Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry
Abstract
1. Introduction
2. Si-, Ge- and Sn-Complexes of α-Amino Carboxylic Acid-Derived (O,N)-Chelating Ligands
2.1. Si-, Ge- and Sn-Complexes Derived from α-Amino Monocarboxylic Acids
2.1.1. Si-Complexes Derived from α-Amino Monocarboxylic Acids
2.1.2. Ge-Complexes Derived from α-Amino Monocarboxylic Acids
2.1.3. Sn-Complexes Derived from α-Amino Monocarboxylic Acids
2.1.4. Concluding Remarks Regarding Si-, Ge- and Sn-Complexes Derived from α-Amino Monocarboxylic Acids
2.2. Si-, Ge- and Sn-Complexes Derived from Dipeptides
2.2.1. Si-Complexes Derived from Dipeptides
2.2.2. Ge-Complexes Derived from Dipeptides
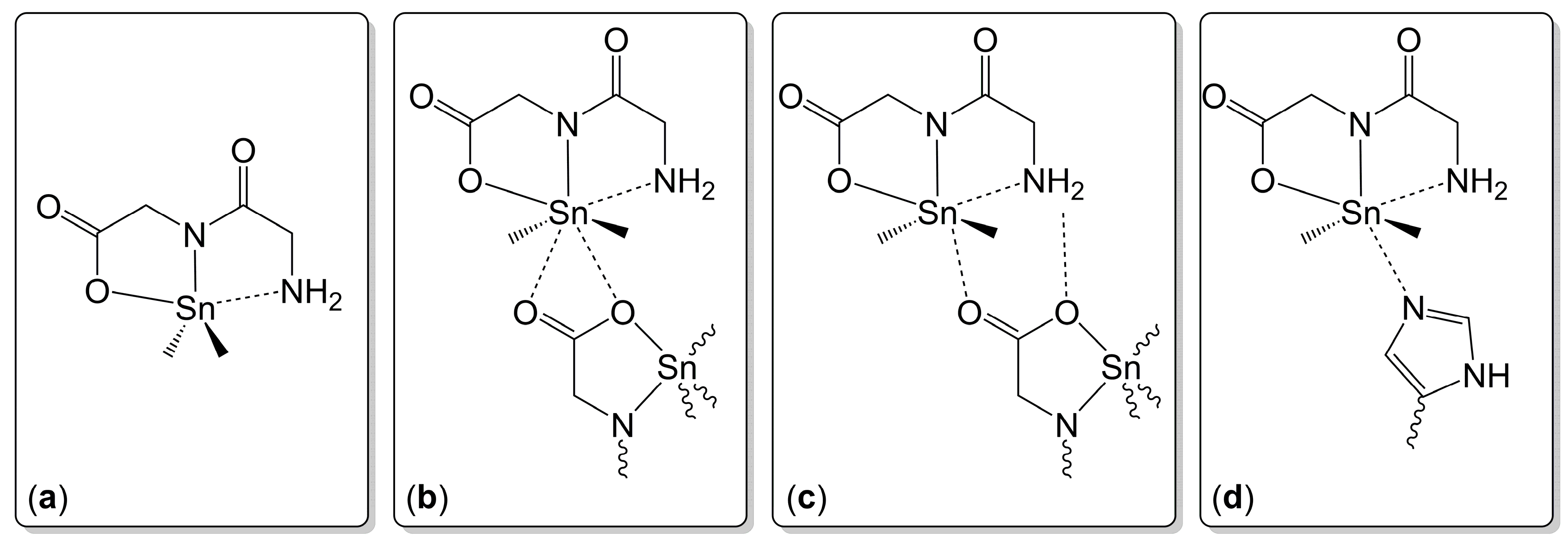
2.2.3. Sn-Complexes Derived from Dipeptides
2.2.4. Concluding Remarks Regarding Si-, Ge- and Sn-Complexes Derived from Dipeptides
2.3. Si-, Ge- and Sn-Complexes Derived from Amineoligoacetic Acids and Related Hydroxyalkylamineacetic Acids
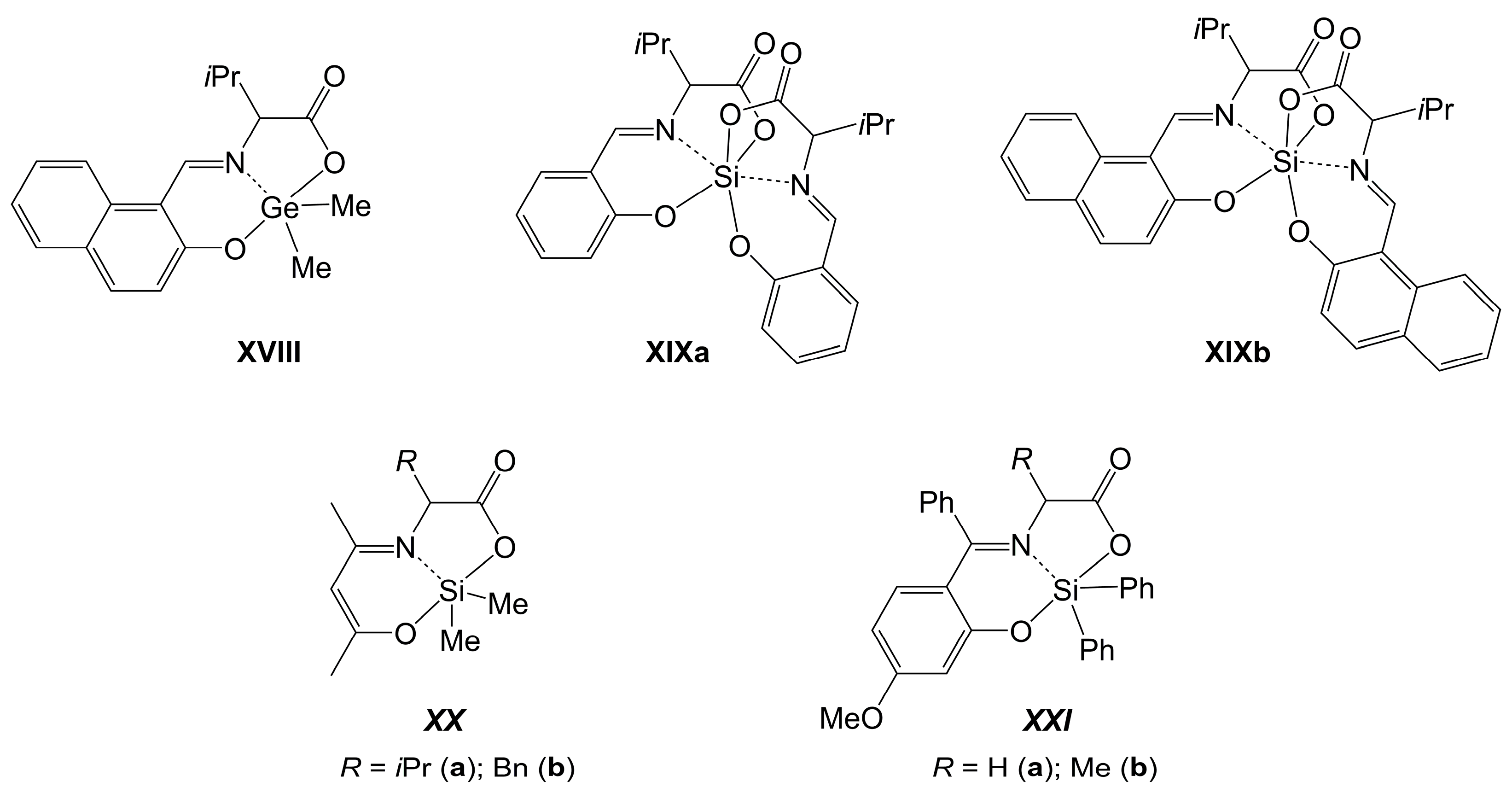
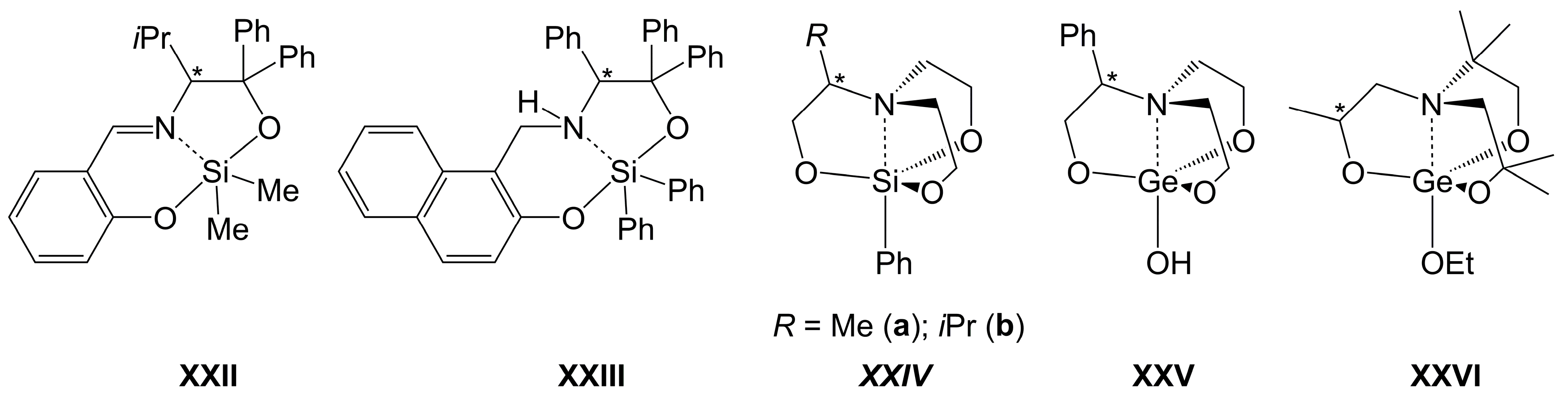
2.3.1. Si-Complexes Derived from Amineoligoacetic Acids and Related Hydroxyalkylamineacetic Acids
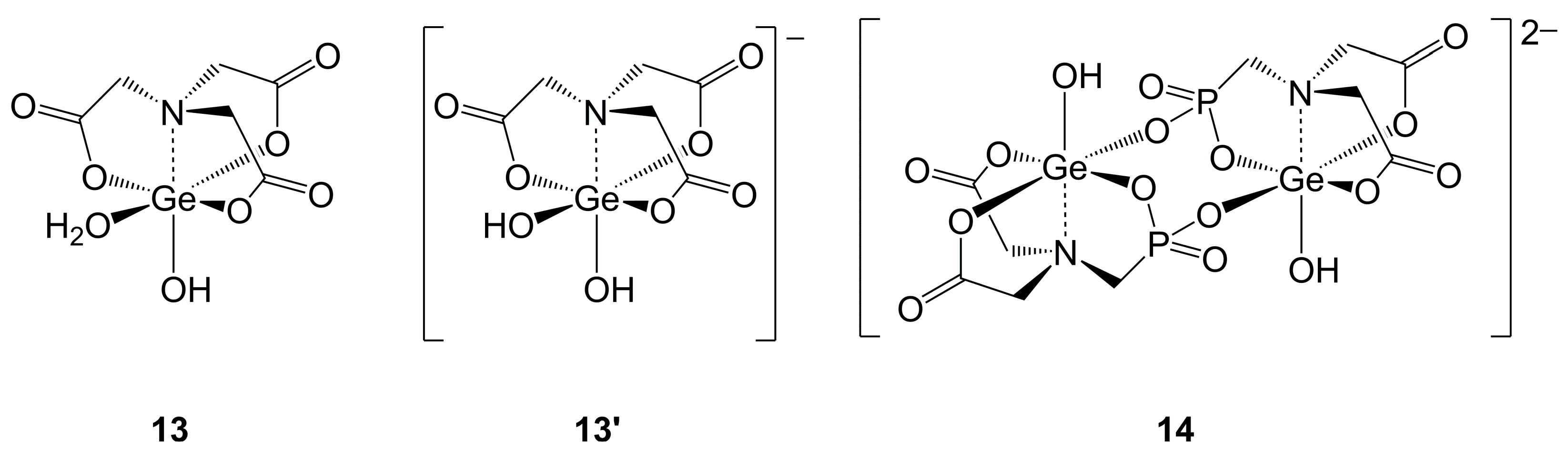
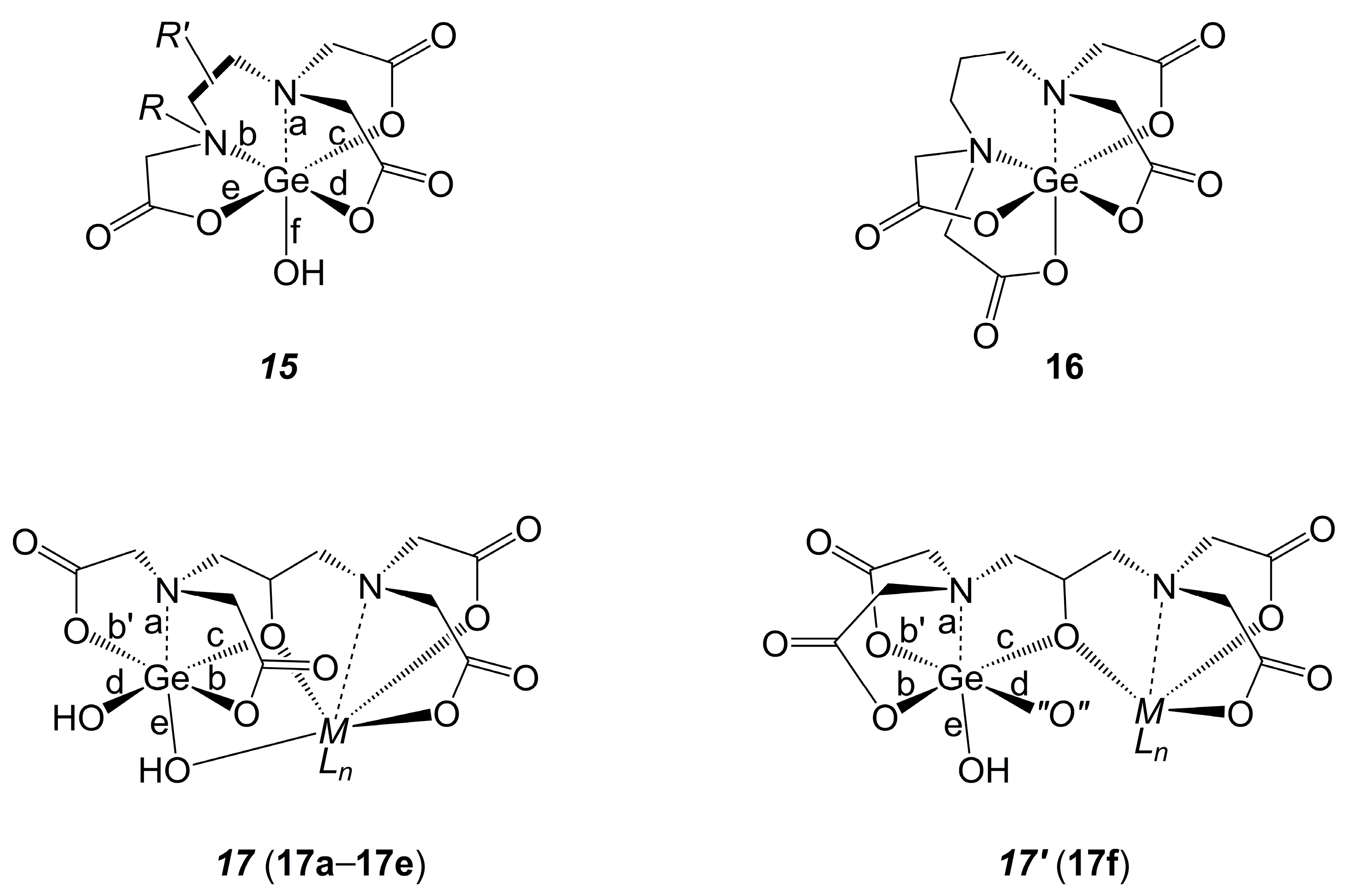
2.3.2. Ge-Complexes Derived from Amineoligoacetic Acids and Related Hydroxyalkylamineacetic Acids
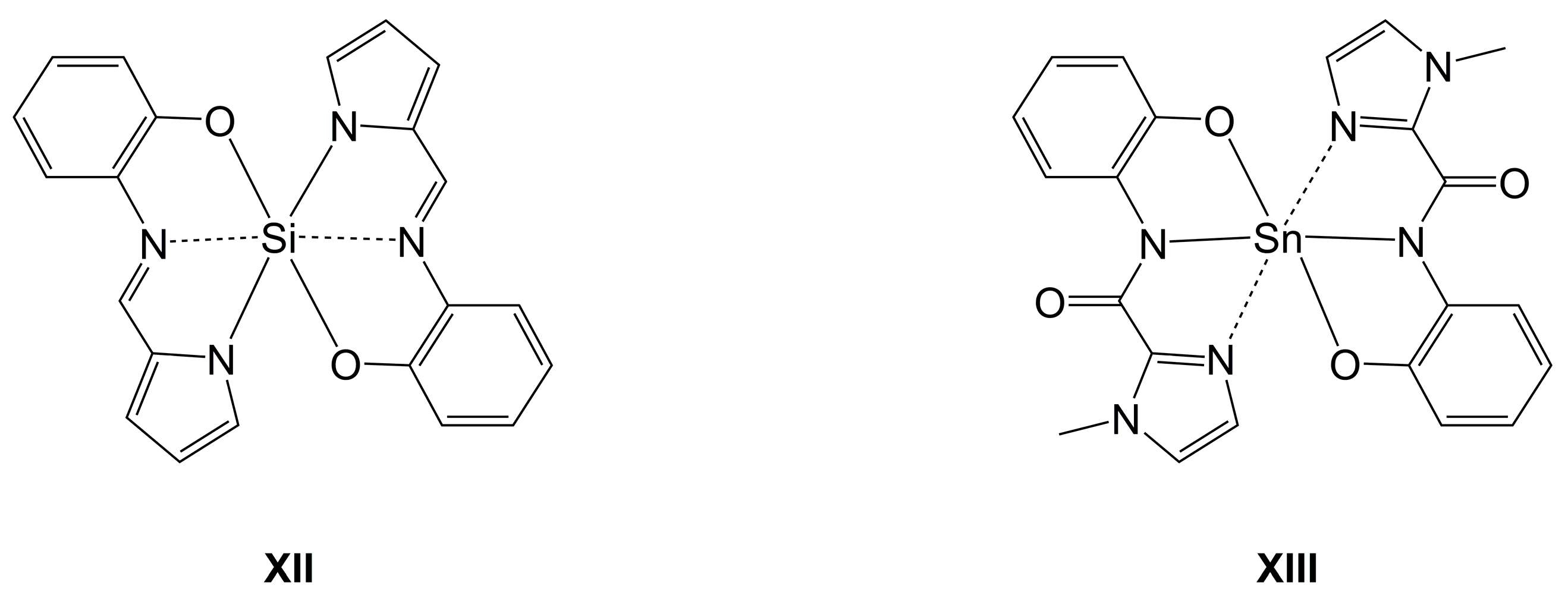
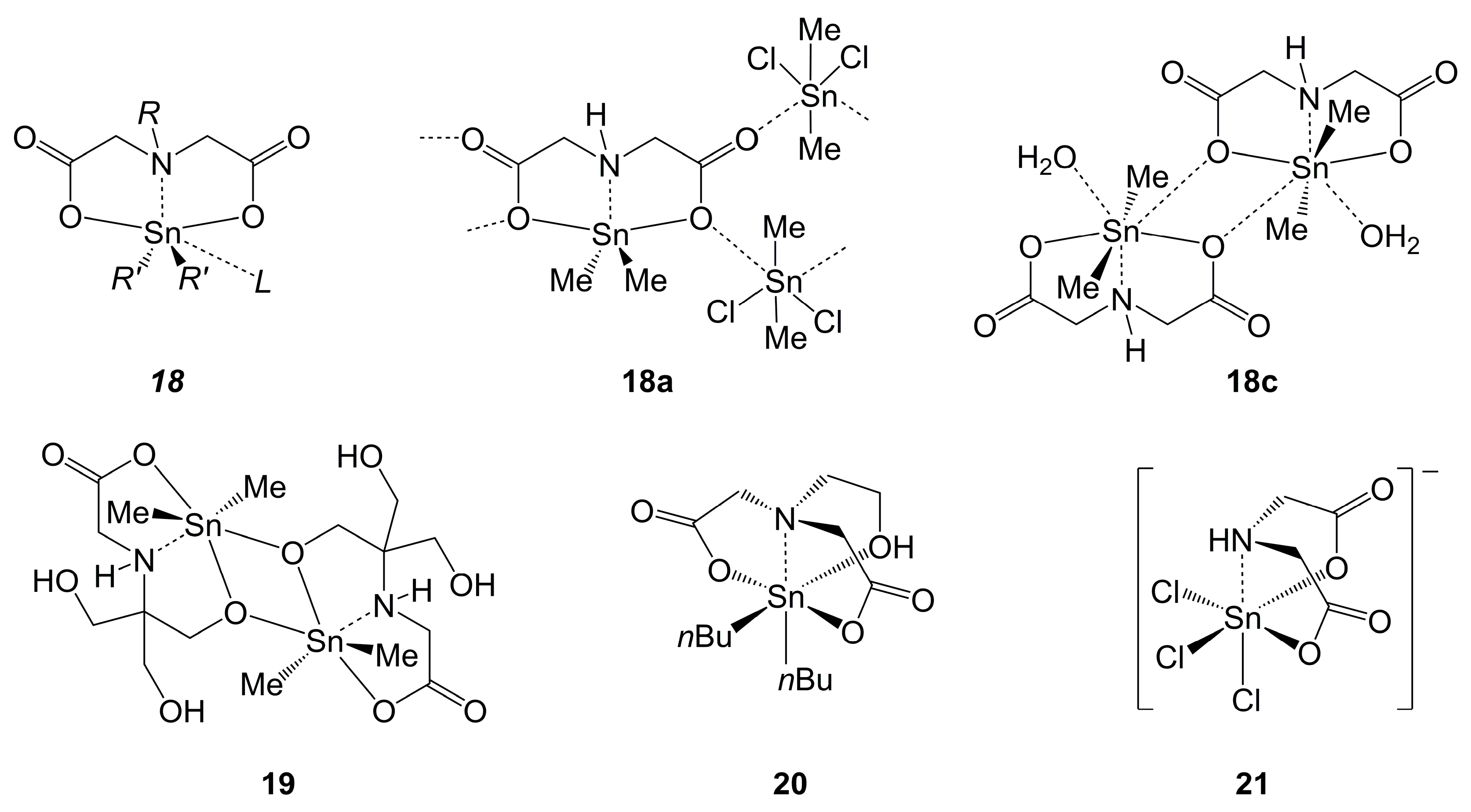
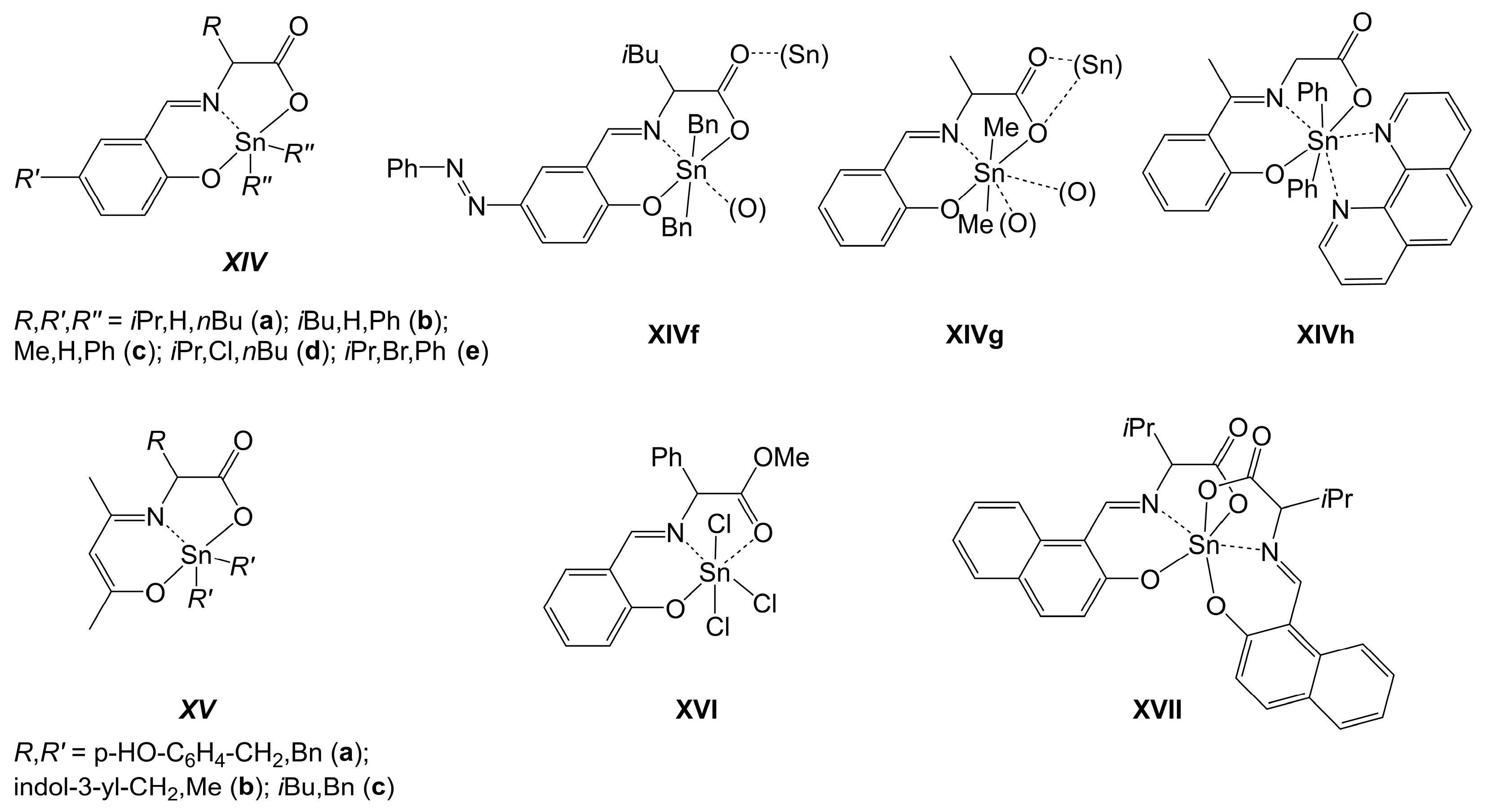
2.3.3. Sn-Complexes Derived from Amineoligoacetic Acids and Related Hydroxyalkylamineacetic Acids
2.3.4. Concluding Remarks Regarding Si-, Ge- and Sn-Complexes Derived from Amineoligoacetic Acids and Related Hydroxyalkylamineacetic Acids
3. Perspectives Outside the Box
3.1. α-Amino Acid-Derived Schiff Bases as Ligands
3.2. α-Amino Acid-Derived Alcohols as Ligands
3.3. Ligands Derived from Non-Carboxylic α-Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Compound | Ref. | CSD Refcode | Compound | Ref. | CSD Refcode | Compound | Ref. | CSD Refcode |
|---|---|---|---|---|---|---|---|---|
| I | [26] | IHAFAY | XIVe | [151] | KAXVIP | XXIII | [167] | FEYMON |
| II | [27] | EYASOO | XIVf | [155] | OGOCIZ | XXIVa | [168] | RABLUB |
| III | [28] | MOCCIT | XIVg | [156] | NEYDED | XXIVb | [168] | RABMAI |
| IV | [29] | DMSNQN | XIVh | [157] | JATHUI | XXV | [169] | IJAXEW |
| V | [30] | FEDYOC | XVa | [152] | ZEHQIP | XXVI | [170] | OGABAD |
| VI | [31] | FICNUA | XVb | [153] | KALYEE | XXVII | [173] | QIFBIT |
| VII | [32] | CEWJAP | XVc | [154] | ESOMAC | XXVIIIa | [174] | UGEKOK |
| VIII | [33] | WONQOG | XVI | [158] | JILXAH | XXVIIIb | [174] | UGELEB |
| IX | [34] | CEKWAR | XVII | [159] | ZIGWIY | XXVIIIc | [174] | UGELAX |
| X | [35] | WEQRIU | XVIII | [159] | ZIGTUH | XXVIIId | [174] | UGELIF |
| XI | [36] | VUKMAS | XIXa | [161] | ACIJED | XXVIIIe | [174] | UGEKUQ |
| XII | [89] | VUNKAR | XIXb | [159] | ZIGWAG 1 | XXIX | [179] | KISROT |
| XIII | [90] | IQATOK | XXa | [162] | MUFQAH | XXX | [180] | QIHWEJ |
| XIVa | [148] | JUNHAB | XXb | [163] | OFOYOZ | XXXI | [181] | JIPYEP 2 |
| XIVb | [149] | TOZNEB | XXIa | [164] | JICTAU | XXXII | [184] | SUPHOC |
| XIVc | [150] | EHIZOK | XXIb | [165] | YILVOI | XXXIII | [185] | GIJJIT |
| XIVd | [151] | KAXVEL | XXII | [166] | WEKFAU | XXXIV | [185] | GIJJAL |
| Compound | Ref. | CSD Refcode | Compound | Ref. | CSD Refcode | Compound | Ref. | CSD Refcode |
|---|---|---|---|---|---|---|---|---|
| 1 | [48] | WUYTIU | 11g | [78] | KIHYAB | 17e | [114] | GELJUE |
| 2a | [48] | WUYTOA | 11h | [79] | VUPRON | 17f | [116] | RAGHIS |
| 2b | [48] | WUYTOG | 11i | [80] | IGUQIL | 18a | [119] | OWEYAS |
| 2c | [48] | WUYVAO | 11j | [81] | MOCRAY | 18b | [120] | IGEYOI |
| 2d | [48] | WUYVES | 11k | [81] | MOCREC | 18c | [121] | KELLAO |
| 2e | [48] | WUYVIW | 11l | [82] | VUPDAL | 18d | [122] | TOSRIC |
| 3a | [49] | GAGJEE | 11m | [83] | PATHEX | 18e | [123] | GAMYUP |
| 3b | [49] | GAGJII | 11n | [83] | PATHIB | 18f | [124] | SARREL |
| 3c | [49] | GAGJOO | 11o | [84] | SASZUH | 18g | [125] | LUKDEB01 |
| 3d | [50] | JAZHAU | 11p | [85] | GLDPSN | 19 | [126] | VAZHAG |
| 3e | [50] | JAZHEY | 12a | [93] | CASYOK | 20 | [127] | VIPDAZ |
| 3f | [50] | JAZHOI | 12b | [94] | CECXEN | 21 | [128] | QOVVEE |
| 5 | [52] | UYAYEZ | 12c | [95] | FMESIA | 22 | [129] | QINWOZ |
| 6a | [39] | JAPZOT | 12d | [95] | FMESIB | 23 | [130] | HAWBOW |
| 6b | [37] | HIVJOP | 12e | [96] | FOFXED | 24 | [131] | LOFJEV |
| 6c | [37] | HIVJUV | 12f | [97] | GEKXID | 25 1 | [132] | FIBWAO |
| 7a | [57] | GLYMSN10 | 12g | [98] | DIRSAY | 25 2 | [132] | FIBWOC |
| 7b | [58] | BIGSUG | 13 | [102] | VUHCIK | 25 3 | [133] | IWEROR |
| 8a | [59] | ECYSSN | 13′ | [103] | LAFHIL | 25 4 | [133] | IWERIL |
| 8b | [60] | BONYAE | 14 | [104] | TEBJOA | 26 5 | [134] | HIVZUJ |
| 8c | [61] | VUKLIW | 15a | [106] | VEFGOC | 26 6 | [135] | SNEDTA |
| 9a | [39] | JAPZUZ | 15b | [107] | VOCPIN | 26 7 | [136] | NUQZEE |
| 9b | [51] | QANSOR | 15c | [108] | YUFYIH | 26 8 | [137] | QELZUC |
| 10a | [70] | VASWER | 15d | [109] | XOHHEH | 26 9 | [137] | QEMBAL |
| 10b | [71] | VAWHEG | 15e | [110] | VOCPOT | 26 10 | [138] | TORTUP |
| 11a | [73] | DOHBIL | 15f | [108] | YUFYON | 26 11 | [139] | WEVNAP |
| 11b | [74] | NOCQOM | 16 | [111] | XUZZEZ | 27 12 | [140] | KIJWUV |
| 11c | [75] | QOBXOU | 17a | [103] | GEMPEV | 27 12 | [141] | KIJWUV01 |
| 11d | [76] | VUKHAK | 17b | [113] | ELOYIO | 27 13 | [142] | LOHLEZ |
| 11e | [76] | VUKHEO | 17c | [114] | GELJOY | 28 | [143] | EDTASN |
| 11f | [77] | ZUQBOC | 17d | [115] | XAVRUL | 29 | [144] | HEDTAT |
| Entry | Ref. | CSD Refcode | Entry | Ref. | CSD Refcode | Entry | Ref. | CSD Refcode |
|---|---|---|---|---|---|---|---|---|
| A3a | [66] | BOHNES | A3h | [122] | TOSRUO | A3o | [177] | PEGRUQ |
| A3b | [67] | ODEWEA | A3i | [146] | EDTAFE01 | A3p | [178] | ZAYRIC |
| A3c | [68] | RIRRUG | A3j | [147] | CERFEK | A3q | [182] | TASSAH |
| A3d | [69] | NAGZAZ | A3k | [171] | TIRYAU | A3r | [183] | TUPMEW |
| A3e | [112] | DANRUG | A3l | [171] | TIRXUN | A3s | [186] | IGICEG |
| A3f | [117] | XAVROF | A3m | [172] | AMSOCU | A3t | [187] | RODLOL |
| A3g | [118] | TEKSEJ | A3n | [176] | JOVXAV |
References
- Stolba, F. Über die Bedeutung der Kieselflusssäure für die chemische Analyse. Z. Anal. Chem. 1864, 3, 298–313. [Google Scholar] [CrossRef]
- Davy, J. XVIII. An Account of some Experiments on different Combinations of Fluoric Acid. Philos. Trans. R. Soc. Lond. 1812, 102, 352–369. [Google Scholar] [CrossRef][Green Version]
- Cohen, E. Physikalisch-chemische Studien am Zinn. Z. Phys. Chem. Stoechiom. Verwandtschafts. 1900, 35, 588–597. [Google Scholar] [CrossRef]
- Laubengayer, A.W.; Billings, O.B.; Newkirk, A.E. Chlorogermanic Acid and the Chlorogermanates. Properties and Crystal Structure of Cesium Hexachlorogermanate. J. Am. Chem. Soc. 1940, 62, 546–548. [Google Scholar] [CrossRef]
- Winkler, C. Germanium, Ge, ein neues, nichtmetallisches Element. Chem. Ber. 1886, 19, 210–211. [Google Scholar] [CrossRef]
- Sivaramakrishna, A.; Pete, S.; Mhaskar, C.M.; Ramann, H.; Ramanaiah, D.V.; Arbaaz, M.; Niyaz, M.; Janardan, S.; Suman, P. Role of hypercoordinated silicon(IV) complexes in activation of carbon–silicon bonds: An overview on utility in synthetic chemistry. Coord. Chem. Rev. 2023, 485, 215140. [Google Scholar] [CrossRef]
- Lemière, G.; Millanvois, A.; Ollivier, C.; Fensterbank, L. A Parisian Vision of the Chemistry of Hypercoordinated Silicon Derivatives. Chem. Rec. 2021, 21, 1119–1129. [Google Scholar] [CrossRef]
- Singh, G.; Kaur, G.; Singh, J. Progressions in hyper–coordinate silicon complexes. Inorg. Chem. Commun. 2018, 88, 11–20. [Google Scholar] [CrossRef]
- Peloquin, D.M.; Schmedake, T.A. Recent advances in hexacoordinate silicon with pyridine-containing ligands: Chemistry and emerging applications. Coord. Chem. Rev. 2016, 323, 107–119. [Google Scholar] [CrossRef]
- Wagler, J.; Böhme, U.; Kroke, E. Higher-Coordinated Molecular Silicon Compounds. In Functional Molecular Silicon Compounds I—Regular Oxidation States; Scheschkewitz, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 115, pp. 29–105. [Google Scholar] [CrossRef]
- Puri, J.K.; Singh, R.; Kaur Chahal, V. Silatranes: A review on their synthesis, structure, reactivity and applications. Chem. Soc. Rev. 2011, 40, 1791–1840. [Google Scholar] [CrossRef]
- Kost, D.; Kalikhman, I. Hydrazide-based hypercoordinate silicon compounds. Adv. Organomet. Chem. 2004, 50, 1–106. [Google Scholar] [CrossRef]
- Chuit, C.; Corriu, R.J.P.; Reye, C.; Young, J.C. Reactivity of Penta- and Hexacoordinate Silicon Compounds and Their Role as Reaction Intermediates. Chem. Rev. 1993, 93, 1371–1448. [Google Scholar] [CrossRef]
- Sidorkin, V.F.; Pestunovich, V.A.; Voronkov, M.G. The Physical Chemistry of Silatranes. Russ. Chem. Rev. 1980, 49, 414–427. [Google Scholar] [CrossRef]
- Kadomtseva, A.V.; Mochalov, G.M.; Kuzina, O.V. Biologically Active Coordination Compounds of Germanium. Synthesis and Physicochemical Properties. Russ. J. Org. Chem. 2021, 57, 879–888. [Google Scholar] [CrossRef]
- Levason, W.; Reid, G.; Zhang, W. Coordination complexes of silicon and germanium halides with neutral ligands. Coord. Chem. Rev. 2011, 255, 1319–1341. [Google Scholar] [CrossRef]
- Singh, R.V.; Gupta, P.; Chaudhary, P.; Deshmukh, C.N. Coordination Compounds of Germanium(IV) Formed with Soft and Hard Donor Atoms: A Look into the Past and Present Work. Main Group Met. Chem. 2005, 28, 93–118. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Germanium Organometallic Compounds: Classification and Analysis of Crystallographic and Structural Data. Main Group Met. Chem. 2002, 25, 185–266. [Google Scholar] [CrossRef]
- Lavigne, A.A.; Tancrede, J.M.; Pike, R.M. Coordination Compounds of Germanium. Coord. Chem. Rev. 1968, 3, 497–508. [Google Scholar] [CrossRef]
- Devi, J.; Kumar, B.; Taxak, B. Recent advancements of organotin(IV) complexes derived from hydrazone and thiosemicarbazone ligands as potential anticancer agents. Inorg. Chem. Commun. 2022, 139, 109208. [Google Scholar] [CrossRef]
- Srivastav, N.; Kumar, K.; Singh, R.; Kaur, V. Tricyclic tin(iv) cages: Synthetic aspects and intriguing features of stannatranes and pseudostannatranes. New J. Chem. 2020, 44, 3168–3184. [Google Scholar] [CrossRef]
- Manju; Mishra, N.; Kumar, D. Coordination Chemistry of Schiff Base Tin Complexes. Russ. J. Coord. Chem. 2014, 40, 343–357. [Google Scholar] [CrossRef]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological activity studies on organotin(IV)n+ complexes and parent compounds. J. Oranomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Pettinari, C. Organotin(IV) Derivatives of Imidazoles, Pyrazoles and Related Pyrazolyl and Imidazolyl Ligands. Main Group Met. Chem. 1999, 22, 661–692. [Google Scholar] [CrossRef]
- Holloway, C.E.; Melnik, M. Tin Coordination Compounds: Classification and Analysis of Crystallographic and Structural Data. Main Group Met. Chem. 1998, 21, 371–488. [Google Scholar] [CrossRef]
- Wagler, J.; Schley, M.; Gerlach, D.; Böhme, U.; Brendler, E.; Roewer, G. Surprising Insights in the Various Molecular Structures of Hypercoordinate Bis(oxinato)silicon Complexes. Z. Naturforsch. B 2005, 60, 1054–1064. [Google Scholar] [CrossRef]
- Wächtler, E.; Kämpfe, A.; Krupinski, K.; Gerlach, D.; Kroke, E.; Brendler, E.; Wagler, J. New Insights into Hexacoordinated Silicon Complexes with 8-Oxyquinolinato Ligands: 1,3-Shift of Si-Bound Hydrocarbyl Substituents and the Influence of Si-Bound Halides on the 8-Oxyquinolinate Coordination Features. Z. Naturforsch. B 2014, 69, 1402–1418. [Google Scholar] [CrossRef]
- Cai, Y.P. Germanium-8-Hydroxyquinoline hydroxides: Synthesis, structure and luminescence properties. Inorg. Chim. Acta 2019, 489, 211–216. [Google Scholar] [CrossRef]
- Schlemper, E.O. Crystal and molecular structure of dimethyltin bis(8-hydroxyquinolinate). Inorg. Chem. 1967, 6, 2012–2017. [Google Scholar] [CrossRef]
- Archer, S.J.; Koch, K.R.; Schmidt, S. Nitrogen donor chelates of bivalent tin chloride. Part II. The crystal structures of SnCl2·(2,2′-bipyridyl) and SnCl2·(1,10-phenanthroline). Inorg. Chim. Acta 1987, 126, 209–218. [Google Scholar] [CrossRef]
- Shi, D.; Hu, S. Structural studies of chloro(quinoline-8-olato)diethyltin(IV),[SnEt2Cl(C8H6NO)]. Chin. J. Struct. Chem. 1987, 6, 193–197. [Google Scholar]
- Schürmann, M.; Schmiedgen, R.; Huber, F.; Silvestri, A.; Ruisi, G.; Barbieri Paulsen, A.; Barbieri, R. Mono-aryltin(IV) and mono-benzyltin(IV) complexes with pyridine-2-carboxylic acid and 8-hydroxyquinoline. X-ray structure of p-chloro-phenyl-tris(8-quinolinato)tin(IV)·2CHCl3. J. Organomet. Chem. 1999, 584, 103–117. [Google Scholar] [CrossRef]
- Hagemann, M.; Mix, A.; Berger, R.J.F.; Pape, T.; Mitzel, N.W. Strong Intramolecular Si−N Interactions in the Chlorosilanes Cl3−nHnSiOCH2CH2NMe2 (n = 1−3). Inorg. Chem. 2008, 47, 10554–10564. [Google Scholar] [CrossRef]
- Khrustalev, V.N.; Portnyagin, I.A.; Borisova, I.V.; Zemlyansky, N.N.; Ustynyuk, Y.A.; Antipin, M.Y.; Nechaev, M.S. Donor-Stabilized Germyl Cations. Stable Pentacoordinate Germanium Chloride [PhGe(OCH2CH2NMe2)2][Cl]. Organometallics 2006, 25, 2501–2504. [Google Scholar] [CrossRef]
- Portnyagin, I.A.; Nechaev, M.S.; Khrustalev, V.N.; Zemlyansky, N.N.; Borisova, I.V.; Antipin, M.Y.; Ustynyuk, Y.A.; Lunin, V.V. An Unusual Reaction of (β-Dimethylaminoethoxy)triethyltin with Phenyltin Trichloride. The First X-ray Structural Evidence of the Existence of Complexes R2SnXY·R2SnXY (R = Alkyl, Aryl; X, Y = Hal, OR, X ≠ Y) Both as Unsymmetrical Adducts [R2SnX2·R2SnY2] and Symmetrical Dimers [R2SnXY]2. Eur. J. Inorg. Chem. 2006, 2006, 4271–4277. [Google Scholar] [CrossRef]
- Verchère, A.; Mishra, S.; Jeanneau, E.; Guillon, H.; Decams, J.-M.; Daniele, S. Heteroleptic Tin(IV) Aminoalkoxides and Aminofluoroalkoxides as MOCVD Precursors for Undoped and F-Doped SnO2 Thin Films. Inorg. Chem. 2020, 59, 7167–7180. [Google Scholar] [CrossRef]
- Seidel, A.; Gericke, R.; Kutzner, B.; Wagler, J. Lewis Acid-Base Adducts of α-Amino Acid-Derived Silaheterocycles and N-Methylimidazole. Molecules 2023, 28, 7816. [Google Scholar] [CrossRef]
- Kuß, S.; Brendler, E.; Wagler, J. Molecular Structures of the Pyridine-2-olates PhE(pyO)3 (E = Si, Ge, Sn)—[4+3]-Coordination at Si, Ge vs. Heptacoordination at Sn. Crystals 2022, 12, 1802. [Google Scholar] [CrossRef]
- Kowalke, J.; Brendler, E.; Wagler, J. Valinate and SiMe2—An interesting couple in pentacoordinate Si-complexes: Templated generation of the dipeptide val-val and formation of an organosilicon-ammonia-adduct. J. Organomet. Chem. 2021, 956, 122126. [Google Scholar] [CrossRef]
- Kämpfe, A.; Brendler, E.; Kroke, E.; Wagler, J. Tp*Cu(I)–CN–SiL2–NC–Cu(I)Tp*—A hexacoordinate Si-complex as connector for redox active metals via π-conjugated ligands. Dalton Trans. 2015, 44, 4744–4750. [Google Scholar] [CrossRef] [PubMed]
- Kämpfe, A.; Brendler, E.; Kroke, E.; Wagler, J. 2-Acylpyrroles as Mono-anionic O,N-Chelating Ligands in Silicon Coordination Chemistry. Chem. Eur. J. 2014, 20, 9409–9418. [Google Scholar] [CrossRef] [PubMed]
- Schöne, D.; Gerlach, D.; Wiltzsch, C.; Brendler, E.; Heine, T.; Kroke, E.; Wagler, J. A Distorted Trigonal Antiprismatic Cationic Silicon Complex with Ureato Ligands: Syntheses, Crystal Structures and Solid State 29Si NMR Properties. Eur. J. Inorg. Chem. 2010, 2010, 461–467. [Google Scholar] [CrossRef]
- Brendler, E.; Wächtler, E.; Wagler, J. Hypercoordinate Silacycloalkanes: Step-by-Step Tuning of N→Si Interactions. Organometallics 2009, 28, 5459–5465. [Google Scholar] [CrossRef]
- Wagler, J.; Böhme, U.; Brendler, E.; Roewer, G. Equilibrium between Tetra-, Penta-, and Hexacoordinate Imine and Enamine Chelates of Silicon: Crystal Structure and Variable-Temperature NMR. Organometallics 2005, 24, 1348–1350. [Google Scholar] [CrossRef]
- Nath, M.; Goyal, S. Triorganosilicon(IV) Derivatives of Amino Acids. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2002, 32, 1205–1221. [Google Scholar] [CrossRef]
- Nath, M.; Goyal, S. Dimethylsilicon(IV) Derivatives of Amino Acids. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 841–851. [Google Scholar] [CrossRef]
- Kelusky, E.C.; Fyfe, C.A. Molecular motions of alkoxysilanes immobilized on silica surfaces: A deuterium NMR study. J. Am. Chem. Soc. 1986, 108, 1746–1749. [Google Scholar] [CrossRef]
- Cota, S.; Beyer, M.; Bertermann, R.; Burschka, C.; Götz, K.; Kaupp, M.; Tacke, R. Neutral Penta- and Hexacoordinate Silicon(IV) Complexes Containing Two Bidentate Ligands Derived from the α-Amino Acids (S)-Alanine, (S)-Phenylalanine, and (S)-tert-Leucine. Chem. Eur. J. 2010, 16, 6582–6589. [Google Scholar] [CrossRef] [PubMed]
- Tacke, R.; Bertermann, R.; Burschka, C.; Dragota, S.; Penka, M.; Richter, I. Spirocyclic Zwitterionic λ5Si-Silicates with Two Bidentate Ligands Derived from α-Amino Acids or α-Hydroxycarboxylic Acids: Synthesis, Structure, and Stereodynamics. J. Am. Chem. Soc. 2004, 126, 14493–14505. [Google Scholar] [CrossRef] [PubMed]
- Dragota, S.; Bertermann, R.; Burschka, C.; Penka, M.; Tacke, R. Diastereo- and Enantiomerically Pure Zwitterionic Spirocyclic λ5Si-[(Ammonio)methyl]silicates with an SiO2N2C Skeleton Containing Two Bidentate Chelate Ligands Derived from α-Amino Acids. Organometallics 2005, 24, 5560–5568. [Google Scholar] [CrossRef]
- Hattori, T.; Yamamoto, H. Synthesis of Silacyclic Dipeptides: Peptide Elongation at Both N- and C-Termini of Dipeptide. J. Am. Chem. Soc. 2022, 144, 1758–1765. [Google Scholar] [CrossRef] [PubMed]
- Soto-Cairoli, B.; Kock, I.; de Pomar, J.J.; Yang, G.; Guzmán, J.M.; González, J.R.; Antomattei, A.; Soderquist, J.A. Selective Triisopropylsilylation of α-Amino Acids: Protection Without Racemization. Heterocycles 2010, 80, 409–426. [Google Scholar] [CrossRef]
- Denmark, S.E. The Interplay of Invention, Discovery, Development, and Application in Organic Synthetic Methodology: A Case Study. J. Org. Chem. 2009, 74, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Lavayssiere, H.; Dousse, G.; Satge, J. Heterocycles du germanium, du phosphore (III), de l’arsenic (III) et du soufre a ligand aminoacide ou hydroxyacide. J. Organomet. Chem. 1977, 137, C37–C42. [Google Scholar] [CrossRef]
- Lavayssiere, H.; Dousse, G.; Satge, J. Oxazolidones et dioxolonnes du germanium (IV) et (II) du phosphore (III), de l’arsenic (III) et du soufre. Phosphorus Sulfur Silicon Relat. Elem. 1990, 53, 411–422. [Google Scholar] [CrossRef]
- Ignatyev, I.S.; Kondratenko, Y.A.; Lezov, D.V.; Ugolkov, V.L.; Kochina, T.A. Hexacoordinate germanium compounds with BIS-TRIS and amino acid ligands. Mendeleev Commun. 2023, 33, 601–604. [Google Scholar] [CrossRef]
- Ho, B.Y.K.; Molloy, K.C.; Zuckerman, J.J.; Reidinger, F.; Zubieta, J.A. The crystal structure and variable temperature 119mSn Mössbauer study of trimethyltin glycinate, a one-dimensional, amino-bridged polymer. J. Organomet. Chem. 1980, 187, 213226. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, J.; Zhang, R. Syntheses and Characterizations of Diorganotin(IV) Complexes with L-Cysteine: Crystal Structure of [(CH3)2Sn(l-C3H5NO2S)·H2O]. Heteroat. Chem. 2003, 14, 636–641. [Google Scholar] [CrossRef]
- Domazetis, G.; MacKay, M.F.; Magee, R.J.; James, B.D. Interaction of Organotin Compounds with Biological Ligands and the Molecular Structure of Ethyl L-Cysteinato S,N.(chlorodimethyl)stahyl)stannate. Inorg. Chim. Acta 1979, 34, L247–L248. [Google Scholar] [CrossRef]
- Calogero, S.; Valle, G.; Cusack, P.A.; Smith, P.J.; Donaldson, J.D. The X-ray Crystal Structure of Dichloro-S,S’-Bis(O-methylcysteinato)tin(IV): The First Determination of an Inorganic Tin-Amino Acid Derivative. Inorg. Chim. Acta 1982, 67, 95–98. [Google Scholar] [CrossRef]
- Anderson, J.E.; Sawtelle, S.M.; Thompson, J.S.; Kretchmar Nguyen, S.A.; Calabrese, J. Ligand Addition Reactions and the Electron-Transfer Properties of SnCl2·2H2O and SnCl4·5H2O. Molecular Structure of Bis(ethylcysteinato)tin(II). Inorg. Chem. 1992, 31, 2778–2785. [Google Scholar] [CrossRef]
- Djurdjevic, P.; Djokic, D. Protein Interactions with Bivalent Tin. 1. Hydrolysis and Complexation of Tin(II) Ion with Glycine. J. Inorg. Biochem. 1996, 62, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Yadaf, R. Studies of Organotin(IV) derivatives of DL-Methionine and L-Asparagine. Bull. Chem. Soc. Jpn. 1998, 71, 1355–1362. [Google Scholar] [CrossRef]
- Cashion, J.D.; Domazetis, G.; James, B.D. Mössbauer spectra of organotin amino-acid and glutathione derivatives. J. Organomet. Chem. 1980, 185, 433–441. [Google Scholar] [CrossRef]
- Nath, M.; Jairath, R.; Eng, G.; Song, X.; Kumar, A. Synthesis, spectral characterization and biological studies of some organotin(IV) complexes of l-proline, trans-hydroxy-l-proline and l-glutamine. Spectrochim. Acta A 2005, 62, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Gasque, L.; Verhoeven, M.A.; Bernès, S.; Barrios, F.; Haasnoot, J.G.; Reedijk, J. The Added Value of Solid-State Pb NMR Spectroscopy to Understand the 3D Structures of Pb Amino Acid Complexes. Eur. J. Inorg. Chem. 2008, 2008, 4395–4403. [Google Scholar] [CrossRef]
- Marandi, F.; Shahbakhsh, N. Synthesis and Crystal Structure of [Pb(phe)2]n: A 2D Coordination Polymer of Lead(II) Containing Phenylalanine. Z. Anorg. Allg. Chem. 2007, 633, 1137–1139. [Google Scholar] [CrossRef]
- Marandi, F.; Shahbakhsh, N. Lead(II) complexes of proline. J. Coord. Chem. 2007, 60, 2589–2595. [Google Scholar] [CrossRef]
- Nair, R.M.; Dhanya, V.S.; Suma, S.; Sudhadevi Antharjanam, P.K.; Sudarsanakumar, M.R. A novel 2D ladder shaped metal–organic framework based on lead-aspartate system with hydrophobic channels. Main Group Chem. 2015, 14, 91–103. [Google Scholar] [CrossRef]
- Preut, H.; Vornefeld, M.; Huber, F. Dimethylgermanium Glycyl-l-methionate. Acta Crystallogr. C 1989, 45, 1504–1506. [Google Scholar] [CrossRef]
- Vornefeld, M.; Huber, F.; Preut, H.; Brunner, H. Synthesis and spectroscopic characterization of dimethylgermanium derivatives of dipeptides, crystal structure of dimethylgermanium glycylglycinate and in vivo effects of dimethylgermanium glycylglycinate against murine leukemia P388. Appl. Organomet. Chem. 1989, 3, 177–182. [Google Scholar] [CrossRef]
- Giuffrida, S.; Fontana, A.; Magio, F.; Duca, D. Synthesis, characterization and conformational analysis of chloro-bis(glycylglycinate)germanium(IV) chloride. New J. Chem. 2011, 35, 807–819. [Google Scholar] [CrossRef]
- Preut, H.; Mundus, B.; Huber, F.; Barbieri, R. Dimethyltin Glyeylmethionate. Acta Crystallogr. C 1986, 42, 536–538. [Google Scholar] [CrossRef]
- Nath, M.; Singh, H.; Eng, G.; Song, X. New di- and triorganotin(IV) derivatives of tyrosinylphenylalanine as models for metal–protein interactions: Synthesis and structural characterization. Crystal structure of Me2Sn(Tyr-Phe) · MeOH. J. Organomet. Chem. 2008, 693, 2541–2550. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Pizzino, T.; Mansueto, C.; Valle, G.; Stocco, G.C. Spectroscopic Characterization and Biological Activity of L-Methionyl-L Histidinato Complexes of R2Sn(IV) Ions (R = Me, nBu, Ph) and X-ray Structure of Me2SnMetHis·0.5MeOH. Appl. Organomet. Chem. 2000, 14, 197–211. [Google Scholar] [CrossRef]
- Stocco, G.; Gulì, G.; Valle, G. Structures of (L-Methionyl-L-methioninato)dimethyltin(IV) and (L-Alanyl-L-histidinato)dimethyltin(IV). A Class of Potential Antitumour Agents. Acta Crystallogr. C 1992, 48, 2116–2120. [Google Scholar] [CrossRef]
- Girasolo, M.A.; Pellerito, L.; Stocco, G.C.; Valle, G. Crystal and molecular structure of dimethyl(L-tryptophyl-L-alaninato)tin(IV)-methanol (l/l) and Mössbauer spectroscopy studies of lattice dynamics of diorganotin(IV) dipeptide complexes. J. Chem. Soc. Dalton Trans. 1996, 25, 1195–1201. [Google Scholar] [CrossRef]
- Preut, H.; Vornefeld, M.; Huber, F. Bis[diethyl(glycylhistidinato)tinl-Methanol (1/1). Acta Crystallogr. C 1991, 47, 264–267. [Google Scholar] [CrossRef]
- Vornefeld, M.; Huber, F.; Preut, H.; Ruisi, G.; Barbieri, R. Synthesis and spectroscopic characterization of diethyltin(1V) derivatives of dipeptides: Crystal and molecular structure of diethyltin glycyltyrosinate. Appl. Organomet. Chem. 1992, 6, 75–82. [Google Scholar] [CrossRef]
- Math, M.; Singh, H.; Kumar, P.; Kumar, A.; Song, X.; Eng, G. Organotin(IV) tryptophanylglycinates: Potential non-steroidal anti-inflammatory agents; crystal structure of dibutyltin(IV) tryptophanylglycinate. Appl. Organomet. Chem. 2009, 23, 347–358. [Google Scholar] [CrossRef]
- Katsoulakou, E.; Tiliakos, M.; Papaefstathiou, G.; Terzis, A.; Raptopoulou, C.; Geromichalos, G.; Papazisis, K.; Papi, R.; Pantazaki, A.; Kyriakidis, D.; et al. Diorganotin(IV) complexes of dipeptides containing the a-aminoisobutyryl residue (Aib): Preparation, structural characterization, antibacterial and antiproliferative activities of [(n-Bu)2Sn(H–1L)] (LH = H-Aib-L-Leu-OH, H-Aib-L-Ala-OH). J. Inorg. Biochem. 2008, 102, 1397–1405. [Google Scholar] [CrossRef]
- Mundus-Glowacki, B.; Huber, F.; Preut, H.; Guisi, G.; Barbieri, R. Synthesis and spectroscopic characterization of dimethyl-, di-n-butyl-, di-t-butyl- and diphenyl-tin(lV) derivatives of dipeptides: Crystal and molecular structure of di-nbutyltin(IV) glycylvalinate. Appl. Organomet. Chem. 1992, 6, 83–94. [Google Scholar] [CrossRef]
- Huber, F.; Vornefeld, M.; Preut, H.; von Angerer, E.; Ruisi, G. Synthesis and spectroscopic characterization of dicyclohexyltin derivatives of dipeptides, and in vitro effects against MDA-MB 231 breast cancer cells: Crystal structures of dicyclohexyltin glycylglycinate and gIycylalaninate. Appl. Organomet. Chem. 1992, 6, 597–606. [Google Scholar] [CrossRef]
- Preut, H.; Mundus, B.; Huber, F.; Barbieri, R. Di-tert-butyl(glycylglycinato)tin(IV) Monohydrate. Acta Crystallogr. C 1989, 45, 728–730. [Google Scholar] [CrossRef]
- Huber, F.; Haupt, H.J.; Preut, H.; Barbieri, R.; Lo Giudice, M.T. Darstellung, Kristall- und Molekülstruktur von Diphenylzinnglycylglycinat, (C6H6)2SnC4H6N2O3. Z. Anorg. Allg. Chem. 1977, 432, 51–57. [Google Scholar] [CrossRef]
- Ho, B.Y.K.; Zuckerman, J.J. Trialkyltin derivatives of amino acids and dipeptides. Inorg. Chem. 1973, 12, 1552–1561. [Google Scholar] [CrossRef]
- Ho, B.Y.K.; Zuckerman, J.J. Structural variations in trimethyl- and tricyclohexyltin derivatives of amino acids. Preference for association by nitrogen bridging. Inorg. Nucl. Chem. Lett. 1973, 9, 849–855. [Google Scholar] [CrossRef]
- Pellerito, L.; Lo Giudice, M.T.; Ruisi, G.; Bertazzi, N.; Barbieri, R.; Huber, F. Complexes of organometallic compounds. XLIV. Synthesis and Mössbauer spectroscopic studies of glylcylglycinato-O,N,N(2−)diorganotin (IV) complexes. Inorg. Chim. Acta. 1976, 17, L21–L22. [Google Scholar] [CrossRef]
- Gerlach, D.; Ehlers, A.W.; Lammertsma, K.; Wagler, J. Silicon(IV) Chelates of an (ONN’)-Tridentate Pyrrole-2-Carbaldimine Ligand: Syntheses, Structures and UV/Vis Properties. Z. Naturforsch. B 2009, 64, 1571–1579. [Google Scholar] [CrossRef]
- Brendler, E.; Wächtler, E.; Heine, T.; Zhechkov, L.; Langer, T.; Pöttgen, R.; Hill, A.F.; Wagler, J. Stannylene or Metallastanna(IV)ocane: A Matter of Formalism. Angew. Chem. Int. Ed. 2011, 50, 4696–4700. [Google Scholar] [CrossRef]
- Hattori, T.; Yamamoto, H. Peptide Bond Formation Between Unprotected Amino Acids: Convergent Synthesis of Oligopeptides. J. Am. Chem. Soc. 2024, 146, 25738–25744. [Google Scholar] [CrossRef]
- Hattori, T.; Yamamoto, H. Trimethylaluminum-mediated one-pot peptide elongation. Chem. Sci. 2023, 14, 5795–5801. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhang, J.; Wu, Y. Chin. X-ray diffraction study on crystals of heteroazotricyclic compounds Ⅱ, crystal and molecular structure of 1-chloropropyl-2,8,9-trioxa-5-aza-1-silicon tricyclo [3,3,3,01.5]undecane-3-one (In Chinese). J. Struct. Chem. 1983, 2, 107. [Google Scholar]
- Dai, J.; Zhang, J.; Wu, Y.; Wu, G. Chin. X-ray study on azotricyclic compounds Ⅲ. Structural determination of 1-chloromethyl-2,8,9-trioxa-5-aza-1-silicon tricyclo [3,3,3,01,5]decane-3-one (In Chinese). J. Struct. Chem. 1983, 2, 207. [Google Scholar]
- Párkányi, L.; Hencsei, P.; Popowski, E. The Crystal Structures of m-Trifluoromethylphenylsilatranone and p-Fluorophenylsilatranone. J. Organomet. Chem. 1980, 197, 275–283. [Google Scholar] [CrossRef]
- Párkányi, L.; Hencsei, P.; Csonka, G.; Kovács, I. The molecular structure of 1-phenylsilatranone. J. Organomet. Chem. 1987, 329, 305–311. [Google Scholar] [CrossRef]
- Fülöp, V.; Kálmán, A.; Hencsei, P.; Csonka, G.; Kovács, I. Structure of l-Methylsilatranone, CH3Si(OCOCH2)(OCH2CH2)2N. Acta Crystallogr. C 1988, 44, 720723. [Google Scholar] [CrossRef]
- Kemme, A.; Bleidelis, J.; Lapsina, A.; Fleisher, M.; Zelcans, G.; Lukevics, E. Molecular and crystal structure of 1-methylsilatrane-3,7-dione. Latv. PSR Zinat. Akad. Vestis Kim. Ser. 1985, 2, 242–245. [Google Scholar]
- Lyssenko, K.A.; Korlyukov, A.A.; Antipin, M.Y.; Knyazev, S.P.; Kirin, V.N.; Alexeev, N.V.; Chernyshev, E.A. The nature of the intramolecular transannular Si⋅⋅⋅N interaction in crystalline 1-methylsilatrane, as found from X-ray diffraction data. Mendeleev. Commun. 2000, 10, 88–90. [Google Scholar] [CrossRef]
- Párkányi, L.; Simon, K.; Nagy, J. Crystal and molecular structure of [beta]-1-phenylsilatrane, C12H17O3NSi. Acta Crystallogr. B 1974, 30, 2328–2332. [Google Scholar] [CrossRef]
- Kupče, E.; Liepiņš, E.; Lapsina, A.; Zelchan, G.; Lukevics, E. Synthesis and 1H, 13C, 15N, 29Si NMR spectra of sil- and germ-atranones. J. Organomet. Chem. 1983, 251, 15–29. [Google Scholar] [CrossRef]
- Ilyukhin, A.B.; Shkol’nikova, L.M.; Seifullina, I.I.; Batalova, T.P.; Dyatlova, N.M. Preparation, structure and properties of germanium(IV) with nitrilotriacetic acid [Ge(Nta)(H2O)(OH)]·2H2O. Koord. Khim. 1991, 17, 795–800. [Google Scholar]
- Martsinko, E.E.; Seifullina, I.I.; Minacheva, L.K.; Syvak, T.A.; Sergienko, V.S. Synthesis, properties, crystal, and molecular structure of potassium (nitrilotriacetato)dihydroxogermanate(IV) hydrate K[Ge(Nta)(OH)2]·H2O. Zh. Neorg. Khim. 2009, 54, 1428–1434. [Google Scholar]
- Mafra, L.; Almeida Paz, F.A.; Shi, F.-N.; Rocha, J.; Trindade, T.; Fernandez, C.; Makal, A.; Wozniak, K.; Klinowski, J. X-ray Diffraction and Solid-State NMR Studies of a Germanium Binuclear Complex. Chem. Eur. J. 2006, 12, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.G. Solid complexes with tetravalent metal ions and ethylenediamime tetra-acetic acid (EDTA). J. Inorg. Nucl. Chem. 1964, 26, 59–72. [Google Scholar] [CrossRef]
- Mizuta, T.; Yoshida, T.; Miyoshi, K. A Novel Structure of Spontaneously Resolved Germanium(IV) Complex with Ethylenediaminetriacetatemonoacetic Acid (Hedta). Inorg. Chim. Acta 1989, 165, 65–71. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Seifullina, I.I.; Minacheva, L.K.; Shchur, T.A.; Sergienko, V.S. Diphenylguanidinium (ethylendiaminetetraacetato)hydroxogermanate hydrate (HDphg)[Ge(OH)(Edta)]·H2O: Synthesis, physicochemical characterization, and crystal structure. Zh. Neorg. Khim. 2007, 52, 2023–2029. [Google Scholar]
- Zhu, J.; Huang, T.-S.; Huang, Y.-Q.; Hong, M.-S.; Hu, S.-Z. Chin.Synthesis and structure of aminopolycarboxylic acid germanium(IV) chelates [Ge(OH)(Hpdta)] and [Ge(OH)(H2dtpa)]·H2O (In Chinese). J. Struct. Chem. 1995, 14, 191. [Google Scholar]
- Chen, M.-D.; Zhu, J.; Hu, S.-Z. Synthesis, crystal structure and quantum chemical calculations of [Ge(OH)(Hcdta)]·H2O (H4cdta = trans-cyclohexane-1,2-diaminetetraacetic acid). Main Group Met. Chem. 1999, 22, 105–109. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Seifullina, I.I.; Minacheva, L.K.; Shchur, T.A.; Sergienko, V.S. Synthesis, properties, and crystal structure of {N-(2-hydroxyethyl)ethylenediaminetriacetato}hydroxogermanium(IV) sesquihydrate. Zh. Neorg. Khim. 2007, 52, 1621–1628. [Google Scholar]
- Sergienko, V.S.; Martsinko, E.E.; Seifullina, I.I.; Churakov, A.V.; Chebanenko, E.A. Synthesis and the Crystal and Molecular Structure of the Germanium(IV) Complex with Propylene-1,3-diaminetetraacetic Acid [Ge(Pdta)]. Crystallogr. Rep. 2015, 60, 677–681. [Google Scholar] [CrossRef]
- Sergienko, V.S.; Aleksandrov, G.G.; Seifullina, I.I.; Martsinko, E.E. Synthesis and the crystal and molecular structures of a germanium(IV)-copper(II) heteronuclear Diethylenetriaminepentaacetate Complex, [Cu(μ-HDtpa)2{Ge(OH)}2]·12H2O. Crystallogr. Rep. 2004, 49, 788–791. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Smola, S.S.; Minacheva, L.K.; Seifullina, I.I.; Sergienko, V.S. Neodymium(III) triaquatrihydroxo(2-hydroxy-1,3-diaminopropanetetraacetato)germanium(IV) hydrate [Ge(OH)(μ-HHpdta)(μ-OH)Nd(OH)(H2O)3]·H2O: Synthesis and crystal and molecular structure. Russ. J. Inorg. Chem. 2009, 54, 1041–1048. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Minacheva, L.K.; Smola, S.S.; Seifullina, I.I.; Sergienko, V.S. Synthesis and characterization of heteronuclear germanium(IV) lanthanide 1,3-diamino-2-propanoltetraacetates: Crystal and molecular structure of the [Ge(OH)(μ-Hpdta)(μ-OH)Ln(H2O)3]·2H2O complexes (Ln = Tb, Yb). Russ. J. Inorg. Chem. 2011, 56, 1034–1042. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Seifullina, I.I.; Chebanenko, E.A.; Dyakonenko, V.V.; Shishkina, S.V.; Odessa, I.I. СИНТЕЗ І СТРУКТУРА БІЯДЕРНОГО КОМПЛЕКСУ ГЕРМАНІЮ(IV)-ТУЛІЮ(III) З 1,3-ДІАМІНО-2-ГІДРОКСИПРОПАН-N,N,N’,N’-ТЕТРАОЦТОВОЮ КИСЛОТОЮ. Mechnikov Natl. Univ. Bull.—Chem. 2020, 25, 24–31. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Minacheva, L.K.; Sergienko, V.S.; Chebanenko, E.A.; Seifullina, I.I. A new binuclear germanium(IV) and copper(II) complex with 1,3-diamino-2-propanoltetraacetic acid: Crystal and molecular structure of [(H2O)(OH)Ge(μ-Hpdta)Cu(H2O)]·3H2O. Russ. J. Inorg. Chem. 2010, 55, 1874–1881. [Google Scholar] [CrossRef]
- Seifullina, I.I.; Martsinko, E.E.; Chebanenko, E.A.; Dyakonenko, V.V.; Shishkina, S.V.; Pesaroglo, A.G. Synthesis, molecular and crystal structure of a heterometallic Cu(II)-Ge(IV) complex with 2-hydroxy-1,3-diaminopropane-n,n,n′,n′-tetracetic acid and 2,2′′-bipyridine. Vopr. Khim. Khim. Tekhnol. 2020, 159–164. [Google Scholar] [CrossRef]
- Seifullina, I.I.; Minacheva, L.K.; Martsinko, E.E.; Sergienko, V.S.; Churakov, A.V. Tetrameric complexes of germanium(IV) and cobalt(II), Nickel(II), or Zinc(II) with 1,3-Diamino-2-propanol-tetraacertic acid: Crystal and molecular structures of [(OH)2Ge2(μ-Hpdta)2Zn2(H2O)4]·12H2O. Russ. J. Inorg. Chem. 2012, 57, 1545–1552. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, S.; Shahzadi, S.; Tahir, M.N.; Shahid, M.; Munawar, K.S.; Abbas, S.M. Synthesis, spectroscopy, single crystal XRD and biological studies of multinuclear organotin dicarboxylates. Polyhedron 2016, 117, 64–72. [Google Scholar] [CrossRef]
- Mancilla, T.; Carrillo, L.; Zamudio Rivera, L.S.; Camacho, C.; De Vos, D.; Kiss, R.; Darro, F.; Mahieu, B.; Tiekink, E.R.T.; Rahier, H.; et al. Di-n-butyltin(IV) derivatives of bis(carboxymethyl)benzylamines: Synthesis, NMR and X-ray structure characterization and in vitro antitumour properties. Appl. Organomet. Chem. 2001, 15, 593–603. [Google Scholar] [CrossRef]
- Lee, F.L.; Gabe, E.J.; Khoo, L.E.; Leong, W.H.; Eng, G.; Smith, F.E. Synthesis and structural studies of diorganotin iminodiacetates. Inorg. Chim. Acta 1989, 166, 257–261. [Google Scholar] [CrossRef]
- Aizawa, S.; Natsume, T.; Hatano, K.; Funahashi, S. Complexation equilibria and structures of dimethyltin (IV) complexes with N-methyliminodiacetate, pyridine-2,6-dicarboxylate, ethylenediamine-N,N′-diacetate and ethylenediamine-N,N,N′,N′-tetraacetate. Inorg. Chim. Acta 1996, 248, 215–224. [Google Scholar] [CrossRef]
- Di Nicola, C.; Galindo, A.; Hanna, J.V.; Marcetti, F.; Pettinari, C.; Pettinari, R.; Rivarola, E.; Skelton, B.W.; White, A.H. Synthesis and Spectroscopic and X-ray Structural Characterization of R2SnIV−Oxydiacetate and −Iminodiacetate Complexes. Inorg. Chem. 2005, 44, 3094–3102. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Ali, S.; Shahzadi, S.; Rizzoli, C.; Shahid, M. Diorganotin(IV) Complexes with Monohydrate Disodium Salt of Iminodiacetic Acid: Synthesis, Characterization, Crystal Structure and Biological Activities. J. Chin. Chem. Soc. 2015, 62, 793–802. [Google Scholar] [CrossRef]
- Henling, L.M.; Marsh, R.E. Some more space-group corrections. Acta Crystallogr. C 2014, 70, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.-N.; Luo, Q.-Y.; Zeng, X.-R.; Sui, Y.; Li, X.-F. Bis{μ-N-[bis(hydroxymethyl)(oxidomethyl)methyl]glycinato}bis[dimethyltin(IV)] dioxane hemisolvate. Acta Crystallogr. E 2005, 61, m2604–m2606. [Google Scholar] [CrossRef]
- Meriem, A.; Willem, R.; Meunier-Piret, J.; Gielen, M. Diorganotin(IV) derivatives of N-(2-hydroxyethyl)- and N-(carbamoylmethyl)iminodiacetic acid: Synthesis, spectroscopic characterization, x-ray structure analysis and in vitro antitumor activity. Main Group Met. Chem. 1989, 12, 187–198. [Google Scholar]
- Arjmand, F.; Yousuf, I.; Zaidib, Y.; Toupetc, L. Crystal structure determination, spectroscopic characterization and biological profile of a tailored ionic molecular entity, Sn(IV) iminodiacetic acid–piperazinediium conjugate: In Vitro DNA/RNA binding studies, Topo I inhibition activity, cytotoxic and systemic toxicity studies. RSC Adv. 2015, 5, 16250–16264. [Google Scholar] [CrossRef]
- Clark, A.M.; Rickard, C.E.F.; Roper, W.R.; Woodman, T.J.; Wright, L.J. Stepwise Conversion of an Osmium Trimethylstannyl Complex to a Triiodostannyl Complex and Nucleophilic Substitution Reactions at the Tin−Iodine Bonds. Organometallics 2000, 19, 1766–1774. [Google Scholar] [CrossRef]
- Dakternieks, D.; Dyson, G.; Jurkschat, K.; Tozer, R.; Tiekink, E.R.T. Utilization of hypervalently activated organotin compounds in synthesis. Preparation and reactions of Me2N(CH2)3SnPh3. J. Organomet. Chem. 1993, 458, 29–38. [Google Scholar] [CrossRef]
- Ilyukhin, A.B.; Davidovich, R.L.; Logvinova, V.B. Nitrilotriacetate complexes with acid and normal ligands: Crystal structure of Cs[SnIV(Nta)(HNta)]·H2O and K2[Bi(Nta)(HNta)]·H2O. Zh. Neorg. Khim. 1999, 44, 1931–1934. [Google Scholar]
- Logvinova, V.B.; Davidovich, R.L.; Ilyukhin, A.B.; Sergienko, V.S.; Tkachev, V.V.; Atovmyan, L.O. Crystal structure of Cat2[M(Nta)2]·2H2O (Cat = K+, or Rb+ and M = Sn or 0.5Sn+0.5Zr). Russ. J. Coord. Chem. 1998, 24, 530–532. [Google Scholar]
- Haussühl, E.; Haussühl, S.; Tillmanns, E. Structural and physical properties of bis(guanidinium) X bis(nitrilotriacetate) hydrate, (C[NH2]3)2X(N[CH2COO]3)2·H2O (X = Zr, Sn, Hf) and bis(methylammonium) X bis(nitrilotriacetate dihydrate, (CH3NH3)2X(N[CH2COO]3)2·2 H2O (X = Sn, Zr). Z. Kristallogr.—Cryst. Mater. 2009, 218, 626–635. [Google Scholar] [CrossRef]
- Martsinko, E.E.; Ilyukhin, A.B.; Seifullina, I.I.; Chebanenko, E.A.; Sergienko, V.S. Synthesis, properties, and crystal structure of the tin(IV) complex with N-(2-hydroxyethyl)ethylenediaminetriacetic acid [Sn(μ-Hedtra)(μ-OH)SnCl3(H2O)]·3H2O. Russ. J. Coord. Chem. 2013, 39, 505–509. [Google Scholar] [CrossRef]
- Van Remoortere, F.P.; Flynn, J.J.; Boer, F.P. Crystal structure of stannic ethylenediaminetetraacetate monohydrate. Inorg. Chem. 1971, 10, 2313–2319. [Google Scholar] [CrossRef]
- Poznyak, A.L.; Ilyukhin, A.B. Crystal structure of Ba[Sn(Edta)(OH)]Cl·4H2O. Kristallografiya 1997, 42, 932–933. [Google Scholar]
- Ilyukhin, A.B.; Logvinova, V.B.; Davidovich, R.L.; Poznyak, A.L. Crystal structure of ethylenediaminetetraacetato complexes of tetravalent tin. Zh. Neorg. Khim. 2000, 45, 1339–1343. [Google Scholar]
- Brouca-Cabarrecq, C.; Marrot, B.; Mosset, A. Crystal structure of aquo (hydroxo) (ethylenediaminetetraacetato) sodium(I) tin(IV), NaSn(OH)(edta)(H2O). J. Chem. Crystallogr. 1996, 26, 503–508. [Google Scholar] [CrossRef]
- Fu, Z.; Chen, B.; He, A.; Lv, X.; Wei, X.; Feng, X.; Huang, C.; Xia, C.; Jin, Y. The crystal structure of oxonium chlorido-ethylenediaminetetraactetotin(IV) hydrate, C10H17ClN2O10Sn. Z. Kristallogr.-New Cryst. Struct. 2017, 232, 941–942. [Google Scholar] [CrossRef]
- Iyer, R.K.; Deshpande, S.G.; Amirthalingam, V. Preparation, properties and molecular structure of monohydrogen diethylenetriaminepentaacetatostannate(IV) trihydrate–an eight coordinated 1:1 TIN(IV) chelate. Polyhedron 1984, 3, 1099–1104. [Google Scholar] [CrossRef]
- Ilyukhin, A.B.; Sergienko, V.S.; Davidovich, R.L.; Logvinova, V.B. Synthesis and structure of dioxonium diethylenetriaminepentaacetatostannate(IV) and hafnate(IV) H5O2[MDtpa]·H2O. Zh. Neorg. Khim. 1997, 42, 1474–1478. [Google Scholar]
- Ilyukhin, A.B.; Davidovich, R.L.; Samsonova, I.N.; Teplukhina, L.V. Eightfold-coordinated diethylenetriaminepentaacetates. Crystal structures of K[M(Dtpa)]·3H2O (M = Zr, Hf) and NH4[Sn(Dtpa)]·H2O. Kristallografiya 2000, 45, 45–49. [Google Scholar] [CrossRef]
- Van Remoortere, F.P.; Flynn, J.J.; Boer, F.P.; North, P.P. Crystal structure of distannous ethylenediaminetetraacetate dihydrate. Inorg. Chem. 1971, 10, 1511–1518. [Google Scholar] [CrossRef]
- Shields, K.G.; Seccombe, R.C.; Kennard, C.H.L. Stereochemistry of flexible-chelate–metal complexes. Part III. Crystal structure of dihydrogen ethylenediaminetetra-acetatostannate(II). J. Chem. Soc. Dalton Trans. 1973, 2, 741–743. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mizuta, T.; Yamamoto, T.; Miyoshi, K.; Kushi, Y. The ligand field stabilization effect on the metal-ligand bond distances in octahedral metal complexes with edta-type ligands. Redetermination of the molecular structure of (ethylenediaminetriacetatoacetic acid)-(aqua)iron(III), [Fe(Hedta)(H2O)]. Inorg. Chim. Acta 1990, 175, 121–126. [Google Scholar] [CrossRef]
- Solans, X.; Font Altaba, M.; Garcia-Oricain, J. Crystal structures of ethylenediaminetetraacetato metal complexes. V. Structures containing the [Fe(C10H12N2O8)(H2O)]– anion. Acta Crystallogr. C 1984, 40, 635–638. [Google Scholar] [CrossRef]
- Smith, F.E.; Hynes, R.C.; Ang, T.T.; Khoo, L.E.; Eng, G. Synthesis and structural characterization of a series of pentacoordinate diorganotin(IV) N-arylidene-α-amino acid complexes. Can. J. Chem. 1992, 70, 1114–1120. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.; Cheng, J.; Xu, Y.; Liu, B.; Wang, H.; Zhang, D. Thermodynamic α-CH acidities of amino acid fragments in five-co-ordinate bicycloazastannoxides in Me2SO. J. Chem. Soc. Dalton Trans. 1996, 25, 3889–3891. [Google Scholar] [CrossRef]
- Beltrán, H.I.; Zamudio-Rivera, L.S.; Mancilla, T.; Santillan, R.; Farfán, N. One-Step Preparation, Structural Assignment, and X-ray Study of 2,2-Di-nbutyl-and 2,2-Diphenyl-6-aza-1,3-dioxa-2-stannabenzocyclononen-4-ones Derived from Amino Acids. Chem. Eur. J. 2003, 9, 2291–2306. [Google Scholar] [CrossRef]
- Tian, L.; Qian, B.; Sun, Y.; Zheng, X.; Yang, M.; Li, H.; Liu, X. Synthesis, structural characterization and cytotoxic activity of diorganotin(IV) complexes of N-(5-halosalicylidene)-α-amino acid. Appl. Organomet. Chem. 2005, 19, 980–987. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Kehie, P.; Duthie, A.; Wang, R.; Englert, U.; Hopfl, H. Synthesis, characterization, crystal structures and supramolecular features of bicycloazastannoxides derived from Schiff bases with L-tyrosine. J. Organomet. Chem. 2017, 828, 96–105. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Kehie, P.; Duthie, A.; Guchhait, N.; Raviprakash, N.; Mokhamatam, R.B.; Manna, S.K.; Armata, N.; Scopelliti, M.; Wang, R.; et al. Synthesis, photophysical properties and structures of organotin-Schiff bases utilizing aromatic amino acid from the chiral pool and evaluation of the biological perspective of a triphenyltin compound. J. Inorg. Biochem. 2017, 168, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Basu Baul, T.S.; Kehie, P.; Hoepfl, H.; Duthie, A.; Eng, G.; Linden, A. Organotin(IV) complexes derived from proteinogenic amino acid: Synthesis, structure and evaluation of larvicidal efficacy on Anopheles stephensi mosquito larvae. Appl. Organomet. Chem. 2017, 31, e3547. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Chaurasiya, A.; Rabha, M.; Khatua, S.; Lycka, A.; Schollmeyer, D.; Jurkschat, K. Diorganotin Compounds Containing α-Aminoacidato Schiff Base Ligands Derived from Functionalized 2-Hydroxy-5-(aryldiazenyl)benzaldehyde. Syntheses, Structures and Sensing of Hydrogen Sulfide. Eur. J. Inorg. Chem. 2020, 2020, 1803–1813. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, K.; Srivastav, N.; Singh, R.; Kaur, V.; Jasinski, J.P.; Butcher, R.J. Exploration of fluorescent organotin compounds of α-amino acid Schiff bases for the detection of organophosphorous chemical warfare agents: Quantification of diethylchlorophosphate. New J. Chem. 2018, 42, 8756–8764. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Masharing, C.; Willem, R.; Biesemans, M.; Holcapek, M.; Jirasko, R.; Linden, A. Self-assembly of diorganotin(IV) 2-{[(E)-1-(2-oxyaryl)alkylidene]amino}acetates: An investigation of structures by X-ray diffraction, solution and solid-state tin NMR, and electrospray ionization MS. J. Organomet. Chem. 2005, 690, 3080–3094. [Google Scholar] [CrossRef]
- Böhme, U.; Weling, G. The crystal structure of trichlorido[N-[(2-oxyphenyl)methylidene]phenylglycinemethylester-κ3 O,N,O′]- tin(IV)–methylene chloride (1/1), C16H14Cl3NO3Sn·CH2Cl2. Z. Kristallogr.-New Cryst. Struct. 2023, 238, 391–393. [Google Scholar] [CrossRef]
- Schwarzer, S.; Böhme, U.; Fels, S.; Günther, B.; Brendler, E. (S)-N-[(2-hydroxynaphthalen-1-yl)methylidene]valine—A valuable ligand for the preparation of chiral complexes. Inorg. Chim. Acta 2018, 483, 136–147. [Google Scholar] [CrossRef]
- Nath, M.; Goyal, S. Synthesis, Characteristic Spectral Studies and in Vitro Antimicrobial Activity of Organosilicon(IV) Complexes of N-(2-Hydroxynaphthalidene)-Amino Acid Schiff Bases. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 447–463. [Google Scholar] [CrossRef]
- Warncke, G.; Böhme, U.; Günther, B.; Kronstein, M. Racemization versus retention of chiral information during the formation of silicon and tin complexes with chiral Schiff base ligands. Polyhedron 2012, 47, 46–52. [Google Scholar] [CrossRef]
- Schwarzer, A.; Fels, S.; Böhme, U. Two reversible enantiotropic phase transitions in a pentacoordinate silicon complex with an O,N,O’-tridentate valinate ligand. Acta Crystallogr. C 2015, 71, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Böhme, U.; Fels, F. A new type of chiral pentacoordinated silicon compounds with azomethine ligands made from acetylacetone and amino acids. Inorg. Chim. Acta 2013, 406, 251–255. [Google Scholar] [CrossRef]
- Böhme, U.; Fels, S. {N-[(4-Methoxy-2-oxidophenyl)(phenyl)methylidene]glycinato}diphenylsilicon(IV). IUCrData 2023, 8, x230306. [Google Scholar] [CrossRef] [PubMed]
- Böhme, U.; Fels, S. The crystal structure of [N-{[2-(oxy)-4-methoxyphenyl](phenyl)methylidene}alaninato]-diphenyl-silicon(IV) –chloroform (1/1),C29H25NO4Si·CHCl3. Z. Kristallogr.-N. Cryst. Struct. 2023, 238, 603–605. [Google Scholar] [CrossRef]
- Böhme, U.; Wiesner, S.; Günther, B. Easy access to chiral penta- and hexacoordinate silicon compounds. Inorg. Chem. Commun. 2006, 9, 806–809. [Google Scholar] [CrossRef]
- Schlecht, S.; Finze, M.; Bertermann, R.; Frank, W.; Domann, A.; Braun, M. Cocrystallising Rotamers of a Pentacoordinate Silicon Complex with a Chiral Aminodiol Ligand. Eur. J. Inorg. Chem. 2013, 2013, 1488–1492. [Google Scholar] [CrossRef]
- Tasaka, M.; Hirotsu, M.; Kojima, M.; Utsuno, S.; Yoshikawa, Y. Syntheses, Characterization, and Molecular Mechanics Calculations of Optically Active Silatrane Derivatives. Inorg. Chem. 1996, 35, 6981–6986. [Google Scholar] [CrossRef] [PubMed]
- Gauchenova, E.V.; Karlov, S.S.; Selina, A.A.; Chernyshova, E.S.; Churakov, A.V.; Howard, J.A.K.; Troitsky, N.A.; Tandura, S.N.; Lorberth, J.; Zaitseva, G.S. Synthesis and characterization of 3- and 4-phenylgermatranes: X-ray crystal structures of N(CH2CH2O)2(CH2CHPhO)GeZ (Z=F, OSiMe3, CCPh) and N(CH2CH2O)2(CHPhCH2O)GeOH. J. Organomet. Chem. 2003, 676, 8–21. [Google Scholar] [CrossRef]
- Glowacki-Pallach, B.; Lutter, M.; Schollmeyer, D.; Hiller, W.; Jouikov, V.; Jurkschat, K. Extending Chirality in Group XIV Metallatranes. Inorg. Chem. 2023, 62, 7662–7680. [Google Scholar] [CrossRef] [PubMed]
- Rohovec, J.; Lukeš, I.; Vojtíšek, P.; Císařová, I.; Hermann, P. Complexing properties of phosphinic analogues of glycine. J. Chem. Soc. Dalton Trans. 1996, 25, 2685–2691. [Google Scholar] [CrossRef]
- Cameron, T.S.; Prout, C.K.; Rossotti, F.J.C.; Steele, D. Crystal and molecular structure of bis(aminomethanesulphonato)copper(II). J. Chem. Soc. Dalton Trans. 1973, 2, 1590–1592. [Google Scholar] [CrossRef]
- Lutter, M.; Jurkschat, K. Aryl(dimethylaminomethyl)phosphinic Acid Esters: Syntheses, Structures, and Reactions with Halogen Hydrogen Acids, Tin Halides, and Trimethyl Halosilanes. Eur. J. Inorg. Chem. 2018, 2018, 3481–3490. [Google Scholar] [CrossRef]
- Knerr, S.; Brendler, E.; Zuber, J.; Kroke, E.; Wagler, J. A new class of silatranes derived from nitrilotris(methylenephenylphosphinic) acid. J. Organomet. Chem. 2025, 1023, 123402. [Google Scholar] [CrossRef]
- Knerr, S.; Brendler, E.; Gericke, R.; Kroke, E.; Wagler, J. Two Modifications of Nitrilotris(methylenephenylphosphinic) Acid: A Polymeric Network with Intermolecular (O=P–O–H)3 vs. Monomeric Molecules with Intramolecular (O=P–O–H)3 Hydrogen Bond Cyclotrimers. Crystals 2024, 14, 662. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, J.; Sun, Z.-G.; Zhu, Y.-Y.; Jiao, C.-Q.; Shi, S.-P.; Dai, L.-L.; Sun, T.; Li, W.-Z.; Ma, M.-X.; et al. Synthesis, crystal structures, and luminescent properties of tin(II) and lead(II) carboxyphosphonates with 3D framework structures. Inorg. Chem. Commun. 2014, 47, 37–41. [Google Scholar] [CrossRef]
- Perry, H.P.; Gagnon, K.J.; Law, J.; Teat, S.; Clearfield, A. Divalent metal phosphonate coordination polymers constructed from a dipiperidine-based bisphosphonate ligand. Dalton Trans. 2012, 41, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Sun, Z.; Zhu, Y.; Chen, K.; Zhu, J.; Li, C.; Wang, C.; Sun, S.; Tian, H.; Chu, W.; et al. Hydrothermal syntheses, crystal structures and luminescence properties of three new metal diphosphonates with layered structure. Inorg. Chim. Acta 2012, 387, 186–194. [Google Scholar] [CrossRef]
- Lorberth, J.; Shin, S.-H.; Otto, M.; Wocadlo, S.; Massa, W.; Yashina, N.S. {Me2NCH[(EtO)2P=O]2 − Me2SnCl2}-dimer: Crystal structure of a binuclear bis(chelate) complex of tin(IV) with cis-linked octahedra. J. Organomet. Chem. 1991, 407, 313–318. [Google Scholar] [CrossRef]
- Khoo, L.E.; Hazell, A. Polymeric [μ3-(N-phosphonomethyl)-glycinato]tris(tri-n-butyltin). Acta Crystallogr. C 2001, 57, 254–256. [Google Scholar] [CrossRef] [PubMed]
- An, B.-H.; Zhang, R.-F.; Li, Q.-L.; Du, X.-M.; Ru, J.; Zhang, S.-L.; Ma, C.-L. Syntheses, structures and in vitro cytostatic activity of four novel homochiral organotin(IV) phosphonates. J. Organomet. Chem. 2019, 881, 51–57. [Google Scholar] [CrossRef]
- Lorberth, J.; Wocadlo, S.; Massa, W.; Grigoriev, E.V.; Yashina, N.S.; Petrosyan, V.S.; Finocchiaro, P. Synthesis and structure of 1:1 complex of dimethyltin dichloride with meso-1,2-bis(α-diethoxyphosphorylbenzylamino)ethane. J. Organomet. Chem. 1996, 510, 287–290. [Google Scholar] [CrossRef]
- Dakternieks, D.; Jurkschat, K.; Tiekink, E.R.T. Crystal structure of 1-methyl-5-methyl-5-(O-tert-butylphosphonic acid)-1-aza-5-stannabicyclo [3.3.01,5]octane, (C8H17N)SnOPO2C(CH3)3. Z. Kristallogr. 1996, 211, 755–756. [Google Scholar] [CrossRef]
- Thamyongkit, P.; Bhise, A.D.; Taniguchi, M.; Lindsey, J.S. Alkylthio Unit as an r-Pyrrole Protecting Group for Use in Dipyrromethane Synthesis. J. Org. Chem. 2006, 71, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Purdy, A.P.; Gilardi, R.; Luther, J.; Butcher, R.J. Synthesis, crystal structure, and reactivity of alkali and silver salts of sulfonated imidazoles. Polyhedron 2007, 26, 3930–3938. [Google Scholar] [CrossRef]
- Rutsch, P.; Renner, G.; Huttner, G.; Sandhöfner, S. Organometallically Protected Indates and Germanates: Synthesis, Structure and Properties of [{(CO)5M}EX3]2- (M = Cr, Mo, W.; E = In; X = Cl, Br), [(CO5Cr)InBr(μ2-Br)]22- and [{(CO)5Cr}E(oxinat)2]n- (E = In, n = 2; E = Ge, n = 1). Z. Naturforsch. B 2002, 57, 757–772. [Google Scholar] [CrossRef]
- Kircher, P.; Huttner, G.; Schiemenz, B.; Heinze, K.; Zsolnai, L.; Walter, O.; Jacobi, A.; Driess, A. Divalent Tin and Lead Compounds EX2 Acting as Bridging Ligands in [{(CO)5M}2EX2]2− (E = Sn, Pb; M = Cr, Mo, W): Preparation and Properties. Chem. Ber. 1997, 130, 687–699. [Google Scholar] [CrossRef]


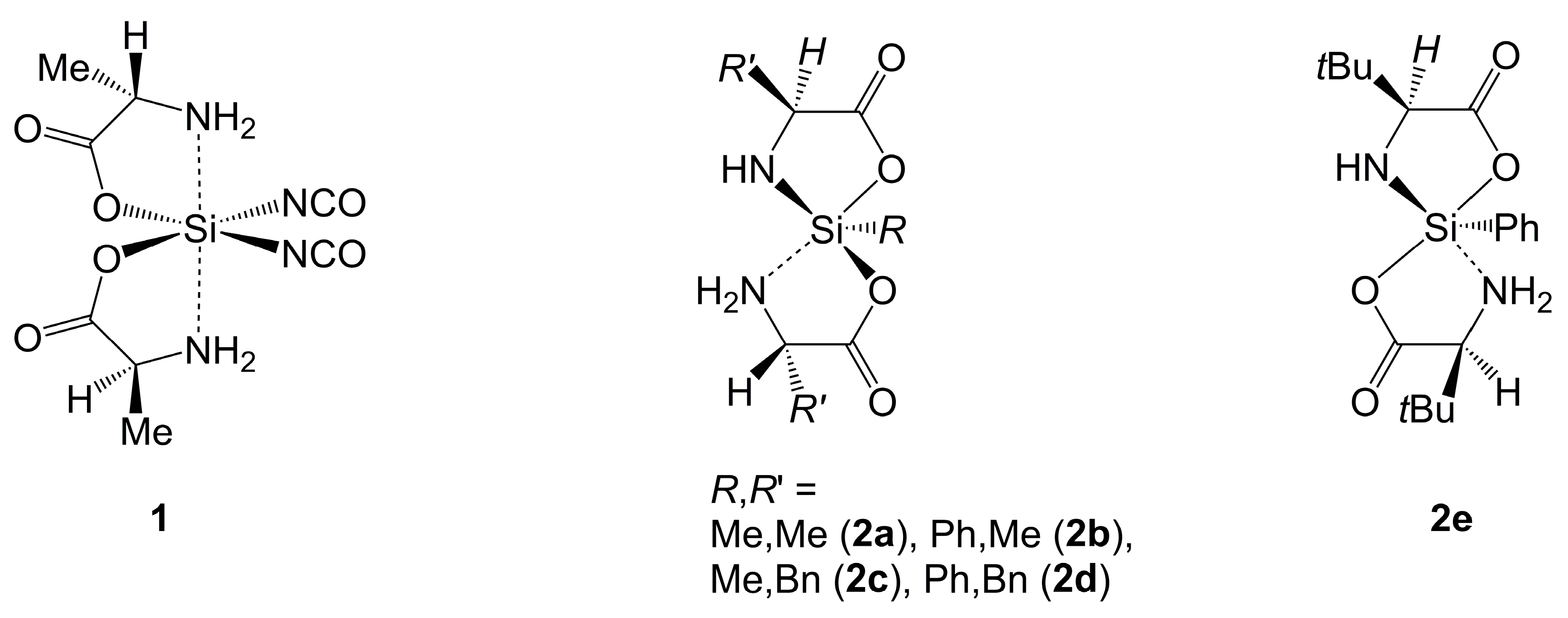

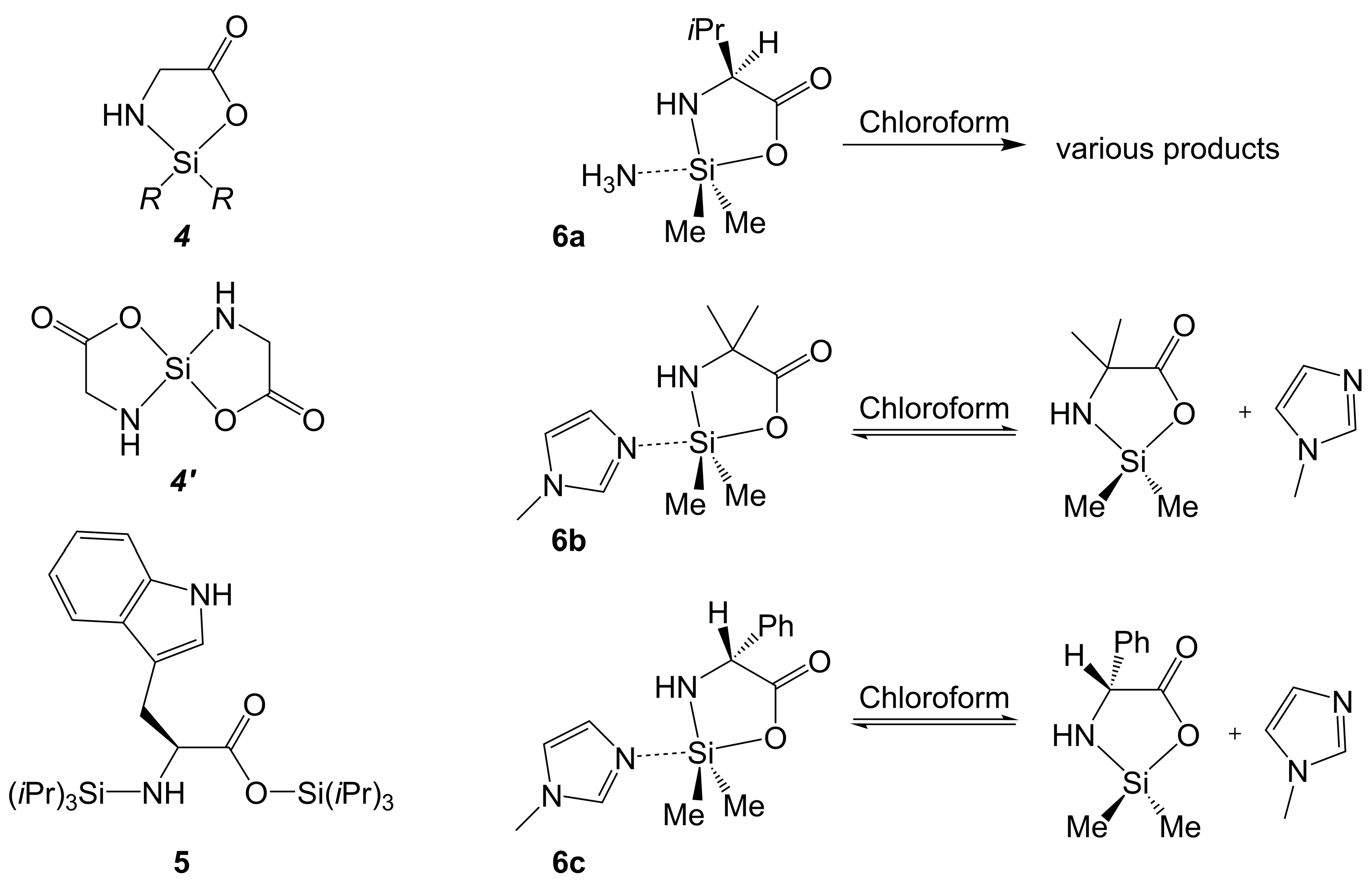
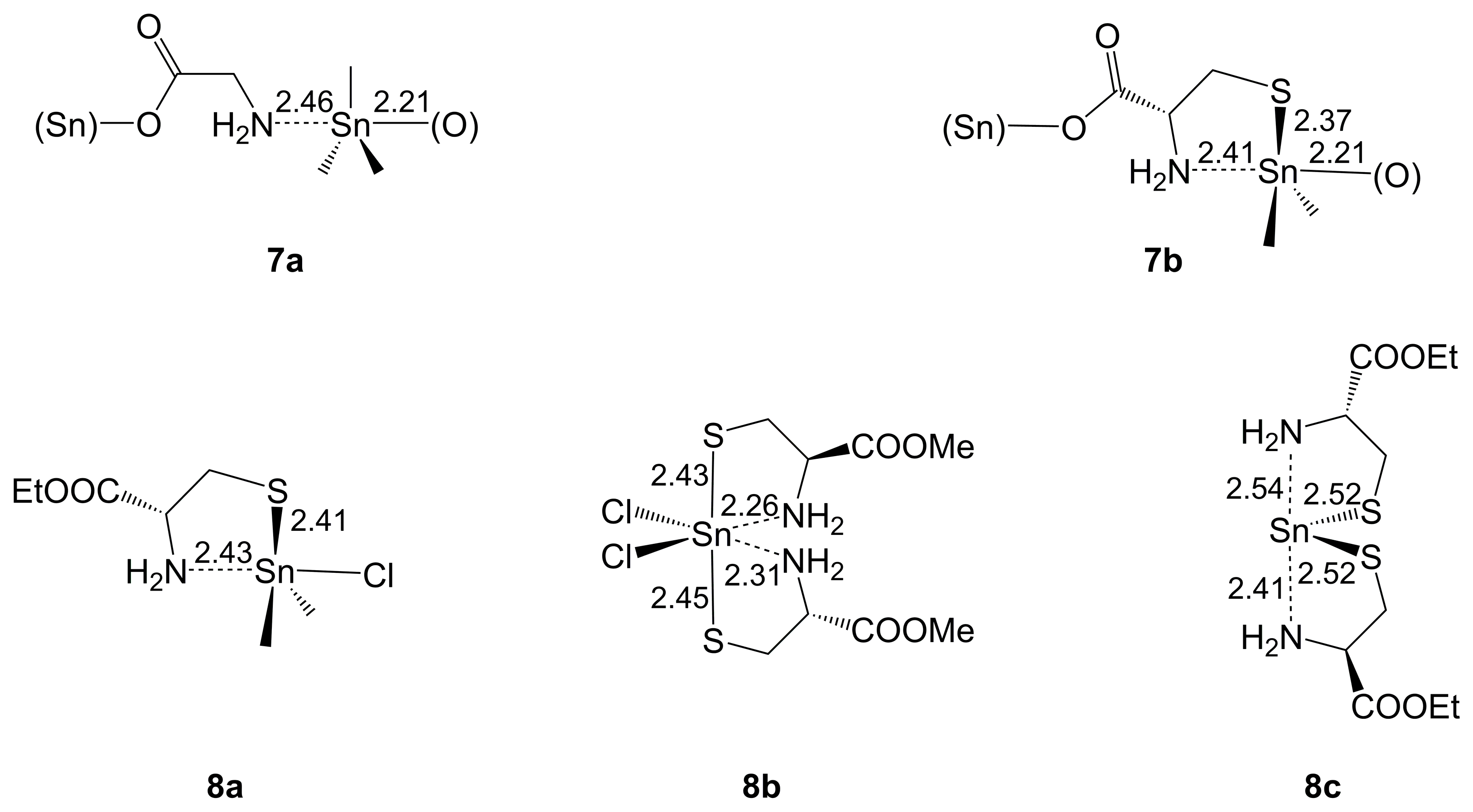
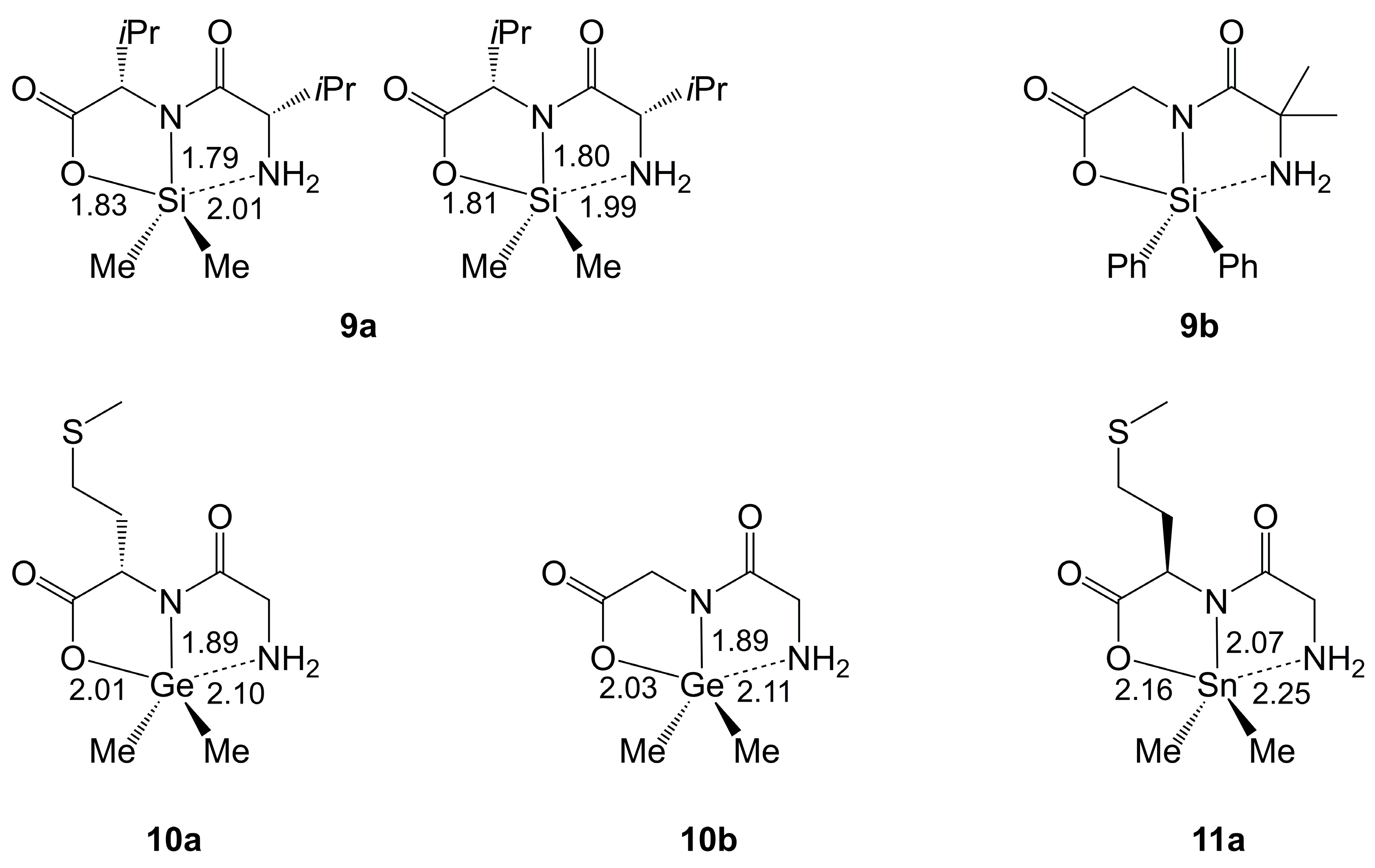



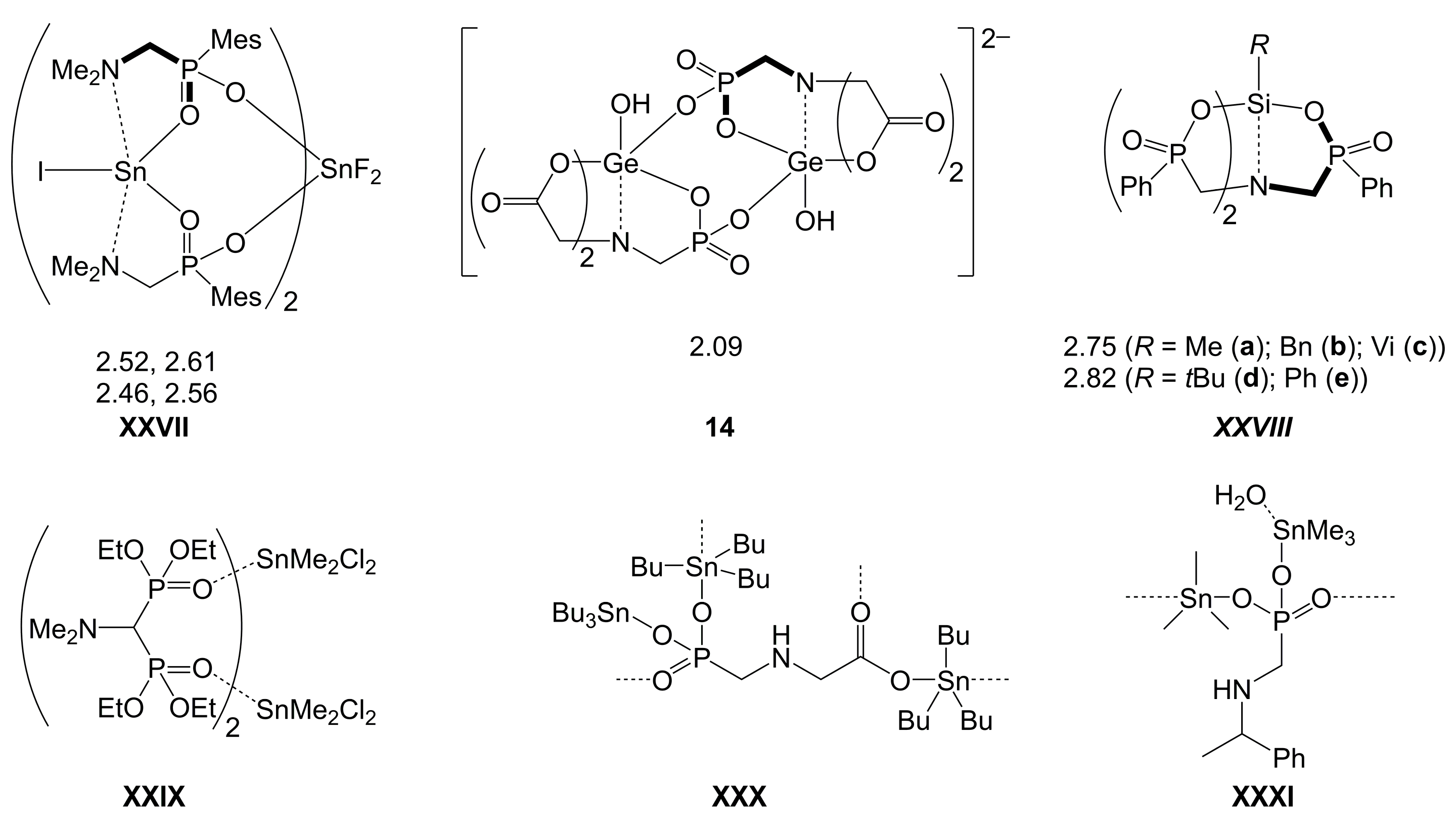
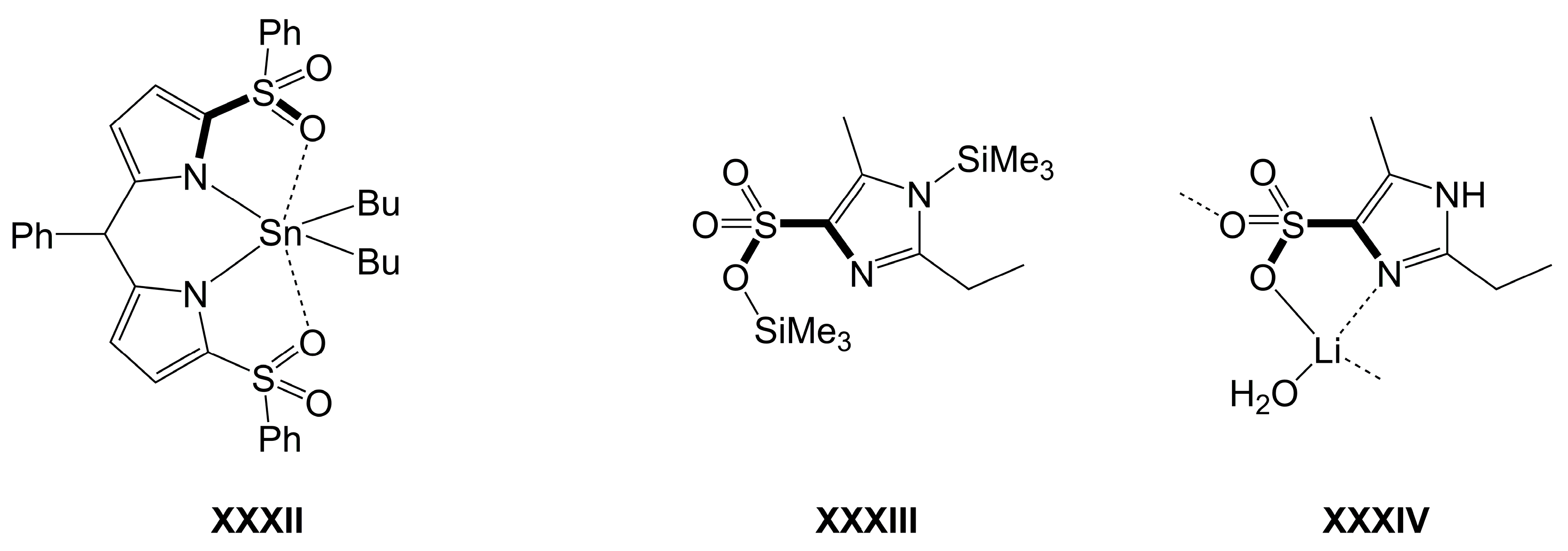
| Axis | Axial Angle | Si–N 1,2 | Si⋯N 1,2 | Si–O 1,2 | Si–O 1,2 | |
|---|---|---|---|---|---|---|
| 1 | H2N-Si-NH2 | 172.1 | n/a | 1.89 3 | n/a | 1.80 3 |
| O-Si-(NCO) | 176.8 3 | |||||
| 2a | O-Si-NH2 | 167.6 | 1.69 e | 1.99 a | 1.79 a | 1.71 e |
| 2b | O-Si-NH2 | 168.3 | 1.69 e | 1.97 a | 1.79 a | 1.71 e |
| 2c 4 | O-Si-NH2 | 169.0, 170.6 | 1.71, 1.71 e | 1.97, 1.98 a | 1.80, 1.80 a | 1.72, 1.72 e |
| 2d | O-Si-NH2 | 169.4 | 1.70 e | 1.97 a | 1.80 a | 1.70 e |
| 2e | O-Si-O | 164.9 | 1.70 e | 1.88 e | 1.79 a | 1.82 a |
| 3a | O-Si-O | 176.3 | 1.71, 1.72 e | n/a | 1.84, 1.82 a | n/a |
| 3b | O-Si-O | 178.5 | 1.71, 1.72 e | n/a | 1.83, 1.81 a | n/a |
| 3c | O-Si-O | 175.8 | 1.73, 1.73 e | n/a | 1.84, 1.82 a | n/a |
| 3d 5 | O-Si-O | 173.1–174.5 | 1.71–1.72 e | n/a | 1.81–1.83 a | n/a |
| 3e | O-Si-O | 176.8 | 1.71, 1.71 e | n/a | 1.81, 1.83 a | n/a |
| 3f 5 | O-Si-O | 177.6–179.3 | 1.73–1.74 e | n/a | 1.81–1.85 a | n/a |
| 6a | O-Si-NH3 | 171.1 | 1.72 e | 2.01 | 1.88 a | n/a |
| 6b | O-Si-NMI | 172.3 | 1.71 e | 2.04 | 1.85 a | n/a |
| 6c | O-Si-NMI | 171.3 | 1.72 e | 2.01 | 1.87 a | n/a |
| Ref. | Route | (a-b)R 2 | O-Sn-N | C-Sn-C | Si–O | Si–N | Si⋯N | Motif | |
|---|---|---|---|---|---|---|---|---|---|
| 11a | [73] | B | (met-gly)Me | 153.0 | 123.8 | 2.16 | 2.07 | 2.25 | a |
| 11b | [74] | C | (phe-tyr)Me | 149.5 | 136.5 | 2.20 | 2.10 | 2.25 | c 3 |
| 11c 1 | [75] | A | (his-met)Me | 147.0 | 124.7 | 2.14 | 2.12 | 2.30 | a |
| 152.3 | 126.1 | 2.18 | 2.07 | 2.24 | a | ||||
| 11d | [76] | A | (met-met)Me | 148.8 | 132.0 | 2.19 | 2.11 | 2.26 | c 3 |
| 11e | [76] | A | (his-ala)Me | 146.4 | 143.9 | 2.22 | 2.14 | 2.28 | b 3 |
| 11f | [77] | A | (ala-trp)Me | 151.5 | 123.8 | 2.18 | 2.06 | 2.27 | a |
| 11g 1 | [78] | A | (his-gly)Et | 151.9 | 128.8 | 2.18 | 2.10 | 2.27 | a |
| 150.0 | 148.5 | 2.24 | 2.13 | 2.27 | d 3 | ||||
| 11h | [79] | C | (tyr-gly)Et | 152.2 | 131.4 | 2.19 | 2.08 | 2.29 | a |
| 11i | [80] | C | (gly-trp)nBu | 147.9 | 151.9 | 2.25 | 2.12 | 2.29 | b 3 |
| 11j | [81] | A | (leu-aib)nBu | 148.7 | 127.8 | 2.20 | 2.11 | 2.28 | a |
| 11k | [81] | A | (ala-aib)nBu | 147.6 | 129.8 | 2.24 | 2.12 | 2.24 | c 3 |
| 11l | [82] | A,B,C | (val-gly)nBu | 151.3 | 125.3 | 2.14 | 2.10 | 2.27 | a |
| 11m | [83] | A | (gly-gly)Cy | 151.4 | 123.6 | 2.17 | 2.10 | 2.28 | a |
| 11n | [83] | A | (ala-gly)Cy | 150.8 | 123.0 | 2.16 | 2.09 | 2.30 | a |
| 11o | [84] | C | (gly-gly)tBu | 149.6 | 121.7 | 2.20 | 2.09 | 2.29 | a |
| 11p | [85] | A | (gly-gly)Ph | 153.2 | 117.5 | 2.16 | 2.08 | 2.27 | a |
| Ref. | R′ 1 | Ge–N (a) | Ge–N (b) | Ge–O (c) | Ge–O (d) | Ge–O (e) | Ge–O (f) | |
|---|---|---|---|---|---|---|---|---|
| 15a | [106] | CH2CH2 | 2.08 | 2.11 | 1.88 | 1.88 | 1.89 | 1.77 |
| 15b | [107] | CH2CH2 | 2.09 | 2.10 | 1.89 | 1.88 | 1.90 | 1.76 |
| 15c | [108] | CH2CHMe2 | 2.09 | 2.10 | 1.88 | 1.87 | 1.89 | 1.78 |
| 15d | [109] | 1,2-C6H103 | 2.07 | 2.13 | 1.88 | 1.86 | 1.89 | 1.77 |
| 15e 4 | [110] | CH2CH2 | 2.09 | 2.09 | 1.88 | 1.90 | 1.91 | 1.76 |
| 2.07 | 2.11 | 1.87 | 1.88 | 1.91 | 1.77 | |||
| 15f | [108] | CH2CH2 | 2.09 | 2.11 | 1.89 | 1.88 | 1.89 | 1.75 |
| Ref. | MLn | Ge–N (a) | Ge–O (b) | Ge–O (b’) | Ge–O (c) | Ge–O (d) | Ge–O (e) | |
|---|---|---|---|---|---|---|---|---|
| 17a | [103] | La(H2O)4 | 2.10 | 1.93 | 1.93 | 1.86 | 1.81 | 1.80 |
| 17b | [113] | Nd(H2O)4 | 2.10 | 1.93 | 1.92 | 1.86 | 1.81 | 1.80 |
| 17c | [114] | Tb(H2O)3 | 2.10 | 1.93 | 1.92 | 1.87 | 1.81 | 1.81 |
| 17d | [115] | Tm(H2O)3 | 2.10 | 1.93 | 1.91 | 1.87 | 1.81 | 1.81 |
| 17e | [114] | Yb(H2O)3 | 2.10 | 1.93 | 1.92 | 1.86 | 1.81 | 1.81 |
| 17f | [116] | Cu(H2O) | 2.09 | 1.91 | 1.91 | 1.84 | 1.90 1 | 1.79 |
| Ref. | L,R,R′ | C-Sn-C | Sn–N | Sn–O 1 | Sn–O 2 | Sn–O(L) | Sn⋯O | |
|---|---|---|---|---|---|---|---|---|
| 18a | [119] | -,H,Me | 134.3 | 2.27 | 2.17 | 2.17 | n/a | n/a |
| 18b | [120] | -,mTol,nBu | 132.4 | 2.23 | 2.13 | 2.14 | n/a | n/a |
| 18c | [121] | H2O,H,Me | 161.1 | 2.29 | 2.20 | 2.37 | 2.38 | 2.79 |
| 18d | [122] | H2O,Me,Me | 162.5 | 2.36 | 2.19 | 2.35 | 2.36 | 2.74 |
| 18e | [123] | MeOH,H,Me | 159.5 | 2.28 3 | 2.16 | 2.35 | 2.37 | 2.95 |
| 18f | [124] | H2O,H,nBu | 162.7 | 2.32 | 2.21 | 2.35 | 2.36 | 2.77 |
| 18g | [125] | H2O,H,nBu | 163.4 | 2.30 | 2.20 | 2.33 | 2.31 | 2.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidel, A.; Wagler, J. Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry. Molecules 2025, 30, 834. https://doi.org/10.3390/molecules30040834
Seidel A, Wagler J. Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry. Molecules. 2025; 30(4):834. https://doi.org/10.3390/molecules30040834
Chicago/Turabian StyleSeidel, Anne, and Jörg Wagler. 2025. "Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry" Molecules 30, no. 4: 834. https://doi.org/10.3390/molecules30040834
APA StyleSeidel, A., & Wagler, J. (2025). Anions of α-Amino Acids as (O,N)-Donor Ligands in Si-, Ge- and Sn-Coordination Chemistry. Molecules, 30(4), 834. https://doi.org/10.3390/molecules30040834








