 2018, 43, 14944–14973. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen Production from Biomass Gasification Using Biochar as a Catalyst/Support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The Influence of Catalysts in Biomass Steam Gasification and Catalytic Potential of Coal Bottom Ash in Biomass Steam Gasification: A Review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, Z.; Wei, D.; Zhang, H.; Lu, W. Application of Fe Based Composite Catalyst in Biomass Steam Gasification to Produce Hydrogen Rich Gas. Front. Chem. 2022, 10, 882787. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, X.; Gu, J.; Shi, J. Co-Gasification of Coal and Biomass Blends Using Dolomite and Olivine as Catalysts. Renew. Energy 2019, 132, 509–514. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Dong, L.; Vassallo, A.; Williams, P.T.; Huang, J. Characteristics and Catalytic Properties of Ni/CaAlOx Catalyst for Hydrogen-Enriched Syngas Production from Pyrolysis-Steam Reforming of Biomass Sawdust. Appl. Catal. B Environ. 2016, 183, 168–175. [Google Scholar] [CrossRef]
- Xu, C.; Chen, S.; Soomro, A.; Sun, Z.; Xiang, W. Hydrogen Rich Syngas Production from Biomass Gasification Using Synthesized Fe/CaO Active Catalysts. J. Energy Inst. 2018, 91, 805–816. [Google Scholar] [CrossRef]
- Taipabu, M.I.; Viswanathan, K.; Wu, W.; Nagy, Z.K. Production of Renewable Fuels and Chemicals from Fats, Oils, and Grease (FOG) Using Homogeneous and Heterogeneous Catalysts: Design, Validation, and Optimization. Chem. Eng. J. 2021, 424, 130199. [Google Scholar] [CrossRef]
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen Production from Catalytic Steam Reforming of Biodiesel Byproduct Glycerol: Issues and Challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Wang, Y.; Liang, T.; Li, X.; Yang, Z.; Chen, M.; Wang, J. Effects of Attapulgite-Supported Transition Metals Catalysts on Glycerol Steam Reforming for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 20451–20464. [Google Scholar] [CrossRef]
- Dahdah, E.; Aouad, S.; Gennequin, C.; Estephane, J.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Glycerol Steam Reforming over Ru-Mg-Al Hydrotalcite-Derived Mixed Oxides: Role of the Preparation Method in Catalytic Activity. Int. J. Hydrogen Energy 2018, 43, 19864–19872. [Google Scholar] [CrossRef]
- Wang, R.; Rohr, D. Natural Gas Processing Strategies for Large Scale Solid Oxide Fuel Cells; American Chemical Society (ACS): Boston, MA, USA, 2002; Volume 47, pp. 506–507. [Google Scholar]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and Strategies for Optimization of Glycerol Steam Reforming Process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef]
- New Jersey Department of Health and Senior Services. Glycerol-Alpha-Monochlorohydrin; CAS: Hammonton, NJ, USA, 1999. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/2453.pdf (accessed on 9 October 2024).
- INCHEM IPCS Glycerol CAS No. 56-81-5. 2006. Available online: https://www.inchem.org/documents/icsc/icsc/eics0624.htm (accessed on 9 October 2024).
- WebMD Vitamins&Supplements. Glycerol—Uses, Side Effects, and More. 2024. Available online: https://www.webmd.com/vitamins/ai/ingredientmono-4/glycerol (accessed on 9 October 2024).
- Busca, G. Critical Aspects of Energetic Transition Technologies and the Roles of Materials Chemistry and Engineering. Energies 2024, 17, 3565. [Google Scholar] [CrossRef]
- Ghaffari Saeidabad, N.; Noh, Y.S.; Alizadeh Eslami, A.; Song, H.T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- Naranje, V.; Swarnalatha, R.; Batra, O.; Salunkhe, S. Technological Assessment on Steam Reforming Process of Crude Glycerol to Produce Hydrogen in an Integrated Waste Cooking-Oil-Based Biodiesel Production Scenario. Processes 2022, 10, 2670. [Google Scholar] [CrossRef]
- Avasthi, K.S.; Reddy, R.N.; Patel, S. Challenges in the Production of Hydrogen from Glycerol—A Biodiesel Byproduct Via Steam Reforming Process. Procedia Eng. 2013, 51, 423–429. [Google Scholar] [CrossRef]
- Lin, K.-H.; Chang, A.C.-C.; Lin, W.-H.; Chen, S.-H.; Chang, C.-Y.; Chang, H.-F. Autothermal Steam Reforming of Glycerol for Hydrogen Production over Packed-Bed and Pd/Ag Alloy Membrane Reactors. Int. J. Hydrogen Energy 2013, 38, 12946–12952. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.; Feik, C.; Chornet, E. Hydrogen by Catalytic Steam Reforming of Liquid Byproducts from Biomass Thermoconversion Processes. Ind. Eng. Chem. Res. 2002, 41, 4209–4215. [Google Scholar] [CrossRef]
- New Jersey Department of Health and Senior Services. Platinum. CAS No 7440-06-4. Hazardous Substances Fact Sheet. 2002. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1547.pdf (accessed on 9 October 2024).
- Safety Data Sheet. CarlRoth Platinum 98,2-98,7%. CAS No 7440-06-4. 2024. Available online: https://www.carlroth.com/medias/SDB-8408-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyMzg0NDJ8YXBwbGljYXRpb24vcGRmfGFEUXhMMmhqWlM4NU1UYzRPVEUzTURFeE5EZzJMMU5FUWw4NE5EQTRYMEZWWDBWT0xuQmtaZ3xiNDg3MmY0YjVmOGFhM2EyOGNhMzA5MjcwNmIzYmUxYzE5ODc2ZTY4YmRlNWE3Y2I5YzkwNzNhYWNmYjI3MzI1 (accessed on 9 October 2024).
- CAMEO. Chemicals ERG Guide 170. Metals (Powders, Dusts, Shavings, Borings, Turnings, or Cuttings, Etc.). 2024. Available online: https://cameochemicals.noaa.gov/erg_guides/en/Guide_170.pdf (accessed on 9 October 2024).
- Gebel, T.; Lantzsch, H.; Pleßow, K.; Dunkelberg, H. Genotoxicity of Platinum and Palladium Compounds in Human and Bacterial Cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1997, 389, 183–190. [Google Scholar] [CrossRef] [PubMed]
- New Jersey Department of Health and Senior Services. Rhodium. CAS No 7440-16-6. Hazardous Substances Fact Sheet. 2002. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1635.pdf (accessed on 9 October 2024).
- Lenntech Rhodium—Rh. Chemical Properties of Rhodium. 2024. Available online: https://www.lenntech.com/periodic/elements/rh.htm (accessed on 9 October 2024).
- ProPlate What Environmental and Safety Considerations Are Associated with Rhodium Electroplating? ProPlate. Medical Device. 2024. Available online: https://www.proplate.com/what-environmental-and-safety-considerations-are-associated-with-rhodium-electroplating/ (accessed on 9 October 2024).
- INCHEM IPCS Rhodium. Cas No 7440-16-6. 2004. Available online: https://www.inchem.org/documents/icsc/icsc/eics1247.htm (accessed on 9 October 2024).
- Lenntech Rhenium—Re. Chemical Properties of Rhenium. 2024. Available online: https://www.lenntech.com/periodic/elements/re.htm (accessed on 9 October 2024).
- Rhenium Alloys. Rhenium Powder (99.99%). CAS No 7440-15-5. Material Safety Data Sheet. 2011. Available online: https://rhenium.com/assets/rhenium-powder-sds.pdf (accessed on 9 October 2024).
- Oak Ridge National Laboratory. Rhenium. CAS No 7440-15-5. Safety Data Sheet. 2019. Available online: https://www.physics.purdue.edu/primelab/safety/MSDS/SDS/rhenium%20187%20metal%20-%20stable%20isotope.pdf (accessed on 9 October 2024).
- Dierks, S. Rhenium Oxide Re2O7. Material Safety Data Sheet. 2012. Available online: https://www.espimetals.com/index.php/msds/630-Rhenium%20Oxide%20Re2O7 (accessed on 9 October 2024).
- Lenntech Palladium—Pd. Chemical Properties of Palladium. 2024. Available online: https://www.lenntech.com/periodic/elements/pd.htm (accessed on 9 October 2024).
- Carl Roth Palladium ROTI METIC 99,999 % (5N) Powder. CAS Number 7440-05-3. Safety Data Sheet. 2024. Available online: https://www.carlroth.com/medias/SDB-5648-GB-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyNjM1MDZ8YXBwbGljYXRpb24vcGRmfGFEa3hMMmhpT1M4NU1UZ3hNekV3T1RFd05EazBMMU5FUWw4MU5qUTRYMGRDWDBWT0xuQmtaZ3w0OGFhMjNkMjI3YmY2ZWViNGNjM2RiNTM4ZDBiZjM4YmM3YWYzZDA4YmVkMzEyZjZlMjNkNGM5MzhhNWFhNDgx (accessed on 9 October 2024).
- Angstrem Sciences. Palladium Aloy. CAS No 7440-05-3. Material Safety Data Sheet. 2000. Available online: https://louisville.edu/micronano/files/documents/safety-data-sheets-sds/Palladium1.pdf (accessed on 9 October 2024).
- ChemicalBook. ChemicalBook. Inorganic Chemistry, 10 September 2019. Available online: https://www.chemicalbook.com/article/palladium-hazard-and-toxicity.htm (accessed on 9 October 2024).
- Lenntech Ruthenium—Ru. Chemical Properties of Ruthenium. 2024. Available online: https://www.lenntech.com/periodic/elements/ru.htm (accessed on 9 October 2024).
- Sigma-Aldrich. Ruthenium. CAS-No. 7440-18-8. Safety Data Sheet. 2017. Available online: https://www.geneseo.edu/sites/default/files/users/247/Ruthenium.pdf (accessed on 9 October 2024).
- Lenntech Iridium—Ir. Chemical Properties of Iridium. 2024. Available online: https://www.lenntech.com/periodic/elements/ir.htm (accessed on 9 October 2024).
- McGough, M. Iridium Scarcity Challenges Clean Hydrogen Scale-Up. N. Am. Clean Energy 2023, 9/10. Available online: https://www.nacleanenergy.com/energy-storage/iridium-scarcity-challenges-clean-hydrogen-scale-up (accessed on 9 October 2024).
- New Jersey Department of Health and Senior Services. Cobalt. CAS No 7440-48-4. Hazardous Substances Fact Sheet. 2005. Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/0520.pdf (accessed on 9 October 2024).
- Chen, R.J.; Lee, V.R. Cobalt Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jensen, A.A.; Tuchsen, F. Cobalt Exposure and Cancer Risk. Crit. Rev. Toxicol. 1990, 20, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. ToxFAQs for Copper. CAS No. 7440-50-8. 2022. Available online: https://wwwn.cdc.gov/Tsp/ToxFAQs/ToxFAQsDetails.aspx?faqid=205&toxid=37 (accessed on 9 October 2024).
- New Jersey Department of Health Copper—Hazardous Substance Fact Sheet. 2016. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0528.pdf (accessed on 9 October 2024).
- Agency for Toxic Substances and Disease Registry. Copper. CAS No. 7440-50-8. Public Health Statement. 2004. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp132-c1-b.pdf (accessed on 9 October 2024).
- National Institutes of Health Copper. Fact Sheet for Consumers. 2022. Available online: https://ods.od.nih.gov/factsheets/Copper-Consumer/ (accessed on 9 October 2024).
- Lenntech Copper—Cu. Chemical Properties of Copper. 2024. Available online: https://www.lenntech.com/periodic/elements/cu.htm (accessed on 9 October 2024).
- ThermoFisher Scientific Raney Nickel, Activated Catalyst, 50% Slurry in Water. Safety Data Sheet. 2024. Available online: https://www.fishersci.fi/store/msds?partNumber=10172953&countryCode=FI&language=en (accessed on 9 October 2024).
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen Production through Glycerol Steam Reforming Using Co Catalysts Supported on SBA-15 Doped with Zr, Ce and La. J. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- New Jersey Department of Health and Senior Services Potassium. CAS No. 7440-09-7. Hazardous Substances Fact Sheet. 2003. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1555.pdf (accessed on 9 October 2024).
- MayoClinic Drugs$Supplements. Potassium Supplement (Oral Route, Parenteral Route). 2007. Available online: https://www.mayoclinic.org/drugs-supplements/potassium-supplement-oral-route-parenteral-route/side-effects/drg-20070753?p=1 (accessed on 9 October 2024).
- Oria, M.; Harrison, M.; Stallings, V.A. (Eds.) 5, Potassium: Dietary Reference Intakes for Toxicity. In Dietary Reference Intakes for Sodium and Potassium; National Academies Press: Washington, DC, USA, 2019. [Google Scholar]
- New Jersey Department of Health and Senior Services Calcium. CAS No. 7440-70-2. Hazardous Substances Fact Sheet. 2010. Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/0309.pdf (accessed on 9 October 2024).
- The Office of Dietary Supplements (ODS) of the National Institutes of Health (NIH). Calcium. Fact Sheet for Consumers. 2023. Available online: https://ods.od.nih.gov/factsheets/Calcium-Consumer/ (accessed on 9 October 2024).
- Lee, H. Can You Eat Calcium? University of Illinois Urbana-Champaign. The Grainger College of Engineering. Physics Van. 2011. Available online: https://van.physics.illinois.edu/ask/listing/17093 (accessed on 9 October 2024).
- MountSinai Calcium Carbonate Overdose. 2023. Available online: https://www.mountsinai.org/health-library/poison/calcium-carbonate-overdose (accessed on 9 October 2024).
- New Jersey Department of Health and Senior Services Strontium. CAS No. 7440-24-6. Hazardous Substances Fact Sheet. 1998. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1739.pdf (accessed on 9 October 2024).
- Agency for Toxic Substances and Disease Registry; Division of Toxicology Strontium. CAS NO. 7440-24-6. Toxicological Profile for Strontium. ToxFAQs. 2004. Available online: https://www.atsdr.cdc.gov/toxfaqs/tfacts159.pdf (accessed on 9 October 2024).
- WHO; ILO. Chemical Safety Strontium. CAS No. 7440-24-6. Chemical Safety. 2004. Available online: https://chemicalsafety.ilo.org/dyn/icsc/showcard.display?p_lang=en&p_card_id=1534&p_version=2 (accessed on 9 October 2024).
- AmesLaboratory. Cerium. CAS No. 7440-45-1. Safety Data Sheet. 2016. Available online: https://www.ameslab.gov/sites/default/files/inline-files/58_Cerium_SDS.pdf (accessed on 9 October 2024).
- ESPI Metals. Cerium Oxide. CAS No. 1306-38-3. Material Safety Data Sheet. 2015. Available online: https://www.espimetals.com/index.php/msds/489-Cerium%20Oxide (accessed on 9 October 2024).
- Lenntech Cerium—CE. Chemical Properties of Cerium. 2024. Available online: https://www.lenntech.com/periodic/elements/ce.htm (accessed on 9 October 2024).
- García, A.; Espinosa, R.; Delgado, L.; Casals, E.; González, E.; Puntes, V.; Barata, C.; Font, X.; Sánchez, A. Acute Toxicity of Cerium Oxide, Titanium Oxide and Iron Oxide Nanoparticles Using Standardized Tests. Desalination 2011, 269, 136–141. [Google Scholar] [CrossRef]
- AmesLaboratory Lanthanum. CAS No. 7439-91-0. Safety Data Sheet. 2016. Available online: https://www.ameslab.gov/sites/default/files/inline-files/57_Lanthanum_SDS.pdf (accessed on 9 October 2024).
- Hutchison, A.J.; Wilson, R.J.; Garafola, S.; Copley, J.B. Lanthanum Carbonate: Safety Data after 10 Years. Nephrology 2016, 21, 987–994. [Google Scholar] [CrossRef] [PubMed]
- MayoClinic Drugs&Supplements. Lanthanum Carbonate (Oral Route). 2024. Available online: https://www.mayoclinic.org/drugs-supplements/lanthanum-carbonate-oral-route/side-effects/drg-20064467?p=1 (accessed on 9 October 2024).
- GoodRx Lanthanum. What Are the Risks and Warnings for Lanthanum (Fosrenol)? 2021. Available online: https://www.goodrx.com/lanthanum/what-is (accessed on 9 October 2024).
- U.S. Department of Labor Hexavalent Chromium. Health Effects. Occupational Safety and Health Administration. 2024. Available online: https://www.osha.gov/hexavalent-chromium/health-effects (accessed on 9 October 2024).
- Agency for Toxic Substances and Disease Registry Chromium Toxicity. What Are the Physiologic Effects of Chromium Exposure? 2023. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf (accessed on 9 October 2024).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Experientia Supplementum; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar] [CrossRef]
- PChrome. The Dangers of Traditional Chrome Plating. 2024. Available online: https://pchrome.com/dangers-traditional-chrome-plating/ (accessed on 9 October 2024).
- Medeiros, M.G. Elevated Levels of DNA-Protein Crosslinks and Micronuclei in Peripheral Lymphocytes of Tannery Workers Exposed to Trivalent Chromium. Mutagenesis 2003, 18, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Chromium Toxicity. 2011. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf (accessed on 9 October 2024).
- Fisher Scientific Iron. CAS No. 7439-89-6. Material Safety Data Sheet. 2007. Available online: https://www.fishersci.com/store/msds?partNumber=AC197815000&productDescription=IRON%2C+POWDER%2C-70+MESH+99%25&vendorId=VN00032119&countryCode=US&language=en (accessed on 9 October 2024).
- O’Malley, G.F.; O’Malley, R. Iron Poisoning. MSD Manual Consumer Version. 2022. Available online: https://www.msdmanuals.com/home/injuries-and-poisoning/poisoning/iron-poisoning (accessed on 9 October 2024).
- CDH Iridium CAS No.: 7439-88-5. 2024. Available online: https://www.cdhfinechemical.com/images/product/msds/51_147563125_IRIDIUM-CASNO-7439-88-5-MSDS.pdf (accessed on 9 October 2024).
- Liu, W.; Liu, C.; Gogoi, P.; Deng, Y. Overview of Biomass Conversion to Electricity and Hydrogen and Recent Developments in Low-Temperature Electrochemical Approaches. Engineering 2020, 6, 1351–1363. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, D.; Wang, J.; Tu, W.; Wu, D.; Koh, S.W.; Gao, P.; Xu, Z.J.; Deng, S.; Zhou, Y.; et al. Raw Biomass Electroreforming Coupled to Green Hydrogen Generation. Nat. Commun. 2021, 12, 2008. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Energy. Hydrogen Production: Biomass Gasification. 2020. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-biomass-gasification (accessed on 9 October 2024).
- Reigstad, G.A.; Roussanaly, S.; Straus, J.; Anantharaman, R.; De Kler, R.; Akhurst, M.; Sunny, N.; Goldthorpe, W.; Avignon, L.; Pearce, J.; et al. Moving toward the Low-Carbon Hydrogen Economy: Experiences and Key Learnings from National Case Studies. Adv. Appl. Energy 2022, 8, 100108. [Google Scholar] [CrossRef]
- Ariful Islam, M.; Chowdhury, A.; Jahan, I.; Farrok, O. Mitigation of Environmental Impacts and Challenges during Hydrogen Production. Bioresour. Technol. 2025, 415, 131666. [Google Scholar] [CrossRef]
- Cleveland Clinic Oxygen Treatment. 2022. Available online: https://my.clevelandclinic.org/health/treatments/23194-oxygen-therapy (accessed on 9 October 2024).
- New Jersey Department of Health and Senior Services. Oxygen CAS Number 7782-44-7. 2007. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/1448.pdf (accessed on 9 October 2024).
- Airgas USA, LLC. Oxygen CAS Number 7782-44-7. 2020. Available online: https://www.airgas.com/msds/001043.pdf (accessed on 9 October 2024).
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life Cycle Assessment of Hydrogen Production via Electrolysis—A Review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water Electrolysis Based on Renewable Energy for Hydrogen Production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM Water Electrolysis for Hydrogen Production: Fundamentals, Advances, and Prospects. Carb Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wu, X.; Jiaqiang, E.J.; Lu, N.; Wang, T.; Zuo, H. System Development and Environmental Performance Analysis of a Pilot Scale Microbial Electrolysis Cell for Hydrogen Production Using Urban Wastewater. Energy Convers. Manag. 2019, 193, 52–63. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Zhang, Z.; Du, X.; Yu, L.; Deng, Y. A Self-Powered Electrolytic Process for Glucose to Hydrogen Conversion. Commun Chem 2019, 2, 67. [Google Scholar] [CrossRef]
- Liu, F.; Moustafa, H.; Hassouna, M.S.E.-D.; He, Z. Enhancing the Performance of a Microbial Electrochemical System with Carbon-Based Dynamic Membrane as Both Anode Electrode and Filtration Media. Environ. Sci. Water Res. Technol. 2021, 7, 870–878. [Google Scholar] [CrossRef]
- Dange, P.; Pandit, S.; Jadhav, D.; Shanmugam, P.; Gupta, P.K.; Kumar, S.; Kumar, M.; Yang, Y.-H.; Bhatia, S.K. Recent Developments in Microbial Electrolysis Cell-Based Biohydrogen Production Utilizing Wastewater as a Feedstock. Sustainability 2021, 13, 8796. [Google Scholar] [CrossRef]
- New Jersey Department of Health and Senior Services. Chlorine CAS No.: 7782-50-5. 2015. Available online: https://nj.gov/health/eoh/rtkweb/documents/fs/0367.pdf (accessed on 9 October 2024).
- Liu, W.; Cui, Y.; Du, X.; Zhang, Z.; Chao, Z.; Deng, Y. High Efficiency Hydrogen Evolution from Native Biomass Electrolysis. Energy Environ. Sci. 2016, 9, 467–472. [Google Scholar] [CrossRef]
- Yang, L.; Liu, W.; Zhang, Z.; Du, X.; Gong, J.; Dong, L.; Deng, Y. Hydrogen Evolution from Native Biomass with Fe3+/Fe2+ Redox Couple Catalyzed Electrolysis. Electrochim. Acta 2017, 246, 1163–1173. [Google Scholar] [CrossRef]
- Hibino, T.; Kobayashi, K.; Ito, M.; Ma, Q.; Nagao, M.; Fukui, M.; Teranishi, S. Efficient Hydrogen Production by Direct Electrolysis of Waste Biomass at Intermediate Temperatures. ACS Sustain. Chem. Eng. 2018, 6, 9360–9368. [Google Scholar] [CrossRef]
- Murugan, A.; Brown, A.S. Review of Purity Analysis Methods for Performing Quality Assurance of Fuel Cell Hydrogen. Int. J. Hydrogen Energy 2015, 40, 4219–4233. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium Membranes Applications in Reaction Systems for Hydrogen Separation and Purification: A Review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Meng, B.; Tan, X.; Loh, K.S.; Sunarso, J.; Liu, S. Perovskite-Based Mixed Protonic–Electronic Conducting Membranes for Hydrogen Separation: Recent Status and Advances. J. Ind. Eng. Chem. 2018, 60, 297–306. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M.; Ghorbani, B. A Novel Integrated Structure for Hydrogen Purification Using the Cryogenic Method. J. Clean. Prod. 2021, 278, 123872. [Google Scholar] [CrossRef]
- Nor Azira, A.M.; Umi Aisah, A. Purification of Biohydrogen from Fermentation Gas Mixture Using Two-Stage Chemical Absorption. E3S Web Conf. 2019, 90, 01012. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A State of the Art Review on Biomass Processing and Conversion Technologies to Produce Hydrogen and Its Recovery via Membrane Separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Amin, M.; Butt, A.S.; Ahmad, J.; Lee, C.; Azam, S.U.; Mannan, H.A.; Naveed, A.B.; Farooqi, Z.U.R.; Chung, E.; Iqbal, A. Issues and Challenges in Hydrogen Separation Technologies. Energy Rep. 2023, 9, 894–911. [Google Scholar] [CrossRef]
- Buffi, M.; Prussi, M.; Scarlat, N. Energy and Environmental Assessment of Hydrogen from Biomass Sources: Challenges and Perspectives. Biomass Bioenergy 2022, 165, 106556. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass Pyrolysis: A Review on Recent Advancements and Green Hydrogen Production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Biofuel Production from Supercritical Water Gasification of Sustainable Biomass. Energy Convers. Manag. X 2022, 14, 100164. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen Production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–83. ISBN 978-0-12-814853-2. [Google Scholar] [CrossRef]
- Patonia, A.; Poudineh, R. Cost-Competitive Green Hydrogen: How to Lower the Cost of Electrolysers? 2022. Available online: https://www.researchgate.net/publication/365188839_Cost-competitive_green_hydrogen_how_to_lower_the_cost_of_electrolysers (accessed on 9 October 2024).
- IEA. Global Hydrogen Review. 2023. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 9 October 2024).
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-Economic and Environmental Impact Assessment of Hydrogen Production Processes Using Bio-Waste as Renewable Energy Resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-Economic Analysis of a Novel Bioprocess Combining Solid State Fermentation and Dark Fermentation for H2 Production from Food Waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef]
- Alam, M.; Nayan, N.F.; Fatema Nayan, N. Techno-Economic Assessment of Biohydrogen Production from Dark Fermentation of Wastewater Sludge. 2024. Available online: https://www.researchgate.net/publication/378002057_Techno-Economic_Assessment_of_Biohydrogen_Production_from_Dark_Fermentation_of_Wastewater_Sludge (accessed on 9 October 2024).
- Szima, S.; Cormos, C.-C. Techno—Economic Assessment of Flexible Decarbonized Hydrogen and Power Co-Production Based on Natural Gas Dry Reforming. Int. J. Hydrogen Energy 2019, 44, 31712–31723. [Google Scholar] [CrossRef]
- Cormos, C.-C.; Cormos, A.-M.; Petrescu, L.; Dragan, S. Techno-Economic Assessment of Decarbonized Biogas Catalytic Reforming for Flexible Hydrogen and Power Production. Appl. Therm. Eng. 2022, 207, 118218. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Mohamadi-Baghmolaei, M.; Cata Saady, N.M.; Zendehboudi, S. Hydrogen Production from Biomass through Integration of Anaerobic Digestion and Biogas Dry Reforming. Appl. Energy 2022, 309, 118442. [Google Scholar] [CrossRef]
- Byun, J.; Han, J. Economic Feasible Hydrogen Production System from Carbohydrate-Rich Food Waste. Appl. Energy 2023, 340, 121044. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-Economic Comparison of Biomass-to-Transportation Fuels via Pyrolysis, Gasification, and Biochemical Pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Brown, T.R.; Thilakaratne, R.; Brown, R.C.; Hu, G. Techno-Economic Analysis of Biomass to Transportation Fuels and Electricity via Fast Pyrolysis and Hydroprocessing. Fuel 2013, 106, 463–469. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Marker, T.L.; Roberts, M.J. Direct Production of Gasoline and Diesel Fuels from Biomass via Integrated Hydropyrolysis and Hydroconversion Process—A Techno-economic Analysis. Environ. Prog. Sustain. Energy 2014, 33, 609–617. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Liu, P.; Wang, Z.; Sun, T.; Wu, S.; Wu, Y.; Lei, T. Oriented Pyrolysis of Biomass for Hydrogen-Rich Gas and Biochar Production: An Energy, Environment, and Economic Assessment Based on Life Cycle Assessment Method. Int. J. Hydrogen Energy 2024, 62, 979–993. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-Economic Analysis and Life Cycle Assessment of Hydrogen Production from Different Biomass Gasification Processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Wang, Q.; Han, L.; Feng, Y.; Sharif, Z.; Li, Z.; Cen, J.; Kumar, S. Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. Sustainability 2022, 14, 2189. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Liu, Z.; Cui, P.; Zhu, Z.; Yang, S. Techno-Economic Analysis of Biomass-to-Hydrogen Process in Comparison with Coal-to-Hydrogen Process. Energy 2019, 185, 1063–1075. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Parkinson, B.; Hellgardt, K.; Shah, N.; Guillen-Gosalbez, G. Uncovering the True Cost of Hydrogen Production Routes Using Life Cycle Monetisation. Appl. Energy 2021, 281, 115958. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Techno-Economic Evaluation and Sensitivity Analysis of a Conceptual Design for Supercritical Water Gasification of Soybean Straw to Produce Hydrogen. Bioresour. Technol. 2021, 331, 125005. [Google Scholar] [CrossRef] [PubMed]
- Cook, B.; Hagen, C. Techno-Economic Analysis of Biomass Gasification for Hydrogen Production in Three US-Based Case Studies. Int. J. Hydrogen Energy 2024, 49, 202–218. [Google Scholar] [CrossRef]
- Tanyi, R.J.; Mensah, L.D.; Ntiamoah, A.; Quansah, D.A.; Adaramola, M.S. Techno-Economic Assessment of Hydrogen Production in Ghana through PV Electrolysis and Biomass Gasification. Oxf. Open Energy 2024, 3, oiae014. [Google Scholar] [CrossRef]
- Shin, H.; Jang, D.; Lee, S.; Cho, H.-S.; Kim, K.-H.; Kang, S. Techno-Economic Evaluation of Green Hydrogen Production with Low-Temperature Water Electrolysis Technologies Directly Coupled with Renewable Power Sources. Energy Convers. Manag. 2023, 286, 117083. [Google Scholar] [CrossRef]
- María Villarreal Vives, A.; Wang, R.; Roy, S.; Smallbone, A. Techno-Economic Analysis of Large-Scale Green Hydrogen Production and Storage. Appl. Energy 2023, 346, 121333. [Google Scholar] [CrossRef]
- Zainal, B.S.; Ker, P.J.; Mohamed, H.; Ong, H.C.; Fattah, I.M.R.; Rahman, S.M.A.; Nghiem, L.D.; Mahlia, T.M.I. Recent Advancement and Assessment of Green Hydrogen Production Technologies. Renew. Sustain. Energy Rev. 2024, 189, 113941. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.-Q.; Zhao, L.; Chen, C.; Guo, W.-Q.; Cao, G.-L. A Review on Bioconversion of Lignocellulosic Biomass to H2: Key Challenges and New Insights. Bioresour. Technol. 2016, 215, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-W.; Lin, C.-Y. Using Social Network Analysis to Examine the Technological Evolution of Fermentative Hydrogen Production from Biomass. Int. J. Hydrogen Energy 2016, 41, 21573–21582. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of Dark Fermentative Bio-Hydrogen Production: A Review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Bragado, D.; Calderón, M. Investigation on the Reactions Influencing Biomass Air and Air/Steam Gasification for Hydrogen Production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-Electrohydrolysis as a Pretreatment Strategy to Catabolize Complex Food Waste in Closed Circuitry: Function of Electron Flux to Enhance Acidogenic Biohydrogen Production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-Economic Analysis of Hydrogen Production Using Biomass Gasification -A Small Scale Power Plant Study. Energy Procedia 2016, 101, 806–813. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Alnazha, A.A.; Ahmed, U.; El-Adawy, M.; Habib, M.A. Review on Techno-Economics of Hydrogen Production Using Current and Emerging Processes: Status and Perspectives. Results Eng. 2024, 21, 101890. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, R.; Man, Y.; Ren, J. Recent Developments of Hydrogen Production from Sewage Sludge by Biological and Thermochemical Process. Int. J. Hydrogen Energy 2019, 44, 19676–19697. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic Membrane Bioreactors for Biohydrogen Production: Recent Developments, Challenges and Perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen Production: Current Perspectives and the Way Forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark Fermentative Biohydrogen Production from Lignocellulosic Biomass: Technological Challenges and Future Prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Zhang, X.; Guo, J.; Chang, S.W.; Nguyen, D.D.; Wang, J. Biohydrogen Production from Anaerobic Digestion and Its Potential as Renewable Energy. Renew. Energy 2018, 129, 754–768. [Google Scholar] [CrossRef]
- Rai, P.K.; Singh, S.P. Integrated Dark- and Photo-Fermentation: Recent Advances and Provisions for Improvement. Int. J. Hydrogen Energy 2016, 41, 19957–19971. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Memon, S.; Ur Rahman, Z.; Lee, K.; Han, J.-I. Current Status, Barriers and Developments in Biohydrogen Production by Microalgae. Renew. Sustain. Energy Rev. 2013, 22, 571–579. [Google Scholar] [CrossRef]
- Zhou, Y.; Swidler, D.; Searle, S.; Baldino, C. Life-Cycle Greenhouse Gas Emissions of Bio-Methane and Hydrogen Pathways in the European Union. 2021. Available online: https://theicct.org/sites/default/files/publications/lca-biomethane-hydrogen-eu-oct21.pdf (accessed on 9 October 2024).
- Camacho, C.I.; Estévez, S.; Conde, J.J.; Feijoo, G.; Moreira, M.T. Dark Fermentation as an Environmentally Sustainable WIN-WIN Solution for Bioenergy Production. J. Clean. Prod. 2022, 374, 134026. [Google Scholar] [CrossRef]
- Barghash, H.; AlRashdi, Z.; Okedu, K.; Desmond, P. Life-Cycle Assessment Study for Bio-Hydrogen Gas Production from Sewage Treatment Plants Using Solar PVs. Energies 2022, 15, 8056. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Huang, J.; Xu, X.; Tang, J.; Hou, P.; Han, W.; Li, H. Environmental Impact Assessment of a Combined Bioprocess for Hydrogen Production from Food Waste. Waste Manag. 2024, 173, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, X.; Li, G.; Qiu, X.; Yao, D.; Zhu, Z.; Wang, Y.; Gao, J.; Cui, P. Energy Consumption, Environmental Performance, and Techno-Economic Feasibility Analysis of the Biomass-to-Hydrogen Process with and without Carbon Capture and Storage. J. Environ. Chem. Eng. 2021, 9, 106752. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zuo, H.; Wu, X.; Jiaqiang, E.; Wang, T.; Zhang, F.; Lu, N. System Development and Environmental Performance Analysis of a Solar-Driven Supercritical Water Gasification Pilot Plant for Hydrogen Production Using Life Cycle Assessment Approach. Energy Convers. Manag. 2019, 184, 60–73. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Z.; Wu, S.; Xiao, R. Negative Net Global Warming Potential Hydrogen Production through Biomass Gasification Combined with Chemical Looping: Environmental and Economic Assessments. Int. J. Hydrogen Energy 2024, 66, 24–32. [Google Scholar] [CrossRef]
- Cormos, C.-C. Decarbonized Green Hydrogen Production by Sorption-Enhanced Biomass Gasification: An Integrated Techno-Economic and Environmental Evaluation. Int. J. Hydrogen Energy 2024, 95, 592–603. [Google Scholar] [CrossRef]
- Honarmandrad, Z.; Kucharska, K.; Gębicki, J. Processing of Biomass Prior to Hydrogen Fermentation and Post-Fermentative Broth Management. Molecules 2022, 27, 7658. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Skrzypczak, D.; Mikula, K.; Witek-Krowiak, A.; Izydorczyk, G.; Kuligowski, K.; Bandrów, P.; Kułażyński, M. Progress in Sustainable Technologies of Leather Wastes Valorization as Solutions for the Circular Economy. J. Clean. Prod. 2021, 313, 127902. [Google Scholar] [CrossRef]
- Rostocki, A.; Obraniak, A.; Bandrow, P.; Wachulak, M.; Olejnik, T.; Bartyzel, J.; Ławińska, K.; Modrzewski, R.; Szufa, S.; Orczykowska, M. Granulation of Waste Tannery Shavings. Technol. I Jakość Wyr. 2023, 68, 202–228. [Google Scholar] [CrossRef]
- Wrzesińska-Jędrusiak, E.; Czarnecki, M.; Kazimierski, P.; Bandrów, P.; Szufa, S. The Circular Economy in the Management of Waste from Leather Processing. Energies 2023, 16, 564. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Piotrowski, K.; Szufa, S.; Sklodowska, M.; Naliwajski, M.; Emmanouil, C.; Kungolos, A.; Zorpas, A.A. Valorization of Spirodela Polyrrhiza Biomass for the Production of Biofuels for Distributed Energy. Sci. Rep. 2023, 13, 16533. [Google Scholar] [CrossRef] [PubMed]
- Unyay, H.; Piersa, P.; Perendeci, N.A.; Wielgosinski, G.; Szufa, S. Valorization of Anaerobic Digestate: Innovative Approaches for Sustainable Resource Management and Energy Production—Case Studies from Turkey and Poland. Int. J. Green Energy 2024, 21, 1928–1943. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Junga, R.; Błaszczuk, A.; Modliński, N.; Sobek, S.; Marczak-Grzesik, M.; Adrian, Ł.; Dzikuć, M. Numerical Modeling of the Co-Firing Process of an in Situ Steam-Torrefied Biomass with Coal in a 230 MW Industrial-Scale Boiler. Energy 2023, 263, 125918. [Google Scholar] [CrossRef]
- Szufa, S.; Piersa, P.; Adrian, Ł.; Sielski, J.; Grzesik, M.; Romanowska-Duda, Z.; Piotrowski, K.; Lewandowska, W. Acquisition of Torrefied Biomass from Jerusalem Artichoke Grown in a Closed Circular System Using Biogas Plant Waste. Molecules 2020, 25, 3862. [Google Scholar] [CrossRef] [PubMed]
- Szufa, S.; Wielgosiński, G.; Piersa, P.; Czerwińska, J.; Dzikuć, M.; Adrian, Ł.; Lewandowska, W.; Marczak, M. Torrefaction of Straw from Oats and Maize for Use as a Fuel and Additive to Organic Fertilizers—TGA Analysis, Kinetics as Products for Agricultural Purposes. Energies 2020, 13, 2064. [Google Scholar] [CrossRef]

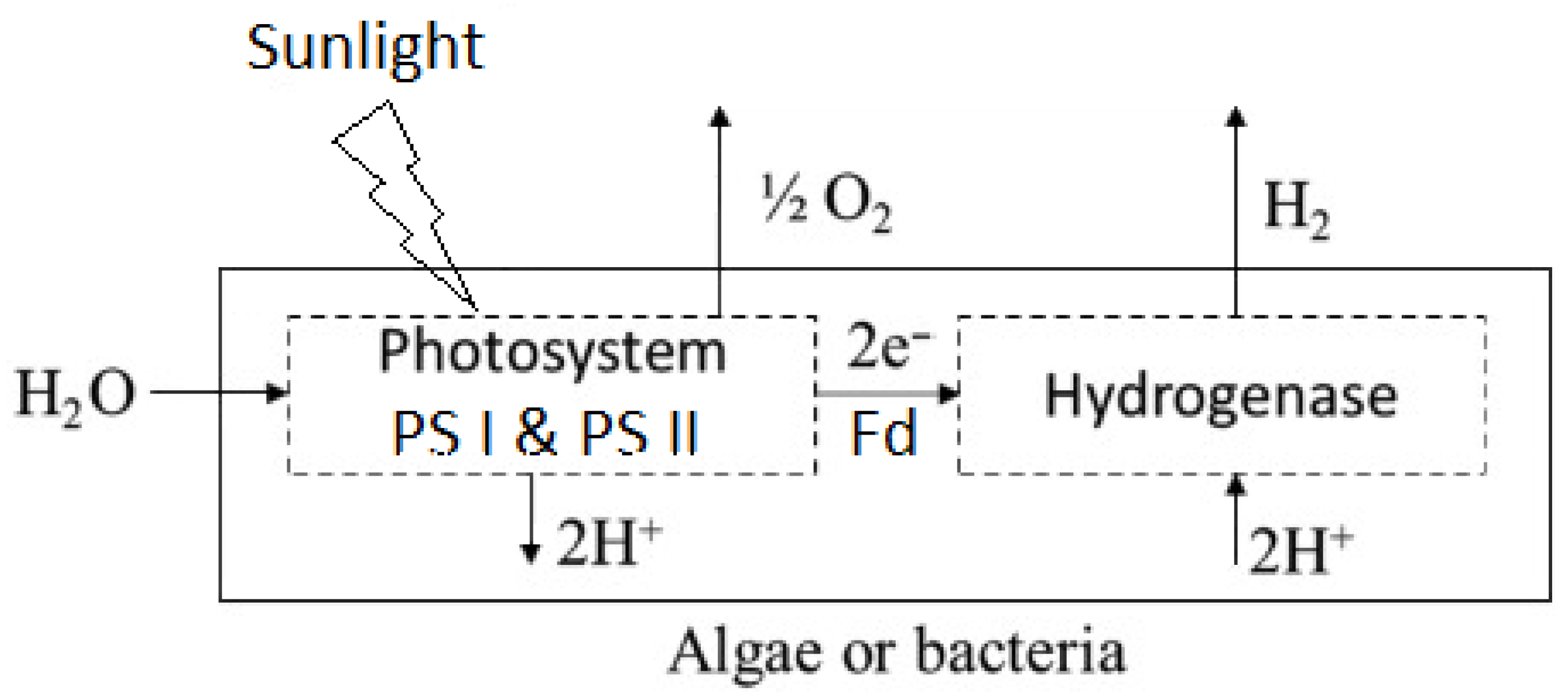
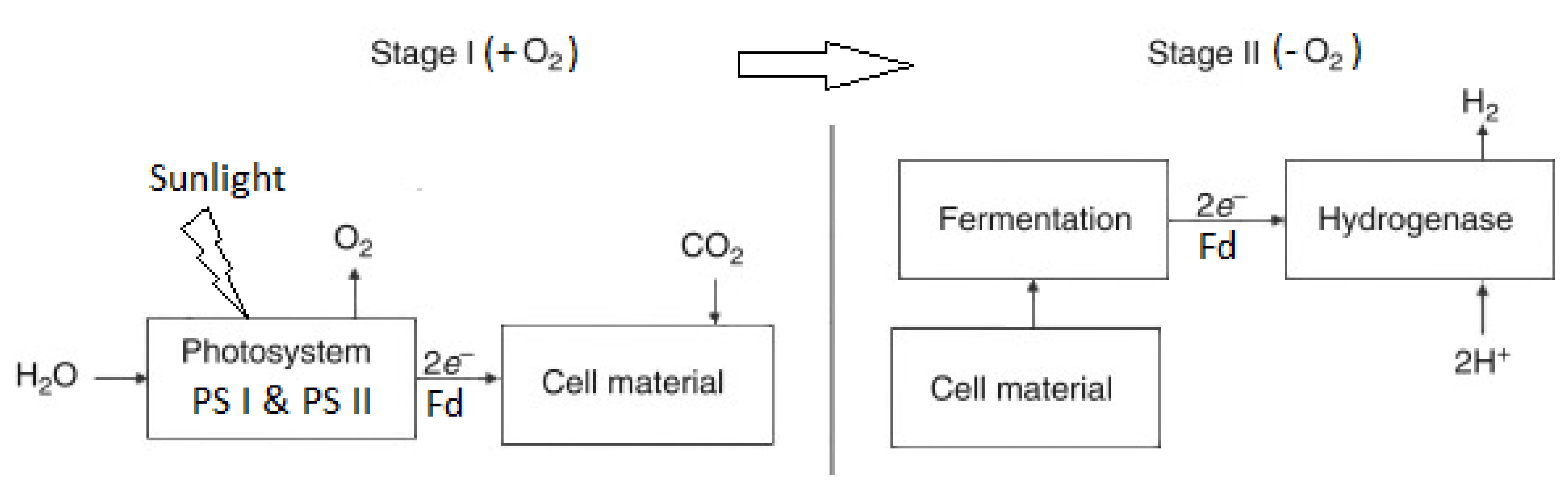
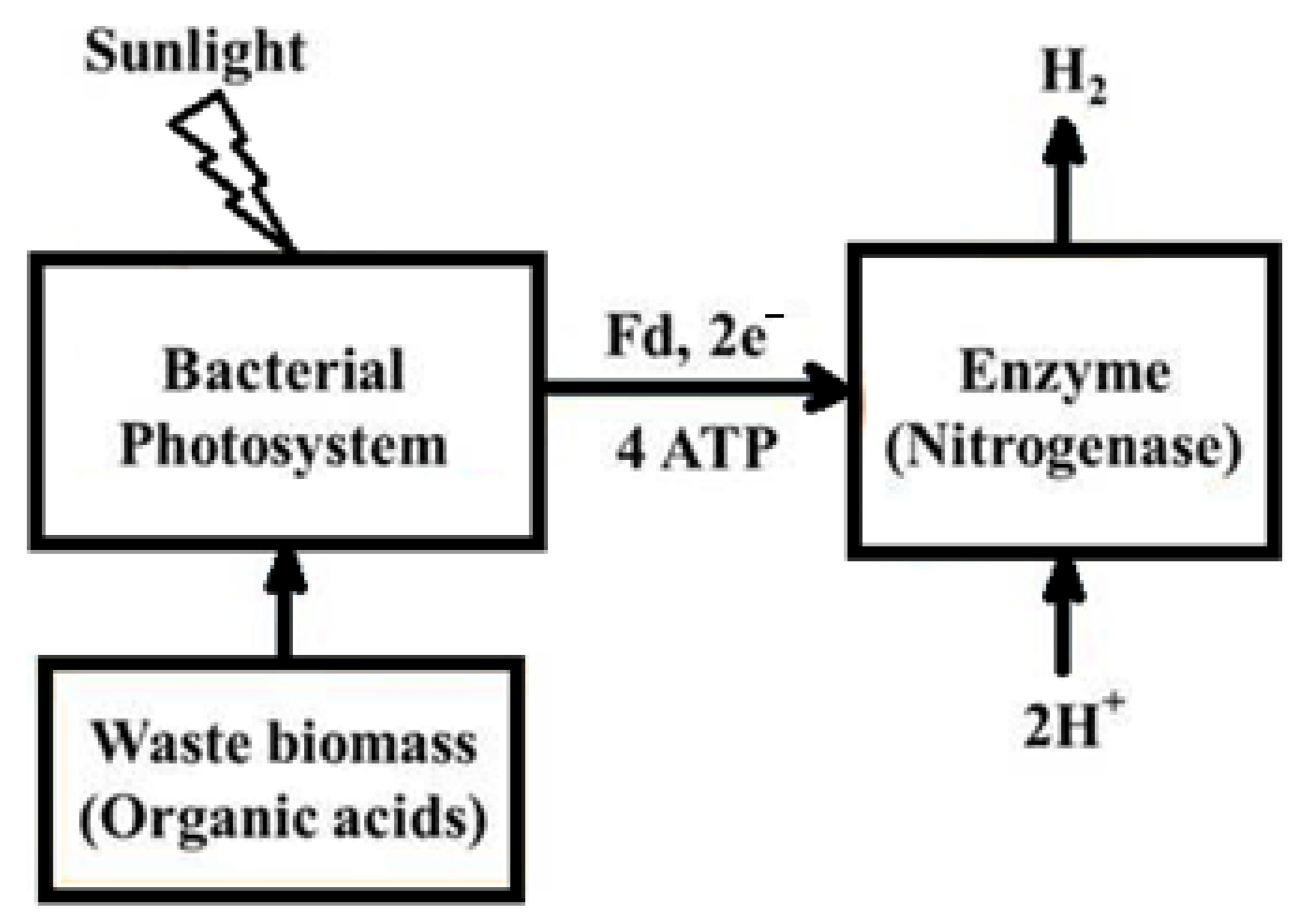

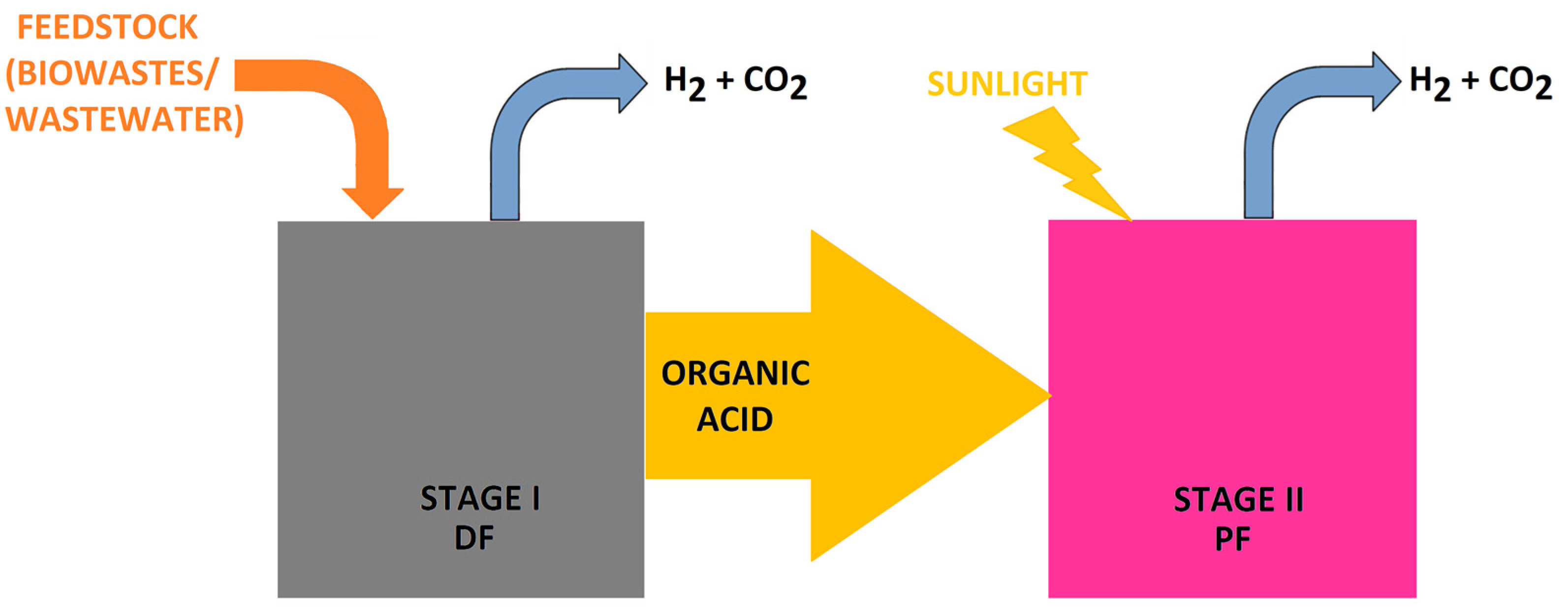

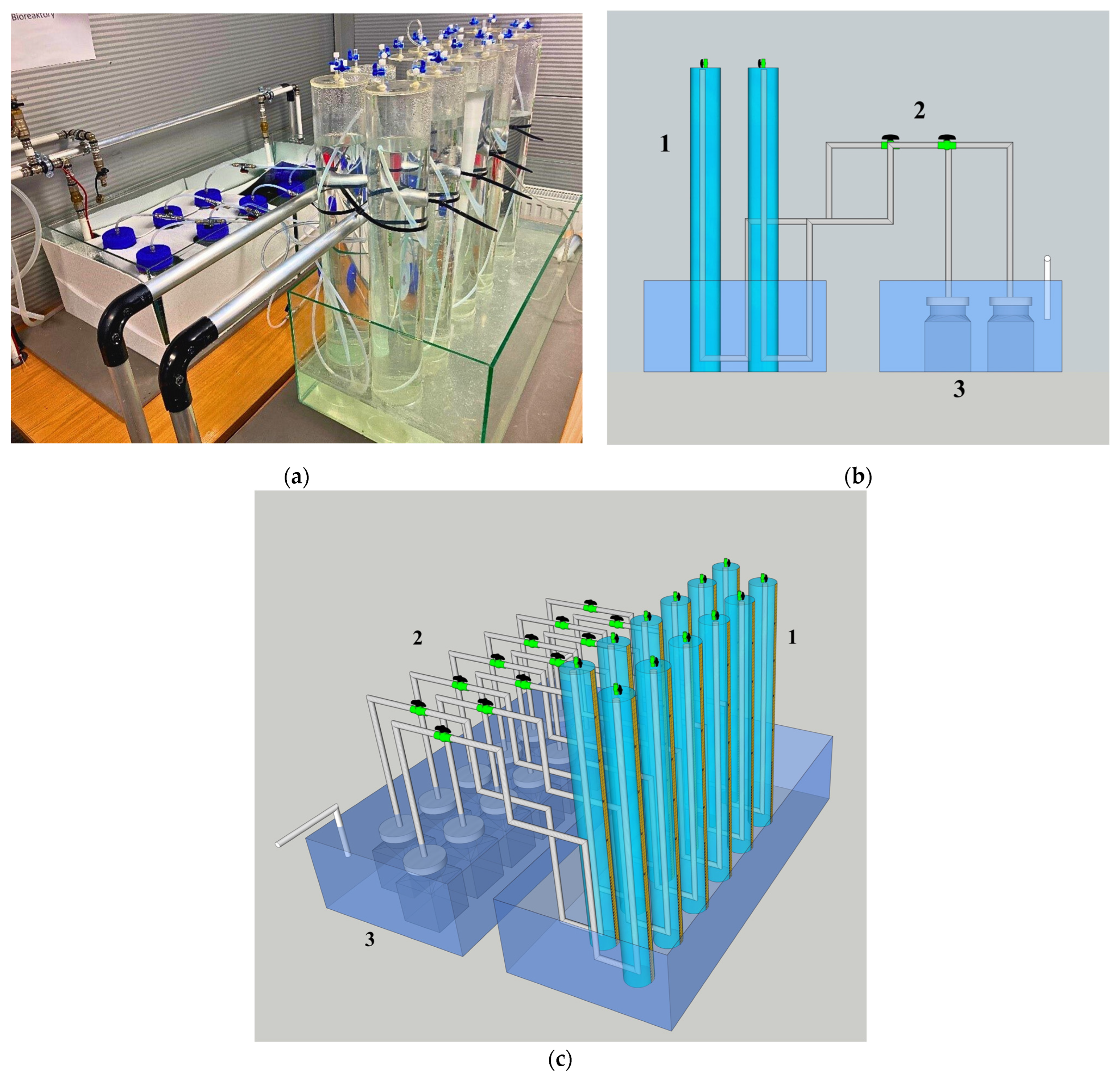

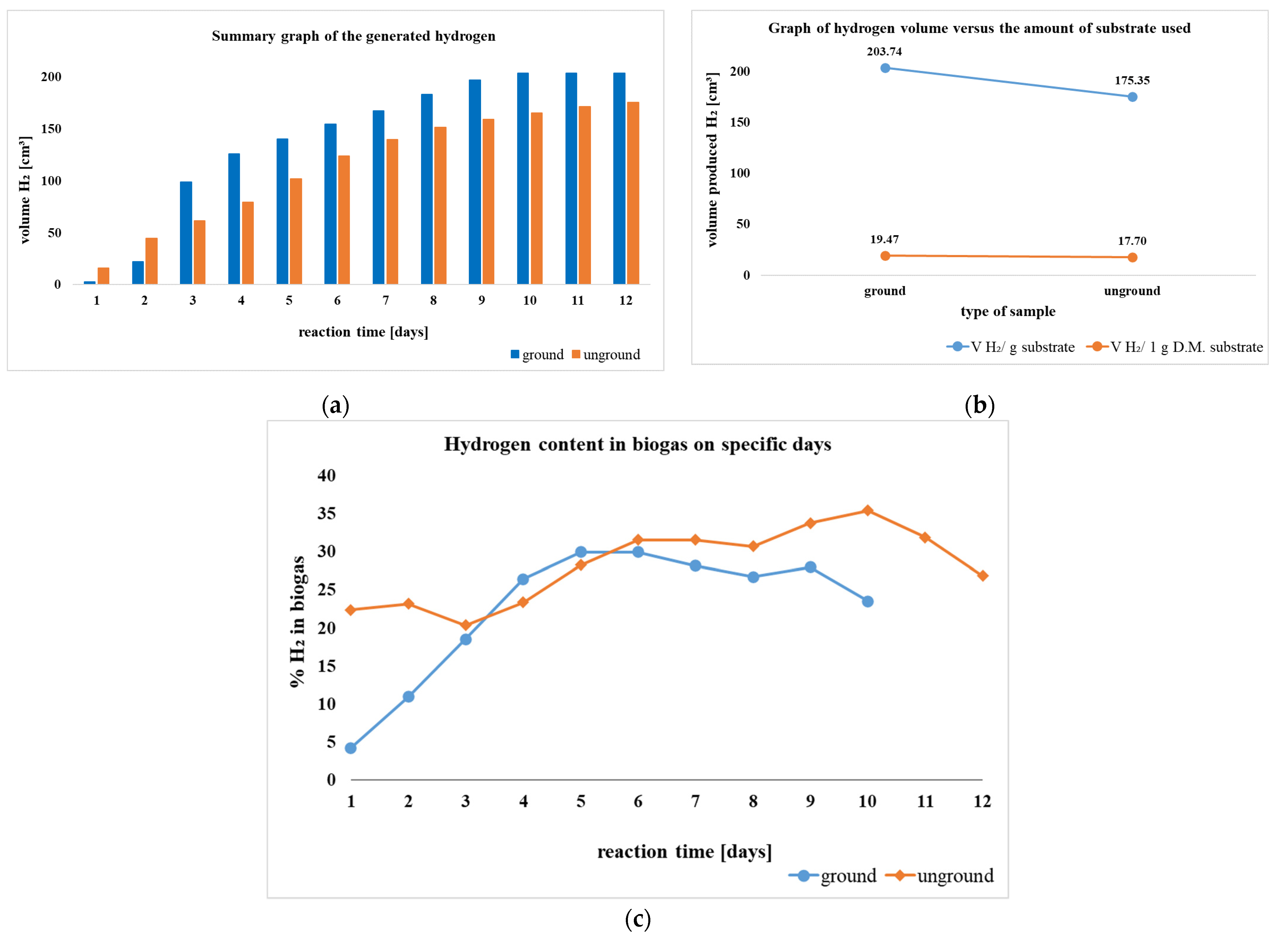
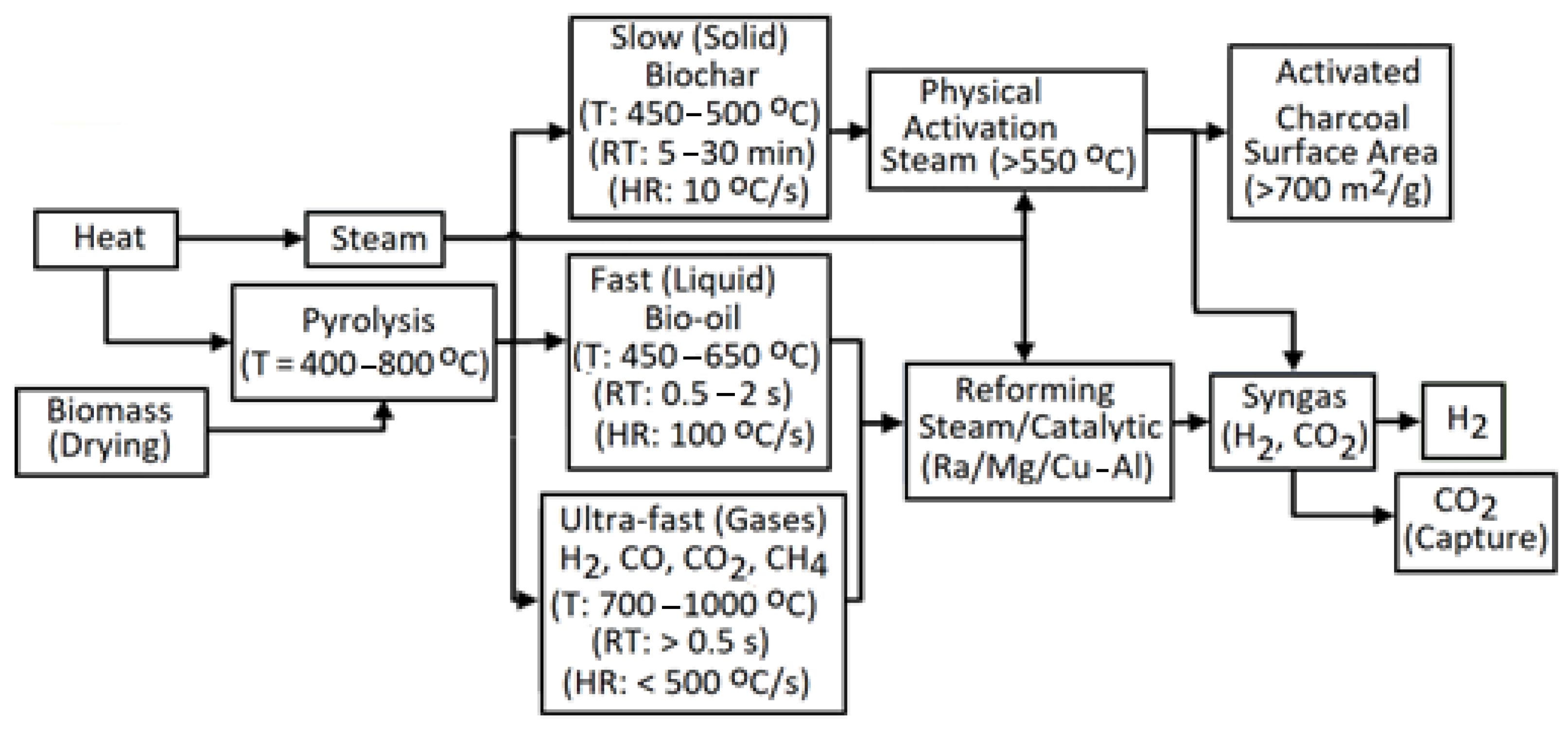
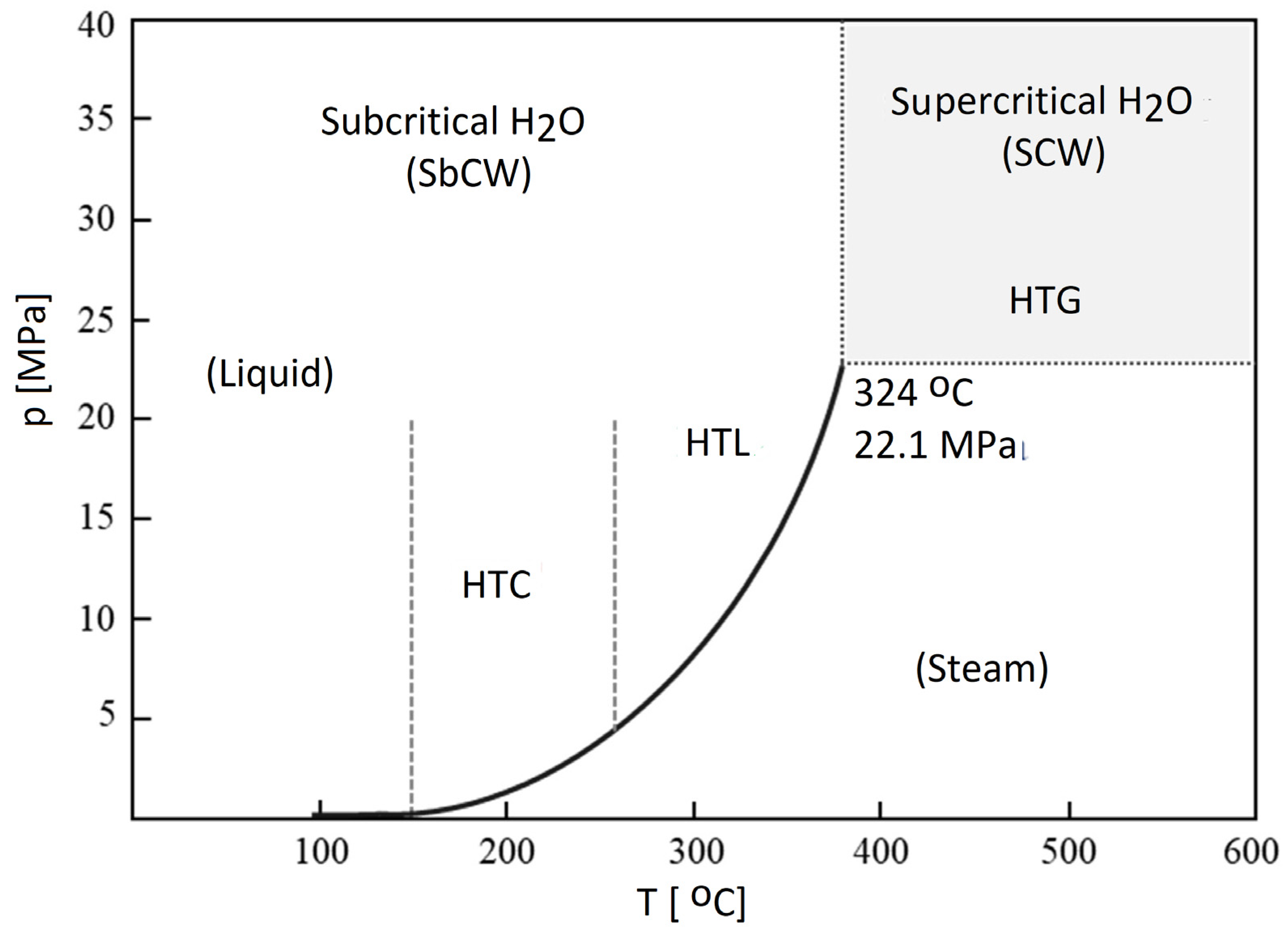
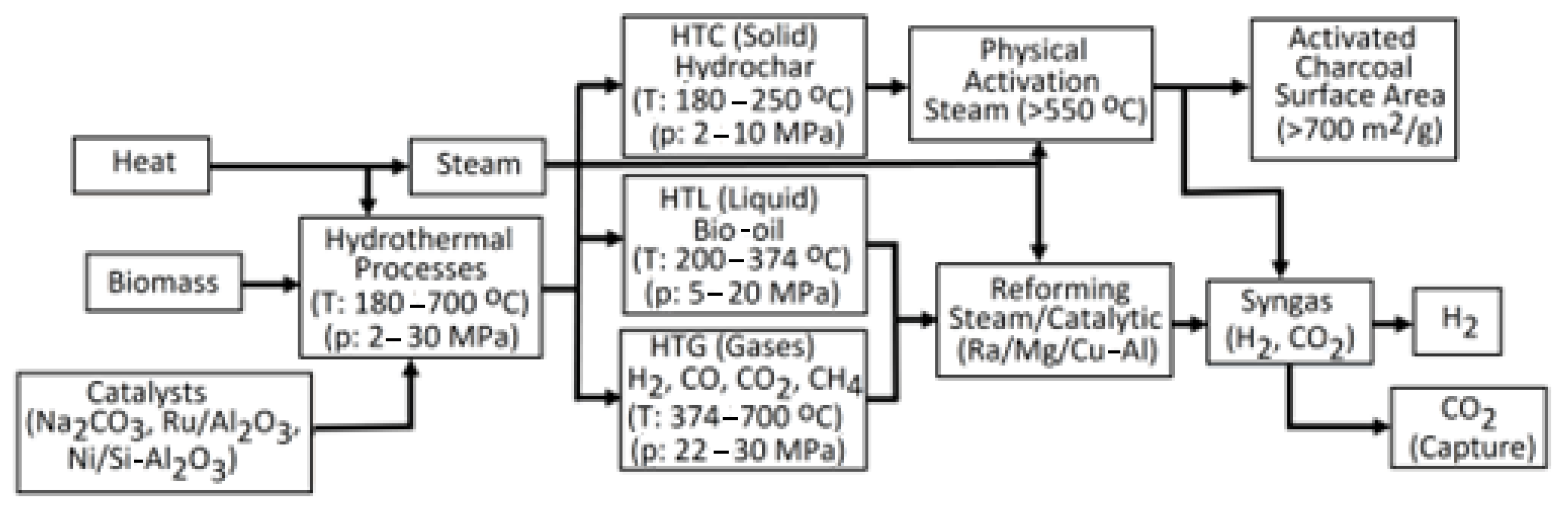
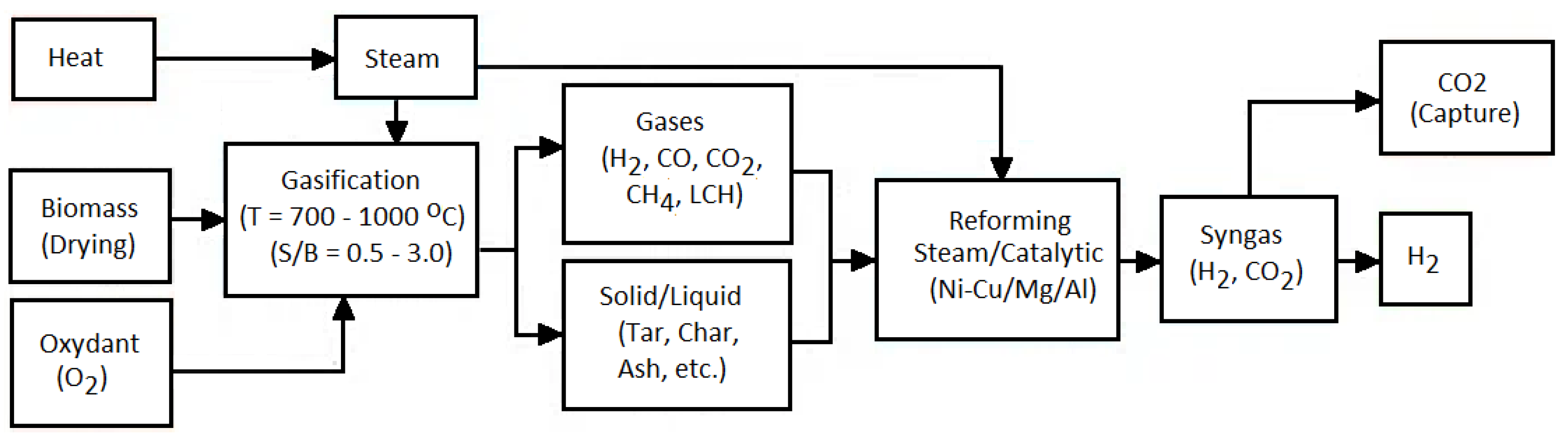
| Methods | Pros | Cons |
|---|---|---|
| DbP | High efficacy, renewable, and sustainable | O2 sensitivity of H2ase, low catalyst availability |
| i-DbP | Separation of H2 and O2 generation, utilization of both H2ase and N2ase | Low efficacy, environmental sensitivity |
| PF | Uses a wide range of organic substrates, sustainable | Great energy expense, O2 sensitivity of N2ase |
| DF | Endless generation, wide substrate availability, cheap | Low substrate conversion efficacy, occurrence of H2 consumers, volatile fatty acid drainage |
| IS | Increased biomass conversion | Capital-intensive, needs specialized framework |
| Oxidizing Agent in GY Technique | O2 | Air | Steam |
|---|---|---|---|
| Products | CO, H2, LHC (CH4, C2H4), CO2 | N2, CO, H2, CO2, LHC (CH4, C2H4), H2O | H2, CO, CO2, LHC (CH4, C2H4) |
| Tar [g/kg] | 2.2–46 | 3.7–61.9 | 60–95 |
| Average H2/(steam–O2 mixture) ratio | 40% | 15% | 40% |
| H2/CO ratio | 1 | 0.75 | 1.6 |
| Heating Value [MJ/Nm3] | 12–28 | 4–7 | 10–18 |
| Pros |
|
|
|
| Cons |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nieścioruk, M.J.; Bandrow, P.; Szufa, S.; Woźniak, M.; Siczek, K. Biomass-Based Hydrogen Extraction and Accompanying Hazards—Review. Molecules 2025, 30, 565. https://doi.org/10.3390/molecules30030565
Nieścioruk MJ, Bandrow P, Szufa S, Woźniak M, Siczek K. Biomass-Based Hydrogen Extraction and Accompanying Hazards—Review. Molecules. 2025; 30(3):565. https://doi.org/10.3390/molecules30030565
Chicago/Turabian StyleNieścioruk, Mariusz J., Paulina Bandrow, Szymon Szufa, Marek Woźniak, and Krzysztof Siczek. 2025. "Biomass-Based Hydrogen Extraction and Accompanying Hazards—Review" Molecules 30, no. 3: 565. https://doi.org/10.3390/molecules30030565
APA StyleNieścioruk, M. J., Bandrow, P., Szufa, S., Woźniak, M., & Siczek, K. (2025). Biomass-Based Hydrogen Extraction and Accompanying Hazards—Review. Molecules, 30(3), 565. https://doi.org/10.3390/molecules30030565







