Safirinium Fluorescent “Click” Molecular Probes: Synthesis, CuAAC Reactions, and Microscopic Imaging
Abstract
:1. Introduction
2. Results and Discussion
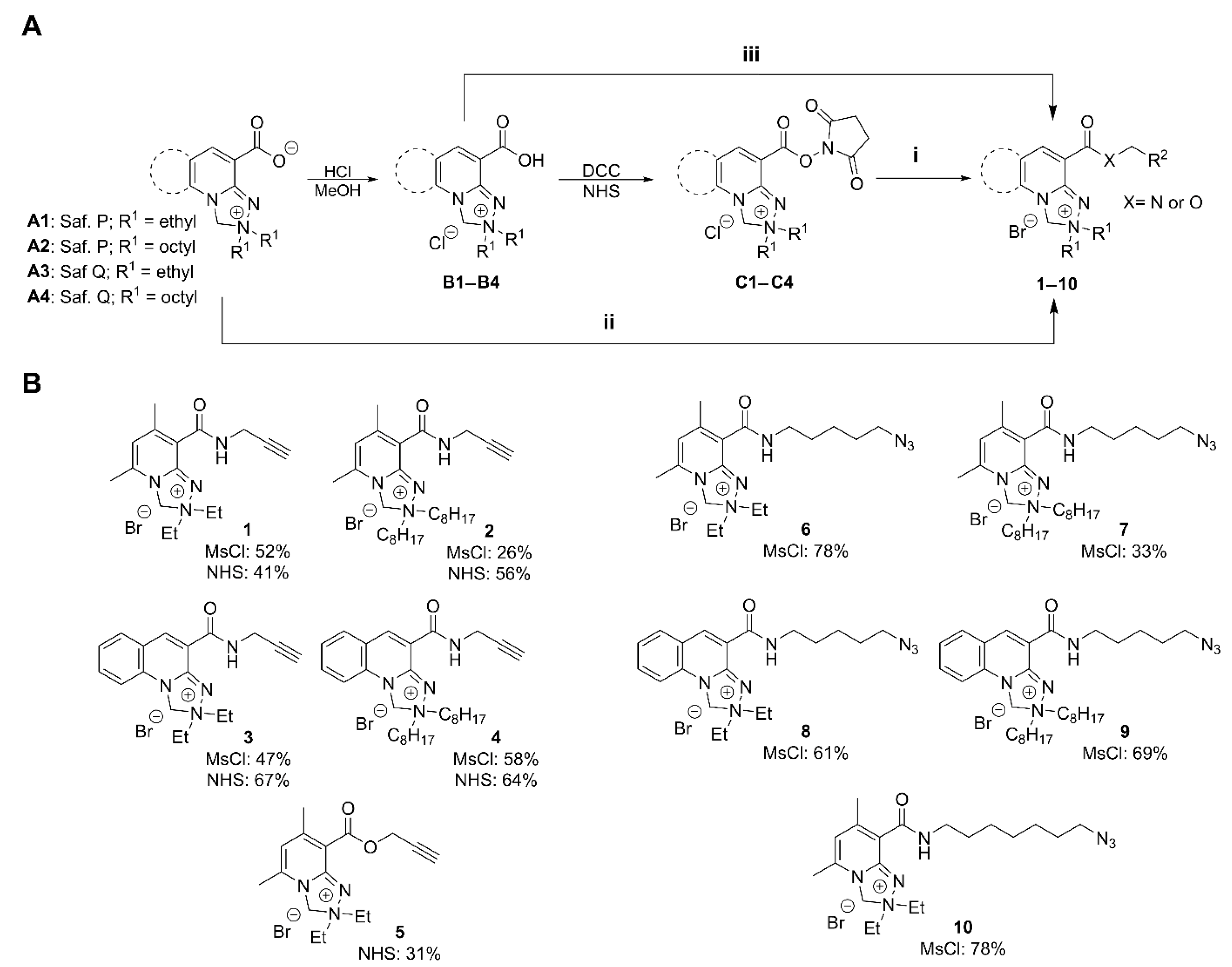
2.1. Synthesis of Safirinium-Based Azide and Alkyne—Functionalized Fluorescent Probes
2.2. Chromatographic Purification of the Safirinium-Based Probes
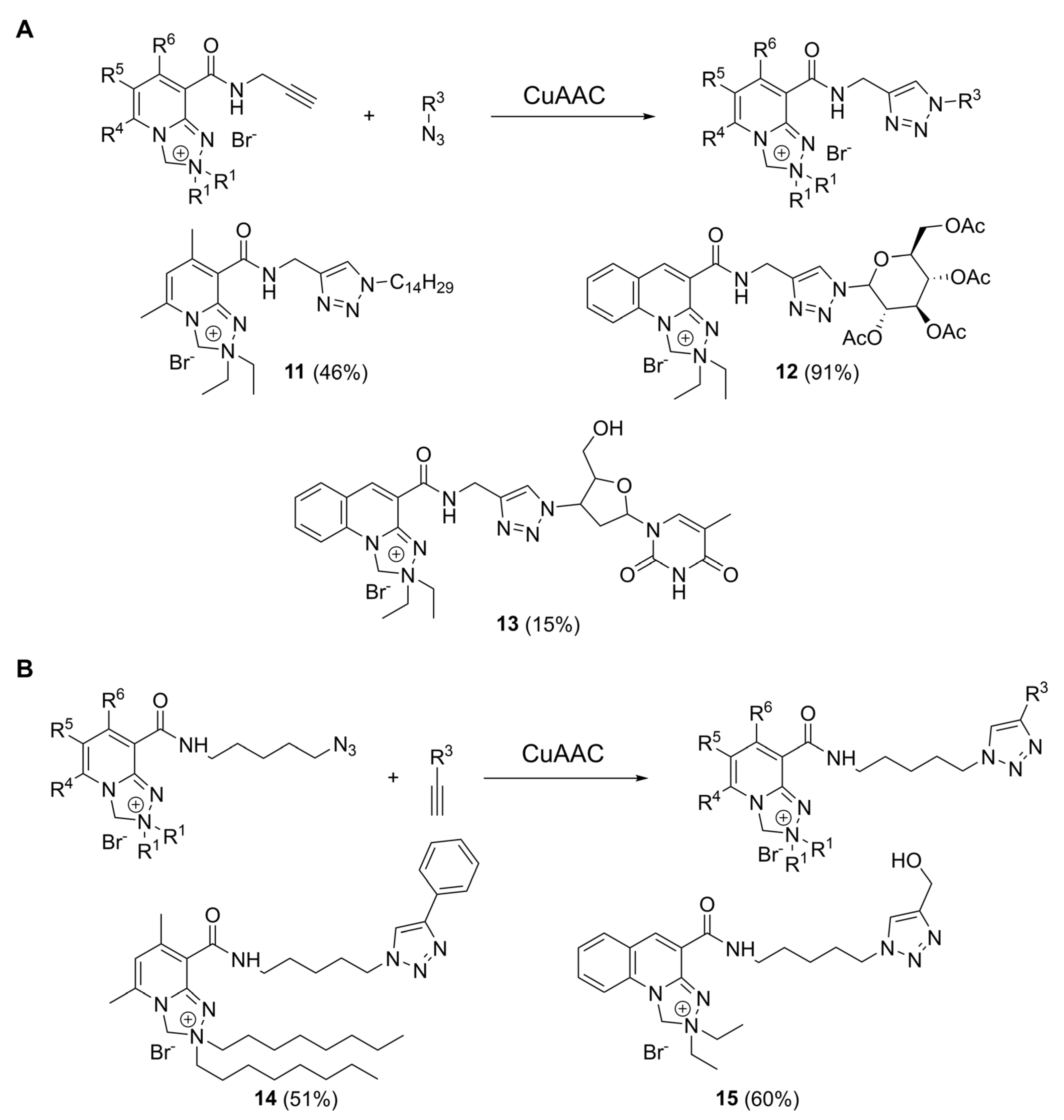
2.3. CuAAC Reactions with Safirinium-Based Alkyne- and Azide-Functionalized Probes
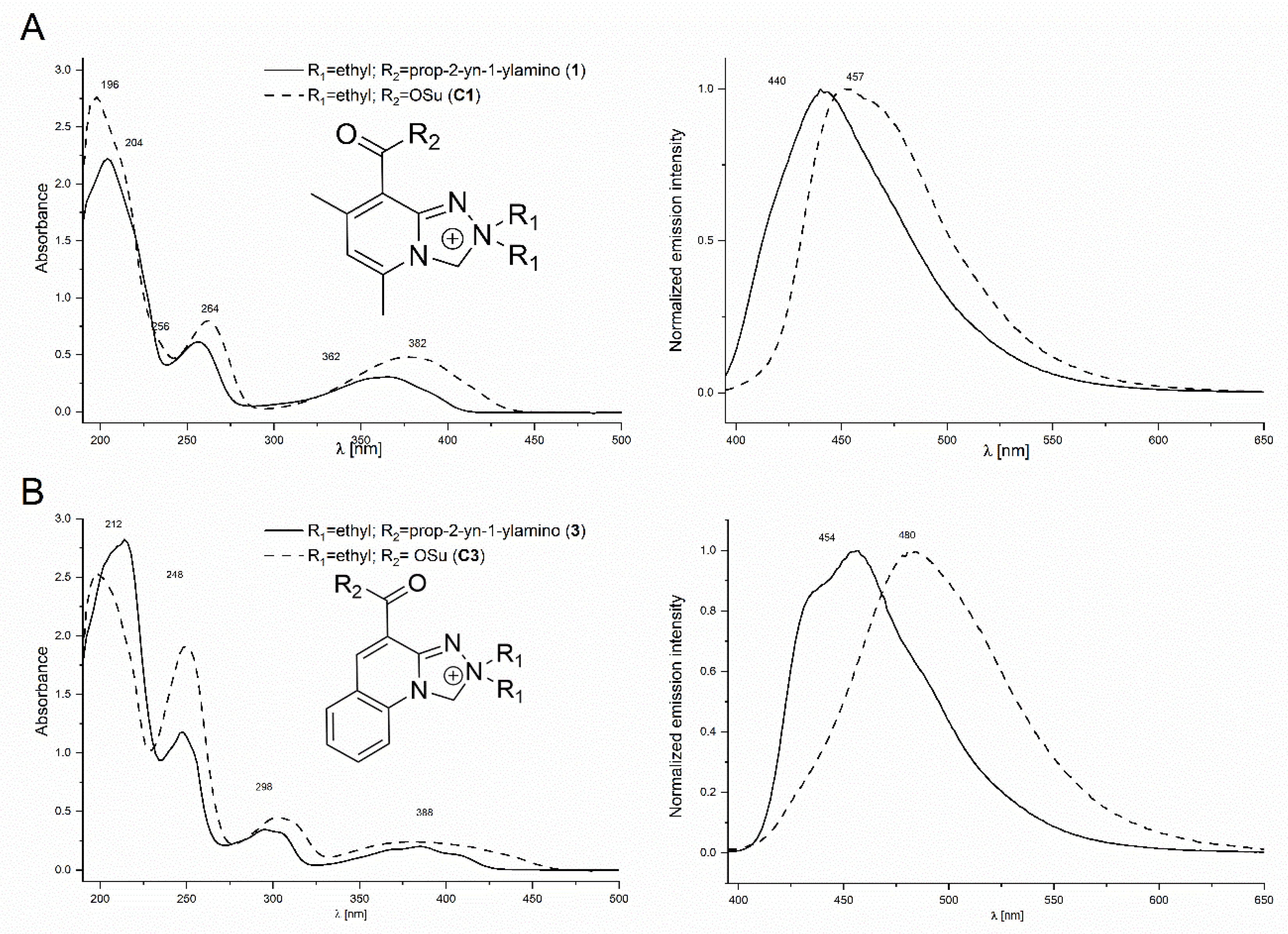
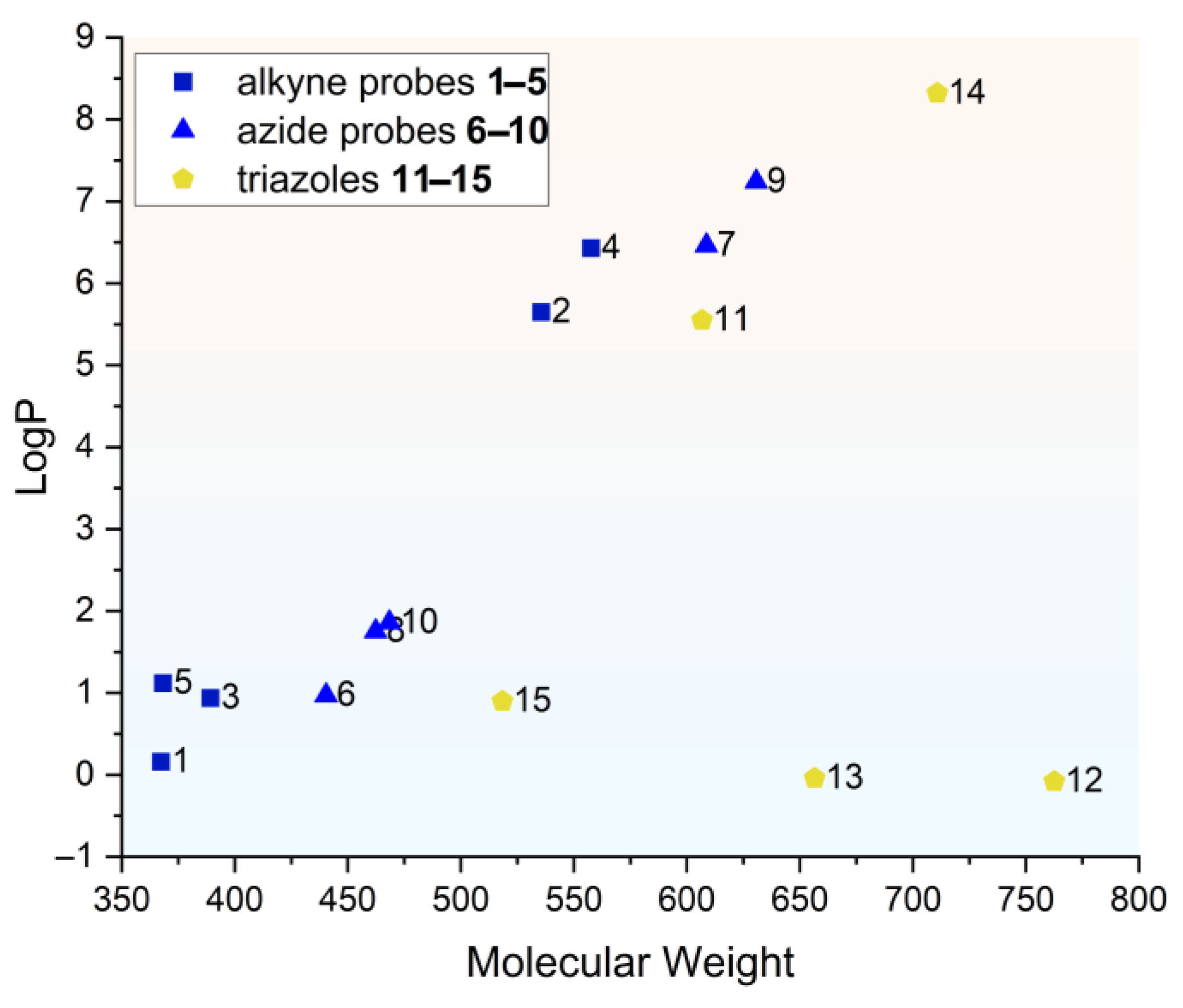
2.4. Spectral and Physicochemical Properties of the Safirinium-Based Probes and Their Triazole Derivatives
2.5. Cellular Uptake and Distribution of Probes and Triazole Derivatives Observed Using Fluorescence Microscopy
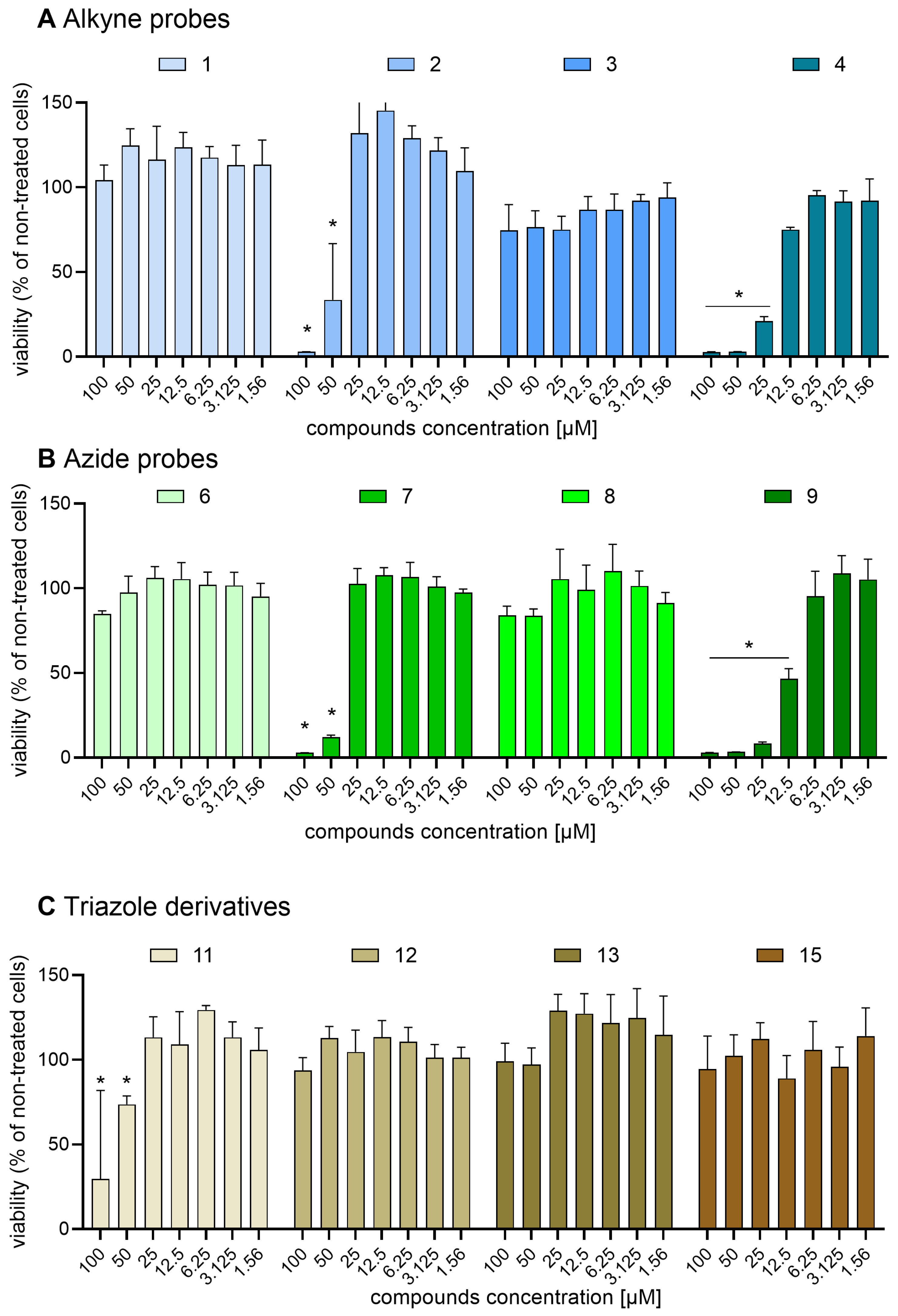
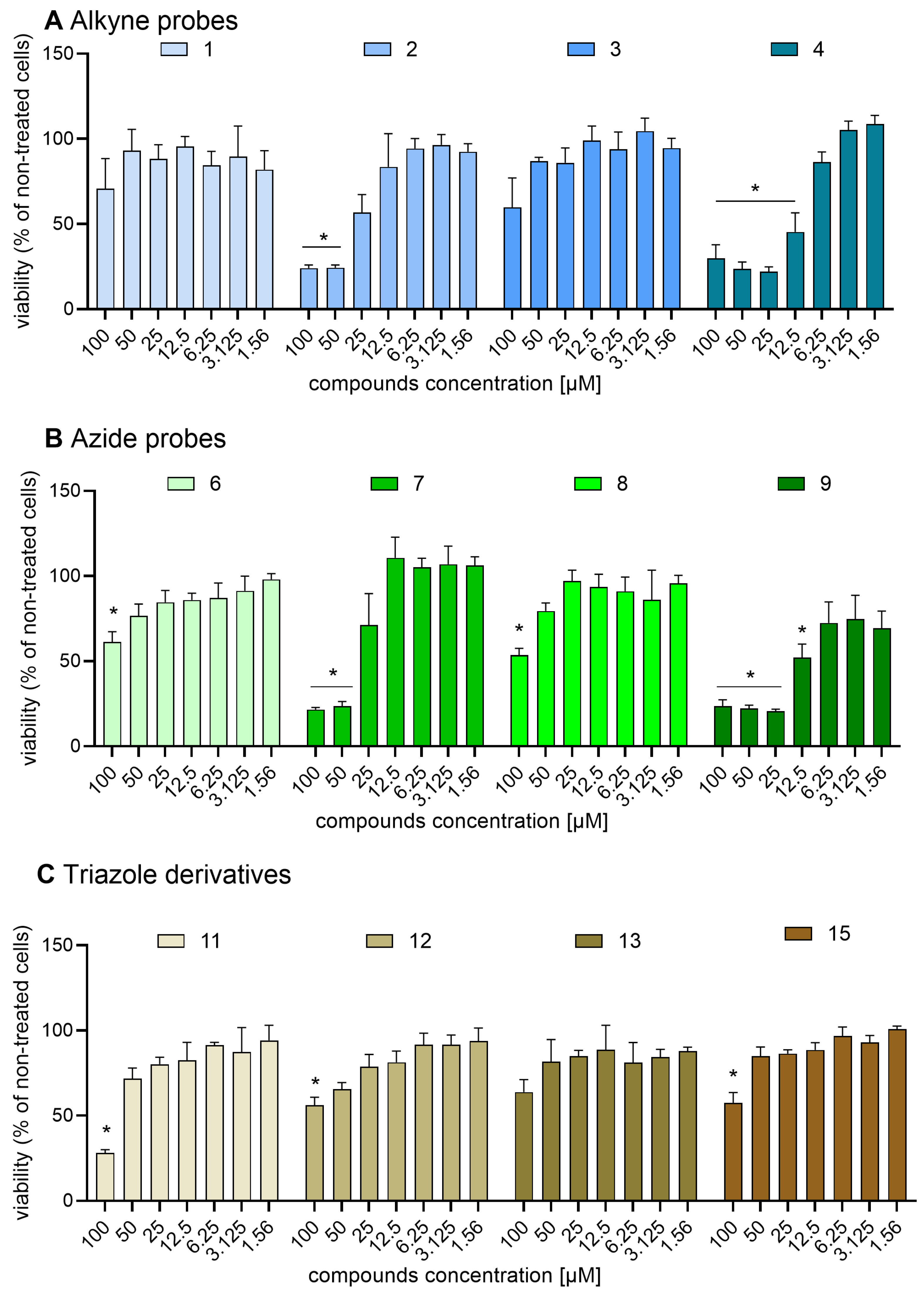
2.6. Safirinium Azide/Alkyne Probes and Their Conjugates Are Safe in CHO-K1 and NIH-3T3 Cells
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Synthesis of Safirinium Carboxylic Acids, Zwitterions, and NHS Esters
4.3. Synthesis of Non-Fluorescent Azides and Alkynes
4.4. Azide-Related Safety Measures
4.5. Synthesis and Purification of Safirinium Azide and Alkyne Probes 1–10
4.6. CuAAC Reactions: Synthesis of Triazoles 11–15
4.7. UV–VIS Absorption and Emission Measurement, and Fluorescence Quantum Yields
4.8. RP-HPLC Analysis
4.9. Cell Culture
4.10. MTT Test
4.11. Microscopic Visualization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maeda, Y.; Kishikawa, N.; Ohyama, K.; Wada, M.; Ikeda, R.; Kuroda, N. Fluorescence Derivatization Method for Sensitive Chromatographic Determination of Zidovudine Based on the Huisgen Reaction. J. Chromatogr. A 2014, 1355, 206–210. [Google Scholar] [CrossRef]
- Ahmed, S.; Abdallah, N.A. Dansyl Azide as a Selective Fluorescence Tagging Probe for Click Chemistry Reactions and Its Application to Monitor Rasagiline in Pharmacokinetic Studies. J. Pharm. Biomed. Anal. 2019, 165, 357–365. [Google Scholar] [CrossRef]
- Abd El-Hay, S.S.; Colyer, C.L.; Hassan, W.S.; Shalaby, A. Utility of 4-Chloro-7-Nitrobenzofurazan (NBD-Cl) for the Spectrophotometric and Spectrofluorometric Determination of Several Antihistamine and Antihypertensive Drugs. J. AOAC Int. 2013, 96, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Darwish, I.A.; Amer, S.M.; Abdine, H.H.; Al-Rayes, L.I. Spectrofluorimetric Determination of Fluvoxamine in Dosage Forms and Plasma Via Derivatization with 4-Chloro-7-Nitrobenzo-2-Oxa-1,3-Diazole. J. Fluoresc. 2009, 19, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Freed, E.O. “Expand and Click”: A New Method for Labeling HIV-1 Envelope Glycoproteins. Cell Chem. Biol. 2017, 24, 548–550. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Chang, Y.Y.; Hu, L.; Yang, Y.; Chao, A.; Du, Z.Y.; Tanner, J.A.; Chye, M.L.; Qian, C.; Ng, K.M.; et al. Rapid Labeling of Intracellular His-Tagged Proteins in Living Cells. Proc. Natl. Acad. Sci. USA 2015, 112, 2948–2953. [Google Scholar] [CrossRef]
- Sahoo, H. Fluorescent Labeling Techniques in Biomolecules: A Flashback. RSC Adv. 2012, 2, 7017–7029. [Google Scholar] [CrossRef]
- Sameiro, M.; Gonçalves, T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chemie Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chemie Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Kaur, J.; Saxena, M.; Rishi, N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjug. Chem. 2021, 32, 1455–1471. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Bauer, D.; Sarrett, S.M.; Lewis, J.S.; Zeglis, B.M. Click Chemistry: A Transformative Technology in Nuclear Medicine. Nat. Protoc. 2023, 18, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Szkółka, A.; Szafrański, P.W.; Kasza, P.; Talik, P.; Krośniak, M.; Cegła, M.; Zajdel, P. THETA as an Efficient Cu-Binding Ligand for Manual and Automated “Click” Synthesis: The Rufinamide Case. Org. Process Res. Dev. 2024, 28, 3257–3266. [Google Scholar] [CrossRef]
- Zhang, J.; Campbell, R.E.; Ting, A.Y.; Tsien, R.Y. Creating New Fluorescent Probes for Cell Biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Li, K.; Wang, Y.; Gruebele, M.; Ferguson, A.L.; Zimmerman, S.C. Polymeric “Clickase” Accelerates the Copper Click Reaction of Small Molecules, Proteins, and Cells. J. Am. Chem. Soc. 2019, 141, 9693–9700. [Google Scholar] [CrossRef]
- Gibney, A.; de Paiva, R.E.F.; Singh, V.; Fox, R.; Thompson, D.; Hennessy, J.; Slator, C.; McKenzie, C.J.; Johansson, P.; McKee, V.; et al. A Click Chemistry-Based Artificial Metallo-Nuclease. Angew. Chemie Int. Ed. 2023, 62, e202305759. [Google Scholar] [CrossRef] [PubMed]
- Szafrański, P.W.; Kasza, P.; Kępczyński, M.; Cegła, M.T. Fluorescent 1,2,3-Triazole Derivative of 3′-Deoxy-3-Azidothymidine: Synthesis and Absorption/Emission Spectra. Heterocycl. Commun. 2015, 21, 263–267. [Google Scholar] [CrossRef]
- Schulz, D.; Rentmeister, A. Current Approaches for RNA Labeling In Vitro and in Cells Based on Click Reactions. ChemBioChem 2014, 15, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verwilst, P.; Li, M.; Ma, D.; Singh, N.; Yoo, J.; Kim, Y.; Yang, Y.; Zhu, J.H.; Huang, H.; et al. Theranostic Fluorescent Probes. Chem. Rev. 2024, 124, 2699–2804. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Lo, Y.; Song, H.; Lu, J. Fluorescent Probes Based on Bioorthogonal Reactions: Construction Strategies and Applications. TrAC—Trends Anal. Chem. 2023, 169, 117388. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.; Chung, J.; Yin, J.; Yoon, J. Recent Progress in Fluorescent Probes for Bacteria. Chem. Soc. Rev. 2021, 50, 7725–7744. [Google Scholar] [CrossRef] [PubMed]
- Sączewski, J.; Hinc, K.; Obuchowski, M.; Gdaniec, M. The Tandem Mannich-Electrophilic Amination Reaction: A Versatile Platform for Fluorescent Probing and Labeling. Chemistry 2013, 19, 11531–11535. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Drażba, Z.; Wiśniewska, P.; Gdaniec, M.; Wicher, B.; Suwiński, G.; Jalińska, A. Synthesis and Fluorescence of Dihydro-[1,2,4]Triazolo[4,3-a]Pyridin-2-Ium-Carboxylates: An Experimental and TD-DFT Comparative Study. Dye. Pigment. 2019, 161, 347–359. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Wierzbicka, M.; Cebrat, M.; Wiśniewska, P.; Piątek, R.; Zalewska-Piątek, B.; Szewczuk, Z.; Sączewski, J. Application of Safirinium N-Hydroxysuccinimide Esters to Derivatization of Peptides for High-Resolution Mass Spectrometry, Tandem Mass Spectrometry, and Fluorescent Labeling of Bacterial Cells. Int. J. Mol. Sci. 2020, 21, 9643. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Cebrat, M.; Wierzbicka, M.; Wiśniewska, P.; Jalińska, A.; Dziomba, S.; Gdaniec, M.; Jaremko, M.; Jaremko, Ł.; Chandra, K.; et al. Synthesis and Evaluation of Dihydro-[1,2,4]Triazolo[4,3-a]Pyridin-2-Ium Carboxylates as Fixed Charge Fluorescent Derivatization Reagents for MEKC and MS Proteomic Analyses. J. Mol. Struct. 2020, 1217, 128426. [Google Scholar] [CrossRef]
- Kraft, O.; Kozubek, M.; Hoenke, S.; Serbian, I.; Major, D.; Csuk, R. Cytotoxic Triterpenoid–Safirinium Conjugates Target the Endoplasmic Reticulum. Eur. J. Med. Chem. 2021, 209, 112920. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Sączewski, J.; Konopacka, A.; Waleron, K.; Lejnowski, D.; Ciura, K.; Tomašič, T.; Skok, Ž.; Savijoki, K.; Morawska, M.; et al. Synthesis and Biological Evaluation of Hybrid Quinolone-Based Quaternary Ammonium Antibacterial Agents. Eur. J. Med. Chem. 2019, 179, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K.; Fedorowicz, J.; Kapica, H.; Adamkowska, A.; Sawicki, W.; Sączewski, J. Affinity of Fluoroquinolone–Safirinium Dye Hybrids to Phospholipids Estimated by IAM-HPLC. Processes 2020, 8, 1148. [Google Scholar] [CrossRef]
- Fedorowicz, J.; Cruz, C.D.; Morawska, M.; Ciura, K.; Gilbert-Girard, S.; Mazur, L.; Mäkkylä, H.; Ilina, P.; Savijoki, K.; Fallarero, A.; et al. Antibacterial and Antibiofilm Activity of Permanently Ionized Quaternary Ammonium Fluoroquinolones. Eur. J. Med. Chem. 2023, 254, 115373. [Google Scholar] [CrossRef] [PubMed]
- Fedorowicz, J.; Bazar, D.; Brankiewicz, W.; Kapica, H.; Ciura, K.; Zalewska-Piątek, B.; Piątek, R.; Cal, K.; Mojsiewicz-Pieńkowska, K.; Sączewski, J. Development of Safirinium Dyes for New Applications: Fluorescent Staining of Bacteria, Human Kidney Cells, and the Horny Layer of the Epidermis. Sci. Rep. 2022, 12, 15098. [Google Scholar] [CrossRef]
- Koh, M.; Park, J.; An, H.; Park, S.B. Ratiometric Analysis of Zidovudine (ZDV) Incorporation by Reverse Transcriptases or Polymerases via Bio-Orthogonal Click Chemistry. Chem. Commun. 2011, 47, 7614–7616. [Google Scholar] [CrossRef] [PubMed]
- Gröst, C.; Berg, T. PYRROC: The First Functionalized Cycloalkyne That Facilitates Isomer-Free Generation of Organic Molecules by SPAAC. Org. Biomol. Chem. 2015, 13, 3866–3870. [Google Scholar] [CrossRef] [PubMed]
- Xiaolin, Z.; Yi, X.; Xuhong, Q. Highly Efficient Energy Transfer in the Light Harvesting System Composed of Three Kinds of Boron-Dipyrromethene Derivatives. Org. Lett. 2008, 10, 29–32. [Google Scholar] [CrossRef]
- Bluhm, L.H.; Li, T. Chromatographic Purification of Quaternary Ammonium and Pyridinium Compounds on Normal Phase Silica Gel. Tetrahedron Lett. 1998, 39, 3623–3626. [Google Scholar] [CrossRef]
- Urban, N.D.; Schenkel, M.R.; Robertson, L.A.; Noble, R.D.; Gin, D.L. Modified Normal-Phase Ion-Pair Chromatographic Methods for the Facile Separation and Purification of Imidazolium-Based Ionic Compounds. Tetrahedron Lett. 2012, 53, 3456–3458. [Google Scholar] [CrossRef]
- Kasza, P.; Pociecha, K.; Wójcik-Pszczoła, K.; Canale, V.; Wyska, E.; Zajdel, P.; Szafrański, P.W.; Cegła, M. Ligand Assisted CuAAC Labelling and RP-HPLC Analysis of Zidovudine and Retrovir Using Propargyl-Fmoc Probe. Eur. J. Pharm. Sci. 2022, 178, 106293. [Google Scholar] [CrossRef] [PubMed]
- Szafrański, P.W.; Kasza, P.; Cegła, M.T. A New Water-Soluble Ligand for Efficient Copper-Catalyzed Huisgen Cycloaddition of Aliphatic Azides and Alkynes. Tetrahedron Lett. 2015, 56, 6244–6247. [Google Scholar] [CrossRef]
- Marvin, version 22.22; ChemAxon Ltd.: Budapest, Hungary, 2022.
- Topa-Skwarczyńska, M.; Szymaszek, P.; Fiedor, P.; Chachaj-Brekiesz, A.; Galek, M.; Kasprzyk, W.; Koczurkiewicz-Adamczyk, P.; Petko, F.; Pękala, E.; Tyszka-Czochara, M.; et al. Pyridine Derivatives as Candidates for Selective and Sensitive Fluorescent Biosensors for Lung Cancer Cell Imaging and Iron Ions Detection. Dye. Pigment. 2022, 200, 110171. [Google Scholar] [CrossRef]
- Lee, J.W.; Jun, S.I.; Kim, K. An Efficient and Practical Method for the Synthesis of Mono-N-Protected α,ω-Diaminoalkanes. Tetrahedron Lett. 2001, 42, 2709–2711. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Biswas, J. Vesicle and Stable Monolayer Formation from Simple “Click” Chemistry Adducts in Water. Langmuir 2011, 27, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jia, Z.; Zhen, B.; Han, M. Supported Ni Catalyst for Liquid Phase Hydrogenation of Adiponitrile to 6-Aminocapronitrile and Hexamethyenediamine. Molecules 2018, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Berthold, H.J.; Franke, S.; Thiem, J.; Schotten, T. Ex Post Glycoconjugation of Phthalocyanines. J. Org. Chem. 2010, 75, 3859–3862. [Google Scholar] [CrossRef] [PubMed]
- Bräse, S.; Banert, K. (Eds.) Organic Azides; Wiley: Hoboken, NJ, USA, 2009; ISBN 9780470519981. [Google Scholar]
- Treitler, D.S.; Leung, S. How Dangerous Is Too Dangerous? A Perspective on Azide Chemistry. J. Org. Chem. 2022, 87, 11293–11295. [Google Scholar] [CrossRef] [PubMed]
- Łapok, Ł.; Cyza, M.; Gut, A.; Kępczyński, M.; Szewczyk, G.; Sarna, T.; Nowakowska, M. Synthesis, Spectroscopic Properties and Interaction with a Liposomal Membrane of a Novel Iodinated Magnesium Phthalocyanine. J. Photochem. Photobiol. A Chem. 2014, 286, 55–63. [Google Scholar] [CrossRef]


| Compound | λ Absorption [nm] | λ Emission [nm] | Fluorescence Quantum Yield | |||
|---|---|---|---|---|---|---|
| H2O | Acetonitrile | H2O | Acetonitrile | H2O | Acetonitrile | |
| 1 | 210; 246; 351 | 204; 256; 365 | 446 | 443 | 1.00 | 0.86 |
| 3 | 210; 247; 297; 379 | 213; 246; 294; 303; 370; 385 | 462 | 455 | 0.62 | 0.71 |
| 8 | 213; 247; 297; 385 | 212; 246; 294; 303; 368; 382 | 459 | 452 | 0.60 | 0.68 |
| 11 | 254; 351 | 203; 256; 363 | 446 | 443 | 0.28 | 0.86 |
| 13 | 212; 249; 295; 387 | 248; 294; 303; 368; 381 | 462 | 452 | 0.59 | 0.45 |
| 15 | 213; 247; 297; 385 | 212; 246; 294; 302; 369; 382 | 459 | 454 | 0.55 | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasza, P.; Szafrański, P.W.; Fedorowicz, J.; Krzysztofiak, F.; Pociecha, K.; Wójcik-Pszczoła, K.; Koczurkiewicz-Adamczyk, P.; Kępczynski, M.; Sączewski, J.; Zajdel, P.; et al. Safirinium Fluorescent “Click” Molecular Probes: Synthesis, CuAAC Reactions, and Microscopic Imaging. Molecules 2025, 30, 731. https://doi.org/10.3390/molecules30030731
Kasza P, Szafrański PW, Fedorowicz J, Krzysztofiak F, Pociecha K, Wójcik-Pszczoła K, Koczurkiewicz-Adamczyk P, Kępczynski M, Sączewski J, Zajdel P, et al. Safirinium Fluorescent “Click” Molecular Probes: Synthesis, CuAAC Reactions, and Microscopic Imaging. Molecules. 2025; 30(3):731. https://doi.org/10.3390/molecules30030731
Chicago/Turabian StyleKasza, Patryk, Przemysław W. Szafrański, Joanna Fedorowicz, Faustyna Krzysztofiak, Krzysztof Pociecha, Katarzyna Wójcik-Pszczoła, Paulina Koczurkiewicz-Adamczyk, Mariusz Kępczynski, Jarosław Sączewski, Paweł Zajdel, and et al. 2025. "Safirinium Fluorescent “Click” Molecular Probes: Synthesis, CuAAC Reactions, and Microscopic Imaging" Molecules 30, no. 3: 731. https://doi.org/10.3390/molecules30030731
APA StyleKasza, P., Szafrański, P. W., Fedorowicz, J., Krzysztofiak, F., Pociecha, K., Wójcik-Pszczoła, K., Koczurkiewicz-Adamczyk, P., Kępczynski, M., Sączewski, J., Zajdel, P., & Cegła, M. (2025). Safirinium Fluorescent “Click” Molecular Probes: Synthesis, CuAAC Reactions, and Microscopic Imaging. Molecules, 30(3), 731. https://doi.org/10.3390/molecules30030731






