Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes
Abstract
1. Introduction
2. Results and Discussions
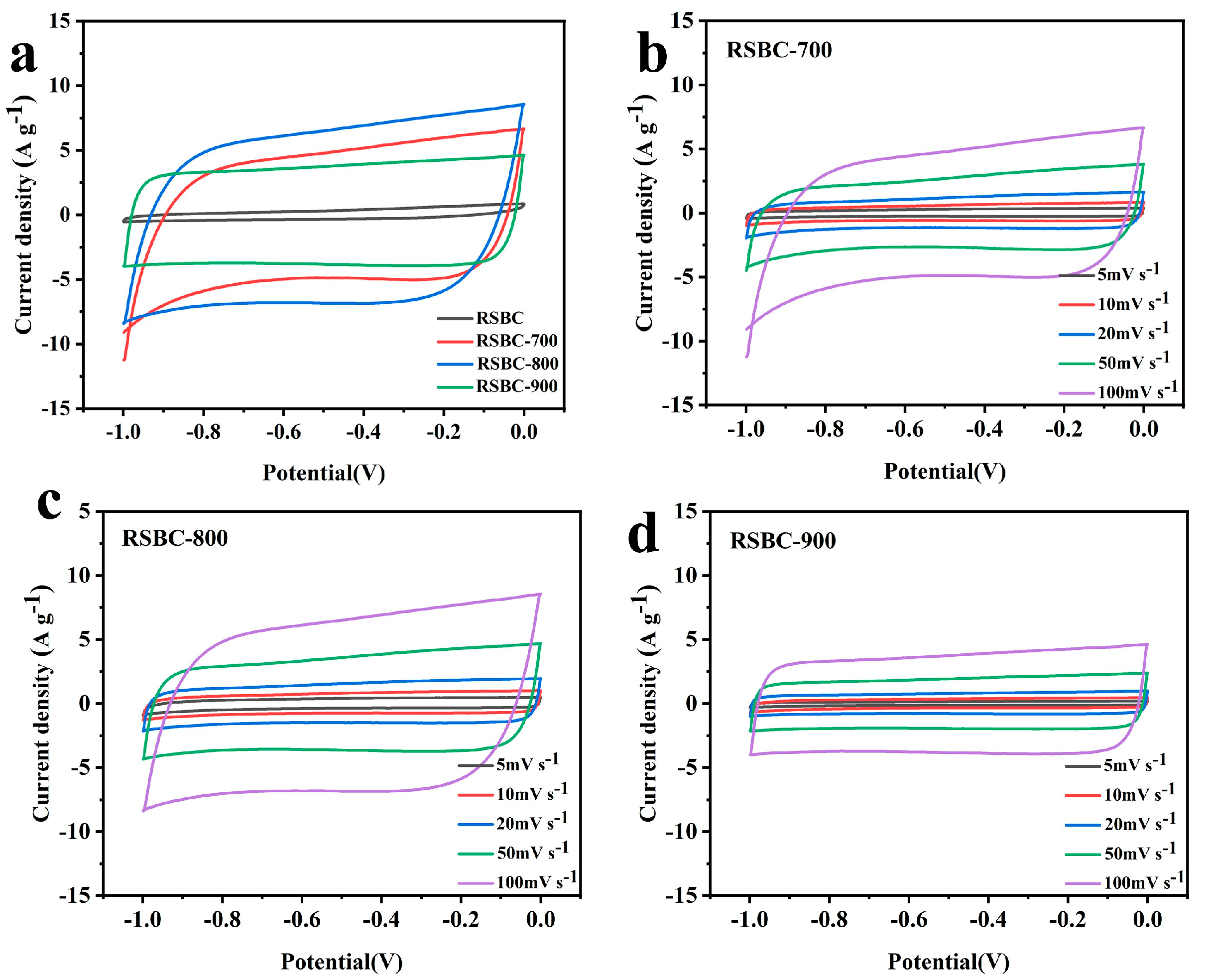
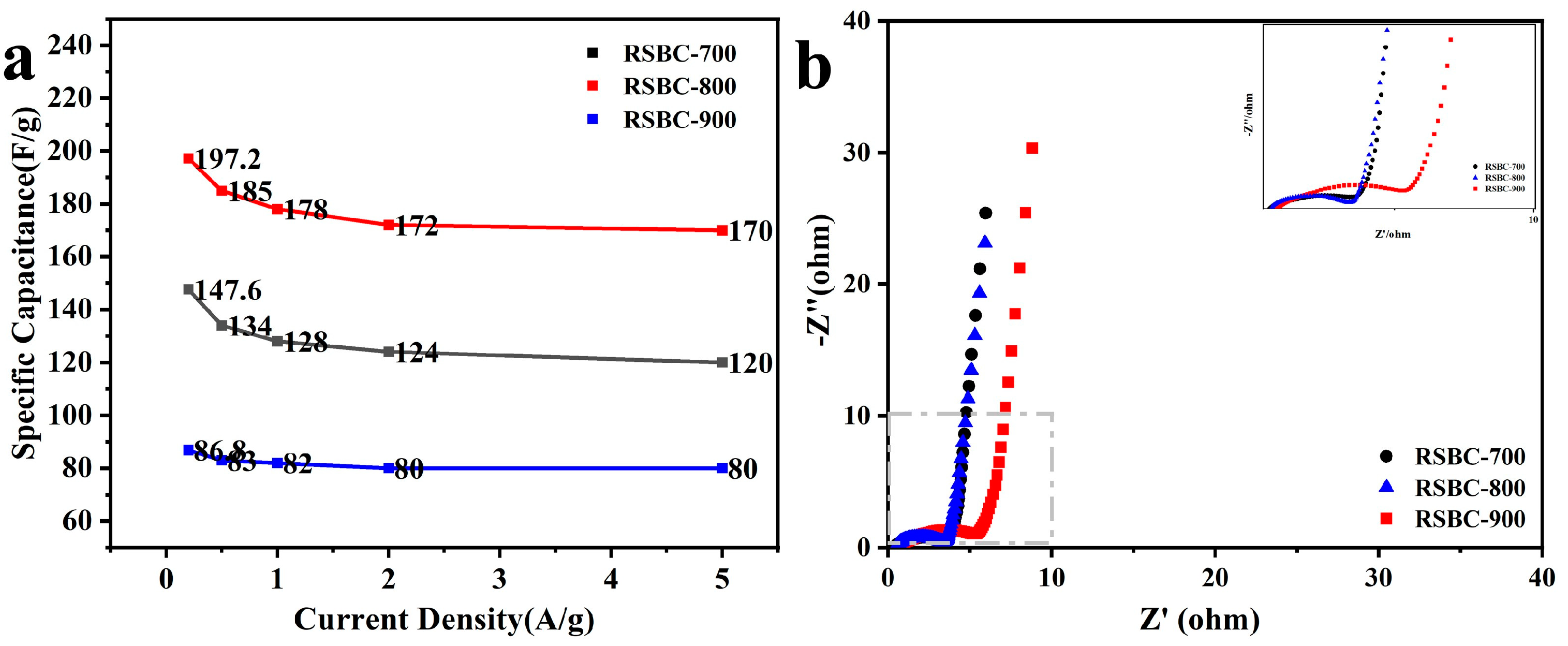
2.1. Effects of Activation Temperature
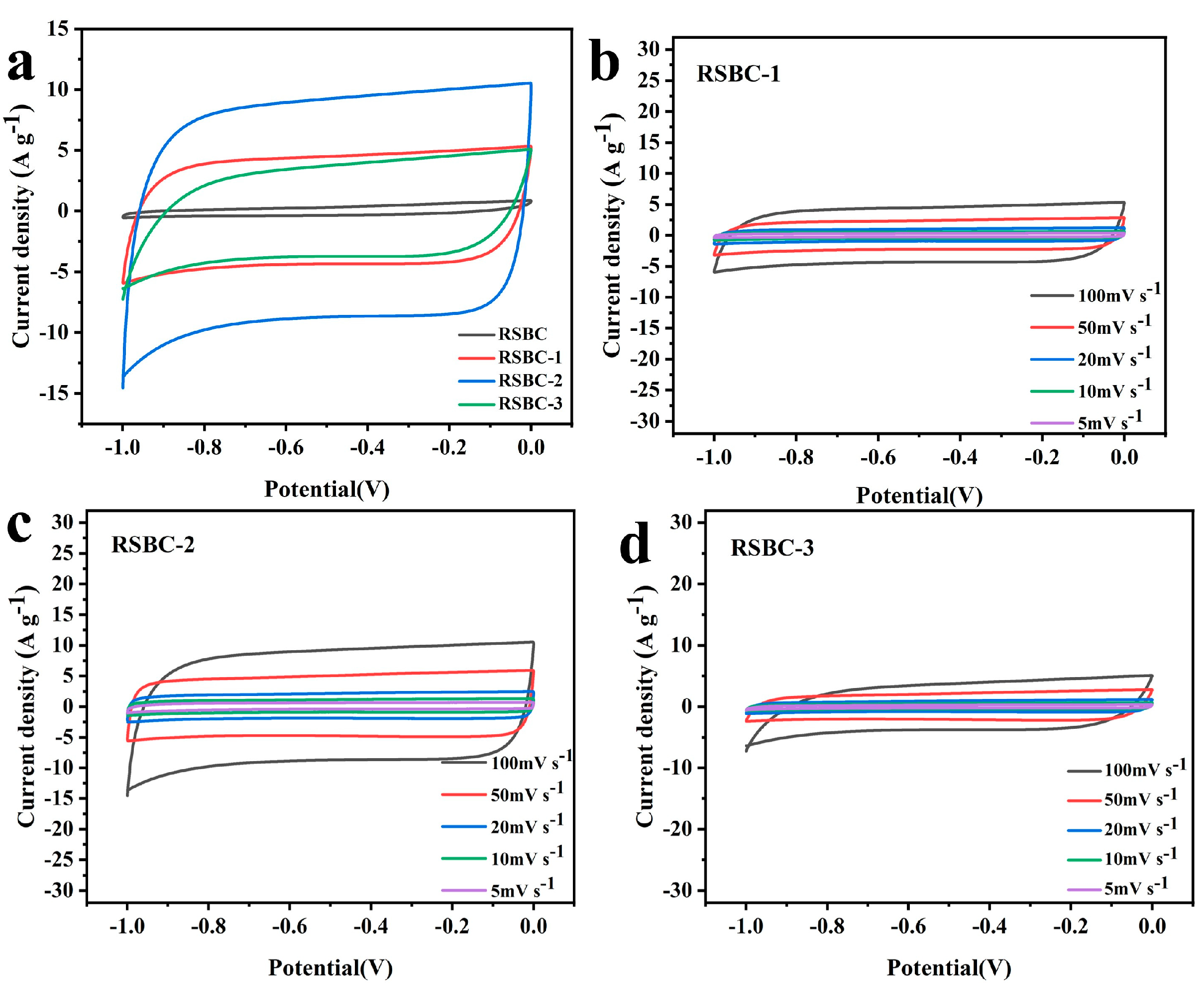
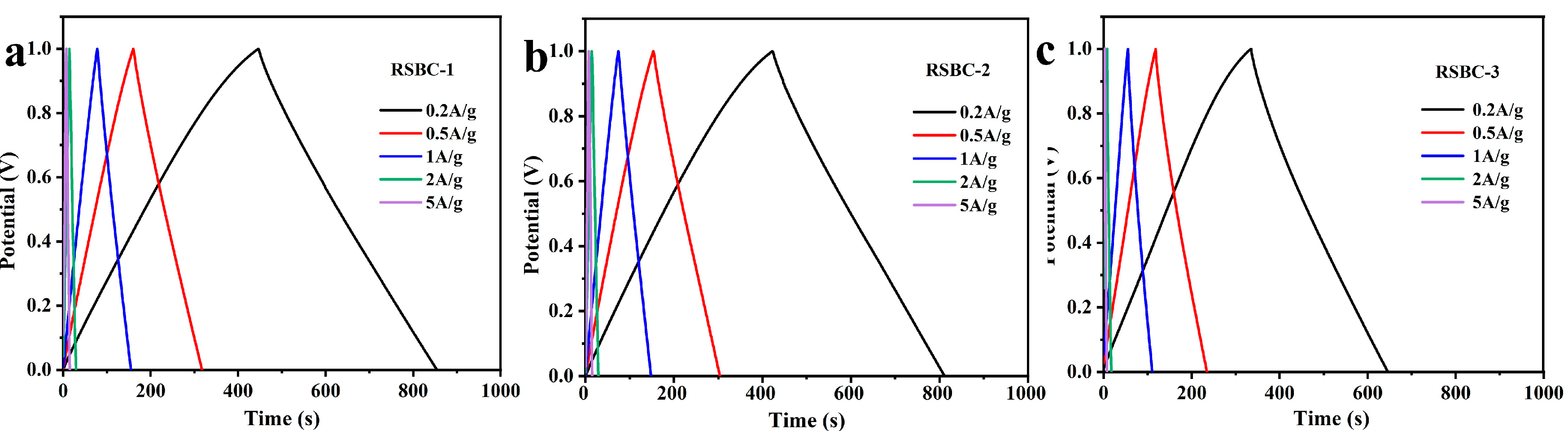
2.2. Effects of Activation Ratio
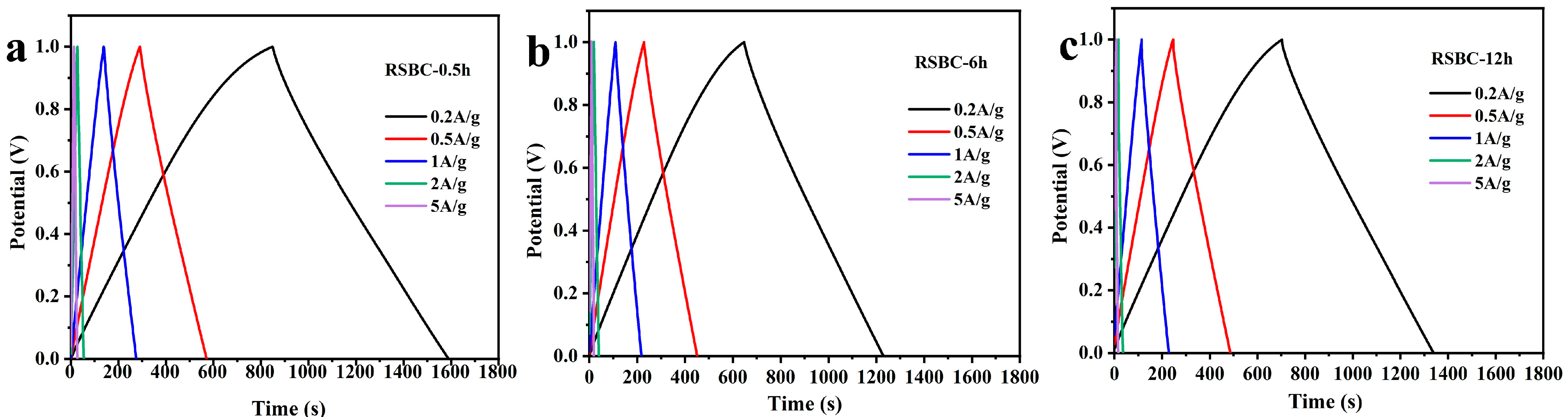
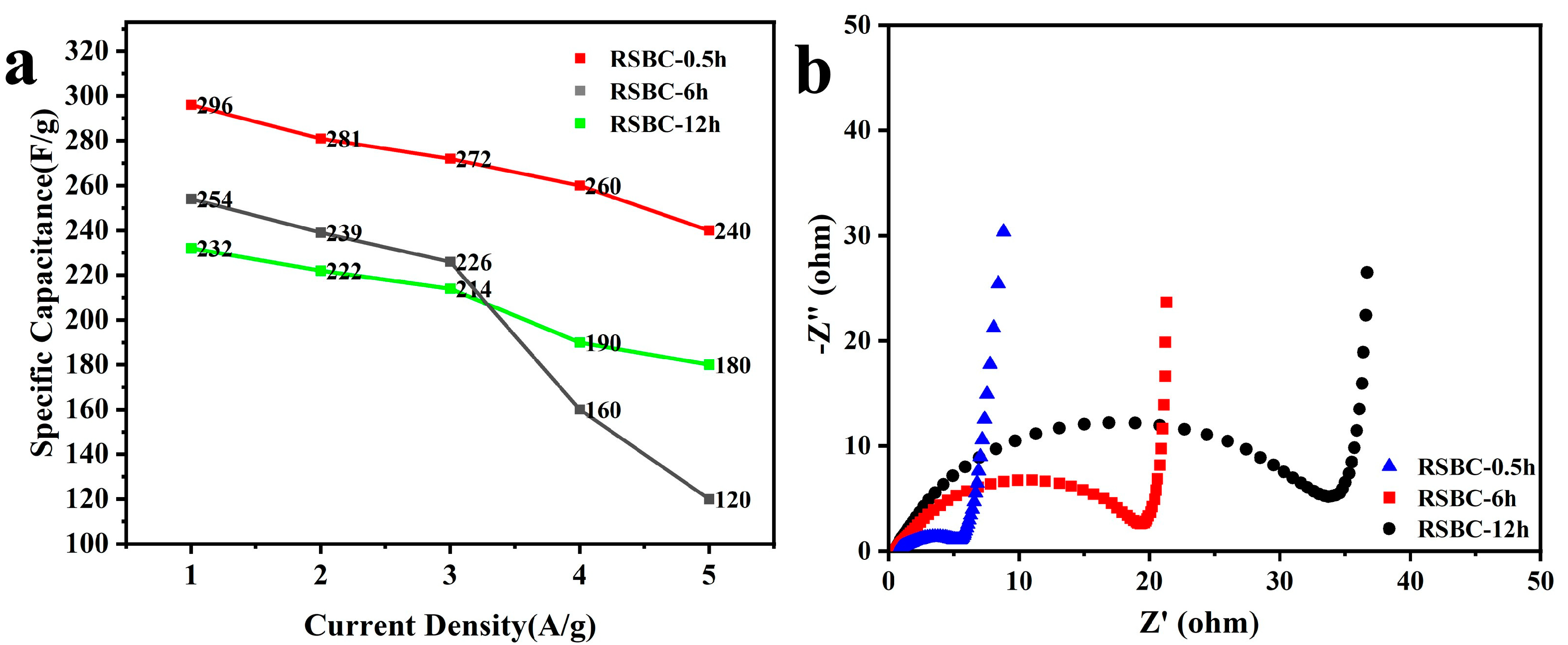
2.3. Effects of Activation Time
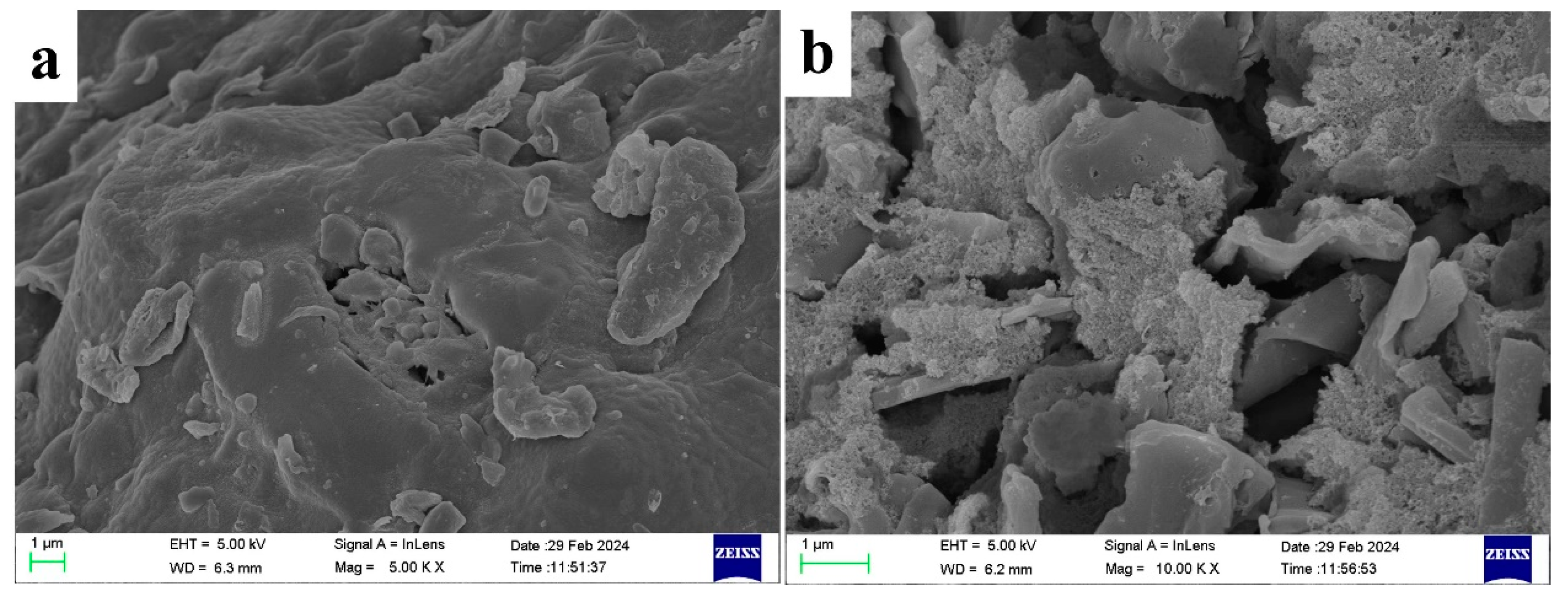
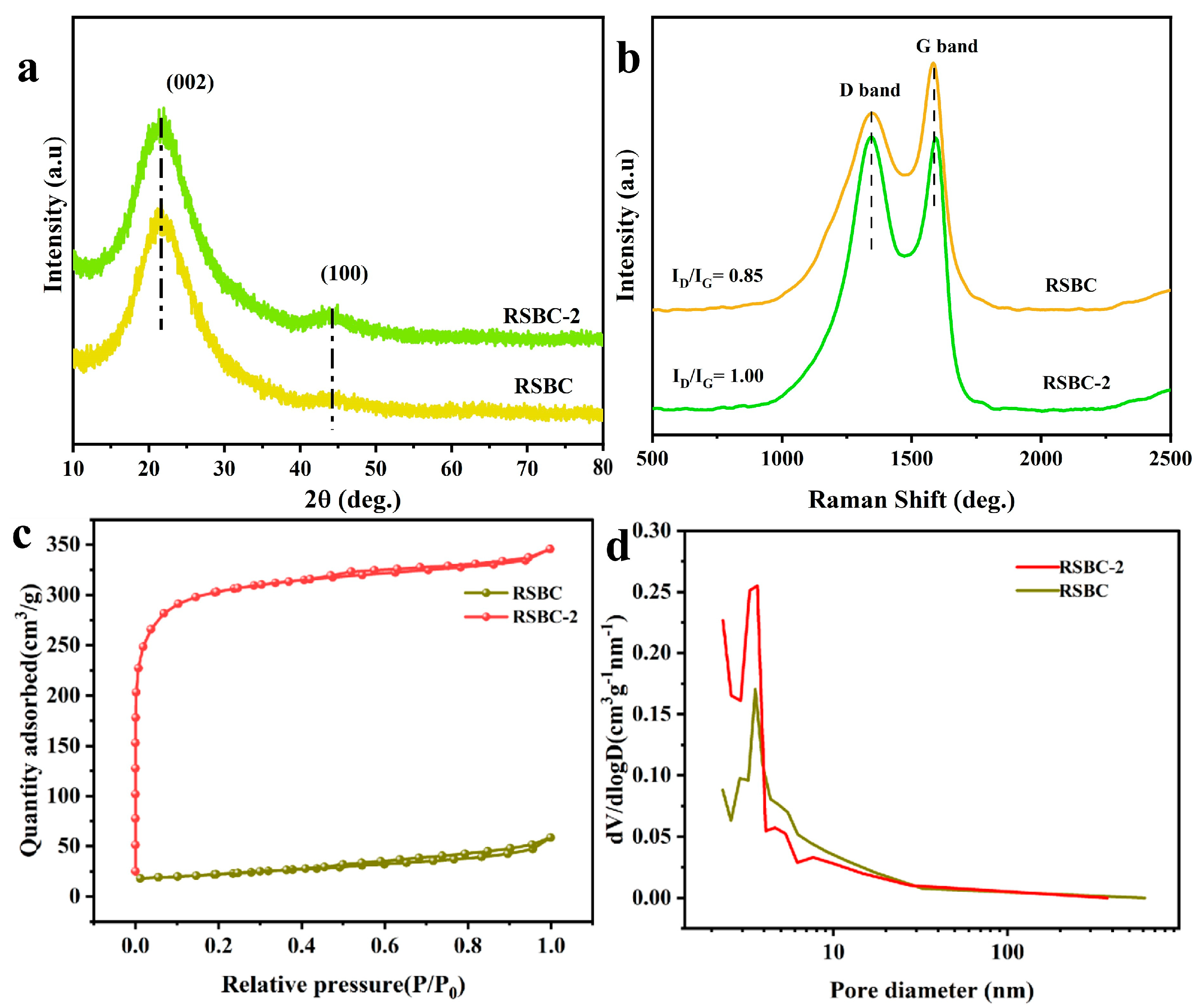
2.4. Morphological and Structural Characterization of Rice Straw Biochar Before and After Activation
3. Experimental
3.1. Materials Preparation
3.2. Materials Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, Z.Z.; Zhang, L.H.; Du, T.M.; Ren, B.; Xu, Y.L.; Wang, S.S.; Miao, J.F.; Liu, Z.F. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Zhu, Q.C.; Zhao, D.Y.; Cheng, M.Y.; Zhou, J.Q.; Owusu, K.A.; Mai, L.Q.; Yu, Y. A New view of supercapacitors: Integrated supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Xue, Q.; Sun, J.F.; Huang, Y.; Zhu, M.S.; Pei, Z.X.; Li, H.F.; Wang, Y.K.; Li, N.; Zhang, H.Y.; Zhi, C.Y. Recent progress on flexible and wearable supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Hussain, C.M. Green supercapacitors: Latest developments and perspectives in the pursuit of sustainability. Renew. Sustain. Energy Rev. 2024, 195, 114324. [Google Scholar] [CrossRef]
- Gao, M.M.; Wang, F.X.; Yang, S.; Ricciardulli, A.G.; Yu, F.; Li, J.K.; Sun, J.H.; Wang, R.H.; Huang, Y.; Zhang, P.P.; et al. Engineered 2D MXene-based materials for advanced supercapacitors and micro-supercapacitors. Mater. Today 2024, 72, 318–358. [Google Scholar] [CrossRef]
- Sahin, M.; Blaabjerg, F.; Sangwongwanich, A. A comprehensive review on supercapacitor applications and developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Naseri, F.; Karimi, S.; Farjah, E.; Schaltz, E. Supercapacitor management system: A comprehensive review of modeling, estimation, balancing, and protection techniques. Renew. Sustain. Energy Rev. 2022, 155, 111913. [Google Scholar] [CrossRef]
- Lv, T.; Liu, M.X.; Zhu, D.Z.; Gan, L.H.; Chen, T. Nanocarbon-based materials for flexible all-solid-state supercapacitors. Adv. Mater. 2018, 30, 1705489. [Google Scholar] [CrossRef] [PubMed]
- Zhong, M.Z.; Zhang, M.; Li, X.F. Carbon nanomaterials and their composites for supercapacitors. Carbon Energy 2022, 4, 950–985. [Google Scholar] [CrossRef]
- Han, R.; Liu, F.; Geng, S.; Yao, M.Q.; Luo, L.; Wang, X.D. N, O self-doped porous carbon derived from distiller’s grains for high performance supercapacitors. Ind. Crops Prod. 2024, 214, 118550. [Google Scholar] [CrossRef]
- Shao, Y.L.; El-Kady, M.F.; Wang, L.J.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z.; Mousavi, M.F.; Kaner, R.B. Graphene-based materials for flexible supercapacitors. Chem. Soc. Rev. 2015, 44, 3639–3665. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Paul, R.; Dai, L.M. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Wang, G.M.; Wang, H.Y.; Lu, X.H.; Ling, Y.C.; Yu, M.H.; Zhai, T.; Tong, Y.X.; Li, Y. Solid-state supercapacitor based on activated carbon cloths exhibits excellent rate capability. Adv. Mater. 2014, 26, 2676–2682. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Li, Z.J.; Guo, D.F.; Liu, Y.Y.; Wang, H.Y.; Wang, L.L. Recent advances and challenges in biomass -derived porous carbon nanomaterials for supercapacitors. Chem. Eng. J. 2020, 397, 125418. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Sathish, M. Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and non-aqueous electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Irfan, O.M.; Moustafa, H.M. H3PO4/KOH activation agent for high performance rice husk activated carbon electrode in acidic media supercapacitors. Molecules 2023, 28, 296. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.U.; Nanaji, K.; Rao, T.N.; Deshpande, A.S. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J. Power Sources 2020, 471, 228387. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Mishra, A.K. Performance optimization of the symmetric supercapacitors based on paddy straw-derived porous activated carbon. J. Energy Storage 2024, 79, 110167. [Google Scholar] [CrossRef]
- Jin, H.; Hu, J.P.; Wu, S.C.; Wang, X.L.; Zhang, H.; Xu, H.; Lian, K. Three-dimensional interconnected porous graphitic carbon derived from rice straw for high performance supercapacitors. J. Power Sources 2018, 384, 270–277. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hua, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.; Idris, A.; Abidin, Z.Z. Batch adsorption of basic dye using acid treated kenaf fibre char: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2012, 181, 449–457. [Google Scholar] [CrossRef]
- Kim, M.I.; Bai, B.C. Effect of nitric acid treatment on the pitch properties and preparation of activated carbon. Carbon Lett. 2022, 32, 99–107. [Google Scholar] [CrossRef]
- Pietrzak, R.; Nowicki, P.; Kazmierczak, J.; Kuszynska, I.; Goscianska, J.; Przepiórski, J. Comparison of the effects of different chemical activation methods on properties of carbonaceous adsorbents obtained from cherry stones. Chem. Eng. Res. Des. 2014, 92, 1187–1191. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; Gyenge, E.; Ellis, N. A novel method to tailor the porous structure of KOH-activated biochar and its application in capacitive deionization and energy storage. Biomass Bioenergy 2016, 87, 107–121. [Google Scholar] [CrossRef]
- Pastor-Villegas, J.; Durán-Valle, C.J. Pore structure of activated carbons prepared by carbon dioxide and steam activation at different temperatures from extracted rockrose. Carbon 2002, 40, 397–402. [Google Scholar] [CrossRef]
- Mei, B.A.; Munteshari, O.; Lau, J.; Dunn, B.; Pilon, L. Physical interpretations of nyquist plots for EDLC electrodes and devices. J. Phys. Chem. C 2018, 122, 194–206. [Google Scholar] [CrossRef]
- Thongpat, W.; Taweekun, J.; Maliwan, K. Synthesis and characterization of microporous activated carbon from rubberwood by chemical activation with KOH. Carbon Lett. 2021, 31, 1079–1088. [Google Scholar] [CrossRef]
- Lee, H.M.; Kim, H.G.; An, K.H.; Kim, B.J. The Effect of CO2 activation on the electrochemical performance of coke-based activated carbons for supercapacitors. J. Nanosci. Nanotechnol. 2015, 15, 8797–8802. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Yang, K.S.; Yang, D.J. Electrochemical behavior of activated carbon nanofiber-vanadium pentoxide composites for double-layer capacitors. Electrochim. Acta 2013, 109, 859–865. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, B.; Chen, D.; Chen, J.X.; Zhang, Q.; Jiang, L.J.; Lan, T.C.; Zhang, C.M.; Yang, W.S.; He, S.J. Free-standing carbon network with enhanced capacitive performance synthesized via green H2O2 activation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131425. [Google Scholar] [CrossRef]
- Qin, Y.; Chai, B.; Wang, C.; Yan, J.; Fan, G.; Song, G. Removal of tetracycline onto KOH-activated biochar derived from rape straw: Affecting factors, mechanisms and reusability inspection. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128466. [Google Scholar] [CrossRef]
- Ding, Y.; Qiao, Z. Carbon surface chemistry: New insight into the old story. Adv. Mater. 2022, 34, 2206025. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Gu, Q.; Klyushin, A.; Huang, X.; Choudhury, S.; Spanos, I.; Song, F.; Mom, R.; Düngen, P.; Mechler, A.; et al. Dynamic carbon surface chemistry: Revealing the role of carbon in electrolytic water oxidation. J. Energy Chem. 2020, 47, 155–159. [Google Scholar] [CrossRef]
- Zhu, M.N.; Liu, H.; Cao, Q.P.; Zheng, H.; Xu, D.; Guo, H.Y.; Wang, S.M.; Li, Y.; Zhou, J.H. Electrospun lignin-based carbon nanofibers as supercapacitor electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841. [Google Scholar] [CrossRef]



| Samples | 0.2 A/g | 0.5 A/g | 1 A/g | 2 A/g | 5 A/g |
|---|---|---|---|---|---|
| RSBC-700 | 147.6 F g−1 | 134 F g−1 | 128 F g−1 | 124 F g−1 | 120 F g−1 |
| RSBC-800 | 197.2 F g−1 | 185 F g−1 | 178 F g−1 | 172 F g−1 | 170 F g−1 |
| RSBC-900 | 86.8 F g−1 | 83 F g−1 | 82 F g−1 | 80 F g−1 | 80 F g−1 |
| Samples | 0.2 A/g | 0.5 A/g | 1 A/g | 2 A/g | 5 A/g |
|---|---|---|---|---|---|
| RSBC-1 | 222 F g−1 | 209 F g−1 | 198 F g−1 | 160 F g−1 | 137 F g−1 |
| RSBC-2 | 296 F g−1 | 281 F g−1 | 272 F g−1 | 260 F g−1 | 240 F g−1 |
| RSBC-3 | 164 F g−1 | 158 F g−1 | 147 F g−1 | 135 F g−1 | 125 F g−1 |
| Samples | 0.2 A/g | 0.5 A/g | 1 A/g | 2 A/g | 5 A/g |
|---|---|---|---|---|---|
| RSBC-0.5h | 296 F g−1 | 281 F g−1 | 272 F g−1 | 260 F g−1 | 240 F g−1 |
| RSBC-6h | 254 F g−1 | 239 F g−1 | 226 F g−1 | 160 F g−1 | 120 F g−1 |
| RSBC-12h | 232 F g−1 | 222 F g−1 | 214 F g−1 | 190 F g−1 | 180 F g−1 |
| Samples | SBET (m2 g−1) | Vt (cm3 g−1) | Average Pore Size (nm) |
|---|---|---|---|
| RSBC | 75.339 | 0.087 | 4.630 |
| RSBC-2 | 939.404 | 0.533 | 2.268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Lu, Y.; Sun, Y.; Deng, S.; Yang, H.; Zhang, M.; Wang, C.; Yan, J. Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes. Molecules 2025, 30, 632. https://doi.org/10.3390/molecules30030632
Cheng J, Lu Y, Sun Y, Deng S, Yang H, Zhang M, Wang C, Yan J. Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes. Molecules. 2025; 30(3):632. https://doi.org/10.3390/molecules30030632
Chicago/Turabian StyleCheng, Jialuo, Yumeng Lu, Ya Sun, Sunhua Deng, Heng Yang, Manman Zhang, Chunlei Wang, and Juntao Yan. 2025. "Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes" Molecules 30, no. 3: 632. https://doi.org/10.3390/molecules30030632
APA StyleCheng, J., Lu, Y., Sun, Y., Deng, S., Yang, H., Zhang, M., Wang, C., & Yan, J. (2025). Impact of Activation Conditions on the Electrochemical Performance of Rice Straw Biochar for Supercapacitor Electrodes. Molecules, 30(3), 632. https://doi.org/10.3390/molecules30030632








