Optimization of Gelatin and Crosslinker Concentrations in a Gelatin/Alginate-Based Bioink with Potential Applications in a Simplified Skin Model
Abstract
:1. Introduction
2. Results
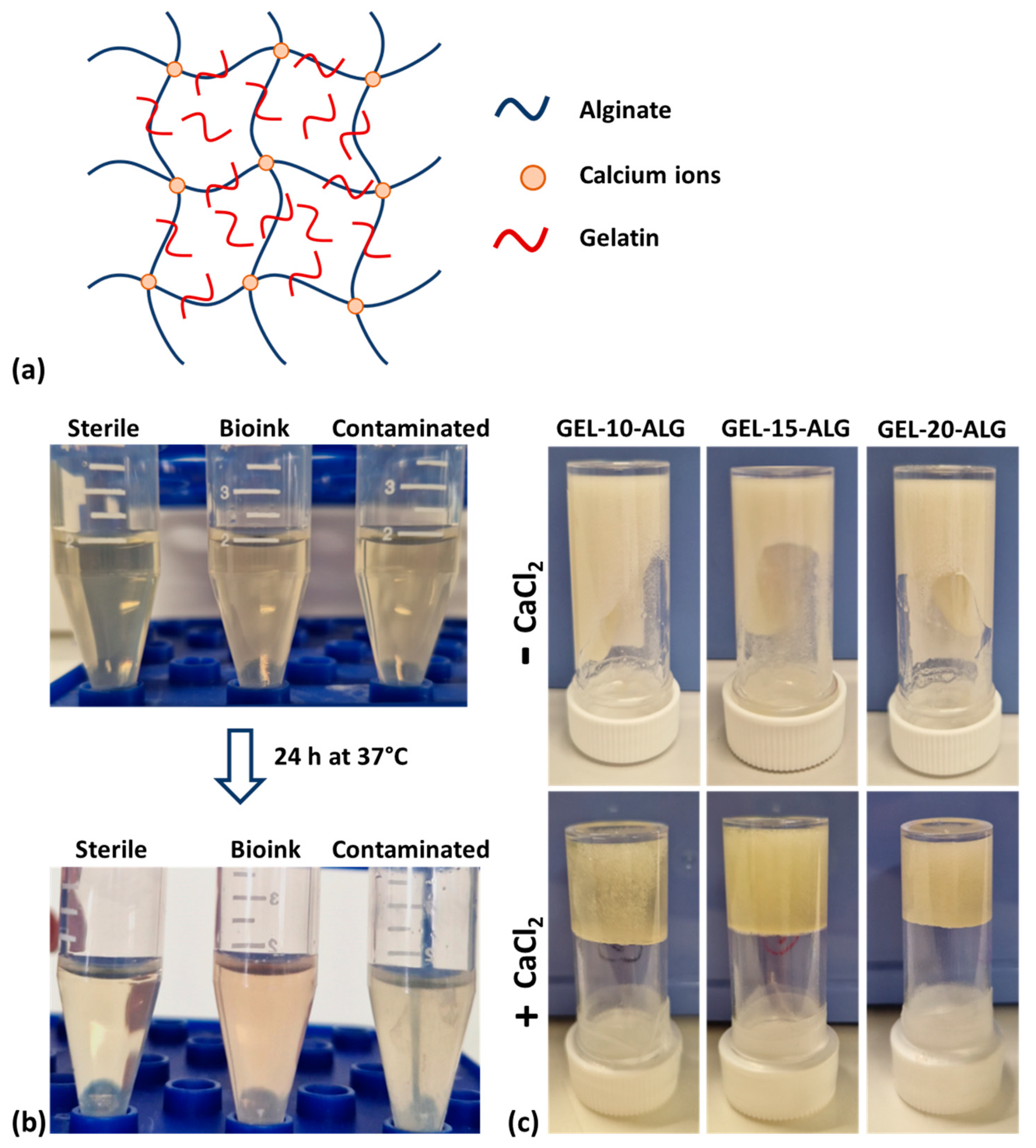
2.1. Bioink Sterility Assessment
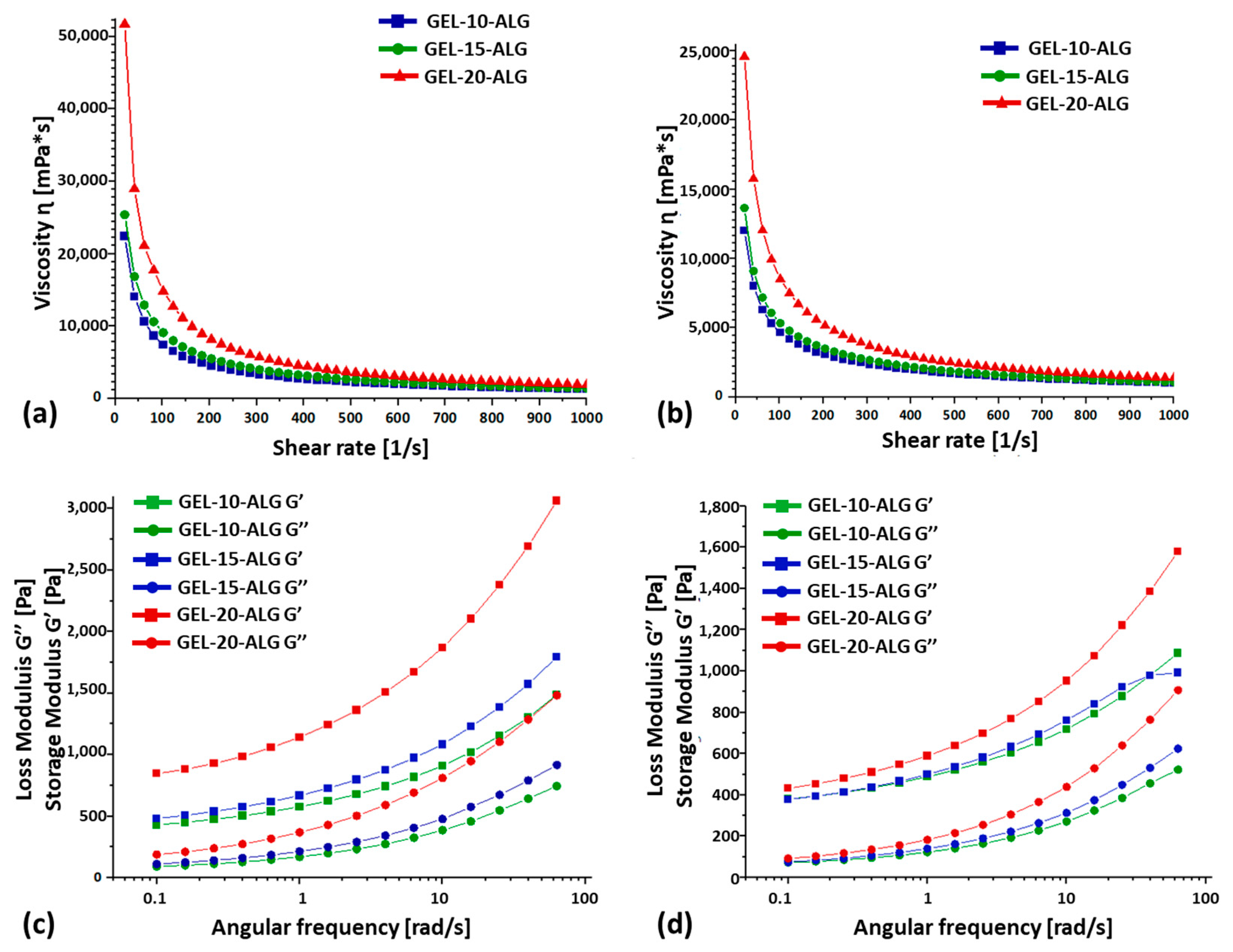
2.2. Rheological Properties and Homogeneity
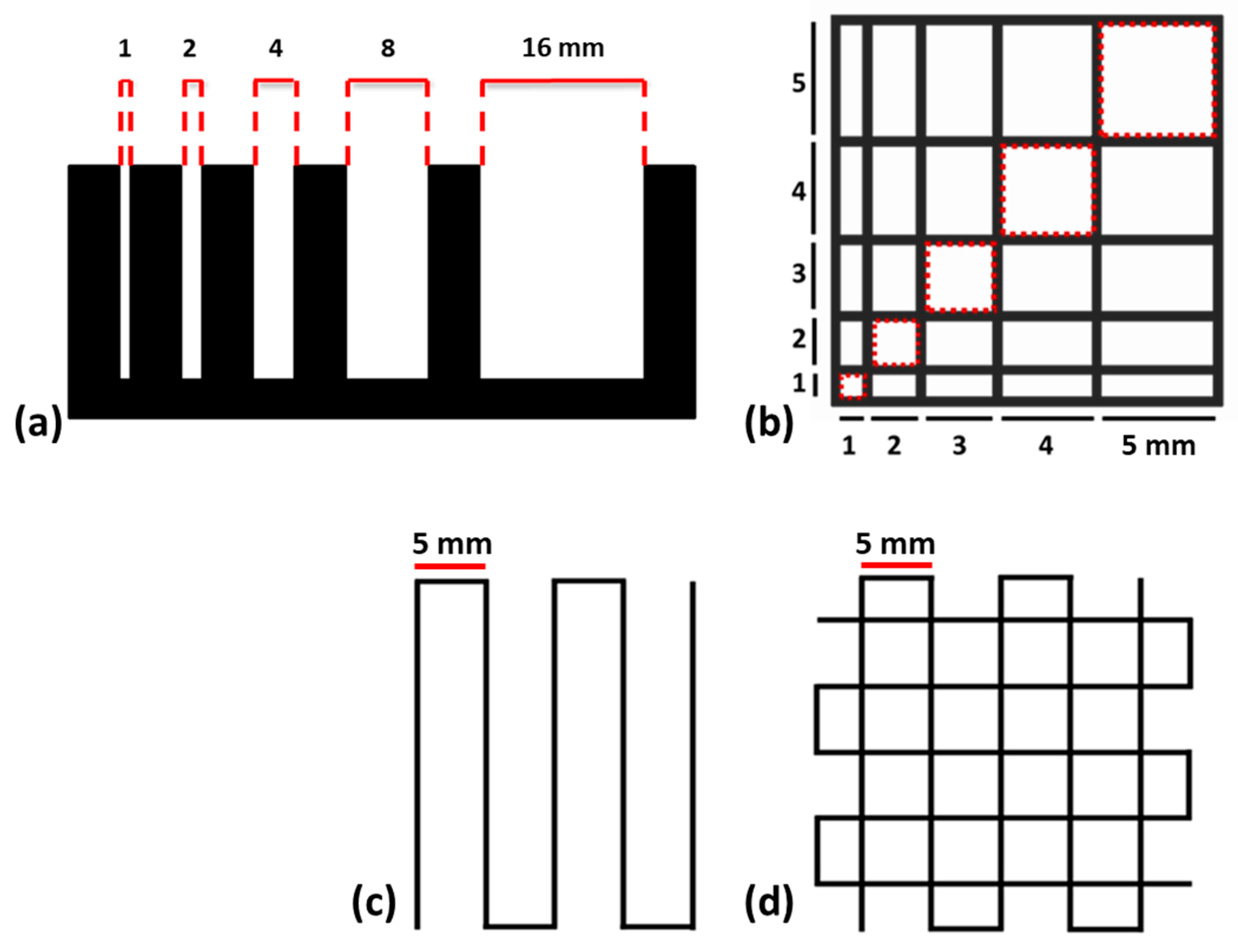
2.3. Printability
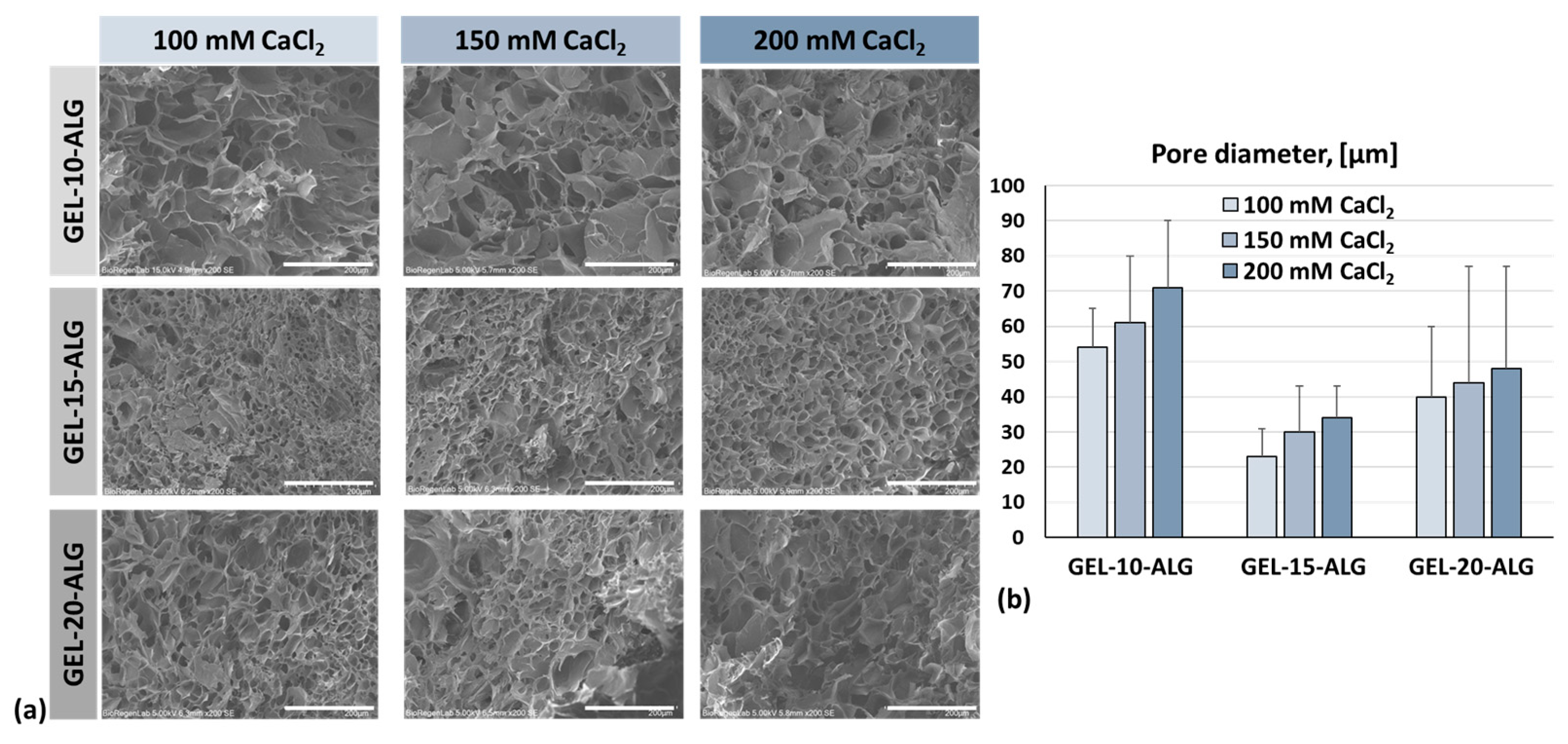
2.4. Morphology
2.5. Degradation and Swelling
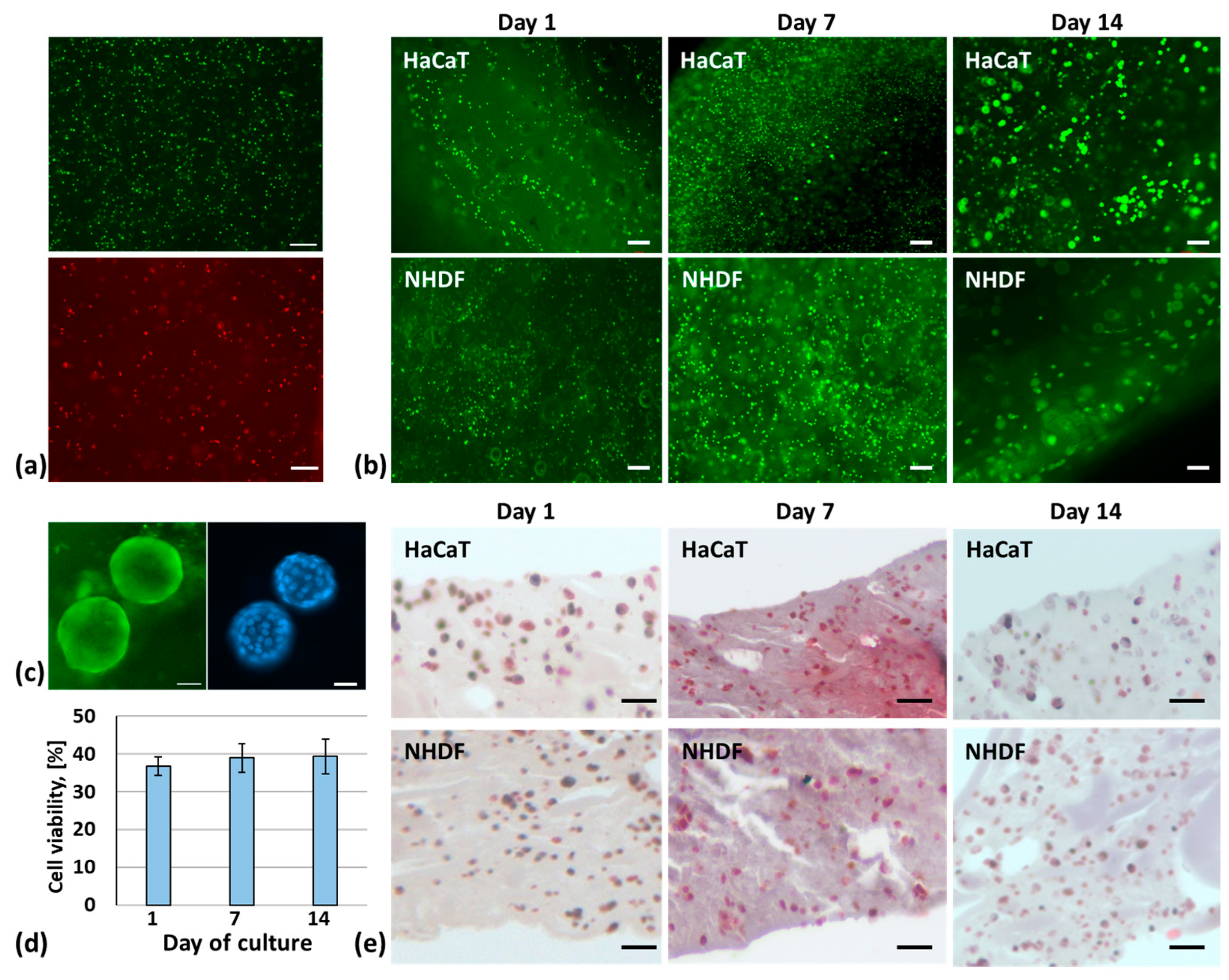
2.6. Three-Dimensional Bioprinting of a Skin Model
3. Discussion
4. Materials and Methods
4.1. Bioink Preparation
4.2. Sterility Evaluation
4.3. Rheological Properties and Homogeneity
4.4. Printability
4.5. Morphology
4.6. Degradation and Swelling
4.7. Three-Dimensional Bioprinting of a Skin Model
4.8. Cell Viability and Proliferation
4.9. Histological Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, W.M. The Development of the Three Rs Concept. Altern. Lab. Anim. 1995, 23, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zuo, X.; Yuan, J.; Xie, Z.; Yin, L.; Pu, Y.; Chen, Z.; Zhang, J. Research Progress in the Development of 3D Skin Models and Their Application to in Vitro Skin Irritation Testing. J. Appl. Toxicol. 2024, 44, 1302–1316. [Google Scholar] [CrossRef]
- Bhar, B.; Das, E.; Manikumar, K.; Mandal, B.B. 3D Bioprinted Human Skin Model Recapitulating Native-Like Tissue Maturation and Immunocompetence as an Advanced Platform for Skin Sensitization Assessment. Adv. Healthc. Mater. 2024, 13, e2303312. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.; Escamez, M.J.; Carretero, M.; Mirones, I.; Martinez-Santamaria, L.; Navarro, M.; Jorcano, J.L.; Meana, A.; Del Rio, M.; Larcher, F. Modeling Normal and Pathological Processes through Skin Tissue Engineering. Mol. Carcinog. 2007, 46, 741–745. [Google Scholar] [CrossRef]
- Hansen, K.; Khanna, C. Spontaneous and Genetically Engineered Animal Models: Use in Preclinical Cancer Drug Development. Eur. J. Cancer 2004, 40, 858–880. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Jang, J.; Jo, H.J.; Kim, W.H.; Kim, B.; Chun, H.J.; Lim, D.; Han, D.W. Advances and Innovations of 3D Bioprinting Skin. Biomolecules 2022, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Varpe, A.; Sayed, M.; Mane, N.S. A Comprehensive Literature Review on Advancements and Challenges in 3D Bioprinting of Human Organs: Ear, Skin, and Bone. Ann. Biomed. Eng. 2025, 53, 14–33. [Google Scholar] [CrossRef]
- Asim, S.; Tabish, T.A.; Liaqat, U.; Ozbolat, I.T.; Rizwan, M. Advances in Gelatin Bioinks to Optimize Bioprinted Cell Functions. Adv. Healthc. Mater. 2023, 12, e2203148. [Google Scholar] [CrossRef]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Pisani, A.; Foffa, I.; Vecoli, C.; Buscemi, M.; Guazzelli, L.; Soldani, G.; et al. Marine Collagen-Based Bioink for 3D Bioprinting of a Bilayered Skin Model. Pharmaceutics 2023, 15, 1331. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Guazzelli, L.; Soldani, G.; Losi, P. Fibrinogen-Based Bioink for Application in Skin Equivalent 3D Bioprinting. J. Funct. Biomater. 2023, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Bramhe, P.; Rarokar, N.; Kumbhalkar, R.; Saoji, S.; Khedekar, P. Natural and Synthetic Polymeric Hydrogel: A Bioink for 3D Bioprinting of Tissue Models. J. Drug Deliv. Sci. Technol. 2024, 101, 106204. [Google Scholar] [CrossRef]
- Yang, J.; He, H.; Li, D.; Zhang, Q.; Xu, L.; Ruan, C. Advanced Strategies in the Application of Gelatin-Based Bioink for Extrusion Bioprinting. Bio-Des. Manuf. 2023, 6, 586–608. [Google Scholar] [CrossRef]
- Sakai, S.; Moriyama, K.; Kawakami, K. Controlling Thermo-Reversibility of Gelatin Gels through a Peroxidase-Catalyzed Reaction under Mild Conditions for Mammalian Cells. J. Biomater. Sci. Polym. Ed. 2011, 22, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.J.; Ou, Y.C. The Micro Patterning of Glutaraldehyde (GA)-Crosslinked Gelatin and Its Application to Cell-Culture. Lab Chip 2005, 5, 979–984. [Google Scholar] [CrossRef]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking Structures of Gelatin Hydrogels Crosslinked with Genipin or a Water-Soluble Carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An Injectable, in Situ Enzymatically Gellable, Gelatin Derivative for Drug Delivery and Tissue Engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef]
- Gaglio, C.G.; Baruffaldi, D.; Pirri, C.F.; Napione, L.; Frascella, F. GelMA Synthesis and Sources Comparison for 3D Multimaterial Bioprinting. Front. Bioeng. Biotechnol. 2024, 12, 1383010. [Google Scholar] [CrossRef] [PubMed]
- Cuvellier, M.; Ezan, F.; Oliveira, H.; Rose, S.; Fricain, J.C.; Langouët, S.; Legagneux, V.; Baffet, G. 3D Culture of HepaRG Cells in GelMa and Its Application to Bioprinting of a Multicellular Hepatic Model. Biomaterials 2021, 269, 120611. [Google Scholar] [CrossRef]
- Liu, W.; Heinrich, M.A.; Zhou, Y.; Akpek, A.; Hu, N.; Liu, X.; Guan, X.; Zhong, Z.; Jin, X.; Khademhosseini, A.; et al. Extrusion Bioprinting of Shear-Thinning Gelatin Methacryloyl Bioinks. Adv. Healthc. Mater. 2017, 6, 1601451. [Google Scholar] [CrossRef]
- Veiga, A.; Silva, I.V.; Duarte, M.M.; Oliveira, A.L. Current Trends on Protein Driven Bioinks for 3D Printing. Pharmaceutics 2021, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Eswaramoorthy, S.D.; Dhiman, N.; Joshi, A.; Rath, S.N. 3D Bioprinting of Mesenchymal Stem Cells and Endothelial Cells in an Alginate-Gelatin-Based Bioink. J. 3D Print. Med. 2021, 5, 23–36. [Google Scholar] [CrossRef]
- Chawla, D.; Kaur, T.; Joshi, A.; Singh, N. 3D Bioprinted Alginate-Gelatin Based Scaffolds for Soft Tissue Engineering. Int. J. Biol. Macromol. 2020, 144, 560–567. [Google Scholar] [CrossRef]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-Dimensional printing alginate/gelatin scaffolds as dermal substitutes for skin tissue engineering. Polym. Eng. Sci. 2018, 58, 1782–1790. [Google Scholar] [CrossRef]
- Huang, S.; Yao, B.; Xie, J.; Fu, X. 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 2016, 32, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D Bioprinting of a Gelatin-Alginate Hydrogel for Tissue-Engineered Hair Follicle Regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Kim, J.-H.; Kim, M.; Marinho, P.A.; Nho, Y.; Kang, S.; Yun, W.-S.; Shim, J.-H.; Jin, S. 3D Bioprinting of in Vitro Full-Thickness Skin Model with a Rete Ridge Structure. Int. J. Bioprint. 2024, 10, 3961. [Google Scholar] [CrossRef]
- Ahn, M.; Cho, W.W.; Park, W.; Lee, J.S.; Choi, M.J.; Gao, Q.; Gao, G.; Cho, D.W.; Kim, B.S. 3D Biofabrication of Diseased Human Skin Models in Vitro. Biomater. Res. 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Augello, F.R.; Ciafarone, A.; Ciummo, V.; Altamura, S.; Cinque, B.; Palumbo, P. 3D Models Currently Proposed to Investigate Human Skin Aging and Explore Preventive and Reparative Approaches: A Descriptive Review. Biomolecules 2024, 14, 1066. [Google Scholar] [CrossRef]
- Zoratto, N.; Matricardi, P. Semi-IPN- and IPN-Based Hydrogels. Adv. Exp. Med. Biol. 2018, 1059, 155–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. An Interpenetrating Alginate/Gelatin Network for Three-Dimensional (3D) Cell Cultures and Organ Bioprinting. Molecules 2020, 25, 756. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Ou, K.-L.; Liu, C.-M.; Chu, S.-F.; Huang, B.-H.; Cho, Y.-C.; Saito, T.; Tsai, C.-H.; Hung, K.-S.; Lan, W.-C.A.; et al. A Three-Dimensional Bioprinted Copolymer Scaffold with Biocompatibility and Structural Integrity for Potential Tissue Regeneration Applications. Polymers 2022, 14, 3415. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.D.; Onofrillo, C.; Duchi, S.; Li, X.; Zhang, Y.; Tian, P.; Lu, L.; Trengove, A.; Quigley, A.; Gambhir, S.; et al. Evaluation of Sterilisation Methods for Bio-Ink Components: Gelatin, Gelatin Methacryloyl, Hyaluronic Acid and Hyaluronic Acid Methacryloyl. Biofabrication 2019, 11, 035003. [Google Scholar] [CrossRef]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; Espona-Noguera, A.; Galvez-Martin, P.; López-Ruiz, E.; Marchal, J.A.; López-Donaire, M.L.; Zabala, A.; Ciriza, J.; Saenz-del-Burgo, L.; et al. Development, Characterization and Sterilisation of Nanocellulose-Alginate-(Hyaluronic Acid)- Bioinks and 3D Bioprinted Scaffolds for Tissue Engineering. Mater. Sci. Eng. C 2021, 126, 112160. [Google Scholar] [CrossRef] [PubMed]
- Lorson, T.; Ruopp, M.; Nadernezhad, A.; Eiber, J.; Vogel, U.; Jungst, T.; Lühmann, T. Sterilization Methods and Their Influence on Physicochemical Properties and Bioprinting of Alginate as a Bioink Component. ACS Omega 2020, 5, 6481–6486. [Google Scholar] [CrossRef] [PubMed]
- Sheth, N.C.; Rathod, Y.V.; Shenoi, P.R.; Shori, D.D.; Khode, R.T.; Khadse, A.P. Evaluation of new technique of sterilization using biological indicator. J. Conserv. Dent. 2017, 20, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Ma, Z.; Dimitrov, A.; Kunze, M.; Mulet-Sierra, A.; Ansari, K.; Osswald, M.; Seikaly, H.; Boluk, Y.; Adesida, A.B. Double Crosslinked Hyaluronic Acid and Collagen as a Potential Bioink for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2024, 273, 132819. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and Bioprinting Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater. Today Bio 2019, 1, 100008. [Google Scholar] [CrossRef]
- Falcone, G.; Mazzei, P.; Piccolo, A.; Esposito, T.; Mencherini, T.; Aquino, R.P.; Del Gaudio, P.; Russo, P. Advanced Printable Hydrogels from Pre-Crosslinked Alginate as a New Tool in Semi Solid Extrusion 3D Printing Process. Carbohydr. Polym. 2022, 276, 118746. [Google Scholar] [CrossRef]
- Feng, H.; Song, Y.; Lian, X.; Zhang, S.; Bai, J.; Gan, F.; Lei, Q.; Wei, Y.; Huang, D. Study on Printability Evaluation of Alginate/Silk Fibroin/Collagen Double-Cross-Linked Inks and the Properties of 3D Printed Constructs. ACS Biomater. Sci. Eng. 2024, 10, 6581–6593. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Palma, J.H.; Bertuola, M.; Hermida, É.B. Modeling Calcium Diffusion and Crosslinking Dynamics in a Thermogelling Alginate-Gelatin-Hyaluronic Acid Ink: 3D Bioprinting Applications. Bioprinting 2024, 38, e00329. [Google Scholar] [CrossRef]
- Skopinska-Wisniewska, J.; Tuszynska, M.; Ka’zmierski, Ł.K.; Bartniak, M.; Bajek, A. Gelatin–Sodium Alginate Hydrogels Cross-Linked by Squaric Acid and Dialdehyde Starch as a Potential Bio-Ink. Polymers 2024, 16, 2560. [Google Scholar] [CrossRef] [PubMed]
- Serafin, A.; Culebras, M.; Collins, M.N. Synthesis and evaluation of alginate, gelatin, and hyaluronic acid hybrid hydrogels for tissue engineering applications. Int. J. Biol. Macromol. 2023, 233, 123438. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Richter, S.; Berger, S. Diffusion NMR as a New Method for the Determination of the Gel Point of Gelatin. J. Phys. Chem. B 2006, 110, 15853–15857. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.A.M.; da Silva, V.A.; Scheck, K.; Garay, T.; Sharma, R.; Willerth, S.M. 3D Bioprinting a Novel Skin Co-Culture Model Using Human Keratinocytes and Fibroblasts. J. Biomed. Mater. Res. A 2024, 113, e37831. [Google Scholar] [CrossRef]
- Sekar, M.P.; Budharaju, H.; Sethuraman, S.; Sundaramurthi, D. Carboxymethyl Cellulose-Agarose-Gelatin: A Thermoresponsive Triad Bioink Composition to Fabricate Volumetric Soft Tissue Constructs. SLAS Technol. 2023, 28, 183–198. [Google Scholar] [CrossRef]
- Galocha-León, C.; Antich, C.; Voltes-Martínez, A.; Marchal, J.A.; Mallandrich, M.; Halbaut, L.; Souto, E.B.; Gálvez-Martín, P.; Clares-Naveros, B. Human mesenchymal stromal cells-laden crosslinked hyaluronic acid-alginate bioink for 3D bioprinting applications in tissue engineering. Drug Deliv. Transl. Res. 2025, 15, 291–311. [Google Scholar] [CrossRef]
- Galocha-León, C.; Antich, C.; Clares-Naveros, B.; Voltes-Martínez, A.; Marchal, J.A.; Gálvez-Martín, P. Design and Characterization of Biomimetic Hybrid Construct Based on Hyaluronic Acid and Alginate Bioink for Regeneration of Articular Cartilage. Pharmaceutics 2024, 16, 1422. [Google Scholar] [CrossRef]
- Majumder, N.; Mishra, A.; Ghosh, S. Effect of Varying Cell Densities on the Rheological Properties of the Bioink. Bioprinting 2022, 28, e00241. [Google Scholar] [CrossRef]
- Gillispie, G.J.; Han, A.; Uzun-Per, M.; Fisher, J.; Mikos, A.G.; Niazi, M.K.K.; Yoo, J.J.; Lee, S.J.; Atala, A. The Influence of Printing Parameters and Cell Density on Bioink Printing Outcomes. Tissue Eng. Part A 2020, 26, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Pitarresi, G.; Palumbo, F.S.; Barberi, G.; Fiorica, C.; Giammona, G. Correlating Rheological Properties of a Gellan Gum-Based Bioink: A Study of the Impact of Cell Density. Polymers 2022, 14, 1844. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, A.M.; Graf, R.; Lim, P.J.; Zhang, J.; Schädli, G.N.; Peterhans, S.; Rohrbach, M.; Giunta, C.; Rüger, M.; Rubert, M.; et al. Physiological cell bioprinting density in human bone-derived cell-laden scaffolds enhances matrix mineralization rate and stiffness under dynamic loading. Front. Bioeng. Biotechnol. 2024, 12, 1310289. [Google Scholar] [CrossRef]
- Jorgensen, A.M.; Gorkun, A.; Mahajan, N.; Willson, K.; Clouse, C.; Jeong, C.G.; Varkey, M.; Wu, M.; Walker, S.J.; Molnar, J.A.; et al. Multicellular bioprinted skin facilitates human-like skin architecture in vivo. Sci. Transl. Med. 2023, 15, eadf7547. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef] [PubMed]

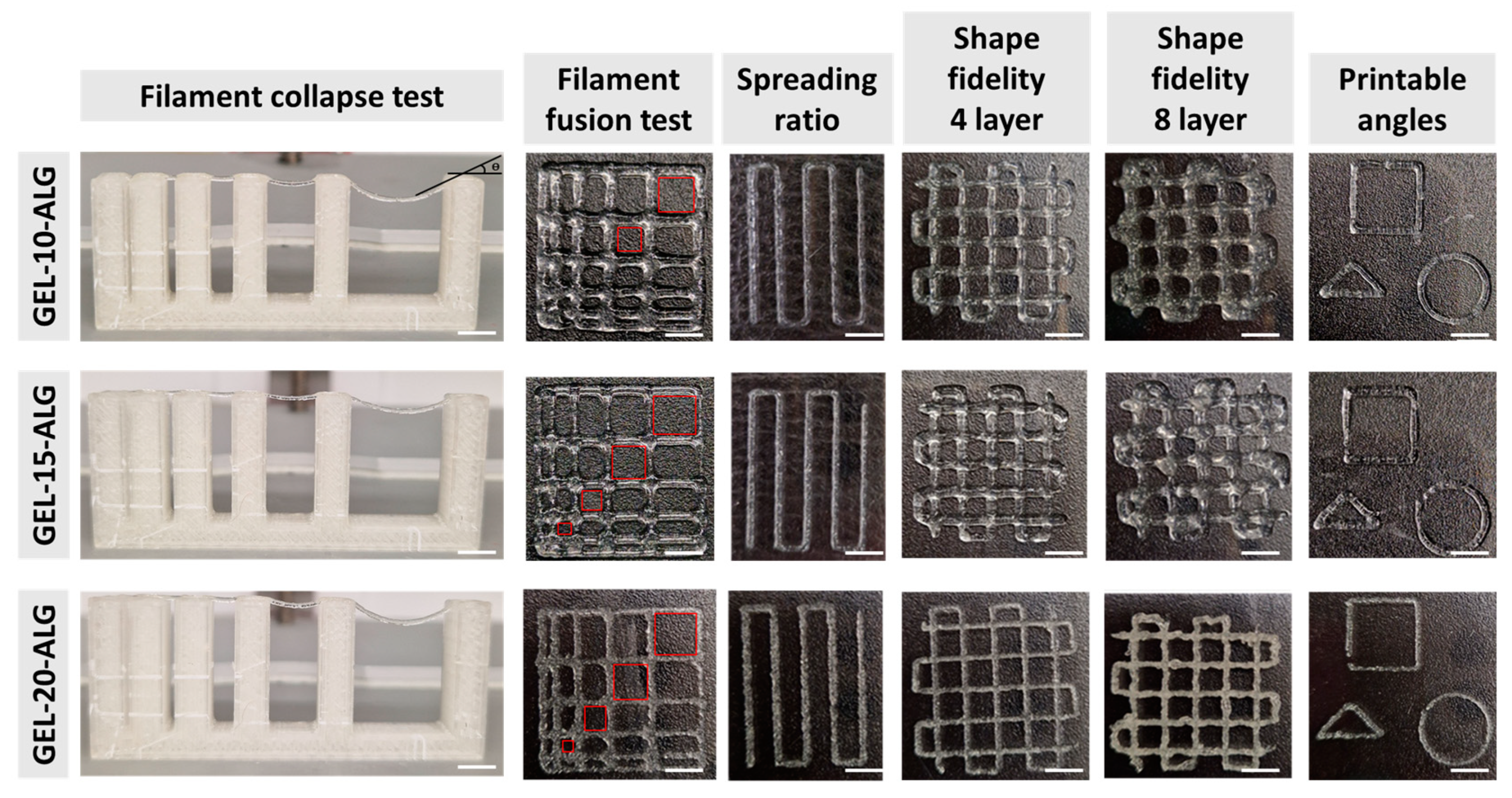
| Bioink | Extrusion Pressure, [KPa] | Printing Speed, [mm/s] | SR | Pr 4 Layers | Pr 8 Layers |
|---|---|---|---|---|---|
| GEL-10-ALG | 10 | 4 | 2 ± 0.3 | 0.97 ± 0.2 | 0.94 ± 0.1 |
| GEL-15-ALG | 17 | 6 | 1.2 ± 0.2 | 0.95 ± 0.3 | 0.92 ± 0.2 |
| GEL-20-ALG | 32 | 9 | 1.5 ± 0.1 | 0.99 ± 0.1 | 1.12 ± 0.1 |
| Bioink | Gelatin, [%] | Alginate, [%] | CaCl2, [mM] |
|---|---|---|---|
| GEL-10-ALG | 7.4 | 4.4 | 26 |
| GEL-15-ALG | 11 | 4.4 | 26 |
| GEL-20-ALG | 14.8 | 4.4 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallo, A.; Radaelli, G.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Guazzelli, L.; Soldani, G.; Losi, P. Optimization of Gelatin and Crosslinker Concentrations in a Gelatin/Alginate-Based Bioink with Potential Applications in a Simplified Skin Model. Molecules 2025, 30, 649. https://doi.org/10.3390/molecules30030649
Cavallo A, Radaelli G, Al Kayal T, Mero A, Mezzetta A, Guazzelli L, Soldani G, Losi P. Optimization of Gelatin and Crosslinker Concentrations in a Gelatin/Alginate-Based Bioink with Potential Applications in a Simplified Skin Model. Molecules. 2025; 30(3):649. https://doi.org/10.3390/molecules30030649
Chicago/Turabian StyleCavallo, Aida, Giorgia Radaelli, Tamer Al Kayal, Angelica Mero, Andrea Mezzetta, Lorenzo Guazzelli, Giorgio Soldani, and Paola Losi. 2025. "Optimization of Gelatin and Crosslinker Concentrations in a Gelatin/Alginate-Based Bioink with Potential Applications in a Simplified Skin Model" Molecules 30, no. 3: 649. https://doi.org/10.3390/molecules30030649
APA StyleCavallo, A., Radaelli, G., Al Kayal, T., Mero, A., Mezzetta, A., Guazzelli, L., Soldani, G., & Losi, P. (2025). Optimization of Gelatin and Crosslinker Concentrations in a Gelatin/Alginate-Based Bioink with Potential Applications in a Simplified Skin Model. Molecules, 30(3), 649. https://doi.org/10.3390/molecules30030649









