Decomposition Reaction Mechanism of Ammonium Perchlorate on N-Doped Graphene Surfaces: A Density Functional Theory Study
Abstract
:1. Introduction
2. Results and Discussion
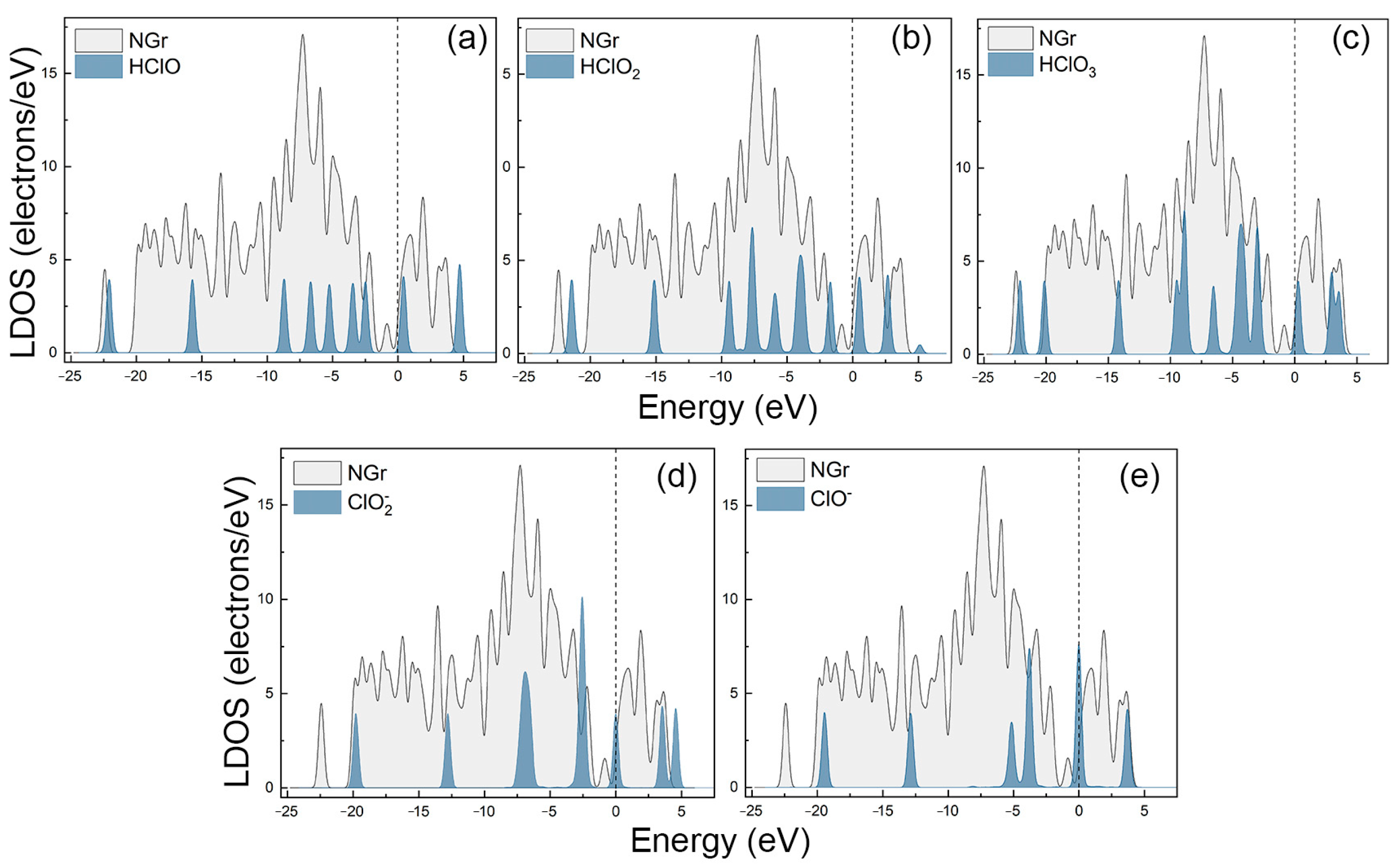
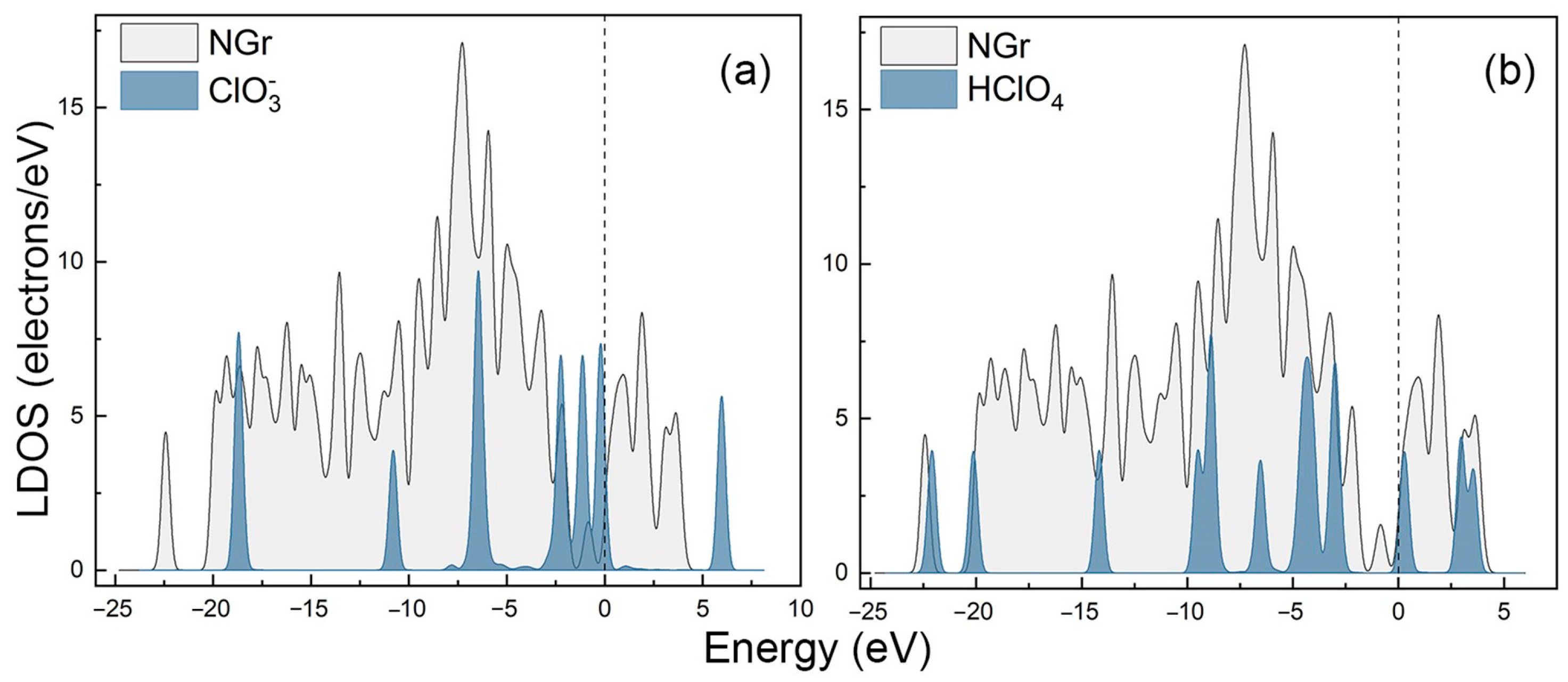
2.1. The Adsorption of HClO4 and Its Decomposition Products on the N-Gr Surface
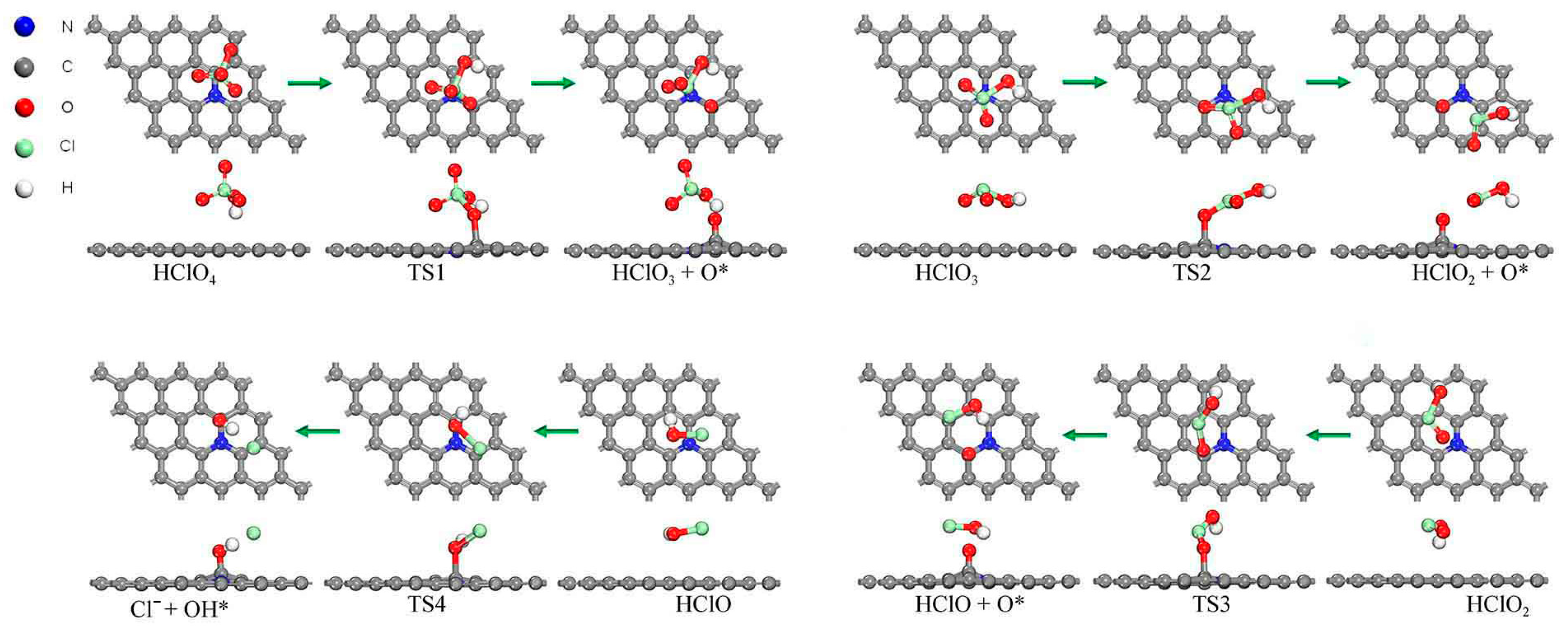
2.2. Reaction Pathway for the First Step of Losing Hydroxyl Group (Acid Root Path)
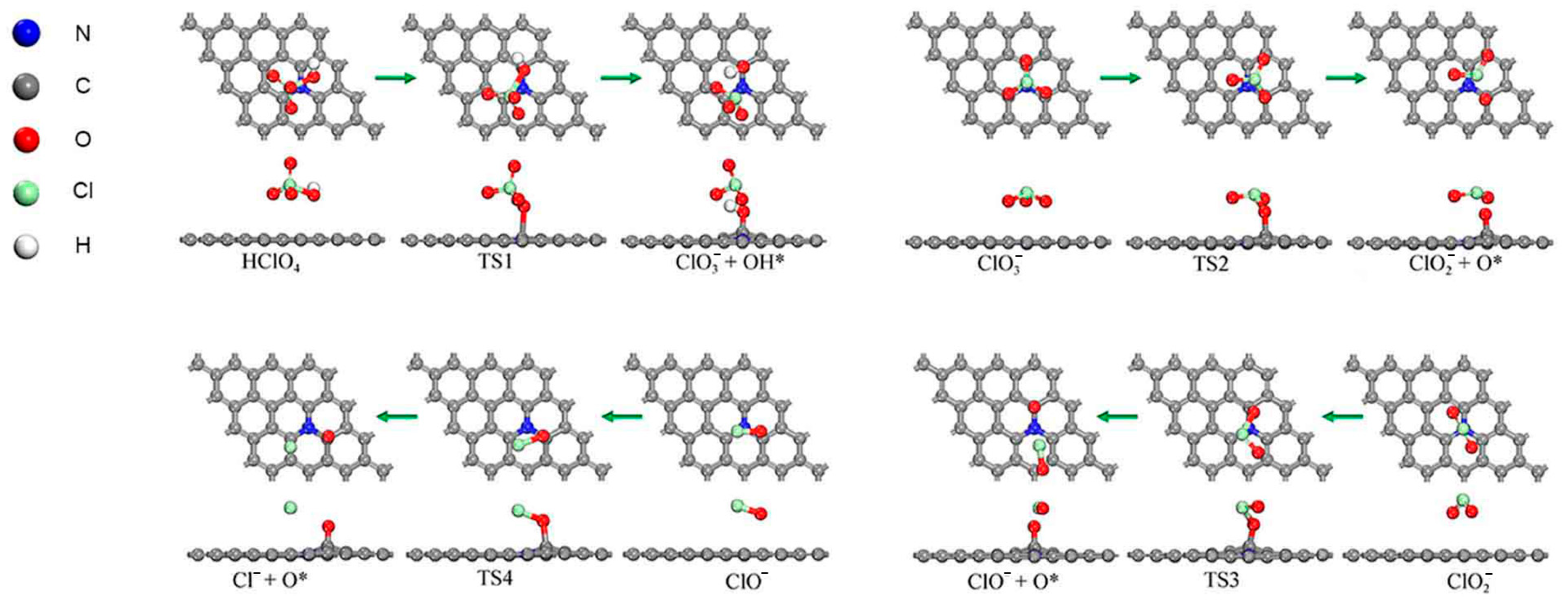
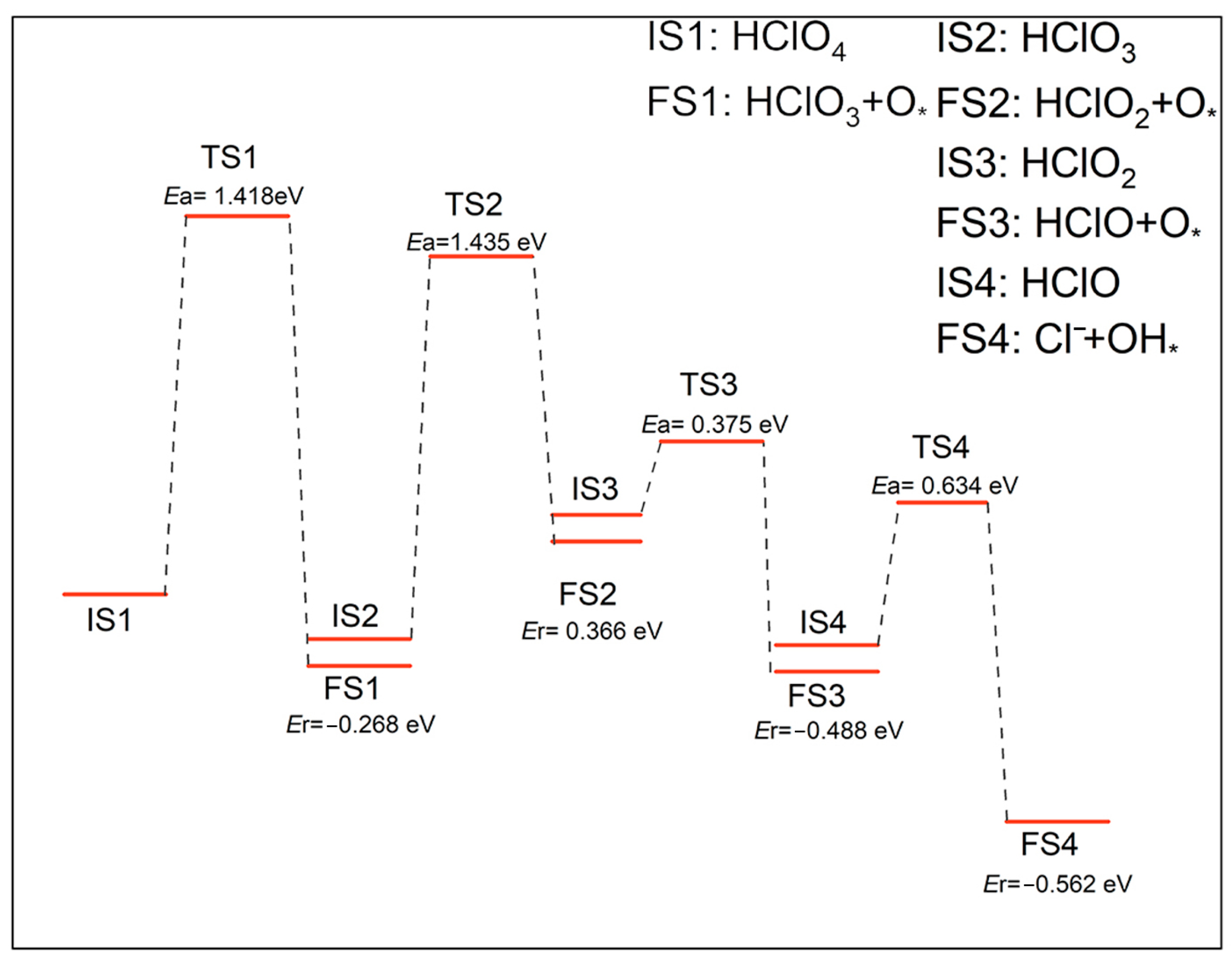
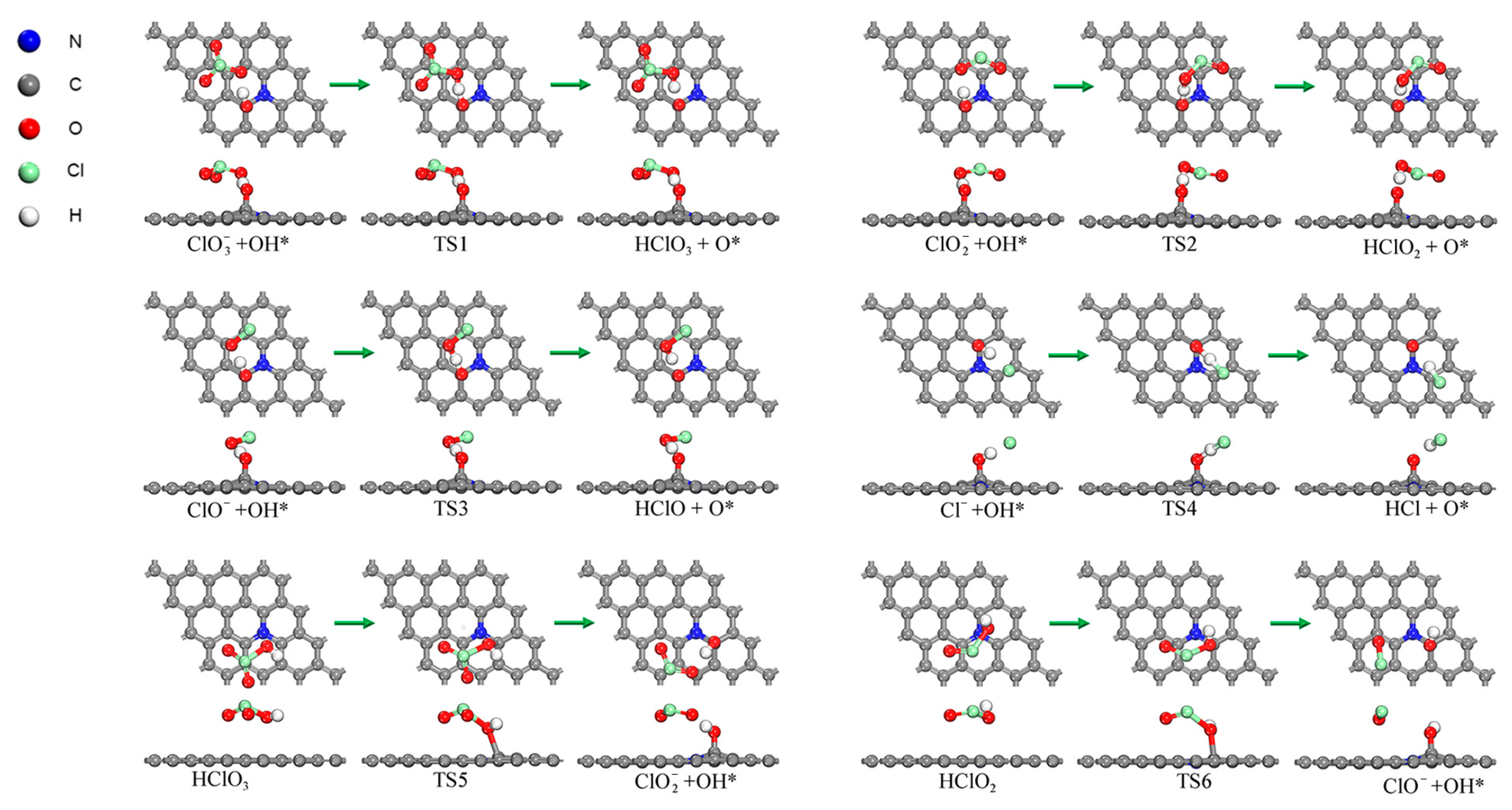
2.3. Reaction Pathway for the First Step of Losing O Atoms (Oxygenated Chlorate Pathways)
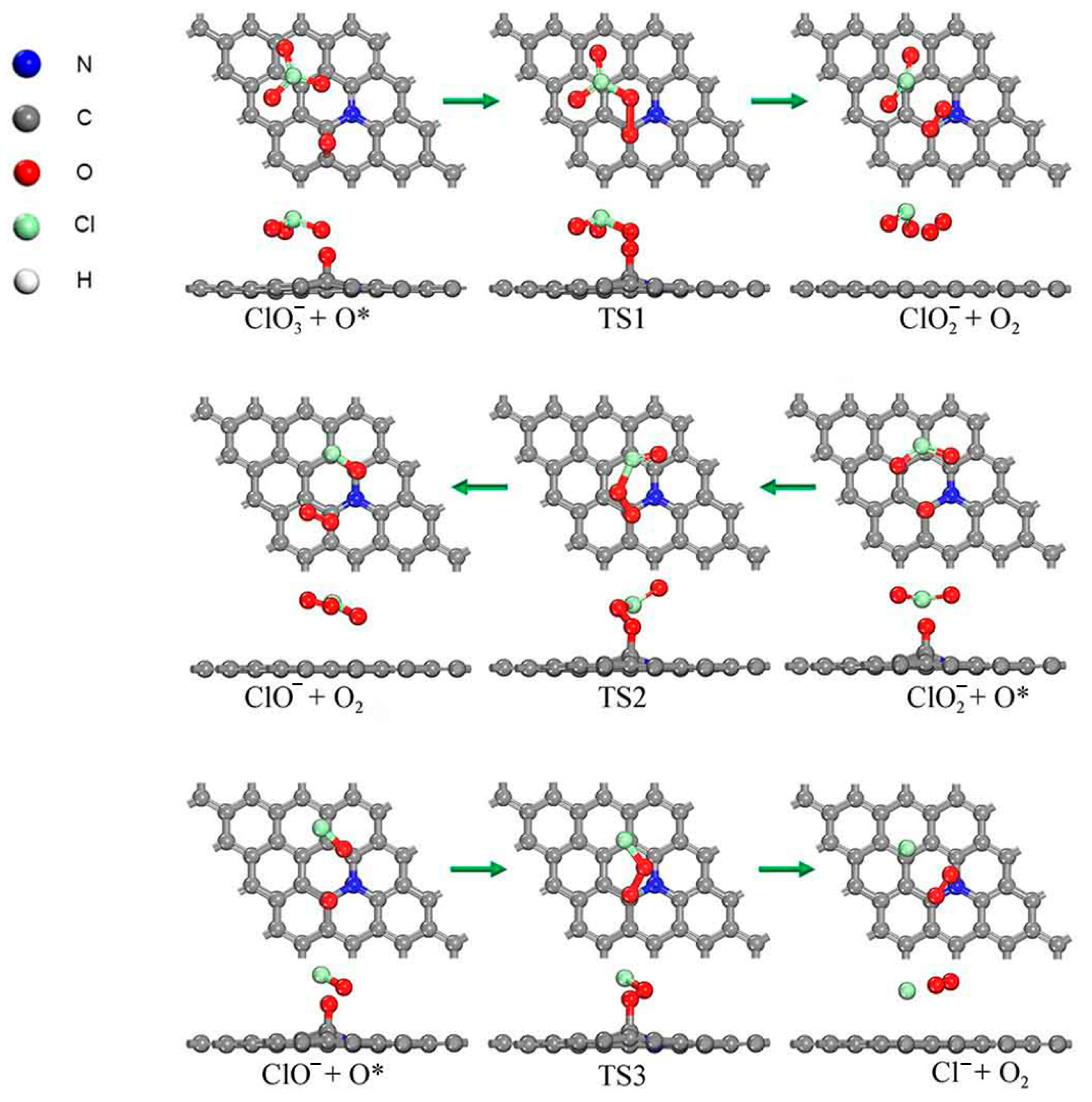
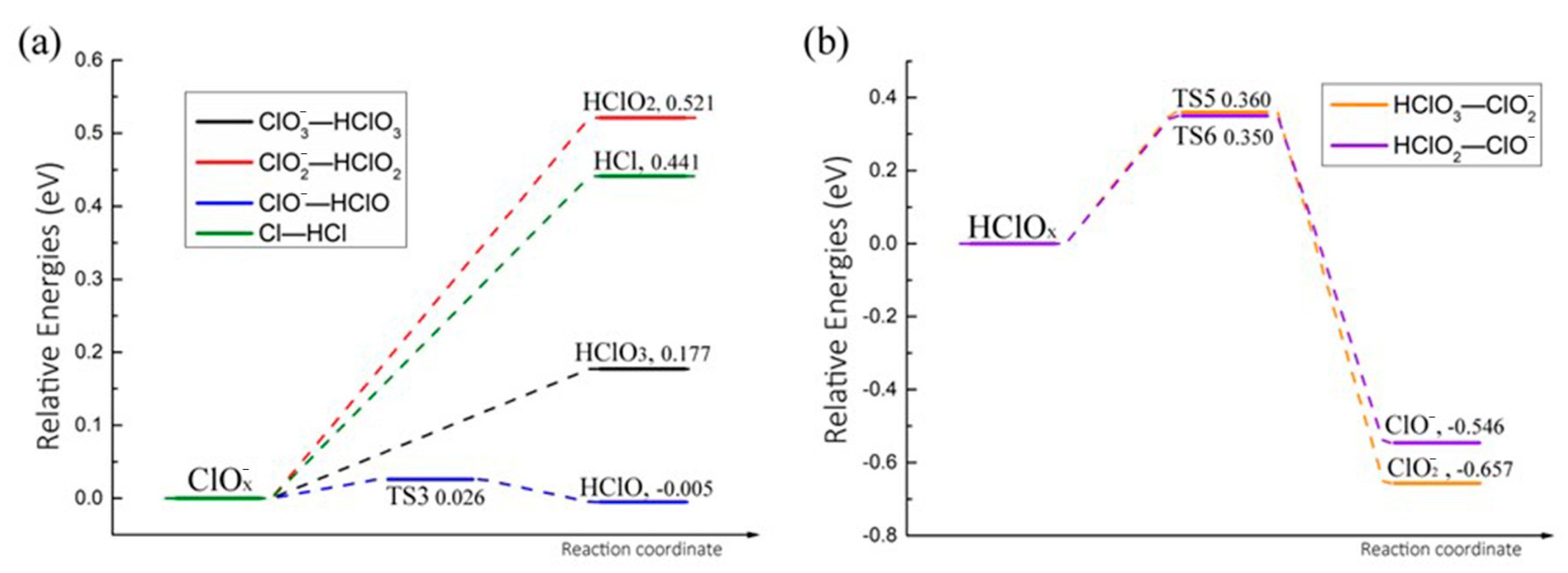
2.4. Conversion Between Two Different Paths Above
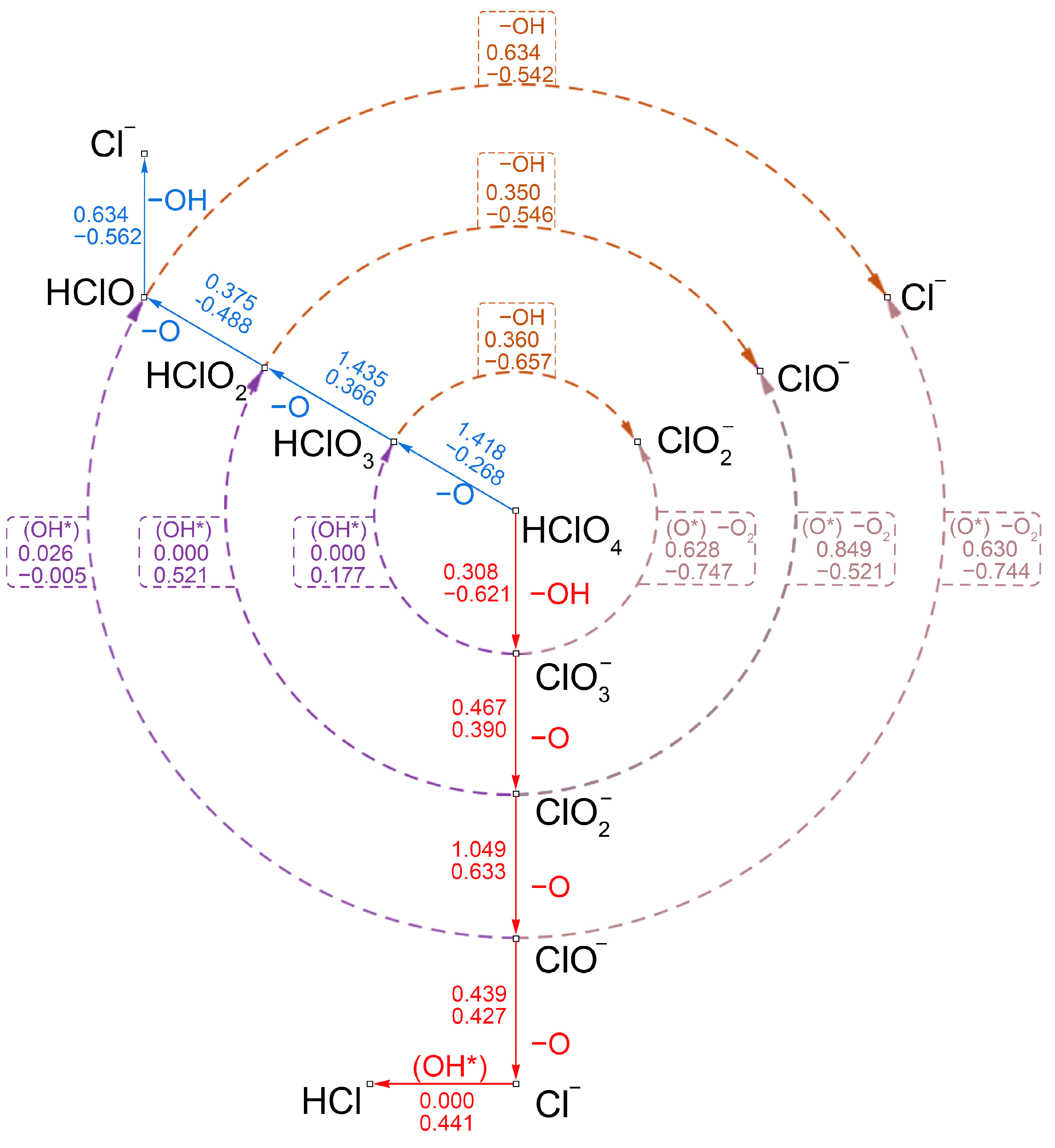
2.5. Mechanistic Analysis of the Decomposition Reaction of HClO4 on the N-Gr Surface
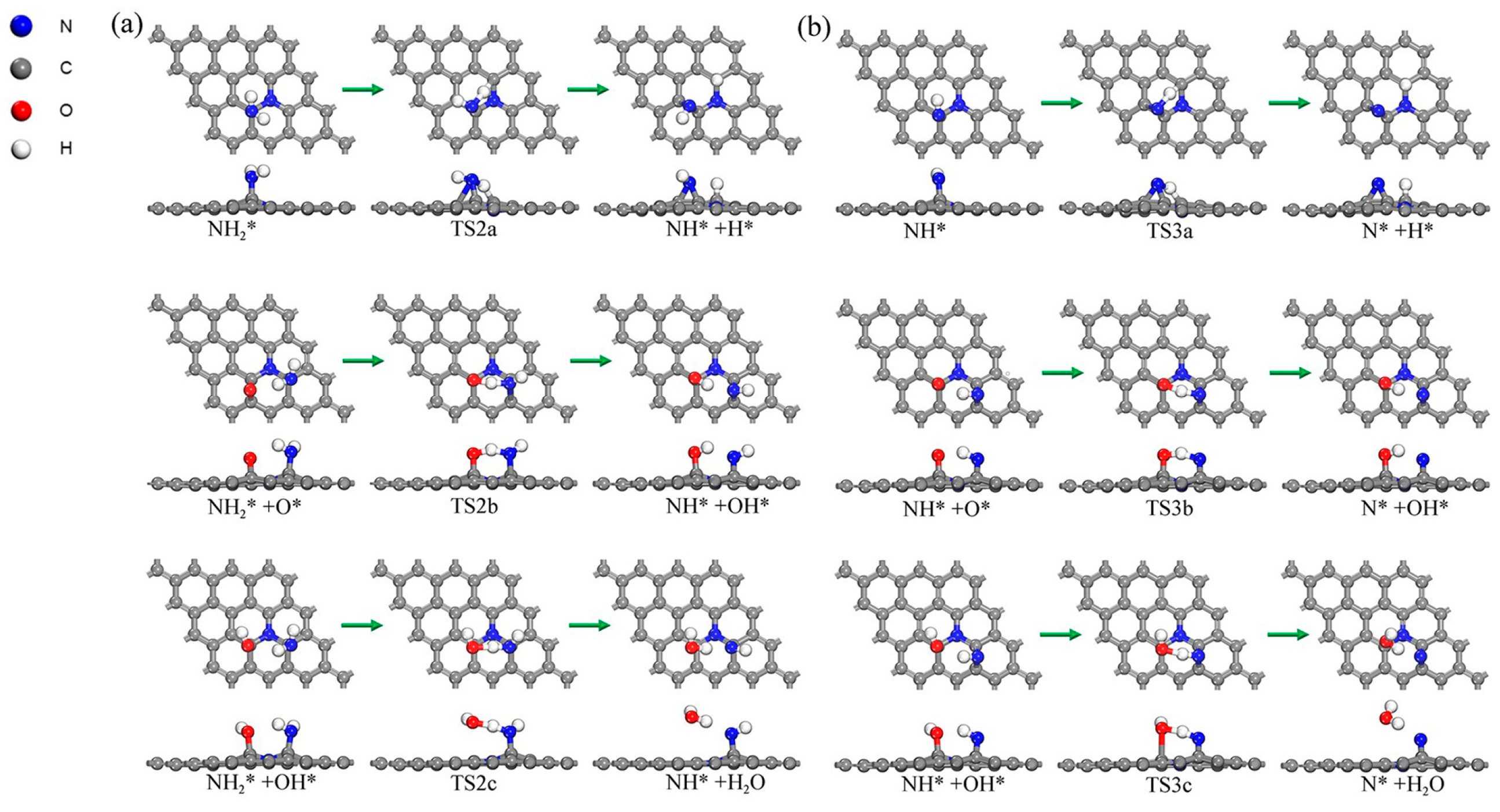
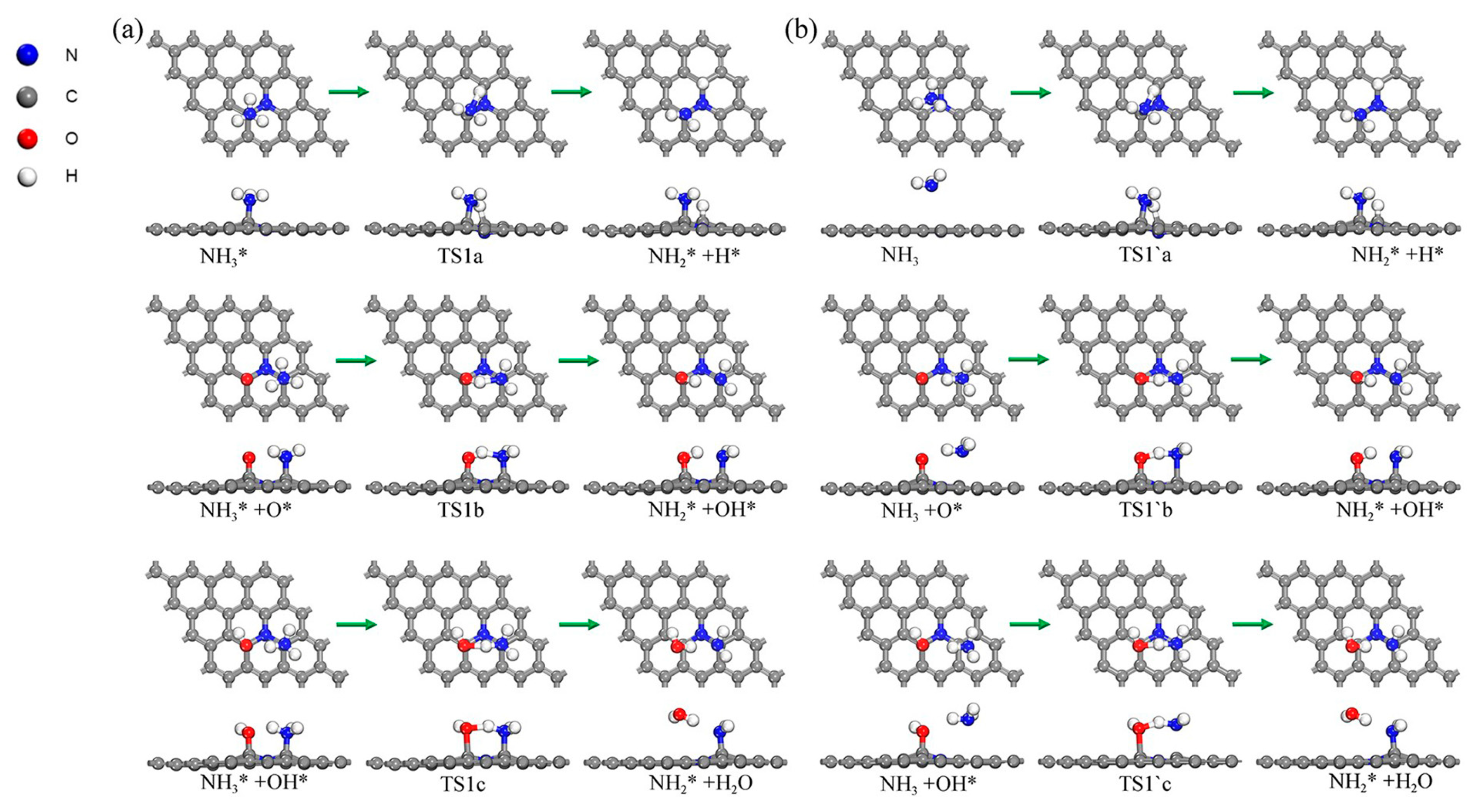
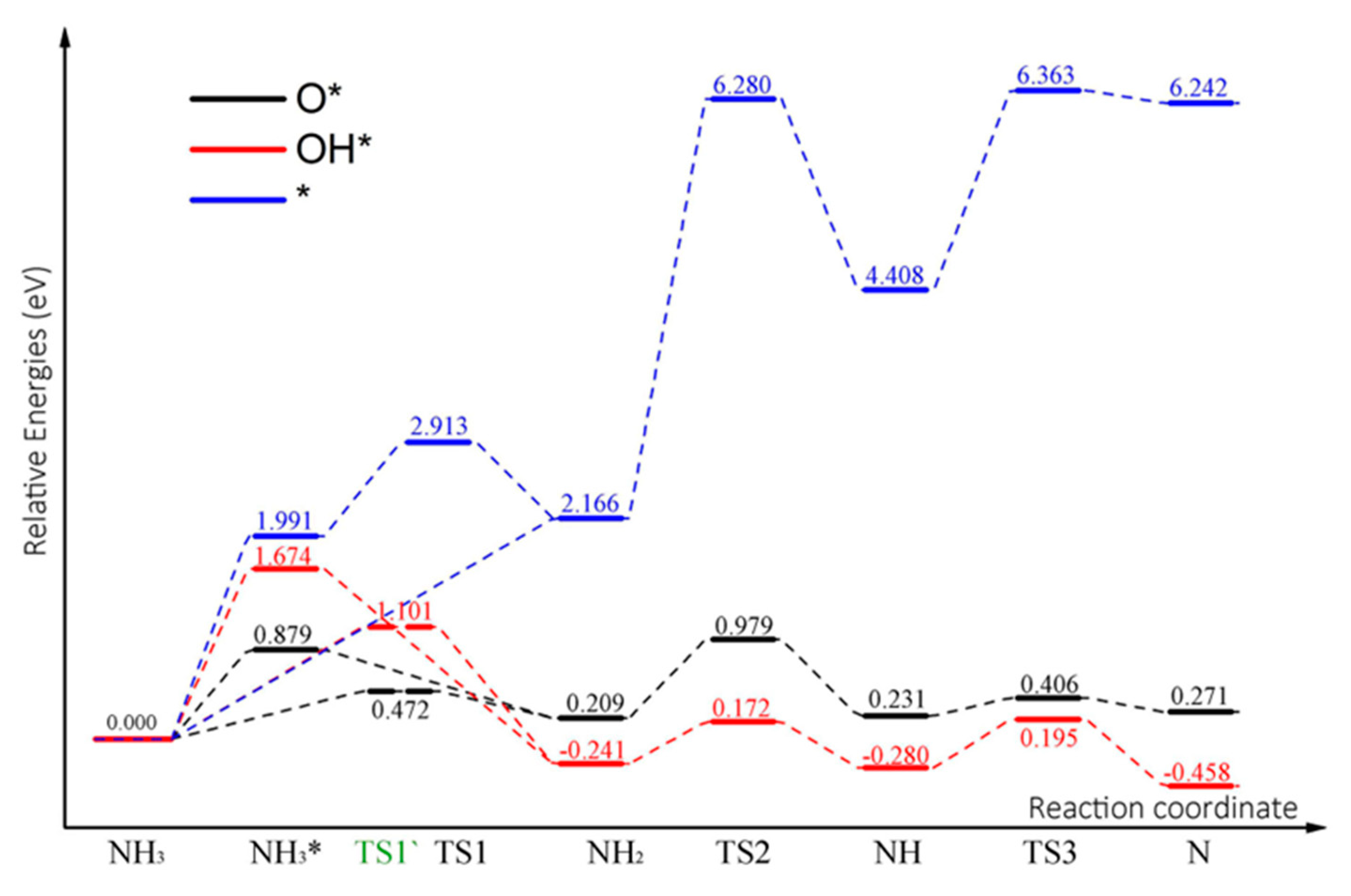
2.6. Sequential Dehydrogenation of NH3 on the N-Gr Surface
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
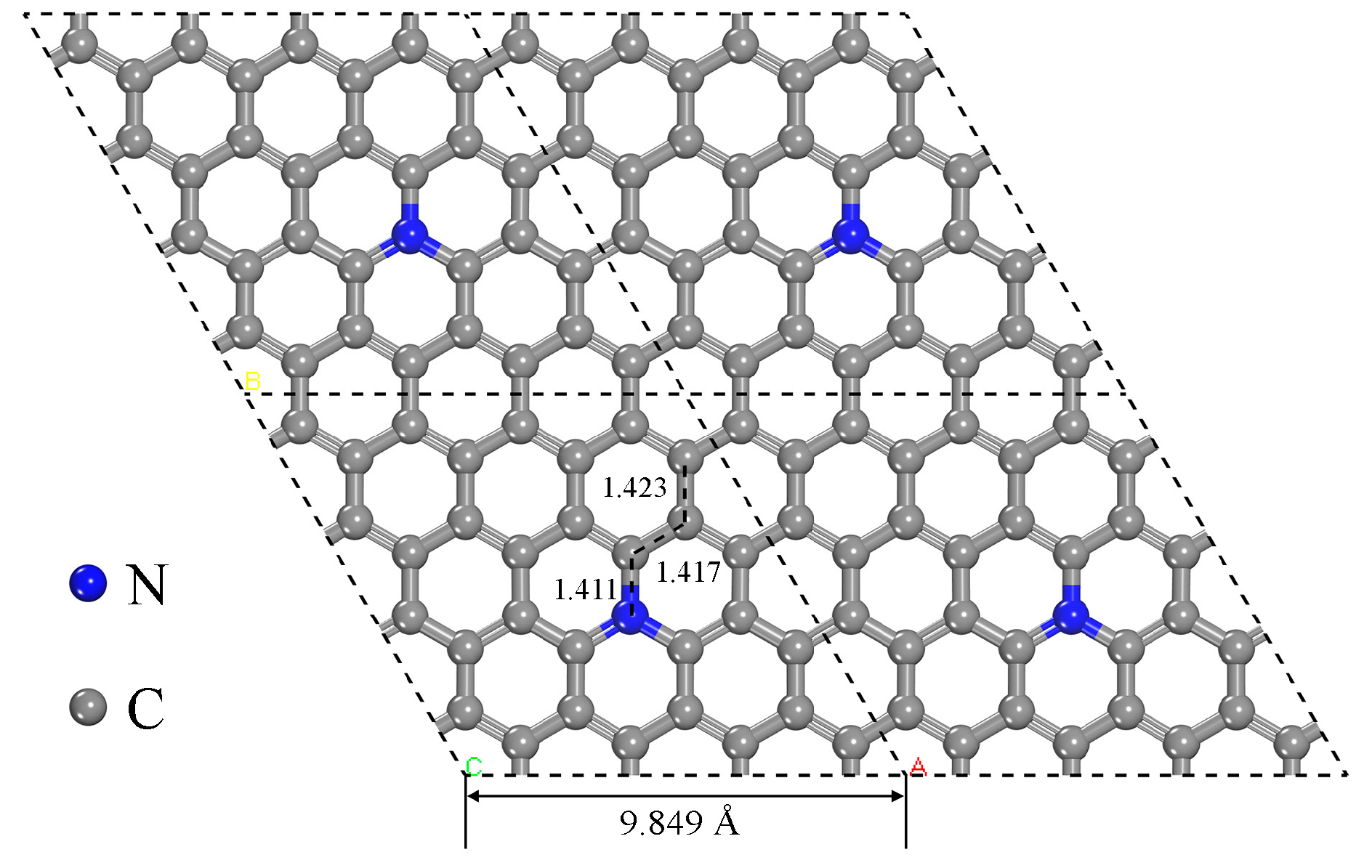
The Structure of N-Doped Graphene
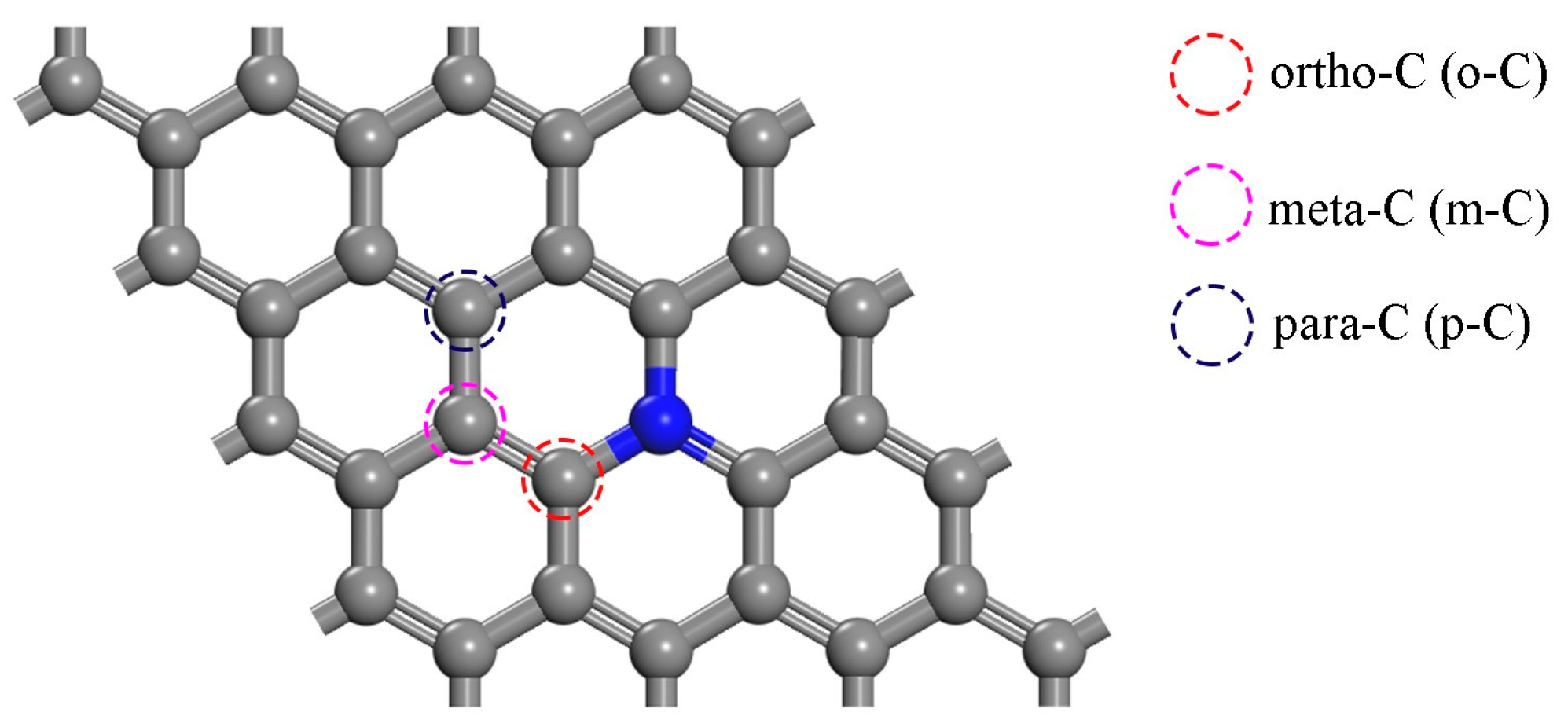
| Species | Mulliken Population Analysis (e) |
|---|---|
| o-C | 0.174 |
| m-C | −0.007 |
| p-C | 0.033 |
References
- Dong, S.; Hu, J.; Zhu, Y.Q.; Qin, Z.; Chen, S.; Zhao, F.Q.; Xu, K.Z. Thermal recombination strategy designed R-CN as active shell to improve catalyst activity. Surf. Interfaces 2024, 51, 104639. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Meng, C.G. Facile fabrication of Fe3O4 and Co3O4 microspheres and their influence on the thermal decomposition of ammonium perchlorate. J. Alloys Compd. 2016, 674, 259–265. [Google Scholar] [CrossRef]
- Zhang, W.C.; Jiang, B.; Ma, X.H.; Wang, J.X.; Liu, J.Q.; Wu, R.H.; Zheng, Z.L.; Liu, J.P.; Ma, K.F. Controllable synthesis of multi-shelled NiCo2O4 hollow spheres catalytically for the thermal decomposition of ammonium perchlorate. RSC Adv. 2019, 9, 23888–23893. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Liu, J.; Liu, H.; Xiao, L.; Qiao, Y.; Gao, H.; Jiang, W.; Zhao, F.Q. Cu–Cr–Pb nanocomposites. J. Therm. Anal. Calorim. 2016, 123, 263–272. [Google Scholar] [CrossRef]
- Razdan, M.K.; Kuo, K.K. Erosive burning study of composite solid propellants by turbulent boundary layer approach. AIAA J. 1979, 17, 1225. [Google Scholar] [CrossRef]
- Bagalkote, V.; Grinstein, D.; Natan, B. Energetic nanocomposites as burn rate catalyst for composite solid propellants. Propellants Explos. Pyrotech. 2017, 28, 136–143. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.; Liu, Z.; Li, L.; Zhao, F.; Xu, S. Thermal decomposition mechanism of Co-ANPyO/CNTs nanocomposites and their application to the thermal decomposition of ammonium perchlorate. RSC Adv. 2015, 5, 50278–50288. [Google Scholar] [CrossRef]
- Cao, S.B.; Zhou, L.Y.; Zhang, C.; Zhang, L.L.; Xiang, G.L.; Wang, J.X.; Chen, J.F. Core-shell structured AP/Fe3O4 composite with enhanced catalytic thermal decomposition property: Fabrication and mechanism study. Chem. Eng. Sci. 2022, 247, 116899. [Google Scholar] [CrossRef]
- Li, Y.; Dang, J.; Ma, Y.Q.; Ma, H.X. Hematite: A Good Catalyst for the Thermal Decomposition of Energetic Materials and the Application in Nano-Thermite. Molecules 2023, 28, 2035. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, J.Z.; Chen, S.H.; Wang, W.M.; Xu, K.Z. In situ synthesis and catalytic decomposition mechanism of CuFe2O4/g-C3N4 nanocomposite on AP and RDX. J. Anal. Appl. Pyrolysis 2021, 160, 105372. [Google Scholar] [CrossRef]
- Jain, S.; Chakraborty, S.; Qiao, L. Burn rate enhancement of ammonium perchlorate–nitrocellulose composite solid propellant using copper oxide–graphene foam micro-structures. Combust. Flame 2019, 206, 282–291. [Google Scholar] [CrossRef]
- Yan, Y.J.; Jin, B.; Peng, R.F. Simple preparation of C (CS)/g-C3N4/Co carbon aerogel and its catalytic performance for ammonium perchlorate. Def. Technol. 2024, 33, 186–196. [Google Scholar] [CrossRef]
- Hu, Y.H.; Tao, B.W.; Shang, F.; Zhou, M.X.; Hao, D.Y.; Fan, R.Q.; Xia, D.B.; Yang, Y.L.; Pang, A.M.; Lin, K.F. Thermal decomposition of ammonium perchlorate over perovskite catalysts: Catalytic decomposition behavior, mechanism and application. Appl. Surf. Sci. 2020, 513, 145849. [Google Scholar] [CrossRef]
- Cheng, J.; Shen, X.Y.; Wang, R.; Zhang, R.X.; Liu, Z.L.; Hao, Y.G.; Su, H.P. Preparation and thermal decomposition behavior of energetic functionalized graphene oxide and its catalytic effect on the thermal decomposition of AP. Chin. J. Explos. Propellants 2020, 43, 180–187. [Google Scholar]
- Eslami, A.; Juibari, N.M.; Hosseini, S.G. Fabrication of ammonium perchlorate/copper-chromium oxides core-shell nanocomposites for catalytic thermal decomposition of ammonium perchlorate. Mater. Chem. Phys. 2016, 181, 12–20. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, K.F.; Wang, J.X.; Gao, Y.; Zhu, X.F.; Zhang, W.C. Catalytic activities of two different morphological nano-MnO2 on the thermal decomposition of ammonium perchlorate. Mater. Res. Bull. 2018, 101, 56–60. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Nano-metal oxide: Potential catalyst on thermal decomposition of ammonium perchlorate. J. Exp. Nanosci. 2012, 7, 205–231. [Google Scholar] [CrossRef]
- Han, K.; Zhang, X.; Deng, P.; Jiao, Q.; Chu, E. Study of the thermal catalysis decomposition of ammonium perchlorate-based molecular perovskite with titanium carbide MXene. Vacuum 2020, 180, 109572. [Google Scholar] [CrossRef]
- Lu, T.; Chen, J.; Wang, R.; Xu, P.; Zhang, G.B.; Guo, C.P.; Yang, G.C. Bio-inspired Cu-alginate to smartly enhance safety performance and the thermal decomposition of ammonium perchlorate. Appl. Surf. Sci. 2019, 470, 269–275. [Google Scholar] [CrossRef]
- Yang, F.; Pei, J.Y.; Zhao, H.Y. First-Principles Investigation of Graphene and Fe2O3 Catalytic Activity for Decomposition of Ammonium Perchlorate. Langmuir 2022, 28, 3844–3851. [Google Scholar] [CrossRef] [PubMed]
- Inami, S.H.; Rosser, W.A.; Wise, H. Dissociation pressure of ammonium perchlorate1. J. Phys. Chem. 1963, 67, 1077–1079. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Whitehead, H.M. Decomposition and combustion of ammonium perchlorate. Chem. Rev. 1969, 69, 551–590. [Google Scholar] [CrossRef]
- Jacobs, P.W.M.; Pearson, G.S. Mechanism of the decomposition of ammonium perchlorate. Combust. Flame 1969, 13, 419–430. [Google Scholar] [CrossRef]
- Khairetdinov, E.F.; Boldyrev, V.V. The mechanism of the low-temperature decomposition of NH4ClO4. Thermochim. Acta 1980, 41, 63–86. [Google Scholar] [CrossRef]
- Cao, S.B.; Han, S.G.; Zhang, L.L.; Wang, J.X.; Luo, Y.; Zou, H.K.; Chen, J.F. Facile and scalable preparation of α-Fe2O3 nanoparticle by high-gravity reactive precipitation method for catalysis of solid propellants combustion. Powder Technol. 2019, 353, 444–449. [Google Scholar] [CrossRef]
- Chandru, R.A.; Patra, S.; Oommen, C.; Munichandraiah, N.; Raghunandan, B.N. Exceptional activity of mesoporous β-MnO2 in the catalytic thermal sensitization of ammonium perchlorate. J. Mater. Chem. 2022, 22, 6536–6538. [Google Scholar] [CrossRef]
- Zhou, L.; Cao, S.; Zhang, L.; Xiang, G.; Wang, J.; Zeng, X.F.; Chen, J.F. Facet effect of Co3O4 nanocatalysts on the catalytic decomposition of ammonium perchlorate. J. Hazard. Mater. 2020, 392, 122358. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Tian, S.Q.; Zhou, Z.X.; Wen, Y.W.; Pang, A.M.; Zhang, Y.G.; Zeng, D.W.; Li, H.; Shan, B.; Xie, C.S. ZnO Micro/Nanocrystals with Tunable Exposed (0001) Facets for Enhanced Catalytic Activity on the Thermal Decomposition of Ammonium Perchlorate. J. Phys. Chem. C 2014, 118, 11833–11841. [Google Scholar] [CrossRef]
- Zhai, P.; Shi, C.; Zhao, S.; Liu, W.; Wang, W.; Yao, L. Thermal decomposition of ammonium perchlorate-based molecular perovskite from TG-DSC-FTIR-MS and ab initio molecular dynamics. RSC Adv. 2021, 11, 16388–16395. [Google Scholar] [CrossRef] [PubMed]
- Gobi, S.; Zhao, L.; Xu, B.; Ablikim, U.; Ahmed, M.; Kaiser, R.I. A vacuum ultraviolet photoionization study on the thermal decomposition of ammonium perchlorate. Chem. Phys. Lett. 2018, 691, 250–257. [Google Scholar] [CrossRef]
- Li, Q.; He, Y.; Peng, R. Graphitic carbon nitride (g-C3N4) as a metal-free catalyst for thermal decomposition of ammonium perchlorate. RSC Adv. 2015, 5, 24507–24512. [Google Scholar] [CrossRef]
- Li, H.; Xu, S.; Song, L.; Wang, Y.; Pang, A.; Liao, G. Thermal decomposition of AP catalyzred by nano ZnO cube and its application in HIPE propellants. Chin. J. Explos. Propellants 2021, 44, 89–95. [Google Scholar]
- Wang, Y.Q.; Yao, Z.H.; Deng, S.W.; Mao, C.L.; Wang, J.G. Unravelling the reaction mechanism for the AP decomposition over MgO: A density functional theory study and microkinetic simulation. Mater. Today Commun. 2024, 39, 108658. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-Doped Carbon Materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Xu, H.; Ma, H.L.; Jin, Z. Nitrogen-Doped Graphene: Synthesis, Characterizations and Energy Applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Goudarzi, M.; Parhizgar, S.S.; Beheshtian, J. Electronic and optical properties of vacancy and B, N, O and F doped graphene: DFT study. Opto-Electron. Rev. 2019, 27, 130–136. [Google Scholar] [CrossRef]
- Esrafili, M.D. Boron and Nitrogen Co-Doped Graphene Nanosheets for NO and NO2 Gas Sensing. Phys. Lett. A 2019, 383, 1607–1614. [Google Scholar] [CrossRef]
- Doronin, S.V.; Budkov, Y.A.; Itkis, D.M. Electrocatalytic Activity of Doped Graphene: Quantum-Mechanical Theory View. Carbon 2021, 175, 202–214. [Google Scholar] [CrossRef]
- Sui, Y.; Meng, N.; Xiao, W.; Zhao, Q.; Wei, X.; Han, H. Nitrogen-Doped Graphene Aerogels as Efficient Supercapacitor Electrodes and Gas Adsorbents. ACS Appl. Mater. Interfaces 2015, 7, 1431–1438. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Khodadadipoor, Z.; Mahyari, M.; Zinab, J.M. Copper Chromite Decorated on Nitrogen-Doped Graphene Aerogel as an Efficient Catalyst for Thermal Decomposition of Ammonium Perchlorate Particles. J. Therm. Anal. Calorim. 2019, 138, 963–972. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Gholami, S.; Mahyari, M. Highly Dispersed Ni–Mn Bimetallic Nanoparticles Embedded in 3D Nitrogen-Doped Graphene as an Efficient Catalyst for the Thermal Decomposition of Ammonium Perchlorate. New J. Chem. 2018, 42, 5889–5899. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Gholami, S.; Mahyari, M. Superb Catalytic Properties of Nickel Cobalt Bimetallic Nanoparticles Immobilized on 3D Nitrogen-Doped Graphene for Thermal Decomposition of Ammonium Perchlorate. Res. Chem. Intermed. 2019, 45, 1527–1543. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Q.; Liu, H.; Shen, S.; Guo, Z.; Jin, B.; Peng, R.F. Understanding the Synergistically Enhanced Thermocatalytic Decomposition of Ammonium Perchlorate Using Cobalt Nanoparticle-Embedded Nitrogen-Doped Graphitized Carbon. Mater. Adv. 2023, 4, 2332–2339. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.R.; Zhang, Z.H.; Li, J.C.; Guo, Z.Q.; Ma, H.X. The Effect of Oxidation of Al(111) Surface on the Catalytic Efficiency to the Continuous Decomposition of Ammonium Perchlorate: A Density Functional Theory Study. Appl. Surf. Sci. 2022, 602, 154381. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.R.; Zhang, Z.H.; Zhao, Z.H.; Li, J.C.; Ma, H.X. Density Functional Theory Study on the Decomposition Mechanism of Ammonium Perchlorate on the Surface of Fluorographene. Chin. J. Explos. Propellants 2023, 46, 134–147. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Predew, P.; Bruke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1969, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, X.R.; Zhang, Z.H.; Li, J.C.; Jin, W.J.; Ma, H.X. Density Functional Theory Calculation of Reaction Pathways for AP Decomposition over g-C3N4 Surface. Surf. Interfaces 2022, 35, 102425. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate molecular van der waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 2009, 102, 0730051–0730054. [Google Scholar] [CrossRef]
- Monkhorst, J.; Pack, D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]


| Adsorption Sites | Ead/eV | d(C-O)/Å | |
|---|---|---|---|
| O a | Bridge, o, m-C | −3.225 | 1.437, 1.542 |
| Bridge, m, p-C | −2.823 | 1.487, 1.487 | |
| Top, o-C | −3.442 | 1.318 | |
| Top, N | −1.537 | 1.396 | |
| OH b | Top, o-C | −2.393 | 1.457 |
| Top, m-C | −1.77 | 1.499 | |
| Top, p-C | −1.969 | 1.494 |
| Pre-Absorbed Species | - | O Absorbed | OH Absorbed | |||
|---|---|---|---|---|---|---|
| Ea | Er | Ea | Er | Ea | Er | |
| NH3 | - | 2.166 | 0.472 | 0.209 | 1.101 | −0.241 |
| NH3,ad | 0.922 | 0.176 | - | −0.669 | - | −1.915 |
| NH2,ad | 4.114 | 2.242 | 0.770 | 0.021 | 0.412 | −0.039 |
| NHad | 1.955 | 1.834 | 0.175 | 0.040 | 0.475 | −0.178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhang, C.; Mu, X.; Li, M.; Ren, Y.; Li, J.; Zhao, F.; Ma, H. Decomposition Reaction Mechanism of Ammonium Perchlorate on N-Doped Graphene Surfaces: A Density Functional Theory Study. Molecules 2025, 30, 837. https://doi.org/10.3390/molecules30040837
Zhao Z, Zhang C, Mu X, Li M, Ren Y, Li J, Zhao F, Ma H. Decomposition Reaction Mechanism of Ammonium Perchlorate on N-Doped Graphene Surfaces: A Density Functional Theory Study. Molecules. 2025; 30(4):837. https://doi.org/10.3390/molecules30040837
Chicago/Turabian StyleZhao, Zihang, Chi Zhang, Xiaogang Mu, Meng Li, Yinghui Ren, Jiachen Li, Fengqi Zhao, and Haixia Ma. 2025. "Decomposition Reaction Mechanism of Ammonium Perchlorate on N-Doped Graphene Surfaces: A Density Functional Theory Study" Molecules 30, no. 4: 837. https://doi.org/10.3390/molecules30040837
APA StyleZhao, Z., Zhang, C., Mu, X., Li, M., Ren, Y., Li, J., Zhao, F., & Ma, H. (2025). Decomposition Reaction Mechanism of Ammonium Perchlorate on N-Doped Graphene Surfaces: A Density Functional Theory Study. Molecules, 30(4), 837. https://doi.org/10.3390/molecules30040837





