Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy
Abstract
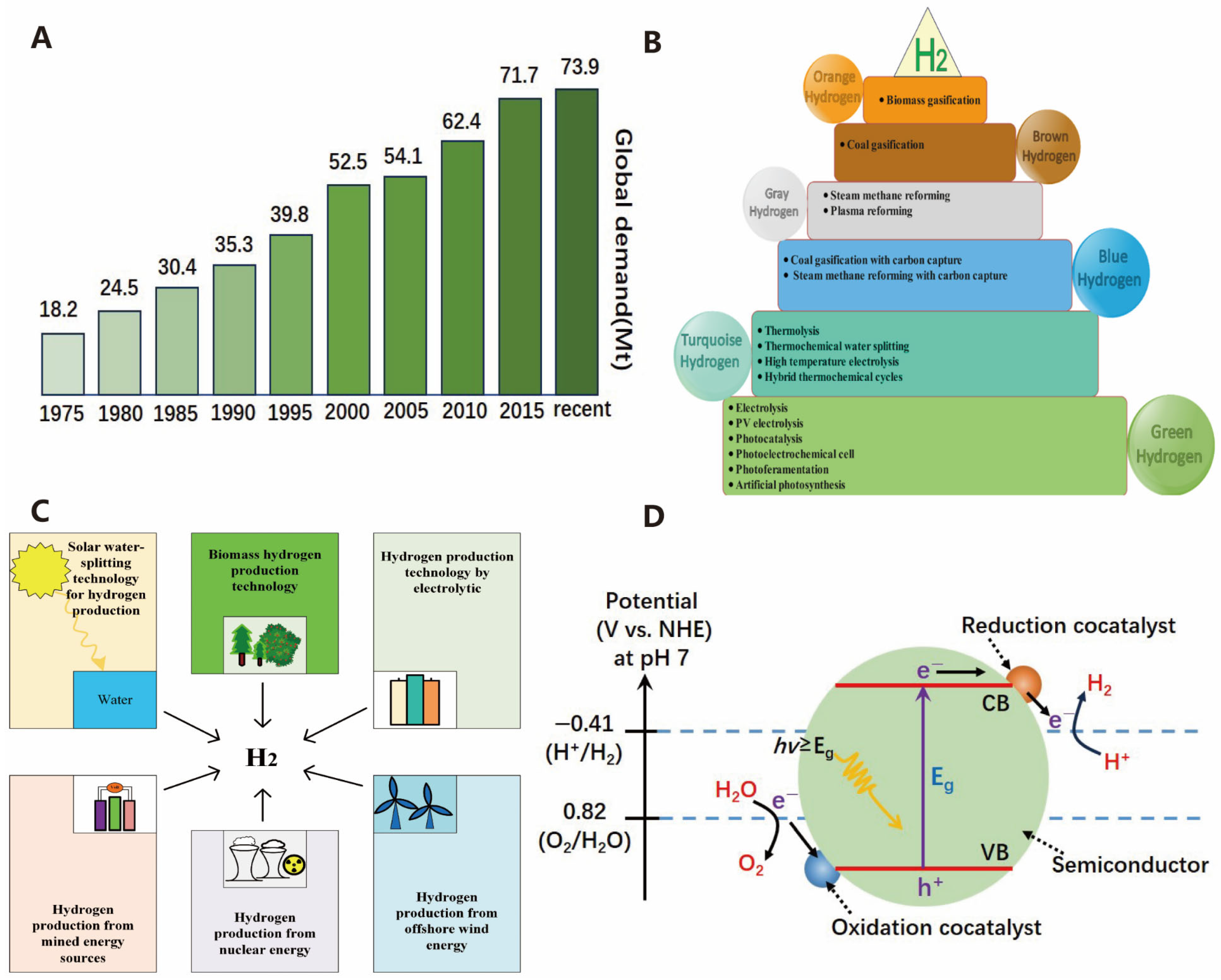
1. Introduction
2. Mechanism
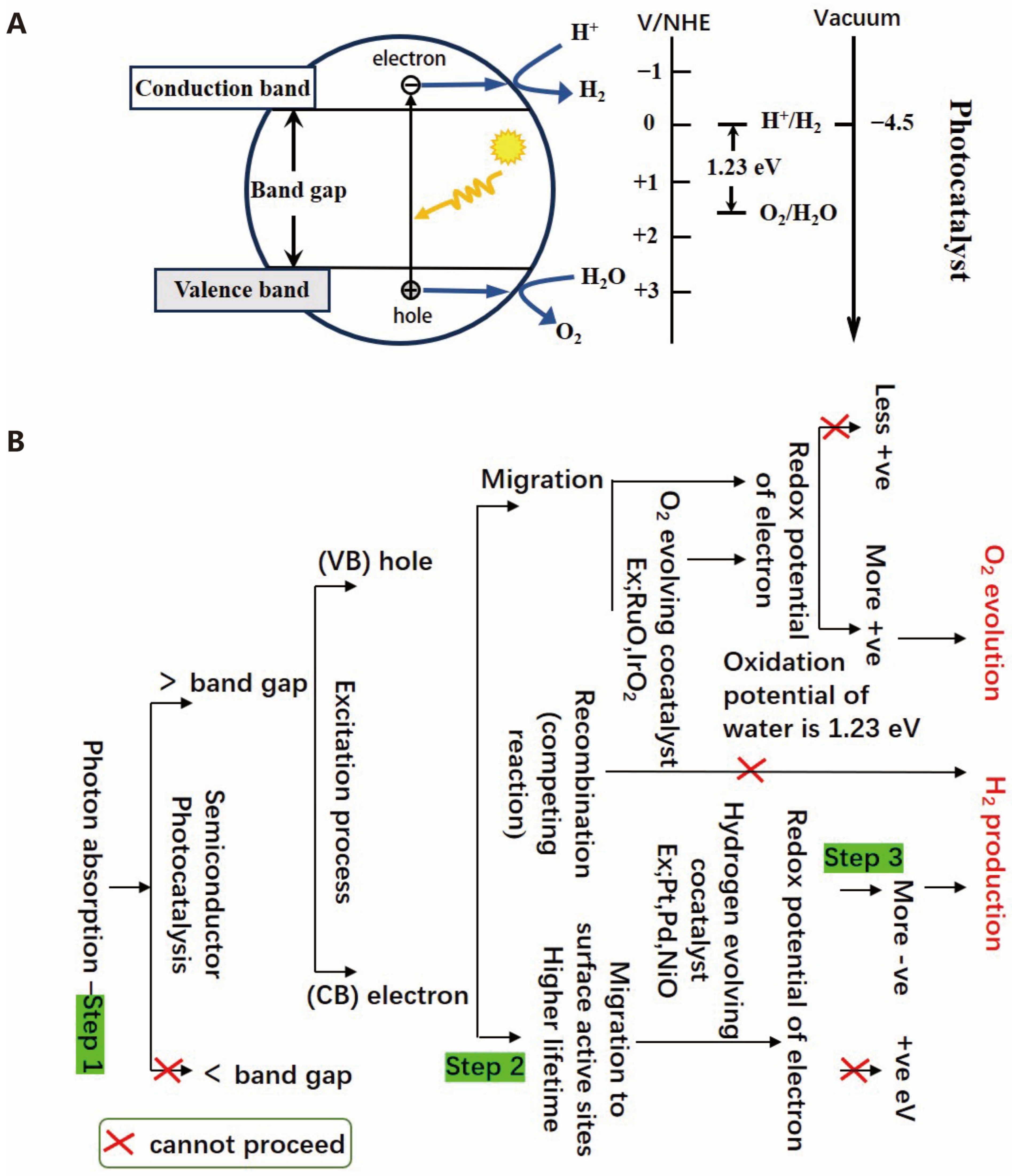
2.1. Photocatalysis
2.1.1. Mechanism of Photocatalytic Reaction
2.1.2. Steps of Photocatalytic Reaction
2.2. Electrocatalysis
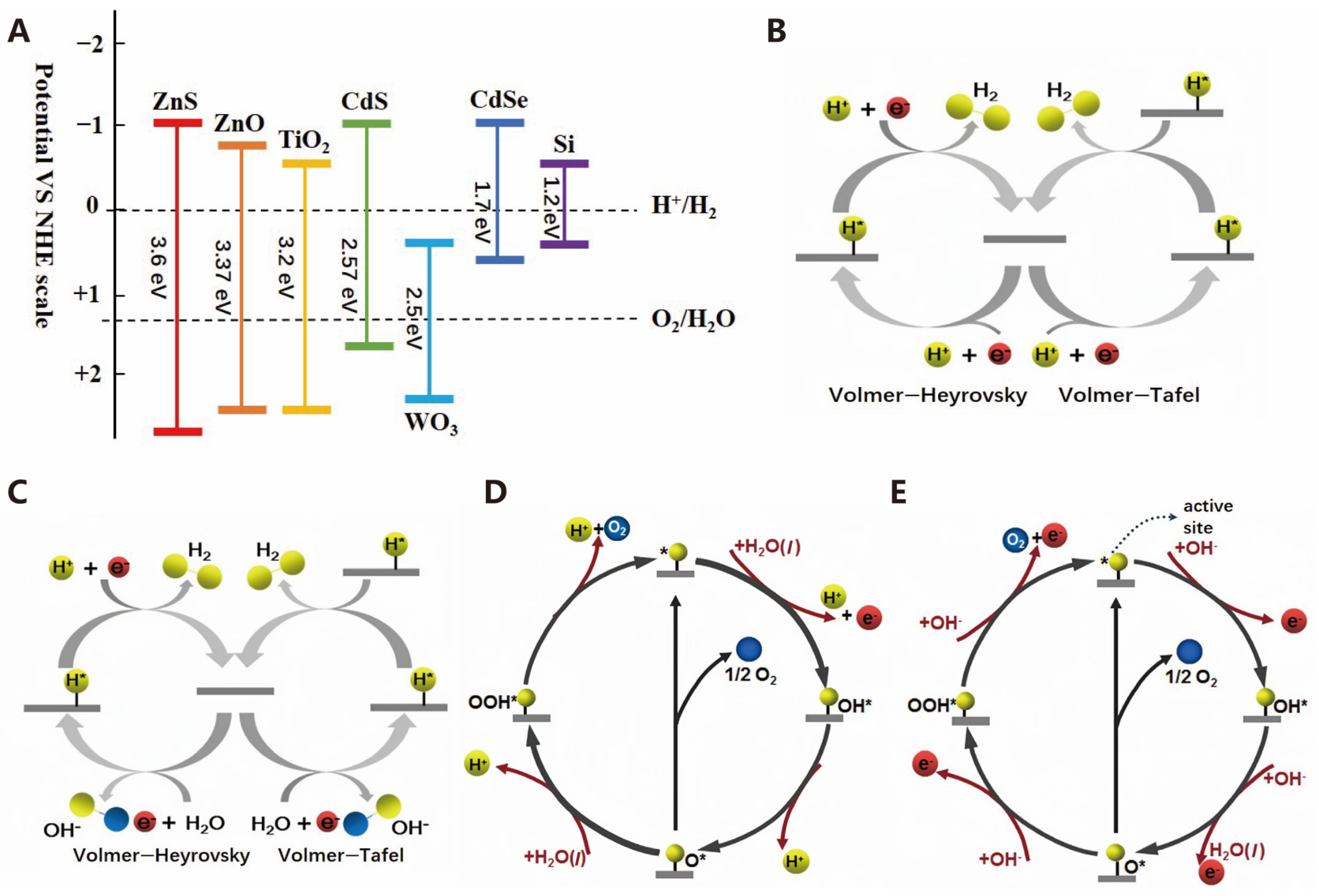
2.2.1. Mechanism of Electrocatalytic Hydrogen Evolution Reaction
2.2.2. Mechanism of Electrocatalytic Oxygen Evolution Reaction
3. Classification of Photo(electro)catalytic Materials
3.1. Photocatalysis
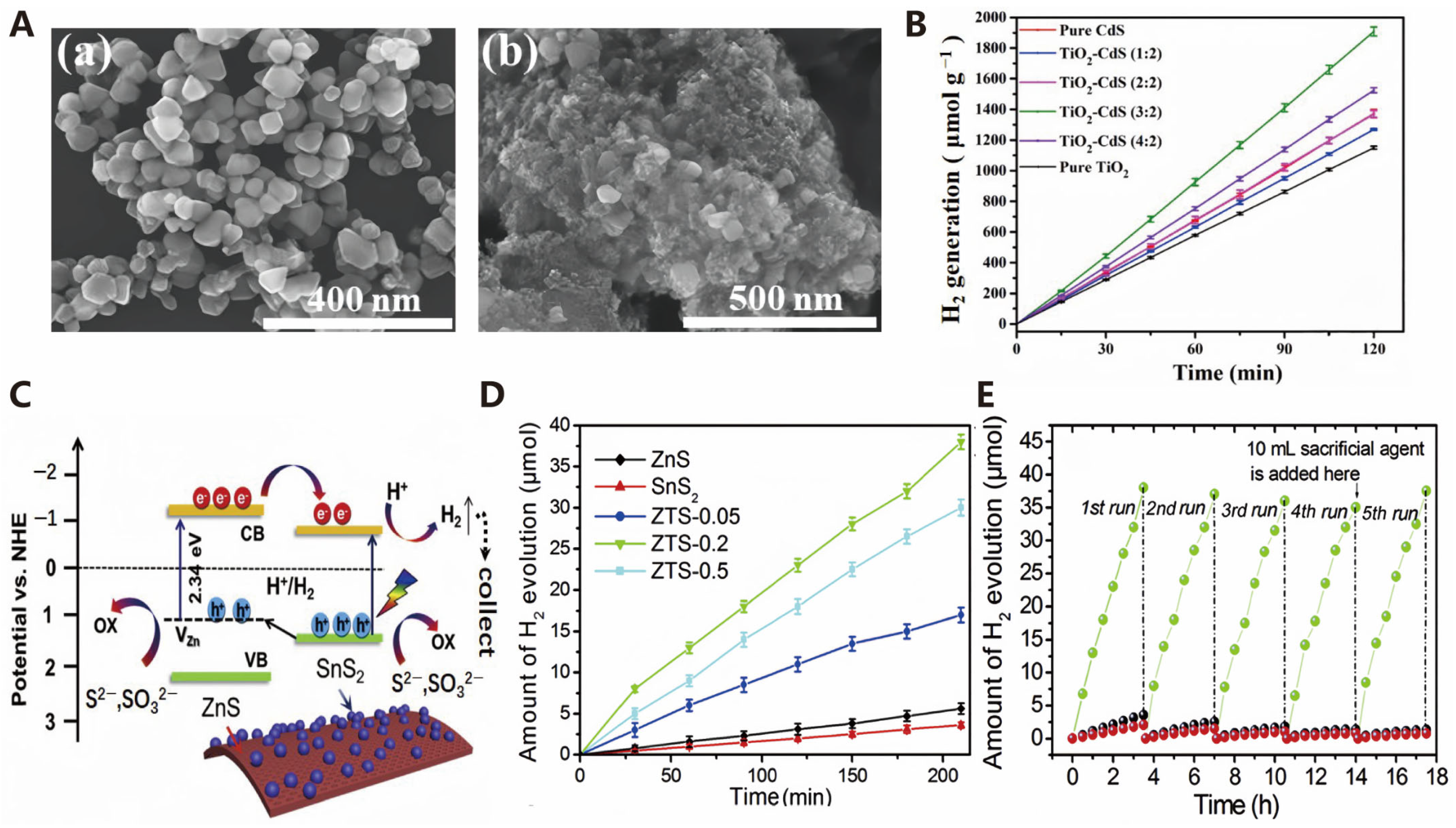
3.1.1. Oxides
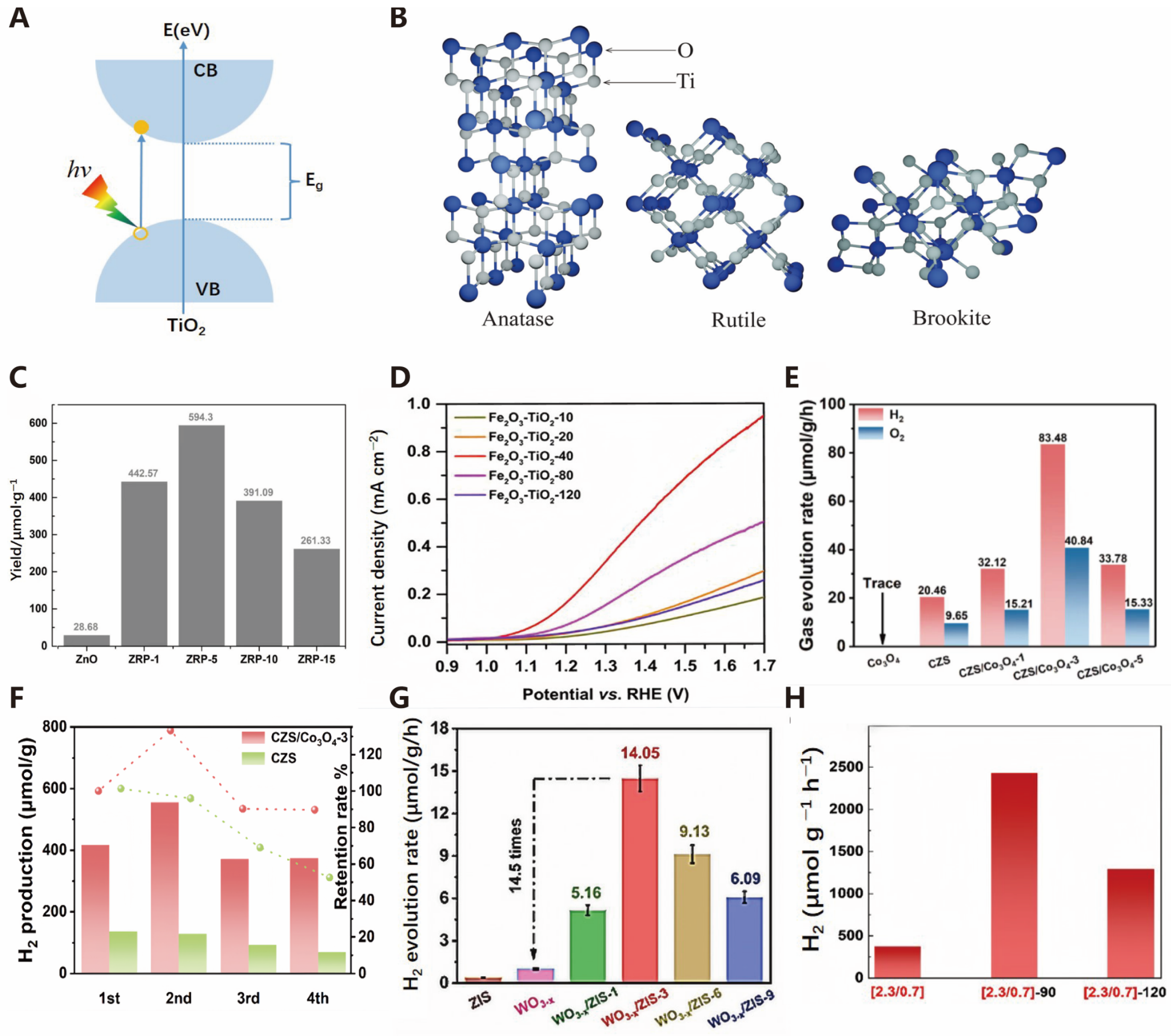
3.1.2. Sulfides
3.1.3. Nitrides
3.1.4. Novel Photocatalyst
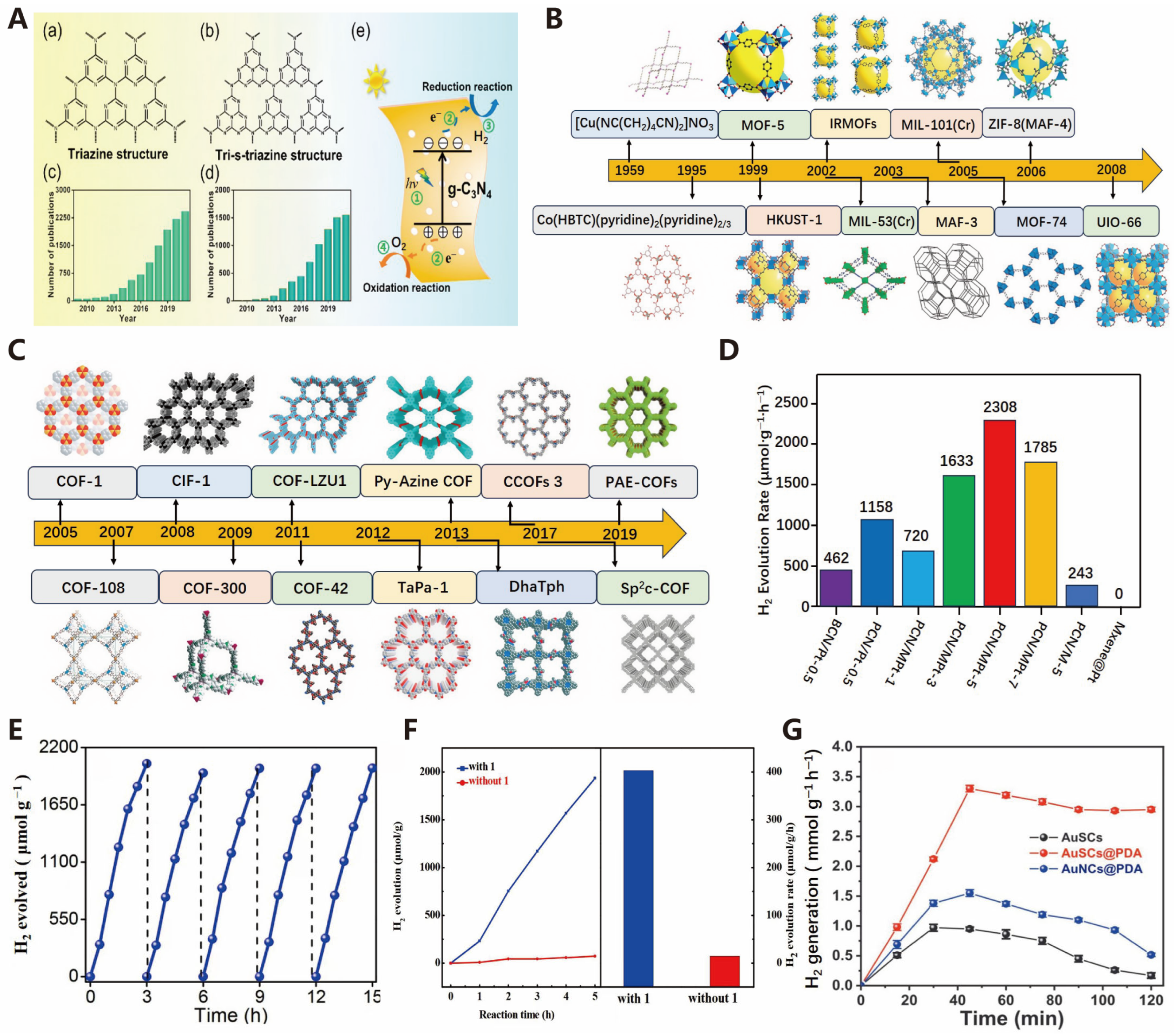
3.2. Electrocatalysis
3.2.1. Noble Metals and Their Composites
3.2.2. Non-Noble Metal Catalyst
3.2.3. Novel Electrocatalyst
4. Design and Optimization of Photo(electro)catalysis
4.1. Catalyst Design and Optimization
4.1.1. Material Structure
Crystal Facet Adjustment
Dimension Change
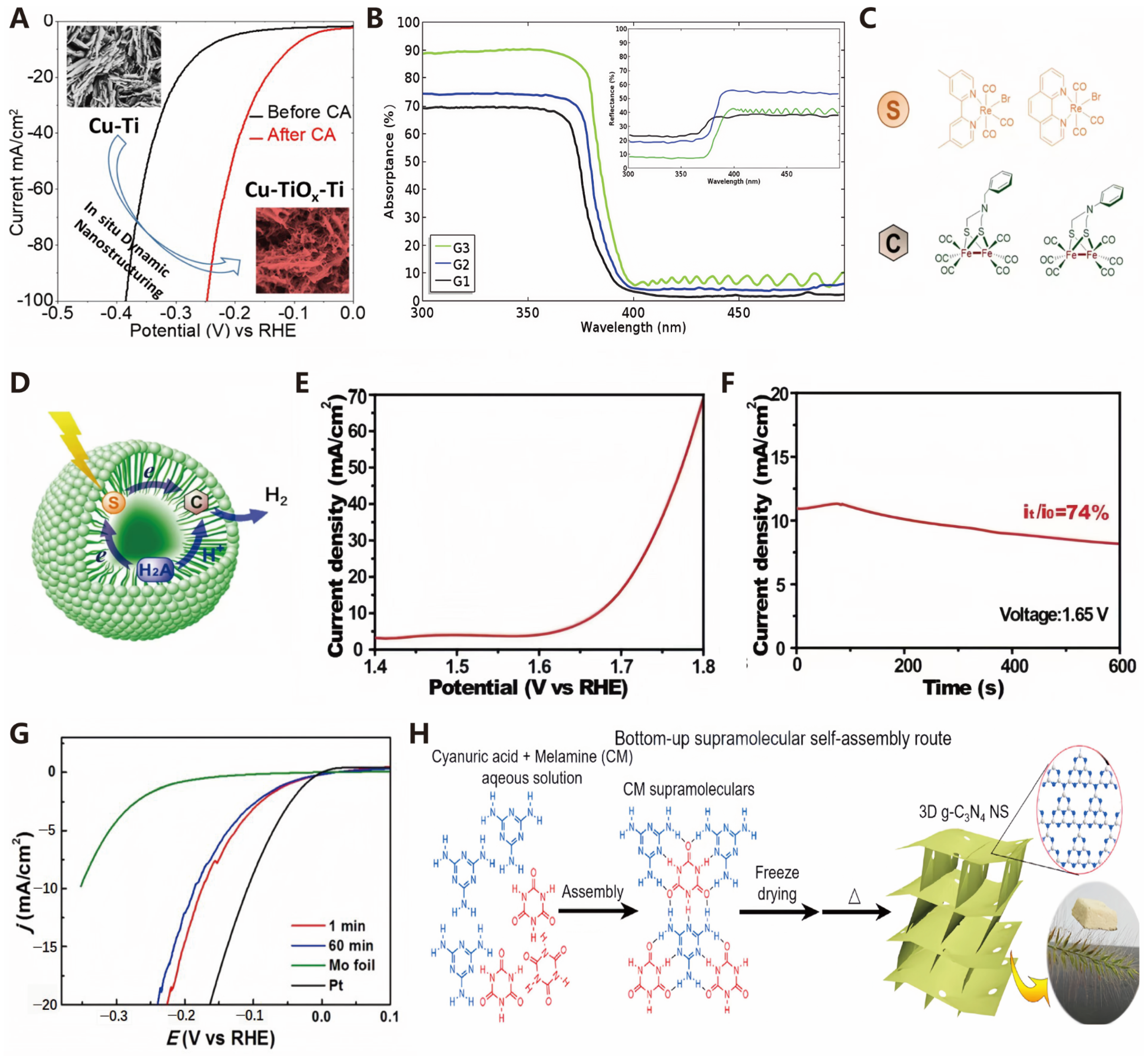
Defect Engineering
4.1.2. Composition Optimization
Doping
Alloying

4.1.3. Surface Modification
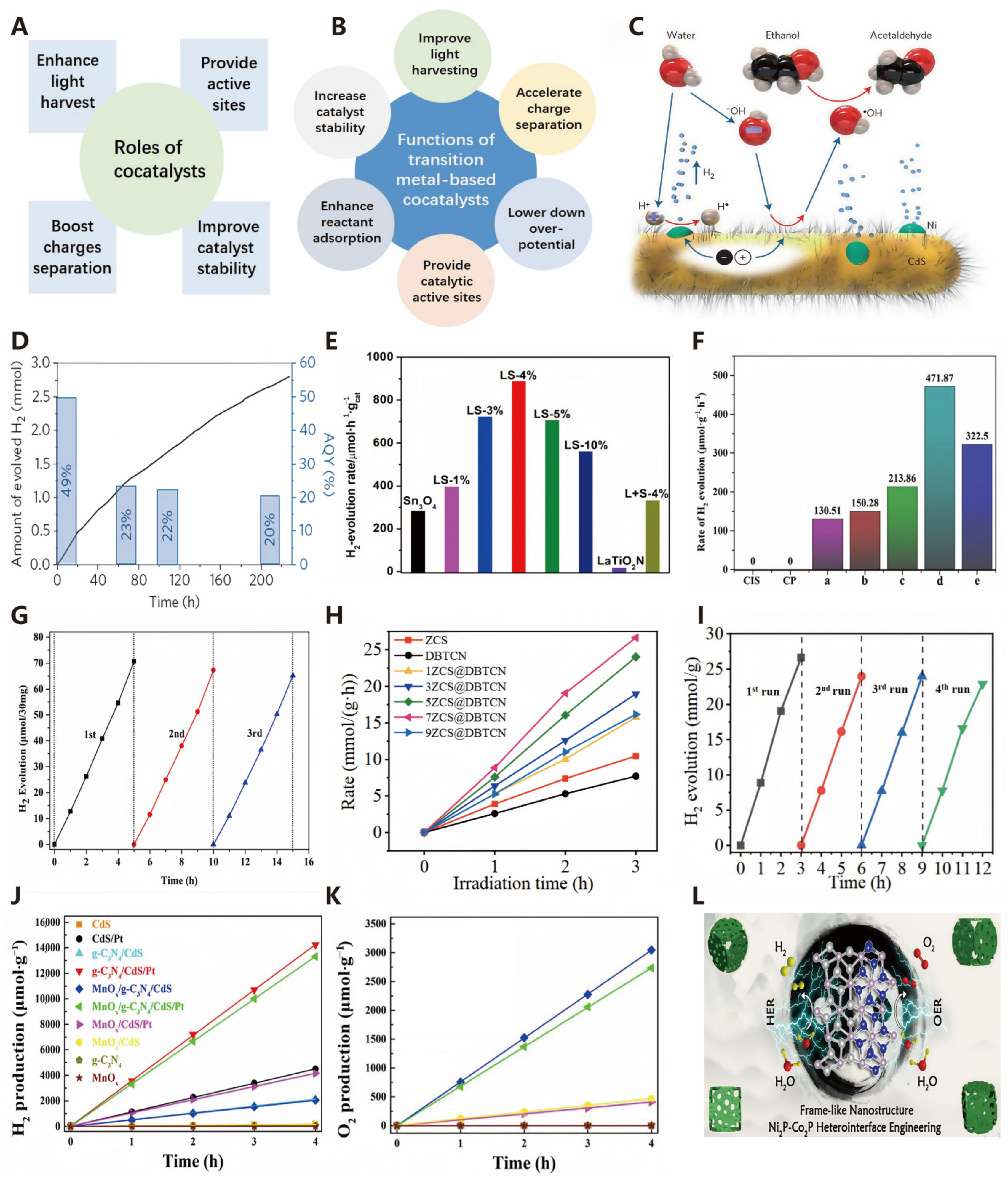
Co-Catalyst Decoration
Heterojunction Construction
| Photocatalysts | Light Source | H2 generation Rate (μmol g−1h−1) | Ref. |
|---|---|---|---|
| O-doped CN/TiO2 | λ > 400 nm | 587 | [200] |
| CoTiO3/CN | λ > 420 nm | 858 | [201] |
| FeOx/CN | 780 > λ > 420 nm | 1080 | [202] |
| S-CN/CN | λ > 420 nm | 4765 | [203] |
| CN/CoO | λ > 400 nm | 651 | [204] |
| p-n homojunction CN | λ > 400 nm | 4020 | [205] |
| O-CN/CN | λ > 420 nm | 6970 | [192] |
4.2. Improvement of Photo(electro)catalysis System
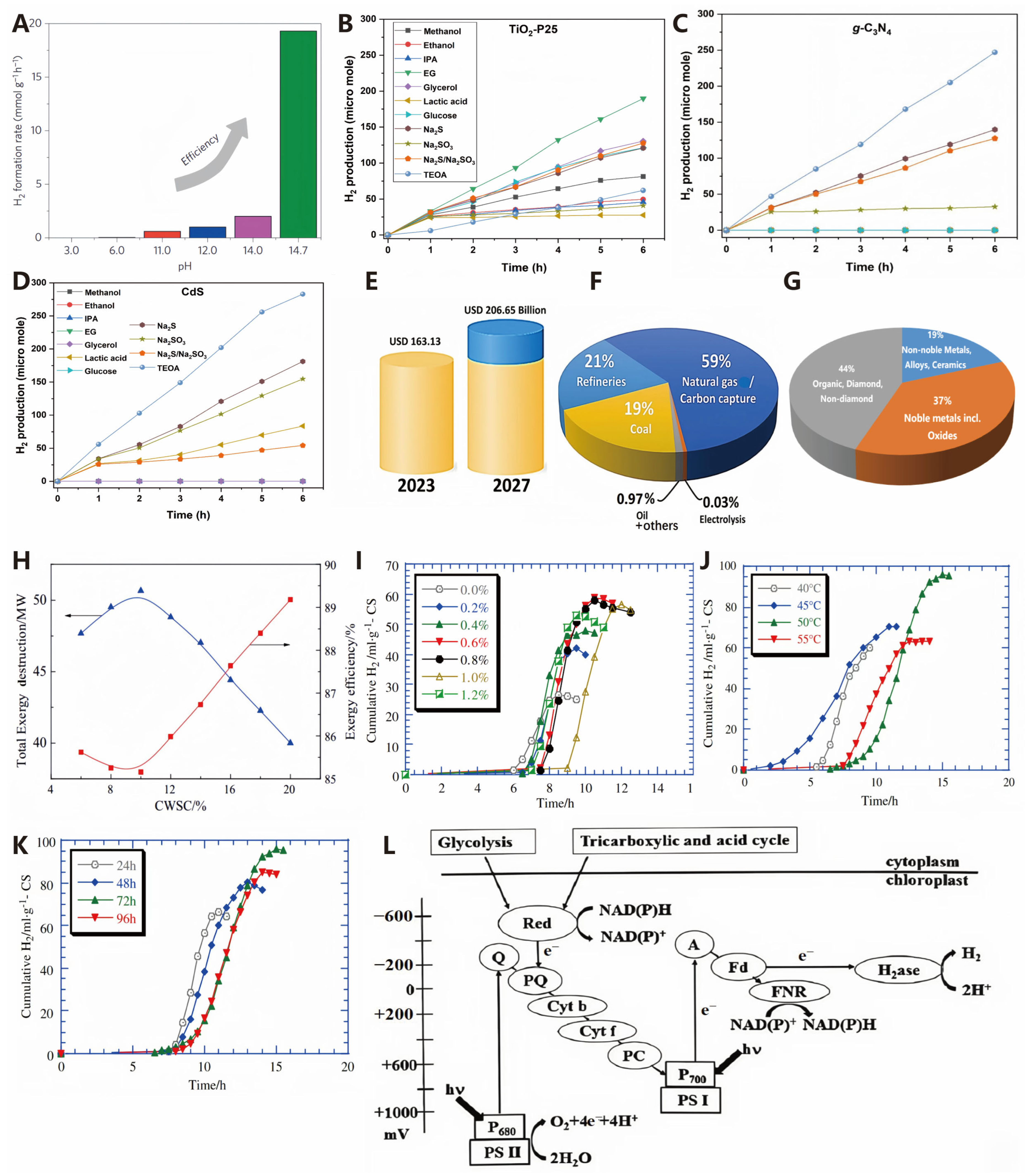
4.2.1. pH of Reaction System
4.2.2. Addition of Sacrificial Agents
4.2.3. Synergistic Effects Between Catalyst and Electrode
5. Economics of Hydrogen Production Technologies
5.1. Water Splitting for Hydrogen Production
5.1.1. Photocatalytic Hydrogen Production
5.1.2. Electrocatalytic Hydrogen Production
5.2. Hydrogen Production from Fossil Fuels
5.3. Hydrogen Production from Bioenergy
6. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Alharthi, M.; Zhang, J.; Khan, I. The Need for Energy Efficiency and Economic Prosperity in a Sustainable Environment. Gondwana Res. 2024, 127, 22–35. [Google Scholar] [CrossRef]
- Sikiru, S.; Oladosu, T.L.; Amosa, T.I.; Olutoki, J.O.; Ansari, M.N.M.; Abioye, K.J.; Rehman, Z.U.; Soleimani, H. Hydrogen-Powered Horizons: Transformative Technologies in Clean Energy Generation, Distribution, and Storage for Sustainable Innovation. Int. J. Hydrogen Energy 2024, 56, 1152–1182. [Google Scholar] [CrossRef]
- Sohail, M.; Rauf, S.; Irfan, M.; Hayat, A.; Alghamdi, M.M.; El-Zahhar, A.A.; Ghernaout, D.; Al-Hadeethi, Y.; Lv, W. Recent Developments, Advances and Strategies in Heterogeneous Photocatalysts for Water Splitting. Nanoscale Adv. 2024, 6, 1286–1330. [Google Scholar] [CrossRef]
- Azizimehr, B.; Armaghani, T.; Ghasemiasl, R.; Kaabi Nejadian, A.; Javadi, M.A. A Comprehensive Review of Recent Developments in Hydrogen Production Methods Using a New Parameter. Int. J. Hydrogen Energy 2024, 72, 716–729. [Google Scholar] [CrossRef]
- Afanasev, P.; Askarova, A.; Alekhina, T.; Popov, E.; Markovic, S.; Mukhametdinova, A.; Cheremisin, A.; Mukhina, E. An Overview of Hydrogen Production Methods: Focus on Hydrocarbon Feedstock. Int. J. Hydrogen Energy 2024, 78, 805–828. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.-J.; Thorat, H.N.; Bodkhe, G.A.; Ramanavicius, A.; Ramanavicius, S.; Shirsat, M.D.; Ha, T.-J. Advanced Energy Materials: Current Trends and Challenges in Electro- and Photo-Catalysts for H2O Splitting. J. Ind. Eng. Chem. 2023, 119, 90–111. [Google Scholar] [CrossRef]
- Tuluhong, A.; Chang, Q.; Xie, L.; Xu, Z.; Song, T. Current Status of Green Hydrogen Production Technology: A Review. Sustainability 2024, 16, 9070. [Google Scholar] [CrossRef]
- Chen, S.; Qi, Y.; Li, C.; Domen, K.; Zhang, F. Surface Strategies for Particulate Photocatalysts toward Artificial Photosynthesis. Joule 2018, 2, 2260–2288. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Yin, Z.; Cao, S. Recent Advances in Synthesis and Applications of Carbon-Doped TiO2 Nanomaterials. Catalysts 2020, 10, 1431. [Google Scholar] [CrossRef]
- Shiraz, H.G.; Crispin, X.; Berggren, M. Transition metal sulfides for electrochemical hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 24060–24077. [Google Scholar] [CrossRef]
- Salehabadi, A.; Zanganeh, J.; Moghtaderi, B. Mixed metal oxides in catalytic ammonia cracking process for green hydrogen production: A review. Int. J. Hydrogen Energy 2024, 63, 828–843. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yang, L.; Lan, T.; Wang, H.; Hu, C.; Han, X.; Liu, Q.; Chen, J.; Feng, Z.; et al. Porous Framework Materials for Energy & Environment Relevant Applications: A Systematic Review. Green Energy Environ. 2024, 9, 217–310. [Google Scholar]
- Gnanasekaran, L.; Rajendran, S.; Karimi-Maleh, H.; Priya, A.K.; Qin, J.; Soto-Moscoso, M.; Ansar, S.; Bathula, C. Surface Modification of TiO2 by Adding V2O5 Nanocatalytic System for Hydrogen Generation. Chem. Eng. Res. Des. 2022, 182, 114–119. [Google Scholar] [CrossRef]
- Zhang, N.; Li, H.; Yu, K.; Zhu, Z. Differently Structured MoS2 for the Hydrogen Production Application and a Mechanism Investigation. J. Alloys Compd. 2016, 685, 65–69. [Google Scholar] [CrossRef]
- Wu, H.; Huang, Q.; Shi, Y.; Chang, J.; Lu, S. Electrocatalytic Water Splitting: Mechanism and Electrocatalyst Design. Nano Res. 2023, 16, 9142–9157. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of Hydrogen Energy and Recent Progress in Electrocatalytic Water Splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liu, J.; Wu, H.; Wu, K.; Lyu, C.; Wu, J.; Lau, W.-M.; Wu, Q.; Zheng, J. The Role of Manganese-Based Catalyst in Electrocatalytic Water Splitting: Recent Research and Progress. Mater. Today Phys. 2023, 36, 101169. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing Single-Atom Catalysts toward Improved Alkaline Hydrogen Evolution Reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Zhou, W.; Zhu, Z.; Su, C.; Liu, M.; Shao, Z. A Perovskite Electrocatalyst for Efficient Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6442–6448. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V.; Horikoshi, S.; Kuznetsov, V.N.; Ryabchuk, V.K. On the Genesis of Heterogeneous Photocatalysis: A Brief Historical Perspective in the Period 1910 to the Mid-1980s. Photochem. Photobiol. Sci. 2012, 11, 1121–1150. [Google Scholar] [CrossRef]
- Wang, Z.; Hisatomi, T.; Li, R.; Sayama, K.; Liu, G.; Domen, K.; Li, C.; Wang, L. Efficiency Accreditation and Testing Protocols for Particulate Photocatalysts toward Solar Fuel Production. Joule 2021, 5, 344–359. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [PubMed]
- Rajaambal, S.; Sivaranjani, K.; Gopinath, C.S. Recent Developments in Solar H2 Generation from Water Splitting. J. Chem. Sci. 2015, 127, 33–47. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Takata, T.; Hara, M.; Saito, N.; Toda, K.; Inoue, Y.; Kobayashi, H.; Domen, K. Overall water splitting on (Ga1-xZnx) (N1-xOx) solid solution photocatalyst: Relationship between physical properties and photocatalytic activity. J. Phys. Chem. B 2005, 109, 20504–20510. [Google Scholar] [CrossRef]
- Takanabe, K. Addressing Fundamental Experimental Aspects of Photocatalysis Studies. J. Catal. 2019, 370, 480–484. [Google Scholar] [CrossRef]
- Van Troostwijk, A.P.; Deiman, J.R.; Lettre à, M. De la Mètherie, sur une manière de dècompose l’eau en air inflammable et en air vital. J. Phys. Chim. L’histoire Nat. 1789, 35, 369–378. [Google Scholar]
- Muellerlanger, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S. Techno-Economic Assessment of Hydrogen Production Processes for the Hydrogen Economy for the Short and Medium Term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Ci, L.; Wang, C.; Ajayan, P.M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 2010, 48, 1124–1130. [Google Scholar] [CrossRef]
- Conway, B.E.; Tilak, B.V. Interfacial Processes Involving Electrocatalytic Evolution and Oxidation of H2, and the Role of Chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. [Google Scholar] [CrossRef]
- Su, P.; Pei, W.; Wang, X.; Ma, Y.; Jiang, Q.; Liang, J.; Zhou, S.; Zhao, J.; Liu, J.; Lu, G.Q. (Max) Exceptional Electrochemical HER Performance with Enhanced Electron Transfer between Ru Nanoparticles and Single Atoms Dispersed on a Carbon Substrate. Angew. Chem. Int. Ed. 2021, 60, 16044–16050. [Google Scholar] [CrossRef]
- Sun, Y.; Li, R.; Chen, X.; Wu, J.; Xie, Y.; Wang, X.; Ma, K.; Wang, L.; Zhang, Z.; Liao, Q.; et al. A-Site Management Prompts the Dynamic Reconstructed Active Phase of Perovskite Oxide OER Catalysts. Adv. Energy Mater. 2021, 11, 2003755. [Google Scholar] [CrossRef]
- Joo, J.; Kim, T.; Lee, J.; Choi, S.; Lee, K. Morphology-Controlled Metal Sulfides and Phosphides for Electrochemical Water Splitting. Adv. Mater. 2019, 31, 1806682. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Litter, M.I.; Navio, J.A. Photocatalytic properties of iron-doped titania semiconductors. J. Photochem. Photobiol. A Chem. 1996, 98, 171–181. [Google Scholar] [CrossRef]
- Zhang, P.; Yu, L.; Lou, X.W. (David) Construction of Heterostructured Fe2O3–TiO2 Microdumbbells for Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 15076–15080. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.Q.; Pan, W.G.; Zhu, Q.Z.; Wu, J.; Guan, Z.Z. Research progress on photocatalytic hydrogen production catalysts. Appl. Chem. Ind. 2024, 53, 228–232+237. [Google Scholar]
- Chen, J.; Huang, S.; Long, Y.; Wu, J.; Li, H.; Li, Z.; Zeng, Y.-J.; Ruan, S. Fabrication of ZnO/Red Phosphorus Heterostructure for Effective Photocatalytic H2 Evolution from Water Splitting. Nanomaterials 2018, 8, 835. [Google Scholar] [CrossRef]
- Lin, Z.; Du, C.; Yan, B.; Yang, G. Amorphous Fe2O3 for Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2019, 9, 5582–5592. [Google Scholar] [CrossRef]
- Peiris, S.; De Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Yu, K.; Zhang, T.; Wang, Y.; Wu, J.; Huang, H.; Yin, K.; Liao, F.; Liu, Y.; Kang, Z. Anchoring Co3O4 on CdZnS to accelerate hole migration for highly stable photocatalytic overall water splitting. Appl. Catal. B Environ. 2023, 324, 122228. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Sun, K.; Tan, L.; Guo, F.; Du, X.; Shi, W. Plasmonic coupling-boosted photothermal composite photocatalyst for achieving near-infrared photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 661, 12–22. [Google Scholar] [CrossRef]
- Deligiannakis, Y.; Mantzanis, A.; Zindrou, A.; Smykala, S.; Solakidou, M. Control of Monomeric Vo’s versus Vo Clusters in ZrO2−x for Solar-Light H2 Production from H2O at High-Yield (millimoles gr−1 h−1). Sci. Rep. 2022, 12, 15132. [Google Scholar] [CrossRef]
- Xin, X.; Li, Y.; Zhang, Y.; Wang, Y.; Chi, X.; Wei, Y.; Diao, C.; Su, J.; Wang, R.; Guo, P.; et al. Large electronegativity differences between adjacent atomic sites activate and stabilize ZnIn2S4 for efficient photocatalytic overall water splitting. Nat. Commun. 2024, 15, 337. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. Photochemical splitting of water for hydrogen production by photocatalysis: A review. Sol. Energy Mater. Sol. Cells 2014, 128, 85–101. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from Photo-Catalytic Water Splitting Process: A Review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Zubair, M.; Svenum, I.-H.; Ronning, M.; Yang, J. Facile synthesis approach for core-shell TiO2-CdS nanoparticles for enhanced photocatalytic H2 generation from water. Catal. Today 2019, 328, 15–20. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Liang, B.; Liu, Z.; Chen, D.; Shen, G. ZnS Nanostructures: Synthesis, Properties, and Applications. Crit. Rev. Solid State Mater. Sci. 2013, 38, 57–90. [Google Scholar] [CrossRef]
- Wang, L.; Jin, G.; Shi, Y.; Zhang, H.; Xie, H.; Yang, B.; Sun, H. Co-catalyst-free ZnS-SnS2 porous nanosheets for clean and recyclable photocatalytic H2 generation. J. Alloys Compd. 2018, 753, 60–67. [Google Scholar] [CrossRef]
- Kato, H.; Asakura, K.; Kudo, A. Highly Efficient Water Splitting into H2 and O2 over Lanthanum-Doped NaTaO3 Photocatalysts with High Crystallinity and Surface Nanostructure. J. Am. Chem. Soc. 2003, 125, 3082–3089. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO Solid Solution as a Photocatalyst for Visible-Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef]
- Ahmed, M.; Xinxin, G. A Review of Metal Oxynitrides for Photocatalysis. Inorg. Chem. Front. 2016, 3, 578–590. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst Releasing Hydrogen from Water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Kubota, J.; Domen, K. Photocatalytic water splitting using oxynitride and nitride semiconductor powders for production of solar hydrogen. Electrochem. Soc. Interface 2013, 22, 57. [Google Scholar] [CrossRef]
- Chen, W.; Chu, M.; Gao, L.; Mao, L.; Yuan, J.; Shangguan, W. Ni(OH)2 Loaded on TaON for Enhancing Photocatalytic Water Splitting Activity under Visible Light Irradiation. Appl. Surf. Sci. 2015, 324, 432–437. [Google Scholar] [CrossRef]
- Kalia, R.; Pirzada, B.M.; Kunchala, R.K.; Naidu, B.S. Noble Metal Free Efficient Photocatalytic Hydrogen Generation by TaON/CdS Semiconductor Nanocomposites under Natural Sunlight. Int. J. Hydrogen Energy 2023, 48, 16246–16258. [Google Scholar] [CrossRef]
- Tang, H.; Wang, R.; Zhao, C.; Chen, Z.; Yang, X.; Bukhvalov, D.; Lin, Z.; Liu, Q. Oxamide-Modified g-C3N4 Nanostructures: Tailoring Surface Topography for High-Performance Visible Light Photocatalysis. Chem. Eng. J. 2019, 374, 1064–1075. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, L.; Ma, T.; Zhang, Y.; Huang, H. 2D Graphitic Carbon Nitride for Energy Conversion and Storage. Adv. Funct. Mater. 2021, 31, 2102540. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniec, M.; Qiao, S.Z. Graphitic Carbon Nitride Materials: Controllable Synthesis and Applications in Fuel Cells and Photocatalysis. Energy Environ. Sci. 2012, 5, 6717. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on g-C3N4-Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Maeda, K.; Domen, K.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Sulfur-mediated synthesis of carbon nitride: Band-gap engineering and improved functions for photocatalysis. Energy Environ. Sci. 2011, 4, 675–678. [Google Scholar] [CrossRef]
- Song, X.-L.; Chen, L.; Gao, L.-J.; Ren, J.-T.; Yuan, Z.-Y. Engineering g-C3N4 Based Materials for Advanced Photocatalysis: Recent Advances. Green Energy Environ. 2024, 9, 166–197. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.; Cui, Y.; Zhou, W.; Qian, G.; Chen, B. Emerging Multifunctional Metal–Organic Framework Materials. Adv. Mater. 2016, 28, 8819–8860. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Matsubara, I.; Higuchi, T.; Saito, Y. The Crystal Structure of Bis(adiponitrilo)copper(I) Nitrate. Bull. Chem. Soc. Jpn. 1959, 32, 1221–1226. [Google Scholar] [CrossRef]
- Wang, C.; deKrafft, K.E.; Lin, W. Pt Nanoparticles@Photoactive Metal–Organic Frameworks: Efficient Hydrogen Evolution via Synergistic Photoexcitation and Electron Injection. J. Am. Chem. Soc. 2012, 134, 7211–7214. [Google Scholar] [CrossRef]
- Toyao, T.; Saito, M.; Dohshi, S.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Horiuchi, Y.; Matsuoka, M. Development of a Ru Complex-Incorporated MOF Photocatalyst for Hydrogen Production under Visible-Light Irradiation. Chem. Commun. 2014, 50, 6779. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal-organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- El-Kaderi, H.M.; Hunt, J.R.; Mendoza-Cortés, J.L.; Côté, A.P.; Taylor, R.E.; O’Keeffe, M.; Yaghi, O.M. Designed Synthesis of 3D Covalent Organic Frameworks. Science 2007, 316, 268–272. [Google Scholar] [CrossRef]
- Perry, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, crystalline, covalent organic frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent Organic Frameworks (COFs) for Environmental Applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ. 2020, 276, 119174. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, L.; Xu, H.; Wu, X.; Yang, J. A Simple Molecular Design Strategy for Two-Dimensional Covalent Organic Framework Capable of Visible-Light-Driven Water Splitting. J. Am. Chem. Soc. 2020, 142, 4508–4516. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Zhang, F.-M.; Sheng, J.-L.; Yang, Z.-D.; Sun, X.-J.; Tang, H.-L.; Lu, M.; Dong, H.; Shen, F.-C.; Liu, J.; Lan, Y.-Q. Rational Design of MOF/COF Hybrid Materials for Photocatalytic H2 Evolution in the Presence of Sacrificial Electron Donors. Angew. Chem. Int. Ed. 2018, 57, 12106–12110. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3 AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Hong, L.; Guo, R.; Yuan, Y.; Ji, X.; Li, Z.; Lin, Z.; Pan, W. Recent Progress of Two-Dimensional MXenes in Photocatalytic Applications: A Review. Mater. Today Energy 2020, 18, 100521. [Google Scholar] [CrossRef]
- Huang, K.; Lv, C.; Li, C.; Bai, H.; Meng, X. Ti3C2 MXene Supporting Platinum Nanoparticles as Rapid Electrons Transfer Channel and Active Sites for Boosted Photocatalytic Water Splitting over g-C3N4. J. Colloid Interface Sci. 2023, 636, 21–32. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, R.; Huang, J.; Jiang, Y.; Zhao, C.; Yang, X. In situ construction of protonated g-C3N4/Ti3C2 MXene Schottky heterojunctions for efficient photocatalytic hydrogen production. Chin. J. Catal. 2021, 42, 107–114. [Google Scholar] [CrossRef]
- Zhuang, S.; Chen, D.; Fan, W.; Yuan, J.; Liao, L.; Zhao, Y.; Li, J.; Deng, H.; Yang, J.; Yang, J.; et al. Single-Atom-Kernelled Nanocluster Catalyst. Nano Lett. 2022, 22, 7144–7150. [Google Scholar] [CrossRef]
- Qu, M.; Zhang, F.-Q.; Zhang, G.-L.; Qiao, M.-M.; Zhao, L.-X.; Li, S.-L.; Walter, M.; Zhang, X.-M. Cocrystallization-driven formation of fcc-based Ag110 nanocluster with Chinese Triple Luban Lock Shape. Angew. Chem. Int. Ed. 2024, 63, e202318390. [Google Scholar] [CrossRef]
- You, Q.; Jiang, X.; Fan, W.; Cui, Y.; Zhao, Y.; Zhuang, S.; Gu, W.; Liao, L.; Xu, C.; Li, J.; et al. Pd8 Nanocluster with Nonmetal-to-Metal- Ring Coordination and Promising Photothermal Conversion Efficiency. Angew. Chem. Int. Ed. 2024, 63, e202313491. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Geng, L.; Kang, Y.; Fang, W.-H.; Zhang, J. Odd-Membered Cyclic Hetero-Polyoxotitanate Nanoclusters with High Stability and Photocatalytic H2 Evolution Activity. Chin. J. Catal. 2021, 42, 1332–1337. [Google Scholar] [CrossRef]
- Bera, D.; Mahata, S.; Biswas, M.; Kumari, K.; Rakshit, S.; Nonappa; Ghosh, S.; Goswami, N. Efficient Photocatalytic Hydrogen Production Using In-situ Polymerized Gold Nanocluster Assemblies. Small 2024, 20, 2406551. [Google Scholar] [CrossRef]
- Devadas, B.; Prokop, M.; Duraisamy, S.; Bouzek, K. Poly (Amidoamine) Dendrimer-Protected Pt Nanoparticles as a Catalyst with Ultra-Low Pt Loading for PEM Water Electrolysis. Electrochim. Acta 2023, 441, 141737. [Google Scholar] [CrossRef]
- Lv, F.; Huang, B.; Feng, J.; Zhang, W.; Wang, K.; Li, N.; Zhou, J.; Zhou, P.; Yang, W.; Du, Y. A highly efficient atomically thin curved PdIr bimetallene electrocatalyst. Natl. Sci. Rev. 2021, 8, nwab019. [Google Scholar] [CrossRef]
- Liu, H.; Yan, Z.; Li, J.; Liu, L.; Liu, F.; Fan, G.; Cheng, F. Electrodeposition of Pt-decorated Ni(OH)2/CeO2 hybrid as superior bifunctional electrocatalyst for water splitting. Research 2020, 2, 9068270. [Google Scholar]
- Zhang, C.; Wang, H.; Yu, H.; Yi, K.; Zhang, W.; Yuan, X.; Huang, J.; Deng, Y.; Zeng, G. Single-Atom Catalysts for Hydrogen Generation: Rational Design, Recent Advances, and Perspectives. Adv. Energy Mater. 2022, 12, 2200875. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, E.; Wan, X.; Pan, R.; Li, Y.; Zhang, X.; Su, M.; Liu, J.; Zhang, J. Ru-Co-Mn Trimetallic Alloy Nanocatalyst Driving Bifunctional Redox Electrocatalysis. Sci. China Mater. 2022, 65, 131–138. [Google Scholar] [CrossRef]
- Li, C.; Qiu, Z.; Sun, H.; Yang, Y.; Li, C. Recent Progress in Covalent Organic Frameworks (COFs) for Electrocatalysis. Chin. J. Struct. Chem. 2022, 41, 2211084–2211099. [Google Scholar]
- Shah, S.H.A.; Shah, A.; Iftikhar, F.J. Nanoporous Metal/Covalent Organic Framework-Based Electrocatalysts for Water Splitting. ACS Appl. Nano Mater. 2024, 7, 8424–8444. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, L.; Liu, Y.; Tang, Z. Atomically precise Au nanoclusters for electrochemical hydrogen evolution catalysis: Progress and perspectives. Polyoxometalates 2023, 2, 9140031. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wu, Z.-S. Recent Advancement and Key Opportunities of MXenes for Electrocatalysis. iScience 2024, 27, 108906. [Google Scholar] [CrossRef]
- Jun, S.E.; Lee, J.K.; Ryu, S.; Jang, H.W. Single Atom Catalysts for Photoelectrochemical Water Splitting. ChemCatChem 2023, 15, e202300926. [Google Scholar]
- Hou, M.; Zheng, L.; Zhao, D.; Tan, X.; Feng, W.; Fu, J.; Wei, T.; Cao, M.; Zhang, J.; Chen, C. Microenvironment reconstitution of highly active Ni single atoms on oxygen-incorporated Mo2C for water splitting. Nat. Commun. 2024, 15, 1342. [Google Scholar] [CrossRef]
- Liu, H.; Yan, X.; Yang, F.; Che, S.; Wang, J.; Qian, J.; Zhang, X.; Sun, S.; Wu, N.; Wang, S.; et al. Electronic synergy between CoP/NiCoP heterostructure and Co, Ni single atoms for efficient hydrogen evolution and overall water splitting. Int. J. Hydrogen Energy 2024, 56, 725–734. [Google Scholar] [CrossRef]
- Zhang, H.; An, P.; Zhou, W.; Guan, B.Y.; Zhang, P.; Dong, J.; Lou, X.W. Dynamic Traction of Lattice-Confined Platinum Atoms into Mesoporous Carbon Matrix for Hydrogen Evolution Reaction. Sci. Adv. 2018, 4, eaao6657. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Li, M.; Zhang, Y.Q.; Sun, Y.F.; Luo, J.L. All-In-One Perovskite Catalyst: Smart Controls of Architecture and Composition toward Enhanced Oxygen/Hydrogen Evolution Reactions. Adv. Energy Mater. 2017, 7, 1700666. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Deng, Y.; Qian, Y.; Jia, X.; Ma, M.; Yang, C.; Liu, K.; Wang, Z.; Qu, S.; et al. The Application of Perovskite Materials in Solar Water Splitting. J. Semicond. 2020, 41, 011701. [Google Scholar] [CrossRef]
- Beall, C.E.; Fabbri, E.; Schmidt, T.J. Perovskite Oxide Based Electrodes for the Oxygen Reduction and Evolution Reactions: The Underlying Mechanism. ACS Catal. 2021, 11, 3094–3114. [Google Scholar] [CrossRef]
- Lopes, P.P.; Chung, D.Y.; Rui, X.; Zheng, H.; He, H.; Martins, P.F.B.D.; Strmcnik, D.; Stamenkovic, V.R.; Zapol, P.; Mitchell, J.F.; et al. Dynamically stable active sites from surface evolution of perovskite materials during the oxygen evolution reaction. J. Am. Chem. Soc. 2021, 143, 2741–2750. [Google Scholar] [PubMed]
- Arandiyan, H.; Mofarah, S.S.; Sorrell, C.C.; Doustkhah, E.; Sajjad, B.; Hao, D.; Wang, Y.; Sun, H.; Ni, B.-J.; Rezaei, M.; et al. Defect engineering of oxide perovskites for catalysis and energy storage: Synthesis of chemistry and materials science. Chem. Soc. Rev. 2021, 50, 10116–10211. [Google Scholar] [CrossRef]
- Si, C.; Zhang, W.; Lu, Q.; Guo, E.; Yang, Z.; Chen, J.; He, X.; Luo, J. Recent Advances in Perovskite Catalysts for Efficient Overall Water Splitting. Catalysts 2022, 12, 601. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Wang, Y.; Zhu, X.; Xiao, W.; Xu, H.; Li, G.; Li, Y.; Fan, D.; Zeng, H.; et al. Solar-Driven Interfacial Evaporation Accelerated Electrocatalytic Water Splitting on 2D Perovskite Oxide/MXene Heterostructure. Adv. Funct. Mater. 2023, 33, 2215061. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct Evidence of Boosted Oxygen Evolution over Perovskite by Enhanced Lattice Oxygen Participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, J.; Cheng, B.; Yu, J.; Xu, J. Nickel-Based Cocatalysts for Photocatalysis: Hydrogen Evolution, Overall Water Splitting and CO2 Reduction. Mater. Today Phys. 2020, 15, 100279. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Zhang, J.; Dai, K. Overall Utilization of Photoexcited Charges for Simultaneous Photocatalytic Redox Reactions. Acta Phys. Chim. Sin. 2022, 0, 2209037. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Wang, J.; Cabot, A.; Zhu, Y. Boosting Hydrogen Evolution by Methanol Oxidation Reaction on Ni-Based Electrocatalysts: From Fundamental Electrochemistry to Perspectives. ACS Energy Lett. 2024, 9, 853–879. [Google Scholar] [CrossRef]
- Zhai, Y.; Han, P.; Yun, Q.; Ge, Y.; Zhang, X.; Chen, Y.; Zhang, H. Phase Engineering of Metal Nanocatalysts for Electrochemical CO2 Reduction. eScience 2022, 2, 467–485. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Hellstern, T.R.; Nielander, A.C.; Chakthranont, P.; King, L.A.; Willis, J.J.; Xu, S.; MacIsaac, C.; Hahn, C.; Bent, S.F.; Prinz, F.B.; et al. Nanostructuring Strategies To Increase the Photoelectrochemical Water Splitting Activity of Silicon Photocathodes. ACS Appl. Nano Mater. 2019, 2, 6–11. [Google Scholar] [CrossRef]
- Shinde, D.V.; Dang, Z.; Petralanda, U.; Palei, M.; Wang, M.; Prato, M.; Cavalli, A.; De Trizio, L.; Manna, L. In Situ Dynamic Nanostructuring of the Cu–Ti Catalyst-Support System Promotes Hydrogen Evolution under Alkaline Conditions. ACS Appl. Mater. Interfaces 2018, 10, 29583–29592. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Li, D.; Wu, A.; Ruan, R. A Nanoporous GaN Photoelectrode on Patterned Sapphire Substrates for High-Efficiency Photoelectrochemical Water Splitting. J. Alloys Compd. 2019, 803, 748–756. [Google Scholar] [CrossRef]
- Kirch, M.; Lehn, J.; Sauvage, J. Hydrogen Generation by Visible Light Irradiation of Aqueous Solutions of Metal Complexes. An Approach to the Photochemical Conversion and Storage of Solar Energy. Helv. Chim. Acta 1979, 62, 1345–1384. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Ziessel, R. Photochemical Generation of Carbon Monoxide and Hydrogen by Reduction of Carbon Dioxide and Water under Visible Light Irradiation. Proc. Natl. Acad. Sci. USA 1982, 79, 701–704. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Wang, W.-G.; Si, G.; Wang, F.; Tung, C.-H.; Wu, L.-Z. Photocatalytic Hydrogen Evolution from Rhenium(I) Complexes to [FeFe] Hydrogenase Mimics in Aqueous SDS Micellar Systems: A Biomimetic Pathway. Langmuir 2010, 26, 9766–9771. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Peng, S.; Zhang, L.; Al-Enizi, A.M.; Zhang, H.; Sun, X.; Zheng, G. Co–Ni-Based Nanotubes/Nanosheets as Efficient Water Splitting Electrocatalysts. Adv. Energy Mater. 2016, 6, 1501661. [Google Scholar] [CrossRef]
- Merki, D.; Hu, X. Recent Developments of Molybdenum and Tungsten Sulfides as Hydrogen Evolution Catalysts. Energy Environ. Sci. 2011, 4, 3878. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum Sulfides—Efficient and Viable Materials for Electro- and Photoelectrocatalytic Hydrogen Evolution. Energy Environ. Sci. 2012, 5, 5577. [Google Scholar] [CrossRef]
- Voiry, D.; Salehi, M.; Silva, R.; Fujita, T.; Chen, M.; Asefa, T.; Shenoy, V.B.; Eda, G.; Chhowalla, M. Conducting MoS2 Nanosheets as Catalysts for Hydrogen Evolution Reaction. Nano Lett. 2013, 13, 6222–6227. [Google Scholar] [CrossRef]
- Bonde, J.; Moses, P.G.; Jaramillo, T.F.; Nørskov, J.K.; Chorkendorff, I. Hydrogen Evolution on Nano-Particulate Transition Metal Sulfides. Faraday Discuss. 2009, 140, 219–231. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jørgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of Active Edge Sites for Electrochemical H2 Evolution from MoS 2 Nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef]
- Hu, T.; Bian, K.; Tai, G.; Zeng, T.; Wang, X.; Huang, X.; Xiong, K.; Zhu, K. Oxidation-Sulfidation Approach for Vertically Growing MoS2 Nanofilms Catalysts on Molybdenum Foils as Efficient HER Catalysts. J. Phys. Chem. C 2016, 120, 25843–25850. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, Y.; Zhang, H.; Liu, Y.; Zhao, Y.; Qiu, J.; Dong, G. 3D Foam Strutted Graphene Carbon Nitride with Highly Stable Optoelectronic Properties. Adv. Funct. Mater. 2017, 27, 1703711. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-dimensional porous g-C3N4 for highly efficient photocatalytic overall water splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Fang, Z.; Bueken, B.; De Vos, D.E.; Fischer, R.A. Defect-Engineered Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 7234–7254. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, N.; Gao, C.; Xiong, Y. Defect Engineering in Photocatalytic Materials. Nano Energy 2018, 53, 296–336. [Google Scholar] [CrossRef]
- Gao, L.-J.; Chen, L.; Ren, J.-T.; Weng, C.-C.; Tian, W.-W.; Yuan, Z.-Y. Mesoporous Cd Zn S with Abundant Surface Defects for Efficient Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2021, 589, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Tian, N.; Li, Z.; Li, J.; Yao, X.; Vakili, M.; Lu, Y.; Zhang, T. Intrinsic Defect Engineering in Graphitic Carbon Nitride for Photocatalytic Environmental Purification: A Review to Fill Existing Knowledge Gaps. Chem. Eng. J. 2021, 421, 127729. [Google Scholar] [CrossRef]
- Brown, J.J.; Ke, Z.; Ma, T.; Page, A.J. Defect Engineering for Photocatalysis: From Ternary to Perovskite Oxynitrides. ChemNanoMat 2020, 6, 708–719. [Google Scholar] [CrossRef]
- Li, G.; Blake, G.R.; Palstra, T.T.M. Vacancies in Functional Materials for Clean Energy Storage and Harvesting: The Perfect Imperfection. Chem. Soc. Rev. 2017, 46, 1693–1706. [Google Scholar] [CrossRef]
- Zhang, Y.; Afzal, N.; Pan, L.; Zhang, X.; Zou, J. Structure-Activity Relationship of Defective Metal-Based Photocatalysts for Water Splitting: Experimental and Theoretical Perspectives. Adv. Sci. 2019, 6, 1900053. [Google Scholar] [CrossRef]
- Fuertes, A. Synthetic Approaches in Oxynitride Chemistry. Prog. Solid State Chem. 2018, 51, 63–70. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with Oxygen Vacancies: Synthesis, Properties and Photocatalytic Applications. Nanoscale 2013, 5, 3601. [Google Scholar] [CrossRef] [PubMed]
- Cushing, S.K.; Meng, F.; Zhang, J.; Ding, B.; Chen, C.K.; Chen, C.-J.; Liu, R.-S.; Bristow, A.D.; Bright, J.; Zheng, P.; et al. Effects of Defects on Photocatalytic Activity of Hydrogen-Treated Titanium Oxide Nanobelts. ACS Catal. 2017, 7, 1742–1748. [Google Scholar] [CrossRef]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6 -Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and Near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- Kong, X.Y.; Choo, Y.Y.; Chai, S.-P.; Soh, A.K.; Mohamed, A.R. Oxygen Vacancy Induced Bi2 WO6 for the Realization of Photocatalytic CO2 Reduction over the Full Solar Spectrum: From the UV to the NIR Region. Chem. Commun. 2016, 52, 14242–14245. [Google Scholar] [CrossRef]
- Li, H.; Shang, J.; Zhu, H.; Yang, Z.; Ai, Z.; Zhang, L. Oxygen vacancy structure associated photocatalytic water oxidation of BiOCl. ACS Catal. 2016, 6, 8276–8285. [Google Scholar] [CrossRef]
- Li, H.; Qin, F.; Yang, Z.; Cui, X.; Wang, J.; Zhang, L. New Reaction Pathway Induced by Plasmon for Selective Benzyl Alcohol Oxidation on BiOCl Possessing Oxygen Vacancies. J. Am. Chem. Soc. 2017, 139, 3513–3521. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Zhu, W.; Coker, E.N.; Li, B.; Zheng, M.; Yu, W.; Fan, H.; Sun, Z. Oxygen Vacancy Enhanced Photocatalytic Activity of Pervoskite SrTiO3. ACS Appl. Mater. Interfaces 2014, 6, 19184–19190. [Google Scholar] [CrossRef]
- Fang, Z.; Weng, S.; Ye, X.; Feng, W.; Zheng, Z.; Lu, M.; Lin, S.; Fu, X.; Liu, P. Defect engineering and phase junction architecture of wide-bandgap ZnS for conflicting visible light activity in photocatalytic H2 evolution. ACS Appl. Mater. Interfaces 2015, 7, 13915–13924. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, Y.; Li, C.; Guo, C.; Du, Z.; Wu, B. Thermodynamic Assessments of the Yb–Bi and the Yb–Te Systems. Calphad 2015, 51, 306–313. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-Small Yellow Defective TiO2 Nanoparticles for Co-Catalyst Free Photocatalytic Hydrogen Production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Wang, S.; Pan, L.; Song, J.-J.; Mi, W.; Zou, J.-J.; Wang, L.; Zhang, X. Titanium-Defected Undoped Anatase TiO2 with p-Type Conductivity, Room-Temperature Ferromagnetism, and Remarkable Photocatalytic Performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Liu, G.; Cheng, H.-M. Nitrogen Vacancy-Promoted Photocatalytic Activity of Graphitic Carbon Nitride. J. Phys. Chem. C 2012, 116, 11013–11018. [Google Scholar] [CrossRef]
- Niu, P.; Yin, L.; Yang, Y.; Liu, G.; Cheng, H. Increasing the Visible Light Absorption of Graphitic Carbon Nitride (Melon) Photocatalysts by Homogeneous Self-Modification with Nitrogen Vacancies. Adv. Mater. 2014, 26, 8046–8052. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Li, Z.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Holey graphitic carbon nitride nanosheets with carbon vacancies for highly improved photocatalytic hydrogen production. Adv. Funct. Mater. 2015, 25, 6885–6892. [Google Scholar] [CrossRef]
- Li, S.; Dong, G.; Haillili, R.; Yang, L.; Li, Y.; Wang, F.; Zeng, Y.; Wang, C. Effective photocatalytic H2O2 production under visible light irradiation at g-C3N4 modulated by carbon vacancies. Appl. Catal. B Environ. 2016, 190, 26–35. [Google Scholar] [CrossRef]
- Du, X.; Huang, J.; Zhang, J.; Yan, Y.; Wu, C.; Hu, Y.; Yan, C.; Lei, T.; Chen, W.; Fan, C.; et al. Modulating Electronic Structures of Inorganic Nanomaterials for Efficient Electrocatalytic Water Splitting. Angew. Chem. Int. Ed. 2019, 58, 4484–4502. [Google Scholar] [CrossRef]
- Zhang, A.; Liang, Y.; Zhang, H.; Geng, Z.; Zeng, J. Doping Regulation in Transition Metal Compounds for Electrocatalysis. Chem. Soc. Rev. 2021, 50, 9817–9844. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of Graphitic Carbon Nitride for Photocatalysis: A Review. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Ren, T.-Z.; Yuan, Z.-Y. Mesoporous Phosphorus-Doped g-C3N4 Nanostructured Flowers with Superior Photocatalytic Hydrogen Evolution Performance. ACS Appl. Mater. Interfaces 2015, 7, 16850–16856. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent Advances in Anion Doped g-C3N4 Photocatalysts: A Review. Carbon 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous Carbon Nitride with in Situ Sulfur Doping for Enhanced Photocatalytic Hydrogen Evolution from Water under Visible Light. J. Mater. Chem. 2012, 22, 15006. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Wang, Y.; Hu, S.; Wang, B.; Liao, Q.; Li, H.; Bao, J.; Ge, G.; Jia, S. Three-Dimensional Flower-like Phosphorus-Doped g-C3N4 with a High Surface Area for Visible-Light Photocatalytic Hydrogen Evolution. J. Mater. Chem. A 2018, 6, 16485–16494. [Google Scholar] [CrossRef]
- Qu, X.; Hu, S.; Bai, J.; Li, P.; Lu, G.; Kang, X. A Facile Approach to Synthesize Oxygen Doped g-C3N4 with Enhanced Visible Light Activity under Anoxic Conditions via Oxygen-Plasma Treatment. New J. Chem. 2018, 42, 4998–5004. [Google Scholar] [CrossRef]
- She, X.; Liu, L.; Ji, H.; Mo, Z.; Li, Y.; Huang, L.; Du, D.; Xu, H.; Li, H. Template-Free Synthesis of 2D Porous Ultrathin Nonmetal-Doped g-C 3 N 4 Nanosheets with Highly Efficient Photocatalytic H 2 Evolution from Water under Visible Light. Appl. Catal. B Environ. 2016, 187, 144–153. [Google Scholar] [CrossRef]
- Thaweesak, S.; Wang, S.; Lyu, M.; Xiao, M.; Peerakiatkhajohn, P.; Wang, L. Boron-Doped Graphitic Carbon Nitride Nanosheets for Enhanced Visible Light Photocatalytic Water Splitting. Dalton Trans. 2017, 46, 10714–10720. [Google Scholar] [CrossRef]
- Jiao, Z.; Zhang, Y.; Ouyang, S.; Yu, H.; Lu, G.; Ye, J.; Bi, Y. BiAg Alloy Nanospheres: A New Photocatalyst for H2 Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 19488–19493. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhao, M.; Zhang, L.; Wang, H.; Gu, J.; Sun, Y.; Ji, W.; Fu, Z. Self- Supported High-Entropy Alloy Electrocatalyst for Highly Efficient H2 Evolution in Acid Condition. J. Mater. 2020, 6, 736–742. [Google Scholar] [CrossRef]
- Ahmad, S.; Egilmez, M.; Abuzaid, W.; Mustafa, F.; Kannan, A.M.; Alnaser, A.S. Efficient Medium Entropy Alloy Thin Films as Bifunctional Electrodes for Electrocatalytic Water Splitting. Int. J. Hydrogen Energy 2024, 52, 1428–1439. [Google Scholar] [CrossRef]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical Alloying Synthesis and Spark Plasma Sintering Consolidation of CoCrFeNiAl High-Entropy Alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Jin, Z.; Lv, J.; Jia, H.; Liu, W.; Li, H.; Chen, Z.; Lin, X.; Xie, G.; Liu, X.; Sun, S.; et al. Nanoporous Al-Ni-Co-Ir-Mo high-entropy alloy for record-high water splitting activity in acidic environments. Small. 2019, 15, 1904180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, W.; Abu Baker, A.; Egilmez, M.; Abuzaid, W.; Orhan, M.F.; Ibrahim, T.; Khamis, M.; Alnaser, A.S. Enhancement of the Corrosion Resistance of Mild Steel with Femtosecond Laser- Nanostructuring and CrCoNi Medium Entropy Alloy Coating. Appl. Surf. Sci. Adv. 2022, 12, 100321. [Google Scholar] [CrossRef]
- Mohamed, O.; Hassan, M.; Egilmez, M.; Abuzaid, W.; Ibrahim, T.; Khamis, M. Corrosion Behavior of CoCrNi/Mild Steel Medium Entropy Alloy Thin Films. Mater. Today Commun. 2022, 30, 103015. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Han, L.; Luo, J.; Yu, S.-H.; Jiang, H.-L. Integration of plasmonic effects and Schottky junctions into metal-organic framework composites: Steering charge flow for enhanced visible-light photocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1103–1107. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Y.; Zhou, Y.; Fang, J.; Wang, Y.; Zhang, C.; Chen, W. Fabrication of Sandwich-Structured g-C3N4/Au/BiOCl Z-Scheme Photocatalyst with Enhanced Photocatalytic Performance under Visible Light Irradiation. J. Mater. Sci. 2018, 53, 6008–6020. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, H.; Yu, W.; Wang, X.; Zhao, Y.; Zong, X.; Li, C. Dynamic interaction between methylammonium lead iodide and TiO2 nanocrystals leads to enhanced photocatalytic H2 evolution from HI splitting. ACS Energy Lett. 2018, 3, 1159–1164. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, P.; Zhu, X.; Zhang, Q.; Wang, Z.; Liu, Y.; Zou, G.; Dai, Y.; Whangbo, M.; Huang, B. Composite of CH3NH3PbI3 with Reduced Graphene Oxide as a Highly Efficient and Stable Visible-Light Photocatalyst for Hydrogen Evolution in Aqueous HI Solution. Adv. Mater. 2018, 30, 1704342. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Xie, J.; Yang, Z.; Shen, R.; Fang, Y.; Ma, S.; Chen, X.; Li, X. Earth-Abundant WC Nanoparticles as an Active Noble-Metal-Free Co-Catalyst for the Highly Boosted Photocatalytic H2 Production over g-C3N4 Nanosheets under Visible Light. Catal. Sci. Technol. 2017, 7, 1193–1202. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, D.; Sun, Z.; Irfan, R.M.; Zhang, L.; Du, P. Cobalt nitride as an efficient cocatalyst on CdS nanorods for enhanced photocatalytic hydrogen production in water. Catal. Sci. Technol. 2017, 7, 1515–1522. [Google Scholar] [CrossRef]
- Zhao, H.; Jian, L.; Gong, M.; Jing, M.; Li, H.; Mao, Q.; Lu, T.; Guo, Y.; Ji, R.; Chi, W.; et al. Transition-Metal-Based Cocatalysts for Photocatalytic Water Splitting. Small Struct. 2022, 3, 2100229. [Google Scholar] [CrossRef]
- Simon, T.; Bouchonville, N.; Berr, M.J.; Vaneski, A.; Adrović, A.; Volbers, D.; Wyrwich, R.; Döblinger, M.; Susha, A.S.; Rogach, A.L.; et al. Redox Shuttle Mechanism Enhances Photocatalytic H2 Generation on Ni-Decorated CdS Nanorods. Nat. Mater. 2014, 13, 1013–1018. [Google Scholar] [CrossRef]
- Wu, A.; Xie, Y.; Ma, H.; Tian, C.; Gu, Y.; Yan, H.; Zhang, X.; Yang, G.; Fu, H. Integrating the Active OER and HER Components as the Heterostructures for the Efficient Overall Water Splitting. Nano Energy 2018, 44, 353–363. [Google Scholar] [CrossRef]
- Li, A.; Chang, X.; Huang, Z.; Li, C.; Wei, Y.; Zhang, L.; Wang, T.; Gong, J. Thin Heterojunctions and Spatially Separated Cocatalysts to Simultaneously Reduce Bulk and Surface Recombination in Photocatalysts. Angew. Chem. 2016, 128, 13938–13942. [Google Scholar] [CrossRef]
- Kim, D.; Zhang, Z.; Yong, K. Synergistic Doping Effects of a ZnO:N/BiVO4: Mo Bunched Nanorod Array Photoanode for Enhancing Charge Transfer and Carrier Density in Photoelectrochemical Systems. Nanoscale 2018, 10, 20256–20265. [Google Scholar] [CrossRef] [PubMed]
- Ledezma-Yanez, I.; Wallace, W.D.Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J.M.; Koper, M.T.M. Interfacial Water Reorganization as a pH-Dependent Descriptor of the Hydrogen Evolution Rate on Platinum Electrodes. Nat. Energy 2017, 2, 17031. [Google Scholar] [CrossRef]
- Park, S.; Shao, Y.; Liu, J.; Wang, Y. Oxygen Electrocatalysts for Water Electrolyzers and Reversible Fuel Cells: Status and Perspective. Energy Environ. Sci. 2012, 5, 9331. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Liang, J.; Yu, Q.; Yang, X.; Zhang, T.; Li, J. A Systematic Theoretical Study on FeOx-Supported Single-Atom Catalysts: M1/FeOx for CO Oxidation. Nano Res. 2018, 11, 1599–1611. [Google Scholar] [CrossRef]
- Zong, S.; Tian, L.; Guan, X.; Zhang, Y.; Cheng, C.; Geng, J.; Jiang, S.; Shi, J. Hierarchical LaTiO2N/Sn3O4 Heterojunction with Intimate Interface Contact for Enhanced Photocatalytic Water Splitting. Surf. Interfaces 2024, 48, 104285. [Google Scholar] [CrossRef]
- Ma, X.; Li, W.; Li, H.; Dong, M.; Geng, L.; Wang, T.; Zhou, H.; Li, Y.; Li, M. Novel Noble-Metal-Free Co2P/CdIn2S4 Heterojunction Photocatalysts for Elevated Photocatalytic H2 Production: Light Absorption, Charge Separation and Active Site. J. Colloid Interface Sci. 2023, 639, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Hou, L.; Guo, X.; Fu, R.; Sun, J. Construction of Covalent Bonding Oxygen-Doped Carbon Nitride/Graphitic Carbon Nitride Z-Scheme Heterojunction for Enhanced Visible-Light-Driven H2 Evolution. Chem. Eng. J. 2020, 383, 123132. [Google Scholar] [CrossRef]
- Li, H.; Tao, S.; Wan, S.; Qiu, G.; Long, Q.; Yu, J.; Cao, S. S-scheme heterojunction of ZnCdS nanospheres and dibenzothiophene modified graphite carbon nitride for enhanced H2 production. Chin. J. Catal. 2023, 46, 167–176. [Google Scholar] [CrossRef]
- Pan, J.; Wang, P.; Wang, P.; Yu, Q.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The photocatalytic overall water splitting hydrogen production of g-C3N4/CdS hollow core-shell heterojunction via the HER/OER matching of Pt/MnOx. Chem. Eng. J. 2021, 405, 126622. [Google Scholar] [CrossRef]
- Ji, L.; Wei, Y.; Wu, P.; Xu, M.; Wang, T.; Wang, S.; Liang, Q.; Meyer, T.J.; Chen, Z. Heterointerface engineering of Ni2P-Co2P nanoframes for efficient water splitting. Chem. Mater. 2021, 33, 9165–9173. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Bakandritsos, A.; Zbofil, R. Bimetallic single-atom catalysts for water splitting. Nano-Micro Lett. 2025, 17, 1. [Google Scholar] [CrossRef]
- Liu, X.; Liu, R.; Wang, J.; Liu, Y.; Li, L.; Yang, W.; Feng, X.; Wang, B. Synergizing High Valence Metal Sites and Amorphous/Crystalline Interfaces in Electrochemical Reconstructed CoFeOOH Heterostructure Enables Efficient Oxygen Evolution Reaction. Nano Res. 2022, 15, 8857–8864. [Google Scholar] [CrossRef]
- Lin, Y.; Pan, Y.; Liu, S.; Sun, K.; Cheng, Y.; Liu, M.; Wang, Z.; Li, X.; Zhang, J. Construction of Multi-Dimensional Core/Shell Ni/NiCoP Nano-Heterojunction for Efficient Electrocatalytic Water Splitting. Appl. Catal. B Environ. 2019, 259, 118039. [Google Scholar] [CrossRef]
- Xiao, N.; Li, S.; Li, X.; Ge, L.; Gao, Y.; Li, N. The Roles and Mechanism of Cocatalysts in Photocatalytic Water Splitting to Produce Hydrogen. Chin. J. Catal. 2020, 41, 642–671. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, Z.; Yi, H.; Zeng, L.; Tang, C.; Huang, L.; Gu, M. Covalently Bonded 2D/2D O-g-C3N4/TiO2 Heterojunction for Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2018, 237, 1130–1138. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.-Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef]
- Cheng, R.; Zhang, L.; Fan, X.; Wang, M.; Li, M.; Shi, J. One-Step Construction of FeOx Modified g-C3N4 for Largely Enhanced Visible-Light Photocatalytic Hydrogen Evolution. Carbon 2016, 101, 62–70. [Google Scholar] [CrossRef]
- Qin, H.; Lv, W.; Bai, J.; Zhou, Y.; Wen, Y.; He, Q.; Tang, J.; Wang, L.; Zhou, Q. Sulfur-Doped Porous Graphitic Carbon Nitride Heterojunction Hybrids for Enhanced Photocatalytic H2 Evolution. J. Mater. Sci. 2019, 54, 4811–4820. [Google Scholar] [CrossRef]
- Mao, Z.; Chen, J.; Yang, Y.; Wang, D.; Bie, L.; Fahlman, B.D. Novel g-C3N4/CoO Nanocomposites with Significantly Enhanced Visible-Light Photocatalytic Activity for H 2 Evolution. ACS Appl. Mater. Interfaces 2017, 9, 12427–12435. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, G.; Zhou, W.; Liu, Y.; Pang, H.; Zhang, H.; Hao, D.; Meng, X.; Li, P.; Kako, T.; et al. In situ bond modulation of graphitic carbon nitride to construct p-n homojunctions for enhanced photocatalytic hydrogen production. Adv. Funct. Mater. 2016, 26, 6822–6829. [Google Scholar] [CrossRef]
- Kumaravel, V.; Imam, M.D.; Badreldin, A.; Chava, R.K.; Do, J.Y.; Kang, M.; Abdel-Wahab, A. Photocatalytic Hydrogen Production: Role of Sacrificial Reagents on the Activity of Oxide, Carbon, and Sulfide Catalysts. Catalysts 2019, 9, 276. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Han, W.; Gao, L.; Li, S. Exergy Analysis on the Process with Integrated Supercritical Water Gasification of Coal and Syngas Separation. Appl. Therm. Eng. 2018, 128, 1003–1008. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Feng, X.; Wang, X.; Huang, J. Biohydrogen Production from Cornstalk Wastes by Anaerobic Fermentation with Activated Sludge. Int. J. Hydrogen Energy 2010, 35, 3092–3099. [Google Scholar] [CrossRef]
- Melis, A.; Happe, T. Hydrogen production: Green algae as a source of energy. Plant Physiol. 2001, 127, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorrendorf, J.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Song, M.; Zhang, Q.; An, L.; Shen, T.; Wang, S.; Hu, H.; Huang, X.; Wang, D. Advances of synergistic electrocatalysis between single atoms and nanoparticles/clusters. Nano-Micro Lett. 2024, 16, 241. [Google Scholar] [CrossRef]
- Sheng, B.; Cao, D.; Liu, C.; Chen, S.; Song, L. Support effects in electrocatalysis and their synchrotron radiation-based characterizations. J. Phys. Chem. Lett. 2021, 12, 11543–11554. [Google Scholar] [CrossRef] [PubMed]
- Freund, S.; Sánchez, D. Chapter 15—Green hydrogen market and growth. Mach. Energy Syst. Hydrog. Econ. 2022, 605–635. [Google Scholar]
- Semente, A.B.; Rodrigues, C.B.M.; Mariano, M.A.; Gaspar, M.B.; Sljukić, B.; Santos, D.M.F. Chapter 19—Prospects and challenges for the green hydrogen market. Sol.-Driven Green Hydrog. Gener. Storage 2023, 381–415. [Google Scholar] [CrossRef]
- Yusuf, M.; Alnarabiji, M.S.; Abdullah, B. Clean hydrogen production technologies. Adv. Sustain. Energy 2021, 159–170. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gao, J.; Chen, C.; Zhao, W.; Zhang, Z.; Li, D.; Chen, Y.; Wang, C.; Zhu, C.; Ke, X.; et al. PtNi-W/C with atomically dispersed tungsten sites toward boosted ORR in proton exchange membrane fuel cell devices. Nano-Micro Lett. 2023, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Ariga, K.; Yamauchi, Y. Single-atom catalysts. Small 2021, 17, 2101584. [Google Scholar] [CrossRef] [PubMed]
- Teitsworth, T.S.; Hill, D.J.; Litvin, S.R.; Ritchie, E.T.; Park, J.-S.; Custer Jr, J.P.; Taggart, A.D.; Bottum, S.R.; Morley, S.E.; Kim, S.; et al. Water splitting with silicon p-i-n superlattices suspended in solution. Nature 2023, 614, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Zhao, Y.; Li, H.; Xu, Y.; Chen, X. 2D BiVO4/g-C3N4 Z-scheme photocatalyst for enhanced overall water splitting. J. Mater. Sci. 2019, 54, 10836–10845. [Google Scholar] [CrossRef]
- Raziq, F.; Hayat, A.; Humayun, M.; Mane, S.K.B.; Faheem, M.B.; Ali, A.; Zhao, Y.; Han, S.; Cai, C.; Li, W.; et al. Photocatalytic solar fuel production and environmental remediation through experimental and DFT based research on CdSe-QDs-coupled P-doped-g-C3N4 composites. Appl. Catal. B Environ. 2020, 270, 118867. [Google Scholar] [CrossRef]
- Sepahvand, H.; Sharifnia, S. Photocatalytic overall water splitting by Z-scheme g-C3N4/BiFeO3 heterojunction. Int. J. Hydrogen Energy 2019, 44, 23658–23668. [Google Scholar] [CrossRef]
- Shi, W.; Li, M.; Huang, X.; Ren, H.; Yan, C.; Guo, F. Facile synthesis of 2D/2D Co3(PO4)2/g-C3N4 heterojunction for highly photocatalytic overall water splitting under visible light. Chem. Eng. J. 2020, 382, 122960. [Google Scholar] [CrossRef]
- Ai, Z.; Shao, Y.; Chang, B.; Zhang, L.; Shen, J.; Wu, Y.; Huang, B.; Hao, X. Rational Modulation of P-n Homojunction in P-Doped g-C3N4 Decorated with Ti3C2 for Photocatalytic Overall Water Splitting. Appl. Catal. B Environ. 2019, 259, 118077. [Google Scholar] [CrossRef]
- Tanaka, A.; Teramura, K.; Hosokawa, S.; Kominami, H.; Tanaka, I. Visible light-induced water splitting in an aqueous suspension of a plasmonic Au/TiO2 photocatalyst with metal co-catalysts. Chem. Sci. 2017, 8, 2574–2580. [Google Scholar] [CrossRef] [PubMed]
- Townsend, T.K.; Browning, N.D.; Osterloh, F.E. Nanoscale Strontium Titanate Photocatalysts for Overall Water Splitting. ACS Nano 2012, 6, 7420–7426. [Google Scholar] [CrossRef]
- Cai, X.; Mao, L.; Fujitsuka, M.; Majima, T.; Kasani, S.; Wu, N.; Zhang, J. Effects of Bi-Dopant and Co-Catalysts upon Hole Surface Trapping on La2Ti2O7 Nanosheet Photocatalysts in Overall Solar Water Splitting. Nano Res. 2022, 15, 438–445. [Google Scholar] [CrossRef]
- Dhanasekaran, P.; Gupta, N.M. Factors Affecting the Production of H2 by Water Splitting over a Novel Visible-Light-Driven Photocatalyst GaFeO3. Int. J. Hydrogen Energy 2012, 37, 4897–4907. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H. Effect of lanthanide-doping into NaTaO3 photocatalysts for efficient water splitting. Chem. Phys. Lett. 2000, 331, 373–377. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Ning, X.; Zhen, W.; Wu, Y.; Lu, G. Inhibition of CdS Photocorrosion by Al2O3 Shell for Highly Stable Photocatalytic Overall Water Splitting under Visible Light Irradiation. Appl. Catal. B Environ. 2018, 226, 373–383. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Ren, J.; Wang, S.; Wang, R.; Fu, G.; Hu, Y. Passivation of Defect States in Anatase TiO2 Hollow Spheres with Mg Doping: Realizing Efficient Photocatalytic Overall Water Splitting. Appl. Catal. B Environ. 2017, 202, 127–133. [Google Scholar] [CrossRef]
- Konieczny, A.; Mondal, K.; Wiltowski, T.; Dydo, P. Catalyst Development for Thermocatalytic Decomposition of Methane to Hydrogen. Int. J. Hydrogen Energy 2008, 33, 264–272. [Google Scholar] [CrossRef]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.-C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable Hydrogen Production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef]
- Lu, C.; Trancas, D.; Zhang, J.; Rodriguez Hernandez, F.; Su, Y.; Zhuang, X.; Zhang, F.; Seifert, G.; Feng, X. Molybdenum carbide-embedded nitrogen-doped porous carbon nanosheets as electrocatalysts for water splitting in alkaline media. ACS Nano 2017, 11, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xiong, Y.; Xie, L.; Zhang, Z.; Lu, X.; Wang, Y.; Yuan, X.-Z.; Fan, J.; Li, H.; Wang, H. Tungsten carbide encapsulated in grape-like N-doped carbon nanospheres: One-step facile synthesis for low-cost and highly active electrocatalysts in proton exchange membrane water electrolyzers. ACS Appl. Mater. Interfaces 2019, 11, 25123–25132. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yang, C.; Sheng, M.; Yin, X.; Que, W. Synergistically coupling phosphorus-doped molybdenum carbide with MXene as a highly efficient and stable electrocatalyst for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2020, 8, 12990–12998. [Google Scholar] [CrossRef]
- Geng, D.; Zhao, X.; Chen, Z.; Sun, W.; Fu, W.; Chen, J.; Liu, W.; Zhou, W.; Loh, K.P. Direct synthesis of large-area 2D Mo2C on in situ grown graphene. Adv. Mater. 2017, 29, 1700072. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Chao, D.; Fan, Z.; Guan, C.; Cao, X.; Zhang, H.; Fan, H.J. A new type of porous graphite foams and their integrated composites with oxide/polymer core/shell nanowires for supercapacitors: Structural design, fabrication, and full supercapacitor demonstrations. Nano Lett. 2014, 14, 1651–1658. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhang, Y.; Jiang, W.-J.; Zhang, X.; Dai, Z.; Wan, L.-J.; Hu, J.-S. Pomegranate-like N,P-Doped Mo2C@C Nanospheres as Highly Active Electrocatalysts for Alkaline Hydrogen Evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Ye, C.; Liu, H.; Xu, M.; Bao, S.-J. Nanosized metal phosphides embedded in nitrogen-doped porous carbon nanofibers for enhanced hydrogen evolution at all pH values. Angew. Chem. Int. Ed. 2018, 57, 1963–1967. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.-Q.; Xie, J.-Y.; Zhang, W.-W.; Dong, B.; Qin, J.-F.; Zhang, X.-Y.; Lin, J.-H.; Chai, Y.-M.; Liu, C.-G. N-Doped sandwich-structured Mo2C@C@Pt interface with ultralow Pt loading for pH-universal hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2019, 11, 4047–4056. [Google Scholar] [CrossRef]
- Hao, Y.-R.; Xue, H.; Sun, J.; Guo, N.; Song, T.; Sun, J.; Wang, Q. Tuning the electronic structure of CoP embedded in N-doped porous carbon nanocubes via Ru doping for efficient hydrogen evolution. ACS Appl. Mater. Interfaces 2021, 13, 56035–56044. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; He, X.; Yin, F.; Liang, X.; Chen, B.; Li, G.; Yin, H. β-Mo2C/N, P-co-doped carbon as highly efficient catalyst for hydrogen evolution reaction. J. Mater. Sci. 2019, 54, 4589–4600. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Su, D.; Sun, B.; Zhu, J.; Wang, G. 3D mesoporous hybrid NiCo2O4@graphene nanoarchitectures as electrode materials for supercapacitors with enhanced performances. J. Mater. Chem. A 2014, 2, 8103–8109. [Google Scholar] [CrossRef]
- Namioka, T.; Saito, A.; Inoue, Y.; Park, Y.; Min, T.; Roh, S.; Yoshikawa, K. Hydrogen-Rich Gas Production from Waste Plastics by Pyrolysis and Low-Temperature Steam Reforming over a Ruthenium Catalyst. Appl. Energy 2011, 88, 2019–2026. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Minowa, T.; Potic, B.; Kersten, S.; Prins, W.; Vanswaaij, W.; Vandebeld, B.; Elliott, D.; Neuenschwander, G.; Kruse, A. Biomass Gasification in Near- and Super-Critical Water: Status and Prospects. Biomass Bioenergy 2005, 29, 269–292. [Google Scholar] [CrossRef]
- Hu, B.; Wang, S.; Yan, J.; Zhang, H.; Qiu, L.; Liu, W.; Guo, Y.; Shen, J.; Chen, B.; Shi, C.; et al. Review of Waste Plastics Treatment and Utilization: Efficient Conversion and High Value Utilization. Process Saf. Environ. Prot. 2024, 183, 378–398. [Google Scholar]
- Liu, J.; Jiang, Z.; Yu, H.; Tang, T. Catalytic Pyrolysis of Polypropylene to Synthesize Carbon Nanotubes and Hydrogen through a Two-Stage Process. Polym. Degrad. Stab. 2011, 96, 1711–1719. [Google Scholar] [CrossRef]
- Yao, D.; Zhang, Y.; Williams, P.T.; Yang, H.; Chen, H. Co-production of hydrogen and carbon nanotubes from real-world waste plastics: Influence of catalyst composition and operational parameters. Appl. Catal. B Environ. 2018, 221, 584–597. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Ahmed, U.; Ahmad, N.; Abdul Jameel, A.G.; Zahid, U.; Naqvi, S.R. A review of hydrogen generation through gasification and pyrolysis of waste plastic and tires: Opportunities and challenges. Int. J. Hydrogen Energy 2024, 77, 1185–1204. [Google Scholar] [CrossRef]
- Athia, N.; Pandey, M.; Sen, M.; Saxena, S. Factors Affecting the Production Cost of Green Hydrogen and Its Challenge for Sustainable Development. Environ. Dev. Sustain. 2024. [CrossRef]
- Farhana, K.; Shadate Faisal Mahamude, A.; Kadirgama, K. Comparing Hydrogen Fuel Cost of Production from Various Sources—A Competitive Analysis. Energy Convers. Manag. 2024, 302, 118088. [Google Scholar] [CrossRef]
- Xu, S.; Yu, B. Current development and prospect of hydrogen energy technology in China. J. Beijing Inst. Technol. (Soc. Sci. Ed.) 2021, 23, 1–12. [Google Scholar]
- He, C.; He, D.; Lv, Q.; Peng, B.; Wang, H.; Zhang, P.; Yuan, J.-H.; Wang, J.; Lv, H. Highly Efficient Overall Photocatalytic Water Splitting in 2D Heterostructure GaSe/ScGaSe3. FlatChem 2025, 49, 100798. [Google Scholar] [CrossRef]
- Molaei, M.J. Recent Advances in Hydrogen Production through Photocatalytic Water Splitting: A Review. Fuel 2024, 365, 131159. [Google Scholar] [CrossRef]
- Li, R.; Luan, J.; Zhang, Y.; Jiang, L.; Yan, H.; Chi, Q.; Yan, Z. A Review of Efficient Photocatalytic Water Splitting for Hydrogen Production. Renew. Sustain. Energy Rev. 2024, 206, 114863. [Google Scholar] [CrossRef]
- Morshedy, A.S.; El-Fawal, E.M.; Zaki, T.; El-Zahhar, A.A.; Alghamdi, M.M.; El Naggar, A.M.A. A Review on Heterogeneous Photocatalytic Materials: Mechanism, Perspectives, and Environmental and Energy Sustainability Applications. Inorg. Chem. Commun. 2024, 163, 112307. [Google Scholar] [CrossRef]
- Chen, W.; Wang, D.; Wang, W.; Liu, X.; Liu, Y.; Wang, C.; Kang, Y.; Fang, S.; Yang, X.; Gu, W.; et al. Enhanced Solar Hydrogen Production via Reconfigured Semi-Polar Facet/Cocatalyst Heterointerfaces in GaN/Si Photocathodes. Nat. Commun. 2025, 16, 879. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.Y.; Temiz, M.; Erdemir, D.; Dincer, I. The Role of Effective Catalysts for Hydrogen Production: A Performance Evaluation. Energy 2025, 315, 134257. [Google Scholar] [CrossRef]
- Ayub, H.M.U.; Alnouri, S.Y.; Stijepovic, M.; Stijepovic, V.; Hussein, I.A. A Cost Comparison Study for Hydrogen Production between Conventional and Renewable Methods. Process Saf. Environ. Prot. 2024, 186, 921–932. [Google Scholar] [CrossRef]
- Chong, W.-K.; Ng, B.-J.; Kong, X.Y.; Low, J.; Lee, H.W.; Tan, L.-L.; Chai, S.-P. Unleashing the Solar-Driven Overall Water-Splitting Potential for Green ZnIn2S4. Chem. Catal. 2024, 101227. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.-S. Scalable Production of High-Performance Electrocatalysts for Electrochemical Water Splitting at Large Current Densities. eScience 2024, 100334. [Google Scholar] [CrossRef]
- Bora, L.V.; Bora, N.V. Photoelectrocatalytic Water Splitting for Efficient Hydrogen Production: A Strategic Review. Fuel 2025, 381, 133642. [Google Scholar] [CrossRef]
- Zheng, D.; Xue, Y.; Wang, J.; Varbanov, P.S.; Klemeš, J.J.; Yin, C. Nanocatalysts in Photocatalytic Water Splitting for Green Hydrogen Generation: Challenges and Opportunities. J. Clean. Prod. 2023, 414, 137700. [Google Scholar] [CrossRef]
- Imran, S.; Hussain, M. Emerging Trends in Water Splitting Innovations for Solar Hydrogen Production: Analysis, Comparison, and Economical Insights. Int. J. Hydrogen Energy 2024, 77, 975–996. [Google Scholar] [CrossRef]



| Catalyst | Sacrificial Agent | Exposure Condition | HER (μmol∙g−1∙h−1) |
|---|---|---|---|
| Cu3Mo2O9/TiO2 | TEOA | 300 W xenon lamp | 3401.90 |
| NiO/TiO2 | methyl alcohol | 300 W xenon lamp | 228.00 |
| TiO2@SiO2 | methyl alcohol | Ultraviolet ray | 410.61 |
| Cu2O/TiO2 | methyl alcohol | 100 MW/cm2 xenon lamp | 11,000.00 |
| Photocatalyst | Dopants and Fabrication Methods | Activity Enhancement (Higher than Pure g-C3N4) | Ref. |
|---|---|---|---|
| S, SiO2-CN | thiourea and SiO2 nanoparticles, simple calcination | 30 times | [162] |
| P-CN | phosphoric acid and cyanuric acid–melamine complex (12:1), thermal condensation | 24 times | [163] |
| O-CN | H2SO and HNO3, chemical oxidation | 17 times | [164] |
| B-CN | ammoniotrihydroborate(H3NBH3), one-pot thermal polycondensation | 12 times | [166] |
| Photocatalyst | HER (μmol∙g−1∙h−1) | OER (μmol∙g−1∙h−1) | Stability (h/Times) | Ref. |
|---|---|---|---|---|
| MnOx/g-C3N4/CdS/Pt | 1303.4 | 641.6 | 24 h (6 times) cycles | [194] |
| Pt/Ti-MOF-NH2 | 11.7 | — | 9 h (3 times) cycles | [72] |
| Ni-CdS | 16,700 | — | 80–220 h | [182] |
| LaTiO2N/Sn3O4 | 887 | — | 18 h (3 times) cycles | [190] |
| BiVO4/g-C3N4 | 15.6 | 7.3 | 20 h (5 times) cycles | [220] |
| CdSe/P-g-C3N4 | 113.0 | 55.5 | 42 h (6 times) cycles | [221] |
| BiFeO3/g-C3N4 | 160.8 | 80.1 | 15 h (3 times) cycles | [222] |
| Co3(PO4)2/g-C3N4 | 375.6 | 177.4 | 12 h (4 times) cycles | [223] |
| P-g-C3N4/Ti3C2 | 627.1 | 305.4 | 40 h (8 times) cycles | [224] |
| Au-NiOx/TiO2 | 5.5 | 2.7 | 35 h (3 times) cycles | [225] |
| NiO-SrTiO3 | 28 | — | 24 h | [226] |
| Co-Pi/Bi-La2Ti2O7/Pt | 66.6 | 32.1 | 7.5 h (3 times) cycles | [227] |
| Co2P/CdIn2S4 | 471.9 | — | 15 h (3 times) cycles | [191] |
| GaFeO3 | 9.0 | 4.5 | 12 h | [228] |
| NiO/NaTaO3: La | 5900 | 2900 | 12 h (4 times) cycles | [229] |
| BiVO4-Ru/SrTiO3: Rh | 40.1 | 18.6 | 11 h | [230] |
| Pt/CdS@Al2O3 | 62.1 | — | 30 h (10 times) cycles | [231] |
| Pt-loaded Mg/TiO2 | 850 | 425 | 30 h | [232] |
| RuO2/GaN:ZnO | 1000 | 200 | 15 h (3 times) cycles | [55] |
| ZnCdS@DBTg-C3N4 | 8.87 | — | 12 h (4 times) cycles | [193] |
| O-CN/g-C3N4 | 6.97 | — | 25 h (5 times) cycles | [192] |
| Electrocatalyst | HER Overpotential (10 mA cm−2) | HER Exchange Current Density | Stability (h/cycles) | Surface Area (or ECSA) | Tafel Slope (mVdec−1) | Ref. |
|---|---|---|---|---|---|---|
| Mo2C@2D-NPCs | 45 mV | 0.0014 A cm−2 | 20 h | 110.2 m2∙g−1 | 46 | [235] |
| WC@NC | 141 mV | 0.78 A cm−2 | Over 20 h | 308.4 m2∙g−1 | 78.7 | [236] |
| P-Mo2C/Ti3C2@NC | 177 mV | — | 60 h | 20.4 mF∙cm−2 | 57.3 | [237] |
| 2D Mo2C/G | 236 mV | — | 1000 cycles | — | 73 | [238] |
| 3D graphene foam | — | — | 20,000 cycles | 980 m2∙g−1 | — | [239] |
| N, P-doped Mo2C@C | 47 mV | 2.042 mA cm−2 | 1000 cycles | 156 m2∙g−1 | 71 | [240] |
| Ni2P@NPCNFs | 63.2 mV | — | 3000 cycles | 520 m2∙g−1 | 56.7 | [241] |
| Mo2C@C@Pt | 47 mV | — | 1000 cycles | 128.4 m2∙g−1 | 28 | [242] |
| Ru-CoP/NCs | 22 mV | — | 20 h | 178 m2∙g−1 | 56 | [243] |
| β-Mo2C/N, P | 181 mV | 0.015 mA cm−2 | 2000 cycles | 9.83 m2∙g−1 | 65.3 | [244] |
| 3D NiCo2O4@graphene | — | — | 10,000 cycles | 194.5 m2∙g−1 | — | [245] |
| Technologies | Main Cost Factor | Advantages | Disadvantages | Application Phase |
|---|---|---|---|---|
| Electrocatalysis | Equipment, Electricity, Electrode material | Environmentally friendly, High purity | Low service life, High costs of noble metal catalyst | Initial commercialization |
| Photocatalysis | Efficiency, Photocatalytic material | Simple device, Low cost, Solar power, Clean energy | Poor efficiency | Laboratory |
| Fossil fuel | Raw material, Equipment, Carbon emissions | Mature and low cost 6.8–12 RMB/kg (Coal) 16–24 RMB/kg (Coal + CCS) 8–16 RMB/kg (Natural gas) | Carbon emissions 10–19 kg (CO2)/kg (H2) for coal and natural gas | Mature industrial application |
| Biomass | Raw material, Efficiency, Equipment | Renewable, Rich source, Clean | Impure product | Preliminary industrial demonstration |
| Waste | Product purity; Processing | Renewable, Resource recycling | Not mature | Preliminary industrial demonstration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, C.; Geng, J.; Zong, S.; Wang, P. Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules 2025, 30, 630. https://doi.org/10.3390/molecules30030630
Li X, Zhang C, Geng J, Zong S, Wang P. Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules. 2025; 30(3):630. https://doi.org/10.3390/molecules30030630
Chicago/Turabian StyleLi, Xingpeng, Chenxi Zhang, Jiafeng Geng, Shichao Zong, and Pengqian Wang. 2025. "Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy" Molecules 30, no. 3: 630. https://doi.org/10.3390/molecules30030630
APA StyleLi, X., Zhang, C., Geng, J., Zong, S., & Wang, P. (2025). Photo(electro)catalytic Water Splitting for Hydrogen Production: Mechanism, Design, Optimization, and Economy. Molecules, 30(3), 630. https://doi.org/10.3390/molecules30030630








