Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation
Abstract
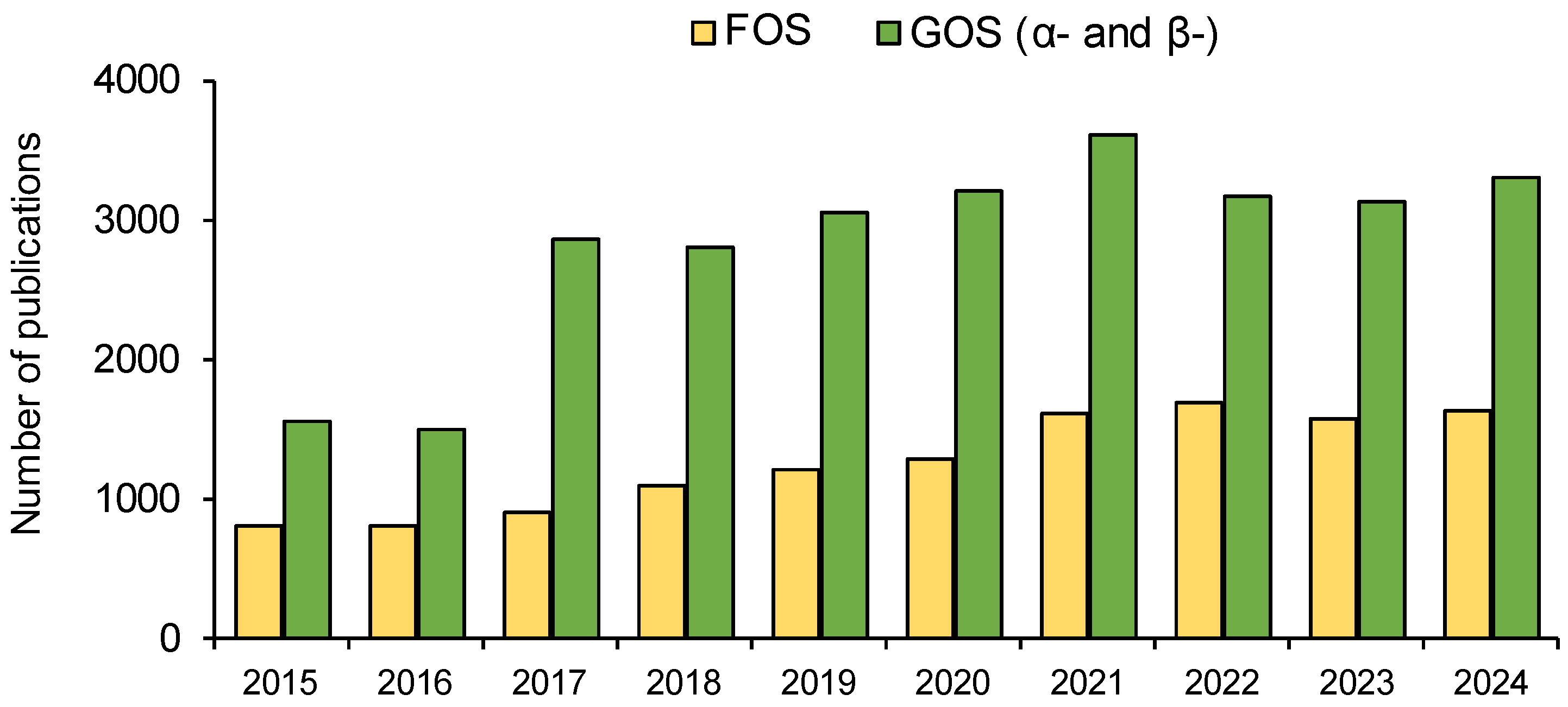
1. Introduction
2. Chemical Structure of GOS, Natural Sources, and Industrial Synthesis
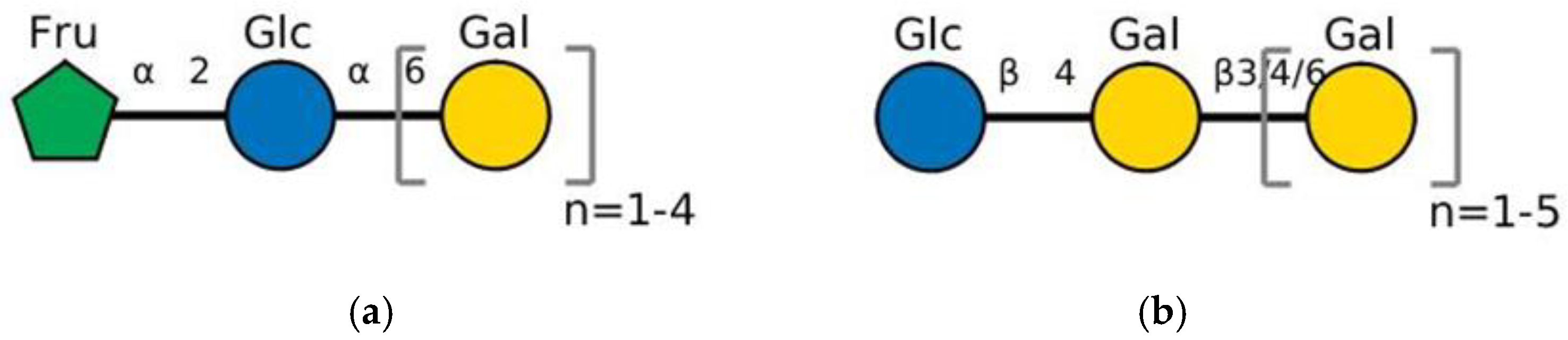
2.1. Raffinose Family Oligosaccharides (RFO, α-GOS)
2.2. Lactose Family Oligosaccharides (β-GOS)
3. Beneficial Effects of GOS Consumption
4. Enzyme Interactions Between Probiotic Strains and GOS
4.1. α-Galactosidases of Probiotic Bacteria
4.2. β-Galactosidases of Probiotic Bacteria
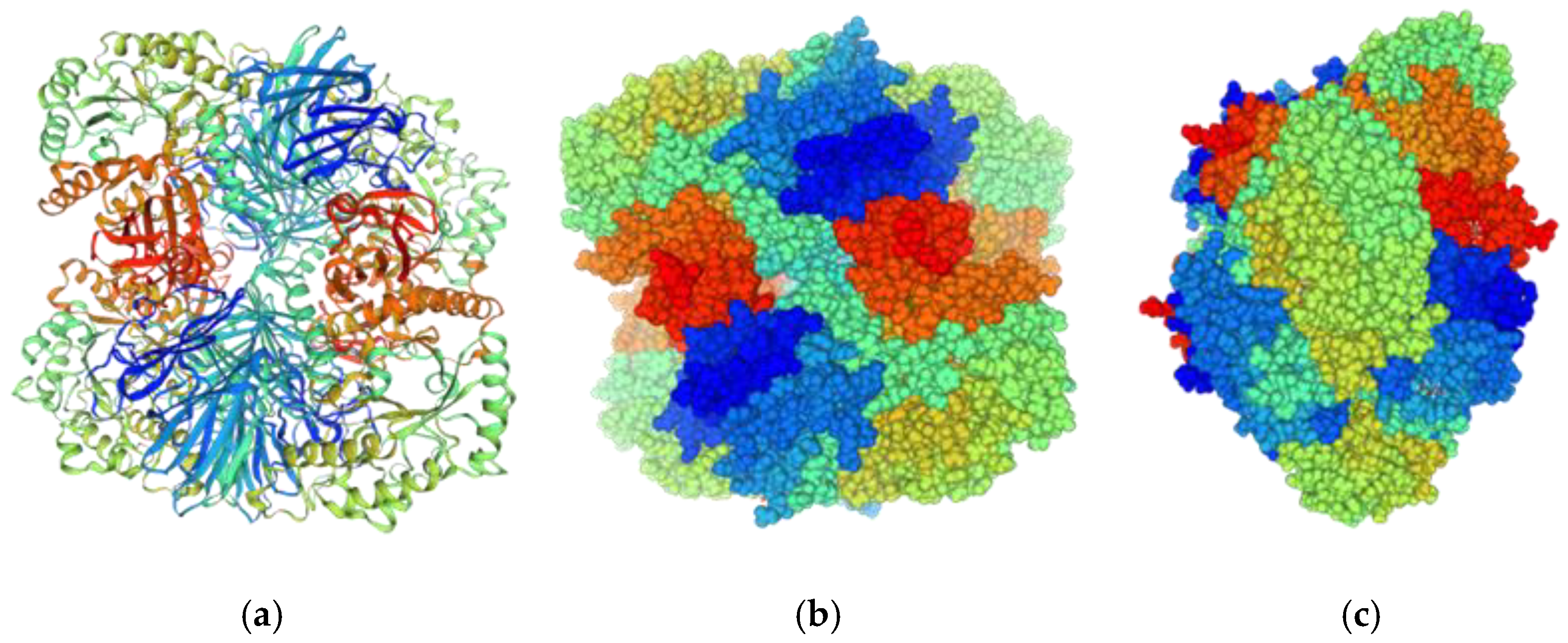
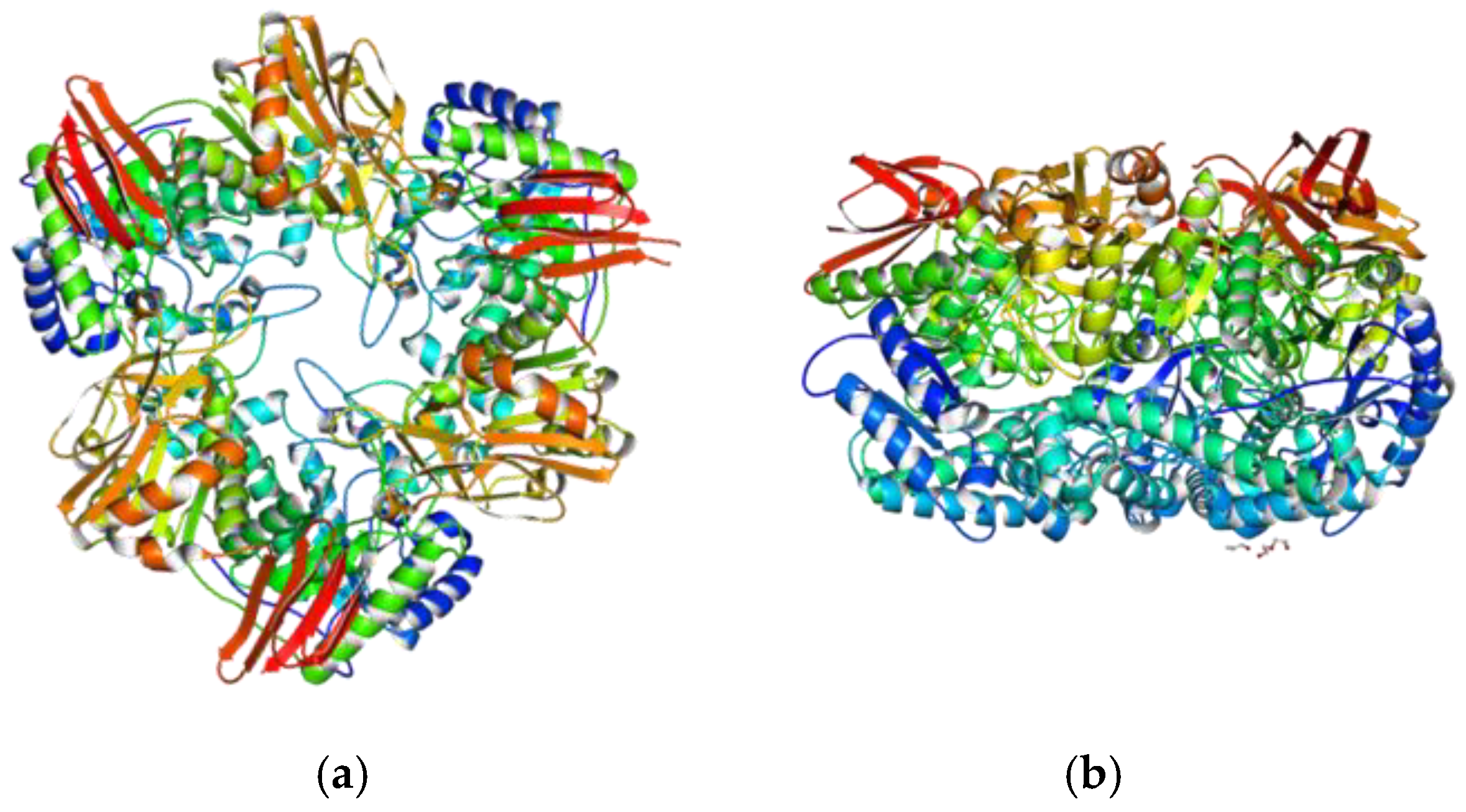
4.2.1. Multimeric Organization, Active Site, Influence of Cations
4.2.2. Catalytic Mechanism and Linkage Preference
5. Trends in GOS Production Enhancement
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, N.; Al-Khalili, M.; Haque, S.A.; Elias, M.; Olqi, N.A.; Al Uraimi, T. Health Benefits of Prebiotics, Probiotics, Synbiotics, and Postbiotics. Nutrients 2024, 16, 3955. [Google Scholar] [CrossRef] [PubMed]
- Fiore, W.; Arioli, S.; Guglielmetti, S. The Neglected Microbial Components of Commercial Probiotic Formulations. Microorganisms 2020, 8, 1177. [Google Scholar] [CrossRef]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Lovillo, A.; Romero-Luna, H.E.; Jiménez-Fernández, M. Health promoting microbial metabolites produced by gut microbiota after prebiotics metabolism. Food Res. Int. 2020, 136, 109473. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Wang, W.; Yang, S.T. A novel inulin-mediated ethanol precipitation method for separating endo-inulinase from inulinases for inulooligosaccharides production from inulin. Front. Bioeng. Biotechnol. 2021, 9, 679720. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.O.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Prebiotics Market Size, Share & Trends Analysis Report by Ingredients (FOS, Inulin, GOS, MOS), By Application (Food & Beverages, Dietary Supplements, Animal Feed), By Region, And Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/prebiotics-market (accessed on 8 December 2024).
- Galacto-oligosaccharide (GOS) Market Size, Share & Trends Analysis by Application (Food & Beverage, Dietary Supplements), By Region (Europe, Asia Pacific, North America), And Segment Forecasts, 2023–2030. Available online: https://www.grandviewresearch.com/industry-analysis/galacto-oligosaccharides-gos-market (accessed on 8 December 2024).
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.K.; Lee, J.C.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in understanding and applying prebiotics in research and practice—An ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef] [PubMed]
- Fructooligosaccharides Fos. Available online: https://scholar.google.com/scholar?q=fructooligosaccharides+fos&hl=bg&as_sdt=0%2C5&as_ylo=2023&as_yhi=2023 (accessed on 1 December 2024).
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-oligosaccharides and Colorectal Cancer: Feeding our Intestinal Probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Klostermann, C.E.; Endika, M.F.; Kouzounis, D.; Buwalda, P.L.; de Vos, P.; Zoetendal, E.G.; Bitter, J.H.; Schols, H.A. Presence of digestible starch impacts fermentation of resistant starch. Food Funct. 2024, 15, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Alander, M.; Mättö, J.; Kneifel, W.; Johansson, M.; Kögler, B.; Crittenden, R.; Mattila-Sandholm, T.; Saarela, M. Effect of galacto-oligosaccharide supplementation on human faecal microflora and on survival and persistence of Bifidobacterium lactis Bb-12 in the gastrointestinal tract. Int. Dairy J. 2001, 11, 817–825. [Google Scholar] [CrossRef]
- Sangwan, V.; Tomar, S.K.; Singh, R.R.; Singh, A.K.; Ali, B. Galactooligosaccharides, novel components of designer foods. J. Food Sci. 2011, 76, R103–R111. [Google Scholar] [CrossRef]
- Torres, D.P.M.; Gonçalves, M.D.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef]
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms 2021, 9, 480. [Google Scholar] [CrossRef] [PubMed]
- Searle, L.E.; Cooley, W.A.; Jones, G.; Nunez, A.; Crudgington, B.; Weyer, U.; Dugdale, A.H.; Tzortzis, G.; Collins, J.W.; Woodward, M.J.; et al. Purified galactooligosaccharide, derived from a mixture produced by the enzymic activity of Bifidobacterium bifidum, reduces Salmonella enterica serovar typhimurium adhesion and invasion in vitro and in vivo. J. Med. Microbiol. 2010, 59, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Liu, X.; Li, Y.; Han, D.; Pi, Y.; Whitmore, M.A.; Lu, X.; Zhang, G.; Zheng, J.; et al. Strain specificity of lactobacilli with promoted colonization by galactooligosaccharides administration in protecting intestinal barriers during Salmonella infection. J. Adv. Res. 2024, 56, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Quintero, M.; Maldonado, M.; Perez-Munoz, M.; Jimenez, R.; Fangman, T.; Rupnow, J.; Wittke, A.; Russell, M.; Hutkins, R. Adherence inhibition of Cronobacter sakazakii to intestinal epithelial cells by prebiotic oligosaccharides. Curr. Microbiol. 2011, 62, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Altomare, A.; Emerenziani, S.; Di Rosa, C.; Ribolsi, M.; Balestrieri, P.; Iovino, P.; Rocchi, G.; Cicala, M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients 2020, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Rattanaprasert, M.; van Pijkeren, J.-P.; Ramer-Tait, A.E.; Quintero, M.; Kok, C.R.; Walter, J.W.; Hutkins, R.W. Genes Involved in Galactooligosaccharide Metabolism in Lactobacillus reuteri and Their Ecological Role in the Gastrointestinal Tract. Appl. Environ. Microbiol. 2019, 85, e01788-19. [Google Scholar] [CrossRef]
- Ivanov, I.; Petrov, K.; Lozanov, V.; Hristov, I.; Wu, Z.; Liu, Z.; Petrova, P. Bioactive Compounds Produced by the Accompanying Microflora in Bulgarian Yoghurt. Processes 2021, 9, 114. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K. Prebiotic–probiotic relationship: The genetic fundamentals of polysaccharides conversion by Bifidobacterium and Lactobacillus genera. In Handbook of Food Bioengineering, 1st ed.; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier Inc.: San Diego, CA, USA, 2017; Volume 2, pp. 237–278. [Google Scholar] [CrossRef]
- Velikova, P.; Petrov, K.; Lozanov, V.; Tsvetanova, F.; Stoyanov, A.; Wu, Z.; Liu, Z.; Petrova, P. Microbial diversity and health-promoting properties of the traditional Bulgarian yogurt. Biotechnol. Biotechnol. Equip. 2018, 32, 1205–1217. [Google Scholar] [CrossRef]
- Fara, A.; Sabater, C.; Palacios, J.; Requena, T.; Montilla, A.; Zárate, G. Prebiotic galactooligosaccharides production from lactose and lactulose by Lactobacillus delbrueckii subsp. bulgaricus CRL450. Food Funct. 2020, 11, 5875–5886. [Google Scholar] [CrossRef]
- Zu, H.; Yan, X.; Wu, J.; Zhao, J.; Mayo, K.H.; Zhou, Y.; Cui, L.; Cheng, H.; Sun, L. Application of an α-galactosidase from Bacteroides fragilis on structural analysis of raffinose family oligosaccharides. Carbohydr. Polym. 2024, 346, 122661. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Ren, D.; Kanwal, W.; Ding, M.; Su, J.; Shang, X. The potential role of nondigestible Raffinose family oligosaccharides as prebiotics. Glycobiol. 2023, 33, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Henriksson, A.; Mitchell, H. Selected prebiotics support the growth of probiotic monocultures in vitro. Anaerobe 2007, 13, 134–139. [Google Scholar] [CrossRef]
- Ambrogi, V.; Bottacini, F.; Cao, L.; Kuipers, B.; Schoterman, M.; Van Sinderen, D. Galacto-Oligosaccharides as Infant Prebiotics: Production, Application, Bioactive Activities and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2023, 63, 753–766. [Google Scholar] [CrossRef]
- Wang, K.; Xu, Y.; Xuan, Z.; Xiao, X.; Gu, G.; Lu, L. Enzymatic synthesis of prebiotic galactooligosaccharides from galactose derived from gum arabic. Food Chem. 2023, 429, 136987. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Whelan, K. Prebiotic inulin-type fructans and galacto-oligosaccharides: Definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017, 32, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, Y.H.; Jin, J.J.; Stull, G.W.; Bruneau, A.; Cardoso, D.; De Queiroz, L.P.; Moore, M.J.; Zhang, S.D.; Chen, S.Y.; et al. Exploration of Plastid Phylogenomic Conflict Yields New Insights into the Deep Relationships of Leguminosae. Syst. Biol. 2020, 69, 613–622, Erratum in Syst. Biol. 2021, 70, 202. [Google Scholar] [CrossRef] [PubMed]
- Kotiguda, G.; Peterbauer, T.; Mulimani, H.V. Isolation and structural analysis of ajugose from Vigna mungo L. Carbohydr. Res. 2006, 341, 2156–2160. [Google Scholar] [CrossRef]
- Explore Chemistry. Quickly Find Chemical Information from Authoritative Sources. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 12 December 2024).
- Sprenger, N.; Keller, F. Allocation of raffinose family oligosaccharides to transport and storage pools in Ajuga reptans: The roles of two distinct galactinol synthases. Plant J. 2000, 21, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kaur, R.; Singh, R.S.; Kennedy, J.F. Biocatalytic Strategies in the Production of Galacto-Oligosaccharides and Its Global Status. Int. J. Biol. Macromol. 2018, 111, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Lyu, W.; Xiang, X.; Tang, Y.; Hu, B.; Ou, S.; Zeng, X. Immunomodulatory Effects of Enzymatic-Synthesized α-Galactooligosaccharides and Evaluation of the Structure-Activity Relationship. J. Agric. Food Chem. 2018, 66, 9070–9079. [Google Scholar] [CrossRef]
- Chen, X.Y.; Gänzle, M.G. Lactose and Lactose-Derived Oligosaccharides: More than Prebiotics? Int. Dairy J. 2017, 67, 61–72. [Google Scholar] [CrossRef]
- Botvynko, A.; Bednářová, A.; Henke, S.; Shakhno, N.; Čurda, L. Production of Galactooligosaccharides Using Various Combinations of the Commercial β-Galactosidases. Biochem. Biophys. Res. Commun. 2019, 517, 762–766. [Google Scholar] [CrossRef]
- Logtenberg, M.J.; Akkerman, R.; Hobé, R.G.; Donners, K.M.H.; Van Leeuwen, S.S.; Hermes, G.D.A.; De Haan, B.J.; Faas, M.M.; Buwalda, P.L.; Zoetendal, E.G.; et al. Structure-Specific Fermentation of Galacto-Oligosaccharides, Isomalto-Oligosaccharides and Isomalto/Malto-Polysaccharides by Infant Fecal Microbiota and Impact on Dendritic Cell Cytokine Responses. Mol. Nutr. Food Res. 2021, 65, 2001077. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.; Vera, C.; Conejeros, R.; Illanes, A. Transgalactosylation and Hydrolytic Activities of Commercial Preparations of β-Galactosidase for the Synthesis of Prebiotic Carbohydrates. Enzym. Microb. Technol. 2015, 70, 9–17. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Orrego, D.; Klotz-Ceberio, B.F.; Palanca, C.; Tortajada-Serra, M. Galactooligosaccharides: Food technological applications, prebiotic health benefits, microbiome modulation, and processing considerations. JSFA Rep. 2022, 2, 578–590. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van Den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef] [PubMed]
- Maráz, A.; Kovács, Z.; Benjamins, E.; Pázmándi, M. Recent Developments in Microbial Production of High-Purity Galacto-Oligosaccharides. World J. Microbiol. Biotechnol. 2022, 38, 95. [Google Scholar] [CrossRef] [PubMed]
- Baek, Y.; Ahn, Y.; Shin, J.; Suh, H.J.; Jo, K. Evaluation of Safety through Acute and Subacute Tests of Galacto-Oligosaccharide (GOS). Prev. Nutr. Food Sci. 2021, 26, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Yuan, J.; Li, D. Biological Activity of Galacto-Oligosaccharides: A Review. Front. Microbiol. 2022, 13, 993052. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Méndez, L.A.; Gurrola-Díaz, C.M.; Zepeda-Nuño, J.S.; Vega-Magaña, N.; Lopez-Roa, R.I.; Íñiguez-Gutiérrez, L.; García-López, P.M.; Fafutis-Morris, M.; Delgado-Rizo, V. In Vivo Healthy Benefits of Galacto-Oligosaccharides from Lupinus albus (LA-GOS) in Butyrate Production through Intestinal Microbiota. Biomolecules 2021, 11, 1658. [Google Scholar] [CrossRef]
- Trompette, A.; Gollwitzer, E.S.; Yadava, K.; Sichelstiel, A.K.; Sprenger, N.; Ngom-Bru, C.; Blanchard, C.; Junt, T.; Nicod, L.P.; Harris, N.L.; et al. Gut Microbiota Metabolism of Dietary Fiber Influences Allergic Airway Disease and Hematopoiesis. Nat. Med. 2014, 20, 159–166. [Google Scholar] [CrossRef]
- Devereux, G. The Increase in the Prevalence of Asthma and Allergy: Food for Thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar] [CrossRef]
- Karakan, T.; Tuohy, K.M.; Janssen-van Solingen, G. Low-Dose Lactulose as a Prebiotic for Improved Gut Health and Enhanced Mineral Absorption. Front. Nutr. 2021, 8, 672925. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.C.; Van Neerven, R.J.J.; Nauta, A.; Van Sinderen, D. Selective Carbohydrate Utilization by Lactobacilli and Bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef]
- Gerbino, E.; Ghibaudo, F.; Tymczyszyn, E.E.; Gomez-Zavaglia, A.; Hugo, A.A. Probiotics, Galacto-Oligosaccharides, and Zinc Antagonize Biological Effects of Enterohaemorrhagic Escherichia coli on Cultured Cells and Brine Shrimp Model. LWT 2020, 128, 109435. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Histone Deacetylases and Cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.-G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef] [PubMed]
- Nakaji, S.; Sugawara, K.; Saito, D.; Yoshioka, Y.; MacAuley, D.; Bradley, T.; Kernohan, G.; Baxter, D. Trends in Dietary Fiber Intake in Japan over the Last Century. Eur. J. Nutr. 2002, 41, 222–227. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.-J. Review Article: The Role of Butyrate on Colonic Function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.; Gibson, G.R.; Rastall, R.A. Development of a Quantitative Tool for the Comparison of the Prebiotic Effect of Dietary Oligosaccharides. Lett. Appl. Microbiol. 2003, 37, 281–284. [Google Scholar] [CrossRef]
- Rajendran, S.R.C.K.; Okolie, C.L.; Udenigwe, C.C.; Mason, B. Structural Features Underlying Prebiotic Activity of Conventional and Potential Prebiotic Oligosaccharides in Food and Health. J. Food Biochem. 2017, 41, e12389. [Google Scholar] [CrossRef]
- Böger, M.; Van Leeuwen, S.S.; Lammerts Van Bueren, A.; Dijkhuizen, L. Structural Identity of Galactooligosaccharide Molecules Selectively Utilized by Single Cultures of Probiotic Bacterial Strains. J. Agric. Food Chem. 2019, 67, 13969–13977. [Google Scholar] [CrossRef]
- Gänzle, M.G.; Follador, R. Metabolism of Oligosaccharides and Starch in Lactobacilli: A Review. Front. Microbiol. 2012, 3, 340. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Kataoka, F.; Nagaoka, S.; Kawai, Y.; Kitazawa, H.; Itoh, H.; Kimura, K.; Taketomo, N.; Yamazaki, Y.; Tateno, Y.; et al. β-Galactosidase, Phospho-β-Galactosidase and Phospho-β-Glucosidase Activities in Lactobacilli Strains Isolated from Human Faeces. Lett. Appl. Microbiol. 2007, 45, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, E.; Sakurama, H.; Kiyohara, M.; Nakajima, M.; Kitaoka, M.; Ashida, H.; Hirose, J.; Katayama, T.; Yamamoto, K.; Kumagai, H. Bifidobacterium longum subsp. infantis Uses Two Different β-Galactosidases for Selectively Degrading Type-1 and Type-2 Human Milk Oligosaccharides. Glycobiology 2012, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Mulualem, D.M.; Agbavwe, C.; Ogilvie, L.A.; Jones, B.V.; Kilcoyne, M.; O’Byrne, C.; Boyd, A. Metagenomic Identification, Purification and Characterisation of the Bifidobacterium adolescentis BgaC β-Galactosidase. Appl. Microbiol. Biotechnol. 2021, 105, 1063–1078. [Google Scholar] [CrossRef]
- Böger, M.; Hekelaar, J.; Van Leeuwen, S.S.; Dijkhuizen, L.; Lammerts Van Bueren, A. Structural and Functional Characterization of a Family GH53 β-1,4-Galactanase from Bacteroides thetaiotaomicron That Facilitates Degradation of Prebiotic Galactooligosaccharides. J. Struct. Biol. 2019, 205, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Gao, F.; Xia, Z.; Zhang, Z.; Addai, F.P.; Zhu, Y.; Chen, J.; Lin, F.; Chen, D. β-Galactosidase: Insights into Source Variability, Genetic Engineering, Immobilisation and Diverse Applications in Food, Industry and Medicine. Int. J. Dairy Technol. 2024, 77, 651–679. [Google Scholar] [CrossRef]
- Rocha, J.M.; Guerra, A. On the Valorization of Lactose and Its Derivatives from Cheese Whey as a Dairy Industry By-Product: An Overview. Eur. Food Res. Technol. 2020, 246, 2161–2174. [Google Scholar] [CrossRef]
- McCarter, J.D.; Stephen Withers, G. Mechanisms of Enzymatic Glycoside Hydrolysis. Curr. Opin. Struct. Biol. 1994, 4, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-M.; Kim, S.-Y.; Song, J.; Lee, Y.-S.; Sim, J.-S.; Hahn, B.-S. Isolation and characterization of a halotolerant and protease-resistant α-galactosidase from the gut metagenome of Hermetia illucens. J. Biotechnol. 2018, 279, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Leder, S.; Hartmeier, W.; Marx, S. α-Galactosidase of Bifidobacterium adolescentis DSM 20083. Curr. Microbiol. 1999, 38, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lu, L.; Xiao, M.; Wang, Q.; Lu, Y.; Liu, C.; Wang, P.; Kumagai, H.; Yamamoto, K. Cloning and characterization of a novel α-galactosidase from Bifidobacterium breve 203 capable of synthesizing Gal-α-1,4 linkage. FEMS Microbiol. Lett. 2008, 285, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Goulas, T.; Goulas, A.; Tzortzis, G.; Gibson, G.R. A novel alpha-galactosidase from Bifidobacterium bifidum with transgalactosylating properties: Gene molecular cloning and heterologous expression. Appl. Microbiol. Biotechnol. 2009, 82, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Singh, A.; Batra, N.; Singh, J. Microbial Production and Biotechnological Applications of α-Galactosidase. Int. J. Biol. Macromol. 2020, 150, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Anisha, G.S. Microbial α-Galactosidases: Efficient Biocatalysts for Bioprocess Technology. Bioresour. Technol. 2022, 344, 126293. [Google Scholar] [CrossRef]
- Modrego, A.; Amaranto, M.; Godino, A.; Mendoza, R.; Barra, J.L.; Corchero, J.L. Human α-Galactosidase A Mutants: Priceless Tools to Develop Novel Therapies for Fabry Disease. Int. J. Mol. Sci. 2021, 22, 6518. [Google Scholar] [CrossRef]
- Lenders, M.; Boutin, M.; Auray-Blais, C.; Brand, E. Effects of Orally Delivered Alpha-Galactosidase A on Gastrointestinal Symptoms in Patients With Fabry Disease. Gastroenterology 2020, 159, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Anisha, G.S. Biopharmaceutical Applications of α-galactosidases. Biotechnol. Appl. Biochem. 2023, 70, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Pandurangan Maragatham, V.; Samuel, M.; Arunraj, R. Properties and Applications of α-galactosidase in Agricultural Waste Processing and Secondary Agricultural Process Industries. J. Sci. Food Agric. 2024, 104, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Fredslund, F.; Abou Hachem, M.; Jonsgaard Larsen, R.; Gerd Sørensen, P.; Coutinho, P.M.; Lo Leggio, L.; Svensson, B. Crystal Structure of α-Galactosidase from Lactobacillus acidophilus NCFM: Insight into Tetramer Formation and Substrate Binding. J. Mol. Biol. 2011, 412, 466–480. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Tauzin, A.S.; Bruel, L.; Laville, E.; Lombard, V.; Esque, J.; André, I.; Vidal, N.; Pompeo, F.; Quinson, N.; et al. α-Galactosidase and Sucrose-Kinase Relationships in a Bi-Functional AgaSK Enzyme Produced by the Human Gut Symbiont Ruminococcus gnavus E1. Front. Microbiol. 2020, 11, 579521. [Google Scholar] [CrossRef] [PubMed]
- Wakinaka, T.; Kiyohara, M.; Kurihara, S.; Hirata, A.; Chaiwangsri, T.; Ohnuma, T.; Fukamizo, T.; Katayama, T.; Ashida, H.; Yamamoto, K. Bifidobacterial α-Galactosidase with Unique Carbohydrate-Binding Module Specifically Acts on Blood Group B Antigen. Glycobiology 2013, 23, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Silvestroni, A.; Connes, C.; Sesma, F.; De Giori, G.S.; Piard, J.-C. Characterization of the melA Locus for α-Galactosidase in Lactobacillus plantarum. Appl. Environ. Microbiol. 2002, 68, 5464–5471. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Shu, G.; Zhu, X.; Zhang, Z.; Lin, X.; Wang, S.; Wang, L.; Zhang, Y.; Jiang, Q. Identification of an Intestine-Specific Promoter and Inducible Expression of Bacterial α-Galactosidase in Mammalian Cells by a Lac Operon System. J. Anim. Sci. Biotechnol. 2012, 3, 32. [Google Scholar] [CrossRef]
- Boucher, I.; Parrot, M.; Gaudreau, H.; Champagne, C.P.; Vadeboncoeur, C.; Moineau, S. Novel Food-Grade Plasmid Vector Based on Melibiose Fermentation for the Genetic Engineering of Lactococcus lactis. Appl. Environ. Microbiol. 2002, 68, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Silva, E.A.; Silvestroni, A.; LeBlanc, J.G.; Piard, J.-C.; De Giori, G.S.; Sesma, F. A Thermostable α-Galactosidase from Lactobacillus fermentum CRL722: Genetic Characterization and Main Properties. Curr. Microbiol. 2006, 53, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.; Hwang, H. Reduction of Soybean Oligosaccharides and Properties of α-d-Galactosidase from Lactobacillus curvatus R08 and Leuconostoc mesenteriodes JK55. Food Microbiol. 2008, 25, 815–823. [Google Scholar] [CrossRef]
- Kandari, S.; Choi, Y.J.; Lee, B.H. Purification and Characterization of Hydrolytic and Transgalactosyl α-Galactosidase from Lactobacillus helveticus ATCC 10797. Eur. Food Res. Technol. 2014, 239, 877–884. [Google Scholar] [CrossRef]
- Sasaki, Y.; Uchimura, Y.; Kitahara, K.; Fujita, K. Characterization of a GH36 α-D-Galactosidase Associated with Assimilation of Gum Arabic in Bifidobacterium longum Subsp. longum JCM7052. J. Appl. Glycosci. 2021, 68, 47–52. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.J.; O’Connell Motherway, M.; O’Callaghan, J.; Fitzgerald, G.F.; Ross, R.P.; Ventura, M.; Stanton, C.; Van Sinderen, D. Metabolism of Four α-Glycosidic Linkage-Containing Oligosaccharides by Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 2013, 79, 6280–6292. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhao, R.; Tu, Y.; Zhang, X.; Deng, L.; Chen, X. A Novel α-Galactosidase from the Thermophilic Probiotic Bacillus coagulans with Remarkable Protease-Resistance and High Hydrolytic Activity. PLoS ONE 2018, 13, e0197067. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hui, W.; Cao, C.; Jin, R.; Ren, C.; Zhang, H.; Zhang, W. Proteomic Analysis of an Engineered Isolate of Lactobacillus plantarum with Enhanced Raffinose Metabolic Capacity. Sci. Rep. 2016, 6, 31403. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Fernandez, P.; Plaza-Vinuesa, L.; Hernandez-Hernandez, O.; De Las Rivas, B.; Corzo, N.; Muñoz, R.; Javier Moreno, F. Unravelling the Carbohydrate Specificity of MelA from Lactobacillus plantarum WCFS1: An α-Galactosidase Displaying Regioselective Transgalactosylation. Int. J. Biol. Macromol. 2020, 153, 1070–1079. [Google Scholar] [CrossRef]
- Panwar, D.; Shubhashini, A.; Chaudhari, S.R.; Prashanth, K.V.H.; Kapoor, M. GH36 α-Galactosidase from Lactobacillus plantarum WCFS1 Synthesize Gal-α-1,6 Linked Prebiotic α-Galactooligosaccharide by Transglycosylation. Int. J. Biol. Macromol. 2020, 144, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Jiao, W.; Wang, Y.; Liang, J.; Liu, G.; Li, L. Cloning and Expression of a Novel α-Galactosidase from Lactobacillus amylolyticus L6 with Hydrolytic and Transgalactosyl Properties. PLoS ONE 2020, 15, e0235687. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Wang, Y.; Zhou, Z.; Liu, N.; Jung, S.; Lee, M.; Li, S. Preventive and Prebiotic Effect of α-Galacto-Oligosaccharide against Dextran Sodium Sulfate-Induced Colitis and Gut Microbiota Dysbiosis in Mice. J. Agric. Food Chem. 2021, 69, 9597–9607. [Google Scholar] [CrossRef] [PubMed]
- Sajnaga, E.; Socała, K.; Kalwasińska, A.; Wlaź, P.; Waśko, A.; Jach, M.E.; Tomczyk, M.; Wiater, A. Response of Murine Gut Microbiota to a Prebiotic Based on Oligosaccharides Derived via Hydrolysis of Fungal α-(1→3)-d-Glucan: Preclinical Trial Study on Mice. Food Chem. 2023, 417, 135928. [Google Scholar] [CrossRef] [PubMed]
- Kurakake, M.; Moriyama, Y.; Sunouchi, R.; Nakatani, S. Enzymatic Properties and Transglycosylation of α-Galactosidase from Penicillium oxalicum SO. Food Chem. 2011, 126, 177–182. [Google Scholar] [CrossRef]
- Kurakake, M.; Okumura, T.; Morimoto, Y. Synthesis of Galactosyl Glycerol from Guar Gum by Transglycosylation of α-Galactosidase from Aspergillus sp. MK14. Food Chem. 2015, 172, 150–154. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Q.; Zhang, R.; Wang, Y.; Wu, Q.; Li, J.; Tang, X.; Xu, B.; Ding, J.; Huang, Z. Characterization of Two Glycoside Hydrolase Family 36 α-Galactosidases: Novel Transglycosylation Activity, Lead–Zinc Tolerance, Alkaline and Multiple pH Optima, and Low-Temperature Activity. Food Chem. 2016, 194, 156–166. [Google Scholar] [CrossRef]
- Juers, D.H.; Matthews, B.W.; Huber, R.E. LacZ Β-galactosidase: Structure and Function of an Enzyme of Historical and Molecular Biological Importance. Protein Sci. 2012, 21, 1792–1807. [Google Scholar] [CrossRef]
- Huber, R.E. Beta (β)-Galactosidase. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780128096338061392. ISBN 978-0-12-809633-8. [Google Scholar]
- Lo, S.; Dugdale, M.L.; Jeerh, N.; Ku, T.; Roth, N.J.; Huber, R.E. Studies of Glu-416 Variants of β-Galactosidase (E. coli) Show That the Active Site Mg2+ Is Not Important for Structure and Indicate That the Main Role of Mg2+ Is to Mediate Optimization of Active Site Chemistry. Protein J. 2010, 29, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.E.; Parfett, C.; Woulfe-Flanagan, H.; Thompson, D.J. Interaction of Divalent Cations with β-Galactosidase (Escherichia coli). Biochemistry 1979, 18, 4090–4095. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, R.W.; Lo, S.; Jancewicz, L.J.; Dugdale, M.L.; Huber, R.E. Structural Explanation for Allolactose (Lac Operon Inducer) Synthesis by lacZ β-Galactosidase and the Evolutionary Relationship between Allolactose Synthesis and the Lac Repressor. J. Biol. Chem. 2013, 288, 12993–13005. [Google Scholar] [CrossRef]
- Wheatley, R.W.; Huber, R.E. An Allolactose Trapped at the lacZ β-Galactosidase Active Site with Its Galactosyl Moiety in a4 H3 Conformation Provides Insights into the Formation, Conformation, and Stabilization of the Transition State. Biochem. Cell Biol. 2015, 93, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Duan, X. A Novel Thermal-Activated β-Galactosidase from Bacillus aryabhattai GEL-09 for Lactose Hydrolysis in Milk. Foods 2022, 11, 372. [Google Scholar] [CrossRef]
- Shafi, A.; Husain, Q. Immobilization of β-Galactosidase on Concanavalin A Modified Silica-Coated Titanium Dioxide Nanocomposite and Its Interaction with Monovalent and Divalent Cations. Mater. Today Commun. 2022, 32, 103828. [Google Scholar] [CrossRef]
- Murphy, J.; Walsh, G. Purification and Characterization of a Novel Thermophilic β-Galactosidase from Picrophilus torridus of Potential Industrial Application. Extremophiles 2019, 23, 783–792. [Google Scholar] [CrossRef]
- Murphy, J.; Ryan, M.P.; Walsh, G. Purification and Characterization of a Novel β-Galactosidase From the Thermoacidophile Alicyclobacillus vulcanalis. Appl. Biochem. Biotechnol. 2020, 191, 1190–1206. [Google Scholar] [CrossRef]
- Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K.; Petrova, P. In Vitro Production of Galactooligosaccharides by a Novel β-Galactosidase of Lactobacillus bulgaricus. Int. J. Mol. Sci. 2022, 23, 14308. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Splechtna, B.; Krasteva, S.; Kneifel, W.; Kulbe, K.D.; Divne, C.; Haltrich, D. Characterization and Molecular Cloning of a Heterodimeric Î2-Galactosidase from the Probiotic Strain Lactobacillus acidophilus R22. FEMS Microbiol. Lett. 2007, 269, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Kong, J.; Lu, W.W.; Kong, W.T.; Tian, H.; Tian, X.Y.; Huo, G.C. β-Galactosidase with Transgalactosylation Activity from Lactobacillus fermentum K4. J. Dairy Sci. 2011, 94, 5811–5820. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Pham, M.-L.; Tran, A.-M.; Nguyen, T.-H. β-Galactosidase from Lactobacillus helveticus DSM 20075: Biochemical Characterization and Recombinant Expression for Applications in Dairy Industry. Int. J. Mol. Sci. 2019, 20, 947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, J.; Tang, Y.; Xie, J.; Wei, D. Expression, Characterization, and Structural Profile of a Heterodimeric β-Galactosidase from the Novel Strain Lactobacillus curiae M2011381. Process Biochem. 2020, 97, 87–95. [Google Scholar] [CrossRef]
- Arifullah; Abbas Bukhari, D.; Bibi, Z.; Ramzan, H.; Younas, S.; Rehman, A. Heterodimeric Studies of β-Galactosidase Genes as Biocatalyst of Lactose from Lactobacillus acidophilus MR-24. Curr. Res. Biotechnol. 2024, 8, 100261. [Google Scholar] [CrossRef]
- Gotoh, A.; Hidaka, M.; Sakurama, H.; Nishimoto, M.; Kitaoka, M.; Sakanaka, M.; Fushinobu, S.; Katayama, T. Substrate Recognition Mode of a Glycoside Hydrolase Family 42 β-Galactosidase from Bifidobacterium longum Subspecies infantis (BiBga42A) Revealed by Crystallographic and Mutational Analyses. Microbiome Res. Rep. 2023, 2, 20. [Google Scholar] [CrossRef]
- Nguyen, T.; Splechtna, B.; Steinböck, M.; Kneifel, W.; Lettner, H.P.; Kulbe, K.D.; Haltrich, D. Purification and Characterization of Two Novel β-Galactosidases from Lactobacillus reuteri. J. Agric. Food Chem. 2006, 54, 4989–4998. [Google Scholar] [CrossRef]
- Iqbal, S.; Nguyen, T.-H.; Nguyen, T.T.; Maischberger, T.; Haltrich, D. β-Galactosidase from Lactobacillus plantarum WCFS1: Biochemical Characterization and Formation of Prebiotic Galacto-Oligosaccharides. Carbohydr. Res. 2010, 345, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Benavente, R.; Pessela, B.; Curiel, J.; De Las Rivas, B.; Muñoz, R.; Guisán, J.; Mancheño, J.; Cardelle-Cobas, A.; Ruiz-Matute, A.; Corzo, N. Improving Properties of a Novel β-Galactosidase from Lactobacillus plantarum by Covalent Immobilization. Molecules 2015, 20, 7874–7889. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Rajagopal, S.N. Isolation and Characterization of β-Galactosidase from Lactobacillus crispatus. Folia Microbiol. 2000, 45, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramírez, S.; Jiménez-Flores, R. Invited Review: Properties of β-Galactosidases Derived from Lactobacillaceae Species and Their Capacity for Galacto-Oligosaccharide Production. J. Dairy Sci. 2023, 106, 8193–8206. [Google Scholar] [CrossRef]
- Godoy, A.S.; Camilo, C.M.; Kadowaki, M.A.; Muniz, H.D.S.; Espirito Santo, M.; Murakami, M.T.; Nascimento, A.S.; Polikarpov, I. Crystal Structure of Β1→6-galactosidase from Bifidobacterium bifidum S17: Trimeric Architecture, Molecular Determinants of the Enzymatic Activity and Its Inhibition by A-galactose. FEBS J. 2016, 283, 4097–4112. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Fushinobu, S.; Ohtsu, N.; Motoshima, H.; Matsuzawa, H.; Shoun, H.; Wakagi, T. Trimeric Crystal Structure of the Glycoside Hydrolase Family 42 β-Galactosidase from Thermus thermophilus A4 and the Structure of Its Complex with Galactose. J. Mol. Biol. 2002, 322, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Mangiagalli, M.; Lapi, M.; Maione, S.; Orlando, M.; Brocca, S.; Pesce, A.; Barbiroli, A.; Camilloni, C.; Pucciarelli, S.; Lotti, M.; et al. The Co-existence of Cold Activity and Thermal Stability in an Antarctic GH42 Β-galactosidase Relies on Its Hexameric Quaternary Arrangement. FEBS J. 2021, 288, 546–565. [Google Scholar] [CrossRef]
- Jahn, M.; Withers, S.G. New Approaches to Enzymatic Oligosaccharide Synthesis: Glycosynthases and Thioglycoligases. Biocatal. Biotransformation 2003, 21, 159–166. [Google Scholar] [CrossRef]
- Brás, N.F.; Fernandes, P.A.; Ramos, M.J. QM/MM Studies on the β-Galactosidase Catalytic Mechanism: Hydrolysis and Transglycosylation Reactions. J. Chem. Theory Comput. 2010, 6, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kittibunchakul, S.; Van Leeuwen, S.S.; Dijkhuizen, L.; Haltrich, D.; Nguyen, T.-H. Structural Comparison of Different Galacto-Oligosaccharide Mixtures Formed by β-Galactosidases from Lactic Acid Bacteria and Bifidobacteria. J. Agric. Food Chem. 2020, 68, 4437–4446. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Gu, G.; Xiao, M.; Wang, F. Separation and Structure Analysis of Trisaccharide Isomers Produced from Lactose by Lactobacillus bulgaricus L3 β-Galactosidase. Food Chem. 2010, 121, 1283–1288. [Google Scholar] [CrossRef]
- Yu, X.; Peng, X.; Liu, F.; Li, Y.; Yan, J.; Li, L. Distinguishing α/β-Linkages and Linkage Positions of Disaccharides in Galactooligosaccharides through Mass Fragmentation and Liquid Retention Behaviour. Food Chem. 2024, 456, 139968. [Google Scholar] [CrossRef]
- Bultema, J.B.; Kuipers, B.J.H.; Dijkhuizen, L. Biochemical Characterization of Mutants in the Active Site Residues of the Β-galactosidase Enzyme of Bacillus Circulans ATCC 31382. FEBS Open Bio. 2014, 4, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Pijning, T.; Meng, X.; Dijkhuizen, L.; Van Leeuwen, S.S. Engineering of the Bacillus Circulans β-Galactosidase Product Specificity. Biochemistry 2017, 56, 704–711. [Google Scholar] [CrossRef]
- Yin, H.; Pijning, T.; Meng, X.; Dijkhuizen, L.; Van Leeuwen, S.S. Biochemical Characterization of the Functional Roles of Residues in the Active Site of the β-Galactosidase from Bacillus circulans ATCC 31382. Biochemistry 2017, 56, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Bultema, J.B.; Dijkhuizen, L.; Van Leeuwen, S.S. Reaction Kinetics and Galactooligosaccharide Product Profiles of the β-Galactosidases from Bacillus circulans, Kluyveromyces lactis and Aspergillus oryzae. Food Chem. 2017, 225, 230–238. [Google Scholar] [CrossRef]
- Kote, N.; Manjula, A.C.; Vishwanatha, T.; Patil, A.G.G. High-Yield Production and Biochemical Characterization of α-Galactosidase Produced from Locally Isolated Penicillium sp. Bull. Natl. Res. Cent. 2020, 44, 168. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Othman, A.M.; Elsayed, M.A.; Ibrahim, G.E.; Hassan, M.M.; Mehanna, N.S. A Statistical Strategy for Optimizing the Production of α-Galactosidase by a Newly Isolated Aspergillus niger NRC114 and Assessing Its Efficacy in Improving Soymilk Properties. J. Genet. Eng. Biotechnol. 2022, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Mulimani, V. α-Galactosidase Production by Aspergillus oryzae in Solid-State Fermentation. Bioresour. Technol. 2007, 98, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Gajdhane, S.B.; Bhagwat, P.K.; Dandge, P.B. Response Surface Methodology-Based Optimization of Production Media and Purification of α-Galactosidase in Solid-State Fermentation by Fusarium moniliforme NCIM 1099. 3 Biotech 2016, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Movahedpour, A.; Ahmadi, N.; Ghalamfarsa, F.; Ghesmati, Z.; Khalifeh, M.; Maleksabet, A.; Shabaninejad, Z.; Taheri-Anganeh, M.; Savardashtaki, A. β-Galactosidase: From Its Source and Applications to Its Recombinant Form. Biotechnol. Appl. Biochem. 2022, 69, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, S.; Jin, L.; Zhang, D.; Li, Y.; Xiao, M. Synthesis of galactosyl sucralose by β-galactosidase from Lactobacillus bulgaricus L3. Food Chem. 2012, 134, 269–275. [Google Scholar] [CrossRef]
- Alazzeh, A.Y.; Ibrahim, S.A.; Song, D.; Shahbazi, A.; AbuGhazaleh, A.A. Carbohydrate and protein sources influence the induction of α- and β-galactosidases in Lactobacillus reuteri. Food Chem. 2009, 117, 654–659. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.W.; Liu, H.C.; Yu, Q.; Pei, X.F. Non-fusion and fusion expression of beta-galactosidase from Lactobacillus bulgaricus in Lactococcus lactis. Biomed Environ Sci. 2008, 21, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.A.; Lee, S.L.; Chou, C.C. Enzymatic Production of Galactooligosaccharides by β-Galactosidase from Bifidobacterium longum BCRC 15708. J. Agric. Food Chem. 2007, 55, 2225–2230. [Google Scholar] [CrossRef]
- Mlichova, Z.; Rosenberg, M. Current Trends of β-Galactosidase Application in Food Technology. J. Food Nutr. Res. 2006, 45, 47–54. [Google Scholar]
- Yu, L.; O’Sullivan, D.J. Production of Galactooligosaccharides Using a Hyperthermophilic β-Galactosidase in Permeabilized Whole Cells of Lactococcus lactis. J. Dairy Sci. 2014, 97, 694–703. [Google Scholar] [CrossRef]
- Lu, L.; Xu, S.; Zhao, R.; Zhang, D.; Li, Z.; Li, Y.; Xiao, M. Synthesis of Galactooligosaccharides by CBD Fusion β-Galactosidase Immobilized on Cellulose. Bioresour. Technol. 2012, 116, 327–333. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Nguyen, H.A.; Arreola, S.L.; Mlynek, G.; Djinović-Carugo, K.; Mathiesen, G.; Nguyen, T.-H.; Haltrich, D. Homodimeric β-Galactosidase from Lactobacillus delbrueckii subsp. bulgaricus DSM 20081: Expression in Lactobacillus plantarum and Biochemical Characterization. J. Agric. Food Chem. 2012, 60, 1713–1721. [Google Scholar] [CrossRef]
- Freitas, F.F.; Marquez, L.D.S.; Ribeiro, G.P.; Brandão, G.C.; Cardoso, V.L.; Ribeiro, E.J. Optimization of the Immobilization Process of β-Galatosidade by Combined Entrapment-Cross-Linking and the Kinetics of Lactose Hydrolysis. Braz. J. Chem. Eng. 2012, 29, 15–24. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Cimen, A.G.; Arica, M.Y. Immobilisation of β-Galactosidase onto Double Layered Hydrophilic Polymer Coated Magnetic Nanoparticles: Preparation, Characterisation and Lactose Hydrolysis. Int. Dairy J. 2023, 138, 105545. [Google Scholar] [CrossRef]
- Selvarajan, E.; Mohanasrinivasan, V.; Subathra Devi, C.; George Priya Doss, C. Immobilization of β-Galactosidase from Lactobacillus plantarum HF571129 on ZnO Nanoparticles: Characterization and Lactose Hydrolysis. Bioprocess Biosyst. Eng. 2015, 38, 1655–1669. [Google Scholar] [CrossRef]
- Mukundan, S.; Melo, J.S.; Sen, D.; Bahadur, J. Enhancement in β-Galactosidase Activity of Streptococcus lactis Cells by Entrapping in Microcapsules Comprising of Correlated Silica Nanoparticles. Colloids Surf. B Biointerfaces 2020, 195, 111245. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.N.; Yoshida, K.; Sakanaka, M.; Jiang, L.; Odamaki, T.; Katayama, T. Ecological and Molecular Perspectives on Responders and Non-Responders to Probiotics and Prebiotics. Curr. Opin. Biotechnol. 2022, 73, 108–120. [Google Scholar] [CrossRef]
- Sotoya, H.; Shigehisa, A.; Hara, T.; Matsumoto, H.; Hatano, H.; Matsuki, T. Identification of Genes Involved in Galactooligosaccharide Utilization in Bifidobacterium breve Strain YIT 4014T. Microbiology 2017, 163, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.M.; Barrangou, R.; Abou Hachem, M.; Lahtinen, S.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional and Functional Analysis of Galactooligosaccharide Uptake by lacS in Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2011, 108, 17785–17790. [Google Scholar] [CrossRef] [PubMed]
- O’Connell Motherway, M.; Fitzgerald, G.F.; Van Sinderen, D. Metabolism of a Plant Derived Galactose-containing Polysaccharide by Bifidobacterium breve UCC2003. Microb. Biotechnol. 2011, 4, 403–416. [Google Scholar] [CrossRef]
- Connell Motherway, M.; Kinsella, M.; Fitzgerald, G.F.; Van Sinderen, D. Transcriptional and Functional Characterization of Genetic Elements Involved in Galacto-oligosaccharide Utilization by Bifidobacterium breve UCC 2003. Microb. Biotechnol. 2013, 6, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Placier, G.; Watzlawick, H.; Rabiller, C.; Mattes, R. Evolved β-Galactosidases from Geobacillus stearothermophilus with Improved Transgalactosylation Yield for Galacto-Oligosaccharide Production. Appl. Environ. Microbiol. 2009, 75, 6312–6321. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xu, L.; Guo, Y.; Zhang, D.; Qi, T.; Jin, L.; Gu, G.; Xu, L.; Xiao, M. Glycosylation of Phenolic Compounds by the Site-Mutated β-Galactosidase from Lactobacillus bulgaricus L3. PLoS ONE 2015, 10, e0121445. [Google Scholar] [CrossRef] [PubMed]
| Name | Formula IUPAC (Condensed) | 2D Structure |
|---|---|---|
| Raffinose | Gal(a1→6)Glc(a1→2b)Fru |  |
| Stachyose | Gal(a1→6)Gal(a1→6)Glc(a1→2b)Fru | 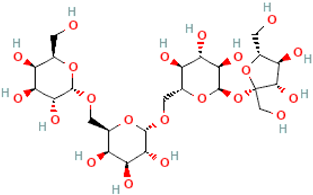 |
| Verbascose | Gal(a1→6)Gal(a1→6)Gal(a1→6)Glc(a1→2b)Fru | 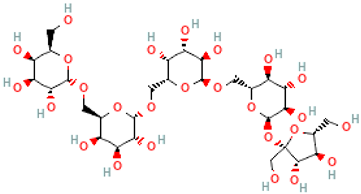 |
| Ajugose | Gal(a1→6)Gal(a1→6)Gal(a1→6)Gal(a1→6)Glc(a1→2b)Fru | 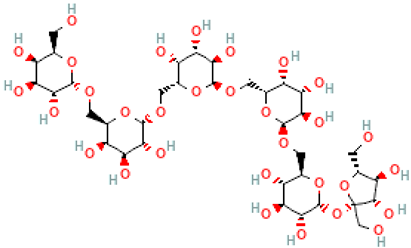 |
| Lychnose | α-D-Gal-(1→1)-β-D-Fru-(2↔1)-α-D-Glc-(6←1)-α-D-Gal (Gal-1-6-Glc-1-2-Fru-1-1-Gal) | 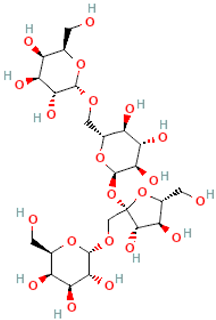 |
| Isolychnose | α-D-Gal-(1→3)-β-D-Fru-(2↔1)-α-D-Glc-(6←1)-α-D-Gal (3F-α-D-Galactosylraffinose) | 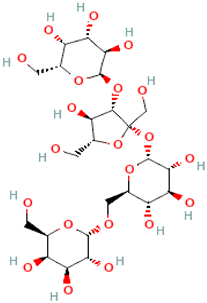 |
| Stellariose | α-D-Gal-(1→1)-β-D-Fru-(2↔1)-α-D-Glc-[(4←1)-α-D-Gal-(6←1)-α-D-Gal | 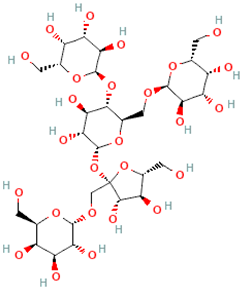 |
| Name | Formula IUPAC (Condensed) | 2D Structure |
|---|---|---|
| 3-Galactosyllactose | Gal(αl→3)-Gal(βl→4)Glc |  |
| 4-Galactosyllactose | β-Gal-(1→4)-β-Gal-(1→4)-Glc |  |
| Species, Strain | Enzyme | pH/ T (°C) | Metal Ions | Substrate | GOS | Reference |
|---|---|---|---|---|---|---|
| B. lactis B94 | Purified | 37 °C | ND | Soymilk | α-(1-6) | [64] |
| S. thermophilus St1342 | Purified | 37 °C | ND | Soymilk | α-(1-6) | [64] |
| L. acidophilus La4962 | Purified | 37 °C | ND | Soymilk | α-(1-6) | [64] |
| B. adolescentis DSM 20083 | 344 kDa (79 kDa monomer) | pH 5.5, 55 °C | ND | Raffinose, stachyose | α-(1-6), α-(1-3), α-(1-4) De novo GOS synthesis | [75] |
| B. breve DSM 20213 | 160 kDa, (80 kDa monomer) | pH 5.5, 37 °C | Cu2+, Hg2+ | Raffinose, melibiose | α-(1-6), α-(1-3), α-(1-4) | [64] |
| B. breve 203 | Aga2, 81.5 kDa monomer | pH 5.5, 50 °C | (NH)4+, EDTA Cu2+, Hg2+, Ag+ | Melibiose | De novo synthesis of a trisaccharide [Gal-α-1, 4-Gal-α-1, 6-Glc] | [76] |
| B. longum, B. pseudocatenulatum | Purified (2 enzymes) | pH 6.0, 40 °C | Zn2+, Na+, Ca2+, Mn2+ Al3+ | Raffinose | GOS of α-D-galactose + L-arabinose, α-D-galactose + sucrose | [64] |
| B. bifidum NCIMB 41171 | MelA, 243 kDa, 85 kDa monomer | pH 6.0 | ND | Melibiose | Synthesis of GOS with DP ≥ 3, the total yield of 20.5% (w/w) | [77] |
| Species, Strain | Mw (kDa) | 3D Structure | pH, T (°C) | Metal Ions | Substrate | β-GOS | Reference |
|---|---|---|---|---|---|---|---|
| L. bulgaricus 43 | 150 | Tetramer | pH 6.5 55 °C | Mn2+, Mg2+, Ca2+ Zn2+, Cu2+ | Lactose | DP3, DP4 | [116] |
| L. acidophilus R22 | 107 | Heterodimer | pH 6.5 55 °C | Mg2+ Mn2+, Cu2+, Zn2+ | Lactose | DP2 | [117] |
| Lim. fermentum K4 | 107 | Heterodimer | pH 6.5–7.0 40–50 °C | Na+, K+, Mg2+ | Lactose | ND | [118] |
| L. helveticus DSM 20075 | 110 | Heterodimer | pH 6.5 55–60 °C | K+, Na+ Mn2+, Mg2+, Ca2+, Zn2+ | Lactose, ONPG 1 | DP2, DP3, DP4 | [119] |
| L. curiae M2011381 | ND | Heterodimer | pH 8.0 55 °C | ND | Lactose | ND | [120] |
| L. acidophilus MR-24 | 110 | Heterodimer | pH 7.0 37 °C | Mg2+, Ca2+ Zn2+, Cu2+ | Lactose | ND | [121] |
| B. longum ssp. infantis BiBga42A | 73.5 | Trimer | pH 7.0 45 °C | ND | Lacto-N-tetraose | ND | [122] |
| L. reuteri L103 | 105 | Heterodimer | pH 6.0 50 °C | Na+, K+, Mn2+ | ONPG 1, Lactose | ND | [123] |
| L. reuteri L461 | 105 | Heterodimer | pH 6.5 55 °C | Na+, K+, Mn2+ | ONPG 1 | ND | [123] |
| L. mucosae OLL 2848 | ND | ND | 37 °C | ND | ONPG 1 | ND | [67] |
| Lpl. plantarum WCFS1 | 107 | Heterodimer | pH 6.5–8.0 30 °C | Mg2+, Mn2+ | ABTG 2 | DP3, DP2 | [124] |
| Lpl. plantarum | ND | ND | pH 6.5 50 °C | Mg2+, Ca2+ carbohydrates | Lactose, Lactulose | DP3, DP4 | [125] |
| L. crispatus ATCC 33820 | ND | ND | pH 6.5 50 °C | Fe2+, Mn2+ Zn2+ | Lactose | ND | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ignatova, I.; Arsov, A.; Petrova, P.; Petrov, K. Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation. Molecules 2025, 30, 803. https://doi.org/10.3390/molecules30040803
Ignatova I, Arsov A, Petrova P, Petrov K. Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation. Molecules. 2025; 30(4):803. https://doi.org/10.3390/molecules30040803
Chicago/Turabian StyleIgnatova, Ina, Alexander Arsov, Penka Petrova, and Kaloyan Petrov. 2025. "Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation" Molecules 30, no. 4: 803. https://doi.org/10.3390/molecules30040803
APA StyleIgnatova, I., Arsov, A., Petrova, P., & Petrov, K. (2025). Prebiotic Effects of α- and β-Galactooligosaccharides: The Structure-Function Relation. Molecules, 30(4), 803. https://doi.org/10.3390/molecules30040803








